Introduction
The major cause of skin aging is both the passage of
time (intrinsic ageing) and cumulative exposure to external
influences (extrinsic ageing), such as UV radiation and smoking
(1,2). Intrinsically aged skin is
characterized by fine wrinkling and reduced elasticity, whereas
extrinsically-aged skin exposed to UV light is associated with the
induction of both deep wrinkles and a significant loss of
elasticity (3,4). Of the range of external factors
inducing skin ageing, UV radiation is considered a key cause of
skin damage, which is characterized by deep wrinkles, roughness,
laxity and pigmentation (5). In
addition, these phenotypical changes in the skin induced by direct
exposure to UV light are tightly associated with a significant
increase in the concentration of reactive oxygen species (ROS)
(6–8). An increase in ROS generation can
help destroy the intracellular antioxidant-defense mechanism, as
well as cause oxidative photodamage and oxidative stress to the
cellular constituents including DNA, lipids or proteins in skin
tissue (9,10). In particular, oxidative stress
plays an important role in initiating and driving the cascade
events that induce the cell responses following exposure to UV
radiation (11,12).
Many of the antioxidants and anti-photoaging
compounds, which act effectively against photodamage of the skin,
are available. The methanol extracts of Corallina pilulifera
exhibit antioxidative activity and have a protective effect on
UVA-induced oxidative stress in human dermal fibroblasts (13). In addition, esculetin isolated
from Fraxinus chinensis was reported to exhibit the
strongest scavenging activity against 1,1-diphenyl-2-picrylhydrazyl
(DPPH) and free radical scavenging activity in UVB-irradiated human
dermal fibroblasts (14). A
series of 2,2′-dithiocinnamate derivatives (DTCD) and 2,2′-dithio
(DTBD) or 2-thiobenzoate derivatives (TBD) were synthesized as new
anti-photoaging agents, which exhibited radical scavenging activity
and MMP-1 inhibitory activity (15). On the other hand, little has been
reported regarding the in vivo effects of specific blends
involving a sea buckthorn fruit extract on the homeostasis and
photodamage of skin although sea buckthorn has shown a potential
therapeutic effect on skin, lung and gastric disease.
Therefore, the present study examined the effects of
the oral intake of SFB on the fundamental properties of hairless
mice skin including wrinkle formation, skin water content, collagen
content and antioxidant status. These results provide strong
evidence of the potential of SFB in the prevention or alleviation
of UV-induced skin aging.
Materials and methods
Preparation of SFB
SFB was prepared from food-grade aqueous extracts of
five components, sea buckthorn fruit extract, blueberry extract,
collagen, hyaluronic acid and pure natural honey. Table I lists the source and content (%)
of SFB. To prepare the SFB solution, the five components were well
mixed at 90°C for 10 min and filtered through 3M paper. Before the
treatment, SFB was diluted with dH2O to make two dilute
solutions (SFB30 and SFB50) with different concentrations (30 and
50%). A Vit which was used as the control, was prepared by adding
only a 21.3% extract of sea buckthorn fruit.
 | Table ISources of sea buckthorn fruit
blends. |
Table I
Sources of sea buckthorn fruit
blends.
| Source | Vendor | Contents (%) |
|---|
| Sea buckthorn fruit
extract | Vitaworld Co.,
Korea | 31.1 |
| Blueberry
extract | ESFood Co.,
Korea | 25.4 |
| Collagen | Amorepacific Co.,
Korea | 8.3 |
| Pure natural
honey | Shennong Honey
Bio-Tech Co., Ltd., China | 5.1 |
| Hyaluronic
acid | Bioland, Korea | 0.1 |
| Total | | 70 |
Care and use of animals
The animal protocol used in this study was reviewed
and approved based on ethical procedures and scientific care by the
Pusan National University-Institutional Animal Care and Use
Committee (PNU-IACUC; approval number PNU-2011-00198). Adult HR-1
hairless mice were purchased from Central Lab. Animal, Inc. (Seoul,
Korea) and handled at the Pusan National University Laboratory
Animal Resources Center accredited by Korea FDA (unit
number-000996). All mice were given a standard irradiated chow diet
(Purina Mills, Seongnam, Korea) ad libitum, and were
maintained in a specific pathogen-free (SPF) state under a strict
light cycle (light on at 06:00 h and off at 18:00 h) at 22±2°C and
50% relative humidity.
Experimental design and UV radiation
Eight-week-old hairless mice (n=35) were assigned to
one of five groups (n=7 per group): a no-radiation group (no
group), vehicle-treated group (vehicle group), vitamin-treated
group (Vit group), SFB30-treated group (SFB30 group) and
SFB50-treated group (SFB50 group). The four groups, either than the
no-radiation group, were exposed to UV light using a UV radiation
device for 6 weeks, and the mice were given access to the various
solutions ad libitum. The vehicle-treated group was given a
consistent volume of water daily, whereas the treatment groups were
given Vit, SFB30 or SFB50 diluted with distilled water. The body
weights of each mouse were measured using a chemical balance every
week. At 6 weeks after commencing the vehicle, Vit and SFB
treatments, the animals were sacrificed immediately using
CO2 gas to acquire blood and skin tissue samples. The
samples were stored in Eppendorf tubes at −70°C until assayed.
UV radiation
The minimal erythemal dose (MED) from the UV
irradiation device was determined using the procedures suggested in
previous studies (16,17). A UV irradiation device was made
from a TL20W/12RS UV lamp and Kodacel filter in a rectangular
parallelepiped box. The UV lamp (Philips, The Netherlands) had an
emission spectrum between 274 and 380 nm, which was composed of
10.2% UVC (275–290 nm), 53.5% UVB (290–320 nm), 25.3% UVA1 (320–340
nm) and 11.2% UVA2 (340–380 nm). Kodacel Sheeting 6805 Product
(Kodak, USA) was used to remove UVC wavelengths <290 nm in front
of the UV lamp. The irradiation intensity was measured at 30 cm
from the light source using a UVX Radiometer (UVP, USA). To
determine the 1MED, the dorsal skin of mice was exposed to
different doses of UV light and the formation of erythema was
detected after 24 h. Skin aging was induced by irradiation of 1MED
three times per week (Monday, Wednesday and Friday) for 6
weeks.
Evaluation of wrinkle formation
Wrinkle formation was measured using the procedure
established by our laboratory using a DETAX System II (MIXPAC) and
Double-Stick Disc (3M Health Care, Germany) (17). After 6 weeks, skin surface
impressions (replica) were prepared by applying silicon rubber in
mixed liquid form secreted from a DETAX System II to the dorsal
skin of the mice. The depth, number of wrinkles on each skin
impression was analyzed and classified into one of the four degrees
suggested by Bissett et al (18). In this analysis, grade 0 indicated
no wrinkle formation, grade 1 indicated some shallow wrinkles,
grade 2 indicated some wrinkles, and grade 3 indicated several deep
wrinkles (19).
Measurement of the skin moisture content,
transepidermal water loss (TEWL) and erythema dose
All three factors related to skin homeostasis were
assessed on the dorsal skin of each mouse using the appropriate
devices (20). TEWL was detected
using a Corneometer CM825 (Courage and Khazaka Electronics,
Cologne, Germany). In addition, the skin moisture content was
measured using a Tewameter TM300, and the erythema dose was
analyzed using a Mexameter MX18 (all were from Courage and Khazaka
Electronics) according to the manufacturer’s protocol. Each
detection was performed 3 times on every site on the dorsal skin of
hairless mice.
Western blotting
The proteins prepared from the skin tissues of the
vehicle, Vit or SFB-treated mice were separated by 4–20% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) for 3
h, and the resolved proteins were transferred to a nitrocellulose
membrane for 2 h at 40 V. Each membrane was incubated separately
with the primary antibody: anti-MMP-1 (SC-30069; Santa Cruz
Biotechnology, Inc., CA, USA), anti-MMP-9 (SC-10737; Santa Cruz
Biotechnology, Inc.), anti-collagen-1 (ab292; Abcam, Cambridge, UK)
and anti-actin (A5316; Sigma-Aldrich, Saint Louis, MO, USA)
overnight at 4°C. The membranes were washed with a washing buffer
(137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM
KH2PO4, and 0.05% Tween-20) and incubated
with horse-radish peroxidase-conjugated goat anti-rabbit IgG (Zymed
Laboratories, San Francisco, CA, USA) at a 1:1,000 dilution at room
temperature for 2 h. The membrane blots were developed using a
Chemiluminescence Reagent Plus kit (Pfizer, New York, NY, USA).
Histological analysis and observation by
optical microscopy
The skin tissues collected from the hairless mice
were fixed with 10% formalin for 12 h, embedded in paraffin wax,
and sectioned into 4-μm slices. The skin sections were then
stained with hematoxylin and eosin (H&E) (Sigma-Aldrich), and
observed by optical microscopy. The thickness of the epidermis and
dermis were measured using a Leica Application Suite (Leica
Microsystems, Switzerland).
Activity analysis of superoxide dismutase
(SOD)
The SOD activity in the skin tissue was detected
using a calorimetric assay and the reagents in the SOD Assay kit
(Dojindo Molecular Technologies, Inc., Japan). First, the skin
tissue (100 mg) was homogenized in 600 μl of sucrose buffer
(0.25 mol/l sucrose, 10 mmol/l HEPES, 1 mmol/l EDTA, pH 7.4) using
a glass homogenizer. The lysate was harvested from the mixture by
centrifugation at 10,000 × g for 60 min and stored at −70°C until
needed for the enzyme activity assay. To measure the SOD activity,
the sample lysate was diluted with the dilution buffer or saline as
follows: 1, 1/5, 1/52, 1/53, 1/54,
1/55, 1/56. Sample solution (25 μl)
was aliquoted into a well of 96-well plate for each blank or
sample, and 200 μl of the WST working solution was added. In
addition, an enzyme working solution (20 μl) was added to
each sample per well and mixed thoroughly. The enzyme reaction was
induced by incubating the mixture plate at 37°C for 20 min, and the
absorbance was measured using a spectrophotometer at 450 nm. The
SOD activity was calculated directly using the following equation:
SOD activity (inhibition rate %) = [Ablank 1 −
Ablank 3) − (Asample − Ablank
2)]/(Ablank 1 − Ablank 3) × 100
(Ablank 1, absorbance of blank 1; Ablank 2,
absorbance of blank 2; blank 3, absorbance of blank 3;
Asample, absorbance of sample).
Serum biochemical analysis
After the final day of drinking the vehicle, Vit and
SFB, all mice were fasted for 24 h and blood was collected from the
abdominal vein. Serum was obtained by centrifuging the blood after
incubation for 30 min at room temperature. Serum biochemical
components were assayed using a model 747 automated serum analyzer
(Hitachi, Tokyo, Japan). All assays were measured with fresh serum
using standard enzymatic methods. The measurements were conducted
in duplicate.
Statistical analysis
One-way ANOVA was used to determine the significant
differences between the no-radiation group and the UV radiation
groups (SPSS for Windows, Release 10.10, Standard Version, Chicago,
IL, USA). In addition, differences in the responses of the
vehicle-treated group and Vit or SFB-treated group in the UV
radiation group were evaluated using a post-hoc test (SPSS for
Windows, Release 10.10, Standard Version) of the variance and
significance levels. All values are reported as the mean ± SEM. A
P-value of <0.05 was considered significant.
Results
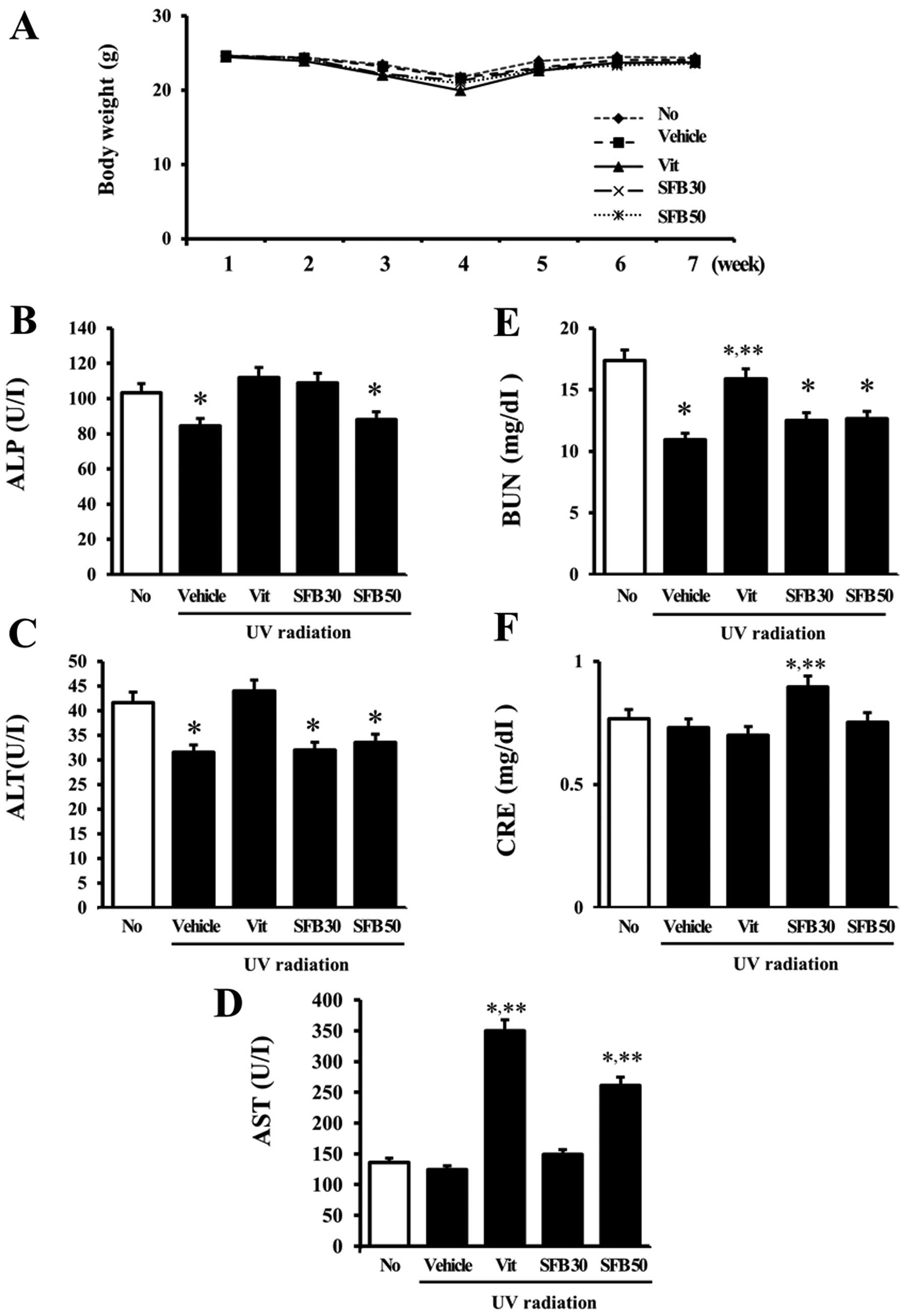
Effects of SFB on body weight and serum
biochemical indicators
The changes in the body weight and serum biochemical
indicators in the hairless mice were detected to determine the
animal toxicity of SFB. Although the body weights of all groups
were slightly lower at 4 weeks, there were no significant
differences in body weight between the treated groups at 6 weeks
(Fig. 1A). The hepatotoxicity of
SFB was determined by measuring the alkaline phosphatase (ALP),
alanine transaminase (ALT) and aspartate transaminase (AST)
concentrations. The ALP concentration was decreased only slightly
in the vehicle and SFB50-treated groups compared to the
no-irradiation group, even though the other groups had constant
levels (Fig. 1B). In addition, a
decreasing pattern for the ALT concentration was detected in all
irradiation groups except for the Vit-treated group (Fig. 1C). In the case of AST, however, a
significant increase was observed only in the Vit- and
SFB50-treated groups (Fig. 1D).
Furthermore, the concentration of BUN and creatine (CRE), which
indicate kidney toxicity, showed a different pattern (Fig. 1E and F). The BUN concentration was
lower in the UV radiation group than in the no radiation group,
even though the concentration in the Vit-treated group was slightly
higher than that in the vehicle-treated group. A significant
increase in CRE concentration was detected only in the
SFB30-treated group. These results support the suggestion that Vit
and SFB50 have low toxicity to the liver, even though SFB30 was
nontoxic to the liver of mice. On the other hand, SFB30 showed
lower toxicity to the kidneys of hairless mice.
Inhibition of UV-induced wrinkle
formation by SFB on the dorsal skin of hairless mice
The depth and number of wrinkles were measured in
the vehicle- or SFB-treated mice after 6 weeks of treatment to
determine if SFB treatment can inhibit wrinkle formation induced by
UV radiation. After UV radiation, the depth and number of wrinkles
were significantly higher in the vehicle-treated group than in the
no-irradiation group. On the other hand, there were significantly
fewer wrinkles induced by UV radiation in the Vit-, SFB30- and
SFB50-treated groups (Fig. 2A and
B). In particular, the largest decrease was detected in the
SFB50-treated group. Therefore, the consumption of Vit and SFB can
effectively inhibit wrinkle formation on the dorsal skin of
hairless mice.
Effects of SFB on the epidermis and
dermis of hairless mice
UV radiation on dorsal skin of mice induced a
significant change in skin histology (21). Therefore, the effects of the oral
intake of SFB on the epidermis and dermis of mice skin were
studied. After UV radiation, the epidermis and dermis were
significantly thicker in the vehicle-treated group than the
no-irradiation group. The number of adipocytes in the subcutaneous
region was also significantly higher in the vehicle-treated group
(Fig. 2C). On the other hand,
these were slightly lower in the Vit- and SFB30-treated group than
that in vehicle-treated group. The largest decrease was detected in
the SFB50-treated group (Fig. 2D and
E). Furthermore, all treated groups (Vit, SFB30 or SFB50)
showed a similar number of adipocytes to the no-irradiation group
(Fig. 2C). These results suggest
that the oral intake of Vit, SFB30 or SFB50 for 6 weeks can induce
a decrease in the thickness of epidermis and dermis, and the number
of adipocytes.
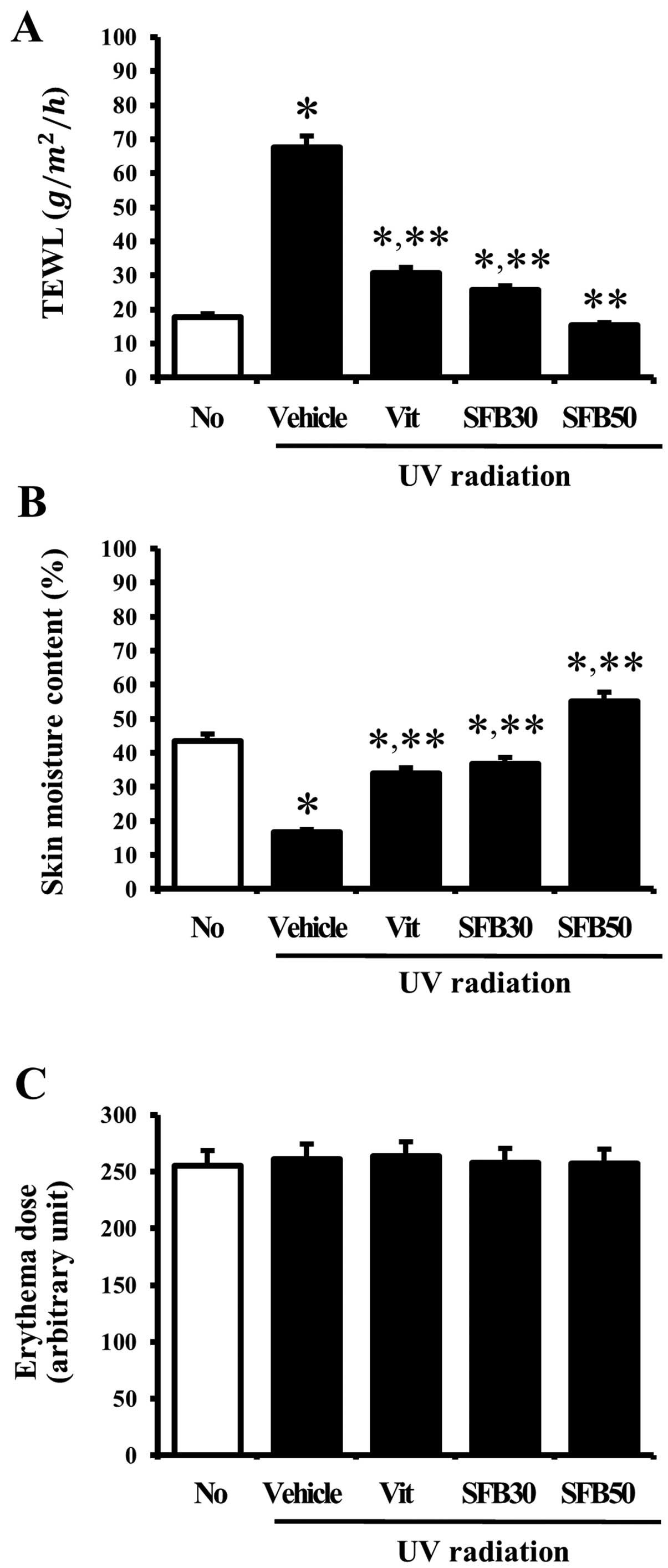
Effects of SFB on the intact skin
To determine the effects of SFB oral intake on
intact skin, the changes in transdermal water loss (TEWL), skin
moisture content and erythema dose were measured after UV radiation
for 6 weeks. The level of TEWL was significantly higher in the mice
exposed to UV light when this level was compared with that of the
no-radiation group. In the case of the Vit, SFB30 or SFB50
treatment, the level was lower than that of the vehicle-treated
group. In particular, the level of TEWL in the SFB50-treated group
was reduced to the level of the no-radiation group (Fig. 3A). In addition, the skin moisture
content showed an opposite pattern to TEWL in the UV radiation
group. The vehicle-treated group showed a lower skin moisture
content than the no-radiation group. On the other hand, their level
was increased significantly in the Vit, SFB30 and SFB50 group. The
greatest increase was observed in the SFB50 group, followed by the
SFB30 and Vit-treated groups (Fig.
3B). Nevertheless, there were no significant differences
between the groups in terms of the erythema dose at 6 weeks
(Fig. 3C). These results showed
that SFB can help maintain the water content in the skin of
hairless mice by preventing water loss and enhancing water
retention.
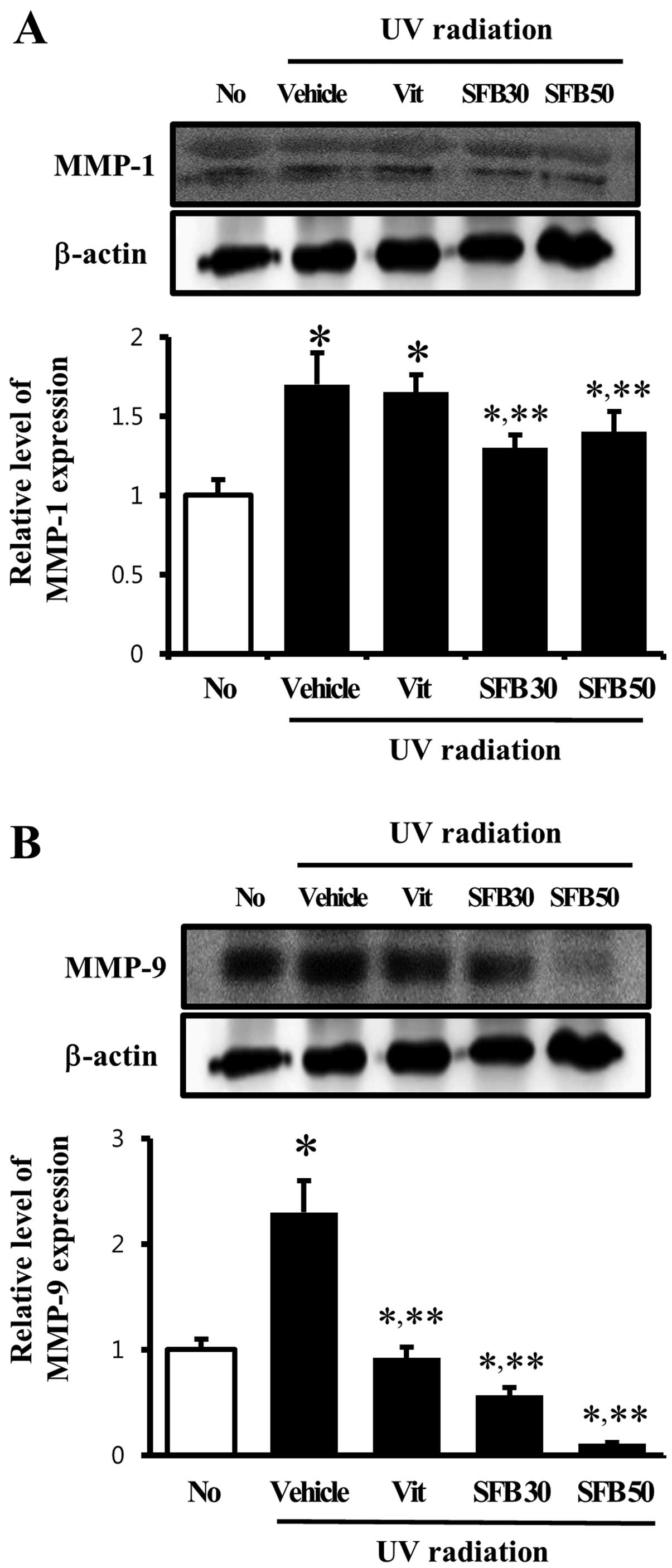
Effect of SFB oral intake on MMP
expression
The level of MMP expression is increased by a range
of factors including UV radiation, oxidative stress and cytokines
(22). Accordingly, the level of
MMP-1 and -9 proteins was examined by western blot analysis to
determine if SFB oral intake can reduce MMPs expression. In the
vehicle-treated group, the level of MMP-1 expression was
significantly higher than that of the no-radiation group. MMP-1
expression, however, was decreased only in the SFB-treated group,
whereas the level was maintained in the Vit-treated group (Fig. 4A). Furthermore, the expression
pattern of MMP-9 was similar to MMP-1 except in the Vit-treated
group. A high level of MMP-9 expression was detected in the
vehicle-treated group and a significant decrease was observed in
the SFB30 and SFB50-treated groups. In the case of the
SFB50-treated group, the level of MMP-9 expression decreased by 70%
(Fig. 4B). These results suggest
that MMP expression induced by UV radiation can be reduced to
normal by the oral intake of SFB.
Effect of SFB on collagen-1 content of
skin tissue
MMPs are the only known mammalian enzymes capable of
degrading a several types of collagen and gelatin (23). Therefore, this study examined
whether the alteration of MMP-1 and -9 expression can be regulated
the content of collagen-1 in skin tissue. Firstly, the collagen-1
level in the vehicle-treated group was lower than in the
no-radiation group. On the other hand, these levels in the Vit-,
SFB30- and SFB50-treated groups were increased significantly (∼2 or
2.5-fold) compared with the vehicle-treated group (Fig. 5). In the SFB30-treated group, the
level of collagen-1 expression was similar to that detected in the
SFB50-treated group. This suggests that the change in MMP-1 and -9
induced by UV radiation can induce a change in the collagen content
in skin tissue.
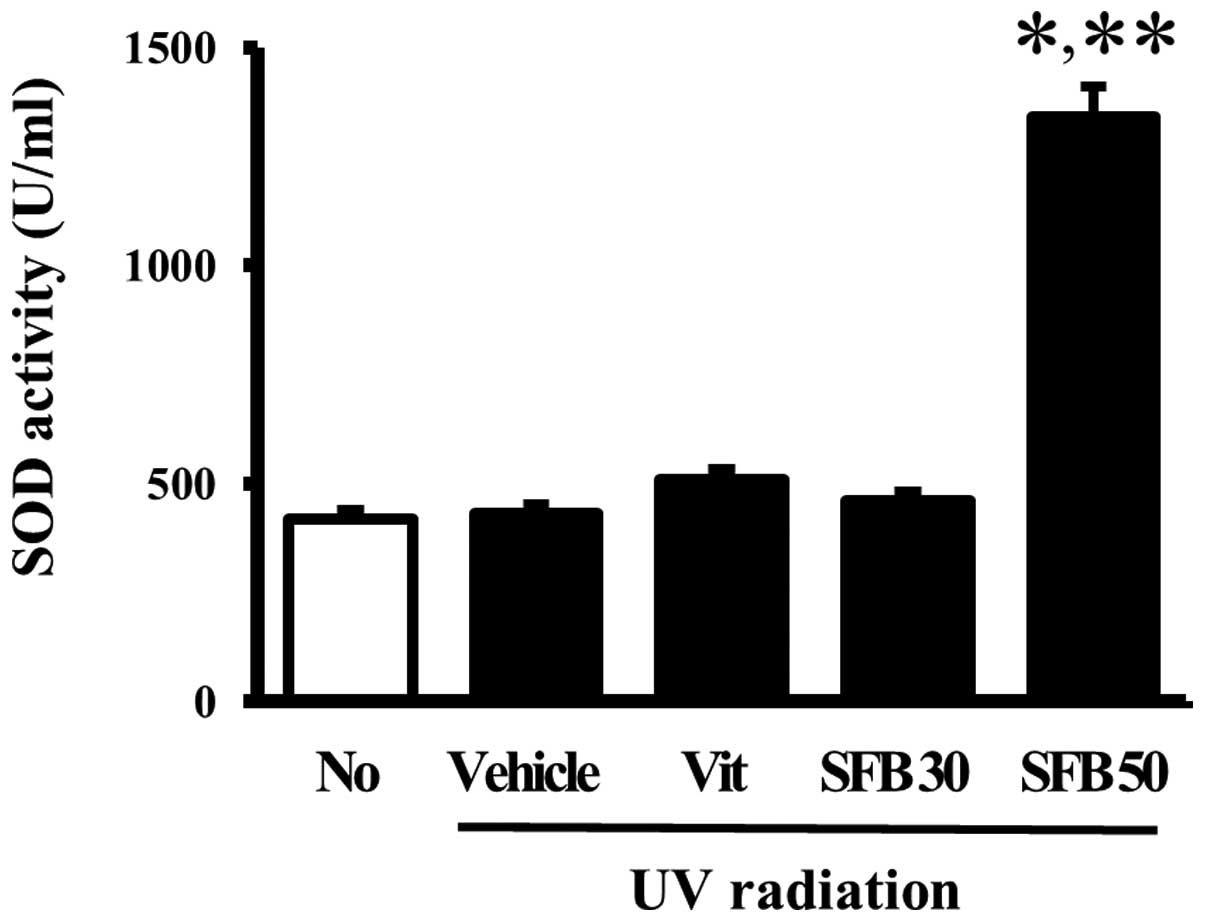
Effects of SFB on the SOD activity of
skin tissue
The effects of the oral intake of SFB on the
antioxidant status of mice were examined by measuring the SOD
activity in the skin tissue of the vehicle- and SFB-treated mice. A
significant difference in SOD activity was not observed between the
no-radiation group and vehicle-treated group. Furthermore, the SOD
activity did not change in the Vit- and SFB30-treated groups. On
the other hand, only the SFB50-treated group showed a high level of
SOD activity compared to vehicle-treated group (Fig. 6). These results implicate SFB50 as
a contributor to the increase in SOD activity in the skin of
hairless mice after a 6 weeks treatment.
Discussion
Sea buckthorn is a wild shrub growing at high
altitude in Asia and Europe and belong to the Elaeaganaceae family
(24). All part of this plant are
widely known as a rich source of biological active compounds such
as flavonoids, carotenoids, steroids, vitamins, tannins and oleic
acid (25,26). The plant had been using to treat
skin diseases, gastric ulcers, asthma and lung disorder for a long
time (27). Especially, the leaf
extracts, and fruit and seed oils of this plant have shown efficacy
on dermal wound healing and burns (28). However, there is no report on the
effect of sea buckthorn fruit on photoaging. In this study, we
investigated the function of sea buckthorn fruit on UV-induced skin
aging. Our results showed that SFB had the ability to prevent skin
aging through MMP-9 suppression and SOD activation.
The connective tissue in the skin is composed to
various types of collagens, elastin, fibronectin, proteoglycan and
other extracellular matrix (ECM) proteins. The normal structure of
the connective tissue is maintained by a balance between the
synthesis and degradation of the extracellular components. After UV
radiation, the excessive deposition of the abnormal elastin complex
and impairment of the collagen fibers were the most prominent
features observed in the skin (16). Therefore, collagen is believed to
be an important component to protect skin photodamage. As a part of
an ongoing study of the collagen function, Zhuang et al
(29) examined the effects of
collagen (JC) and collagen hydrolysate (JCH) from Jellyfish on the
mouse skin exposed to UV radiation. They focused on the abnormal
changes in the antioxidative indicators, such as the SOD activity,
glutathione peroxidase (GSH-Px) activity and catalase activity. The
present study showed the effects of the SFB on of the change in
skin morphology, water content, MMPs expression and collagen
content. Our group previously reported that SFB should be
considered as a potential therapeutic mixture for preventing and
treating skin aging induced by UV radiation. Furthermore, these
results provide evidence supporting the therapeutic potential of
SFB as a novel therapeutic drug.
MMPs including MMP-1, -3 and -9 can degrade all
types of ECM proteins, but can also process a number of bioactive
molecules (30,31). These enzymes participate in the
degradation of the basement membrane and ECM (23,32). In particular, MMP-1 plays
important roles in the degradation of dermal collagens in the ECM,
composed mainly of type-1 collagen during the aging of human skin
(8). Therefore, an analysis of
the MMP-1 expression level is a useful tool for screening
anti-aging components (33,34). In the present study, the level of
MMP-1 expression was measured in the skin of mice in different
groups. Only a significant change was detected in the SFB-treated
group, but it was not changed in the Vit-treated group. This shows
that SFB30 and SFB50 may work better on the anti-aging activity
than a Vit solution. Therefore, SFB 30 and SFB 50 have similar
effects to several compounds, such as tempol (35), compounds isolated from Fraxinus
chinensis (14) and
sulfur-containing cinnamate (15)
reported in previous studies.
MMP-9 can degrade collagen type IV, which is an
important component of the basement membrane during UV radiation in
the skin (36). In addition, this
protein has been associated with wound healing, tumor invasion and
angiogenesis, and can induce the secretion of inflammatory
cytokines (37,38). As shown Fig. 4, the level of MMP-9 expression in
the vehicle-treated group was more than twice that observed in the
no-radiation group. These results are similar to those of a
previous study (5). Significant
results were observed after the Vit, SFB30 and SFB50 treatments. In
particular, SFB50 almost completely inhibited MMP-9 expression in
the dorsal skin of hairless mice. Therefore, SFB is believed to be
a strong candidate for inhibiting MMP-9 expression.
Collagen I and III are distributed abundantly in the
dermis, and polymerized to produce extended mechanically stiff
fibrils, which impart tensile strength to the tissue (39,40). Therefore, wrinkle formation is
correlated with the regulation of collagen synthesis and
degradation (41). Most studies
reported that the level of collagen in the skin is decreased
significantly after UV radiation (35,42). In this study, the collagen level
in the radiation group was significantly lower than that in the
no-radiation group. On the other hand, this level was recovered
dramatically in the Vit-, SFB30- and SFB50-treated group. These
changes were similar to those reported in previous studies
examining the effects of tempol and retinoic acid on the collagen
regulation of skin. The data first suggested that the expression of
collagen might be affected more by the oral take of SFB30 and SFB50
than Vit.
Overall, these results suggest that SFB containing
collagen, sea buckthorn fruit extract and blueberry extract can
contribute to the protection and treatment of photodamaged skin
caused by UV exposure.
Acknowledgements
This study was supported by a grant to
PNU-Wellbeing Product Center from the Ministry of Knowledge Economy
(B0011529).
References
|
1.
|
M YaarBA GilchrestPhotoageing: mechanism,
prevention and therapyBr J
Dermatol157874887200710.1111/j.1365-2133.2007.08108.x17711532
|
|
2.
|
AK LangtonMJ SherrattCEM GriffithsREB
WatsonA new wrinkle on old skin: the role of elastic fibres in skin
ageingInt J Cosmet
Sci32330339201010.1111/j.1468-2494.2010.00574.x20572890
|
|
3.
|
R WarrenV GartsteinAM KligmanW MontagnaRA
AllendorfGM RidderAge, sunlight, and facial skin: a histologic and
quantitative studyJ Am Acad
Dermatol25751760199110.1016/S0190-9622(08)80964-41802896
|
|
4.
|
PG AgacheC MonneurJL LevequeJ
DerigalMechanical-properties and young’s modulus of human-skin in
vivoArch Dermatol Res2692212321980
|
|
5.
|
HK ChoiDH KimJW KimS NgadiranMR SarmidiCS
ParkLabisia pumila extract protects skin cells from
photo-aging caused by UVB irradiationJ Biosci
Bioeng109291296201010.1016/j.jbiosc.2009.08.478
|
|
6.
|
JH KimYH ChoSM ParkKE LeeJJ LeeBC LeeHB
PyoKS SongHD ParkYP YunAntioxidants and inhibitor of matrix
metalloproteinase-1 expression from leaves of Zostera marina
LArch Pharm Res27177183200410.1007/BF0298010315022719
|
|
7.
|
RJ BergeronG HuangWR WeimarRE SmithJ
WiegandJS McManisDesferrithiocin analogue based hexacoordinate iron
(III) chelatorsJ Med Chem461624200310.1021/jm020184n12502356
|
|
8.
|
M BrennanH BhattiKC NerusuN BhagavathulaS
KangGJ FisherJ VaraniJJ VoorheesMatrix metalloproteinase-1 is the
major collagenolytic enzyme responsible for collagen damage in
UV-irradiated human skinPhotochem
Photobiol784348200310.1562/0031-8655(2003)078%3C0043:MMITMC%3E2.0.CO;212929747
|
|
9.
|
H YasuiH SakuraiChemiluminescent detection
and imaging of reactive oxygen species in live mouse skin exposed
to UVABiochem Biophys Res
Commun269131136200010.1006/bbrc.2000.225410694489
|
|
10.
|
KM HansonJD SimonEpidermal trans-urocanic
acid and the UV-A-induced photoaging of the skinProc Natl Acad
Sci951057610578199810.1073/pnas.95.18.105769724745
|
|
11.
|
CD RopkeVV da SilvaCZ KeraDV MirandaRL de
AlmeidaTC SawadaSB BarrosIn vitro and in vivo inhibition of skin
matrix metalloproteinases by Pothomorphe umbellata root
extractPhotochem
Photobiol82439442200610.1562/2005-06-29-RA-59616613496
|
|
12.
|
YS LeeDQ JinSM BeakES LeeJA KimInhibition
of ultraviolet-A-modulated signaling pathways by asiatic acid and
ursolic acid in HaCaT human keratinocytesEur J
Pharmacol476173178200310.1016/S0014-2999(03)02177-012969763
|
|
13.
|
R PallelaNY YoungSK KimAnti-photoaging and
photo-protective compounds derived from marine organismsMar
Drugs811891202201010.3390/md804118920479974
|
|
14.
|
BC LeeSY LeeHJ LeeGS SimJH KimJH KimYH
ChoDH LeeHB PyoTB ChoeAnti-oxidative and photo-protective effects
of coumarins isolated from Fraxinus chinensisArch Pharm
Res3012931301200710.1007/BF0298027018038908
|
|
15.
|
CC ChiangTC ChangHJ TsaiLY HsuSynthesis
and biological evaluation of sulfur-containing cinnamate and
salicy-late derivativesChem Pharm
Bull56369373200810.1248/cpb.56.36918310951
|
|
16.
|
CH ParkMJ LeeJP KimID YooJH
ChungPrevention of UV radiation-induced premature skin aging in
hairless mice by the novel compound Melanocin APhotochem
Photobiol82574578200610.1562/2005-07-26-RA-62316613515
|
|
17.
|
SH NamSE JungYK LeeJE KimEP LeeHW ChoiHS
KimJH LeeYJ JungCY LeeTopical application of selenium can
significantly relieve UV-induced skin aging in hairless miceLab
Anim Res263745201010.5625/lar.2010.26.1.37
|
|
18.
|
DL BissettR ChatterjeeDP
HannonPhotoprotective effect of topical anti-inflammatory agents
against ultraviolet radiation-induced chronic skin damage in the
hairless mousePhotodermatol Photoimmunol
Photomed715315819902076370
|
|
19.
|
K TsukaharaS MoriwakiT FujimuraY
TakemaInhibitory effect of an extract of Sanguisorba
officinalis L. on ultraviolet-B-induced photodamage of rat
skinBiol Pharm Bull249981003200111558584
|
|
20.
|
H BabaA MasuyamaC YoshimuraY AoyamaT
TakanoK OhkiOral intake of Lactobacillus
helveticus-fermented milk whey decreased transepidermal water
loss and prevented the onset of sodium dodecylsulfate-induced
dermatitis in miceBiosci Biotechnol Biochem7418232010
|
|
21.
|
I KoshiishiE HorikoshiH MitaniT
ImanariQuantitative alterations of hyaluronan and dermatan sulfate
in the hairless mouse dorsal skin exposed to chronic UV
irradiationBiochim Biophys
Acta1428327333199910.1016/S0304-4165(99)00081-110434051
|
|
22.
|
GJ FisherSC DattaHS TalwarZQ WangJ VaraniS
KangJJ VoorheesMolecular basis of sun-induced premature skin ageing
and retinoid
antagonismNature379335339199610.1038/379335a08552187
|
|
23.
|
JL Lauer-FieldsD JuskaGB FieldsMatrix
metalloproteinases and collagen
catabolismBiopolymers661932200210.1002/bip.1020112228918
|
|
24.
|
A GuptaR KumarK PalV SinghPK BanerjeeRC
SawhneyInfluence of sea buckthorn (Hippophae rhamnoides L.)
flavone on dermal wound healing in ratsMol Cell
Biochem2901931982006
|
|
25.
|
A RousiThe genus Hippophae L., a
taxonomic studyAnn Bot Fenici81772271971
|
|
26.
|
T BeveridgeTS LiBD OomahA SmithSea
buckthorn products: manufacture and compositionJ Agric Food
Chem4734803488199910.1021/jf981331m10552673
|
|
27.
|
H SuleymanK GumustekinS TaysiS KelesN
OztasanO AktasK AltinkaynakH TimurF AkcayS AkarBeneficial effects
of Hippophae rhamnoides L. on nicotine induced oxidative
stress in rat blood compared with vitamin EBiol Pharm
Bull25113311362002
|
|
28.
|
A GuptaR KumarK PalPK BanerjeeRC SawhneyA
preclinical study of the effects of sea buckthorn (Hippophae
rhamnoides L.) leaf extract on cutaneous wound healing in
albino ratsInt J Low Extrem Wounds488922005
|
|
29.
|
Y ZhuangH HouX ZhaoZ ZhangB LiEffects of
collagen and collagen hydrolysate from jellyfish (Rhopilema
esculentum) on mice skin photoaging induced by UV irradiationJ
Food Sci74183188200910.1111/j.1750-3841.2009.01236.x19723203
|
|
30.
|
AZ EisenJJ JeffreyJ GrossHuman skin
collagenase. Isolation and mechanism of attack on the collagen
moleculeBiochim Biophys
Acta151637645196810.1016/0005-2744(68)90010-74967132
|
|
31.
|
GS LazarusJR DanielsJ LianMC BurleighRole
of granulocyte collagenase in collagen degradationAm J
Pathol6856557819724340976
|
|
32.
|
R VisseH NagaseMatrix metalloproteinases
and tissue inhibitors of metalloproteinases: structure, function,
and biochemistryCirc
Res92827839200310.1161/01.RES.0000070112.80711.3D12730128
|
|
33.
|
SR PinnellCutaneous photodamage, oxidative
stress, and topical antioxidant protectionJ Am Acad
Dermatol48119200310.1067/mjd.2003.1612522365
|
|
34.
|
GJ FisherS DattaZ WangXY LiT QuanJH ChungS
KangJJ Voorheesc-Jun-dependent inhibition of cutaneous procollagen
transcription following ultraviolet irradiation is reversed by
all-trans retinoic acidJ Clin
Invest106663670200010.1172/JCI9362
|
|
35.
|
SX YanXY HongY HuKH LiaoTempol, one of
nitroxides, is a novel ultraviolet-A1 radiation protector for human
dermal fibroblastsJ Dermatol
Sci37137143200510.1016/j.jdermsci.2004.11.00515734282
|
|
36.
|
S AmanoY OguraN AkutsuY MatsunagaK KadoyaE
AdachiT NishiyamaProtective effect of matrix metalloproteinase
inhibitors against epidermal basement membrane damage: skin
equivalents partially mimic photoageing processBr J
Dermatol1533746200510.1111/j.1365-2133.2005.06968.x
|
|
37.
|
S KimY KimY LeeKH ChoKH KimJH
ChungCholesterol inhibits MMP-9 expression in human epidermal
keratinocytes and HaCaT cellsFEBS
Lett58138693874200710.1016/j.febslet.2007.06.07417643435
|
|
38.
|
S OnoueT KobayashiY TakemotoI SasakiH
ShinkaiInduction of matrix metalloproteinase-9 secretion from human
keratinocytes in culture by ultraviolet B irradiationJ Dermatol
Sci33105111200310.1016/j.jdermsci.2003.08.00214581136
|
|
39.
|
J GoslineM LillieE CarringtonP GueretteC
OrtleppK SavageElastic proteins: biological roles and mechanical
propertiesPhilos Trans R Soc Lond B Biol
Sci357121132200210.1098/rstb.2001.102211911769
|
|
40.
|
AJ HeimWG MatthewsTJ KoobDetermination of
the elastic modulus of native collagen fibrils via radial
indentationAppl Phys Lett89181902181903200610.1063/1.2367660
|
|
41.
|
S ChenI KissKM TramposchEffects of
all-trans retinoic acid on UVB-irradiated and non-irradiated
hairless mouse skinJ Invest
Dermatol98248254199210.1111/1523-1747.ep125560661732390
|
|
41.
|
FT VicentiniYM FonsecaDL PitolMM IyomasaMV
BentleyMJ FonsecaEvaluation of protective effect of a water-in-oil
microemulsion incorporating quercetin against UVB-induced damage in
hairless mice skinJ Pharm Pharm Sci13274285201020816012
|