Introduction
The application of medical gases in disease
treatment remains a relatively unexplored field in medicine.
However, accumulating evidence has demonstrated the attractive
achievements of medical gases, especially hydrogen gas, in the
treatment of various types of diseases including ischemic heart
disease, stroke, sepsis, acute lung injury and inflammatory bowel
disease (1–3). Gharib et al placed an animal
model of chronic infectious hepatitis into a hydrogen hyperbaric
chamber and found that the hydrogen significantly reduced liver
injury and fibrosis, improved hemodynamics and increased nitric
oxide synthase 2 and antioxidant enzyme activity (4).
Ischemia/reperfusion (I/R) injury is one of the key
issues encountered during liver transplantation. It is closely
associated with postoperative biliary complications, acute
rejection and occasionally results in fatal injury (5–7).
Therefore, decreasing I/R injury is critical for reducing
complications following liver transplantation. Vascular endothelial
cells are the main targets of I/R injury. Thus, in a preliminary
experiment of the current study, liver endothelial cells were
cultured in vitro to simulate liver transplantation I/R
injury and hydrogen treatment. In addition, the levels of hydrogen
and methane in blood samples from rats following hydrogen gas
inhalation were measured using gas chromatography and it was
successfully demonstrated that hydrogen was able to reach the
target organ. Also, apoptosis of the vascular endothelial cells was
detected using an Annexin V-PE apoptosis detection kit and it was
observed that hydrogen treatment was able to significantly reduce
the I/R injury-induced apoptosis of vascular endothelial cells.
Hydrogen is highly diffusible and may reach subcellular structures
such as nuclear and mitochondrial DNA and sites where reactive
oxygen species are present. Hydrogen may also remove hydroxyl
radicals, protect the mitochondrial membrane potential, maintain
ATP synthesis and protect the DNA in the nucleus (8). Hydrogen is very convenient for patients
to use and inhalation of hydrogen can be easily applied in clinical
practice, particularly for mechanically ventilated patients in the
perioperative period. However, the mechanisms underlying the
protective effect of hydrogen remain obscure and require
elucidation.
Therefore, in the current study, low concentrations
of hydrogen were mixed with air to evaluate its protective effect
against I/R injury associated with liver transplantation.
Materials and methods
Animals
Male Sprague-Dawley (SD) rats, weighing 250–300 g,
were provided by the Experimental Animal Center of the Third
Military Medical University (Chongqing, China). Rats were housed
with free access to food and water under a natural day/night cycle.
Rats were acclimated for seven days prior to any experimental
procedures. All experimental procedures were approved by the
Institutional Animal Care and Use Committee of the Third Military
Medical University.
Inhalation of hydrogen gas
Different concentrations of hydrogen gas (1, 2 and
3%) were established by combining hydrogen with air, and
subsequently compressed into oxygen bottles. During the process of
hydrogen administration, rats were placed into a glass container
which was connected to a pipeline. The hydrogen was administered to
the conscious rats through the pipeline at a rate of 1.5 ml/h. The
duration of hydrogen administration was 1, 3 or 6 h.
Model establishment
Immediately following the administration of
hydrogen, the rats underwent an I/R injury procedure under general
anesthesia with Sevofrane as described by Lord et al
(9). A midline incision was created
and the portal triad to the left lobe and the left middle lobe of
the liver was occluded with a vascular microclamp to induce partial
liver ischemia. Occlusion was verified visually by the color change
of the left side of the liver to a paler shade. The abdominal
muscles and peritoneum were closed with 5.0-nylon sutures.
Following 90 min of ischemia, a second laparotomy was performed to
remove the clamp. The abdomen was closed in the same manner and
followed by 180 min of reperfusion. Then, immediately after I/R
injury, syngeneic orthotopic liver transplants (OLTs) were
performed using livers that were harvested from SD rats and stored
for 18 h in University of Wisconsin (UW) solution, prior to being
transplanted into syngeneic SD recipients with revascularization
without hepatic artery reconstruction. There were three groups of
rats that received liver transplants (each n=6), namely the
hydrogen-treated donor and recipient group in which the donor and
recipient rats both received hydrogen, the hydrogen-treated donor
group in which only the donor rat received hydrogen, and the
hydrogen-treated recipient group in which only the recipient rat
received hydrogen. Right hepatic lobe tissue samples were taken at
1-h intervals following surgery and venous blood samples were
collected after 3 h. Liver tissues were placed in 4% formaldehyde
and stored in liquid nitrogen.
Hematoxylin and eosin (H&E)
staining
Fixed livers were dehydrated and embedded in
paraffin. Tissues were sectioned (4-µm thickness) and stained with
H&E.
Serum alanine aminotransferase (ALT)
and aspartate aminotransferase (AST) activity
Following clotting, each blood sample was
centrifuged at 1,100 × g for 5 min. The clear top layer was
centrifuged again under the same conditions to prepare the serum.
The activities of serum ALT and AST were examined using a Wako
Transaminase CII-Test kit (Wako Pure Chemical Industries Ltd.,
Osaka, Japan).
Quantitative polymerase chain reaction
(qPCR)
The liver tissues were lysed with TRIzol®
(Invitrogen Life Technologies, Carlsbad, CA, USA) and vigorously
mixed with chloroform for 15 sec, then stored at room temperature
for 3 min. Subsequently, they were centrifuged at 12,000 × g for 15
min at 4°C and the RNA was precipitated in the aqueous phase with
isopropanol. The upper aqueous phase was transferred to a new
microcentrifuge tube. The RNA was precipitated by adding 0.75%
ethanol and centrifuged at 12,000 × g for ≤5 min at 4°C. The
supernatant was removed and the RNA was dried at room temperature
for 5–10 min. The mRNA expression levels of zinc finger protein A20
(A20), nuclear factor κB (NF-κB), heme oxygenase-1 (HO-1) and
B-cell lymphoma 2 (Bcl-2) were determined using qPCR with
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) used as a control.
The expression levels of interleukin (IL)-6, tumor necrosis factor
(TNF)-α, early growth response protein 1 (Egr-1) and IL-1β mRNA
were also determined. The qPCR reactions were performed using an
ABI 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA,
USA) with the following conditions: 95°C, 10 min for one cycle; and
then 95°C, 15 sec, 60°C, 1 min for 40 cycles. The expression levels
of mRNA were quantified with 2−∆∆CT. The primer
sequences are listed in Table I.
 | Table I.Primers used in the quantitative
polymerase chain reaction (qPCR). |
Table I.
Primers used in the quantitative
polymerase chain reaction (qPCR).
| Gene | Forward primer | Reverse primer |
|---|
| A20 |
ACCTGTTTCAAAAGGACTACGG |
AAGGTAGCCAGAGGGGACG |
| NF-κB |
CTGCTTACGGTGGGATTGC |
TGTTTCTTTCTCAGGGGGATTC |
| HO-1 |
GCGAAACAAGCAGAACCCA |
CCACCAGCAGCTCAGGATG |
| Bcl-2 |
GTGAACTGGGGGAGGATTGT |
GCATCCCAGCCTCCGTTA |
| IL-6 |
AAGCCAGAGTCATTCAGAGCAA |
TGGATGGTCTTGGTCCTTAGC |
| TNF-α |
CTTCTCATTCCTGCTCGTGG |
ATCTGAGTGTGAGGGTCTGGG |
| Egr-1 |
CAAGGGTGGTTTCCAGGTTC |
GAAGGCTGCTGGGTACGGT |
| IL-1β |
GGGATGATGACGACCTGCTAG |
CCACTTGTTGGCTTATGTTCTGT |
| GAPDH |
CCCATCTATGAGGGTTACGC |
TTTAATGTCACGCACGATTTC |
Enzyme-linked immunosorbent assay
(ELISA)
ELISA (R&D Systems, Minneapolis, MN, USA) was
used to determine the expression levels of IL-6 and TNF-α in serum
according to the manufacturers' instructions. The absorbance was
measured at 450 nm using a microplate reader (Model 680; Bio-Rad,
Hercules, CA, USA).
Transmission electron microscopy
Liver samples were fixed in 2.5% glutaraldehyde for
2 h and then rinsed in phosphate buffer. This was followed by a
postfixation step for 2 h with 1% osmium tetroxide in phosphate
buffer at 4°C. Afterwards, samples were dehydrated and embedded in
resin. The samples were then trimmed and sectioned into slices
(50∼60 nm). Ultrastructural features were observed on a
transmission electron microscope (TEM; Philips CM120 TEM; Philips,
Amsterdam, The Netherlands) at 60 kV.
Western blot analysis
After treatment with different concentrations of
hydrogen (1, 2 and 3%) for different durations (1, 3 and 6 h),
total cell lysates were prepared and subjected to sodium dodecyl
sulfate polyacrylamide gel electrophoresis (SDS-PAGE). For western
blot analysis, the primary antibodies used included anti-A20,
anti-NF-κB, anti-HO-1, anti-Bcl-2 (Cell Signaling Technology, Inc.,
Beverly, MA, USA) and anti-GAPDH (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA). An anti-rabbit or anti-mouse secondary
antibody conjugated with horseradish peroxidase was also used
(Pierce Biotechnology, Inc., Rockford, IL, USA). Immunoreactive
bands were detected with an enhanced chemiluminescence (ECL) kit
for western blot detection using the ChemiGenius Bio Imaging System
(Syngene, Frederick, MD, USA).
Statistical analysis
Data are presented as mean ± standard error of the
mean (SEM) and were statistically analyzed using SPSS software
version 13.0 (SPSS, Inc., Chicago, IL, USA). Comparisons between
groups were made using analysis of variance (ANOVA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of hydrogen gas inhalation on
liver function
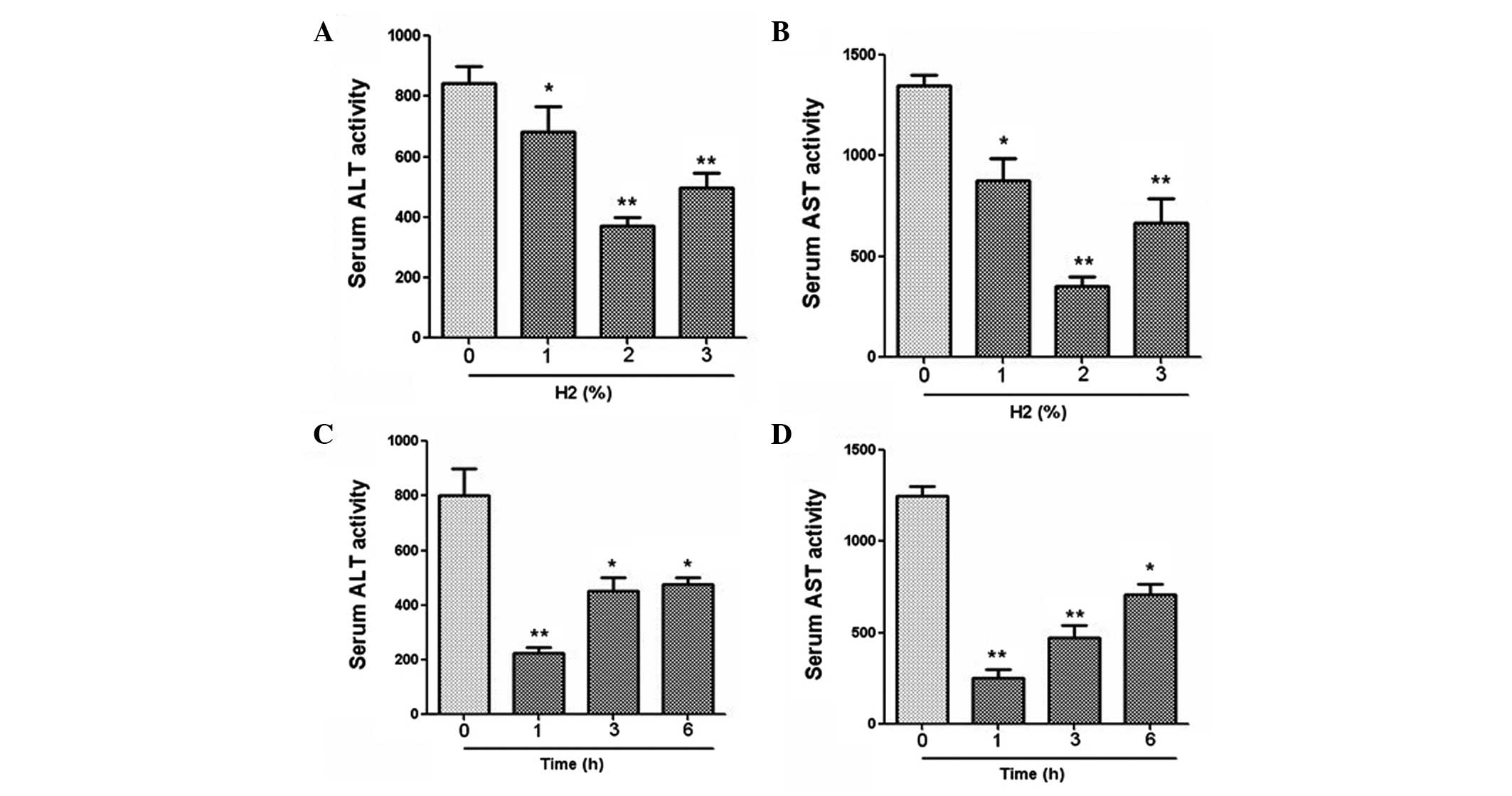
The serum activities of ALT and AST were measured by
ELISA. For rats that were administered different concentrations of
hydrogen gas (1, 2 and 3%), the results revealed that gas
inhalation at all concentrations significantly reduced the ALT and
AST activities compared with those in the control group (Fig. 1). In particular, a gas inhalation
concentration of 2% achieved the optimal effect. Furthermore, for
the rats treated with hydrogen at different time points, the
results demonstrated that 1 h administration of gas prior to
surgery led to a significant reduction in ALT and AST activities
(Fig. 1).
Effect of hydrogen gas inhalation on
cytokine expression
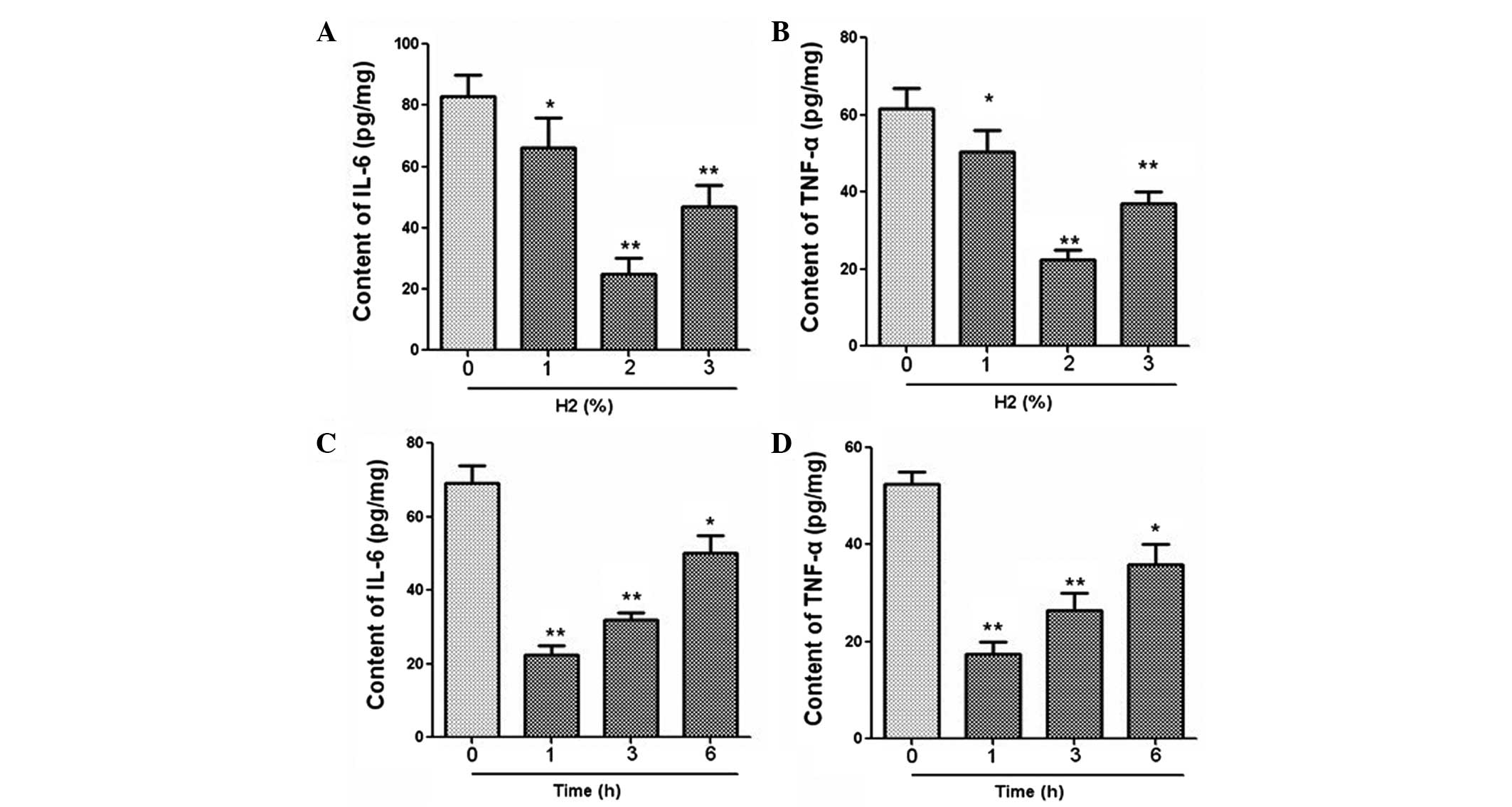
The mRNA expression of IL-6, TNF-α, Egr-1 and IL-1β
was measured using qPCR. Results revealed that hydrogen
administration at 2% concentration significantly downregulated the
mRNA levels of IL-6, TNF-α, Egr-1 and IL-1β (Fig. 2). In addition, the expression levels
of these cytokines were clearly decreased after hydrogen treatment
for a period of 1 h (Fig. 2). The
expression levels of IL-6 and TNF-α in serum were further examined
using an ELISA. As demonstrated in Fig.
3, hydrogen gas inhalation at a concentration of 2% and a
duration of 1 h led to marked reductions in the levels of IL-6 and
TNF-α expression.
Effect of hydrogen gas inhalation on
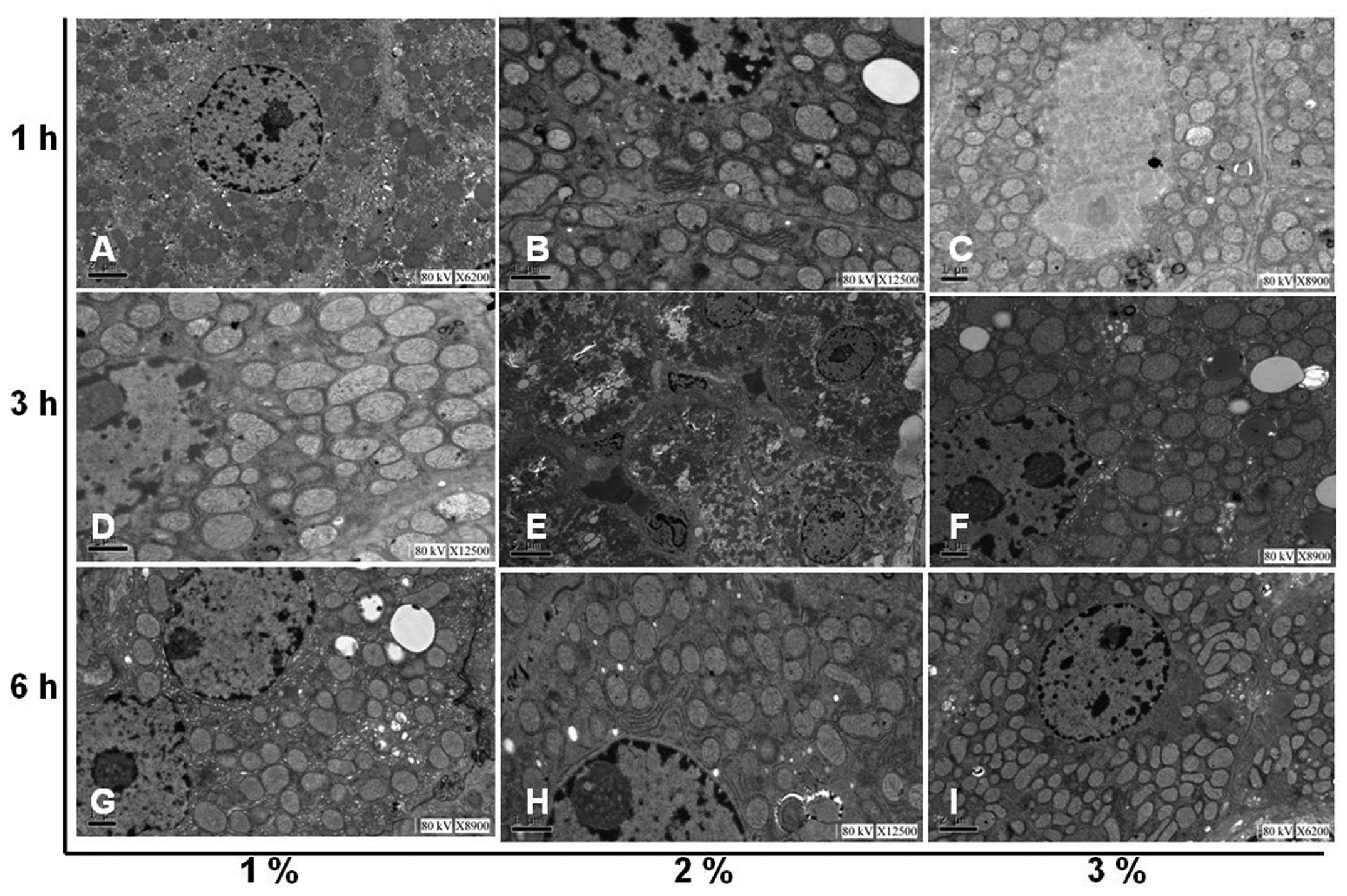
liver morphology changes
The effect of hydrogen gas treatment on
histopathological changes in the livers of rats with I/R is
demonstrated in Fig. 4.
Morphological examination revealed that the liver tissues of rats
with I/R injury were severely damaged in the control group with
severe edema, alveolar hemorrhage and extensive inflammatory cell
infiltration. In the experimental groups, hydrogen treatment
significantly alleviated liver edema, alveolar hemorrhage and
inflammatory cell infiltration, suggesting that the liver injury
induced by I/R was reduced by hydrogen treatment. In addition,
following exposure to hydrogen, the morphological changes in
subcellular structures in the liver tissues were further examined
using a TEM. Gross morphological changes in the liver were clearly
observed in the control group while hydrogen administration
markedly reduced the I/R injury in the experimental groups
(Fig. 5).
Hydrogen gas protects liver I/R injury
by inhibiting the NF-κB signaling pathway
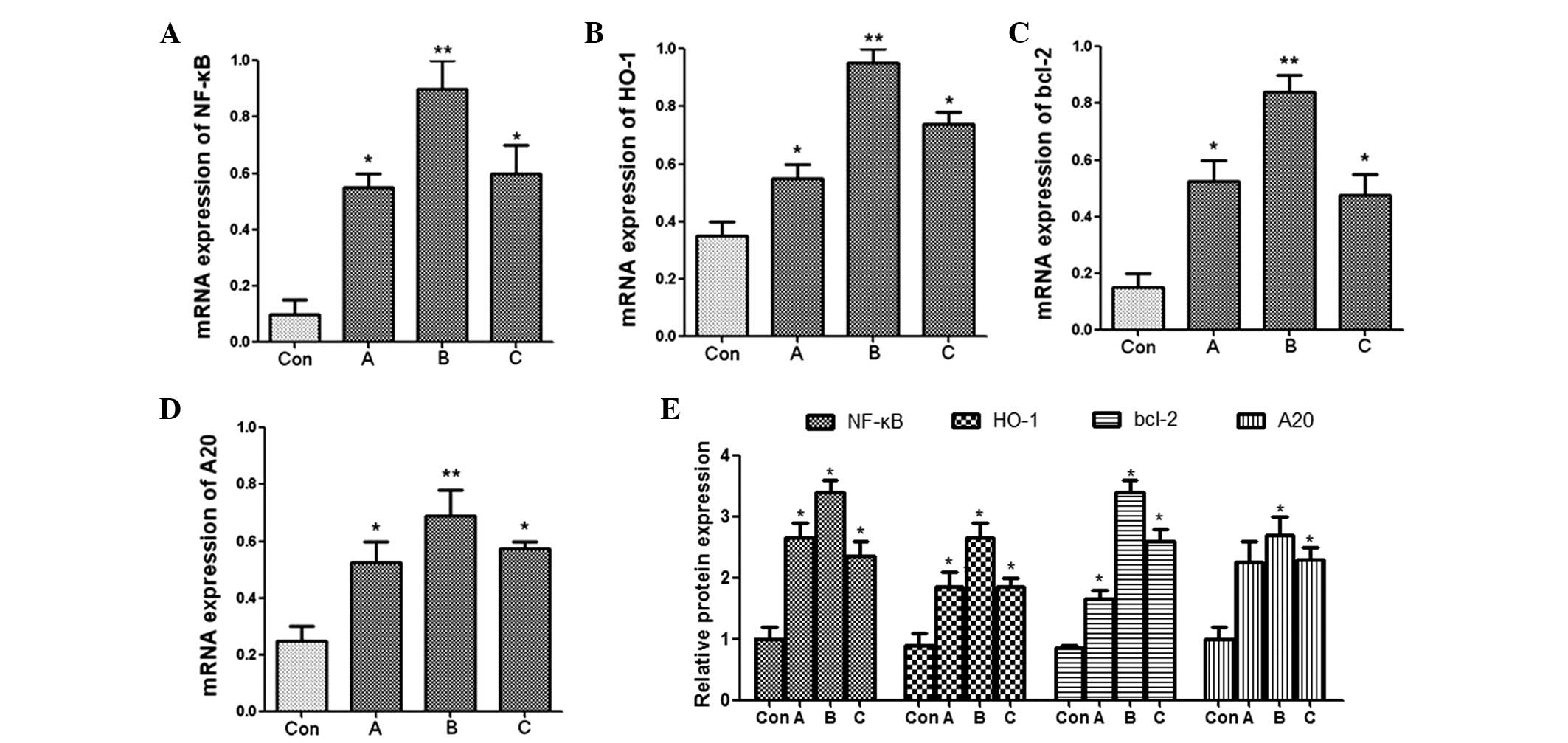
The current study then investigated whether the
NF-κB signaling pathway was involved in initiating the protective
effect of hydrogen on liver I/R injury. The rats were randomly
divided into three groups: hydrogen-treated donor and recipient,
hydrogen-treated donor, and hydrogen-treated recipient. The mRNA
expression levels of NF-κB, HO-1, Bcl-2 and A20 were measured by
qPCR. The results revealed that the mRNA expression levels of
NF-κB, HO-1, Bcl-2 and A20 in the hydrogen-treated donor group were
significantly increased compared with those in the other groups
(Fig. 6). The expression levels of
the corresponding proteins were then determined by western blot and
demonstrated similar results (Fig.
6).
Discussion
In the current study, hydrogen gas inhalation by
rats was used to investigate the protective effect of hydrogen on
I/R injury during liver transplantation and identify the underlying
mechanism. It was demonstrated that hydrogen inhalation was able to
significantly suppress I/R injury in rats by downregulating ALT and
AST activities, cytokine expression and morphological damage. In
addition, the protective effect of hydrogen was identified to be
mediated by the activation of the NF-κB signaling pathway.
Compared with current drug therapy, the inhalation
of hydrogen has several potential advantages. Hydrogen is
physiologically safe for humans and is produced from undigested
carbohydrates in the large intestine during fermentation (∼150
ml/day). Colonic microflora continuously supply low doses of
hydrogen into the blood circulation. Hydrogen is able to be
metabolized by intestinal flora and may be discharged through the
anus (10). It has been shown that
<5% of hydrogen is not explosive and the successful application
of hydrogen to prevent decompression sickness following deep diving
has also been demonstrated to be safe (11). Oxygen produced by macrophages and
neutrophils may kill bacteria, and this function is not affected by
hydrogen. Therefore, hydrogen treatment does not affect cellular
autophagy and other innate immune functions (12). In the present study, the activities
of ALT and AST were markedly reduced following hydrogen treatment,
indicating that hydrogen gas inhalation alleviated I/R injury in
liver transplantation. Hydrogen gas inhaled at a concentration of
2% achieved the optimal effect. Furthermore, the rats were treated
with hydrogen at different time points and it was revealed that 1 h
administration of gas prior to surgery led to significant
reductions in ALT and AST activities. Hydrogen treatment also led
to marked reductions in IL-6, TNF-α, Egr-1 and IL-1β expression.
Analysis of changes to liver morphology following hydrogen
inhalation using H&E staining and TEM revealed that hydrogen
treatment at 2% concentration for 1 h prior to surgery
significantly alleviated the tissue damage induced by I/R
injury.
NF-κB is a nuclear transcription factor present in
almost all animal cells and is involved in the cell response to
stimulation by stress, cytokines and reactive oxygen species. NF-κB
plays a critical role in mediating inflammatory and immune
responses (13,14). Activation of NF-κB regulates an
anti-apoptotic cascade and a number of anti-apoptotic genes
including heme oxygenase, A20 and Bcl-2 (15,16).
HO-1 is an inducible isoform produced in response to stress such as
oxidative stress, hypoxia and cytokines (17). A20 has been identified to be a gene
whose expression is rapidly induced by tumor necrosis factors
(18). The protein encoded by this
gene is a zinc finger protein and it has been shown to inhibit
NF-κB activation as well as TNF-mediated apoptosis (19). The anti-apoptotic protein Bcl-2 has
been demonstrated to prevent disruption of mitochondrial physiology
and block cytochrome c release from mitochondria, which is a
response gene of p53 and involved in p53-regulated apoptosis
(20,21). The current study demonstrated that
hydrogen gas inhalation resulted in a significant increase in NF-κB
expression as well as in the expression levels of the
anti-apoptotic genes HO-1, A20 and Bcl-2, particularly in the
hydrogen-treated donor group compared with those in the
hydrogen-treated donor and recipient group and the hydrogen-treated
recipient group. The protein expression of these genes was
determined by western blot analysis and revealed similar results.
Together, these results demonstrated that the protective effect of
hydrogen gas is dependent on the activation of the NF-κB signaling
pathway.
In summary, the present study demonstrated that
hydrogen gas inhalation at 2% concentration for 1 h prior to liver
transplantation protected rats from I/R injury by activating the
NF-κB signaling pathway.
References
|
1
|
Henderson PW, Singh SP, Belkin D, et al:
Hydrogen sulfide protects against ischemia-reperfusion injury in an
in vitro model of cutaneous tissue transplantation. J Surg Res.
159:451–455. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hoetzel A, Dolinay T, Schmidt R, Choi AM
and Ryter SW: Carbon monoxide in sepsis. Antioxid Redox Signal.
9:2013–2026. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roediger WE: Review article: nitric oxide
from dysbiotic bacterial respiration of nitrate in the pathogenesis
and as a target for therapy of ulcerative colitis. Aliment
Pharmacol Ther. 27:531–541. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gharib B, Hanna S, Abdallahi OM, Lepidi H,
Gardette B and De Reggi M: Anti-inflammatory properties of
molecular hydrogen: investigation on parasite-induced liver
inflammation. C R Acad Sci III. 324:719–724. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Katsuramaki T, Isobe M, Kimura H, et al:
Different changes of endothelin-1 after reperfusion in a warm
ischemia/reperfusion and transplantation model in pig liver.
Transplant Proc. 32:2276–2278. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Beiras-Fernandez A, Chappell D, Hammer C,
Beiras A, Reichart B and Thein E: Impact of polyclonal
anti-thymocyte globulins on the expression of adhesion and
inflammation molecules after ischemia-reperfusion injury. Transpl
Immunol. 20:224–228. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim ES, Lee BJ, Won JY, Choi JY and Lee
DK: Percutaneous transhepatic biliary drainage may serve as a
successful rescue procedure in failed cases of endoscopic therapy
for a post-living donor liver transplantation biliary stricture.
Gastrointest Endosc. 69:38–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fukuda K, Asoh S, Ishikawa M, Yamamoto Y,
Ohsawa I and Ohta S: Inhalation of hydrogen gas suppresses hepatic
injury caused by ischemia/reperfusion through reducing oxidative
stress. Biochem Biophys Res Commun. 361:670–674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lord R, Kamada N, Goto S, et al: Detection
of donor MHC class 1 encoded cells after orthotopic liver
transplantation and retransplantation in the rat. Transplant Proc.
26:2231–2232. 1994.PubMed/NCBI
|
|
10
|
Zheng X, Mao Y, Cai J, et al:
Hydrogen-rich saline protects against intestinal
ischemia/reperfusion injury in rats. Free Radic Res. 43:478–484.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abraini JH, Gardette-Chauffour MC,
Martinez E, Rostain JC and Lemaire C: Psychophysiological reactions
in humans during an open sea dive to 500 m with a
hydrogen-helium-oxygen mixture. J Appl Physiol (1985).
76:1113–1118. 1994.PubMed/NCBI
|
|
12
|
Ohsawa I, Ishikawa M, Takahashi K, et al:
Hydrogen acts as a therapeutic antioxidant by selectively reducing
cytotoxic oxygen radicals. Nat Med. 13:688–694. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chandel NS, Trzyna WC, McClintock DS and
Schumacker PT: Role of oxidants in NF-kappa B activation and
TNF-alpha gene transcription induced by hypoxia and endotoxin. J
Immunol. 165:1013–1021. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ghosh S, May MJ and Kopp EB: NF-kappa B
and Rel proteins: evolutionarily conserved mediators of immune
responses. Annu Rev Immunol. 16:225–260. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ahn KS, Sethi G and Aggarwal BB:
Simvastatin potentiates TNF-alpha-induced apoptosis through the
down-regulation of NF-kappaB-dependent antiapoptotic gene products:
role of IkappaBalpha kinase and TGF-beta-activated kinase-1. J
Immunol. 178:2507–2516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Q, Guo Y, Ou Q, et al: Gene transfer of
inducible nitric oxide synthase affords cardioprotection by
upregulating heme oxygenase-1 via a nuclear
factor-{kappa}B-dependent pathway. Circulation. 120:1222–1230.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen X, Zhang Z, Su C, Gu W, Li H and Zhou
G: Protective effect of heme oxygenase-1 to pancreas islet
xenograft. J Surg Res. 164:336–343. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Opipari AW Jr, Hu HM, Yabkowitz R and
Dixit VM: The A20 zinc finger protein protects cells from tumor
necrosis factor cytotoxicity. J Biol Chem. 267:12424–12427.
1992.PubMed/NCBI
|
|
19
|
Sakai Y, Uchida K and Nakayama H: A20 and
ABIN-3 possibly promote regression of trehalose 6,6′-dimycolate
(TDM)-induced granuloma by interacting with an NF-kappa B signaling
protein, TAK-1. Inflamm Res. 61:245–253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee MH, Han DW, Hyon SH and Park JC:
Apoptosis of human fibrosarcoma HT-1080 cells by
epigallocatechin-3-O-gallate via induction of p53 and caspases as
well as suppression of Bcl-2 and phosphorylated nuclear factor-κB.
Apoptosis. 16:75–85. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dogu Y and Diaz J: Mathematical model of a
network of interaction between p53 and Bcl-2 during
genotoxic-induced apoptosis. Biophys Chem. 143:44–54. 2009.
View Article : Google Scholar : PubMed/NCBI
|