Introduction
Acute myocardial infarction (AMI) is the most common
form of acute cardiac injury. The treatment of AMI has improved,
however, AMI results in ischemic death of a large number of
cardiomyocytes. Although early reperfusion of the ischemic
myocardium in coronary artery infarction can rescue the agonal
cardiac muscle, it causes subsequent myocardial
ischemia-reperfusion (I/R) injury (1,2),
which lowers the protective effect of reperfusion therapy.
Myocardial I/R injury is a complex pathophysiological process that
involves many kinds of factors and pathways. During the initiation
and progression of I/R injury, apoptosis has been identified as
providing an important molecular basis (3). Moreover, the inflammatory response
is still considered to be a major cause of I/R-induced tissue
injury (4).
Toll-like receptor 4 (TLR4) as a member of pattern
recognition receptors is expressed and functional in cells of
myeloid lineage. However, TLR4 is also detectable in
non-professional immunocyte cell types, such as cardiomyocytes and
microvascular endothelial cells (5). Recent studies have shown that TLR4
can induce apoptosis in several cell types (6–8).
In addition, TLR4 plays an important role in the induction of the
inflammatory response by recognition of several endogenous ligands
associated with tissue injury (9). Activation of TLR4 induces the
nuclear factor-κB (NF-κB)-dependent apoptosis and expression of
proinflammatory cytokines (10,11). A previous report demonstrated that
there is reduced myocardial injury and inflammation in
TLR4-deficient mice after I/R (12).
Resveratrol (trans-3,4′,5-trihydroxystilbene), a
naturally occurring polyphenol phytoalexin, is abundant in a wide
variety of plant species, such as grapes, mulberries, peanuts and
so on (13). Resveratrol has
diverse biochemical and physiological actions, including
antiplatelet (14), antiaging
(15), antiapoptosis (16) and anti-inflammatory (17) actions. Recent studies have
demonstrated that resveratrol can provide cardioprotective effects
(18,19). It seems that resveratrol-mediated
cardioprotection is achieved through the preconditioning effect
rather than direct protection. Although preconditioning can
effectively protect the heart from I/R injury, it can hardly be
applied in the clinical setting of AMI because of the requirement
for pretreatment. Therefore, it is significant to determine whether
resveratrol applied at reperfusion can also protect the heart from
I/R injury. If resveratrol is protective when given at reperfusion,
it is interesting to define the potential cellular and molecular
mechanisms underlying the protection.
Youn et al (20) reported that resveratrol could
inhibit NF-κB activation induced by TLR4-mediated signaling in
RAW264.7 cells. It has been demonstrated that the injury of
cardiomyocytes induced by anoxia/reoxygenation (A/R) is a useful
in vitro model to study myocardial I/R injury (21,22). Thus, in the present study, we
first investigated whether resveratrol applied at reoxygenation
could protect cardiomyocytes against A/R injury. Then we explored
if the protective effect is exerted through the TLR4/NF-κB
signaling pathway.
Materials and methods
Animals
Sprague-Dawley rats (1-3-days-old) were purchased
from the Center of Experimental Animal in Wuhan University, China.
All animals used in this study were cared for in accordance with
the Guide for the Care and Use of Laboratory Animals published by
the United States National Institute of Health (NIH publication no.
85-23, revised 1996), and all procedures were approved by the
Committee of Experimental Animals of Wuhan University.
Primary culture of neonatal rat
cardiomyocytes
Primary cultures of neonatal rat cardiomyocytes were
prepared from the ventricles of 1-3-day-old Sprague-Dawley rats, as
described previously (23), with
some modifications. Briefly, the hearts were harvested and placed
in phosphate-buffered saline (calcium- and magnesium-free PBS: NaCl
137 mmol/l, Na2HPO4 10.6 mmol/l,
KH2PO4 2.1 mmol/l,
K2HPO4 1.1 mmol/l, pH 7.4). The ventricles
were minced into pieces approximately 1 mm3. The tissue
fragments were dissociated by treatment with 0.125% trypsin 5 times
at 37°C, then filtered and centrifuged for 10 min (120 × g), and
finally resuspended in the culture medium, which consisted of
Dulbecco’s modified Eagle’s medium (DMEM, Hyclone, Logan, UT)
containing 10% fetal bovine serum (FBS, Invitrogen Corp., Carlsbad,
CA), penicillin (100 U/ml) and streptomycin (100 μg/ml).
Resuspended cells were then plated in a petri dish in a humidified
incubator (5% CO2, 37°C) for 1.5 h to reduce fibroblast
contamination. Non-adherent cells were counted with a hemocytometer
and the final myocyte cultures were found to contain >90%
cardiomyocytes. Subsequently the cells in the culture medium were
transferred into 6-well gelatin-coated plates at a density of
approximately 1×106 cells/ml and incubated for 4 days
before the experiment.
A/R injury model
According to a previously described method (24), the in vitro model of A/R
was used in this study. Briefly, the confluent beating
cardiomyocytes in 6-well plates were exposed to anoxia for 3 h and
then reoxygenated for 2 h. As a control, cardiomyocytes were
initially perfused in normal Tyrode’s solution with a gas mixture
of 95% O2-5% CO2 at 37°C, pH 7.4. To simulate
anoxia, the Tyrode’s solution was switched to pH 6.8 at 37°C
without glucose and then the cells were aerated with a gas mixture
of 95% N2-5% CO2. To simulate reoxygenation,
the cells were treated with normal Tyrode’s solution with a gas
mixture of 95% O2-5% CO2. Anoxic conditions
were obtained by equilibrating a small humidified plexiglass
chamber containing cardiomyocytes with 95% N2 and 5%
CO2 via a gas transfusive apparatus (Changjing Biotech
Co., Beijing, China), which was confirmed by measuring chamber
pO2 (chamber pO2 fell to 0 mmHg within 5 min
after the initiation of perfusion with the anoxic gas).
Reoxygenation was achieved by exposing cells to room air
(CO2 incubator).
Experimental groups and protocols
At the beginning of each experiment, the cells were
rinsed in PBS, and the culture medium was replaced. Eighty percent
confluent cardiomyocytes were randomly distributed into different
experimental groups as follows, and each group included two
parallel wells for three replicate experiments: i) control group:
cardiomyocytes were incubated in aerobic Tyrode’s solution during
the entire experimental period; ii) A/R group: cardiomyocytes were
incubated in anaerobic Tyrode’s solution for 3 h anoxia followed by
2 h reoxygenation; 3) resveratrol group: cardiomyocytes were
subjected to A/R as described above, and resveratrol (final
concentrations: 5, 10 or 20 μM) was applied 5 min after
reoxygenation and maintained throughout the experiment. Resveratrol
(Sigma-Aldrich, St. Louis, MO) was freshly prepared as a 22.8 mg/ml
solution in ethanol and then further diluted in cell culture
medium. Cell viability and lactate dehydrogenase (LDH) activity
were measured at the end of the reoxygenation times. Other
measurements were performed after the cells were incubated at 37°C
in a CO2 incubator for additional 24 h.
Assay of cell viability
Cell viability was determined by the cell counting
kit (CCK)-8 assay (Dojindo, Tokyo, Japan), and the experimental
procedure was based on the manufacturer’s manual. The
cardiomyocytes were seeded in 96-well plates at 1×104
cells/well and incubated with different concentrations of
resveratrol alone (0, 5, 10, 20 μM) for 48 h, or after 3 h anoxia.
These cells were treated with different concentrations of
resveratrol (0, 5, 10, 20 μM) that was applied 5 min after 2 h
reoxygenation. After experimental treatment, 10 μl of WST-8
solution
(2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium,
monosodium salt) was added to each well, and the cardiomyocytes
were incubated for an additional 2 h at 37°C. The absorbance of
each well at 450 nm was measured with a reference at 630 nm using a
microplate reader (Bio-Rad Laboratories, Hercules, CA). The
percentage of cell viability was calculated by the following
formula: % cell viability = (mean absorbance in test wells)/(mean
absorbance in control well) × 100.
Assay of lactate dehydrogenase (LDH)
activity
The extent of cellular injury was monitored by
measuring LDH release. According to the manufacturer’s instruction,
100 μl of culture medium was taken to assess LDH activity using a
commercial kit (JianCheng Bioengineering Institute, Nanjing, China)
with a spectrophotometer.
Flow cytometric analysis of
apoptosis
Apoptosis was assessed by flow cytometric analysis
of Annexin V and propidium lodide (PI) double staining. The
cardiomyocytes were seeded in 6-well plates at approximately
2×104 cells/well. After treatment, cells were
centrifuged to remove the medium, rinsed in PBS, and suspended in
100 μl of 1X binding buffer (10 mM HEPES, 140 mM NaCl, and 2.5 mM
CaCl2) to be stained with Annexin V and PI according to
the manufacturer’s instructions (BioVision, Inc., Palo Alto, CA).
Stained cells were analyzed using a FACStar plus flow cytometer
(Becton-Dickinson, San Jose, CA) in the FL1-H and FL2-H
channels.
Measurement of caspase-3 activity
Caspase-3 activity was evaluated by using a
commercialized caspase-3 assay kit (Biovision, Inc.). Approximately
1×106 cells were harvested by centrifugation, and the
pellet was resuspended in lysis buffer. Protein levels were
determined with the bicinchoninic acid assay (Beyotime
Biotechnology, Shanghai, China). As described in the manufacturer’s
instructions, aliquots of protein (10 μl) were incubated with 10 μl
of synthetic peptide substrate Ac-DEVD-pNA in a total volume of 100
μl at 37°C for 2 h to detect caspase-3 activity. Caspase-3 activity
was expressed as optical density. The absorbance at 405 nm of the
released pNA was monitored in a spectrophotometer.
Quantitative real-time PCR analysis
Total-RNA was prepared from cells with TRIzol
reagent (Invitrogen Corp.) and reversely transcribed to produce
cDNA from total-RNA with oligo(dt). The expression of candidate
genes were measured by quantitative real-time PCR analysis using a
SYBR-Green-based assays kit (Invitrogen Corp.) to amplify the
fragments according to the manufacturer’s instructions. The RT-PCR
conditions were 42°C/15 min, 95°C/2 min for reverse transcription;
95°C/30 sec, 58.9°C (TLR4) or 60°C (GAPDH)/30 sec, and 72°C/60 sec,
over 40 cycles for polymerase chain reaction. Level of TLR4 mRNA
was calculated based on the method of 2−ΔΔCT between the
intervening group and the control group. GAPDH was used as an
internal control, and the comparative threshold method was used to
assess the relative abundance of TLR4 mRNA. The specific primer
sequence of the selected genes were: TLR4, sense,
5′-AGCCATTGCTGCCAACATCA-3′ and antisense primer,
5′-GCCAGAGCTACTCAGAAAC-3′; GAPDH, sense, 5′-GACAACTTTGGCTCGTGGA-3′
and antisense primer, 5′-ATGCAGGGGTTCTGG-3′. Primers were
synthesized by Shanghai Sangon Biological Engineering Technology
Company Limited (China). The correctness of the gene order was
proven in GenBank.
Western blot analysis
Membranous, cytoplasmic and nuclear extracts were
prepared for western blot analysis of TLR4 (membranous), I-κBα
(cytoplasmic) and NF-κBp65 (nuclear) expression, using Membranous,
Cytoplasmic and Nuclear Extraction Reagents (Pierce Biotechnology,
Inc., Rockford, IL). Protein concentration was determined by the
bicinchoninic acid protein assay (Beyotime Biotechnology). Equal
amounts (50 μg) of denatured proteins were separated on 10%
SDS-polyacrylamide gels and transferred to nitrocellulose membrane.
The membranes were blocked with 5% non-fat dry milk in TBST
(containing 0.05% Tween-20), and incubated overnight at 4°C with
the primary antibody (TLR4, 1:1,000, Cell Signaling Technology,
Inc., Beverly, MA; I-κBα, 1:500, Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA; NF-κBp65, 1:500, Santa Cruz Biotechnology, Inc.).
Then the blots were washed and incubated with horseradish
peroxidase-conjugated secondary antibody (goat anti-rabbit IgG,
1:2,000, Beyotime Biotechnology) for 1 h at room temperature.
Immunoreactivity was enhanced with a chemiluminescence kit
(Beyotime Biotechnology) and exposed to film. β-actin was used as
an internal control to correct the variations of different samples.
The density of bands on western blots was quantified by using a
Bio-Rad image system (Hercules, CA).
Enzyme-linked immunosorbent assay
The levels of tumor necrosis factor (TNF)-α and
interleukin (IL)-1β in the culture medium were measured by
enzyme-linked immunosorbent assay (ELISA), using commercially
available kits (Zhong Shan-Golden Bridge Biological Technology Co.,
Beijing, China) according to the manufacturer’s instructions.
Statistical analysis
Data are expressed as means ± SD. Statistical
analyses of data were performed by one-way ANOVA followed by the
Student-Newman-Keuls test. A value of P<0.05 was considered to
be statistically significant. All data analyses were conducted with
the SPSS 13.0 software package (SPSS, Inc., Chicago, IL).
Results
Effect of resveratrol on A/R-induced cell
damage in cardiomyocytes
To observe the cytotoxicity of resveratrol on
cardiomyocytes, the cells were exposed to different concentrations
of resveratrol (0, 5, 10 or 20 μM) for 48 h, and the CCK-8 assay
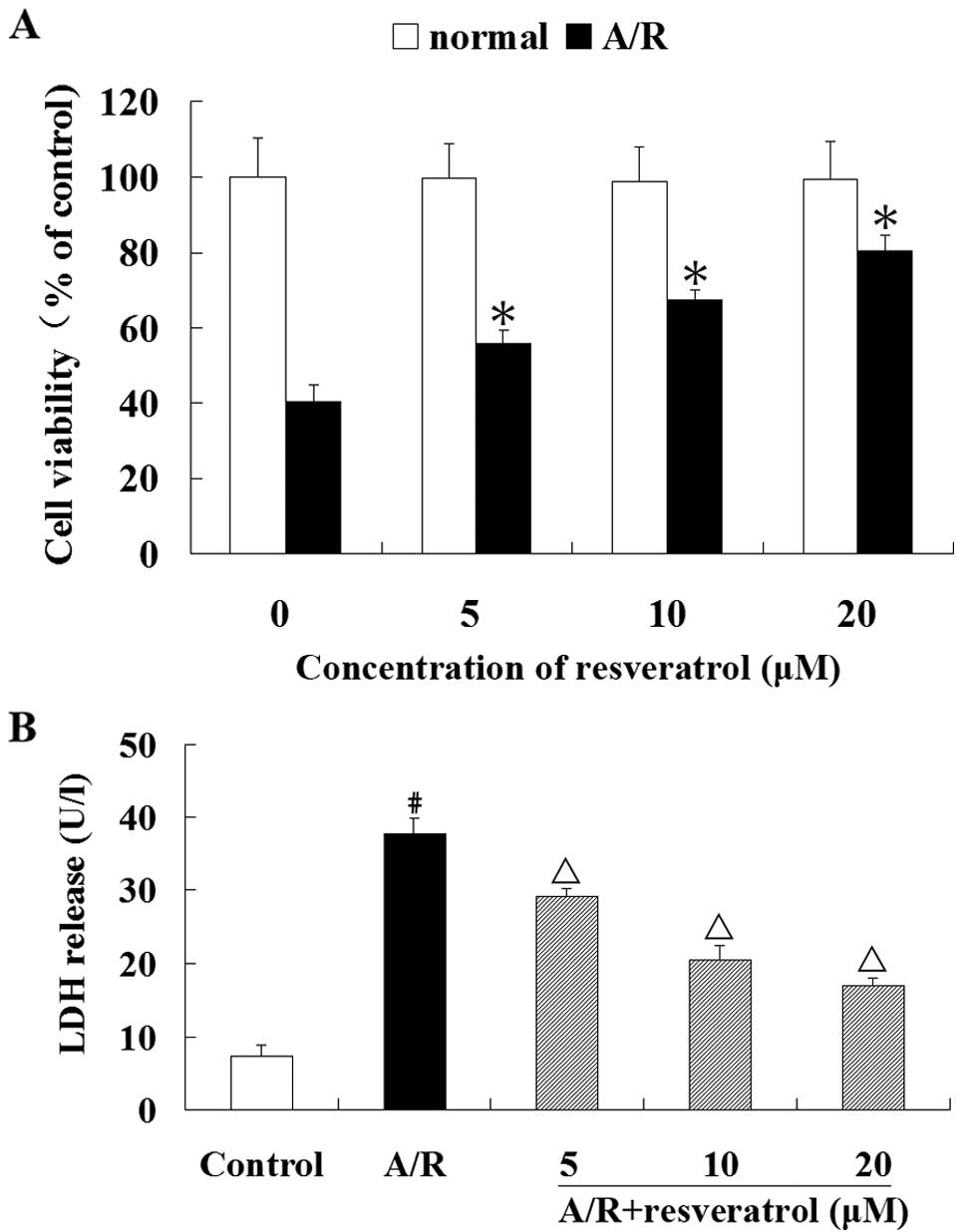
showed no loss of cell viability (Fig. 1A). Therefore, we used resveratrol
at the concentrations of 5, 10 or 20 μM for our subsequent studies.
Resveratrol (5, 10 or 20 μM) significantly prevented the loss of
cardiomyocyte viability that resulted from A/R induction
(P<0.05) (Fig. 1A).
Furthermore, resveratrol (5, 10 or 20 μM) significantly suppressed
the release of LDH in cardiomyocytes that had undergone A/R
(P<0.05) (Fig. 1B).
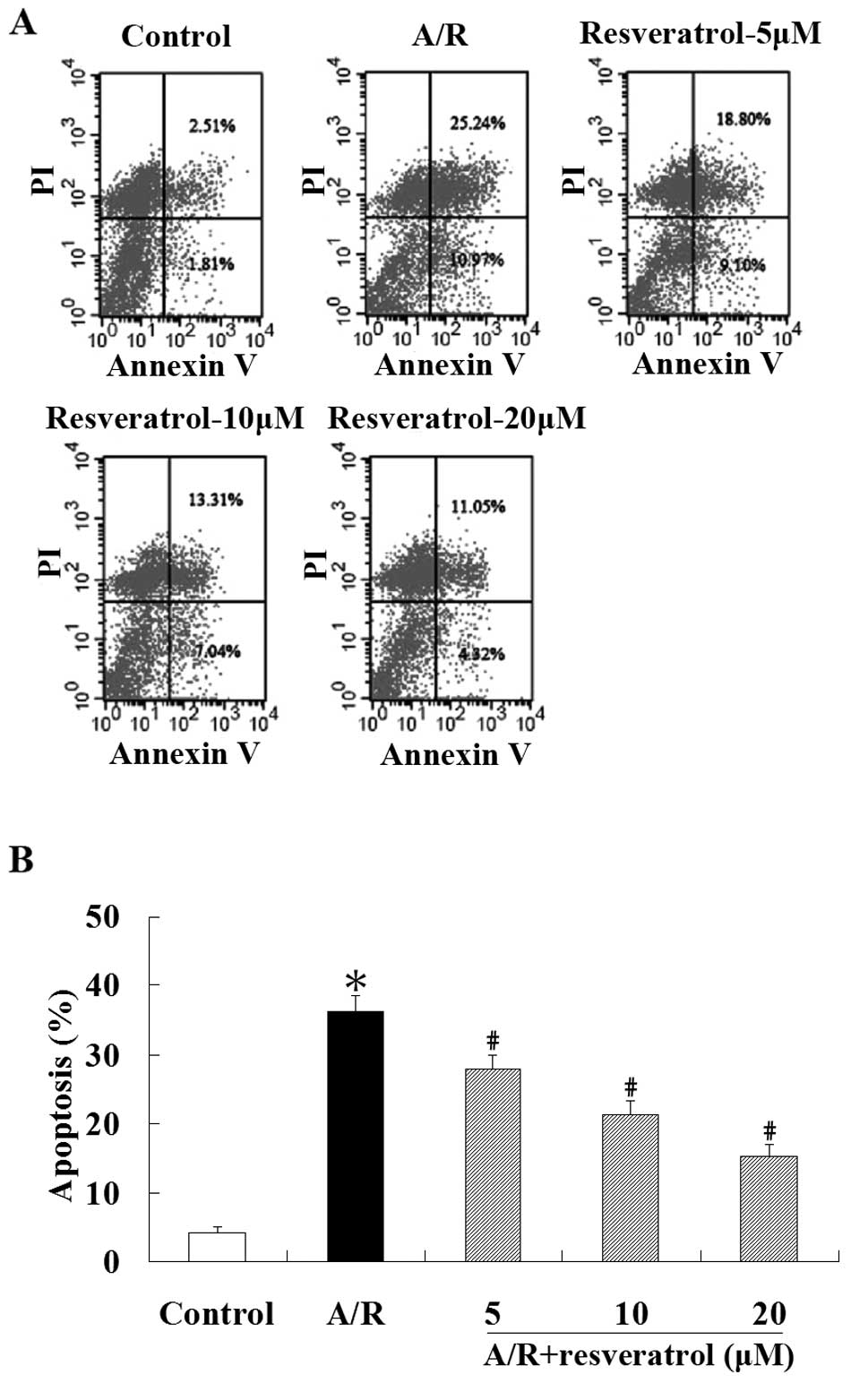
Flow cytometric analysis was used to quantify the
rate of cell apoptosis. Spontaneous apoptosis was low in
cardiomyocytes under control condition, yet stimulation induced by
A/R in cardiomyocytes led to enhanced apoptosis (P<0.05).
Treatment with resveratrol (5, 10 or 20 μM) showed a significant
resistance in apoptosis in cardiomyocytes undergoing A/R
(P<0.05) (Fig. 2). These
results suggest that resveratrol is a potent cardioprotective agent
against A/R injury.
Effect of resveratrol on the activity of
caspase-3 induced by A/R in cardiomyocytes
Caspase-3 activation is unique to apoptosis as it
does not occur in other forms of cell death and provides strong
evidence for the presence of apoptosis. Therefore, we examined the
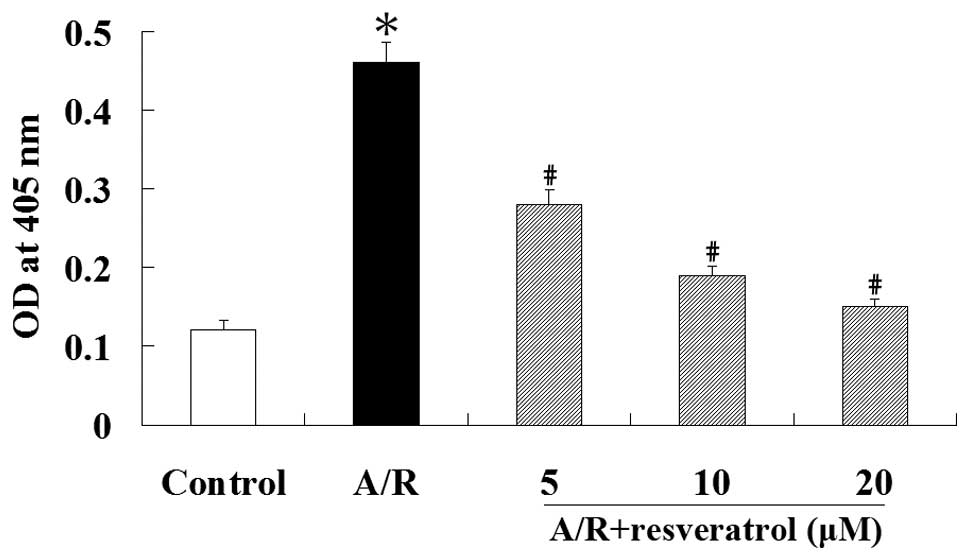
activity of caspase-3 using the synthetic peptide substrate
Ac-DEVD-pNA. Caspase-3 activation was increased during A/R
(P<0.05). However, resveratrol significantly inhibited the
activation of caspase-3 induced by A/R (P<0.05) (Fig. 3). These results suggest that
resveratrol protects against A/R-induced apoptosis associated with
the inhibition of caspase-3 in cardiomyocytes.
Effect of resveratrol on TLR4 expression
in cardiomyocytes undergoing A/R
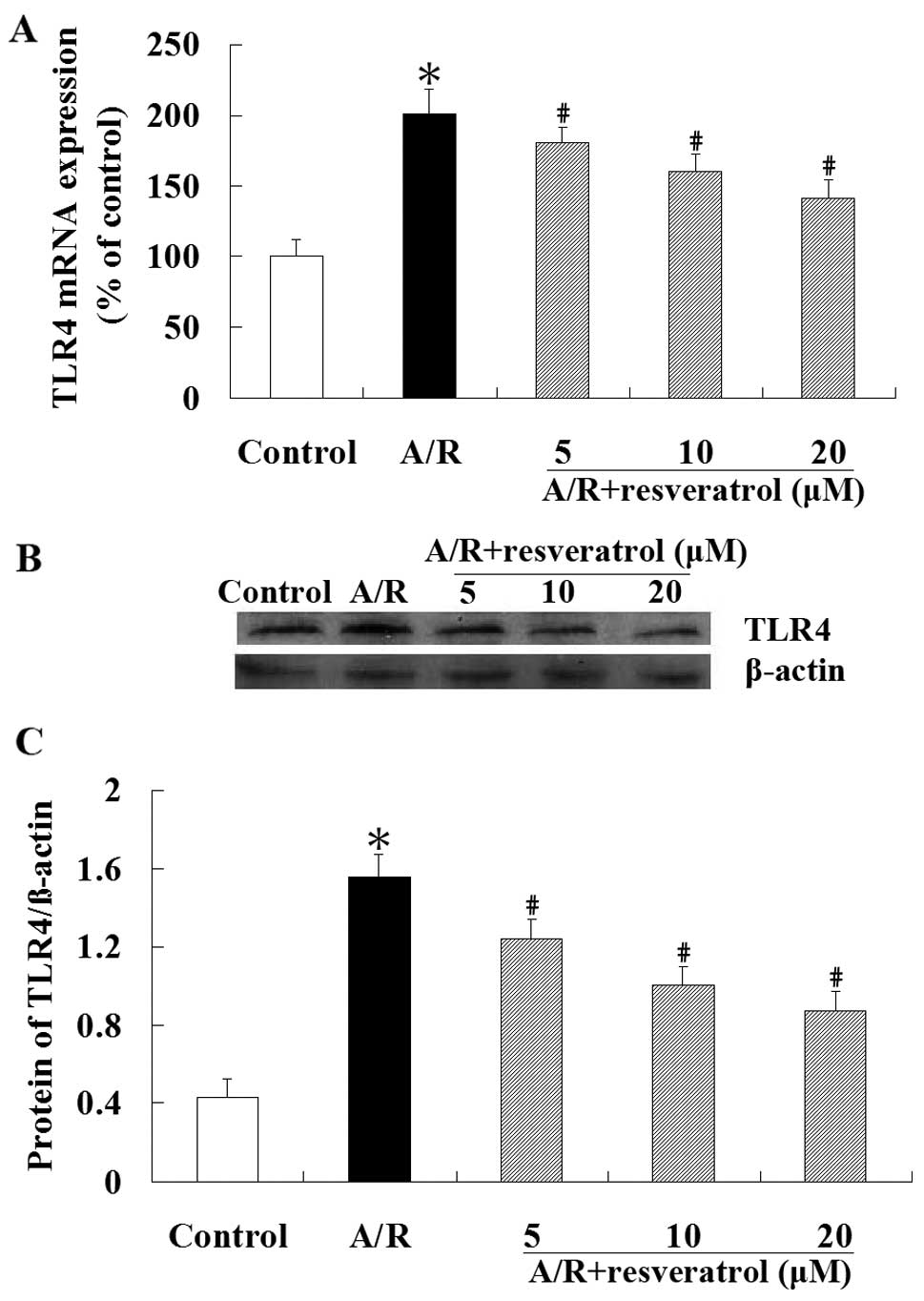
Real-time RT-PCR revealed that the expression of
TLR4 mRNA was significantly increased in cardiomyocytes undergoing
A/R (P<0.05). However, compared with the A/R group, treatment
with resveratrol (5, 10 or 20 μM) produced a significant reduction
of TLR4 mRNA expression (P<0.05) (Fig. 4A). Similar to the qRT-PCR result,
western blot analysis showed that the TLR4 protein level was
significantly lower in resveratrol group compared with the A/R
group (P<0.05) (Fig. 4B).
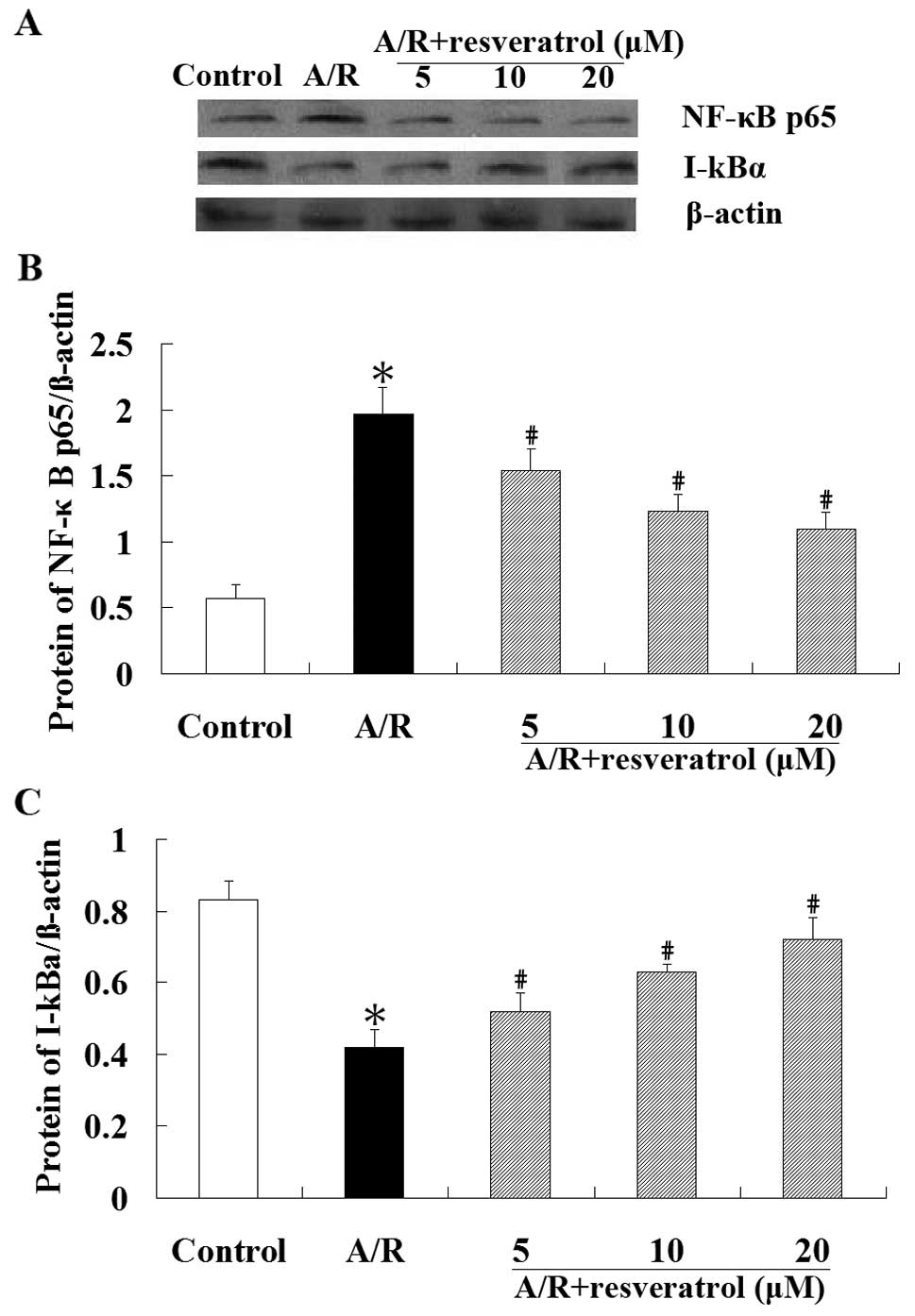
Effect of resveratrol on NF-κB
translocation in cardiomyocytes undergoing A/R
The western blot analysis was performed to explore
whether resveratrol has an effect on NF-κB translocation. These
results showed that A/R induced nuclear translocation of NF-κBp65
protein in cardiomyocytes, which was markedly attenuated by
resveratrol (P<0.05). Cytoplasmic I-κBα protein levels were
significantly higher (P<0.05) in resveratrol groups than those
in A/R group. As demonstrated in Fig.
5, A/R induced an obvious NF-κB nuclear translocation in an
IκBα-dependent manner. Resveratrol (5, 10 or 20 μM) treatment
inhibited the degradation of I-κBα, and blocked the translocation
of NF-κB into the nucleus.
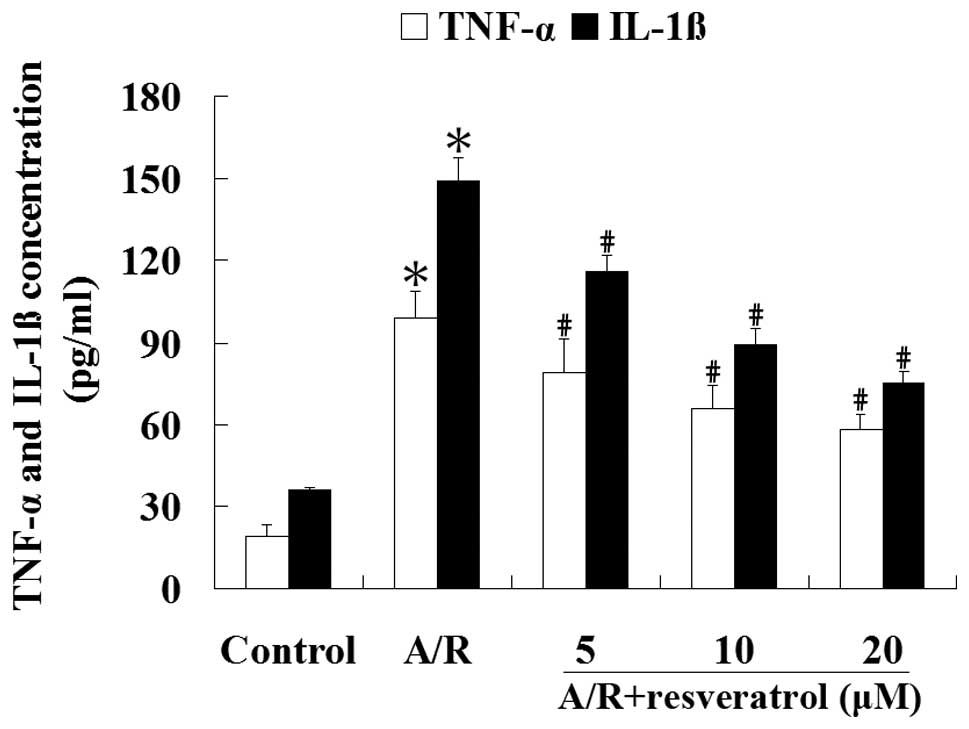
Resveratrol inhibits the production of
TNF-α and IL-1β induced by A/R in cardiomyocytes
ELISA was used to analyze the concentrations of
TNF-α and IL-1β in the culture medium. Cardiomyocytes subjected to
A/R had an increase of TNF-α and IL-1β contentrations in the
culture medium compared with the control group (P<0.05).
Treatment with resveratrol (5, 10 or 20 μM) produced a significant
reduction of TNF-α and IL-1β concentrations in the culture medium
undergoing A/R (P<0.05) (Fig.
6). These results suggest that resveratrol can significantly
attenuate the levels of proinflammatory cytokines induced by A/R in
cardiomyocytes (P<0.05).
Discussion
The principal findings of the present study were
that i) the toxicity of resveratrol (5, 10 or 20 μM) on
cardiomyocytes was negligible and resveratrol applied shortly after
the onset of reoxygenation markedly suppressed the decrease of cell
viability resulting from A/R, and reduced the levels of LDH in the
culture medium of cardiomyocytes; ii) Resveratrol administration
decreased apoptotic cardiomyocytes, caspase-3 activity and
attenuated the level of proinflammatory cytokines (TNF-α and IL-1β)
induced by A/R in cardiomyocytes; iii) treatment with resveratrol
downregulated the TLR4 expression and blocked NF-κB translocation
from the cytoplasm to the nucleus in an IκBα-dependent manner. Our
data showed that resveratrol exerted the protective effects by
inhibiting cell death and inflammation in cardiomyocytes undergoing
A/R, which might be associated with the TLR4/NF-κB signaling
pathway.
The measures of cell viability and level of LDH are
usually used as indicators of cardiomyocyte injury. In the present
study, we found that the cell viability was markedly decreased and
the level of LDH in the culture medium was increased in
cardiomyocytes undergoing A/R, which indicated severe cardiomyocyte
membrane damage. However, resveratrol (5, 10 or 20 μM)
significantly prevented A/R-induced cell damage in cardiomyocytes
confirmed by improved cell viability and reduced LDH activity,
implying that resveratrol can protect cardiomyocytes.
Shortly after the onset of ischemia, the apoptotic
process of cardiomyocytes is initiated and subsequently cells
undergo necrosis. Paradoxically, reperfusion itself may lead to
necrosis and accelerate the process of apoptosis in cardiomyocytes
(2). Extracellular stimuli can
trigger apoptosis via the death receptor and mitochondrial mediated
pathways (25). The activation of
caspase cascade is an important mechanism in regulating cell
apoptosis (26). As one of the
key effectors, the activity of caspase-3 was found to increase
after I/R injury (27).
Consistent with a previous report, in this study, we found that
stimulation induced by A/R in cardiomyocytes resulted in enhanced
apoptosis and increased caspase-3 activation. Treatment with
resveratrol shortly after the onset of reoxygenation significantly
reduced the number of apoptotic cells and attenuated caspase-3
activity, providing a evidence that resveratrol can protect
cardiomyocytes against A/R injury by antiapoptosis.
In addition, the inflammatory response plays a
crucial role in myocardial I/R injury. In our study, we found an
increase of proinflammatory cytokines (TNF-α and IL-1β)
concentrations in the culture medium of cardiomyocytes subjected to
A/R. However, resveratrol applied shortly after the onset of
reoxygenation markedly suppressed the levels of TNF-α and IL-1β in
the culture medium. The result showed that resveratrol can also
protect cardiomyocytes against A/R injury via an anti-inflammatory
response.
In this study, another important finding was that
resveratrol applied shortly after the onset of reoxygenation
decreased TLR4 mRNA and protein expression, which were upregulated
in cardiomyocytes undergoing A/R. Increasing evidence has indicated
that TLR4 has a central role in myocardial I/R injury through TLR4
signaling (28,29). Endogenous ligands released from
damaged cells or tissue fragments seem to initiate cellular
apoptosis and the inflammatory response by inducing TLR4 signaling
(8,30,31). TLR4 signaling could be
downregulated through c-Jun-N-terminal kinases (JNKs), p38 kinases,
and the NF-κB pathway (32). As a
preformed trimeric complex (mainly consisting of the proteins p50
and p65), NF-κB interacts with the inhibitory proteins, I-κBα in
the cytoplasm. IKKβ (a kinase) phosphorylates I-κBα resulting in
the subsequent degradation of I-κBα. When liberated from the
inhibition, NF-κB translocates to the nucleus, where it remains
activated (33). NF-κB is an
important transcription factor in TLR4-mediated signaling pathway
regulating genes encoding proteins implicated in apoptosis and
inflammation (34,35). In our study, we found that A/R
induced NF-κB nuclear translocation in an IκBα-dependent manner,
enhanced cardiomyocyte apoptosis, increased caspase-3 activation
and the production of proinflammatory cytokines (TNF-α, IL-1β)
accompanied with the upregulation of TLR4 expression, implying that
TLR4 may play an important role in triggering apoptosis and the
inflammatory response in the process of myocardial A/R.
Increasing evidence exists supporting that at a
lower dose resveratrol acts as an antiapoptotic agent, providing
cardioprotection by increasing the expression of cell survival
proteins, improving post-ischemic ventricular recovery and reducing
myocardial infarct size and cardiomyocyte apoptosis compared to the
control. However, at higher dose, resveratrol acts as a
pro-apoptotic compound, inducing apoptosis by exerting a death
signal, increases myocardial infarct size and the number of
apoptotic cells (36,37). Consistent with these previous
studies, our data demonstrate that resveratrol protected
cardiomyocytes at a relatively low dose (5–20 μM). Although direct
beneficial effects of resveratrol on cardiomyocytes at a lower dose
have already been reported, the underlying mechanisms remain poorly
understood. Das and Maulik (38)
reported that the antiapoptotic and antiinflammatory effects of
resveratrol in cardioprotection were related to a number of
signaling pathways. Interestingly, our study found that resveratrol
applied shortly after the onset of reoxygenation could improve cell
survival and attenuate A/R-induced inflammatory response through
the downregulation of TLR4/NF-κB signaling pathway. Therefore, we
presumed that resveratrol protects cardiomyocytes against A/R
injury by decreasing the number of apoptotic cardiomyocytes and
attenuating the inflammatory response under A/R conditions as an
in vitro I/R model, which might be mediated in part by
TLR4/NF-κB signaling pathway.
In conclusion, our data provide new insight to
understand the role of resveratrol in protecting cardiomyocytes
against A/R injury. The increased number of apoptotic
cardiomyocytes and the production of TNF-α and IL-1β were
associated with the elevated expression of TLR4 and enhanced NF-κB
activation in the cardiomyocyte A/R model, which could be inhibited
by resveratrol administered shortly after the onset of
reoxygenation in cardiomyocytes. Thus, our results suggest that as
an adjuvant therapeutic approach, resveratrol applied at
reperfusion may constitute a new strategy for myocardial I/R injury
at least in part via the TLR4-mediated NF-κB signaling pathway. In
the future, in vivo studies will be carried out to determine
the potentially protective role of resveratrol for the treatment of
myocardial I/R injury.
Acknowledgements
This project was supported by the National Nature
Science Foundation of China (30900609).
References
|
1
|
E BraunwaldRA KlonerMyocardial
reperfusion: a double-edged sword?J Clin
Invest7617131719198510.1172/JCI1121604056048
|
|
2
|
DM YellonDJ HausenloyMyocardial
reperfusion injuryN Engl J
Med35711211135200710.1056/NEJMra07166717855673
|
|
3
|
X LvJ WanJ YangCytochrome P450
omega-hydroxylase inhibition reduces cardiomyocyte apoptosis via
activation of ERK1/2 signaling in rat myocardial
ischemia-reperfusionEur J
Pharmacol596118126200810.1016/j.ejphar.2008.08.00818771665
|
|
4
|
ML EntmanL MichaelRD RossenInflammation in
the course of early myocardial ischemiaFASEB
J52529253719911868978
|
|
5
|
S FrantzL KobzikYD KimToll4 (TLR4)
expression in cardiac myocytes in normal and failing myocardiumJ
Clin Invest104271280199910.1172/JCI670910430608
|
|
6
|
LC HsuJM ParkK ZhangThe protein kinase PKR
is required for macrophage apoptosis after activation of Toll-like
receptor 4Nature428341345200410.1038/nature0240515029200
|
|
7
|
DY JungH LeeBY JungTLR4, but not TLR2,
signals autoregulatory apoptosis of cultured microglia: a critical
role of IFN-beta as a decision makerJ
Immunol17464676476200510.4049/jimmunol.174.10.646715879150
|
|
8
|
SC KimJP SticeL ChenExtracellular heat
shock protein 60, cardiac myocytes, and apoptosisCirc
Res10511861195200910.1161/CIRCRESAHA.109.20964319875724
|
|
9
|
K MiyakeInnate immune sensing of pathogens
and danger signals by cell surface Toll-like receptorsSemin
Immunol19310200710.1016/j.smim.2006.12.00217275324
|
|
10
|
KJ HanX SuLG XuLH BinJ ZhangHB
ShuMechanisms of the TRIF-induced interferon-stimulated response
element and NF-kappaB activation and apoptosis pathwaysJ Biol
Chem2791565215661200410.1074/jbc.M31162920014739303
|
|
11
|
G BaumgartenP KnuefermannN NozakiN
SivasubramanianDL MannJG VallejoIn vivo expression of
proinflammatory mediators in the adult heart after endotoxin
administration: the role of toll-like receptor-4J Infect
Dis18316171624200110.1086/32071211343210
|
|
12
|
J OyamaC Blais JrX LiuReduced myocardial
ischemia-reperfusion injury in toll-like receptor 4-deficient
miceCirculation109784789200410.1161/01.CIR.0000112575.66565.8414970116
|
|
13
|
JA BaurDA SinclairTherapeutic potential of
resveratrol: the in vivo evidenceNat Rev Drug
Discov5493506200610.1038/nrd206016732220
|
|
14
|
MY ShenG HsiaoCL LiuInhibitory mechanisms
of resveratrol in platelet activation: pivotal roles of p38 MAPK
and NO/cyclic GMPBr J Haematol139475485200717868048
|
|
15
|
JG WoodB RoginaS LavuSirtuin activators
mimic caloric restriction and delay ageing in
metazoansNature430686689200410.1038/nature0278915254550
|
|
16
|
PM BritoNF SimoesLM AlmeidaTC
DinisResveratrol disrupts peroxynitrite-triggered mitochondrial
apoptotic pathway: a role for
Bcl-2Apoptosis1310431053200810.1007/s10495-008-0235-418584328
|
|
17
|
G LanzilliA CottarelliG NicoteraS GuidaG
RavagnanMP FuggettaAnti-inflammatory effect of resveratrol and
polydatin by in vitro IL-17 modulationInflammationMar 32011(Epub
ahead of print)
|
|
18
|
S BradamanteL BarenghiF
PiccininiResveratrol provides late-phase cardioprotection by means
of a nitric oxide- and adenosine-mediated mechanismEur J
Pharmacol465115123200310.1016/S0014-2999(03)01441-912650840
|
|
19
|
S DasGA CordisN MaulikDK
DasPharmacological preconditioning with resveratrol: role of
CREB-dependent Bcl-2 signaling via adenosine A3 receptor
activationAm J Physiol Heart Circ
Physiol288H328H335200510.1152/ajpheart.00453.200415345477
|
|
20
|
HS YounJY LeeKA FitzgeraldHA YoungS
AkiraDH HwangSpecific inhibition of MyD88-independent signaling
pathways of TLR3 and TLR4 by resveratrol: molecular targets are
TBK1 and RIP1 in TRIF complexJ
Immunol17533393346200510.4049/jimmunol.175.5.333916116226
|
|
21
|
T RuiQ FengM LeiErythropoietin prevents
the acute myocardial inflammatory response induced by
ischemia/reperfusion via induction of AP-1Cardiovasc
Res65719727200510.1016/j.cardiores.2004.11.01915664399
|
|
22
|
HP ChenM HeQR HuangD LiuM
HuangSasanquasaponin protects rat cardiomyocytes against oxidative
stress induced by anoxia-reoxygenation injuryEur J
Pharmacol5752127200710.1016/j.ejphar.2007.07.04317761161
|
|
23
|
H ReineckeM ZhangT BartosekCE
MurrySurvival, integration, and differentiation of cardiomyocyte
grafts: a study in normal and injured rat
heartsCirculation100193202199910.1161/01.CIR.100.2.19310402450
|
|
24
|
T KoyamaK TemmaT AkeraReperfusion-induced
contracture develops with a decreasing [Ca2+]i in single
heart cellsAm J Physiol261H1115H112219911928393
|
|
25
|
TM ScarabelliA StephanouE PasiniDifferent
signaling pathways induce apoptosis in endothelial cells and
cardiac myocytes during ischemia/reperfusion injuryCirc
Res90745748200210.1161/01.RES.0000015224.07870.9A
|
|
26
|
N MoorjaniS WestabyJ NarulaEffects of left
ventricular volume overload on mitochondrial and
death-receptor-mediated apoptotic pathways in the transition to
heart failureAm J Cardiol10312611268200919406269
|
|
27
|
TV ArumugamSL ChanDG JoGamma
secretase-mediated Notch signaling worsens brain damage and
functional outcome in ischemic strokeNat
Med12621623200610.1038/nm140316680150
|
|
28
|
W ChaoToll-like receptor signaling: a
critical modulator of cell survival and ischemic injury in the
heartAm J Physiol Heart Circ
Physiol296H1H12200910.1152/ajpheart.00995.200819011041
|
|
29
|
J ChaZ WangL AoCytokines link Toll-like
receptor 4 signaling to cardiac dysfunction after global myocardial
ischemiaAnn Thorac
Surg8516781685200810.1016/j.athoracsur.2008.01.04318442564
|
|
30
|
AJ ChongA ShimamotoCR HamptonToll-like
receptor 4 mediates ischemia/reperfusion injury of the heartJ
Thorac Cardiovasc
Surg128170179200410.1016/j.jtcvs.2003.11.03615282452
|
|
31
|
DJ KaczorowskiA NakaoR
VallabhaneniMechanisms of Toll-like receptor 4 (TLR4)-mediated
inflammation after cold ischemia/reperfusion in the
heartTransplantation8714551463200910.1097/TP.0b013e3181a36e5e19461481
|
|
32
|
Y WangAM AbarbanellJL HerrmannToll-like
receptor signaling pathways and the evidence linking toll-like
receptor signaling to cardiac ischemia/reperfusion
injuryShock34548557201010.1097/SHK.0b013e3181e686f520458266
|
|
33
|
RJ CarmodyYH ChenNuclear factor-kappaB:
activation and regulation during toll-like receptor signalingCell
Mol Immunol43141200717349209
|
|
34
|
B SalaunP RomeroS LebecqueToll-like
receptors’ two-edged sword: when immunity meets apoptosisEur J
Immunol37331133182007
|
|
35
|
A ShimamotoAJ ChongM YadaInhibition of
Toll-like receptor 4 with eritoran attenuates myocardial
ischemia-reperfusion injuryCirculation114Suppl
1S270274200610.1161/CIRCULATIONAHA.105.00090116820585
|
|
36
|
J DudleyS DasS MukherjeeDK DasResveratrol,
a unique phytoalexin present in red wine, delivers either survival
signal or death signal to the ischemic myocardium depending on
doseJ Nutr Biochem20443452200910.1016/j.jnutbio.2008.05.003
|
|
37
|
S MukherjeeJI DudleyDK DasDose-dependency
of resveratrol in providing health benefitsDose
Response8478500201010.2203/dose-response.09-015.Mukherjee21191486
|
|
38
|
DK DasN MaulikResveratrol in
cardioprotection: a therapeutic promise of alternative medicineMol
Interv63647200610.1124/mi.6.1.716507749
|