Introduction
The prevalence of non-alcoholic fatty liver disease
(NAFLD) is considered one of the manifestations of metabolic
syndrome. It is currently recognized that NAFLD is a common
metabolic change that increases the risk of chronic liver diseases
resulting from excessive fat accumulation in the liver. A number of
studies have been published linking adiposity with inflammation and
increased liver disease. Visceral white adipose tissue (AT) is
currently considered the key depot linked with obesity-related
systemic metabolic disturbances. Obesity is associated with chronic
inflammation, evidenced by increased levels of chemokines/cytokines
in AT and liver and increased accumulation and activation of
macrophages in AT and liver (1–3).
NAFLD is closely linked to obesity, diabetes, hyperlipidemia,
insulin resistance and is considered to represent a hepatic
manifestation of metabolic syndrome (4). In addition, excess fat accumulation
in hepatocytes may lead to hepatocellular injury via direct
cellular cytotoxicity mediated by free fatty acids (FFAs),
oxidative stress, lipid peroxidation, mitochondrial impairment and
cytokine-induced hepatotoxicity (5,6).
Furthermore, excessive fat accumulation in the liver damages
mitochondria, which are the primary cellular sites for fatty acid
utilization (7). Mitochondrial
dysfunction may also play a crucial role in the induction of a
‘two-hit’ model, as mitochondria are involved in the β-oxidation of
FFAs and are the most important cellular source of reactive oxygen
species (ROS). In this study, we aimed to find treatments that
would improve mitochondrial function in fatty liver disease in
obese mice.
Recently, a role of the inflammatory response in
obesity and non-alcoholic steatohepatitis (NASH) was established.
Currently, inflammation overactivity in both the liver and AT is
considered to contribute to metabolic disorders. The
pharmacological blockade of the inflammation has shed light on the
treatment of obesity and NASH (8,9) by
acting on the adipose and liver tissue. The polyphenol, curcumin,
is generally regarded as having potent antioxidant,
anti-inflammatory, and anti-carcinogenic properties (10). Curcumin modulates multiple
molecular targets and has potent anti-inflammatory activities,
which may contribute to its therapeutic role in obesity and
obesity-related metabolic diseases. Despite the potential
importance of curcumin in regulating inflammatory signaling and
lipid metabolism, its role in controlling obesity-related hepatic
inflammation has not been examined in vivo. Previous studies
have shown that curcumin reduces the expression of certain
inflammatory molecules in obese animals (11,12). However, these studies did not
establish the curcumin effect associated with lipotoxicity-induced
mitochondrial abnormalities that sensitize the liver to additional
pro-inflammatory insults. Therefore, we conducted the current study
to examine the hypothesis that increased macrophage infiltration in
the liver of obese mice induces inflammatory response and impairs
mitochondrial function in the liver tissue. These results
demonstrate that curcumin is a critical regulator of
obesity-induced inflammation and mitochondrial biogenesis in obese
mice.
Materials and methods
Experimental animals
C57BL/6J ob/ob mice have a mutation that
prevents leptin synthesis, resulting in a phenotype of obesity,
hepatomegaly, severe steatosis and high serum levels of
aminotransferase (13). Male
ob/ob C57BL/6J mice were obtained from the Jackson
Laboratory (Bar Harbor, ME). Mice were housed 5/cage and maintained
under a 12-h light, 12-h dark cycle with ad libitum access
to food and water. Upon receipt at the age of 8 weeks, the mice
were randomized to receive a normal diet. At the age of 13 weeks,
these mice were further randomized as regards an addition to their
predesignated diet of a 1 or 3% by weight admixture of curcumin or
no additive. Curcumin was purchased from Sigma (St. Louis, MO), and
the purity was >94%. Curcumin was dissolved in corn-oil (final
concentration of corn-oil <1%) and mixed with the homogenized
normal diet to form the final concentration of 3% (w/w). This
concentration showed the most protective effect on
obesity-associated inflammation as previously reported (12). The animals were divided into 4
groups: i) wild-type mice fed with a normal diet, ii) ob/ob
mice fed with a normal diet, iii) ob/ob mice fed with a
curcumin-supplemented diet (1%) and iv) ob/ob mice fed with
a curcumin-supplemented diet (3%). Each experimental group
consisted of 5 animals and all mice remained on their assigned diet
until they were sacrificed by CO2 asphyxiation at the
age of 21 weeks. All protocols were conducted in accordance with
the Guide for the Care and Use of Laboratory Animals and were
approved by the Chang Gung University Animal Care and Use
Committee.
Biochemical measurement, histological and
immunohistochemical analysis
Epididymal fat pads and liver tissue were harvested
immediately after euthanization, weighed, flash frozen in liquid
nitrogen, and stored at −80°C. Liver tissue was fixed in 10%
formalin, embedded in paraffin, cut into 5-μm-thick sections, and
stained with hematoxylin and eosin (H&E). Staining was
performed using standard techniques by the Pathology Core of the
Research Center at the Chang Gung Memorial Hospital and examined
under a light microscopy by an experienced pathologist.
Immunostaining for F4/80 (BioLegend, San Diego, CA) and neutrophil
(Abcam, Cambridge, MA) was performed in sections using the specific
antibodies and an avidin-biotin complex immunoperoxidase method.
Changes in glutathione (GSH) levels were determined by
high-performance liquid chromatography (HPLC) (14). The thiobarbituric acid reactive
substances (TBARS) were determined as described previously
(15). To determine the serum
alanine aminotransferase (ALT) levels, commercial kits (Roche
Diagnostics GmbH, Manheim, Germany) were used. The concentrations
of adipose tumor necrocis factor-α (TNF-α), monocyte chemotactic
protein-1 (MCP-1), and interleukin-6 (IL-6) were determined using
sandwich ELISA. The capture and detection antibodies against rat
TNF-α, MCP-1 and IL-6 were purchased from R&D systems
(Minneapolis, MN).
Oil Red O staining and liver triglyceride
(TG) content
To visualize the fat droplet accumulation, frozen
liver tissue sections were stained with Oil Red O. Liver samples
were used to assay TG concentration using the Triglyceride
Quantification kit (Randox, Antrim, UK).
NF-κB activity assay
Nuclear protein extracts were prepared from lean
control liver tissue and curcumin-fed ob/ob mice using the
nuclear extract kit (BioVision, Mountain View, CA) according to the
manufacturer’s instructions. NF-κB activity was determined by the
NF-κB EZ-Transcription Factor assay (Millipore).
Reverse transcription-polymerase chain
reaction (RT–PCR) and real-time PCR analysis
Total RNA was extracted from the epididymal fat and
hepatic tissue using the guanidinium-phenol-chloroform method.
Total RNA (5 μg) was reverse-transcribed using the RevertAid™ First
Strand cDNA Synthesis kit according to the manufacturer’s
instructions. The cDNA was amplified using the TaqDNA polymerase
kit (Fermentas, Vilnius, Lithuania). RT-PCR products were separated
by electrophoresis on a 3% agarose gel and quantified by ImageQuant
5.2 software (Healthcare Bio-Sciences, Philadelphia, PA). Real-time
PCR was performed on a LightCycler 1.5 apparatus (Roche Diagnostics
GmbH) using the LightCycler FastStart DNA MasterPLUS SYBR-Green I
kit according to the manufacturer’s protocol. Mitochondrial DNA
(mtDNA) copy number was determined by real-time PCR as previously
described (16).
Western blot analysis
Liver tissue was lysed with distilled water
containing protease inhibitors and a Bio-Rad Rapid Coomassie kit
was used to determine the total protein concentration. Protein (50
μg) was run on a 10% SDS-polyacrylamide gel and transferred to a
polyvinylidene difluoride membrane. Immunoblotting was performed
with various mouse or rabbit monoclonal or polyclonal antibodies,
followed by the incubation of the appropriate secondary antibody
coupled with horseradish peroxidase. The blot was developed with a
chemiluminescence system (ECL; Amersham, Piscataway, NJ) according
to the manufacturer’s instructions. The optical densities of the
bands were quantified using the GS-700 Imaging Densitometer
(Bio-Rad, Hercules, CA).
Mitochondrial respiratory complexes and
adenosine triphosphate (ATP) measurement
The mitochondrial respiratory complex activity was
analyzed as previously described (17). The ATP contents of liver
homogenates were measured with the ATP bioluminescence assay kit HS
II according to the manufacturer’s instructions (Roche Diagnostics
GmbH). The luminescence value was normalized by the protein
concentration and the luminescence ratio was compared with the
ob/ob mice group.
Statistical analysis
Data were presented as the means ± SEM. The
statistical analysis were performed using a one-way analysis of
variance followed by the Student Newman-Keuls multiple-range test.
A value of P<0.05 was considered to indicated a statistically
significant difference.
Results
Consistent with previous reports (12), curcumin treatment of obese mice
markedly induced weight loss as compared with the vehicle group
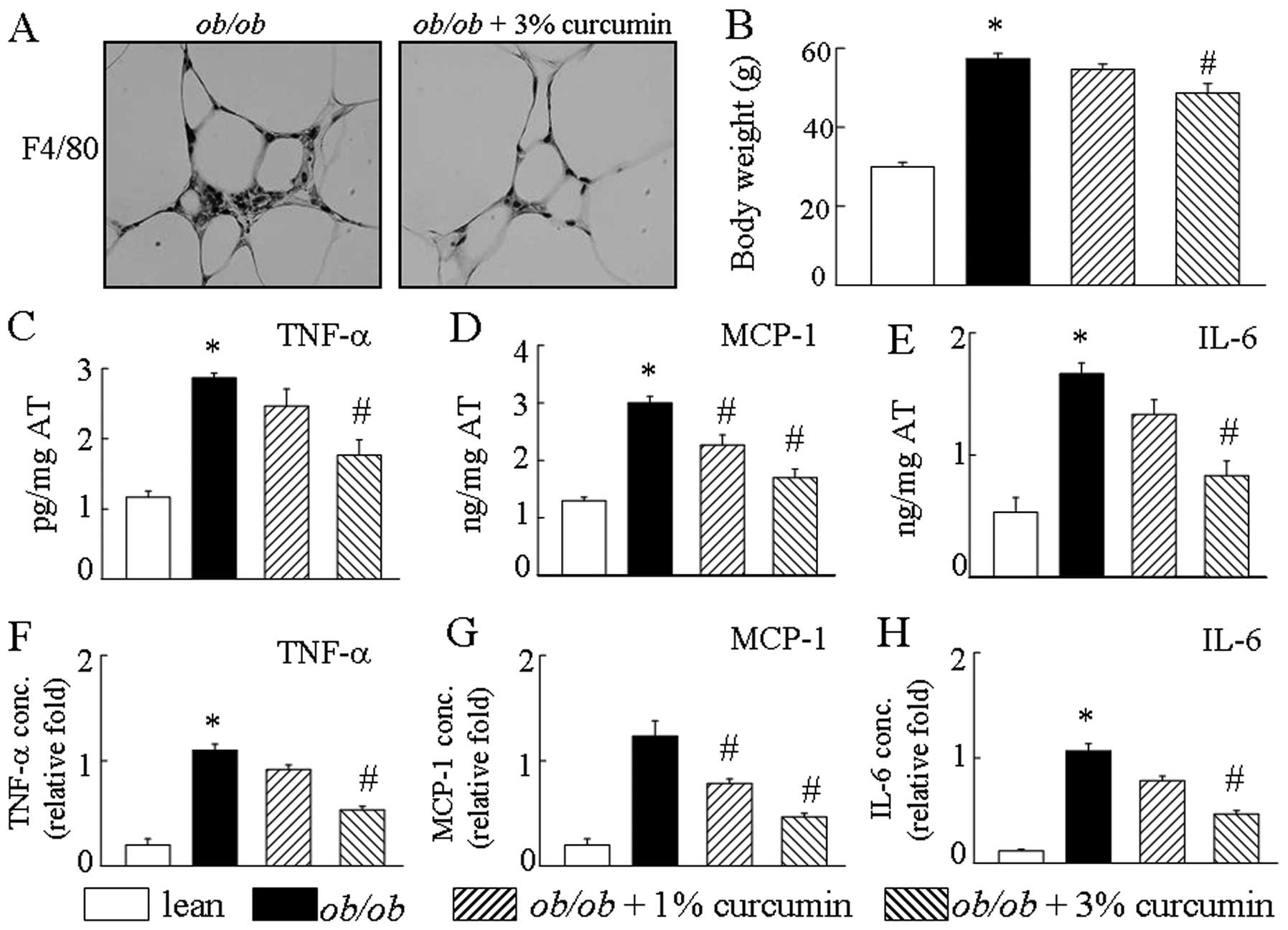
(Fig. 1B). After an 8-week
treatment with curcumin, the mice exhibited lower body weight,
smaller epididymal fat pads, smaller livers and less total fat mass
than obese mice. Compared to the lean mice, the ob/ob mice
had an increased number of F4/80-positive cells in the AT. Compared
to the ob/ob mice, the curcumin-treated groups had
significantly different levels of macrophage cell markers (Fig. 1A). Additionally, obese mice han an
increased production of MCP-1, IL-6 and TNF-α by AT. Curcumin
treatment in obese mice significantly decreased AT TNF-α, IL-6, and
MCP-1 mRNA levels compared to those in the curcumin-free controls
(Fig. 1C–E). Treatment with
curcumin caused a drastic reduction in the secretion of
pro-inflammatory cytokines, including TNF-α, MCP-1 and IL-6 in the
serum of obese mice, in a dose-dependent manner (Fig. 1F–H). Treatment with curcumin
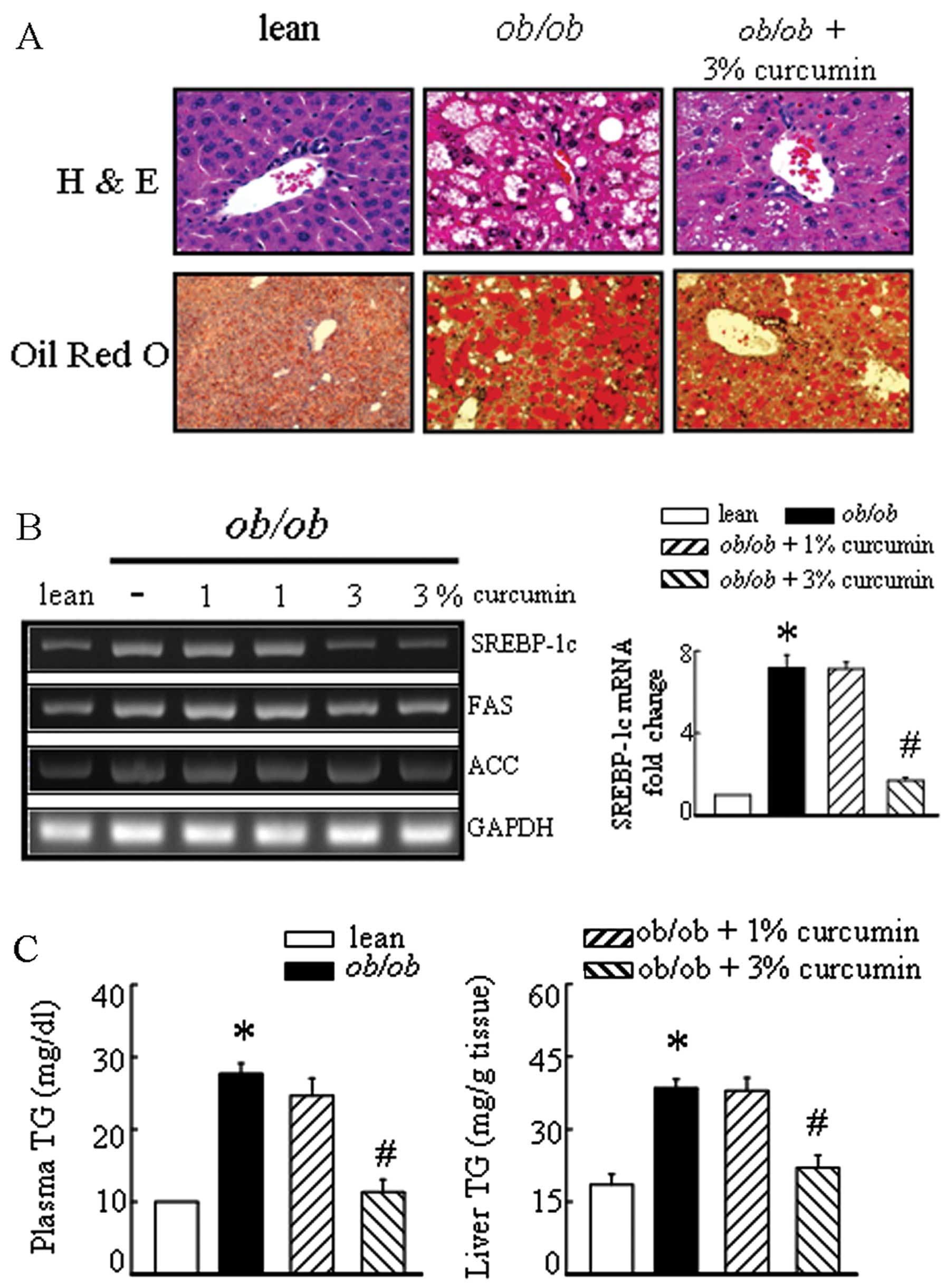
markedly decreased plasma and liver TG levels (Fig. 2C). Significant liver fatty
degeneration and piecemeal necrosis of hepatocytes were observed in
the obese mice (Fig. 2A). There
were many red lipid deposits in the hepatocytes of the obese group.
The number of lipid deposits decreased with curcumin, especially in
the high-dose therapeutic groups (Fig. 2). Sterol regulatory
element-binding protein-1c (SREBP-1c) mRNA levels tended to be
higher in the liver tissue of obese mice than in lean mice
(Fig. 2B). The expressions of the
target genes of SREBP-1c, including fatty acid synthase (FAS) and
acetyl-CoA carboxylase (ACC) were decreased in the liver of
curcumin-treated mice. Since the presence of low-grade inflammation
is linked to the transcriptional activation of NF-κB, we examined
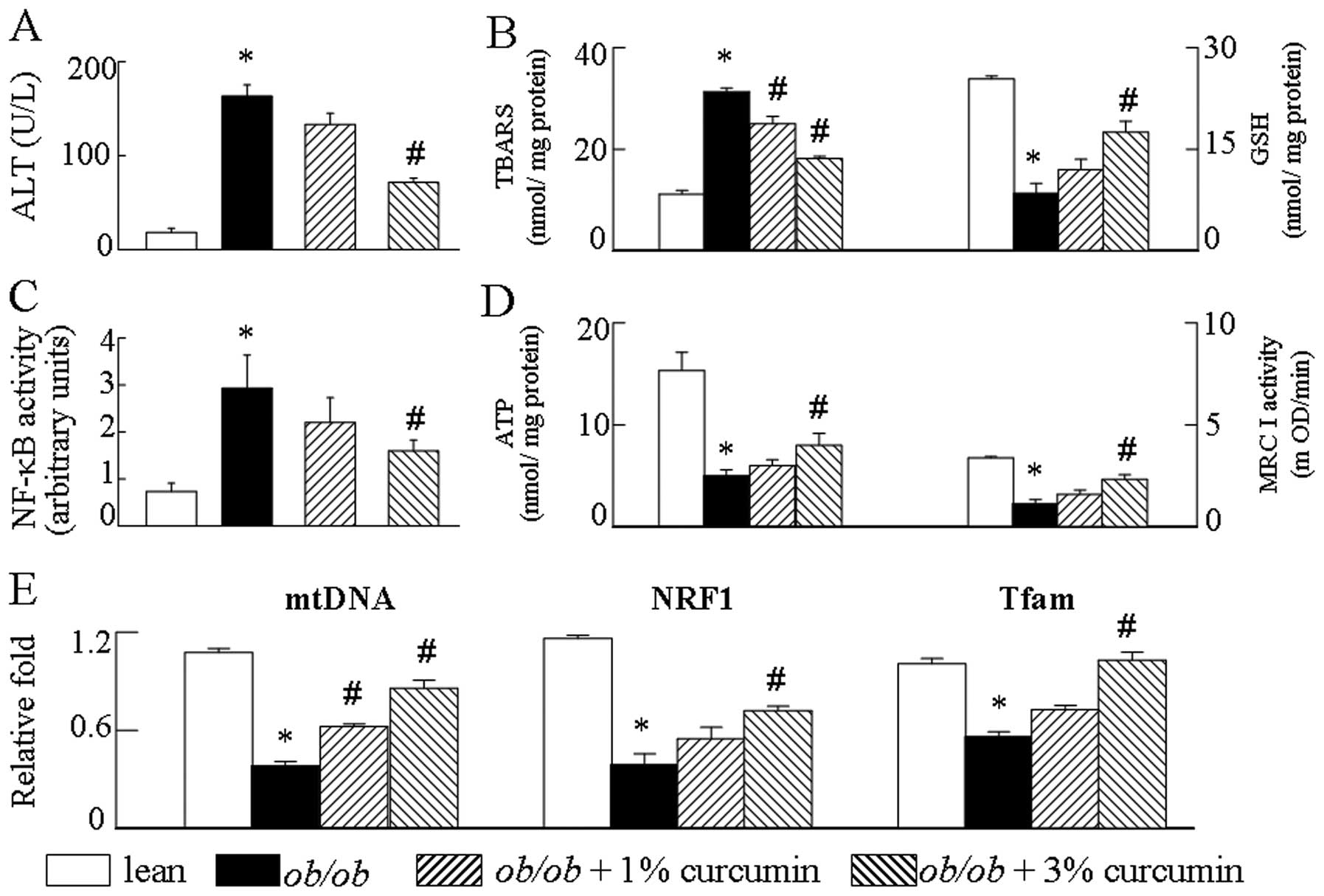
the effects of curcumin on NF-κB activity. Curcumin reduced NF-κB
activity (Fig. 4C) in the liver
tissue and decreased the production of the pro-inflammatory
cytokine MCP-1, IL-6 and TNF-α mRNA levels compared to obese mice
(Fig. 3B). These data suggest
that curcumin has a direct inhibitory effect on the chemokine
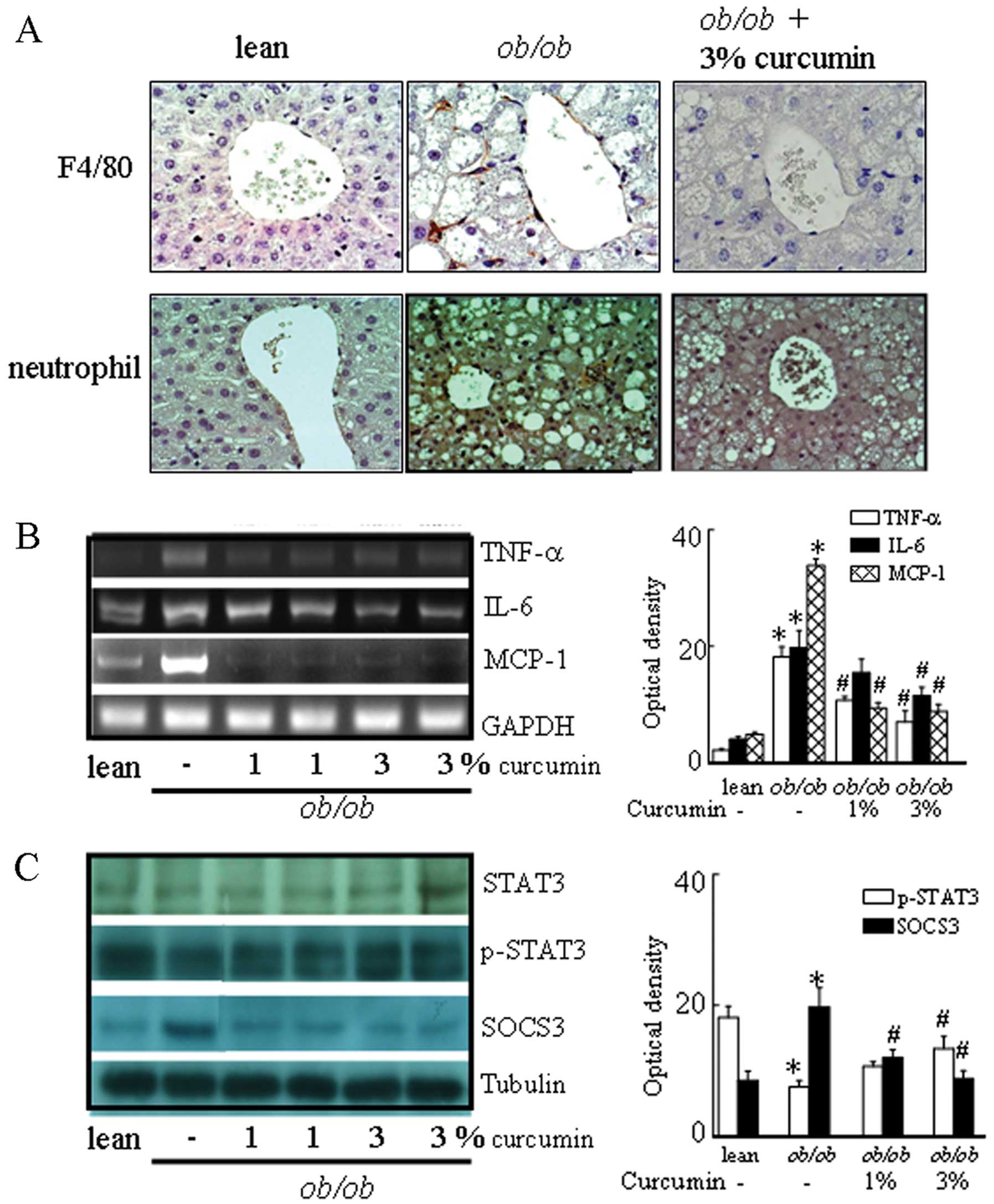
expression in the liver. In addition, obese mice had higher levels
of F4/80-positive cells, and a trend towards lower signal
transducer and activator of transcription 3 (STAT3) phosphorylation
in the liver than lean control mice. Curcumin-treated mice showed
significantly different levels of F4/80 and STAT3 phosphorylation
in the liver compared to obese mice (Fig. 3). In addition, suppressor of
cytokine signaling 3 (SOCS3) protein levels were significantly
decreased in obese mice treated with curcumin.
To verify that the attenuation of mitochondrial
dysfunction signal by curcumin is a relevant molecular mechanism by
which mitochondrial biogenesis is affected, obese mice were treated
with curcumin. Obvious mtDNA copy numbers, boigenesis disorder and
a decrease in mitochondrial respiratory chain (MRC) complex I
activities and ATP levels were observed in the obese group compared
to the lean control group (Fig.
4D). Since mitochondrial biogenesis was affected by curcumin
treatment (Fig. 4E), we
investigated whether curcumin affected the MRC. We found that
complex I activity was altered after curcumin prevented the
reduction of MRC in obese mice (Fig.
4D). We then determined whether obesity may affect both hepatic
TBARS content and GSH levels. TBARS levels in obese mice were much
higher compared to those of the lean control mice. Conversely,
obesity led to a significant reduction in hepatic GSH levels. As
expected, the results demonstrate that the presence of curcumin
suppresses the TBARS generation and upregulates the GSH expression
in obese mice. These observations suggest that curcumin attenuates
obesity-induced aspects of mitochondrial dysfunction, including the
depression of the GSH and the production of TBARS (Fig. 4B).
Discussion
In the present study, we discovered a protective
function of curcumin in hepatic steatosis. The protective role of
curcumin may be observed in various animal models to different
extents. Curcumin modulates multiple molecular targets and has
potent anti-inflammatory activities, which may contribute to its
therapeutic role in obesity and obesity-related metabolic diseases.
To the best of our knowledge, our results demonstrate for the first
time that curcumin-treated obese mice had decreased inflammatory
response in AT, liver and blood. The decreases in inflammation with
curcumin treatment may contribute to its beneficial effects on
mitochondrial dysfunction associated with obesity-induced liver
steatosis.
Weisberg et al (12) found that curcumin treatment of
diabetic mice also decreased inflammatory response in AT,
particularly in subcutaneous fat, as compared with diabetic
controls. Based on the important role of inflammation in the
development of obesity-linked metabolic abnormalities, the reduced
inflammation in curcumin-treated obese mice may contribute to the
improvement in hepatic steatosis observed in these mice. There are
a number of observations in the literature linking adiposity with
inflammation and increased liver disease. Several studies have
conclusively demonstrated that a chronic low-grade systemic
inflammatory state, predominantly arising from AT and liver, plays
a critical role in the development of obesity-related diseases
(2,18,19). Thus, in AT, fat laden adipocytes
initiate the inflammatory response by producing cytokines and
chemokines, including MCP-1 and TNF-α, that promote the recruitment
of pro-inflammatory macrophages and further amplification of local
inflammation (2,18,19). Increased AT inflammation
contributes to insulin resistance by disrupting insulin-dependent
signaling pathways, leading to the increased delivery of FFAs to
the liver, and thereby contributing to hepatic steatosis (20). Moreover, fatty liver itself
induces a local subacute inflammatory state characterized by the
production of inflammatory mediators, such as TNF-α or IL-6, that
directly contribute to hepatic and systemic insulin resistance
(21).
The manifestations of the pro-inflammatory state
include an increased production of TNF-α and MCP-1 and an
upregulation of the NF-κB signaling pathway in the liver tissue.
The inhibition of NF-κB signaling confers protection from
obesity-induced inflammation in mouse models (22). Conversely, the stimulation of
hepatic NF-κB signaling is sufficient to increase the local hepatic
production of pro-inflammatory cytokines (23). The role of curcumin in
obesity-associated inflammation (12,24) and steatohepatitis (25) has been established. Therefore, the
fact that curcumin attenuates the inflammatory response associated
with obesity may prove useful in the medical management of patients
with hepatic steatosis. On the other hand, the majority of studies
have indicated that the STAT3-mediated signaling pathway is
associated with hepatic steatosis. The activation of STAT3 in
hepatocytes is known to ameliorate fatty liver. The overexpression
of a constitutively active form of STAT3 in the liver of db/db mice
reduces the lipid content in the liver (26). The results from our present sudy
show that treatment with curcumin induces STAT3 phosphorylation in
the liver and alleviates steatosis in obese mice. We postulate that
STAT3 activation is implicated in the protective function of
curcumin in hepatic steatosis. In addition, the anti-steatogenic
effect of curcumin is mediated, at least in part, via the
inhibition of SREBP-1c, a master transcription factor that controls
lipid synthesis, and subsequent suppression of hepatic lipogenesis.
Our findings showed that the presence of decreased inhibitory
signaling protein SOCS-3 expression and enhanced levels of its
target, phosphorylated STAT3 in obese mice treated with curcumin.
These significant findings shed light on a previous study by Ueki
et al (27), who reported
that the hepatic expression of SOCS-3 was elevated in the liver of
obese mice and that the exogenous expression of the SOCS-3 protein
induced SREBP-1c expression. Our findings show the concomitant
downregulation of SOCS-3 with decreased SREBP-1c gene expression in
the livers of obese mice treated with curcumin.
In addition to the effects on AT, obese mice also
exhibit fatty liver and liver inflammation, which contributes
substantially to obesity-linked mitochondrial dysfunction. A number
of mechanisms may be considered to explain the mitochondrial
dysfunction found in obese mice and NAFLD patients. One of them is
oxidative stress. ROS-induced depletion in mtDNA may severely
affect mitochondrial function and induces steatosis and liver
lesion (28). Such depletion may
impair the synthesis of complexes I, III, IV and V of the MRC. Our
study demonstrates that TBARS, a marker of oxidative stress, were
markedly increased in the liver of obese mice. Likewise, hepatic
GSH was decreased in these animals. The decreased activity of the
MRC complex I in obese mice is in part attributable to oxidative
stress, as treatment of obese mice with curcumin, improved the
activity of complex I of the MRC and inhibited the decline in ATP
production by affecting energy metabolism in the mitochondria.
Raised serum TNF-α levels have been demonstrated in patients with
fatty liver or NASH. The role of this cytokine in the pathogenesis
of mitochondrial dysfunction is strongly supported by the results
of the current study, as the administration of curcumin to obese
mice resulted in a marked increase in MRC activity. Based on the
highly positive correlation of liver NF-κB activation with
lipogenesis genes expression, the current results suggest that
curcumin ameliorates fatty liver in obese mice and restores liver
mitochondrial biogenesis. We demonstrate that the suppression of
NF-κB enhances the ability of curcumin to rescue liver from
obesity-induced mitochondrial dysfunction, strongly suggesting that
a decrease in NF-κB activity by curcumin is at least partially
responsible for this rescue of mitochondrial function.
Curcumin has been shown to prevent
hypertriglyceridemia and hepatic NF-κB activation (12,25,29). Our current study also indicates
that curcumin activates STAT3 signaling and inhibits hepatic
lipogenesis. We reveal that curcumin protects liver from
obesity-induced steatosis and the possibility that the
anti-inflammatory effect of curcumin is an integral part of the
improvement of mitochondrial function in hepatic steatosis.
Acknowledgements
This study was supported by grant no.
NSC98-2320-B-182-021-MY3 (T.-Y.L.) from the National Science
Council, Taiwan; and grant no. CMRPG5A0041 (H.-H.C.) from the Chang
Gung Memorial Hospital, Linkuo, Taiwan.
References
|
1.
|
JP DesprésI LemieuxAbdominal obesity and
metabolic syndromeNature4448818872006
|
|
2.
|
SP WeisbergD McCannM DesaiM RosenbaumRL
LeibelAW Ferrante JrObesity is associated with macrophage
accumulation in adipose tissueJ Clin
Invest11217961808200310.1172/JCI20031924614679176
|
|
3.
|
H WuXD PerrardQ WangJL PerrardVR PolsaniPH
JonesCW SmithCM BallantyneCD11c expression in adipose tissue and
blood and its role in diet-induced obesityArterioscler Thromb Vasc
Biol30186192201010.1161/ATVBAHA.109.19804419910635
|
|
4.
|
AJ SanyalAGA technical review on
nonalcoholic fatty liver
diseaseGastroenterology12317051725200210.1053/gast.2002.3657212404245
|
|
5.
|
E FabbriniS SullivanS KleinObesity and
nonalcoholic fatty liver disease: biochemical, metabolic, and
clinical
implicationsHepatology51679689201010.1002/hep.2328020041406
|
|
6.
|
H TilgAR MoschenEvolution of inflammation
in nonalcoholic fatty liver disease: the multiple parallel hits
hypothesisHepatology5218361846201010.1002/hep.2400121038418
|
|
7.
|
S WangA Kama tP PergolaA SwamyF TioK
CusiMetabolic factors in the development of hepatic steatosis and
altered mitochondrial gene expression in
vivoMetabolism6010901099201110.1016/j.metabol.2010.12.00121310443
|
|
8.
|
J ChenD LiuQ BaiJ SongJ GuanJ GaoB LiuX
MaY DuCelecoxib attenuates liver steatosis and inflammation in
non-alcoholic steatohepatitis induced by high-fat diet in ratsMol
Med Rep4811816201121643627
|
|
9.
|
PS HsiehKC LuCF ChiangCH ChenSuppressive
effect of COX2 inhibitor on the progression of adipose inflammation
in high-fat-induced obese ratsEur J Clin
Invest40164171201010.1111/j.1365-2362.2009.02239.x20039930
|
|
10.
|
S ShishodiaG SethiBB AggarwalCurcumin:
getting back to the rootsAnn NY Acad
Sci1056206217200510.1196/annals.1352.01016387689
|
|
11.
|
SK YekolluR ThomasB O’SullivanTargeting
curcusomes to inflammatory dendritic cells inhibits NF-κB and
improves insulin resistance in obese
miceDiabetes6029282938201121885868
|
|
12.
|
SP WeisbergR LeibelDV TortorielloDietary
curcumin significantly improves obesity-associated inflammation and
diabetes in mouse models of
diabesityEndocrinology14935493558200810.1210/en.2008-026218403477
|
|
13.
|
A KoteishA Mae DiehlAnimal models of
steatohepatitisBest Pract Res Clin
Gastroenterol16679690200210.1053/bega.2002.0332
|
|
14.
|
TY LeeFY ChenHH ChangHC LinThe effect of
capillarisin on glycochenodexcholic acid-induced apoptosis and heme
oxygenase-1 in rat primary hepatocytesMol Cell
Biochem3255359200910.1007/s11010-008-0019-819132499
|
|
15.
|
TY LeeHH ChangJH ChenML HsuehJJ KuoHerb
medicine Yin-Chen-Hao-Tang ameliorates hepatic fibrosis in bile
duct ligation ratsJ
Ethnopharmacol109318324200710.1016/j.jep.2006.07.04216989967
|
|
16.
|
L TedescoA ValerioC CervinoA CardileC
PaganoR VettorR PasqualiMO CarrubaG MarsicanoB LutzCannabinoid type
1 receptor blockade promotes mitochondrial biogenesis through
endothelial nitric oxide synthase expression in white
adipocytesDiabetes5720282036200810.2337/db07-1623
|
|
17.
|
R GonzálezG FerrínAB HidalgoI RanchalP
López-CilleroM Santos-GónzalezG López-LluchJ BriceñoMA GómezA
PoyatoN-acetylcysteine, coenzyme Q10 and superoxide dismutase
mimetic prevent mitochondrial cell dysfunction and cell death
induced by d-galactosamine in primary culture of human
hepatocytesChem Biol Interact18195106200919523936
|
|
18.
|
GS HotamisligilInflammation and metabolic
disordersNature444860867200610.1038/nature0548517167474
|
|
19.
|
SE ShoelsonL HerreroA NaazObesity,
inflammation, and insulin
resistanceGastroenterology13221692180200710.1053/j.gastro.2007.03.05917498510
|
|
20.
|
C PosticJ GirardContribution of de novo
fatty acid synthesis to hepatic steatosis and insulin resistance:
lessons from genetically engineered miceJ Clin
Invest118829838200810.1172/JCI3427518317565
|
|
21.
|
S ParekhFA AnaniaAbnormal lipid and
glucose metabolism in obesity: implications for nonalcoholic fatty
liver
diseaseGastroenterology13221912207200710.1053/j.gastro.2007.03.05517498512
|
|
22.
|
MC ArkanAL HevenerFR GretenS MaedaZW LiJM
LongA Wynshaw-BorisG PoliJ OlefskyM KarinIKK-beta links
inflammation to obesity-induced insulin resistanceNat
Med11191198200510.1038/nm118515685170
|
|
23.
|
HM ArafaCurcumin attenuates diet-induced
hypercholesterolemia in ratsMed Sci Monit11228234200515990684
|
|
24.
|
A ShehzadT HaF SubhanYS LeeNew mechanisms
and the anti-inflammatory role of curcumin in obesity and
obesity-related metabolic diseasesEur J
Nutr50151161201110.1007/s00394-011-0188-121442412
|
|
25.
|
IA LeclercqGC FarrellC SempouxA dela PeñaY
HorsmansCurcumin inhibits NF-kappaB activation and reduces the
severity of experimental steatohepatitis in miceJ
Hepatol41926934200410.1016/j.jhep.2004.08.01015582125
|
|
26.
|
H InoueW OgawaM OzakiS HagaM MatsumotoK
FurukawaN HashimotoY KidoT MoriH SakaueRole of STAT-3 in regulation
of hepatic gluconeogenic genes and carbohydrate metabolism in
vivoNat Med10168174200410.1038/nm98014716305
|
|
27.
|
K UekiT KadowakiCR KahnRole of suppressors
of cytokine signaling SOCS-1 and SOCS-3 in hepatic steatosis and
the metabolic syndromeHepatol
Res33185192200510.1016/j.hepres.2005.09.032
|
|
28.
|
C DemeilliersC MaisonneuveA GrodetA
MansouriR NguyenM TinelP LettéronC DegottG FeldmannD PessayreB
FromentyImpaired adaptive resynthesis and prolonged depletion of
hepatic mitochondrial DNA after repeated alcohol binges in
miceGastroenterology12312781290200210.1053/gast.2002.3595212360488
|
|
29.
|
JM LiYC LiLD KongQH HuCurcumin inhibits
hepatic protein-tyrosine phosphatase 1B and prevents
hypertriglyceridemia and hepatic steatosis in fructose-fed
ratsHepatology5115551566201010.1002/hep.2352420222050
|