Introduction
Hepatitis C virus (HCV) is a major cause of chronic
liver disease, with an estimated 170 million people infected
worldwide. In Japan, the carrier rate is estimated to be
approximately 1% of the general population. This rate increases
depending on age and reaches approximately 5% in individuals over
70 years of age. The main goal of treatment for chronic hepatitis C
is prevention of cirrhosis and hepatocellular carcinoma by
eradication of the virus. Interferon (IFN)-based therapy was
initiated in 1992, and efficacy of treatment regimens has improved
year by year. Although the HCV viral eradication rate is
approximately 5% following 24 weeks of treatment with conventional
IFN therapy, the therapeutic result of combined pegylated IFN and
ribavirin is ~55%. However, approximately half of patients treated
with pegylated-IFN do not achieve a sustained viral response
(1–3).
Due to the numerous side effects and the high cost
of treatment, it is important to understand the individual
mechanisms involved in non-response to treatment and to predict
therapeutic efficacy prior to treatment. It has been reported that
various viral and host factors are associated with the therapeutic
response.
The role of amino acid (aa) mutations within the
functional regions of non-structural 5A (NS5A) in relation to
therapeutic response has been reported by several researchers. In
1996, it was reported that a high number of mutations in the
IFN-sensitivity-determining region (ISDR) (aa 2209–2248) was
strongly related to the sustained viral response (SVR) to IFN
monotherapy in genotype 1b Japanese patients (4,5).
In 2008, high mutations in the IFN-ribavirin resistance-determining
region (IRRDR) (aa 2334–2379) were also related to the SVR to
combined pegylated-IFN and ribavirin therapy (6). The significance of these mutations
was also confirmed by studies carried out in different populations
in different countries (7).
Based on previous studies, factor analysis and
determination of NS5A viral mutations in relation to SVR of
patients treated with pegylated-IFN and ribavirin combination
therapy for HCV genotype 1b and a high viral load was carried out
in a collaborative study in Kobe, Japan.
Materials and methods
Sample collection
Serum samples were collected from chronic hepatitis
C patients with genotype 1b and a high viral load. A total of 96
patients (age 57.7±8.3 years; 45 males, 51 females) who were
treated by subcutaneous injections of pegylated-IFN-α-2b once every
week (1.5 μg/kg) (Pegintron; Schering-Plough, Innishannon, Country
Cork, Ireland) in combination with oral ribavirin (400–800 mg)
daily for 48 weeks between September, 2006 and June, 2008 were
enrolled. HCV-RNA in serum samples was examined at 4 weeks, at the
end of treatment and 6 months after the end of treatment. Serum
samples were collected and stored at −80°C until virological
examination. The rapid virological response (RVR) was defined as
undetectable HCV-RNA at 4 weeks. Patients who had persistent
undetectable serum HCV-RNA and normal serum alanine
aminotransferase (ALT) levels 6 months after the end of treatment
were considered to have an SVR.
The standard dosage of PEG-IFN (1.5 μg/kg) and
ribavirin (12 mg/kg) was determined depending on the weight-based
dose. Patients treated with >80% of the standard dosage were
considered as high drug adherence and patients treated with at
least one drug at <80% of the standard dosage were categorized
as a low drug adherence group.
This study was conducted by Kobe University Hospital
and 25 affiliated hospitals in Hyogo prefecture. The study protocol
was approved by the Ethics Committee of Kobe University Hospital,
and written informed consent was obtained from each patient before
treatment.
NS5A sequence analysis
HCV-RNA was extracted from 140 μl serum using a
commercial kit according to the manufacturer’s protocol (QIAmp
Viral RNA kit; Qiagen, Tokyo, Japan). The NS5A region of the HCV
genome was amplified and sequenced by nested RT-PCR using primer
sets (6). The aa sequences were
deduced and aligned using GENETYX Win software version 7.0 (Genetyx
Corp., Tokyo, Japan).
Statistical analysis
Differences in parameters, including all available
patient demographic, biochemical, hematological, and virological
data, as well as ISDR and IRRDR sequence variations factors, were
determined between the different patient groups by the Student’s
t-test for numerical variables, and Fisher’s exact probability test
for categorical variables.
Subsequently, univariate and multivariate logistic
analyses were performed to identify variables that independently
predict SVR. The odds ratios (OR) and 95% confidence intervals
(CIs) were also calculated. Positive and negative predictive values
of SVR were computed, and their significance levels were evaluated
using the sign test. All statistical analyses were performed using
the SPSS version 16 software (SPSS Inc., Chicago, IL). Unless
otherwise stated, a P-value of <0.05 was considered to indicate
a statistically significant result.
Results
Baseline characteristics and on-treatment
response in association with SVR
Baseline characteristics and on-treatment response
are summarized in Table I.
Overall, 42 cases out of 96 (44%) achieved an SVR. SVR patients
were significantly younger in age and had a higher rate of RVR than
the non-SVR patients. The prevalence of high drug adherence in SVR
patients (73%) was significantly higher than that in non-SVR
patients (46%) (P=0.03).
 | Table I.Comparison of the base
characteristics of the SVR and the non-SVR groups. |
Table I.
Comparison of the base
characteristics of the SVR and the non-SVR groups.
| Factor | SVR | Non-SVR | P-value |
|---|
| No. of patients
(%) | 42 (44%) | 54 (56%) | |
| Age, years | 55.1±8.6 | 59.7±7.5 | 0.005 |
| Males:Females | 22:20 | 23:31 | |
| BMI
(kg/m2) | 24.0±3.4 | 23.2±3.4 | 0.85 |
| ALT (IU/l) | 72.3±69.4 | 75.8±61.8 | 0.66 |
| PLT
(x104/mm3) | 17.7±4.9 | 17.0±5.3 | 0.68 |
| RVR | 15/38 | 3/49 | <0.001 |
| PPB/ITT | 30/41 (73%) | 25/54 (46%) | 0.03 |
Drug adherence to pegylated interferon
and ribavirin therapy
Due to various side effects, 31 patients were not
treated with a sufficiently high dosage. Table II summarizes the patient groups
with low and high drug adherence. Sixty-five (68%) patients had
high drug adherence to the therapy. Older age women tended to
require dose reductions. The SVR rate (35%) in patients with low
drug adherence was significantly lower than those (46%) with high
drug adherence.
 | Table II.Drug adherence of patients to
pegylated-interferon and ribavirin therapy. |
Table II.
Drug adherence of patients to
pegylated-interferon and ribavirin therapy.
| High drug
adherence | Low drug
adherence | P-value |
|---|
| No. of patients
(%) | 65 (68%) | 31 (32%) | |
| Age, years | 57.4±8.2 | 59.3±7.2 | 0.25 |
| Male:Female | 33:32 | 13:18 | |
| BMI
(kg/m2) | 23.6±2.8 | 23.5±4.3 | NS |
| ALT (IU/l) | 78.2±54.5 | 72.7±68.5 | 0.7 |
| PLT
(×104/mm3) | 16.3±5.6 | 16.7±4.6 | 0.8 |
| SVR | 30/65 (46%) | 11/31 (35%) | NS |
| ISDR ≥1 | 26/50 (52%) | 12/26 (46%) | NS |
| IRRDR ≥6 | 18/50 (36%) | 11/26 (42%) | NS |
Mutations in the NS5A region and
predictive indicators for SVR
Factor analysis in association with the SVR was
performed by per-protocol-based (PPB) analysis. The average number
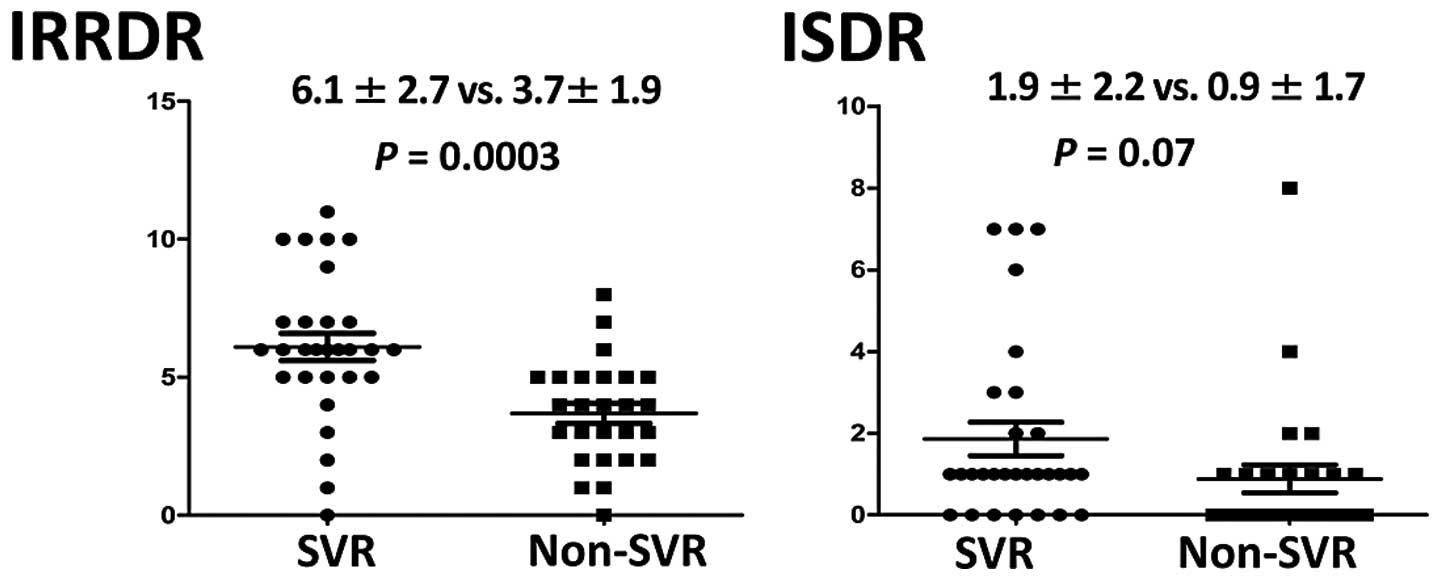
of mutations in IRRDR was significantly higher in the SVR group
(6.1±2.7) than that in the non-SVR group (3.7±1.9) (P=0.0003). The
average number of mutations in ISDR was also higher in the SVR
group (1.9±2.2) than that in the non-SVR group (0.9±1.7), but this
difference did not achieve statistical significance (Fig. 1). The SVR group and the non-SVR
group were compared based on the number of mutations in the NS5A
region. The prevalence of patients with ≥6 aa mutations within
IRRDR in the SVR group (60%) was significantly higher than that in
the non-SVR group (18%) (P= 0.02). Similarly, the prevalence of
patients with ≥1 aa mutation within ISDR in the SVR group (73%) was
higher than that in the non-SVR group (41%), but this difference
was not statistically significant (P=0.06). All patients with ≥6 aa
mutations in IRRDR and ≥1 aa mutation in ISDR achieved an SVR
(Table III). The positive
predictive values of SVR in patients with ≥6 aa mutations in IRRDR
was 78%. The sensitivity and specificity were 64 and 86%,
respectively.
 | Table III.Number of mutations in the NS5A
region in relation to sustained viral response (SVR). |
Table III.
Number of mutations in the NS5A
region in relation to sustained viral response (SVR).
| NS5A | Factor | SVR n (%) | Non-SVR n (%) | P-value |
|---|
| IRRDR | ≥6 | 9/15 (60)a | 3/17 (18)a | 0.02a |
| ≥4 | 13/15 (87) | 9/17 (53) | 0.05 |
| ISDR | ≥4 | 3/15 (20) | 1/17 (6) | 0.25 |
| ≥2 | 5/15 (33) | 3/17 (18) | 0.22 |
| ≥1 | 11/15 (73) | 7/17 (41) | 0.06 |
Factor analysis in association with the
SVR
Univariate and multivariate analyses are summarized
in Table IV. Univariate analysis
showed that ≥6 aa mutations in IRRDR and ≥1 aa mutation in ISDR
were strongly associated with an SVR. In addition, RVR and LVR were
also significant between the two groups. Multivariate analysis
revealed that ≥6 aa mutations in IRRDR (odds ratio 18.1) and RVR
(odds ratio 15.5) were significantly related to the SVR.
 | Table IV.Univariate and multivariate analyses
in relation to the sustained viral response (SVR). |
Table IV.
Univariate and multivariate analyses
in relation to the sustained viral response (SVR).
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|---|
| Factor | P-value | Odds ratio (95%
CI) | P-value |
|---|
| IRRDR (IRRDR ≥6 vs.
IRRDR ≤5) | 0.000 | 18.1
(3.5–94.4) | 0.001 |
| ISDR (ISDR ≥1 vs.
ISDR =0) | 0.000 | | |
| RVR | 0.017 | 15.5
(1.3–179.1) | 0.028 |
| LVR | 0.001 | | |
| HCV-RNA titer
(≥1000 vs. <1000) | 0.099 | | |
| Age (≥60 vs.
<60) | 0.072 | | |
| Gender (male) | 1.000 | | |
| PLT (≥15 vs.
<15) | 0.427 | | |
Discussion
Pegylated-IFN and ribavirin combination therapy has
been a standard treatment for patients with chronic hepatitis C.
However, HCV genotype 1 is more resistant to IFN treatment than
genotypes 2 or 3. In Japan, genotype 1b is the most prevalent and
it is important to predict the therapeutic response for these
patients prior to therapy (7–9).
In general, approximately 50% of patients with genotype 1b do not
achieve SVR even when using a combination of pegylated-IFN plus
ribavirin treatment (10). In the
present study, the overall SVR rate was 44% and this value was
slightly lower than that in a previous study (8). The reason for this is possibly
related to the patient age and drug adherence. The present study
showed that age, drug adherence and RVR in the SVR group were
significantly different than these values in the non-SVR group. The
SVR rate in patients younger than 65 years was 52% and was
significantly higher than that in patients over 65. In addition,
the SVR rate (46%) in patients with high drug adherence was higher
than that (35%) in patients with low drug adherence. There is no
doubt that elder patients have difficulties continuing therapy and
are forced to reduce the dosage or terminate treatment because of
side effects. In the present study, the percentage of patients
having low drug adherence was 32%, and the majority of patients in
this group were aged women. Physically and mentally, it is
frequently difficult to continue therapy for elder patients. The
average age of patients in Japan is older than that in most other
European countries and this is one of the important reasons for the
therapeutic difference among Japanese studies and those carried out
in other countries.
On-treatment response is an important factor for
predicting SVR; RVR 4 weeks following the initiation of treatment
has been reported to be a good predictor of SVR (11–13). In this study, RVR was an important
factor for predicting SVR by multivariate analysis. The positive
predictive value was 82% and RVR was confirmed to be a good
predictor in this study. However, even when patients are predicted
as good responders for IFN/RBV therapy, they do not always achieve
SVR as side effects result in dose reduction or termination of the
planned IFN/RBV treatment. It was also reported that drug adherence
is related to SVR (14). In this
study, 3 patients relapsed after achieving RVR. The first case was
over 65 years of age, the second case had low drug adherence, and
the third was an older patient over 65 years with low drug
adherence. Incomplete treatment is an important factor contributing
to the failure of achieving SVR. This result suggests the necessity
for prolonged therapy or therapeutic modification in patients with
RVR receiving a dosage reduction.
Mutations in several amino acids in the NS5A protein
have been described and are thought to play an important role in
response to IFN treatment. It has been reported that a high number
of mutations in ISDR and IRRDR are significantly associated with
SVR (6). In the present study,
patients with ≥1 aa mutation in ISDR and ≥6 aa mutations in IRRDR
tended to achieve SVR, which was supported by previous data
(6). For ISDR, the mutation
results are similar to previous studies (4,5).
Compared with ISDR, IRRDR was more strongly associated with SVR in
this study. Based on the multivariate analysis, only IRRDR was
associated with an SVR. Patients with more than 6 IRRDR mutations
had a higher SVR rate and it was the same as previous studies
(6). The positive predictive
value and sensitivity was >80%, suggesting it to be a good
predictive marker. All patients with ≥6 aa mutations and ≥1 aa
mutation in ISDR achieved SVR following pegylated-IFN and ribavirin
combination therapy. The importance of the NS5A mutation is still
controversial. It has been reported that a mutation in NS5A is not
related to the IFN response in European and American HCV strains
(15–18). However, the importance of NS5A was
reported in Asian HCV strains including Taiwan and Chinese strains
(19,20). To date, this inconsistency is
unclear but is partly related to the fact that HCV strains are
different depending on geographic distribution (21). Meta-analysis revealed that the
prevalence of a mutation in ISDR was 44.1% in Japanese and 24.8% in
European patients, respectively (21). Mutational studies are sometimes
inconsistent even among Japanese studies, suggesting that mutations
in the NS5A region vary based on different geographical regions
even in Japan.
The NS5A protein has a transcriptional activation
function and represses IFN-induced gene expression (22). In addition, the NS5A protein
interacts with antiviral protein PKR resulting in suppressed PKR
activity (23). It is possible
that mutations in the NS5A protein may affect the structural and/or
biological functions of NS5A and inhibit IFN activity (23,24). Mutations in E2-PePHD (aa 659–670),
PKRBD (aa 2209–2274) and NS5A-V3 (aa 2356–2379) are also reported
to be associated with IFN sensitivity (24,25).
Recent studies have shown that SNPs in the IL28B
region are strongly associated with response to IFN therapy
(26). In this study, genomic
factors in the host were not analyzed due to the pre-treatment
study design and informed consent. Therapeutic prediction can be
more accurate upon examination of host factors as well as viral
factors. In the near future, new drug therapies such as protease
and polymerase inhibitors called new direct-acting antivirals
(DAAs) will become available (27). Standard therapy for hepatitis C
virus will include combination therapies using DAAs and
pegylated-IFN plus ribavirin. However, the SVR rate by
telaprevir-based pegylated-IFN plus ribavirin combination therapy
(REALIZE study; phase III, randomized, double blind,
placebo-controlled study) was found to be as high as 31% in
patients who were non-responders to prior treatment (28). The viral response to pegylated-IFN
and ribavirin combination therapy is important for the development
of future combination therapies.
In conclusion, mutations in the NS5A region,
particularly in patients with more than 6 aa mutations in the IRRDR
region are strongly associated with the therapeutic response to
pegylated-IFN and ribavirin combination therapy.
References
|
1.
|
LB SeeffNatural history of chronic
hepatitis CHepatology36Suppl
1S35S46200210.1002/hep.184036070612407575
|
|
2.
|
D LavanchyThe global burden of hepatitis
CLiver Int297481200910.1111/j.1478-3231.2008.01934.x
|
|
3.
|
P MarcellinHepatitis B and hepatitis C in
2009Liver Int2918200910.1111/j.1478-3231.2008.01947.x
|
|
4.
|
N EnomotoI SakumaY AsahinaMutations in the
nonstructural protein 5A gene and response to interferon in
patients with chronic hepatitis C virus 1b infectionN Engl J
Med3347781199610.1056/NEJM1996011133402038531962
|
|
5.
|
N EnomotoI SakumaY AsahinaComparison of
full length sequences of interferon-sensitive and resistant
hepatitis C virus 1b: sensitivity to interferon is conferred by
amino acid substitutions in the NS5A regionJ Clin
Invest96224230199510.1172/JCI1180257542279
|
|
6.
|
A El-ShamyM Nagano-FujiiN SasaseS ImotoSR
KimH HottaSequence variation in hepatitis C virus nonstructural
protein 5A predicts clinical outcome of pegylated
interferon/ribavirin combination
therapyHepatology483847200810.1002/hep.22339
|
|
7.
|
K KumthipC PantipP ChusriCorrelation
between mutations in the core and NS5A genes of hepatitis C virus
genotypes 1a, 1b, 3a, 3b, 6f and the response to pegylated
interferon and ribavirin combination therapyJ Viral
Hepat18e117e125201110.1111/j.1365-2893.2010.01379.x
|
|
8.
|
MP MannsJG McHutchisonSC
GordonPeginterferon alfa-2b plus ribavirin compared with interferon
alfa-2b plus ribavirin for initial treatment of chronic hepatitis
C: a randomised
trialLancet358958965200110.1016/S0140-6736(01)06102-511583749
|
|
9.
|
MW FriedML ShiffmanKR ReddyPeginterferon
alfa-2a plus ribavirin for chronic hepatitis C virus infectionN
Engl J Med347975982200210.1056/NEJMoa02004712324553
|
|
10.
|
SJ HadziyannisH Sette JrTR
MorganPeginterferon-alpha2a and ribavirin combination therapy in
chronic hepatitis C: a randomized study of treatment duration and
ribavirin doseAnn Intern
Med140346355200410.7326/0003-4819-140-5-200403020-0001014996676
|
|
11.
|
ML YuCY DaiJF HuangRapid virological
response and treatment duration for chronic hepatitis C genotype 1
patients: a randomized
trialHepatology4718841893200810.1002/hep.2231918508296
|
|
12.
|
JW YuGQ WangLJ SunXG LiSC LiPredictive
value of rapid virological response and early virological response
on sustained virological response in HCV patients treated with
pegylated interferon alpha-2a and ribavirinJ Gastroenterol
Hepatol22832836200710.1111/j.1440-1746.2007.04904.x
|
|
13.
|
H JafferbhoyMH MillerZ El WahedJF
DillonPre-treatment prediction of response to pegylated-interferon
plus ribavarin for chronic hepatitis C using RVRJ
Hepatol5511621164201110.1016/j.jhep.2011.04.00521740939
|
|
14.
|
D TaniokaY IwasakiY ArakiFactors
associated with adherence to combination therapy of interferon and
ribavirin for patients with chronic hepatitis C: importance of
patient’s motivation and physician’s treatment experienceLiver
Int297217292009
|
|
15.
|
RT ChungA MontoJL DienstagLM
KaplanMutations in the NS5A region do not predict
interferon–responsiveness in American patients infected with
genotype 1b hepatitis C virusJ Med Virol583533581999
|
|
16.
|
S ZeuzemJH LeeWK RothMutations in the
nonstructural 5A gene of European hepatitis C virus isolates and
response to interferon
alfaHepatology25740744199710.1002/hep.5102503419049228
|
|
17.
|
M PatersonCD LaxtonHC ThomasAM AckrillGR
FosterHepatitis C virus NS5A protein inhibits interferon antiviral
activity, but the effects do not correlate with clinical
responseGastroenterology11711871197199910.1016/S0016-5085(99)70405-110535883
|
|
18.
|
M Torres-PuenteJM CuevasN
Jimenez-HernandezHepatitis C virus and the controversial role of
the interferon sensitivity determining region in the response to
interferon treatmentJ Med
Virol80247253200810.1002/jmv.2106018098147
|
|
19.
|
C ShenT HuL ShenL GaoW XieJ ZhangMutations
in ISDR of NS5A gene influence interferon efficacy in Chinese
patients with chronic hepatitis C virus genotype 1b infectionJ
Gastroenterol
Hepatol2218981903200710.1111/j.1440-1746.2006.04566.x17914967
|
|
20.
|
CH HungCM LeeSN LuMutations in the NS5A
and E2-PePHD region of hepatitis C virus type 1b and correlation
with the response to combination therapy with interferon and
ribavirinJ Viral
Hepat108794200310.1046/j.1365-2893.2003.00414.x12614464
|
|
21.
|
M PascuP MartusM HöhneSustained
virological response in hepatitis C virus type 1b infected patients
is predicted by the number of mutations within the NS5A ISDR: a
meta-analysis focused on geographical
differencesGut5313451351200410.1136/gut.2003.031336
|
|
22.
|
MJ GaleMJ KorthNM TangEvidence that
hepatitis C virus resistance to interferon is mediated through
repression of the PKR protein kinase by the nonstructural 5A
proteinVirology30217227199710.1006/viro.1997.84939143277
|
|
23.
|
MJ GaleMJ KorthMG KatzeRepression of the
PKR protein kinase by the hepatitis C virus NS5A protein: a
potential mechanism of interferon resistanceClin Diagn
Virol10157162199810.1016/S0928-0197(98)00034-89741641
|
|
24.
|
WP HofmannS ZeuzemC SarrazinHepatitis C
virus-related resistance mechanisms to interferon alpha-based
antiviral therapyJ Clin
Virol328691200510.1016/j.jcv.2004.08.00415653410
|
|
25.
|
J GervainA CzibulaJ SimonT
KalmarStructural analysis of the PKR-binding region of HCV 1b
samples from patients with chronic hepatitis C and the correlation
with IFN-sensitivityOrv Hetil144117911842003(In Hungarian).
|
|
26.
|
Y TanakaN NishidaM SugiyamaGenome-wide
association of IL28B with response to pegylated interferon-alpha
and ribavirin therapy for chronic hepatitis CNat
Genet4111051109200910.1038/ng.44919749757
|
|
27.
|
T AsselahY BenhamouP MarcellinProtease and
polymerase inhibitors for the treatment of hepatitis CLiver
Int295767200910.1111/j.1478-3231.2008.01928.x19207967
|
|
28.
|
N ForestierS ZeuzemTriple therapy with
telaprevir: results in hepatitis C virus-genotype 1 infected
relapsers and nonrespondersLiver
Int324450201210.1111/j.1478-3231.2011.02720.x22212571
|