Introduction
Cancer remains a significant cause of morbidity and
mortality in the United States and worldwide, despite advances made
in recent decades on early detection and diagnosis. Increasingly,
among practicing clinicians and research professionals, the
one-time dominant view of seeking ‘magic bullets’ for cancer
eradication has been supplanted by the objective of cancer
prevention and management in order to improve the quality of life
of cancer patients.
A reductionist approach to curtail cancer risks,
thereby preventing cancer, involve the elimination or reduction, of
exposure to sources of carcinogens, together with change in
lifestyle and dietary habits. It is therefore notable that,
although anti-tumorigenic agents abound in the diet, as revealed by
epidemiological/migrant studies reporting an inverse relationship
between diet and cancer incidence rate, evidence indicates that
diet harbors carcinogens or their precursors. This dual
benefit/risk nature of diet with regard to carcinogenesis was
clearly demonstrated in a seminal report by Doll and Peto (1) who suggested that 10–70% of human
cancer mortality may be associated with diet. Furthermore, whereas
studies by Steinmetz and Potter (2,3)
and Willett (4) portend that
dietary factors contribute to one third of all cancer deaths,
evidence indicates that an estimated 20–50% of all cancer types are
preventable using plant-based diet strategies (5,6),
in part attributed to phytochemicals and polyphenols with
chemo-preventive activities (7–11).
The Reishi mushroom Ganoderma lucidum belongs
to the Basidiomycetes class of fungi whose use as an antitumor
agent dated back to the Imperial Court of ancient China (12–14). The major bioactive compounds in
Ganoderma lucidum are polysaccharides, ganoderic,
ganodermic, lucidic acids and their aldehydes and alcohols, and
highly oxidized lanostane-type triterpenes (15–17). I’m-Yunity™ is a proprietary method
procured version of the Basidomycetes mushroom comprising a
heterogeneous family of polysaccharide-protein complexes isolated
from the cultivated mycelia of Trametes (formerly
Coriolus) versicolor, which have been used as a major
ingredient in traditional Chinese medicinal formulations for the
prevention and treatment of chronic diseases including cancer
(18–27). The two mushroom products are
available as dietary supplements, principally as adjunct immune
boosters and prophylactic chemopreventative agents targeting the
initiation and progression of cancers (12,20,23,28–40). Despite their similar biological
activities, differences may exist, both with respect to the nature
of the polysaccharides and the spectrum of synthesized and secreted
secondary metabolites. Accordingly, it may be postulated that the
combination of the two mushrooms elicits distinct as well as
overlapping biological and molecular effects, compared to single
mushroom extracts. However, this possibility has yet to be
investigated. In this study, extracts prepared from I’m-Yunity and
from a combined formulation denoted I’m-Yunity-Too™-containing
extracts derived from I’m-Yunity and Ganoderma lucidum were
investigated with regard to differences in: ) anti-proliferative
activities; ii) cell cycle control and induction of apoptosis; iii)
activities between aqueous and ethanolic extracts.
Materials and methods
Reagents
Fetal calf serum, RPMI-1640, penicillin and
streptomycin were purchased from Cellgro, Inc. (Herndon, VA, USA).
Any other chemicals and solvents were of analytical grade. Primary
antibodies, respectively, against Rb, E2F, NF-κB p50, NF-κB p65,
IκB, caspase 3, bcl 2, Bax, actin, and secondary antibodies were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Primary antibodies against phosphorylated Rb Ser780, Ser795,
Ser807, Thr821 and Thr826 were obtained from Biosource
International, Inc. (Camarillo, CA, USA). Anti-PARP was purchased
from Biomol International, L.P. (Plymouth Meeting, PA, USA).
Source of Coriolus versicolor and
Ganoderma lucidum
I’m-Yunity and I’m-Yunity-Too capsules containing
extracts of Coriolus versicolor, and of Coriolus
versicolor and Ganoderma lucidum, respectively, (lot
3BA03020528) were provided by Integrated Chinese Medicine Holdings,
Ltd. (Hong Kong, China). The mushroom products were extracted and
purified from mycelia of Coriolus versicolor and
Ganoderma lucidum according to Good Manufacturing Practice
(GMP) standards. Quality control assays validating their
authenticity and integrity were performed in government-approved
testing centers in Hong Kong.
Preparation of aqueous and ethanolic
extracts of I’m-Yunity and I’m-Yunity-Too
To prepare aqueous or ethanolic extracts of
I’m-Yunity and I’m-Yunity-Too, the contents of each capsule were
suspended in 6 ml of water or 70% ethanol, intermittently mixed by
vortexing for 60 min at room temperature, and centrifuged to remove
insoluble particles. The clear super-natant was sterilized by
passing through a 0.22 μm filter and stored in aliquots at
4°C.
Cell culture and growth inhibition
assay
Human promyelocytic leukemia cell line (HL-60) was
supplied by American Tissue Culture Collection (Manassas, VA, USA).
Cells were cultured in RPMI-1640 and seeded at a density of
3×105 cells/ml for all the experiments, as previously
described (24, 26, 41– 43). Extracts of I’m-Yunity and
I’m-Yunity-Too were added to the culture media at the final doses
specified. Control and mushroom extract-exposed cells were
harvested at indicated times after treatment. Cell count was
performed using a hemocytometer and viability was determined by
trypan blue exclusion. Harvested cells were washed twice with PBS,
and pellets were stored at −80°C for subsequent analysis.
Cell cycle analysis
Cell cycle phase distribution was assayed by flow
cytometry. Following a 24-, 48- and 72-h treatment of HL-60 cells
with aqueous and ethanol extracts of I’m-Yunity or I’m-Yunity-Too
(10 μl/ml), the cells were stained with 1.0 μg/ml
DAPI containing 100 mM NaCl, 2 mM MgCl2 and 0.1% Triton
X-100 (Sigma-Aldrich Corp., St. Louis, MO, USA) at pH 6.8, as
previously described (24,
26, 41– 43). The DNA contents were collected and
analyzed by a flow cytometer (Ortho Diagnostic, Westwood, MA, USA)
and MultiCycle software from Phoenix Flow Systems (San Diego, CA,
USA) was used to quantify the percentage of cells in the respective
phases (G1, S and G2/M) of the cell cycle, as
previously described (24,
26, 41– 43). Flow cytometry was used to
determine cells undergoing apoptosis, as evident by the appearance
of the sub-G1 peak (44–46).
Preparation of cell extracts and western
blot analysis
For immunoblotting experiments, cells were collected
and lysed in ice-cold RIPA buffer, as described in recent studies
(47–50). The aliquots of lysates (20
μg of protein) were boiled with sample buffer for 5 min, and
resolved by 10% SDS-PAGE. The proteins were transferred to a
nitrocellulose membrane and blocked with TBST buffer (10 mM Tris,
pH 7.5, 100 mM NaCl and 0.05% Tween-20) containing 3% non-fat dried
milk overnight at 4°C. The blots were incubated overnight with
various primary antibodies followed by a 1-h incubation with
secondary antibodies. The blots were detected with an ECL detection
system (LumiGLO Peroxidase Chemiluminescent Substrate kit, KPL
Biotechnology, Inc., Gaithersburg, MD, USA) and quantified by
densitometry. Actin expression was used for normalization of the
sample loading, as previously described (47–50).
Results
Differential inhibition of cell growth by
aqueous and ethanolic extracts of I’m-Yunity and
I’m-Yunity-Too
To determine whether aqueous and ethanolic extracts
of I’m-Yunity and I’m-Yunity-Too exert comparable
anti-proliferative activities, human leukemia HL-60 cells were
exposed to a single dose of either extracts for 24–72 h. Growth and
cell viability were measured as described in Materials and methods.
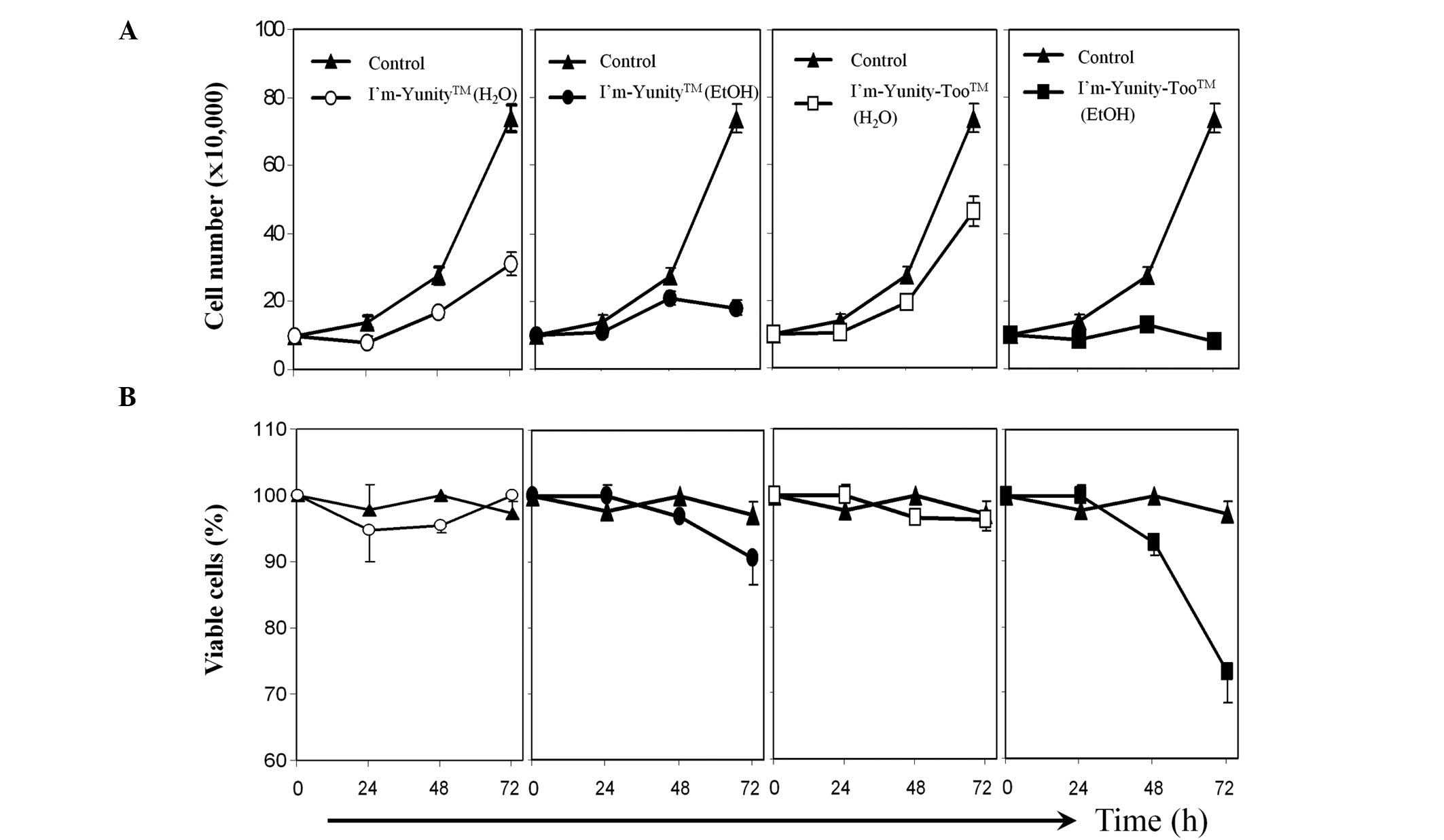
Results in Fig. 1A show that the
aqueous extract of I’m-Yunity was more potent in inhibiting cell
proliferation than the comparable aqueous extract of
I’m-Yunity-Too. Ethanolic extracts of the latter were slightly more
active than the former in suppressing cell growth and,
significantly more effective in inducing cell death (Fig. 1B).
I’m-Yunity and I’m-Yunity-Too extracts
affect NF-κB/IκB expression
To determine whether NF-κB plays a role on cell
growth inhibition by extracts of I’m-Yunity or I’m-Yunity-Too, the
expression of NF-κB/IκB was assayed by western blot analysis.
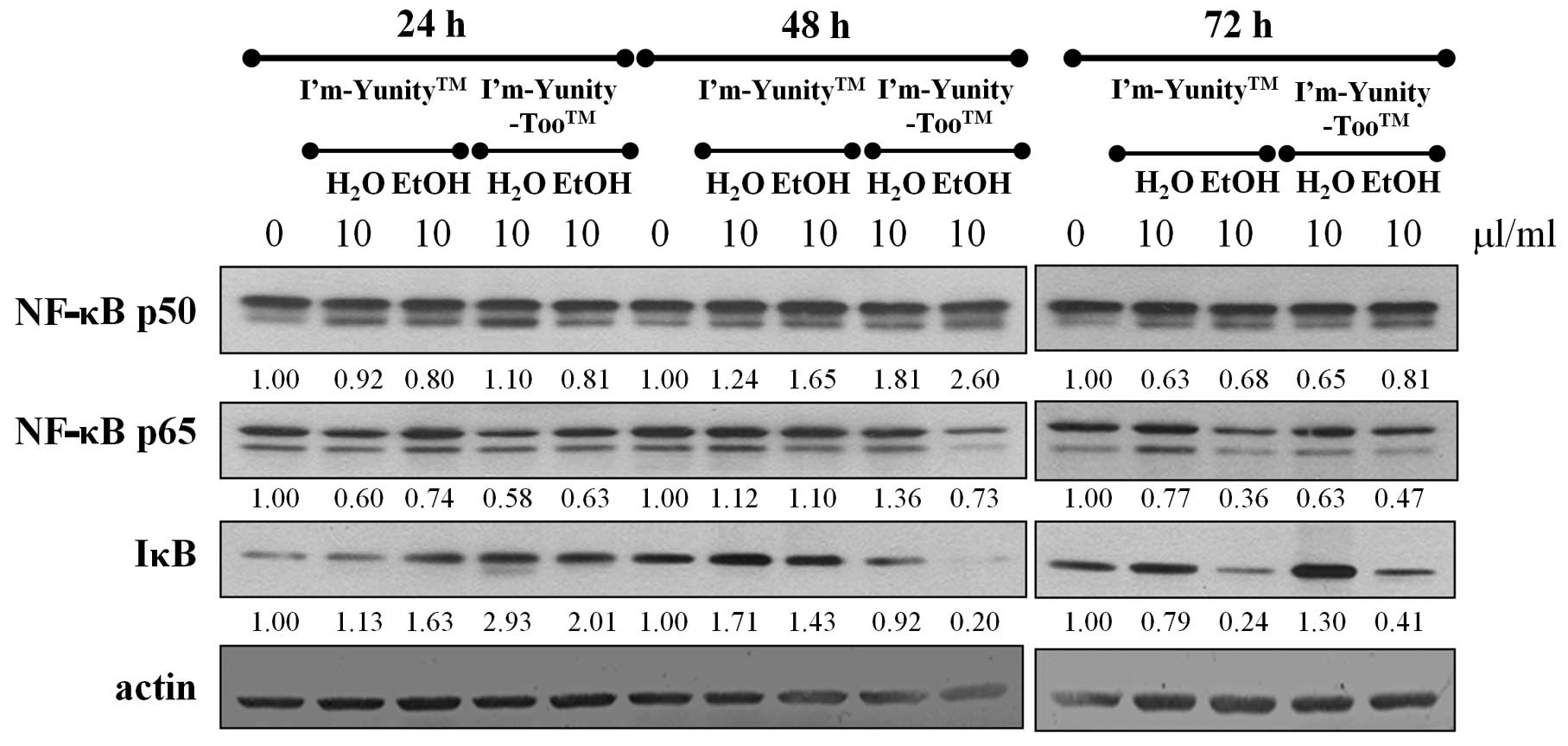
Results in Fig. 2 show that the
ethanolic extracts of I’m-Yunity-Too had a potent suppressive
effect on the expression of NF-κB p65 throughout the duration of
the experiment, and similarly inhibited IκB levels for 48- and 72
h-exposed cells. The NF-κB p65 modulatory effects were only
transiently observed in cells treated for 24 h with aqueous
extracts of I’m-Yunity or I’m-Yunity-Too, while for I’m-Yunity the
ethanolic extract-exposed cell treatment for 72 h also
significantly suppressed NF-κB p65 and IκB expression. These
results suggest that the ethanolic extract of I’m-Yunity-Too has a
significantly more pronounced effect in reducing the level of the
cell survival gene, NF-κB, as compared to extracts derived from
I’m-Yunity, suggesting that differential gene modulatory effects
exist between the aqueous and ethanolic extracts of I’m-Yunity and
I’m-Yunity-Too.
Cell cycle control by extracts of
I’m-Yunity and I’m-Yunity-Too
To examine whether I’m-Yunity- and
I’m-Yunity-Too-induced cell growth inhibition involves changes in
the cell cycle phase transition, flow cytometry was performed in
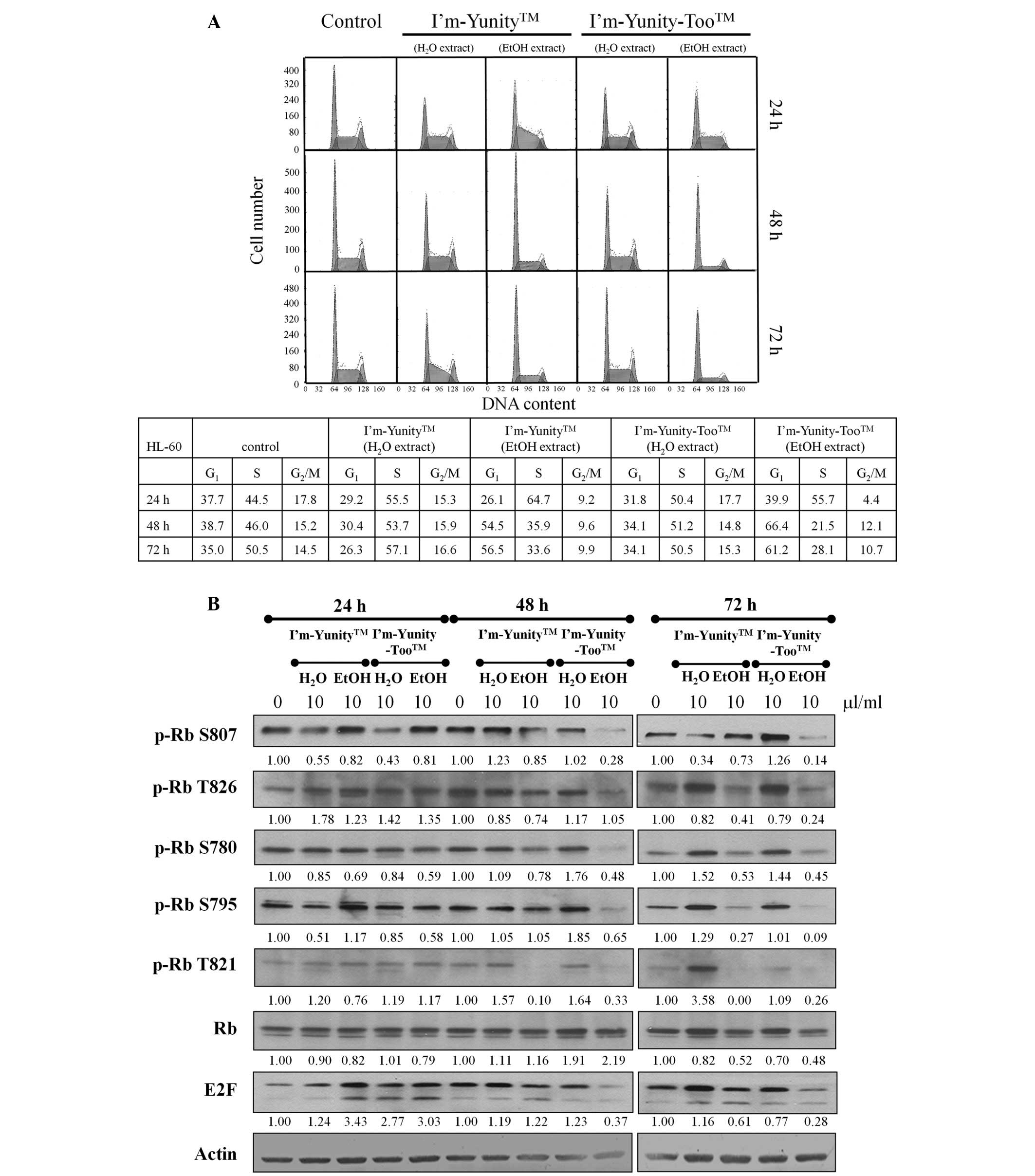
the control and exposed cells. Results in Fig. 3A show that HL-60 cells treated
with ethanolic extracts of I’m-Yunity-Too were restricted in the
G1→S progression. Fig.
3A also shows a decrease in cell fraction accumulated in the
G2M phase of the cell cycle, as compared to the
untreated cells. These effects were most notable in 48–72 h exposed
cells. By contrast, in cells treated for 24 h by aqueous and
ethanolic extracts of I’m-Yunity and I’m-Yunity-Too, the primary
cell cycle involved attenuation in the S→G2M phase
transition.
To elucidate control of G1→S in HL-60
cells treated with aqueous/ethanolic extracts of I’m-Yunity and
I’m-Yunity-Too, changes in Rb/E2F expression and the state of
phosphorylation of Rb were determined. Western blot analysis
demonstrated that while aqueous and ethanolic extracts of
I’m-Yunity and I’m-Yunity-Too did not change the expression of
total Rb, they markedly reduced the levels of Rb phosphorylation at
Ser807, Ser780, Thr821, as well as E2F, in 48 h-exposed cells
(Fig. 3B). Furthermore, the most
pronounced changes occurred in cells treated with ethanolic
extracts of I’m-Yunity-Too for 48 and 72 h, reinforcing that
extracts derived from the combination mushroom have biological
activities that are different from the ethanolic extract prepared
from the single mushroom I’m-Yunity. These findings also suggest
that ethanolic extracts of I’m-Yunity and I’m-Yunity-Too exert
multiple cell cycle effects in HL-60 cells manifested as specific
checkpoint arrest.
Targeting the induction of apoptosis by
I’m-Yunity and I’m-Yunity-Too
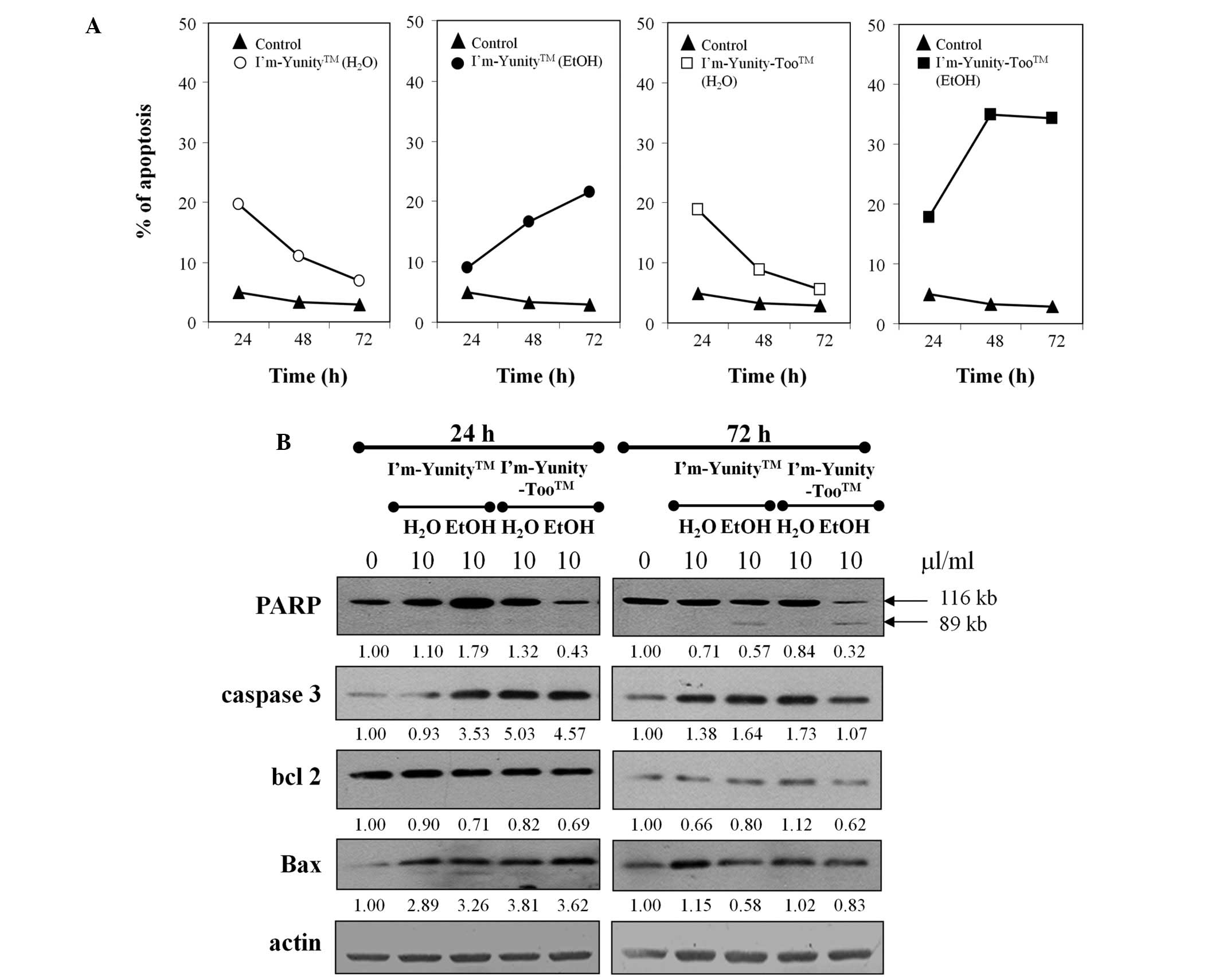
Results in Figs. 1B
and 4A show that the ethanolic extract of I’m-Yunity-Too
induced significantly more effective cell death as compared to the
ethanolic extract of I’m-Yunity, leading to subsequent analysis of
molecular determinants possibly contributing to such effects.
Fig. 4B shows that treatment with
aqueous and ethanolic extracts of I’m-Yunity and I’m-Yunity-Too at
24 and 72 h elevated the expression of caspase 3 as well as Bax,
both integral to the induction of apoptosis. Corroborative evidence
for apoptosis is derived from a reduction in the expression of PARP
in cells treated for 72 h with ethanolic, but not aqueous, extracts
of I’m-Yunity and I’m-Yunity-Too, concomitant with the increase in
the cleaved 89-kDa product from the 112-kDa precursor.
Discussion
The adjunctive potential of Ganoderma lucidum
in the management of cancer patients has been previously reported
(51). However, the mechanisms by
which it exerts efficacy have not been fully elucidated. It also
remains to be determined whether, when administered in combination,
the mechanisms generate novel activities.
In the present study, HL-60 cells cultured in
vitro were used to analyze the effects of I’m-Yunity
(Coriolus versicolor) relative to I’m-Yunity-Too
(Coriolus versicolor combined with Ganoderma lucidum)
with regard to proliferation and induction of apoptosis. We
demonstrated that I’m-Yunity and I’m-Yunity-Too significantly
inhibited HL-60 proliferation, in concordance with the
downregulation of NF-κB p65 expression. We also observed
differences concerning changes in IκB expression between the
aqueous and ethanolic extracts of I’m-Yunity and I’m-Yunity-Too.
Thus, cells treated with ethanolic extracts for 72 h showed the
downregulation of IκB, which was not affected in cells treated with
aqueous extracts.
Studies directed at determining the mechanism
controlling cell cycle progression by I’m-Yunity and I’m-Yunity-Too
revealed ethanolic extracts derived from I’m-Yunity-Too- restricted
cells in the G1 compared to the untreated cells, the
effects of which were most pronounced in cells treated for 48–72 h
and occurred in coordination with profound loss of E2F and the
downregulation of the Rb phosphorylation sites Ser780, Ser795,
Thr821 and Thr826, respectively. In terms of induction of
apoptosis, ethanolic extract of I’m-Yunity-Too caused more cell
death, as compared to those of I’m-Yunity. We also found that
ethanolic extract-treated cells induced more cell death at 72 h as
compared to aqueous extract-treated cells, based on PARP cleavage
which only appeared in ethanolic extract-treated cells.
Taken together, the results suggest that distinct
differences exist regarding the cell and gene regulatory effects of
aqueous and ethanolic extracts of I’m-Yunity-Too, as compared to
comparable extracts derived from I’m-Yunity. In addition, extracts
from the combination mushroom have different biological activity,
compared to ethanolic extract from the single mushroom I’m-Yunity.
Additional experiments and more detailed analysis are required to
determine the underlying mechanisms involved.
Acknowledgements
Research in this report was supported
in part by an unrestricted grant from the Hong Kong Healthcare
Center, Ltd. We acknowledge with thanks the support of Ms. Vivien
Chou and her family on mechanistic studies of medicinal mushrooms
in the prevention and management of chronic diseases in humans.
References
|
1.
|
Doll R and Peto R: The causes of cancer:
quantitative estimates of avoidable risks of cancer in the United
States today. J Natl Cancer Inst. 66:1191–1308. 1981.PubMed/NCBI
|
|
2.
|
Steinmetz KA and Potter JD: Vegetables,
fruit, and cancer. I Epidemiology Cancer Causes Control. 2:325–357.
1991. View Article : Google Scholar
|
|
3.
|
Steinmetz KA and Potter JD: Vegetables,
fruit, and cancer prevention: a review. J Am Diet Assoc.
96:1027–1039. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Willett WC: Diet, nutrition, and avoidable
cancer. Environ Health Perspect. 103(Suppl 8): 165–170. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Link LB and Potter JD: Raw versus cooked
vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev.
13:1422–1435. 2004.PubMed/NCBI
|
|
6.
|
Potter JD: Vegetables, fruit, and cancer.
Lancet. 366:527–530. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Surh YJ: Cancer chemoprevention with
dietary phytochemicals. Nat Rev Cancer. 3:768–780. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Thomasset SC, Berry DP, Garcea G, Marczylo
T, Steward WP and Gescher AJ: Dietary polyphenolic phytochemicals -
promising cancer chemopreventive agents in humans? A review of
their clinical properties. Int J Cancer. 120:451–458. 2007.
View Article : Google Scholar
|
|
9.
|
de Kok TM, van Breda SG and Manson MM:
Mechanisms of combined action of different chemopreventive dietary
compounds: a review. Eur J Nutr. 47(Suppl 2): 51–59.
2008.PubMed/NCBI
|
|
10.
|
Link A, Balaguer F and Goel A: Cancer
chemoprevention by dietary polyphenols: promising role for
epigenetics. Biochem Pharmacol. 80:1771–1792. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Lee KW, Bode AM and Dong Z: Molecular
targets of phytochemicals for cancer prevention. Nat Rev Cancer.
11:211–218. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Sliva D: Ganoderma lucidum (Reishi)
in cancer treatment. Integr Cancer Ther. 2:358–364. 2003.
View Article : Google Scholar
|
|
13.
|
Yuen JW and Gohel MD: Anticancer effects
of Ganoderma lucidum: a review of scientific evidence. Nutr
Cancer. 53:11–17. 2005.
|
|
14.
|
Paterson RR: Ganoderma - a therapeutic
fungal biofactory. Phytochemistry. 67:1985–2001. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Zhou X, Lin J, Yin Y, Zhao J, Sun X and
Tang K: Ganodermataceae: natural products and their related
pharmacological functions. Am J Chin Med. 35:559–574. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Zhong JJ and Xiao JH: Secondary
metabolites from higher fungi: discovery, bioactivity, and
bioproduction. Adv Biochem Eng Biotechnol. 113:79–150.
2009.PubMed/NCBI
|
|
17.
|
Zhang J, Tang Q, Zhou C, et al: GLIS, a
bioactive proteoglycan fraction from Ganoderma lucidum,
displays anti-tumour activity by increasing both humoral and
cellular immune response. Life Sci. 87:628–637. 2010.PubMed/NCBI
|
|
18.
|
Wasser SP and Weis AL: Therapeutic effects
of substances occurring in higher Basidiomycetes mushrooms: a
modern perspective. Crit Rev Immunol. 19:65–96. 1999.PubMed/NCBI
|
|
19.
|
Cui J and Chisti Y: Polysaccharopeptides
of Coriolus versicolor: physiological activity, uses, and
production. Biotechnol Adv. 21:109–122. 2003.
|
|
20.
|
Kidd PM: The use of mushroom glucans and
proteoglycans in cancer treatment. Altern Med Rev. 5:4–27.
2000.PubMed/NCBI
|
|
21.
|
Ho CY, Kim CF, Leung KN, et al:
Coriolus versicolor (Yunzhi) extract attenuates growth of
human leukemia xenografts and induces apoptosis through the
mitochondrial pathway. Oncol Rep. 16:609–616. 2006.
|
|
22.
|
Nicandro JP, Tsourounis C, Frassetto L and
Guglielmo BJ: In vivo effect of I’m-Yunity on hepatic cytochrome
P450 3A4. J Herb Pharmacother. 7:39–56. 2007.
|
|
23.
|
Hui KP, Sit WH and Wan JM: Induction of S
phase cell arrest and caspase activation by polysaccharide peptide
isolated from Coriolus versicolor enhanced the cell
cycle-dependent activity and apoptotic cell death of doxorubicin
and etoposide, but not cytarabine in HL-60 cells. Oncol Rep.
14:145–155. 2005.PubMed/NCBI
|
|
24.
|
Hsieh TC, Kunicki J, Darzynkiewicz Z and
Wu JM: Effects of extracts of Coriolus versicolor
(I’m-Yunity) on cell-cycle progression and expression of
interleukins-1 beta, -6, and -8 in promyelocytic HL-60 leukemic
cells and mitogenically stimulated and nonstimulated human
lymphocytes. J Altern Complement Med. 8:591–602. 2002.PubMed/NCBI
|
|
25.
|
Zeng F, Hon CC, Sit WH, et al: Molecular
characterization of Coriolus versicolor PSP-induced
apoptosis in human promyelotic leukemic HL-60 cells using cDNA
microarray. Int J Oncol. 27:513–523. 2005.
|
|
26.
|
Hsieh TC, Wu P, Park S and Wu JM:
Induction of cell cycle changes and modulation of
apoptogenic/anti-apoptotic and extra-cellular signaling regulatory
protein expression by water extracts of I’m-Yunity (PSP). BMC
Complement Altern Med. 6:302006.PubMed/NCBI
|
|
27.
|
Hsieh TC and Wu JM: Differential control
of growth, cell cycle progression, and gene expression in human
estrogen receptor positive MCF-7 breast cancer cells by extracts
derived from polysaccharopeptide I’m-Yunity and Danshen and their
combination. Int J Oncol. 29:1215–1222. 2006.PubMed/NCBI
|
|
28.
|
Hu H, Ahn NS, Yang X, Lee YS and Kang KS:
Ganoderma lucidum extract induces cell cycle arrest and
apoptosis in MCF-7 human breast cancer cell. Int J Cancer.
102:250–253. 2002. View Article : Google Scholar
|
|
29.
|
Tsang KW, Lam CL, Yan C, et al:
Coriolus versicolor polysaccharide peptide slows progression
of advanced non-small cell lung cancer. Respir Med. 97:618–624.
2003. View Article : Google Scholar
|
|
30.
|
Wong CK, Bao YX, Wong EL, Leung PC, Fung
KP and Lam CW: Immunomodulatory activities of Yunzhi and Danshen in
post-treatment breast cancer patients. Am J Chin Med. 33:381–395.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Wan JM, Sit WH and Louie JC:
Polysaccharopeptide enhances the anticancer activity of doxorubicin
and etoposide on human breast cancer cells ZR-75-30. Int J Oncol.
32:689–699. 2008.PubMed/NCBI
|
|
32.
|
Luk SU, Lee TK, Liu J, et al:
Chemopreventive effect of PSP through targeting of prostate cancer
stem cell-like population. PLoS One. 6:e198042011. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Jiang J, Slivova V, Harvey K,
Valachovicova T and Sliva D: Ganoderma lucidum suppresses
growth of breast cancer cells through the inhibition of
Akt/NF-kappaB signaling. Nutr Cancer. 49:209–216. 2004. View Article : Google Scholar
|
|
34.
|
Yue GG, Fung KP, Tse GM, Leung PC and Lau
CB: Comparative studies of various ganoderma species and their
different parts with regard to their antitumor and immunomodulating
activities in vitro. J Altern Complement Med. 12:777–789. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Jiang J, Grieb B, Thyagarajan A and Sliva
D: Ganoderic acids suppress growth and invasive behavior of breast
cancer cells by modulating AP-1 and NF-κB signaling. Int J Mol Med.
21:577–584. 2008.PubMed/NCBI
|
|
36.
|
Weng CJ, Chau CF, Yen GC, Liao JW, Chen DH
and Chen KD: Inhibitory effects of Ganoderma lucidum on
tumorigenesis and metastasis of human hepatoma cells in cells and
animal models. J Agric Food Chem. 57:5049–5057. 2009.PubMed/NCBI
|
|
37.
|
Thyagarajan A, Jedinak A, Nguyen H, et al:
Triterpenes from Ganoderma lucidum induce autophagy in colon
cancer through the inhibition of p38 mitogen-activated kinase (p38
MAPK). Nutr Cancer. 62:630–640. 2010.
|
|
38.
|
Hsieh TC and Wu JM: Suppression of
proliferation and oxidative stress by extracts of Ganoderma
lucidum in the ovarian cancer cell line OVCAR-3. Int J Mol Med.
28:1065–1069. 2011.PubMed/NCBI
|
|
39.
|
Wu G, Qian Z, Guo J, et al: Ganoderma
lucidum extract induces G1 cell cycle arrest, and apoptosis in
human breast cancer cells. Am J Chin Med. 40:631–642. 2012.
View Article : Google Scholar
|
|
40.
|
Bao PP, Lu W, Cui Y, et al: Ginseng and
Ganoderma lucidum use after breast cancer diagnosis and
quality of life: a report from the Shanghai Breast Cancer Survival
Study. PLoS One. 7:e393432012.PubMed/NCBI
|
|
41.
|
DiPietrantonio AM, Hsieh TC, Olson SC and
Wu JM: Regulation of G1/S transition and induction of apoptosis in
HL-60 leukemia cells by fenretinide (4HPR). Int J Cancer. 78:53–61.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
DiPietrantonio AM, Hsieh TC, Juan G,
Traganos F, Darzynkiewicz Z and Wu JM: Fenretinide-induced caspase
3 activity involves increased protein stability in a mechanism
distinct from reactive oxygen species elevation. Cancer Res.
60:4331–4335. 2000.
|
|
43.
|
Elangovan S and Hsieh TC: Control of
cellular redox status and upregulation of quinone reductase NQO1
via Nrf2 activation by α-lipoic acid in human leukemia HL-60 cells.
Int J Oncol. 33:833–838. 2008.PubMed/NCBI
|
|
44.
|
Bedner E, Burfeind P, Hsieh TC, et al:
Cell cycle effects and induction of apoptosis caused by infection
of HL-60 cells with human granulocytic ehrlichiosis pathogen
measured by flow and laser scanning cytometry. Cytometry. 33:47–55.
1998. View Article : Google Scholar
|
|
45.
|
Smolewski P, Bedner E, Du L, et al:
Detection of caspases activation by fluorochrome-labeled
inhibitors: multiparameter analysis by laser scanning cytometry.
Cytometry. 44:73–82. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Hsieh TC, Aguero-Rosenfeld ME, Wu JM, et
al: Cellular changes and induction of apoptosis in human
promyelocytic HL-60 cells infected with the agent of human
granulocytic ehrlichiosis (HGE). Biochem Biophys Res Commun.
232:298–303. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Hsieh TC, Yang CJ, Lin CY, Lee YS and Wu
JM: Control of stability of cyclin D1 by quinone reductase 2 in
CWR22Rv1 prostate cancer cells. Carcinogenesis. 33:670–677. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Yang CJ, Lin CY, Hsieh TC, Olson SC and Wu
JM: Control of eotaxin-1 expression and release by resveratrol and
its metabolites in culture human pulmonary artery endothelial
cells. Am J Cardiovasc Dis. 1:16–30. 2011.PubMed/NCBI
|
|
49.
|
Hsieh TC: Uptake of resveratrol and role
of resveratrol-targeting protein, quinone reductase 2, in normally
cultured human prostate cells. Asian J Androl. 11:653–661. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Hsieh TC: Antiproliferative effects of
resveratrol and the mediating role of resveratrol targeting protein
NQO2 in androgen receptor-positive, hormone-non-responsive CWR22Rv1
cells. Anticancer Res. 29:3011–3017. 2009.PubMed/NCBI
|
|
51.
|
Lin CN, Tome WP and Won SJ: Novel
cytotoxic principles of Formosan Ganoderma lucidum. J Nat
Prod. 54:998–1002. 1991. View Article : Google Scholar : PubMed/NCBI
|