Introduction
Minerals have been traditionally used in Oriental
herbal medicine treatment, particularly for immune-related diseases
in Korea, China and other Asian countries (1–3).
Among them, chalcanthite is a richly colored blue water-soluble
sulfate mineral, commonly found in the late-stage oxidation zones
of copper deposits (4). The main
ingredient of chalcanthite is CuSO4. CuSO4
exists as a series of compounds that differ in their degrees of
hydration. The anhydrous form occurs as a rare mineral known as
chalcocyanite. The hydrated copper sulfate occurs in nature as
chalcanthite (CuSO4·5H2O) and two of the
rarer ones include bonattite (CuSO4·3H2O) and
boothite (CuSO4·7H2O) (4). According to Oriental medicine,
chalcanthite has sour, acrid, cold and toxic properties, and is
affiliated with the liver meridian. Its main function is to
eliminate toxins. Internally, it functions as a purgative and helps
the body get rid of excess phlegm and toxins. Chalcanthite can also
be applied externally to treat skin abscesses, reduce inflammation
and remove necrotic tissue. The traditionally used dose of
chalcanthite is between 0.3 and 0.6 g of powdered mineral, decocted
in water for oral administration. Larger amounts can be used when
chalcanthite is applied externally. However, chalcanthite is
poisonous and should be used only with extreme caution. To date,
due to safety issues concerning their cytotoxic mechanisms, many
mineral medicines are rarely used.
In addition to being a quality protein source, egg
whites contain many essential minerals including potassium,
magnesium, calcium, phosphorus, copper, zinc and iron. They are
also a good source of riboflavin and selenium along with essential
vitamins such as folate, B12, niacin betaine and choline (5–9).
In Medieval times in the West, egg whites were used for medicinal
purposes such as treating wounds, skin disorders and mending broken
bones. At present, egg whites are considered one of the most
nutrition-rich foods and are widely recommended for children, the
elderly, athletes and patients with a variety of health
conditions.
Egg white-chalcanthite (EWCC) is a mixture of egg
white and chalcanthite and may be prepared by roasting the
chalcanthite in order to dehydrate it. The dehydrated chalcanthite
is then pulverized and the pulverized chalcanthite is mixed to
react with the egg white in order to trigger a chemical reaction.
In the egg white-chalcanthite prepared as noted above, the toxicity
of the chalcanthite is neutralized by egg white, so that the
toxicity is reduced or removed and the pharmaceutical properties
are increased. Recently, it has been reported that EWCC inhibits
the growth of human lung cancer NCI-H460 cells by inducing
apoptotic cell death through caspase-3 activation (10). However, the precise biochemical
mechanisms underlying EWCC-induced anti-inflammatory effects have
not yet been clarified.
Microglia have conventionally been regarded as brain
macrophages and have been proposed to play a major role in host
defense and tissue repair in the central nervous system (CNS)
(11). They are activated in
response to environmental stress and exposure to lipopolysaccharide
(LPS), interferon (IFN)-γ and β-amyloid (12,13). Activation of microglia results in
the release of various bioactive molecules, including nitric oxide
(NO), prostaglandin E2 (PGE2), reactive
oxygen species (ROS), monocyte chemoattractant protein-1 (MCP-1)
and pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-6
and tumor necrosis factor-α (TNF-α) (14,15). Overproduction of these
inflammatory mediators causes various severe neurodegenerative
diseases, such as Alzheimer’s disease, Parkinson’s disease,
cerebral ischemia, multiple sclerosis and trauma (16–18). Therefore, activated microglia have
been shown to be a major cellular source of pro-inflammatory and/or
cytotoxic factors that cause neuronal damage in the CNS. Previous
studies have also demonstrated that a decrease in the number of
pro-inflammatory mediators in microglia attenuates the severity of
these disorders (19–21). Thus, control of microglial
activation may be an effective therapeutic option for the treatment
of various neurodegenerative diseases.
LPS, the main component of endotoxins, initiates a
number of major cellular effects that play critical roles in the
pathogenesis of inflammatory responses and have been employed to
induce microglial activation during infection by Gram-negative
bacteria (22). Therefore, LPS
stimulation of microglia is a useful model with which to study the
mechanisms that underlie neuronal injury through various
pro-inflammatory and neurotoxic factors released by activated
microglia, such as NO, PGE2, ROS, IL-1β, IL-6 and TNF-α
(14,15,23). In addition, inflammation is the
organized response of an organism to injuries of multiple
pathologies and the response involves the speedy upregulation and
activation of many genes. Among them, nuclear factor-κB (NF-κB) is
a critical transcription factor involved in this response and is
normally present in the cytoplasm by virtue of its association with
the inhibitory protein, IκB (24–26). Once activated by inflammatory
stimulants such as LPS, IκBs are rapidly phosphorylated and
degraded by IκB kinase (IKK). IκB degradation liberates NF-κB to
move into the nucleus, resulting in transcriptional induction of
inflammation-associated genes (27,28).
Based on these previous observations, the present
study was designed to evaluate the inhibitory effects of EWCC on
the LPS-stimulated inflammatory mediator production in murine BV2
microglia and the related anti-inflammatory mechanisms. As a result
of our findings, we suggest that EWCC may be a candidate for the
treatment of various neurodegenerative disorders.
Materials and methods
Preparation of EWCC
For the preparation of EWCC, chalcanthite was heated
and dehydrated. During the heating process, chalcanthite was
uniformly heated while carefully being watched for a color change
every 3–5 h until it turned completely grey. The heating duration
(~10–24 h) varied with the amount and quality of the mineral used.
Once the chalcanthite was dehydrated, it was cooled until the
remaining heat was completely released. The moisture content of the
chalcanthite ranged from 0 to 5%. After cooling, chalcanthite was
finely pulverized. The pulverized chalcanthite was stored in a
concealed airtight container in a dry place to prevent moisture
from accumulating. The prepared chalcanthite powder was mixed with
egg whites at ratios of 30:13 to 30:30 for 10 min. The egg whites
needed to be homogeneously mixed with a wooden spatula in a ceramic
vessel so the utensils would not interfere or cause any chemical
reactions during the mixing process. After the chalcanthite and egg
whites were sufficiently mixed, the mixture was cooled until the
reaction heat was completely released. One hundred milligrams of
the obtained substance was then dissolved in 1 liter of deionized
water and centrifuged at 6,000 rpm for 10 min. A portion of the
upper level concentration (0.80 μm) was filtered by syringe filter
after the centrifugation.
Cell culture
The murine BV2 cell line was maintained in DMEM
supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin
and 100 μg/ml streptomycin at 37°C in a humidified incubator with
5% CO2. Confluent cultures were passed by
trypsinization. The cells used in the experiments were washed twice
with warm DMEM (without phenol red) and treated in a serum-free
medium for at least 4 h before treatments. In all experiments, the
cells were treated with various concentrations of EWCC at the
indicated times before the addition of LPS (0.5 μg/ml).
Cell viability assay
Cell viability was measured based on the formation
of blue formazan that was metabolized from colorless MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide;
Sigma, St. Louis, MO] by mitochondrial dehydrogenases, which are
active only in live cells. BV2 cells were plated on 24-well plates
at a density of 2×105 cells/well for 24 h and then
washed. Cells incubated with various concentrations of EWCC were
treated with or without LPS (Escherichia coli 026:B6; Sigma)
for 24 h and then incubated in 0.5 mg/ml MTT solution. Three hours
later, the supernatant was removed and the formation of formazan
was measured at 540 nm using a microplate reader (Dynatech MR-7000;
Dynatech Laboratories).
NO production
Concentrations of NO in the culture supernatants
were determined by measuring nitrite, which is a major stable
product of NO, using the Griess reagent (Sigma). Cells
(5×105 cells/ml) were stimulated in 24-well plates for
24 h and then 100 μl of each culture medium was mixed with an equal
volume of Griess reagent (1% sulfanilamide/0.1%
N-(1-naphthyl)-ethylenediamine dihydrochloride/2.5%
H3PO4). Nitrite levels were determined using
an ELISA plate reader at 540 nm and nitrite concentrations were
calculated by reference to a standard curve generated by known
concentrations of sodium nitrite (29).
Measurement of PGE2
production
BV2 cells were incubated with purpurogallin in
either the presence or absence of LPS (0.5 μg/ml) for 24 h.
Following the manufacturer’s instructions, a volume of 100 μl of
culture-medium supernatant was collected for determination of
PGE2 concentration by ELISA (Cayman, Ann Arbor, MI)
(29).
RNA isolation and RT-PCR
Total RNA was prepared using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA) and primed with random
hexamers for the synthesis of complementary DNA using M-MLV Reverse
Transcriptase (Promega, Madison, WI), according to the
manufacturer’s instructions using DNAse I (1 U/μg RNA) pretreated
total mRNA. Single stranded cDNA was amplified by a polymerase
chain reaction (PCR) with primers for inducible nitric oxide
synthase (iNOS), cyclooxygenase (COX)-2, IL-1β, TNF-α and
glyceraldhyde-3-phosphate dehydrogenase (GAPDH) (Table I). The following PCR conditions
were applied - GAPDH: 18 cycles of denaturation at 94°C for 30 sec,
annealing at 57°C for 30 sec and extension at 72°C for 30 sec;
iNOS, COX-2, IL-1β and TNF-α: 25 cycles of denaturation at 94°C for
30 sec, annealing at 52°C for a 30-sec extension at 72°C for 30
sec. GAPDH was used as an internal control to evaluate the relative
expression of COX-2, iNOS, IL-1β and TNF-α (30).
 | Table ISequence of primers used for RT-PCR
in this study. |
Table I
Sequence of primers used for RT-PCR
in this study.
| Gene name | Sequences |
|---|
| iNOS |
| Sense | 5′-ATG TCC GAA GCA
AAC ATC AC-3′ |
| Antisense | 5′-TAA TGT CCA GGA
AGT AGG TG-3′ |
| COX-2 |
| Sense | 5′-CAG CAA ATC CTT
GCT GTT CC-3′ |
| Antisense | 5′-TGG GCA AAG AAT
GCA AAC ATC-3′ |
| TNF-α |
| Sense | 5′-ATG AGC ACA GAA
AGC ATG ATC-3′ |
| Antisense | 5′-TAC AGG CTT GTC
ACT CGA ATT-3′ |
| IL-1β |
| Sense | 5′-ATG GCA ACT GTT
CCT GAA CTC AAC T-3′ |
| Antisense | 5′-TTT CCT TTC TTA
GAT ATG GAC AGG AC-3′ |
| GAPDH |
| Sense |
5′-CGG-AGT-CAA-CGG-ATT-TGG-TCG-TAT-3′ |
| Antisense |
5′-AGC-CTT-CTC-CAT-GGT-GGT-GAA-GAC-3′ |
Protein extraction and western blot
analysis
Cells were washed with PBS three times, placed at a
temperature of 4°C and lysed for 30 min in lysis buffer (20 mM
sucrose, 1 mM EDTA, 20 μM Tris-Cl, pH 7.2, 1 mM DTT, 10 mM KCl, 1.5
mM MgCl2 and 5 μg/ml aprotinin). Lysates were then
centrifuged at 12,000 rpm at 4°C. The protein concentration was
measured using a Bio-Rad protein assay (Bio-Rad, Hercules, CA)
according to the manufacturer’s instructions. Equal amounts of
protein (30–50 μg) were separated electrophoretically using 8–10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE). The gel was then transferred to 0.45-μm polyvinylidene
fluoride (PVDF; Millipore, Bedford, MA) membranes. Membranes were
soaked in blocking buffer (5% skimmed milk) and then incubated with
the primary antibodies (Table
II). After thorough washing with PBST, horseradish peroxidase
conjugated antibodies were applied and immune complexes were then
visualized using the enhanced chemiluminescence (ECL) detection
system according to the recommended procedure (Amersham). In a
parallel experiment, cells were washed with ice-cold PBS and
scraped; and cytoplasmic and nuclear proteins were then extracted
using NE-PER® Nuclear and Cytoplasmic Extraction
Reagents (Pierce Biotechnology, Inc., Rockford, IL).
 | Table IIList of antibodies used in this
study. |
Table II
List of antibodies used in this
study.
| Antibody | Dilution | Product no. | Species of origin
(supplier) |
|---|
| iNOS | 1:1,000 | 610333 | Rabbit polyclonal
(BD Biosciences) |
| COX2 | 1:500 | SC-1999 | Mouse monoclonal
(Santa Cruz Biotechnology) |
| NF-κB p65 | 1:500 | SC-109 | Mouse monoclonal
(Santa Cruz Biotechnology) |
| IκBα | 1:500 | SC-847 | Rabbit polyclonal
(Santa Cruz Biotechnology) |
| PI3K | 1:500 | 4257 | Mouse monoclonal
(Cell Signaling Technology) |
| p-PI3K | 1:500 | 4228 | Rabbit polyclonal
(Cell Signaling Technology) |
| Akt | 1:500 | SC-8312 | Rabbit polyclonal
(Santa Cruz Biotechnology) |
| p-Akt | 1:500 | 9271S | Rabbit polyclonal
(Cell Signaling Technology) |
| p38 | 1:500 | SC-728 | Rabbit polyclonal
(Santa Cruz Biotechnology) |
| ERK | 1:2,000 | SC-535 | Rabbit polyclonal
(Santa Cruz Biotechnology) |
| JNK | 1:500 | 9252 | Rabbit polyclonal
(Cell Signaling Technology) |
| p-p38 | 1:500 | 9211S | Rabbit polyclonal
(Cell Signaling Technology) |
| p-ERK | 1:500 | 9106S | Mouse monoclonal
(Cell Signaling Technology) |
| p-JNK | 1:500 | 9255S | Mouse monoclonal
(Cell Signaling Technology) |
| Lamin B | 1:1,000 | NA12 | Mouse monoclonal
(Oncogene Science) |
Cytokine assays
The levels of IL-1β and TNF-α (R&D Systems,
Minneapolis, MN) were measured by ELISA kits according to the
manufacturer’s instructions. Briefly, BV2 cells (5×105
cells/ml) were plated in 24-well plates and pretreated with
indicated concentrations of EWCC for 1 h before treatment of 0.5
μg/ml LPS for 24 h. One hundred microliters of culture-medium
supernatants was collected to determine the IL-1β and TNF-α
concentrations by ELISA.
Immunofluorescence analysis
For detection of NF-κB p65 translocation, cells were
grown on glass coverslips for 24 h and then treated with 0.5 μg/ml
LPS, and were either pretreated or not pretreated with EWCC for 60
min. Cells were fixed with 3.7% paraformaldehyde, treated with 0.2%
Triton X-100 and blocked with 2% BSA. Cells were then sequentially
incubated with the anti-NF-κB p65 antibody, FITC-conjugated donkey
anti-rabbit IgG and DAPI solution and examined using a fluorescence
microscope (Carl Zeiss, Germany).
Statistical analyses
Data values represent the means ± SD. Statistical
significance was determined using an analysis of variance that was
followed by the Student’s t-test. A value of P<0.05 was accepted
as statistically significant.
Results
Effect of EWCC on NO and PGE2
production in LPS-stimulated BV2 microglia
In the present study, we aimed to evaluate the
potential anti-inflammatory properties of EWCC regarding the
production of two major inflammatory mediators, NO and
PGE2, in LPS-stimulated BV2 microglia. To determine the
level of NO production, we measured the nitrite released into the
culture medium using Griess reagent. BV2 cells were pretreated with
various concentrations of EWCC (25, 50 and 75 μg/ml) for 1 h before
being stimulated with LPS (0.5 μg/ml). According to the NO
detection assay, LPS alone was able to markedly induce NO
production from the cells as compared to those generated by the
control. However, pretreatment with EWCC significantly repressed
the levels of NO production in LPS-stimulated BV2 microglia in a
concentration-dependent manner of up to 75 μg/ml (Fig. 1A).
PGE2 represents another important
inflammatory mediator that is produced from the conversion of
arachidonic acid by COXs. Therefore, we next evaluated the
inhibitory effects of EWCC on PGE2 levels present in the
supernatant. To assess whether EWCC inhibits production of
LPS-induced PGE2 in BV2 microglia, cells were pretreated
with various concentrations of EWCC for 1 h and then stimulated
with 0.5 μg/ml LPS. After incubation for 24 h, the cell culture
medium was harvested and the production of PGE2 was
measured using an ELISA. As shown in Fig. 1B, the amount of PGE2
present in the culture medium increased from initial levels after
24 h of exposure to LPS alone, however a marked repression was
observed after administration of EWCC in a concentration-dependent
fashion.
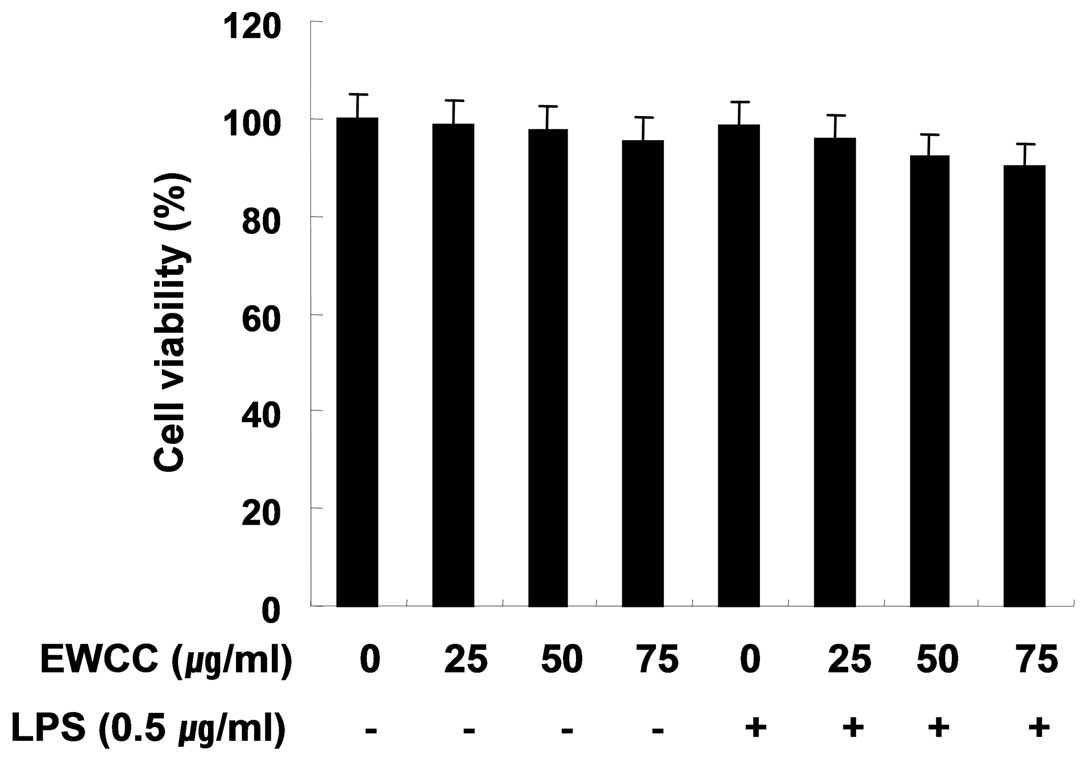
Effect of EWCC on the cell viability in
LPS-stimulated BV2
In order to exclude the cytotoxic effect of EWCC on
the cell growth of BV2 cells, the cells were exposed to various
concentrations of EWCC for 24 h in the presence or absence of LPS,
and cell viability was then measured by an MTT assay. Under the
same condition, we also analyzed whether chromatin condensation, a
hallmark of apoptosis, was induced by EWCC treatment. As indicated
in Fig. 2, the concentrations of
EWCC (25–75 μg/ml) used to inhibit NO and PGE2
production did not affect cell viability. These results clearly
indicated that the inhibition of NO and PGE2 production
in LPS-stimulated BV2 cells was not due to the cytotoxic action of
EWCC.
Effect of EWCC on LPS-stimulated levels
of iNOS and COX-2 mRNA and protein
To ascertain whether the inhibition of NO and the
PGE2 production by EWCC treatment is associated with
decreased levels of iNOS and COX-2, we performed RT-PCR and western
blot analysis for detection of mRNA and protein levels. The levels
of iNOS and COX-2 protein were markedly upregulated after 24 h of
LPS (0.5 μg/ml) treatment, and EWCC significantly inhibited iNOS
and COX-2 protein expression in LPS-stimulated BV2 microglia in a
concentration-dependent manner (Fig.
3A). Next, to investigate whether or not EWCC suppresses the
LPS-mediated induction of iNOS and COX-2 via a pretranslational
mechanism, the effects of EWCC on iNOS and COX-2 mRNA expression
were evaluated (Fig. 3B). RT-PCR
analysis showed that the reduction in iNOS and COX-2 mRNA
correlated with the reduction in the corresponding protein levels.
The results suggest that EWCC-induced reduction in the expression
of iNOS and COX-2 resulted in the inhibition of NO and
PGE2 production.
Effects of EWCC on LPS-induced IL-1β and
TNF-α production and mRNA expression
We next investigated whether EWCC suppresses the
production of pro-inflammatory cytokines, IL-1β and TNF-α, in
LPS-stimulated BV2 cells. BV2 microglia were incubated with EWCC in
the absence or presence of 0.5 μg/ml of LPS for 24 h and the
cytokine levels were evaluated in the culture supernatants. As
indicated in Fig. 4A and B,
induction of IL-1β and TNF-α by LPS treatment was significantly
decreased by EWCC in cell culture media. In a parallel experiment,
using RT-PCR, we studied the effects of EWCC on LPS-induced IL-1β
and TNF-α mRNA expression. As shown in Fig. 5, IL-1β and TNF-α mRNA
transcription also decreased following EWCC treatment. These
results suggest that EWCC is effective in the suppression of
pro-inflammatory cytokine production through the alteration of the
transcription levels of IL-1β and TNF-α in activated microglia.
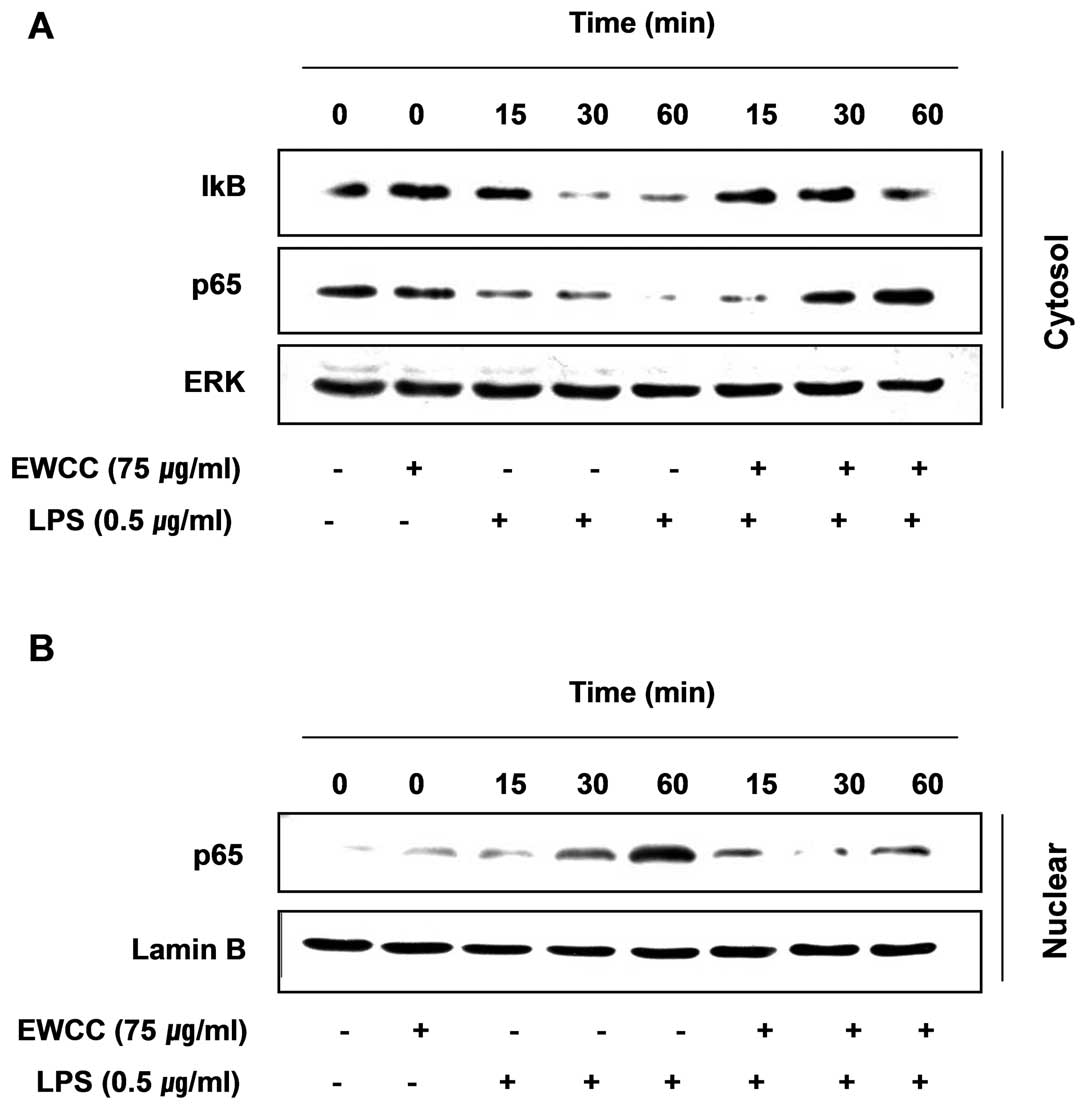
Effect of EWCC on LPS-induced NF-κB
activity
NF-κB is one of the important transcription factors
that regulate gene expression of pro-inflammatory mediators
including iNOS, COX-2, TNF-α and IL-1β in activated BV2 microglia
(24–26); therefore, to further characterize
the mechanism through which EWCC inhibits pro-inflammatory and/or
cytotoxic factor expression, we examined whether EWCC prevents the
translocation of the p65 subunit of NF-κB to the nucleus. Western
blot analysis showed that the amount of NF-κB p65 in the nucleus
was markedly increased after exposure to LPS alone, but the
LPS-induced p65 level in the nuclear fractions was reduced by EWCC
pretreatment (Fig. 6). Similar
results were also found using immunofluorescence microscopy
(Fig. 7). In addition, we
investigated whether EWCC blocks the LPS-stimulated degradation of
IκB-α using western blotting. As shown in Fig. 6, IκB-α was markedly degraded at 30
min after LPS treatment. However, this LPS-induced IκB-α
degradation was significantly reversed by EWCC. These results
suggest that EWCC inhibits NF-κB activation in BV2 microglia cells
by suppression of IκB degradation and nuclear translocation of
NF-κB. Taken together the above findings show the anti-inflammatory
effect of EWCC in the LPS-stimulated BV2 cells involved the NF-κB
pathway.
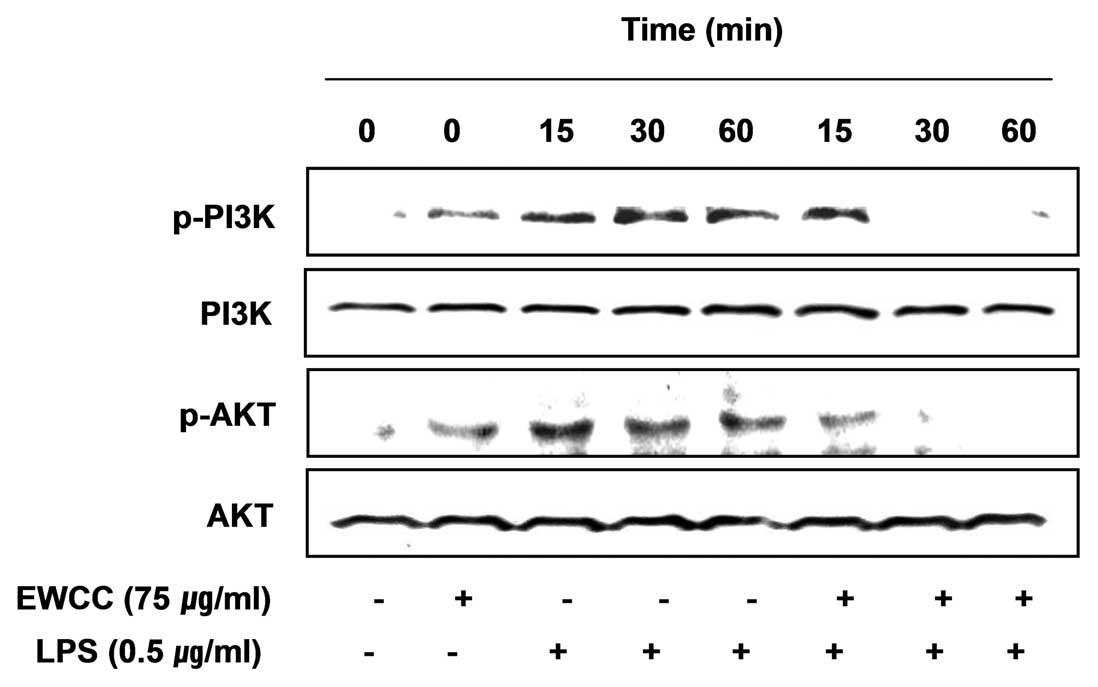
Effects of EWCC on the LPS-induced
activation of the PI3K/Akt pathway in BV2 microglia
Findings from recent studies have indicated that the
phosphoinositide 3-kinase (PI3K)/Akt signaling molecule is able to
trigger NF-κB activation through IκB degradation (31,32). Therefore, we examined the effect
of EWCC on LPS-induced PI3K/Akt activation. As shown in Fig. 8, phosphorylation of PI3K and Akt
showed a marked increase within 15 min after LPS stimulation;
however, EWCC pretreatment resulted in a significant blockage of
LPS-induced PI3K and Akt phosphorylation. The results show that
inhibition of pro-inflammatory mediator expression by EWCC in
LPS-stimulated BV2 microglia was associated with the inactivation
of the PI3K/Akt signaling pathway.
Effects of EWCC on the LPS-induced
activation of MAPKs in BV2 microglia
Mitogen-activated protein kinases (MAPKs) are the
most important signaling molecules with involvement in activated
microglia (33,34). Therefore, we determined the
activation levels of the MAPK pathway at various times after LPS
stimulation of BV2 cells. Stimulation of BV2 cells with LPS led to
the rapid activation of p38MAPK, extracellular signal-regulated
kinase (ERK) and c-Jun N-terminal kinase (JNK), with peak levels of
each phospho-MAPK occurring 15–60 min after the addition of LPS.
However, EWCC pretreatment significantly inhibited phosphorylation
of MAPKs in LPS-stimulated BV2 microglia (Fig. 9). This finding suggests that EWCC
is capable of disrupting key signal transduction pathways activated
by LPS in BV2 microglia, which subsequently prevents the production
of pro-inflammatory mediators.
Discussion
Microglia are brain macrophage cells that reside
within the CNS. They play a pivotal role in the innate immune
response of the CNS and serve as the first line of defense against
invading pathogens (35).
Activation of microglia by LPS induces the release of various
pro-inflammatory mediators, such as IL-1β, IL-6, TNF-α, NO and
PGE2, which have been implicated as important
intermediaries of neuronal injury (14–16). In that regard, the inhibition of a
variety of pro-inflammatory enzymes and cytokines would be an
effective therapeutic approach against many neurodegenerative
diseases. Many recent studies have reported the neuroprotective
effects of natural extracts that are useful therapeutic agents for
the protection of neurons from toxicities associated with exposure
to activated microglia.
The present results demonstrated that EWCC exhibits
pharmacological and biological activities via significant
inhibitory effects on the production of LPS-induced inflammatory
mediators and cytokines in BV2 microglial cells. These mediators
and cytokines include NO, PGE2, IL-1β and TNF-α. The
inhibitory effects were also mediated through the inhibition of
NF-κB activation and the PI3K/Akt and MAPK pathways. These data
suggest that EWCC may be a possible therapeutic agent for the
treatment of inflammatory disease, including neurodegenerative
disorders.
NO is an important regulatory molecule in a wide
range of physiological and pathological processes. NO is produced
from L-arginine by NO synthase (NOS), a three member enzyme family,
including iNOS, which is produced mainly by activated glial cells
(36). NO production can lead to
inflammatory, infectious, ischemic, traumatic and neurodegenerative
diseases (13,37–39). Thus, inflammatory diseases could
be prevented through the inhibition of iNOS and NO. Similarly,
PGE2 is also a well-known inflammatory mediator derived
from arachidonic acid via the action of cyclooxygenases. The
overproduction of PGE2 in response to growth factors,
cytokines and pro-inflammatory molecules is associated with the
upregulation of COX-2 (40,41). COX-2 has emerged as one of the
major players in brain inflammation and increased COX-2 expression
is believed to contribute to neurodegeneration (42,43). Therefore, intense research has
been undertaken in order to search for selective COX-2 inhibitors.
In the present study, EWCC inhibited both PGE2 and NO
production in a concentration-dependent manner (Fig. 1) and these inhibitory effects were
mediated through the downregulation of COX-2 and iNOS expression at
the mRNA and protein levels (Fig.
3) without causing cytotoxicity (Fig. 2). Chronic microglial activation
and consequent overproduction of pro-inflammatory cytokines, such
as IL-1β and TNF-α, are a histopathological hallmark of various
neurological diseases (14,15). The data also show that EWCC
inhibits LPS-induced cytokine expression at the transcriptional
level in BV2 microglia (Fig.
5).
NF-κB, as a result of its key role in several
pathologic conditions, is a major drug target in a variety of
diseases. Activation of the NF-κB protein plays a pivotal role in
inflammation due to its ability to induce transcription of
pro-inflammatory genes. The activity of NF-κB is suppressed in the
cytoplasm, while it is complexed with an inhibitory IκB protein.
The phosphorylation of IκB subsequently leads to its ubiquitination
and degradation (27,28). NF-κB dimers are then free to
translocate to the nucleus and activate target genes, including
iNOS, COX-2 and pro-inflammatory cytokines. It is well known that
the blockade of NF-κB transcriptional activity in the microglial
nucleus can also suppress the expression of iNOS, COX-2 and
pro-inflammatory cytokines, such as IL-1β, IL-6 and TNF-α (24,44). Recently, involvement of the
PI3K/Akt pathway in the expression of inflammatory mediators in
microglia through the activation of NF-κB has also been
demonstrated (31,32,45). Therefore, many current
anti-inflammatory therapies seek to block NF-κB activity. Our study
indicated that EWCC inhibited the LPS-induced IκB-α degradation and
that it inhibited the nuclear translocation of the p65 subunit of
NF-κB in BV2 microglia (Figs. 6
and 7). Therefore, inhibition of
NF-κB signaling pathways in microglia by EWCC might result in the
downregulation of pro-inflammatory mediators, resulting in an
anti-inflammatory effect. Furthermore, EWCC significantly inhibited
PI3K/Akt activation in LPS-stimulated BV2 microglia (Fig. 8), indicating that EWCC inhibits
LPS-induced NF-κB activation via the inactivation of the PI3K/AKT
signaling pathway.
The involvement of various intracellular signaling
pathways, such as MAPK, in the induction of inflammatory mediators
has also been reported (33,34). Therefore, we determined whether
EWCC tightly regulates the expression of MAPKs to induce
anti-inflammatory effects in LPS-stimulated microglia. Our results
indicated that EWCC is a potent inhibitor of the activation of
MAPKs, such as p38MAPK, ERK and JNK, induced by LPS stimulation in
BV2 microglia (Fig. 9),
suggesting that the anti-inflammatory effects were also due to the
inhibition of the MAPK signaling pathway.
In summary, the present results demonstrate that
EWCC significantly attenuated the release of inflammatory mediators
such as NO, PGE2, TNF-α and IL-1β in a
concentration-dependent manner; in particular, it acted at the
level of transcription in activated BV2 microglia. The inhibitory
action of EWCC was mediated by the prevention of NF-κB activation
and the inhibition of IκB degradation. In addition, the activation
of PI3K/Akt and MAPKs was significantly decreased after
pretreatment with EWCC in LPS-stimulated BV2 microglial cells. As a
result of the findings presented in this study, EWCC may provide an
effective treatment for many neurodegenerative diseases, and the
pharmacology and mode of action of its active components must be
further investigated.
Acknowledgements
This study was supported by the Blue-Bio Industry
Regional Innovation Center (RIC08-06-07) at Dongeui University as a
RIC program under the Ministry of Knowledge, Economy and Busan
City.
References
|
1
|
Bielory L, Chiaramonte L, Ehrlich P and
Field J: Alternative treatment for allergy and asthma. J Asthma.
40(Suppl): S47–S53. 2003.
|
|
2
|
Enrico P, Sirca D and Mereu M:
Antioxidants, minerals, vitamins, and herbal remedies in tinnitus
therapy. Prog Brain Res. 166:323–330. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Olaku O and White JD: Herbal therapy use
by cancer patients: a literature review on case reports. Eur J
Cancer. 47:508–514. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nordstrom DK and Alpers CN: Negative pH,
efflorescent mineralogy, and consequences for environmental
restoration at the Iron Mountain Superfund site, California. Proc
Natl Acad Sci USA. 96:3455–3462. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Latshaw JD and Osman M: Distribution of
selenium in egg white and yolk after feeding natural and synthetic
selenium compounds. Poult Sci. 54:1244–1252. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
White HB III: Vitamin-binding proteins in
the nutrition of the avian embryo. J Exp Zool (Suppl). 1:53–63.
1987.PubMed/NCBI
|
|
7
|
Stevens L: Egg white proteins. Comp
Biochem Physiol B. 100:1–9. 1991.
|
|
8
|
Jacobs K, Shen L, Benemariya H and
Deelstra H: Selenium distribution in egg white proteins. Z Lebensm
Unters Forsch. 196:236–238. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamasaki K, Kajimura K, Nakano M, Yokoyama
H, Yoneda K and Umezawa C: Effects of preparations of Chinese
medicinal prescriptions on digestive enzymes in vitro and in vivo.
Biol Pharm Bull. 21:133–139. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Choi EA, Kim KH, Yoo BC and Yoo HS:
Induction of apoptotic cell death by egg white
combined-chalcanthite on NCI-H460 human lung cancer cells. J Korean
Pharmacopuncture Inst. 12:49–59. 2009.(In Korean).
|
|
11
|
Perry VH and Gordon S: Macrophages and
microglia in the nervous system. Trends Neurosci. 11:273–277. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zielasek J and Hartung HP: Molecular
mechanisms of microglial activation. Adv Neuroimmunol. 6:191–222.
1996. View Article : Google Scholar
|
|
13
|
Block ML, Zecca L and Hong JS:
Microglia-mediated neurotoxicity: uncovering the molecular
mechanisms. Nat Rev Neurosci. 8:57–69. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rankine EL, Hughes PM, Botham MS, Perry VH
and Felton LM: Brain cytokine synthesis induced by an
intraparenchymal injection of LPS is reduced in MCP-1-deficient
mice prior to leucocyte recruitment. Eur J Neurosci. 24:77–86.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lynch MA: The multifaceted profile of
activated microglia. Mol Neurobiol. 40:139–156. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gonzalez-Scarano F and Baltuch G:
Microglia as mediators of inflammatory and degenerative diseases.
Annu Rev Neurosci. 22:219–240. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wirenfeldt M, Ladeby R, Dalmau I, Banati
RB and Finsen B: Microglia - biology and relevance to disease.
Ugeskr Laeger. 167:3025–3030. 2005.(In Danish).
|
|
18
|
Garden GA and Möller T: Microglia biology
in health and disease. J Neuroimmune Pharmacol. 1:127–137. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eikelenboom P and van Gool WA:
Neuroinflammatory perspectives on the two faces of Alzheimer’s
disease. J Neural Transm. 111:281–294. 2004.PubMed/NCBI
|
|
20
|
Gao HM, Liu B, Zhang W and Hong JS: Novel
anti-inflammatory therapy for Parkinson’s disease. Trends Pharmacol
Sci. 24:395–401. 2003.
|
|
21
|
Liu B and Hong JS: Role of microglia in
inflammation-mediated neurodegenerative diseases: mechanisms and
strategies for therapeutic intervention. J Pharmacol Exp Ther.
304:1–7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Burroughs M, Cabellos C, Prasad S and
Tuomanen E: Bacterial components and the pathophysiology of injury
to the blood-brain barrier: does cell wall add to the effects of
endotoxin in gram-negative meningitis? J Infect Dis. 165(Suppl 1):
S82–S85. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jung WK, Lee DY, Park C, Choi YH, Choi I,
Park SG, Seo SK, Lee SW, Yea SS, Ahn SC, Lee CM, Park WS, Ko JH and
Choi IW: Cilostazol is anti-inflammatory in BV2 microglial cells by
inactivating nuclear factor-kappaB and inhibiting mitogen-activated
protein kinases. Br J Pharmacol. 159:1274–1285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Baldwin AS Jr: The NF-kappa B and I kappa
B proteins: new discoveries and insights. Annu Rev Immunol.
14:649–683. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee SJ and Lee S: Toll-like receptors and
inflammation in the CNS. Curr Drug Targets Inflamm Allergy.
1:181–191. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Harari OA and Liao JK: NF-κB and innate
immunity in ischemic stroke. Ann NY Acad Sci. 1207:32–40. 2010.
|
|
27
|
Sarkar FH, Li Y, Wang Z and Kong D:
NF-kappaB signaling pathway and its therapeutic implications in
human diseases. Int Rev Immunol. 27:293–319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mankan AK, Lawless MW, Gray SG, Kelleher D
and McManus R: NF-kappaB regulation: the nuclear response. J Cell
Mol Med. 13:631–643. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee YH, Jeon SH, Kim SH, Kim C, Lee SJ,
Koh D, Lim Y, Ha K and Shin SY: A new synthetic chalcone
derivative, 2-hydroxy-3′,5,5′-trimethoxychalcone (DK-139),
suppresses the Toll-like receptor 4-mediated inflammatory response
through inhibition of the Akt/NF-κB pathway in BV2 microglial
cells. Exp Mol Med. 44:369–377. 2012.PubMed/NCBI
|
|
30
|
Lee SH, Kim DW, Back SS, Hwang HS, Park
EY, Kang TC, Kwon OS, Park JH, Cho SW, Han KH, Park J, Eum WS and
Choi SY: Transduced Tat-Annexin protein suppresses
inflammation-associated gene expression in lipopolysaccharide
(LPS)-stimulated Raw 264.7 cells. BMB Rep. 44:484–489. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Madrid LV, Wang CY, Guttridge DC,
Schottelius AJ, Baldwin AS Jr and Mayo MW: Akt suppresses apoptosis
by stimulating the transactivation potential of the RelA/p65
subunit of NF-kappaB. Mol Cell Biol. 20:1626–1638. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wei J and Feng J: Signaling pathways
associated with inflammatory bowel disease. Recent Pat Inflamm
Allergy Drug Discov. 4:105–117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim SH, Smith CJ and Van Eldik LJ:
Importance of MAPK pathways for microglial pro-inflammatory
cytokine IL-1 beta production. Neurobiol Aging. 25:431–439. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Y and Dong C: Regulatory mechanisms
of mitogen-activated kinase signaling. Cell Mol Life Sci.
64:2771–2789. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kreutzberg GW: Microglia: a sensor for
pathological events in the CNS. Trends Neurosci. 19:312–318. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ohshima H and Bartsch H: Chronic
infections and inflammatory processes as cancer risk factors:
possible role of nitric oxide in carcinogenesis. Mut Res.
305:253–264. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Murphy S: Production of nitric oxide by
glial cells: regulation and potential roles in the CNS. Glia.
29:1–13. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zekry D, Epperson TK and Krause KH: A role
for NOX NADPH oxidases in Alzheimer’s disease and other types of
dementia? IUBMB Life. 55:307–313. 2003.
|
|
39
|
Brown GC and Bal-Price A: Inflammatory
neurodegeneration mediated by nitric oxide, glutamate, and
mitochondria. Mol Neurobiol. 27:325–355. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Minghetti L: Cyclooxygenase-2 (COX-2) in
inflammatory and degenerative brain diseases. J Neuropathol Exp
Neurol. 63:901–910. 2004.PubMed/NCBI
|
|
41
|
St-Onge M, Flamand N, Biarc J, Picard S,
Bouchard L, Dussault AA, Laflamme C, James MJ, Caughey GE, Cleland
LG, Borgeat P and Pouliot M: Characterization of prostaglandin
E2 generation through the cyclooxygenase (COX)-2 pathway
in human neutrophils. Biochim Biophys Acta. 1771:1235–1245.
2007.
|
|
42
|
Kawano T, Anrather J, Zhou P, Park L, Wang
G, Frys KA, Kunz A, Cho S, Orio M and Iadecola C: Prostaglandin
E2 EP1 receptors: downstream effectors of COX-2
neurotoxicity. Nat Med. 12:225–229. 2006.
|
|
43
|
Hewett SJ, Uliasz TF, Vidwans AS and
Hewett JA: Cyclooxygenase-2 contributes to
N-methyl-D-aspartate-mediated neuronal cell death in primary
cortical cell culture. J Pharmacol Exp Ther. 293:417–425.
2000.PubMed/NCBI
|
|
44
|
Moon DO, Choi YH, Kim ND, Park YM and Kim
GY: Anti-inflammatory effects of beta-lapachone in
lipopolysaccharide-stimulated BV2 microglia. Int Immunopharmacol.
7:506–514. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee JY, Jhun BS, Oh YT, Lee JH, Choe W,
Baik HH, Ha J, Yoon KS, Kim SS and Kang I: Activation of adenosine
A3 receptor suppresses lipopolysaccharide-induced TNF-alpha
production through inhibition of PI 3-kinase/Akt and NF-kappaB
activation in murine BV2 microglial cells. Neurosci Lett. 396:1–6.
2006. View Article : Google Scholar
|