Introduction
Oxidative stress is considered to be significant in
the pathogenesis of diabetes-induced cardiovascular disease (CVD),
which is associated with an abnormal blood lipid profile, insulin
resistance and metabolic syndrome (1). Oxidative stress occurs due to a
disturbance of the balance between antioxidant defense mechanisms
and the levels of reactive oxygen species (ROS) (2,3).
Numerous studies have identified significant alterations in plasma
antioxidant vitamins (including vitamins C, E and A), antioxidant
enzyme systems [superoxide dismutase (SOD), catalase (CAT) and
glutathione peroxidase (GSH-Px)] and in lipid peroxidation in
diabetes (4,5). In chronic diabetes mellitus (DM), the
stability and reactive capacity of antioxidants seriously affect
the long-term complications caused by oxidative stress (6,7).
Antioxidants have been shown to reduce complications in DM by
preventing free radical-mediated damage (4,8).
Dietary supplementation with antioxidants, including
vitamins and phenolic compounds obtained from plants, may help to
maintain a desirable pro-oxidative/antioxidative balance (9,10). A
variety of dietary sources are presently of considerable interest
due to their potential health benefits in relation to numerous
diseases, including diabetic disorders (11,12).
Flaxseed (also known as linseed and Linum usitatissimum), an
edible oil seed/grain from a traditional arable crop, has been
acknowledged as a functional food (13), and is the focus of considerable
attention due to its unique nutrient components and its potential
to prevent CVD (14). In addition
of being one of the richest plant sources of α-linolenic acid (ALA;
~22% of whole flaxseed), flaxseed is an essential source of lignans
(in particular, secoisolariciresinol diglucoside, SDG) (15). SDG has demonstrated extreme radical
scavenging activity, thereby protecting polyunsaturated fatty acids
from oxidation (16–18). Despite the beneficial effects of
SDG, flaxseed consumption has been found to significantly reduce α-
and γ-tocopherol concentrations in rats (19) and only vitamin E supplementation
was able to restore the plasma concentrations (20).
α-tocopherol supplementation has previously been
demonstrated to be beneficial in increasing the levels of
antioxidant enzymes such as SOD and GSH-Px, and of reduced GSH in
diabetes (21–23); however, little is known about the
effect of adding vitamin E to a flaxseed-enriched diet on oxidative
stress and aortic atherosclerotic lesions in diabetes.
In the present study, the effects of dietary
flaxseed, α-tocopherol and their combination were investigated on
atherosclerotic progression in diabetic hamsters fed a high-fat
diet by examining serum lipid concentrations, oxidative stress
markers, aortic cholesterol content and aortic histological
aspects.
Materials and methods
Animals
A total of 35 male Golden Syrian hamsters were used
in the study. The hamsters were purchased from the Cantacuzino
National Institute for Research and Development in Microbiology and
Immunology (Bucharest, Romania). This study was approved by the
Laboratory Animal Care Committee of Grigore T. Popa University of
Medicine and Pharmacy (Iaşi, Romania), and the hamsters were
maintained in accordance with the general guidelines for the care
and use of laboratory animals recommended by the Council of
European Communities (24).
The hamsters were caged singly and kept in standard
laboratory conditions with a controlled temperature (20±2°C) and a
12 h light/12 h dark cycle. Diabetes was induced in the hamsters
using streptozotocin (STZ; catalog no. S0130; Sigma-Aldrich, St.
Louis, MO, USA) (25). STZ, at a
dose of 50 mg/kg body weight, freshly solved in citrate buffer (50
mM, pH 4.5), was administered intraperitoneally, as a single dose
in the morning, following a night of fasting. Citrate buffer (50
mM) was prepared from sodium citrate and citric acid (S.C. Chemical
Company, Iaşi, Romania) dissolved in distilled water, with
adjustment of the pH to 4.5. The control group (C) received an
equivalent quantity of citrate buffer (50 mM), administered
intraperitoneally. All animals having a blood glucose level >126
mg/dl (7 mmol/l) were considered to be diabetic.
Grouping and diets
Each hamster received 15 g food/day and tap water
ad libitum. The animals were randomly divided into a control
group (C) and four diabetic groups (DM, DM + flax, DM + E and DM +
Flax + E). The hamsters received four different diets for a 20-week
period, as follows: i) Groups C and DM received a high-fat diet
(40% energy as fat), that was deficient in ALA; ii) the DM + Flax
group received a high-fat diet enriched with ground flaxseed (Linum
usitatissimum; 15/100 g of food), that was rich in ALA; Flaxseed of
the Olin variety was provided by the Department of Phytotechny of
the Faculty of Agronomy (Iaşi, Romania). iii) the DM + E group
received a high-fat diet enriched with vitamin E (40 mg
α-tocopherol/100 g of food; Sigma-Aldrich); and iv) the DM + Flax +
E group received a high-fat diet enriched with a combination of
flaxseed and vitamin E. All four diets had similar carbohydrate,
total fiber, protein and fat contents (Table I).
 | Table IComposition of the diets. |
Table I
Composition of the diets.
| Ingredients | High-fat diet (40% of
energy as fat) | High-fat diet
supplemented with flaxseed | High-fat diet
supplemented with vitamin E | High-fat diet
supplemented with flaxseed and vitamin E |
|---|
| Carbohydrates | 330 | 330 | 330 | 330 |
| Fiber | 110 | 110 | 110 | 110 |
| Proteins | 240 | 240 | 240 | 240 |
| Lipids | 160 | 160 | 160 | 160 |
| Saturateda | 96 | 96 | 96 | 96 |
| Monounsaturated | 7 | 7 | 7 | 7 |
| Polyunsaturated | 57 | 57 | 57 | 57 |
| n-6 PUFA (sunflower
oil) | 57 | 23 | 57 | 23 |
| n-3 PUFA
(flaxseedb) | - | 34 | - | 34 |
| Choline | 3 | 3 | 3 | 3 |
| Vitamin A (IU) | 200000 | 200000 | 200000 | 200000 |
| Vitamin C | 1 | 1 | 1 | 1 |
| Vitamin D (IU) | 10000 | 10000 | 10000 | 10000 |
| Vitamin E | 0.05 | 0.05 | 0.4 | 0.4 |
| Calcium | 6.2 | 6.2 | 6.2 | 6.2 |
| Phosphate | 3.9 | 3.9 | 3.9 | 3.9 |
| Potassium
bicarbonate | 20 | 20 | 20 | 20 |
Animal necropsy and processing of
samples
At the end of the experiment, the animals were
sacrificed by cardiac puncture under ketamine anesthesia (100 mg/kg
body weight). Blood samples were collected without anticoagulant.
Aliquots of serum were frozen and kept at −80°C for subsequent
analysis. The liver was immediately removed, rinsed with ice-cold
saline, placed in a sealed container, homogenized for biochemical
analysis and stored at −20°C until analyzed. The aortas were
dissected, rinsed with cold saline and preserved in a phosphate
buffer (pH 7.2).
Serum glucose
Serum glucose was measured by enzymatic colorimetric
methods on a Tecan microplate reader (Tecan SunriseTM Touchscreen,
Männedorf, Switzerland) using the AD1A716 commercially available
kits (Audit Diagnostics, Cork, Ireland).
Serum lipid profile
Serum total cholesterol (TC), high-density
lipoprotein cholesterol (HDL-C) and triglyceride (TG) levels were
measured by enzymatic colorimetric methods on a Tecan microplate
reader using the AD1A704, AD1A602 and AD1A306 commercially
available kits (Audit Diagnostics). Non-HDL-C levels were
calculated by subtracting the level of HDL-C from that of TC.
Evaluation of cholesterol content in the
aorta
The aortas of the hamsters in each group were
removed. A section of the aortic arch of each animal was soaked in
a 10% (v/v) formal saline solution and was stored on ice prior to
being stained and fixed. The remainder of the aorta was soaked in
phosphate-buffered saline (PBS) and homogenized for biochemical
analysis. The lipid content was extracted by treatment with
chloroform and methanol followed by centrifugation (26). The extracted lipid was dissolved in
ethanol and measured using a AD1A704 cholesterol assay kit (Audit
Diagnostics) utilizing absorbance spectrophotometry (Microlab 300,
Vital Scientific, Spankeren, Netherlands) at 500 nm wavelength, and
the amount quantified as μg/mg wet tissue.
Parameters of oxidative stress
Serum and liver thiobarbituric acid reactive
substances (TBARS) are indices of lipid peroxidation and oxidative
stress. TBARS were determined using the method described by Phelps
and Harris (27). The quantity of
the TBARS was measured using a Tecan microplate reader at a
wavelength of 540 nm.
Liver levels of reduced GSH were determined by an
enzymatic reaction, based on the oxidation of GSH by
5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), in the presence of GSH
reductase and methylenetetrahydrofolate reductase, monitored at a
wavelength of 405 nm (28). Liver
SOD levels were determined using the method described by Minami and
Yoshikawa (29).
Morphological study of the aorta
For evaluation of aortic atherosclerotic lesions by
light microscopy, frozen sections of 10 μm thickness were taken
from the region of the proximal aorta. The extent of
atherosclerosis was visually assessed after cutting the frozen
sections at a size of 8–10 mm, fixing them in formalin, briefly
washing them with running tap water for 1–10 mins and rinsing with
60% isopropanol prior to staining the sections with Oil Red O (3
mg/ml Oil Red O in 60% acetone and 40% water; Sigma-Aldrich). The
samples were then counterstained with hematoxylin and the samples
were examined under a light microscope (Olympus BX40, Olympus,
Tokyo, Japan).
Statistical analysis
Data are expressed as the mean ± standard deviation
(SD). Univariate statistical analysis was performed using the
Student’s t-test and Bonferroni’s multiple comparison test
(Statistical Software Package SPSS®, version 13, SPSS
Inc., Chicago, IL, USA).
Results
Serum glucose
The addition of flaxseed or/and vitamin E to the
high-fat diet led to significant reductions of serum glucose levels
compared with those in the DM group (Table II).
 | Table IISerum glucose, serum lipid profile
and aortal cholesterol content in the study groups. |
Table II
Serum glucose, serum lipid profile
and aortal cholesterol content in the study groups.
| Parameter | C | DM | DM + Flax | DM + E | DM + Flax + E |
|---|
| Serum |
| Glucose
(mg/dl) | 62±7 | 362±99a | 139±43a,b | 143±43a,b | 163±47a,b |
| TC (mg/dl) | 232±7 | 275±13a | 206±5a,b | 146±26a,b | 164±1a–c |
| TG (mg/dl) | 217±10 | 331±31a | 313±12a | 159±30a,b | 94±17a–c |
| HDL-C (mg/dl) | 78±7 | 43±5a | 81±10b | 46±7a | 34±5a |
| Non-HDL | 154±5 | 231±13a | 125±11a,b | 100±30a,b | 130±12b |
| Aortal cholesterol
(μg/mg wet tissue) | 3.6±1.4 | 4.8±1.7a | 4.1±1.9b | 4.0±0.9b | 4.2±1.5b |
Serum lipid profile parameters
Diabetes resulted in significant increases in the
serum concentrations of TC, TG and non-HDL-C, and a significant
reduction of HDL-C concentrations compared with those in the
control group. Flaxseed supplementation significantly decreased
serum TC and non-HDL-C levels and increased HDL-C levels (Table II) compared with those in the
unsupplemented DM group. The addition of vitamin E to the
flaxseed-rich diet significantly decreased TC, non-HDL-C and TG
levels but did not improved the HDL-C level in diabetic
hamsters.
Cholesterol content of the aorta
Aortic cholesterol contents were significantly
increased in the DM group fed with a standard diet compared with
those in the control group (Table
II). Flaxseed or/and vitamin E supplementation of the diet
significantly decreased the aortic cholesterol content in the
hamsters with DM (Table II).
Parameters of oxidative stress
The main findings are summarized in Table III. Serum and liver TBARS, as
indices of lipid peroxidation, were significantly increased in the
DM group compared with those in the control group, while the
addition of flaxseed or a combination of flaxseed and α-tocopherol
to the high-fat diet of the hamsters with DM significantly
decreased these two markers compared with those in the
unsupplemented DM group. Liver GSH levels were significantly
diminished in the DM group compared with those in the control
group. Flaxseed, α-tocopherol and their combination added to the
high-fat diet significantly increased the liver GSH levels in the
diabetic hamsters. The increased in liver GSH content was
significantly higher in the DM + Flax + E group compared with that
in the DM + Flax group. No significant differences of SOD activity
in the liver were identified between groups.
 | Table IIIOxidative stress parameters in the
study groups. |
Table III
Oxidative stress parameters in the
study groups.
| Parameters | C | DM | DM + Flax | DM + E | DM + Flax + E |
|---|
| Serum TBARS
(nmol/ml ser) | 1.6±0.5 | 4.3±1.4a | 1.6±0.5b | 2.6±1.0a,b | 2.5±0.9b |
| Liver TBARS
(nmol/mg prot) | 12.0±1.2 | 14.5±2.3a | 11.9±2.2b | 15.4±0.9a | 12.1±1.8b |
| Liver GSH (μM/mg
prot) | 17.3±5.6 | 10.6±2.1a | 15.5±3.3b | 20.7±4.4b | 33.6±3.9a–c |
| Liver SOD (U/mg
prot) | 12.1±0.8 | 11.3±0.7 | 12.4±1.6 | 12.9±0.9b | 12.5±0.9 |
Histological changes in aortic
tissues
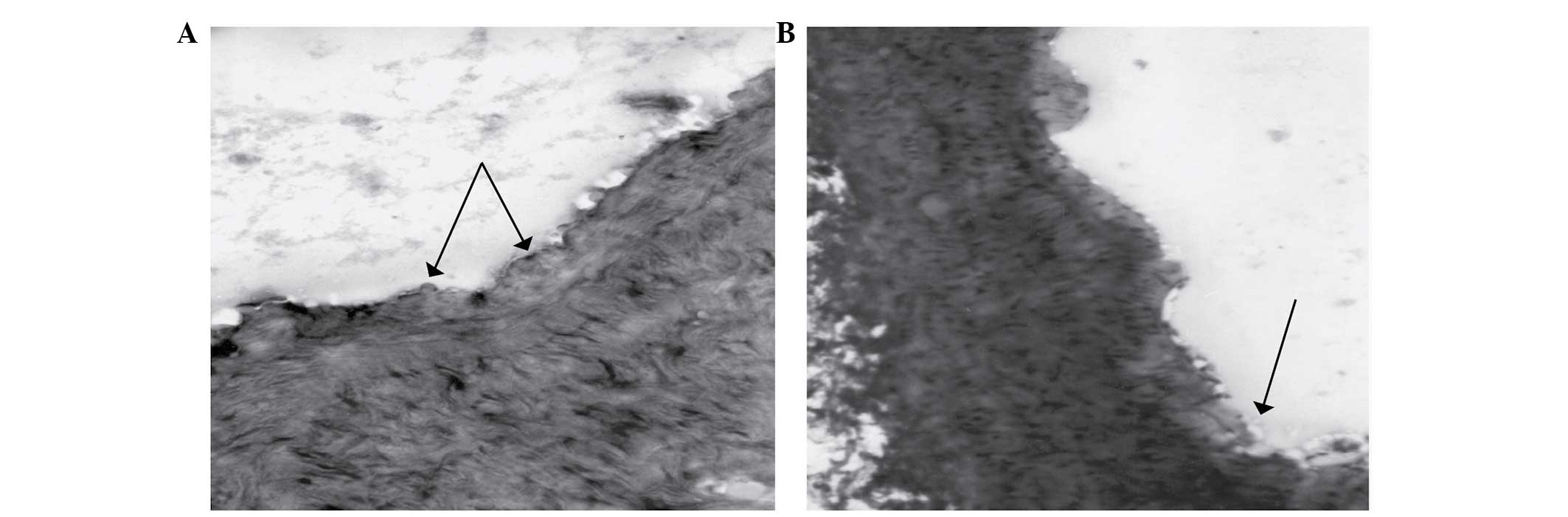
Control group
The histologic examination of aortal fragments with
O-Rd staining revealed discrete thickening of the aortic intimal
layer (Fig. 1A).
Diabetic groups
The histologic examination of aortic fragments with
O-Rd staining from diabetic hamsters revealed morphologic changes
specific to diabetic macroangiopathy (Fig. 1B). Intimal thickening, endothelial
discontinuities, lipid drops in the subintimal space, and
alterations to the middle layer of the arterial wall were observed.
The addition of flaxseed and/or vitamin E to the high-fat diet in
diabetic hamsters improved the histological aspects of aortic
atherosclerosis described in the diabetic hamsters fed an
unsupplemented diet. The aortal tissue in the supplemented groups
was characterized by slightly swollen endothelium, with moderate
intimal thickening and moderate lipid infiltration of the intima
(Fig. 2).
Discussion
In DM, oxidative stress occurs, primarily due to the
increased production of oxygen free radicals and/or a reduction in
the antioxidant defense. Numerous studies have demonstrated that an
increase in oxidative stress is implicated in the pathogenesis and
progression of diabetic vascular lesions (1,4,6).
In the present study, the antioxidative and
antiatherosclerotic effects of supplementation with flaxseed (15 g
Linum usitatissimum/100 g food), α-tocopherol (40 mg
α-tocopherol/100 g food), and their combination were investigated
in an animal model that replicates human atherosclerotic lesions.
Golden Syrian hamsters appear more predisposed to develop
atherosclerotic lesions than rats (30), and present many similarities with
the lipoprotein metabolism in humans (31). In hamsters, a 15%
flaxseed-supplemented diet is similar in energetic load to the 40
g/day dosage used in human trials. In humans, high doses of 40–50 g
flaxseed/day, which corresponds to ~10% of total energy intake have
been used in trials (32).
In the present study, it was hypothesized that
adding vitamin E to the flaxseed diet would lead to improved
results for oxidative stress and the prevention of atherosclerotic
lesions as there is evidence that the consumption of flaxseed
reduces vitamin E (19), and only
vitamin E supplementation is able to restore the plasma
concentration (20).
The present study demonstrated that all three diets
(flaxseed, α-tocopherol or their combination) had an
antiatherogenic effect in diabetic hamsters, suggested by the
prevention of histological lesions (reduced intimal lipid
infiltration and discrete alterations to the middle layer of the
arterial wall) and by the decreased aortic cholesterol content in
supplemented diabetic hamsters compared with those in the
unsupplemented diabetic group. The antiatherogenic mechanism of the
diets used in our experiment may be associated with changes in
metabolic parameters, particularly in glucose concentrations and
the lipid profile, and oxidative stress.
Flaxseed, α-tocopherol, and the combination of
flaxseed and α-tocopherol added to the high-fat diet resulted in
similar reductions in serum lipid peroxidation in the diabetic
hamsters. Liver lipid peroxidation was significantly diminished by
supplementation of the diet by flaxseed alone or in combination
with α-tocopherol, but not by α-tocopherol supplementation alone.
Moreover, all three diets increased the liver GSH content and the
highest concentrations were obtained with the combined diet.
These results suggest that the higher antioxidant
effects of flaxseed plus vitamin E are due to their synergic
antioxidant actions. Flaxseed is the richest source of the lignan
SDG. SDG isolated from flaxseed has oxygen radical-scavenging
properties that have been demonstrated in vitro by direct
hydroxyl radical-scavenging activity (18) or by inhibition of lipid
peroxidation (16). Vitamin E is
known as a major antioxidant in the cellular antioxidant defense
system, which acts by interrupting the propagation of free radical
chain reactions. The effect of vitamin E against the prooxidant
status associated with DM has been demonstrated by the suppression
of diabetes-induced increases in ROS and diminishment of lipid
peroxidation (33,34). The synergistic effect of flaxseed
oil and vitamin E on oxidative stress has also been observed by
Deng et al (35) in male
Wistar rats fed a high-fat diet.
Similar to the findings of previous studies
(36), the diabetic hamsters had
higher serum concentrations of serum glucose, TC, TG and non-HDL-C,
and lower HDL-C concentrations compared with the control group. The
addition of flaxseed, α-tocopherol or their combination to the
high-fat diet reduced serum concentrations of glucose, TC and
non-HDL in diabetic male hamsters (37,38).
The hypocholesterolemic effects of flaxseed may be attributed to
ALA, lignans (SDG) or the fiber content (39). In agreement with other studies
(40), vitamin E significantly
reduced serum TG levels in diabetic hamsters fed an atherogenic
diet. In the present study, the addition of vitamin E alone or in
combination with flaxseed to the high-fat diet resulted in lower
serum concentrations of TC, non-HDL and TG but failed to correct
the HDL-C concentrations in diabetic hamsters. Another study has
reported that in hamsters with experimental STZ-induced DM fed high
a saturated fat and cholesterol diet, supplementation with
α-tocopherol increased TC and did not change HDL-C (40).
The results of the present study suggest that the
antiatherogenic effect of flaxseed, α-tocopherol and their
combination added to a high-fat diet in diabetic hamsters is based
primarily on the antioxidative role of these components. The
improvement of serum glucose and non-HDL were evident and could
contribute to the prevention of diabetic macroangiopathy. The
antioxidant effect of flaxseed was similar to that of α-tocopherol
in diabetic hamsters fed a high-fat diet and the combined diet
(Flax + E) did not seem to confer more benefits than flaxseed
alone. Moreover, the high dose of ground flaxseed alone may have a
better cardioprotective effect than α-tocopherol in diabetic
hamsters due to the reduction of TC and non-HDL levels and increase
in HDL-C levels.
Oxidative stress has been proposed as a pathogenic
mechanism of diabetic macrovascular complications, and dietary
strategies that ameliorate oxidative stress may represent a
promising approach to the prevention of atherosclerosis.
The flaxseed-enriched diet may regulate the
generation of reactive oxygen species and the metabolism of glucose
and lipids, and could consequently play a role in the prevention of
major cardiovascular complications in diabetic states. Ground
flaxseed combined with α-tocopherol does not appear to confer a
clear advantage compared with flaxseed alone in the reduction of
oxidative stress and vascular lesion formation in experimental
diabetes.
References
|
1
|
Rains JL and Jain SK: Oxidative stress,
insulin signaling, and diabetes. Free Radic Biol Med. 50:567–575.
2011. View Article : Google Scholar
|
|
2
|
Gutteridge JM and Halliwell B:
Antioxidants: Molecules, medicines, and myths. Biochem Biophys Res
Commun. 393:561–564. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Halliwell B: The wanderings of a free
radical. Free Radic Biol Med. 46:531–542. 2009. View Article : Google Scholar
|
|
4
|
Lazo-de-la-Vega-Monroy M-L and
Fernández-Mejía C: Oxidative stress in diabetes mellitus and the
role Of vitamins with antioxidant actions. Oxidative Stress and
Chronic Degenerative Diseases - A Role for Antioxidants.
Morales-González JA: InTech; Rijeka, Croatia: pp. 209–232. 2013
|
|
5
|
Likidlilid A, Patchanans N, Poldee S and
Peerapatdit T: Glutathione and glutathione peroxidase in type 1
diabetic patients. J Med Assoc Thai. 90:1759–1767. 2007.PubMed/NCBI
|
|
6
|
Niedowicz DM and Daleke DL: The role of
oxidative stress in diabetic complications. Cell Biochem Biophys.
43:289–330. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
VanderJagt DJ, Harrison JM, Ratliff DM,
Hunsaker LA and Vander Jagt DL: Oxidative stress indices in IDDM
subjects with and without long-term diabetic complications. Clin
Biochem. 34:265–270. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tan SM, Sharma A and de Haan JB: Oxidative
stress and novel antioxidant approaches to reduce diabetic
complications. Oxidative Stress and Diseases. Lushchak V: InTech;
Rijeka, Croatia: pp. 247–280. 2012
|
|
9
|
Papageorgiou N, Tousoulis D, Katsargyris
A, et al: Antioxidant treatment and endothelial dysfunction: is it
time for flavonoids? Recent Pat Cardiovasc Drug Discov. 8:81–92.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Halliwell B, Rafter J and Jenner A: Health
promotion by flavonoids, tocopherols, tocotrienols, and other
phenols: direct or indirect effects? Antioxidant or not? Am J Clin
Nutr. 81:268S–276S. 2005.PubMed/NCBI
|
|
11
|
Sabate J and Wien M: Nuts, blood lipids
and cardiovascular disease. Asia Pac J Clin Nutr. 19:131–136.
2010.PubMed/NCBI
|
|
12
|
Calder PC: The role of marine omega-3
(n-3) fatty acids in inflammatory processes, atherosclerosis and
plaque stability. Mol Nutr Food Res. 56:1073–1080. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thompson LU and Cunnane SC: Flaxseed in
Human Nutrition. 2nd edition. AOCS Publishing; Champaign, IL: 2003,
View Article : Google Scholar
|
|
14
|
Bloedon LT and Szapary PO: Flaxseed and
cardiovascular risk. Nutr Rev. 62:18–27. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pan A, Yu D, Demark-Wahnefried W, Franco
OH and Lin X: Meta-analysis of the effects of flaxseed
interventions on blood lipids. Am J Clin Nutr. 90:288–297. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu C, Yuan YV and Kitts DD: Antioxidant
activities of the flaxseed lignan secoisolariciresinol diglucoside,
its aglycone secoisolariciresinol and the mammalian lignans
enterodiol and enterolactone in vitro. Food Chem Toxicol.
45:2219–2227. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moree SS, Kavishankar GB and Rajesha J:
Antidiabetic effect of secoisolariciresinol diglucoside in
streptozotocin-induced diabetic rats. Phytomedicine. 20:237–245.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Prasad K: Antioxidant activity of
secoisolariciresinol diglucoside-derived metabolites,
secoisolariciresinol, enterodiol, and enterolactone. Int J Angiol.
9:220–225. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Frank J, Eliasson C, Leroy-Nivard D, et
al: Dietary secoisolariciresinol diglucoside and its oligomers with
3-hydroxy-3-methyl glutaric acid decrease vitamin E levels in rats.
Br J Nutr. 92:169–176. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Flis M, Sobotka W, Antoszkiewicz Z,
Lipiński K and Zduńczyk Z: The effect of grain polyphenols and the
addition of vitamin E to diets enriched with α-linolenic acid on
the antioxidant status of pigs. J Anim Feed Sci. 19:539–553.
2010.
|
|
21
|
Peerapatdit T, Likidlilid A, Patchanans N
and Somkasetrin A: Antioxidant status and lipid peroxidation end
products in patients of type 1 diabetes mellitus. J Med Assoc Thai.
89(Suppl 5): S141–S146. 2006.
|
|
22
|
Aksoy N, Vural H, Sabuncu T, Arslanc O and
Aksoy S: Beneficial effects of vitamins C and E against oxidative
stress in diabetic rats. Nutrition Research. 25:625–630. 2005.
View Article : Google Scholar
|
|
23
|
Peerapatdit T, Patchanans N, Likidlilid A,
Poldee S and Sriratanasathavorn C: Plasma lipid peroxidation and
antioxidant nutrients in type 2 diabetic patients. J Med Assoc
Thai. 89(Suppl 5): S147–S155. 2006.
|
|
24
|
General Guidelines for the Care and Use of
Laboratory Animals recommended by the Council of European
Communities. Council instructions about the protection of living
animals used in scientific investigations. Official Journal of
European Communities. Brussels. 1–18. 1986.
|
|
25
|
Like AA and Rossini AA:
Streptozotocin-induced pancreatic insulitis: new model of diabetes
mellitus. Science. 193:415–417. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Folch J, Lees M and Sloane Stanley GH: A
simple method for the isolation and purification of total lipides
from animal tissues. J Biol Chem. 226:497–509. 1957.PubMed/NCBI
|
|
27
|
Phelps S and Harris WS: Garlic
supplementation and lipoprotein oxidation susceptibility. Lipids.
28:475–477. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tietze F: Enzymic method for quantitative
determination of nanogram amounts of total and oxidized
glutathione: applications to mammalian blood and other tissues.
Anal Biochem. 27:502–522. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Minami M and Yoshikawa H: A simplified
assay method of superoxide dismutase activity for clinical use.
Clin Chim Acta. 92:337–342. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alexaki A, Wilson TA, Atallah MT,
Handelman G and Nicolosi RJ: Hamsters fed diets high in saturated
fat have increased cholesterol accumulation and cytokine production
in the aortic arch compared with cholesterol-fed hamsters with
moderately elevated plasma non-HDL cholesterol concentrations. J
Nutr. 134:410–415. 2004.PubMed/NCBI
|
|
31
|
Simionescu MSA: Cellular and molecular
alterations in diabetes-induced cardiovascular complications
studied in an original experimental model. New Insights into
Experimental Diabetes. Cheta DM: Romanian Academy Publishing House;
Bucharest: pp. 179–209. 2002
|
|
32
|
Mocanu V, Haliga RE, Paduraru O, et al: A
diet rich in whole grain flaxseeds has antithrombotic effects
without increasing oxidative stress in experimental
atherosclerosis. Journal of Biologically Active Products From
Nature. 1:144–159. 2011. View Article : Google Scholar
|
|
33
|
Pazdro R and Burgess JR: The role of
vitamin E and oxidative stress in diabetes complications. Mech
Ageing Dev. 131:276–286. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Munteanu A, Zingg JM and Azzi A:
Anti-atherosclerotic effects of vitamin E - myth or reality? J Cell
Mol Med. 8:59–76. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Deng Q, Yu X, Xu J, et al: Effect of
flaxseed oil fortified with vitamin E and phytosterols on
antioxidant defense capacities and lipids profile in rats. J Food
Sci. 77:H135–140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lucas EA, Lightfoot SA, Hammond LJ, et al:
Flaxseed reduces plasma cholesterol and atherosclerotic lesion
formation in ovariectomized Golden Syrian hamsters.
Atherosclerosis. 173:223–229. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Haliga R, Mocanu V, Oboroceanu T, Stitt PA
and Luca VC: The effects of dietary flaxseed supplementation on
lipid metabolism in streptozotocin-induced diabetic hamsters. Rev
Med Chir Soc Med Nat Iasi. 111:472–476. 2007.PubMed/NCBI
|
|
38
|
Soltan S and Soltan AB: The Effects of
Varieties Sources of Omega-3 Fatty Acids on Diabetes in Rats. Food
Nutr Sci. 3:1404–1412. 2012. View Article : Google Scholar
|
|
39
|
Prasad K: Reduction of serum cholesterol
and hypercholesterolemic atherosclerosis in rabbits by
secoisolariciresinol diglucoside isolated from flaxseed.
Circulation. 99:1355–1362. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
El-Swefy S, Schaefer EJ, Seman LJ, et al:
The effect of vitamin E, probucol, and lovastatin on oxidative
status and aortic fatty lesions in hyperlipidemic-diabetic
hamsters. Atherosclerosis. 149:277–286. 2000. View Article : Google Scholar : PubMed/NCBI
|