Introduction
Poor ovarian response to controlled ovarian
hyperstimulation (COH) remains a major problem in assisted
reproduction. Although no clear definition has been agreed, poor
ovarian response in the context of in vitro fertilization
(IVF) treatment is usually defined as failure to achieve ≥3 oocytes
or a certain estradiol concentration in response to ovarian
stimulation. The incidence in a population undergoing assisted
reproduction treatment is estimated to be 9–24% (1,2).
Over the years, numerous techniques and therapies have been
developed in an effort to improve success in the poor responders,
but few have met with success (3,4)
Androgens and their ovarian receptors have been
shown to play an important role in ovarian physiology and
follicular growth by numerous studies (5–7).
Recently, clinicians have attempted to improve the ovarian response
in poor ovarian responders by using androgens or androgen
modulators prior to in vitro fertilization/intracytoplasmic
sperm injection (IVF/ICSI) treatment. The main androgen treatments
involve direct androgen supplementation [including
dehydroepiandrosterone (DHEA) and testosterone] and the indirect
increase of intra-ovarian androgen levels. The latter has been
achieved through aromatase inhibitors (anastrozole and letrozole),
which are able to elevate intra-ovarian androgen levels by blocking
the conversion of androgen substrate to estrogen (8). Among the androgens and androgen
modulators mentioned above, transdermal testosterone treatment is
most attractive due to its easy application, convenience,
painlessness to the patient and most importantly its safety as, in
contrast to oral androgen treatment, androgen is not first
metabolized in the liver. The aim of the present study was to
review the current studies available on the use of transdermal
testosterone in poor ovarian responders undergoing IVF/ICSI.
Materials and methods
Literature search
MEDLINE, EMBASE and the Cochrane library were
searched with no time limit applied to any database. To identify
relevant studies, a combination of medical subject headings (MeSH)
and text words were used as follows: i) poor ovarian response
(‘poor response’, ‘low response’ or ‘bad response’); ii) IVF
(‘in vitro fertilization’ or ‘IVF’, or ‘intracytoplasmic
sperm injection’ or ‘ICSI’); iii) COH (‘controlled ovarian
hyperstimulation’ or ‘ovarian stimulation’); and iv) testosterone
(‘androgen’ or ‘testosterone’). These key words were combined using
‘AND’ to search for studies relevant to the subject under
investigation. The Chinese Biomedical Literature Database (CBM) was
also searched using the same keywords in the Chinese language. In
addition, the reference lists of the studies to be included were
individually searched in order to identify other potentially
eligible studies. Studies published in languages other than English
and Chinese were not considered. The study selection was undertaken
by two of the authors (Xiaohong Li and Lang Qin).
Study selection and data extraction
Studies that were randomized controlled trials
(RCTs) and which compared the clinical outcomes of the pretreatment
of poor ovarian responders, with or without transdermal
testosterone, prior to the IVF/ICSI cycle were selected. No
pre-specified definition for low response was used for the search
strategy in order to include all potentially relevant published
studies and capture the full range of diagnostic criteria. Studies
were selected in two steps. First, the titles and abstracts from
the electronic searches were analyzed independently by the two
reviewers. Full papers of all citations that were likely to meet
the predefined selection criteria were also obtained. In the second
step, the final decisions of whether to include or exclude the
study were made upon examination of the full manuscript. If there
were duplicate studies, the study that reported more detailed data
was included for analysis.
The two reviewers completed the data extraction and
quality assessment. Selected studies were evaluated for
methodological quality using standard forms adapted from the
Cochrane Handbook for Systematic Reviews of Interventions (9) and the quality of reporting of
meta-analyses checklist (10).
When necessary, the reviewers wrote to the authors and obtained
extra information and raw data. Outcome data were extracted from
each study in 2×2 tables.
Definition of outcomes measured
Outcomes measured included: i) the live birth rate;
ii) the clinical pregnancy rate; iii) the number of oocytes
retrieved; iv) total follicle-stimulating hormone (FSH) dose and
total duration of ovarian stimulation; v) the cancellation rate;
and vi) any adverse effects of testosterone. The rates of live
birth, clinical pregnancy and cancellation were calculated on the
basis of the number of initiated cycles.
Risk of bias in individual studies
To ascertain the validity of each study, the
selected trials were assessed for their methodological quality on
the basis of information reported in the original published papers.
The study quality was analyzed by checking the adequacy of
randomization, comparability of the groups at baseline (examining
baseline characteristics for any substantial differences), the use
of blinding, the completeness of the follow-up, and the reliability
with a priori sample size estimation. The level of evidence for
primary research was evaluated in five grades (11): level 1, high-quality RCT with
either statistically significant differences or no statistically
significant differences but with narrow confidence intervals; level
2, lesser quality RCT (e.g. <80% follow-up, no blinding, or
improper randomization), or prospective comparative study; level 3,
case control study or retrospective comparative study; level 4,
case series; level 5, expert opinion.
Statistical analysis
Dichotomous outcomes were expressed as a risk ratio
(RR) with 95% confidence interval (CI) using a fixed effects model.
Continuous outcomes were expressed as the mean difference (MD) with
95% CI. The treatment effects were statistically evaluated using
the I2 statistic to quantify heterogeneity across
studies (12). Statistical
analyses were performed using RevMan 5.0 software (Cochrane
Collaboration, Oxford, UK).
Results
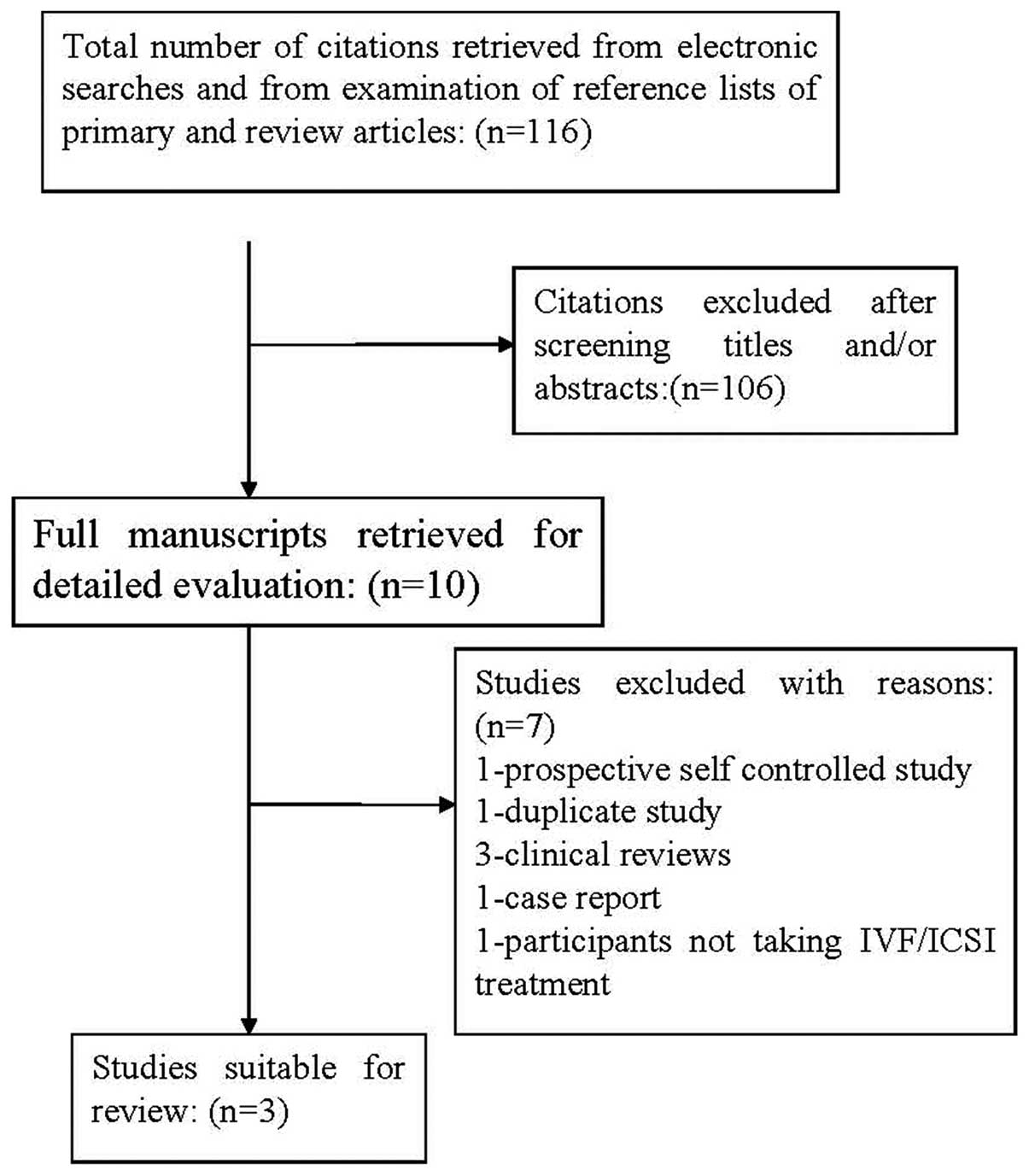
Identification of articles
The combined searches identified 116 studies for
review. The titles and abstracts of the citations were
cross-checked and duplicates were removed by the investigators
independently. Studies were removed at initial screening based on
the title or abstract if it could be determined that the
publication did not meet the inclusion criteria. Ten manuscripts
were selected during the initial screening. By examination of the
full text, seven studies were excluded: one was a prospective
self-controlled study (13), one
was a duplicate study (14,15),
three were clinical reviews (16–18),
one was a case report (19) and
one included females not undergoing IVF/ICSI (20). The flow chart for selection of the
studies included is given in Fig.
1.
Data synthesis
From the identified studies, three RCT studies were
deemed suitable for review (14,21,22).
The main characteristics of the three studies are presented in
Table I. These include: ‘poor
ovarian responder’ inclusion and exclusion criteria, baseline
characteristics, comparison groups, transdermal testosterone dosage
and duration, COH protocol and clinical outcomes. Baseline results
for a total of 221 participants were included across all studies,
with the number of participants in a single study ranging from 49
to 110 individuals. All three studies recruited females with a
history of poor ovarian response in previous IVF/ICSI cycles. The
mean age of participants was 36–37 years. The main type of
transdermal testosterone used was a transdermal testosterone gel or
patch, which was applied prior to the COH. All three studies
reported all the above outcomes.
 | Table ISummary of study characteristics and
clinical outcomes. |
Table I
Summary of study characteristics and
clinical outcomes.
| Study (Ref.) | Design | Inclusion and
exclusion criteria (definition of poor responder) | Participants | Testosterone
protocol | COH protocol | Outcomes
reported |
|---|
| Kim et al
(21) | RCT | Inclusion:
Low responder defined as <3 oocytes retrieved in the first
IVF/ICSI cycle
Exclusion:
History of endocrine disorder | N=110
Testosterone (n=55), age=37.8±3.0
Control (n=55), age=37.9±2.9 | Case: transdermal
testosterone gel 12.5 mg/day (1.25 mg/day nominal delivery rate of
testosterone) for 21 days preceding the ovarian
stimulation
Control: none | Case: GnRH antagonist
protocol
Control: GnRH antagonist protocol | Live birth
rate
Clinical pregnancy rate
No. of oocytes
Total dose of FSH
Duration of FSH used
Cancellation rate
Adverse effects |
| Fábregues et
al (22) | RCT | Inclusion:
History of the first cycle cancelled due to poor follicular
response
Exclusion:
Age >40 years
History of previous ovarian surgery, ovarian endometriosis and
endocrine and metabolic disorders | N=62
Testosterone (n=31), age=36.5±12.5
Control (n=31), age=36.4±2.0 | Case: transdermal
testosterone with a daily single patch of 2.5 mg during the 5 days
preceding ovarian stimulation
Control: none | Cases: standard long
downregulation protocol
Controls: high dose gonadotrophins with a mini dose GnRH agonist
protocol | Live birth
rate
Clinical pregnancy rate
No. of oocytes
Total dose of FSH
Duration of FSH used
Cancellation rate
Adverse effects |
| Massin et al
(14) | RCT | Inclusion:
History of poor ovarian response to ovarian stimulation
Evidence of a decreased ovarian reserve (D3 FSH >12 IU/l, E2
>70 pg/ml and inhibin B <45 pg/ml)
Exclusion:
Age >42 years
History of previous ovarian surgery, ovarian endometriosis and
endocrine and metabolic disorders | N=49
Testosterone (n=24), age=36.9±3.8
Control (n=25), age=37.3±4.0 | Case: transdermal
testosterone gel 1 g/day (10% absorption, 10 mg of testosterone)
for 15–20 days preceding the ovarian stimulation
Control: placebo | The same GnRH agonist
protocol and the same starting dose of r FSH was used for both case
and control group | Live birth
rate
Clinical pregnancy rate
No. of oocytes
Total dose of FSH
Duration of FSH used
Cancellation rate
Adverse effects |
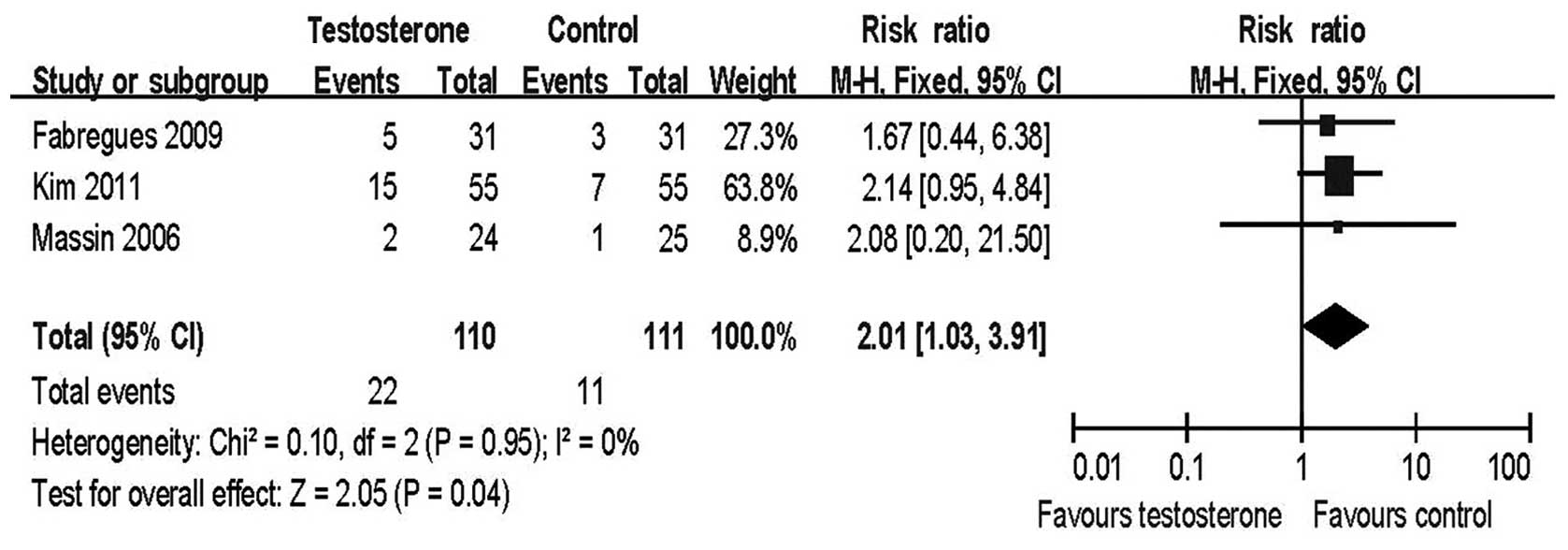
Live birth rate
The live birth rate per initiated cycle was the
index of most interest among the outcomes of IVF/ICSI treatment.
All three studies reported this result and a meta-analysis of these
studies revealed a significant improvement in the testosterone
group compared with the control group [risk ratio (RR)=2.01, 95%
confidence interval (CI) 1.03–3.91; Fig. 2].
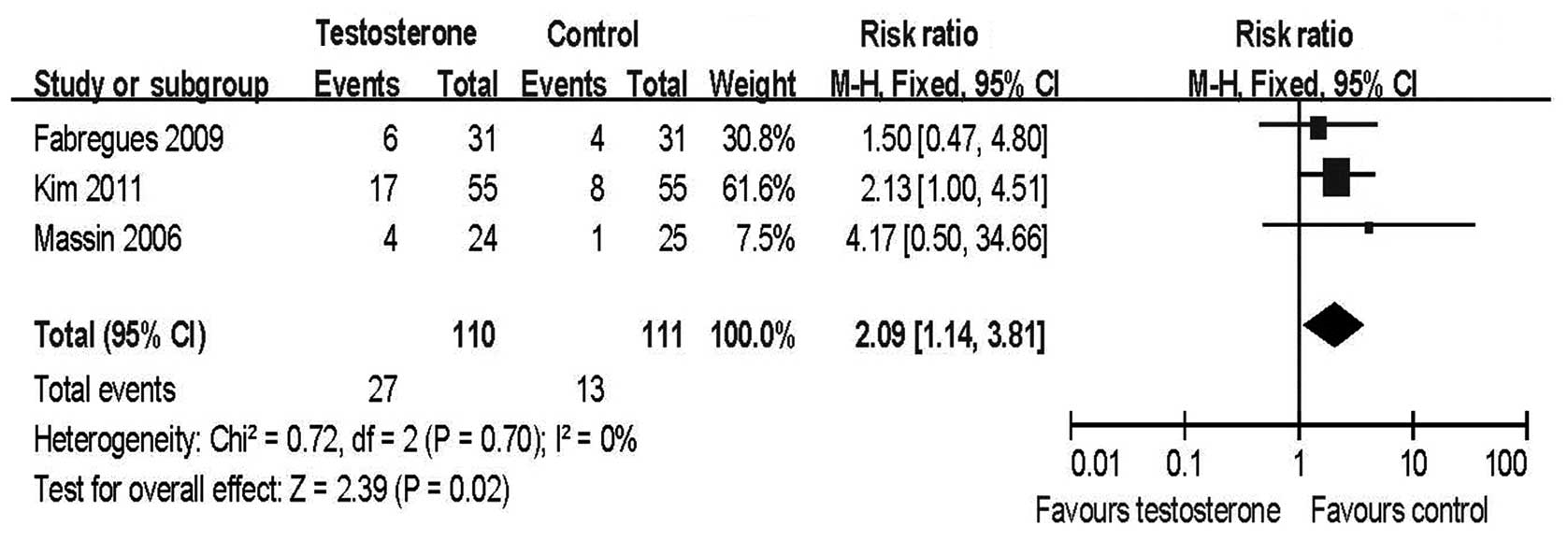
Clinical pregnancy rate
Meta-analysis of the three RCTs revealed that the
clinical pregnancy rate per initiated cycle (RR=2.09, 95% CI
1.14–3.81; Fig. 3) and per cycle
transferred (RR=1.97, 95% CI 1.10–3.52; data not shown) in the
transdermal testosterone groups was significantly higher than in
the control groups.
Number of oocytes retrieved
Low ovarian responders were characterized as those
having ≤3 oocytes retrieved despite the use of a high gonadotropin
dose in previous failed IVF/ICSI cycles. They often faced the risk
of cycle cancellation due to fewer developing follicles following
COH. Thus, the number of oocytes retrieved was the most direct
index used to evaluate the ovarian response to gonadotropin
stimulation. All three studies reported a change in the number of
oocytes retrieved and meta-analysis revealed that more oocytes were
retrieved from the testosterone pretreatment group than from the
control group [mean difference (MD)=1.36, 95% CI 0.82–1.90;
Fig. 4].
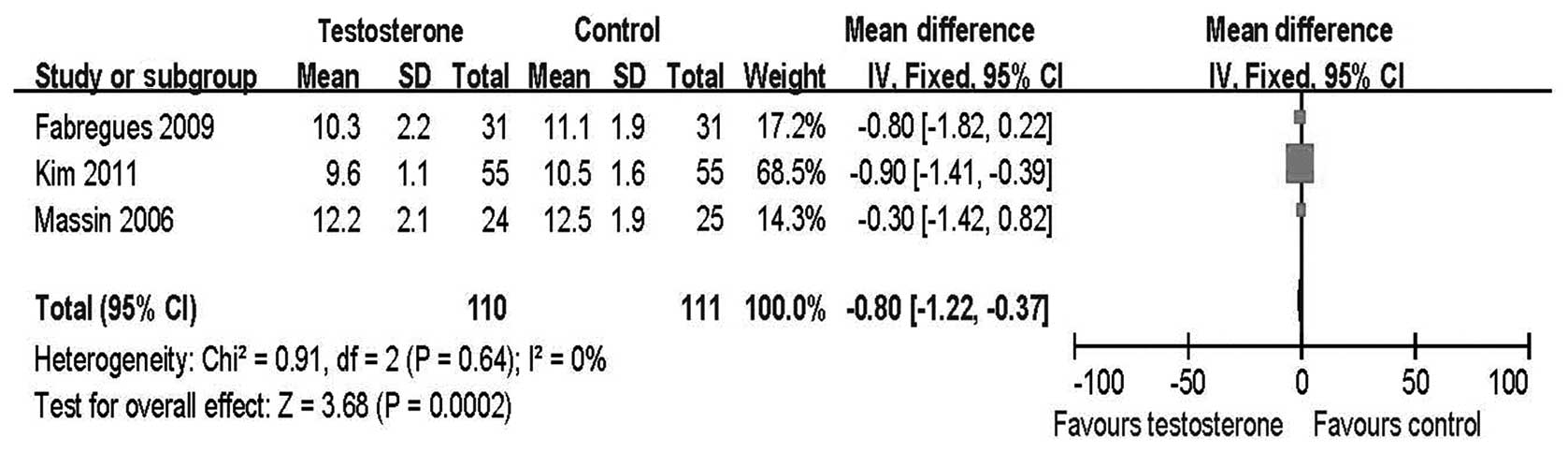
Total dose of FSH and total duration of
FSH stimulation
The total dose of FSH used and the duration of
ovarian stimulation were also effective indices used to evaluate
ovarian response. A higher dose of gonadotropins or dosing over a
longer period of time suggested a poorer ovarian response. A
significantly increased FSH dose and duration were found in the
control group compared with the treatment group [(MD=−459.46, 95%
CI −610.47 to −308.46; Fig. 5) and
(MD=−0.80, 95% CI −1.22 to −0.37; Fig.
6), respectively].
Cancellation rate
Poor ovarian responders often face an increased risk
of cycle cancellation. Meta-analysis revealed no significant
difference in cycle cancellation rates between the treatment group
and the control group (RR=0.76, 95% CI 0.42–1.36; Fig. 7). However, the criteria for the
human chorionic gonadotropin (HCG) injection varied greatly between
the studies. In the study by Massin et al, only those cycles
with at least three follicles reaching 17 mm in diameter could be
retrieved. This criteria for oocyte retrieval was more rigorous
than in the other two studies and resulted in a very high cycle
cancellation rate. This increased the weight of the study by Massin
et al in the meta-analysis and may have interfered with the
final results.
Testosterone adverse effects
No systematic or local adverse effects attributed to
the use of testosterone were reported in any of the three
studies.
Study quality, heterogeneity and
compliance
Table II
summarizes the study quality information across the three studies,
including a brief summary of the strengths and weaknesses of each
study. All three studies described the randomization methods used,
two by computer generated sequencing (21,22)
and one by a randomization list (14). One study was double-blinded
(14). Lack of blinding to the
investigators in the other two studies gave a moderate risk of
performance and detection bias in adjusting FSH dose and evaluating
subjective outcomes, for example, the number of high quality
embryos and embryos suitable for transfer. All three studies were
single-center studies. This avoided any performance bias caused by
subtle differences in clinical and IVF/ICSI laboratory techniques
among the different centers. However, the time frame of the studies
varied from 10 months to 4 years. Studies with a longer time frame
may have incurred bias originating from technical improvements in
IVF/ICSI laboratory techniques.
 | Table IISummary of study quality
characteristics. |
Table II
Summary of study quality
characteristics.
| Study | Design | Level of
evidence | Compliance | Testosterone
protocol | Strengths | Weaknesses |
|---|
| Kim et al
(21) | RCT
N=110
Testosterone (n=55)
Control (n=55)
Recruitment from 2005 to 2009 | 2 | 100% completed | Case: transdermal
testosterone gel
Control: none | RCT
High compliance
Daily dose and duration of testosterone was determined on basis of
previous study
Ovarian stimulation protocol and initial gonadotropin dose were
similar | Small sample
size
Sample size/power calculations not reported
Lack of blinding to investigator
Long time frame of study (4 years) |
| Fábregues et
al (22) | RCT
N=62
Testosterone (n=31)
Control (n=31)
Recruitment from 2006 to 2007 | 2 | 100% completed | Case: transdermal
testosterone patch
Control: none | RCT
No drop-out
Sample size/power calculated on basis of previous study
Short time frame of study (10 months) | Not blinding
design
Down-regulation and ovarian stimulation protocols were different
between the two groups
Test power decreased because of higher than expected rate of
patients undergoing ovum retrieval in control group |
| Massin et al
(14) | RCT
N=53
Testosterone (n=27)
Control (n=26)
Recruitment from 2001 to 2003 | 2 | Drop-out
rate
7.5%; n=53 recruited and randomized; results provided for n=49 | Case: transdermal
testosterone gel
Control: placebo | RCT
Double binding
Down-regulation and ovarian stimulation protocol was comparable in
the two groups | Small sample
size
Relatively high drop-out rate |
Discussion
Pretreatment of poor ovarian responders with
androgen or androgen-modulating agents has been a popular topic for
debate for many years, with one other systematic review already
published in this area. In that review (17), the author included studies which
compared the clinical outcomes between the control group and the
androgen (DHEA or testosterone) or androgen-modulating agent
(letrozole) pretreatment group. The results of the meta-analysis
did not reveal any significant differences in the number of oocytes
retrieved and the ongoing pregnancy/live birth rate with androgen
supplementation or modulation compared with the control group.
Thus, the authors concluded that there was insufficient evidence to
support the use of androgen supplementation or modulation to
improve live birth outcome in poor ovarian responders undergoing
IVF/ICSI treatment. However, due to the lack of high quality
studies available in this area at the time, the review had to use
the data of trials using different types of androgens. Therefore,
the heterogeneity of the including studies, especially the
heterogeneity in androgen supplementation protocol (including
different types, dosage and duration of androgen used), may have
weakened the confidence of the meta-analysis results to some
degree. Based on this consideration, the present study analyzed the
effectiveness of only one type of androgen on poor ovarian
responders. Transdermal testosterone has been considered an ideal
type of androgen to use in treatment due to its safety (no
supraphysiological concentrations of the steroid are released into
the body), easy application, convenience and painlessness to the
patient. Several studies (13,14,21,22)
have compared the clinical effects of poor ovarian responders with
or without transdermal testosterone pretreatment. The present
systematic review aimed to gather the existing evidence to explore
the effect of transdermal testosterone on poor ovarian responders
undergoing IVF/ICSI.
Experimental studies (5–7)
demonstrated that treatment with testosterone increased FSH
receptor expression in granulosa cells, promoting the initiation of
primordial follicle growth and improving the number of growing
preantral and small antral follicles in rhesus monkeys. These
studies suggested that androgen treatment may amplify the effects
of FSH in the ovaries. Barbieri et al (23) evaluated the baseline serum
testosterone levels of 425 females undergoing IVF and observed that
testosterone levels decreased significantly with advancing age.
There was also a positive correlation between serum testosterone
level and the number of oocytes retrieved. Frattarelli et al
(24) reported that females with
higher baseline levels of testosterone required a lower FSH dose, a
shorter duration of ovarian stimulation and were more likely to
achieve a pregnancy than females with lower testosterone levels.
Consistent with these results, the current systematic review
revealed that pretreatment with testosterone in poor ovarian
responders undergoing IVF/ICSI significantly increased their oocyte
number (MD=1.36, 95% CI 0.82–1.90) and achieve a two-fold
improvement in live birth rate per initiated cycle (RR=2.01, 95% CI
1.03–3.91) and clinical pregnancy rate per cycle (RR=2.09, 95% CI
1.14–3.81) compared with the control group. Poor ovarian responders
taking IVF/ICSI treatment often face a high risk of cycle
cancellation due to few oocytes or good quality embryos being
obtained. Thus, the number of oocytes retrieved, clinical pregnancy
rate and the live birth rate per cycle initiated were the primary
outcomes to be considered when evaluating the curative effect of
any treatment. The current study demonstrated that pretreatment
with transdermal testosterone was able to effectively improve the
clinical outcomes of poor ovarian responders. At the same time, a
lower dose of FSH (MD=−459.46, 95% CI −610.47 to −308.46) and a
shorter duration of FSH (MD=−0.80, 95% CI −1.22 to −0.37) used were
found in the transdermal testosterone group compared with the
control group. The results confirmed the effectiveness of
transdermal testosterone in improving the ovarian reactivity of
poor ovarian responders.
The current systematic review provided evidence that
pretreatment with transdermal testosterone prior to IVF/ICSI cycles
may improve the clinical outcomes of poor ovarian responders. The
validity of the present review relied on its methodological rigor
and the components of the primary studies. Great effort and an
appropriate protocol were used to gather the evidence. Two
independent reviewers extracted data and evaluated the quality of
the studies. The level of agreement between the two reviewers was
high.
A major problem frequently encountered when
selecting studies for meta-analysis is the clinical and
methodological heterogeneity of the studies. For the three studies
included in the current review, the characteristics used in the
studies, such as the definitions for poor ovarian response and the
mean ages of the participants, had relatively good homogeneity.
However, heterogeneity was still encountered including: the dose
and duration of the transdermal testosterone used, the ovarian
stimulation protocol, and certain important differences in the
quality features between the studies. In addition, the small sample
size was another limitation. Thus, the results of the current
review should be interpreted with caution. Further good quality
RCTs are required to provide more robust evidence and ensure more
reliable conclusions in the future.
References
|
1
|
Fasouliotis SJ, Simon A and Laufer N:
Evaluation and treatment of low responders in assisted reproductive
technology: a challenge to meet. J Assist Reprod Genet. 17:357–373.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shanbhag S, Aucott L, Bhattacharya S,
Hamilton MA and McTavish AR: Interventions for ‘poor responders’ to
controlled ovarian hyperstimulation (COH) in in-vitro fertilization
(IVF). Cochrane Database Syst Rev. 24:CD0043792007.
|
|
3
|
Kucuk T, Kozinoglu H and Kaba A: Growth
hormone co-treatment within a GnRH agonist long protocol in
patients with poor ovarian response: a prospective, randomized,
clinical trial. J Assist Reprod Genet. 25:123–127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kyrou D, Kolibianakis EM, Venetis CA,
Papanilolaou EG, Bontis J and Tarlatzis BC: How to improve the
probability of pregnancy in poor responders undergoing in vitro
fertilization: a systrmatic review and meta-analysis. Fertil
Steril. 91:749–766. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weil SJ, Vendola K, Zhou J, Adesanya OO,
Wang J, Okafor J and Bondy CA: Androgen receptor gene expression in
the primate ovary: cellular localization, regulation, and
functional correlations. J Clin Endocrinol Metab. 83:2479–2485.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vendola KA, Zhou J, Adesanya OO, Weil SJ
and Bondy CA: Androgens stimulate early stages of follicular growth
in the primate ovary. J Clin Invest. 101:2622–2629. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vendola K, Zhou J, Wang J, Famuyiwa OA,
Bievre M and Bondy CA: Androgens promote oocyte insulin-like growth
factor I expression and initiation of follicle development in the
primate ovary. Biol Reprod. 61:353–357. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garcia-Velasco JA, Moreno L, Pacheco A,
Guillén A, Duque L, Requena A and Pellicer A: The aromatase
inhibitor letrozole increases the concentration of intraovarian
androgens and improves in vitro fertilization outcome in low
responder patients: a pilot study. Fertil Steril. 84:82–87. 2005.
View Article : Google Scholar
|
|
9
|
Higgins JPT and Green S: Cochrane Handbook
for Systematice Reviews of Interventions Version 5.1.0. (updated
March 2011). The Cochrane Collaboration; 2011, https://training.cochrane.org/handbook.
|
|
10
|
Moher D, Cook DJ, Eastwood S, Olkin I,
Rennie D and Stroup DF: Improving the quality of reports of
meta-analyses of randomised controlled trials: the QUOROM
statement. Quality of Reporting of Meta-analyses. Lancet.
354:1896–1900. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sackett DL: Rules of evidence and clinical
recommendations on the use of antithrombotic agents. Chest. 89(2
Suppl): 2S–3S. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Balasch J, Fábregues F, Peñarrubia J,
Carmona F, Casamitjana R, Creus M, Manau D, Casals G and Vanrell
JA: Pretreatment with transdermal testosterone may improve ovarian
response to gonadotrophins in poor-responder IVF patients with
normal basal concentrations of FSH. Hum Reprod. 21:1884–1893. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Massin N, Cedrin-Durnerin I, Coussieu C,
Galey-Fontaine J, Wolf JP and Hugues JN: Effects of transdermal
testosterone application on the ovarian response to FSH in poor
responders undergoing assisted reproduction technique - a
prospective, randomized, double-blind study. Hum Reprod.
21:1204–1211. 2006. View Article : Google Scholar
|
|
15
|
Massin N, Cedrin-Durnerin I,
Galey-Fontaine J, Coussieu C, Bry-Gauillard H and Hugues JN: Is
androgen application beneficial for low responders. Hum Reprod.
19(Suppl 1): 120–121. 2004.
|
|
16
|
Feigenberg T, Simon A, Ben-Meir A,
Gielchinsky Y and Laufer N: Role of androgens in the treatment of
patients with low ovarian response. Reprod Biomed Online.
19:888–898. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sunkara SK, Pundir J and Khalaf Y: Effect
of androgen supplementation or modulation on ovarian stimulation
outcome in poor responders: a meta-analysis. Reprod Biomed Online.
22:545–555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gleicher N and Barad DH: Androgen priming
before ovarian stimulation for IVF. Hum Reprod. 23:2868–2870. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sipe CS and Van Voorhis BJ: Testosterone
patch improves ovarian follicular response to gonadotrophins in a
patient with Kallmann’s syndrome: a case report. Hum Rerod.
22:1380–1383. 2007.PubMed/NCBI
|
|
20
|
Sipe CS, Thomas MR, Stegmann BJ and Van
Voorhis BJ: Effects of exogenous testosterone supplementation in
gonadotrophin stimulated cycles. Hum Reprod. 25:690–696. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim CH, Howles CM and Lee HA: The effect
of transdermal testosterone gel pretreatment on controlled ovarian
stimulation and IVF outcome in low responders. Fertil Steril.
95:679–683. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fábregues F, Peñarrubia J, Creus M, Manau
D, Casals G, Carmona F and Balasch J: Transdermal testosterone may
improve ovarian response to gonadotrophins in low-responder IVF
patients: a randomized, clinical trial. Hum Reprod. 24:349–359.
2009.PubMed/NCBI
|
|
23
|
Barbieri RL, Sluss PM, Powers RD, McShane
PM, Vitonis A, Ginsburg E and Cramer DC: Association of body mass
index, age, and cigarette smoking with serum testosterone levels in
cycling women undergoing in vitro fertilization. Fertil Steril.
83:302–308. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fratarelli JL and Peterson EH: Effect of
androgen levels on in vitro fertilization cycles. Fertil Steril.
81:1713–1714. 2004. View Article : Google Scholar : PubMed/NCBI
|