Introduction
Evidence has demonstrated that individuals with
prehypertension are at an increased risk of developing hypertension
and suffering cardiovascular events, poor cognitive performance,
type 2 diabetes, as well as kidney damage (1–5).
Lifestyle modification is recommended as a basis for all subjects
belonging to this blood pressure (BP) category by current
guidelines for hypertension, and drug therapy is considered for
individuals with hypertension concomitant with diabetes or kidney
disease when a trial of lifestyle modification fails to reduce
their BP to 130/80 mmHg or less (6). However, poor adherence makes its
implementation into clinical settings difficult and relatively
ineffective. Recently published TROPHY and PHARAO trials
demonstrated that prehypertensive treatment with a
renin-angiotensin system (RAS) inhibitor over a relatively short
time reduces the risk of incident hypertension and is well
tolerated (7,8). In addition, studies using
spontaneously hypertensive rats (SHRs) also indicate that early and
transient treatment with an angiotensin-converting-enzyme inhibitor
(ACEI), angiotensin-receptor blockers (ARB) or aldosterone (Aldo)
antagonist attenuates the development of hypertension and exerts
cardiovascular protective effects up to an advanced age (9–11).
The aforementioned studies suggest that the
reninangiotensin-Aldo system (RAAS) blockade may be a feasible and
beneficial option for individuals with prehypertension, which
raises the question whether the effects are restricted only to RAAS
antagonists or whether they may also be extended to other forms of
therapies, such as calcium channel blockers (CCBs). To our
knowledge, this question remains unanswered.
CCBs are a heterogeneous class of drugs used in the
treatment of coronary heart disease and hypertension. Amlodipine, a
third-generation dihydropyridine CCB, is characterized by high
vascular selectivity and relatively low negative inotropic effects.
Previous studies involving animal models have proven that
amlodipine is effective in preventing hypertension-related left
ventricular hypertrophy (LVH) and dysfunction (12,13). However, research concerned with
its use in the prehypertensive stage remains limited.
In the present study, we aimed to compare the
effectiveness of prehypertensive treatment with losartan compared
with amlodipine in SHRs on long-term BP, renal and cardiac
protection, and to investigate the underlying mechanism of their
effects.
Materials and methods
Animals and pharmacological
treatment
A total of 72 four-week-old male SHRs (purchased
from Shanghai Slac Laboratory Animal Co., Ltd.) were randomly
divided into 3 groups and were administered losartan
[losartan-treated SHRs (SHR-Los), 20 mg/kg/day, n=24], amlodipine
[amlodipine-treated SHRs (SHR-Aml), 10 mg/kg/day, n=24] or saline
(SHRs, n=24) by gavage for 6 weeks. Age- and gender-matched
Wistar-Kyoto rats (WKYs, n=24) served as the normal control. Rats
were housed 5/cage under controlled temperature (22±1°C) and
humidity (60%) conditions, and were exposed to a 12-h dark-light
cycle. Standard food and tap water were supplied ad libitum.
All experiments were approved by the Animal Ethics Committee of
Fujian Medical University and performed in accordance with
institutional guidelines.
Experimental protocol
Rats were followed up until week 46. Systolic blood
pressure (SBP) was measured at weeks 4 and 10 and monthly after
treatment was stopped. Cardiac, renal structure and function,
circulating and local RAS components were repeatedly assessed at
weeks 14, 30 and 46, with 8 rats sacrificed/group at each time
point for analysis. Cardiac, renal Ang II type 1 receptor
(AT1R) and type 2 receptor (AT2R) protein
were determined at weeks 14 and 46. Cardiac brain natriuretic
peptide (BNP) mRNA was determined at week 46.
Blood pressure determinations
SBP was measured noninvasively in unanesthetized
rats by the tail-cuff method using a specialized pressure
transducer (PowerLab ML125/R NIBP System; ADInstruments) as
previously described (14). SBP
was determined when the cuff pressure corresponded to the
restoration of the first caudal artery pulse. Three consecutive
readings were averaged for each determination.
Evaluation of cardiac hypertrophy and
fibrosis
Under anesthesia with chloral hydrate (300 mg/100 g,
i.p.), the animals were sacrificed and the hearts were removed and
blotted dry, the left ventricle (LV) was carefully dissected and
weighed, and the left ventricular mass index (LVMI) (LV weight/100
g body weight) was calculated to indicate cardiac hypertrophy.
Then, the hearts were further incubated for 24 h in formalin,
embedded in paraffin and cut into 4-μm sections for Sirius
Red staining. Sections were carefully scanned with a light
microscope (Olympus, Japan) connected to a computer using the Image
Pro Plus 6.0 software for histomorphometry at a magnification of
×100. The collagen volume fraction (CVF), an index of cardiac
fibrosis, was determined as the percentage of the Sirius
Red-stained area/total myocardial tissue area.
Echocardiography
Transthoracic echocardiography was performed on the
animals in the left lateral decubitus position, after being
anesthetized with chloral hydrate (300 mg/100 g, i.p.). A vivid 7
echocardiographic system (GE Healthcare, USA) with a 10-MHz
transducer was used to obtain an M-mode echocardiogram from the
long axis view of the LV. The left ventricular end-diastolic
dimension (LVEDD), end-diastolic interventricular septum thickness
(IVSTd) and left ventricular ejection fraction (LVEF) were measured
following the guidelines of the American Society of
Echocardiography (15).
24-h urinary albumin and creatinine
clearance rate
The 24-h urine excretion was collected in metabolic
cages. These studies were performed during the same weeks as the
echocardiography. Urinary albumin was measured by
immnonephelometric analysis (BN-II analyzer; Dade Behring,
Eschborn, Germany). Creatine in serum and urine were measured by
the sarcosine oxidase enzymic method using an automatic
Biochemistry Analyzer (AU2700; Olympus) to calculate the creatinine
clearance rate (CCr) with the standard formula: CCr = (urinary
creatinine × 24-h urine flow)/(serum creatinine × 1440) mg/min.
Renal histology
The kidneys were removed from the animals under
pentobarbital sodium anesthesia, fixed in 4% formalin and embedded
in paraffin. Central cross-sections of the whole kidney including
the cortex and medulla were prepared for examination. The
glomerulosclerosis index (GSI) was measured in 4-μm
sections, stained with periodic acid Schiff. GSI was calculated in
both superficial and juxtamedullary glomeruli for the whole
interstitial area with a light microscope (Olympus) by a
nephropathologist in a blinded manner using a cumulative
semi-quantitative scoring as previously described (16).
Plasma, renal cortex tissue and
myocardium angiotensin II (Ang II) and Aldo
The heart and renal cortex tissues were weighed (100
mg), boiled and homogenized by Polytron in 1 ml PBS, respectively.
The homogenate was centrifuged at 3,500 rpm for 10 min. The tissue
supernatant and plasma Ang II and Aldo levels were determined by
radioimmunoassay using a commercially available kit (Beijing North
Institute of Biological Technology, Beijing, China).
Western blot analysis for AT1R
and AT2R proteins
Western blot analysis was performed as described by
Xu et al (17) Briefly, LV
tissue (10 mg) and renal cortex tissue (10 mg) were electrophoresed
on SDS-polyacrylamide gels under reducing conditions, respectively.
Proteins were transferred onto nitro-cellulose membranes, blocked
with 5% non-fat milk in TBS containing 0.05% Tween-20 (TBST) and
then incubated with an anti-AT1R antibody (1:500,
ab9391; Abcam), anti-AT2R antibody (1:800, ab19134;
Abcam) or anti-actin antibody (1:1000, sc-1616; Santa Cruz
Biotechnology, Inc.) overnight at 4°C. After 5 washes with TBST and
2 washes with TBS, the membranes were incubated for 1 h at room
temperature with horseradish peroxidase-conjugated secondary
antibody. Following another 2 washes with TBST, labeled proteins
were visualized using ECL (sc-2048; Santa Cruz Biotechnology, Inc.)
on high-performance chemiluminescence film. The intensity of the
bands was quantified by densitometry with image analysis software.
Results for AT1R and AT2R were expressed as a
ratio of AT1R or AT2R over β-actin.
Quantification of cardiac BNP mRNA
Total RNA from ventricular tissue was isolated with
TRIzol reagent (Invitrogen), and the concentration was determined
by measuring the absorbance at 260 nm. The RNA was transcribed into
cDNA using M-MLV reverse transcriptase (Promega) and amplified
(Takara PCR Amplification kit) using primers (Table I) specific to each gene of
interest. The cycle profiles were programed as follows: initial
5-min denaturation at 95°C followed by 35 cycles of denaturation at
95°C for 30 sec, annealing at 59 or 55°C (for BNP and GAPDH,
respectively) for 30 sec and extension at 72°C for 30 sec, with a
final extension at 72°C for 5 min. PCR products were analyzed by
1.5% agarose gel electrophoresis. The photodensity of amplified
cDNA product bands was quantified by densitometry. The BNP mRNA
abundance was defined as a ratio to GAPDH mRNA.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Primer | Sequence 5′ to
3′ | Annealing
temperature (°C) | Amplification
product (bp) |
|---|
| BNP | Up
GCTGCTTTGGGCAGAAGATAGA
Down ACAACCTCAGCCCGTCACA | 59 | 169 |
| GAPDH | Up
CAACGGCACAGTCAAGG
Down CCAGTGGATGCAGGGAT | 55 | 468 |
Statistical analysis
All analyses were performed with SPSS software
version 13.0 (Chicago, IL, USA). Continuous data are presented as
the means ± standard deviation (SD). Differences between groups
were compared by one-way analysis of variance, followed by LSD
t-test for multiple comparisons. P<0.05 values were considered
to indicate a statistically significant difference. For all tests,
a two-tailed P-value <0.05 was considered to indicate a
significant result.
Results
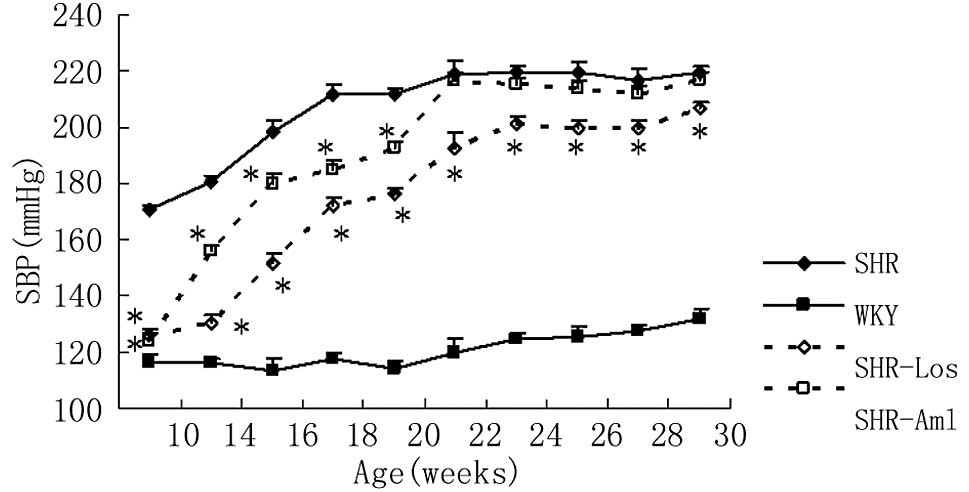
Systolic blood pressure
At week 10, the SBP in the untreated SHRs was
significantly higher compared to that in the WKYs (SHRs vs. WKYs:
170±5 vs. 116±8 mmHg, P<0.05). Prehypertensive treatment with
losatran and amlodipine equally reduced BP to a midway level
between untreated SHRs and WKYs (SHR-Los vs. SHR-Aml: 126±6 vs.
124±7 mmHg; P>0.05). After drug withdrawal, the SBP in SHR-Los
remained significantly lower compared to that in untreated SHRs
until week 46 (P<0.05), whereas SBP gradually increased to
untreated SHR levels in SHR-Aml from 30 weeks onwards (P>0.05)
(Fig. 1).
Evaluation of cardiac hypertrophy and
fibrosis
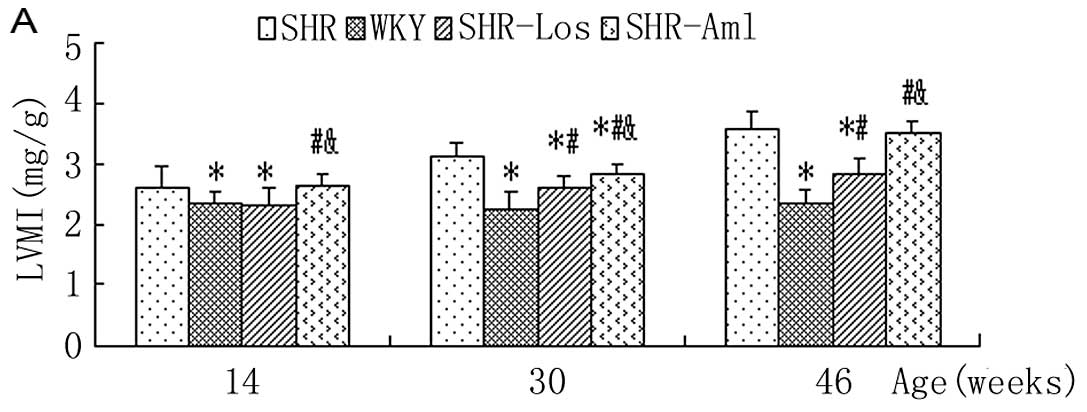
Cardiac hypertrophy and fibrosis, expressed by the
left ventricular hypertrophy index and myocardial CVF,
respectively, were both significantly elevated in untreated SHRs
compared with WKYs throughout the study (P<0.05). The LVMI and
CVF in SHR-Los displayed similar values compared to those in WKYs
at week 14 (LVMI, 2.29±0.34 vs. 2.33±0.21 mg/g; CVF, 2.6±0.7 vs.
2.6±0.4%, both P>0.05) and remained midway between untreated
SHRs and WKYs at weeks 30 and 46 (P<0.05). Unlike losartan
treatment, the LVMI in SHR-Aml was not different from that in
untreated SHRs at weeks 14 and 46 (P>0.05), but was slightly
reduced at week 30 (2.61±0.18 vs. 3.11±0.09 mg/g, P<0.05)
(Fig. 2A); the CVF in SHR-Aml
demonstrated comparable values to untreated SHRs at weeks 14 and 30
(P>0.05), but was significantly higher compared to that in
untreated SHRs at week 46 (12.1±1.3 vs. 7.8±1.5%, P<0.05)
(Fig. 2B).
Echocardiography
As shown in Table
II, the IVSTd in untreated SHRs was significantly higher
compared to that in WKYs throughout the experiment (P<0.05), and
the IVSTd in SHR-Los revealed similar values as in WKYs
(P>0.05), whereas an insignificant reduction of IVSTd was
observed in SHR-Aml compared with untreated SHRs (P>0.05). The
LVEDDs in the WKY and SHR-Los groups remained stable over the
follow-up period, but increased progressively in untreated SHRs and
SHR-Aml at week 46 as compared to previous values. The LVEF
deteriorated significantly in untreated SHRs and even more sharply
in SHR-Aml as compared with WKYs at week 46 (SHR, 79.5±1.9%;
SHR-Aml, 74.4±4.3%; WKY, 84.2±2.3%; both P<0.05), but values
remained similar for the WKY and SHR-Los groups (83.1±2.3%).
 | Table IILongitudinal echocardiographic
evaluation of WKYs, untreated and prehypertensively treated
SHRs. |
Table II
Longitudinal echocardiographic
evaluation of WKYs, untreated and prehypertensively treated
SHRs.
| Group | Age (Weeks) | IVSTd (mm) | LVEDD (mm) | EF (%) |
|---|
| SHR | 14 | 2.52±0.23 | 6.9±0.6 | 83.2±2.9 |
| 30 | 2.64±0.17 | 6.9±0.6 | 83.5±2.7 |
| 46 | 2.60±0.25 | 7.6±0.7d | 79.5±1.9 |
| WKY | 14 | 1.72±0.07a | 7.2±0.6 | 85.1±2.2 |
| 30 | 1.96±0.11a | 7.3±0.4 | 85.1±1.8 |
| 46 | 1.92±0.22a | 7.3±0.5 | 84.2±2.3a |
| SHR-Los | 14 | 1.78±0.12a | 7.2±0.9 | 83.2±3.2 |
| 30 | 1.85±0.13a | 7.2±0.5 | 82.8±2.5 |
| 46 | 1.88±0.16a | 7.3±0.4 | 83.1±2.3a |
| SHR-Aml | 14 |
2.62±0.15b,c | 6.8±0.7 | 82.7±3.1 |
| 30 |
2.54±0.21b,c | 7.0±0.3 | 80.5±4.6 |
| 46 |
2.52±0.22b,c | 7.8±0.7d |
74.4±4.3a–c |
Renal state until advanced age
Compared with WKYs, 24-h albuminuria was increased
in untreated SHRs and prehypertensively treated SHRs throughout the
study period. A steep increase in albumin excretion was noticed in
untreated SHRs (20.51±4.61 mg/day, P<0.05) and SHR-Aml
(18.20±3.24 mg/day, P<0.05) at 30 weeks of age. However, this
surge was blunted in SHR-Los (15.35±5.05 mg/day, P<0.05).
Albuminuria, however, remained significantly higher in these rats
compared to WKYs (9.24±1.40 mg/day). At 46 weeks of age, 24-h
albuminuria remained low in WKYs (10.27±2.19 mg/day), but increased
further in untreated SHRs (45.46±5.55 mg/day, P<0.05) and
SHR-Aml (40.92±4.48 mg/day, P<0.05), whereas the difference
between the two groups with respect to albuminuria was further
pronounced (SHR-Los 22.66±3.16 mg/day, P<0.05). Contrary to 24-h
albuminuria, continual decline in CCr occurred in the untreated SHR
and SHR-Aml groups throughout the study period, whereas the level
was stable in WKYs. A steep increase in CCr was noticed in
untreated SHRs (0.71±0.06 ml/min, P<0.05) and SHR-Aml (1.11±0.10
ml/min, P<0.05) at 46 weeks of age. However, this surge was
blunted in SHR-Los (1.52±0.08 ml/min, P<0.05). The data obtained
are depicted in Table III.
Glomerular morphology at 14 weeks of age demonstrated enhanced
sclerosis in untreated SHRs as compared with WKYs. Intermediate
values were revealed in SHR-Los and SHR-Aml. A rapid progression of
glomerulosclerosis occurred in the untreated SHR and SHR-Aml
groups. There were no differences in SHR-Aml compared to untreated
SHRs, whereas SHR-Los had a significantly attenuated
glomerulosclerosis compared to both groups in 46-week-old animals.
This pattern was most pronounced in juxtamedullary glomeruli
(Fig. 3A and B).
 | Table IIILongitudinal renal function
evaluation of WKYs, untreated and prehypertensively treated SHRs
(n=8). |
Table III
Longitudinal renal function
evaluation of WKYs, untreated and prehypertensively treated SHRs
(n=8).
| Group | Age (Weeks) | Serum creatinine
(μmol/l) | Serum urea nitrogen
(mmol/l) | CCr (ml/min) | Albuminuria (mg/24
h) |
|---|
| SHR | 14 | 30.84±4.34 | 7.79±1.00 | 1.76±0.14 | 11.42±2.60 |
| 30 | 37.80±7.09 | 7.87±1.24 | 1.40±0.12 | 20.51±4.61 |
| 46 | 41.25±5.18 | 8.85±1.30 | 0.71±0.06 | 45.46±5.55 |
| WKY | 14 | 30.61±4.41 | 7.17±1.16 | 1.81±0.12 | 10.65±1.91 |
| 30 | 34.53±5.78 | 7.28±1.26 | 1.89±0.16a | 9.24±1.40a |
| 46 | 34.81±6.50a | 7.92±1.09 | 1.74±0.06a | 10.27±2.19a |
| SHR-Los | 14 | 30.74±2.43 | 7.10±1.02 | 1.74±0.11 | 10.44±1.40 |
| 30 | 34.81±8.20 | 7.29±1.10 |
1.71±0.13a,b |
15.35±5.05a,b |
| 46 | 35.40±5.19a | 8.21±1.21 |
1.52±0.08a,b |
22.66±3.16a,b |
| SHR-Aml | 14 | 31.50±3.53 | 7.46±0.69 | 1.79±0.13 | 10.97±3.06 |
| 30 | 33.96±6.46 | 7.56±0.70 |
1.70±0.11a,b | 18.20±3.24b |
| 46 | 36.44±3.75 | 8.35±1.18 |
1.11±0.10a–c |
40.92±4.48b,c |
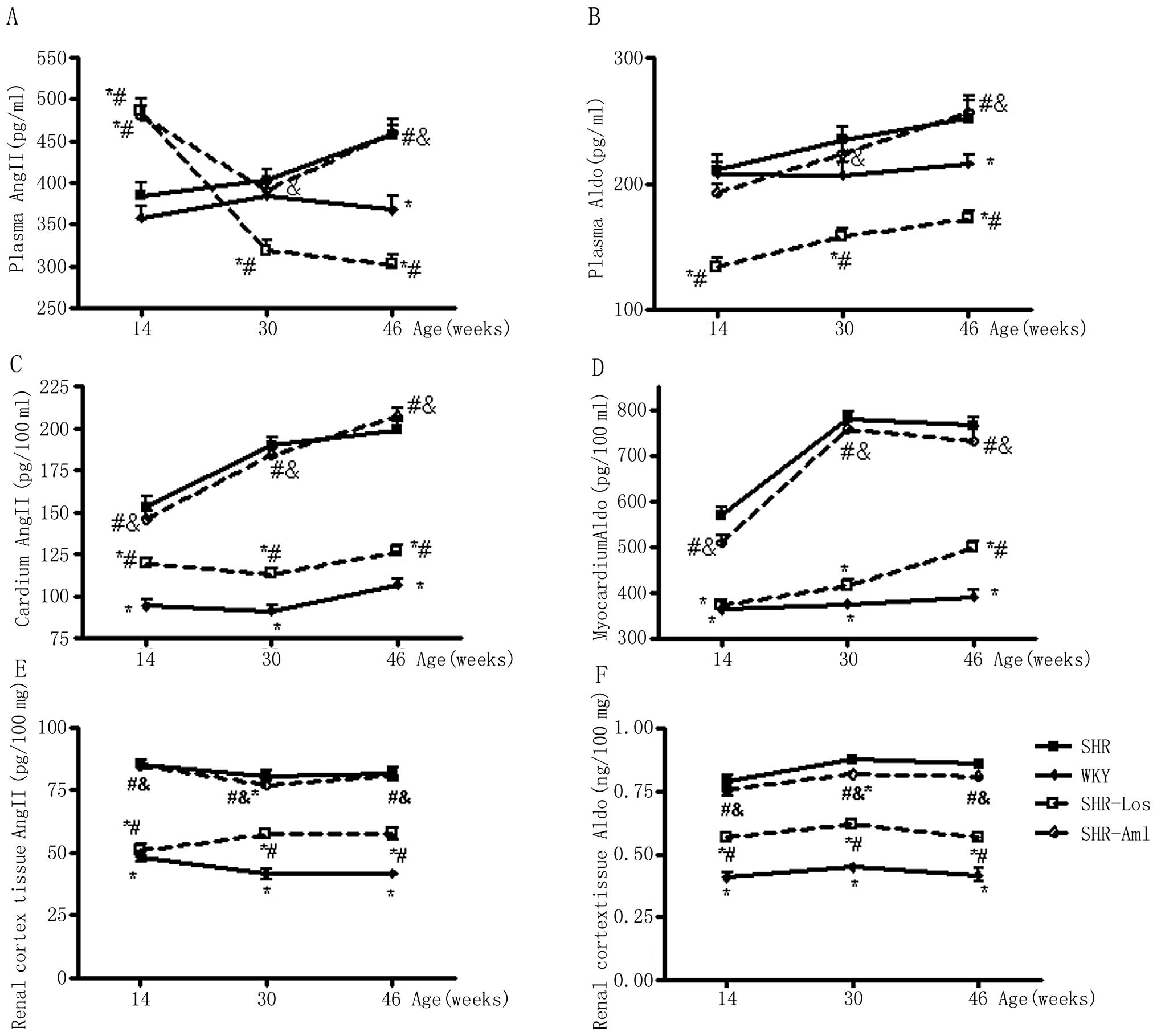
Plasma, renal cortex tissue and
myocardium Ang II and Aldo
The plasma Ang II in untreated SHRs was comparable
to WKYs at weeks 14 and 30, but was significantly higher compared
to that in WKYs at week 46. The plasma Ang II in SHR-Los and
SHR-Aml were both significantly higher compared to those in
untreated SHRs at week 14 (P<0.05). At weeks 30 and 46, the
plasma Ang II in SHR-Los was significantly reduced compared with
the untreated SHR and WKY groups (P<0.05), whereas the plasma
Ang II in SHR-Aml exhibited similar values to untreated SHRs
(P>0.05). The difference in plasma Aldo between untreated SHR
and WKY groups was consistent with that in plasma Ang II throughout
the study period. The plasma Aldo in SHR-Los was permanently
attenuated compared with untreated SHRs (P<0.05), however, no
significant difference of plasma Aldo was observed between the
SHR-Aml and untreated SHR groups (P>0.05) (Fig. 4A and B). The Ang II and Aldo of
renal cortex tissue and myocardium in untreated SHRs were markedly
higher compared to those in WKYs throughout the follow-up period.
The myocardium Ang II and Aldo in SHR-Los were significantly
decreased compared with untreated SHRs (P<0.05), whereas no
significant difference of myocardium Ang II and Aldo was observed
between the SHR-Aml and untreated SHR groups (P>0.05) (Fig. 4C and D). The renal cortex tissue
Ang II and Aldo in SHR-Los were significantly decreased compared
with untreated SHRs (P<0.05), whereas no significant difference
of renal cortex tissue Ang II and Aldo was observed between the
SHR-Aml and untreated SHR groups (P>0.05) (Fig. 4E and F and Table IV).
 | Table IVEvaluation of longitudinal plasma and
renal cortex tissue Ang II and Aldo levels of WKYs, untreated and
prehypertensively treated SHRs (n=8). |
Table IV
Evaluation of longitudinal plasma and
renal cortex tissue Ang II and Aldo levels of WKYs, untreated and
prehypertensively treated SHRs (n=8).
| Group | Age (Weeks) | Plasma Ang II
(pg/ml) | Plasma Aldo
(ng/ml) | Renal cortex tissue
Ang II (pg/100 mg) | Renal cortex tissue
Ang II (ng/100 mg) |
|---|
| SHR | 14 | 385.02±45.24 | 0.21±0.033 | 85.15±5.53 | 0.79±0.076 |
| 30 | 403.23±36.86 | 0.23±0.031 | 80.66±6.54 | 0.88±0.035 |
| 46 | 458.88±32.48 | 0.25±0.041 | 81.34±7.03 | 0.86±0.041 |
| WKY | 14 | 358.18±78.02 | 0.20±0.026 | 48.24±5.71a | 0.41±0.055a |
| 30 | 385.23±41.43 | 0.20±0.029 | 41.64±5.79a | 0.45±0.041a |
| 46 |
367.71±43.69a | 0.21±0.019a | 41.70±5.02a | 0.42±0.071a |
| SHR-Los | 14 |
485.18±94.25a,b |
0.13±0.021a,b | 50.90±6.61a |
0.57±0.035a,b |
| 30 |
319.02±53.78a,b |
0.15±0.020a,b |
57.21±4.17a,b |
0.62±0.042a,b |
| 46 |
302.11±32.36a,b |
0.17±0.020a,b |
57.70±6.31a,b |
0.57±0.056a,b |
| SHR-Aml | 14 |
478.92±73.99a,b | 0.19±0.019 |
84.75±4.96b,c |
0.76±0.071b,c |
| 30 |
391.39±40.56c | 0.22±0.036c |
76.89±3.31a–c |
0.82±0.022a–c |
| 46 |
458.91±46.26b,c |
0.25±0.036b,c |
80.51±4.07b,c |
0.81±0.052b,c |
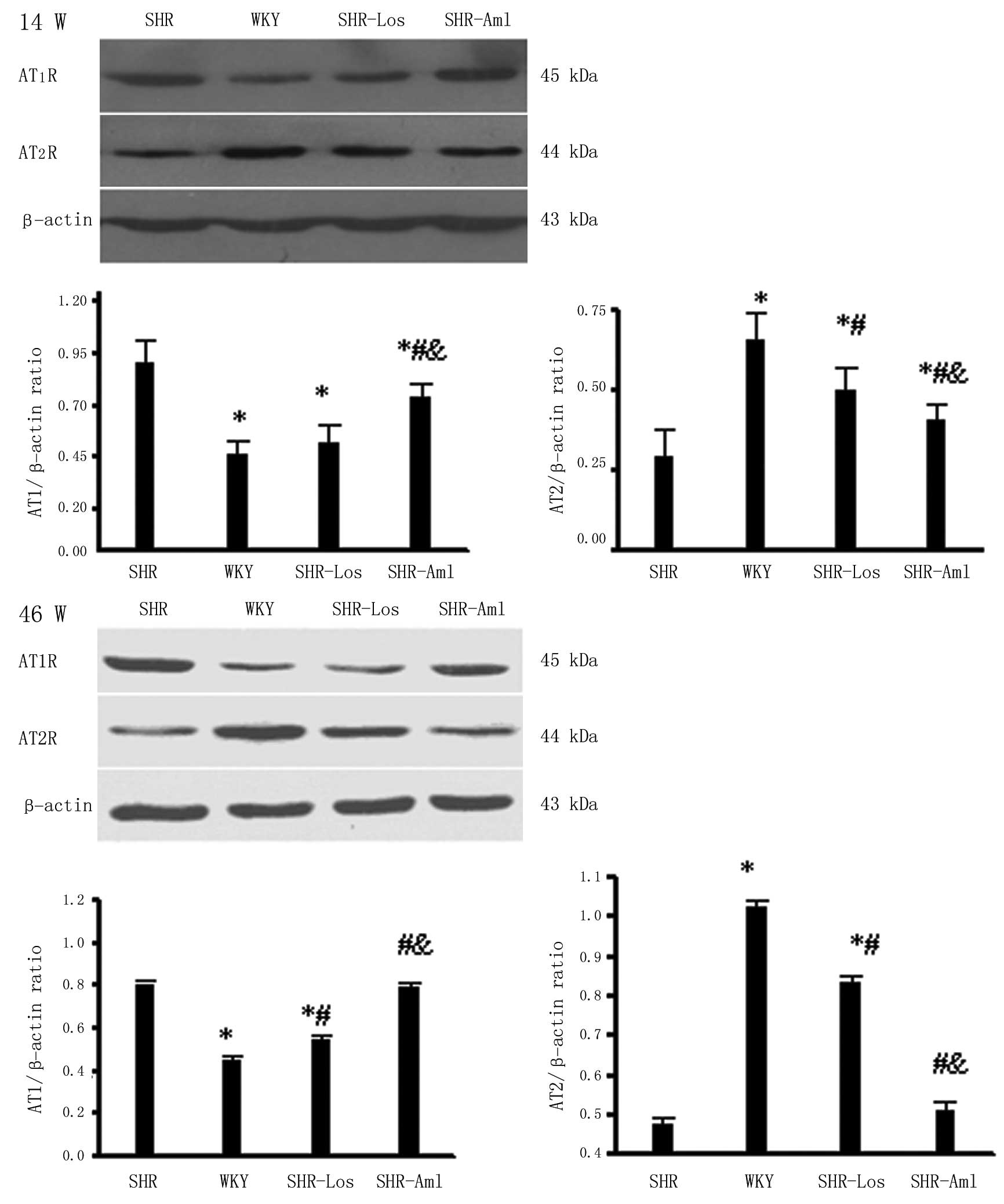
Western blot analysis for AT1R
and AT2R proteins
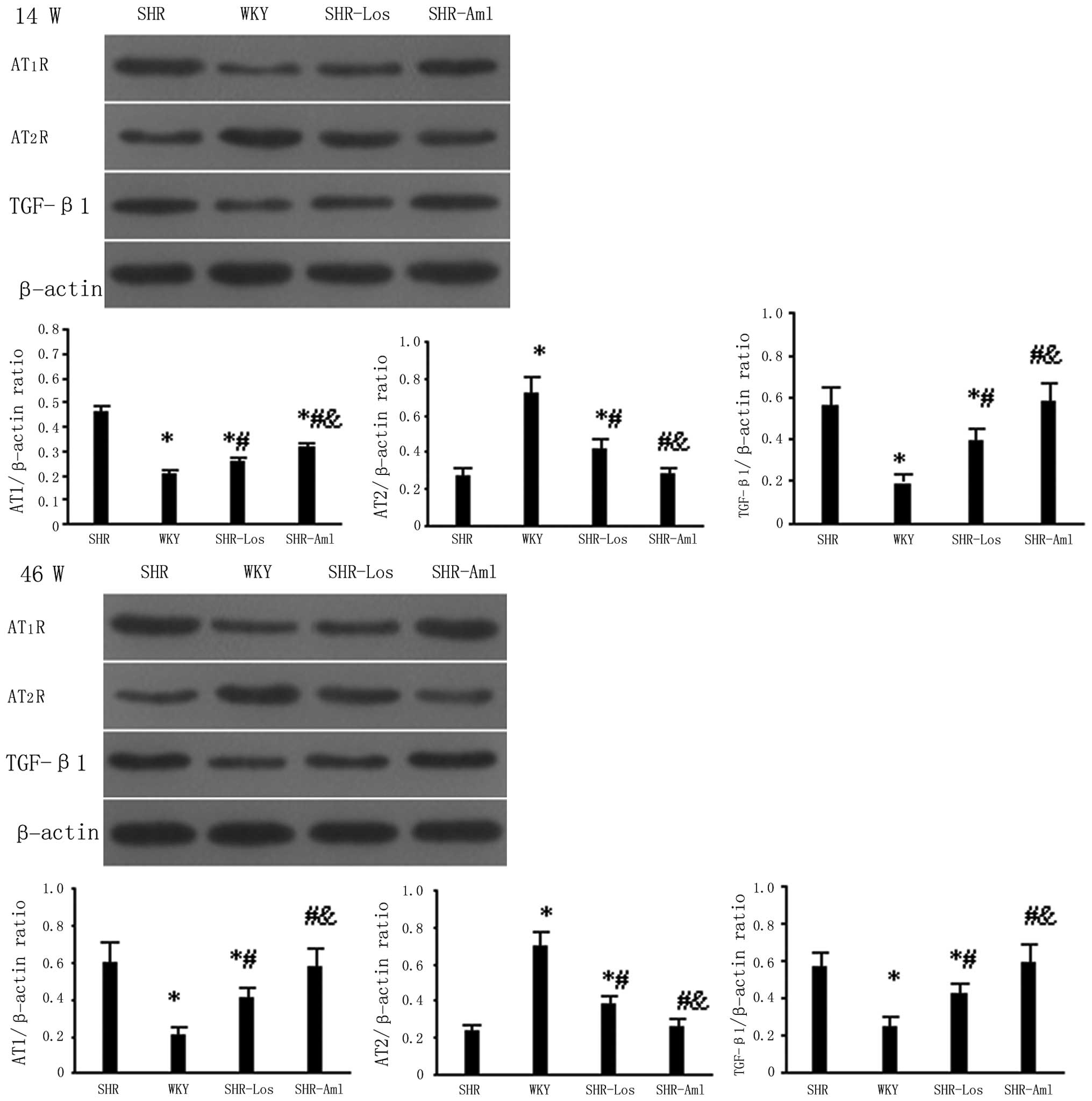
At weeks 14 and 46, the cardiac AT1R
protein expression in untreated SHRs was significantly higher
compared to that in WKYs (P<0.05), and the reverse occurred for
the AT2R protein (P<0.05) (Fig. 5). The AT1R protein
expression was downregulated while the AT2R protein was
upregulated in the SHR-Los group as compared with untreated SHRs;
no significant difference of the two protein levels was observed
between the SHR-Aml and untreated SHR groups (P>0.05). The renal
AT1R and AT2R protein expression levels were
similar to the results of the cardiac levels at weeks 14 and 46,
and TGF-β1 protein expression in untreated SHRs was
significantly higher compared to that in WKYs (P<0.05). The
renal TGF-β1 protein expression was lower in SHR-Los as
compared with untreated SHRs, and no significant difference of the
two protein levels was observed between the SHR-Aml and untreated
SHR group (Fig 6).
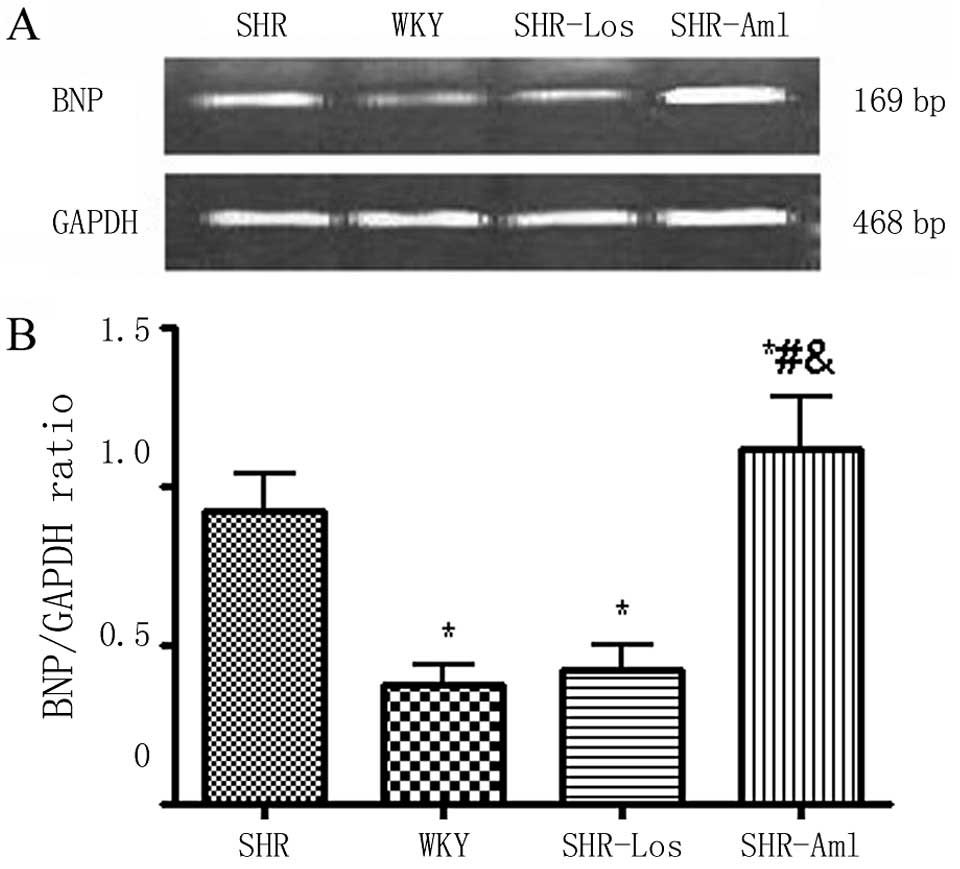
Quantification of cardiac BNP mRNA
At week 46, the cardiac BNP mRNA expression in
untreated SHRs was notably higher compared to that in WKYs
(P<0.05), the cardiac BNP mRNA in SHR-Los was observably
decreased as compared with untreated SHRs, but was still higher
compared to that in WKYs (P<0.05) (Fig. 7). In contrast, the cardiac BNP
mRNA expression in SHR-Aml was slightly and significantly increased
compared with untreated SHRs (P<0.05).
Discussion
In the present study, we demonstrated that transient
prehypertensive treatment with losartan was more effective than
amlodipine in delaying a long-term blood pressure (BP) increase and
ameliorating renal, cardiac structure and function, and that the
mechanism responsible for losartan’s superiority may be due to its
permanent attenuation of both circulating and local
renin-angiotensin systems (RAS).
Our results demonstrated that although the
antihypertensive effects were similar between the two therapeutic
regimens during treatment, BP development differed significantly
after drug withdrawal. BP in losartan-treated SHRs (SHR-Los)
remained significantly lower compared to that in untreated SHRs
until week 46, whereas BP in amlodipine-treated SHRs (SHR-Aml)
returned to untreated SHR levels from 30 weeks onwards. This
finding notably coincides with a previous study by Christensen
et al (18) that SHR
treatment with angiotensin-converting-enzyme inhibitor (ACEI)
rather than β-blocker, vasodilator or calcium antagonist prevented
BP increase after withdrawal of long-term treatment. Whether or not
the ACEI or angiotensin-receptor blockers (ARB) had longer effects
on BP reduction compared to other forms of therapies remains
unclear. One possibility is related to the ‘RAS block memory’ via
suppression of a ‘reno-vascular amplifier’ mechanism (19–21). According to this hypothesis,
during the critical period of hypertension development in
juveniles, increased BP leads to vascular hypertrophy in the
resistance vessels, which decreases glomerular perfusion and
results in increased synthesis of renin and activation of RAS.
These changes cause a further BP increase and initiate a positive
feedback loop. RAS inhibitors may block this vicious cycle by
attenuating the rise in BP and importantly, by decreasing the
vascular hypertrophy. Other possibilities are that alterations in
the central RAS, decreases in plasma AVP levels and alterations in
endothelial function may be involved in the prolonged hypotensive
effect (22–24). Recently Baumann et al
(25) demonstrated that a BP
decrease after transient ARB is associated with an increased
medullary blood flow and a reduced medullary pericyte number. These
possibilities are not mutually exclusive, and it is possible that
multiple mechanisms may be involved in the sustained suppression of
BP. As to the prolonged pressure-lowering effects of amlodipine, BP
development after drug withdrawal in our study was different from
the result reported by Sevilla et al (26). They indicated that treatments of
adult SHRs with 8 and 20 mg/kg/day of amlodipine for six months
yielded persistent hypotensive effects even at three months after
drug withdrawal. A potential reason for this difference was that
amlodipine treatment was administered too early and for a limited
time period to reverse vascular hypertrophy and break the vicious
cycle.
With respect to the effects on cardiac protection,
our results demonstrated that prehypertensive treatment with
losartan permanently reduced cardiac hypertrophy and fibrosis until
week 46. Longitudinal echocardiographic evaluation revealed
identical values of interventricular septum thickness (IVSTd)
between SHR-Los and WKYs, and the left ventricular end-diastolic
dimension (LVEDD) remained stable over the follow-up period.
Besides these, the left ventricular ejection fraction (LVEF) and
cardiac BNP mRNA expression were improved in SHR-Los compared with
untreated SHRs at week 46. All the above changes suggest prolonged
cardioprotective effects by losartan treatment. The mechanism to
determine how transient prehypertensive treatment with losartan
exerts sustained cardiac protection is required. Previous studies
have mainly explained the mechanism through the reduction of gene
expression of cardiac Ang II type 1 receptor (AT1R)
(10) or plasma Ang II
concentration (27) and not by a
BP decrease alone. In our study, we measured the circulating and
local angiotensin II and Aldo levels by radioimmunoassay and
discovered that, besides the suppressant effects on circulating RAS
(at week 14, the plasma Ang II was elevated via negative feedback
regulation), the cardiac RAS was also significantly inhibited,
which has been proven to be involved in the pathogenesis of cardiac
hypertrophy and fibrosis (28,29). Furthermore, western blot analysis
detected a favorably altered AT1R/AT2R
balance with losartan treatment, which consisted of downregulated
cardiac AT1R protein but upregulated AT2R
protein. Although how low levels of local Ang II act on high levels
of AT2R to ameliorate cardiac damage remains unknown,
our results revealed that transient prehypertensive treatment with
losartan may maintain the circulating and local RAS in an initial
low-activity or physiologic state. Unlike the results reported by
Baumann et al (10) the
SBP in our study remained lower compared to the untreated SHRs
during the experimental period; thus, it cannot be precluded that
the beneficial effects of losartan treatment is related to a BP
decrease alone.
Noteworthy, the LVMI in amlodipine-treated SHR was
similar to that in untreated SHRs at weeks 14 and 46, but was
significantly decreased at week 30. The possible reason for this
phenomenon is that the cardiac hypertrophy of SHRs is fully
established between week 14 and 30 (30), and amlodipine treatment causes
hypertrophy regression via its residual pressure reduction during
this period and the antihypertrophy action tapers with the
disappearance of antihypertensive effects from 30 weeks onwards.
Unexpectedly, the SHR-Aml exhibited no difference in CVF from
untreated SHRs at weeks 14 and 30, but an observably higher value
compared to the untreated SHRs at week 46. Moreover, the
echocardiography displayed enlarged LVEDD and declined EF at the
end of follow-up, and RT-PCR assay detected a slightly and
significantly increased expression of BNP mRNA. These results
implied a detrimental effect of amlodipine treatment, which we
speculated may be related to the negative inotropic effects of
amlodipine since the plasma and myocardium Ang II and Aldo levels
were comparable between SHR-Aml and untreated SHRs at week 46. Our
speculation contradicted the commonly accepted properties of
amlodipine of insignificant negative inotropic effects (31). The specific intervention period or
inappropriate dose of amlodipine in our study may be responsible
for this discrepancy, yet a detailed mechanism needs to be further
investigated.
Circulating RAS regulates physiological responses,
whereas the local RAS is activated during tissue injury and
contributes to pathological processes, including cell
proliferation/apoptosis, fibrosis and inflammation. Ang II is one
key factor in the development of renal fibrosis. In this study, our
data demonstrated that transient prehypertensive treatment with
losartan reduces Ang II and Aldo concentrations both in plasma and
renal tissue, upregulates AT2R protein expression while
downregulating AT1R protein expression. It revealed that
transient prehypertensive treatment with losartan may maintain the
circulating and local RAS in a low-activity state. Furthermore,
AT2R upregulation in SHR-Los inhibits progress of
glomerulosclerosis, supporting key effects of
AT1R/AT2R balance in fibrotic states.
Although regulation of the expression of the AT2R is
poorly understood, previous studies have proven the AT2R
actions contribute to ARB effects in the kidney (32).
In summary, our results suggest that transient
AT1R blockade in young SHRs is superior to the calcium
antagonist on delaying long-term BP rise and ameliorating cardiac,
renal structure and function, which corroborated the effectiveness
and feasibility of an RAS inhibitor for prehypertensive treatment
demonstrated by TROPHY and PHARAO trials. However, the superiority
of ARB over a calcium channel blocker (CCB) observed in animal
experiments should be cautiously interpreted when translated into
clinical settings, since the prehypertensive condition of human
beings is far more complicated (e.g., strain difference, attendant
obesity, dyslipidemia or impaired glucose tolerance) as compared
with SHRs. Moreover, since the recently published trial of DHYPP
demonstrated that one year of candesartan treatment in subjects
with high familial risk of future hypertension had no persistent
effect on BP when treatment was discontinued (33), further studies to explore how to
‘apply’ the prolonged cardioprotective effects observed in animals
to human beings is required. Nevertheless, our data have important
clinical implications that ARB may be a better choice compared with
CCB for hypertension prevention and cardiac protection.
Acknowledgements
This study was supported by a grant
from the National Natural Science Foundation of China (81070207).
Amlodipine was generously supplied by Pfizer Pharmaceutical, Inc.
The authors would like to thank Meiyan Lin and Shusheng Liao for
their technical support.
References
|
1.
|
RS VasanMG LarsonEP LeipWB KannelD
LevyAssessment of frequency of progression to hypertension in
non-hypertensive participants in the Framingham Heart Study: a
cohort
studyLancet35816821686200110.1016/S0140-6736(01)06710-111728544
|
|
2.
|
J HsiaKL MargolisCB EatonPrehypertension
and cardiovascular disease risk in the Women’s Health
InitiativeCirculation1158558602007
|
|
3.
|
S KnechtH WerschingH LohmannHigh-normal
blood pressure is associated with poor cognitive
performanceHypertension51663668200810.1161/HYPERTENSIONAHA.107.10557718250360
|
|
4.
|
DR MullicanC LorenzoSM HaffnerIs
prehypertension a risk factor for the development of type 2
diabetes?Diabetes Care3218701872200910.2337/dc09-032819628813
|
|
5.
|
T NinomiyaM KuboY DoiPrehypertension
increases the risk for renal arteriosclerosis in autopsies: the
Hisayama StudyJ Am Soc
Nephrol1821352142200710.1681/ASN.200701006717582160
|
|
6.
|
AV ChobanianGL BakrisHR BlackThe Seventh
Report of the Joint National Committee on Prevention, Detection,
Evaluation, and Treatment of High Blood Pressure: the JNC 7
reportJAMA28925602572200310.1001/jama.289.19.2560
|
|
7.
|
S JuliusSD NesbittBM EganFeasibility of
treating prehypertension with an angiotensin-receptor blockerN Engl
J Med35416851697200610.1056/NEJMoa06083816537662
|
|
8.
|
S LudersJ SchraderJ BergerThe PHARAO
study: prevention of hypertension with the angiotensin-converting
enzyme inhibitor ramipril in patients with high-normal blood
pressure: a prospective, randomized, controlled prevention trial of
the German Hypertension LeagueJ
Hypertens2614871496200810.1097/HJH.0b013e3282ff8864
|
|
9.
|
SB HarrapWM Van der MerweSA GriffinF
MacphersonAF LeverBrief angiotensin converting enzyme inhibitor
treatment in young spontaneously hypertensive rats reduces blood
pressure
long-termHypertension16603614199010.1161/01.HYP.16.6.603
|
|
10.
|
M BaumannBJ JanssenJJ HermansTransient AT1
receptor-inhibition in prehypertensive spontaneously hypertensive
rats results in maintained cardiac protection until advanced ageJ
Hypertens25207215200710.1097/HJH.0b013e3280102bff
|
|
11.
|
M BaumannJJ HermansBJ JanssenTransient
prehypertensive treatment in spontaneously hypertensive rats: a
comparison of spironolactone and losartan regarding long-term blood
pressure and target organ damageJ
Hypertens2525042511200710.1097/HJH.0b013e3282ef84f8
|
|
12.
|
D TomassoniM SabbatiniF AmentaEffect of
different dihydropyridine-type Ca2+ antagonists on left
ventricle hypertrophy and coronary changes in spontaneously
hypertensive ratsJ Cardiovasc Pharmacol41544552200312658055
|
|
13.
|
M WatanabeH KawaguchiH OnozukaChronic
effects of enalapril and amlodipine on cardiac remodeling in
cardiomyopathic hamster heartsJ Cardiovasc
Pharmacol32248259199810.1097/00005344-199808000-000129700987
|
|
14.
|
RE WiddopXC LiA simple versatile method
for measuring tail cuff systolic blood pressure in conscious
ratsClin Sci (Lond)9319119419979337632
|
|
15.
|
RM LangM BierigRB DevereuxRecommendations
for chamber quantification: a report from the American Society of
Echocardiography’s Guidelines and Standards Committee and the
Chamber Quantification Writing Group, developed in conjunction with
the European Association of Echocardiography, a branch of the
European Society of CardiologyJ Am Soc
Echocardiogr1814401463200516376782
|
|
16.
|
J FloegeB HackmannV KliemAge-related
glomerulosclerosis and interstitial fibrosis in Milan normotensive
rats: a podocyte diseaseKidney
Int51230243199710.1038/ki.1997.288995738
|
|
17.
|
Y XuD KumarJR DyckAT(1) and AT(2) receptor
expression and blockade after acute ischemia-reperfusion in
isolated working rat heartsAm J Physiol Heart Circ
Physiol282H1206H1215200210.1152/ajpheart.00839.200011893553
|
|
18.
|
KL ChristensenLT JespersenMJ
MulvanyDevelopment of blood pressure in spontaneously hypertensive
rats after withdrawal of long-term treatment related to vascular
structureJ
Hypertens78390198910.1097/00004872-198902000-000022522478
|
|
19.
|
K IshiguroH SasamuraY SakamakiH ItohT
SarutaDevelopmental activity of the renin-angiotensin system during
the ‘critical period’ modulates later L-NAME-induced hypertension
and renal injuryHypertens Res3063752007
|
|
20.
|
H NakayaH SasamuraM MifunePrepubertal
treatment with angiotensin receptor blocker causes partial
attenuation of hypertension and renal damage in adult Dahl
salt-sensitive ratsNephron91710718200210.1159/00006503512138277
|
|
21.
|
H SasamuraK HayashiK IshiguroH NakayaT
SarutaH ItohPrevention and regression of hypertension: role of
renal microvascular protectionHypertens
Res32658664200910.1038/hr.2009.8519521419
|
|
22.
|
AK KeatonCR WhiteKH BerecekCaptopril
treatment and its withdrawal prevents impairment of
endothelium-dependent responses in the spontaneously hypertensive
ratClin Exp
Hypertens20847866199810.3109/106419698090532519817606
|
|
23.
|
L ZhangDG EdwardsKH BerecekEffects of
early captopril treatment and its removal on plasma angiotensin
converting enzyme (ACE) activity and arginine vasopressin in
hypertensive rats (SHR) and normotensive rats (WKY)Clin Exp
Hypertens18201226199610.3109/106419696090817658869001
|
|
24.
|
JN WuKH BerecekPrevention of genetic
hypertension by early treatment of spontaneously hypertensive rats
with the angiotensin converting enzyme inhibitor
captoprilHypertension22139146199310.1161/01.HYP.22.2.1398340149
|
|
25.
|
M BaumannBJ JanssenJJ Rob HermansR
BartholomeJF SmitsHA Struijker BoudierRenal medullary effects of
transient prehypertensive treatment in young spontaneously
hypertensive ratsActa Physiol
(Oxf)196231237200910.1111/j.1748-1716.2008.01916.x18983459
|
|
26.
|
MA SevillaF VocesR CarronAmlodipine
decreases fibrosis and cardiac hypertrophy in spontaneously
hypertensive rats: persistent effects after withdrawalLife
Sci75881891200410.1016/j.lfs.2004.04.00415183079
|
|
27.
|
K TakemoriH IshidaH ItoContinuous
inhibition of the renin-angiotensin system and protection from
hypertensive end-organ damage by brief treatment with angiotensin
II type 1 receptor blocker in stroke-prone spontaneously
hypertensive ratsLife
Sci7722332245200510.1016/j.lfs.2004.12.048
|
|
28.
|
M BaderRole of the local renin-angiotensin
system in cardiac damage: a minireview focussing on transgenic
animal modelsJ Mol Cell
Cardiol3414551462200210.1006/jmcc.2002.207712431444
|
|
29.
|
K MizunoM TaniS HashimotoEffects of
losartan, a nonpeptide angiotensin II receptor antagonist, on
cardiac hypertrophy and the tissue angiotensin II content in
spontaneously hypertensive ratsLife
Sci51367374199210.1016/0024-3205(92)90589-H
|
|
30.
|
MA AdamsA BobikPI KornerDifferential
development of vascular and cardiac hypertrophy in genetic
hypertension. Relation to sympathetic
functionHypertension14191202198910.1161/01.HYP.14.2.1912527201
|
|
31.
|
R BurgesD MoiseyUnique pharmacologic
properties of amlodipineAm J
Cardiol732A9A199410.1016/0002-9149(94)90268-2
|
|
32.
|
T NaitoLJ MaH YangAngiotensin type 2
receptor actions contribute to angiotensin type 1 receptor blocker
effects on kidney fibrosisAm J Physiol Renal
Physiol298F683F691201010.1152/ajprenal.00503.200920042458
|
|
33.
|
K SkovH EiskjaerHE HansenJK MadsenS
KvistMJ MulvanyTreatment of young subjects at high familial risk of
future hypertension with an angiotensin-receptor
blockerHypertension508995200710.1161/HYPERTENSIONAHA.107.08953217485597
|