Introduction
Endometriosis is a benign, estrogen-dependent,
gynecological disorder that is defined as the presence of
endometrial glands and stroma outside the uterine cavity. Women
with endometriosis may present with chronic pelvic pain,
dysmenorrhea, dyspareunia and/or infertility. Retrograde
menstruation, peritoneal adhesion and outgrowth of endometrial
tissue are major factors in the pathogenesis of endometriosis
according to Sampson’s classic implantation theory (1). However, the stimuli promoting the
attachment and outgrowth of endometrial cells in the peritoneal
cavity have remained largely unknown. Among many factors suggested
to be involved in the pathogenesis of endometriosis, immunological
and inflammatory responses have been suggested to play crucial
roles in the development and progression of endometriosis, and thus
the disease may be considered an immune-related chronic
inflammatory disease (2,3).
Cell adhesion molecules mediate essential cell-cell
interactions and play an important role in cell differentiation,
the organization of the extracellular matrix and in the recruitment
and aggregation of leukocytes from the circulation. Intercellular
adhesion molecule-1 (ICAM-1, CD54) mediates various biological
processes, including adhesive interactions with integrins,
leukocyte extravasation and lymphocyte-mediated cytotoxicity
(4). The expression of ICAM-1,
which is upregulated in response to a number of inflammatory
mediators, such as tumor necrosis factor (TNF)-α, is associated
with a variety of inflammatory diseases and conditions (4). Similar to ICAM-1, vascular cell
adhesion molecule-1 (VCAM-1, CD106) also has a well-characterized
role in immunological responses in humans. VCAM-1 plays an
important role in mediating mononuclear leukocyte-selective
adhesion to vascular endothelial cells, preparing them for
transendothelial migration into inflammatory foci. The upregulation
of VCAM-1 in endothelial cells is induced by pro-inflammatory
cytokines, such as TNF-α, interleukin (IL)-1β and interferon
(IFN)-γ (5). A growing body of
evidence suggests that these critical cell adhesion molecules in
various immune and inflammatory responses also play pivotal roles
in the physiology and pathophysiology of the human endometrium.
ICAM-1 is expressed in a regulated pattern by endometrial stromal
cells during the menstrual cycle (6). ICAM-1 is expressed in cultured human
endometrial stromal cells (HESCs), as well as in endometrial
stromal cells in the human endometrium in situ (7,8).
Furthermore, in patients with endometriosis, ICAM-1 is more
strongly expressed in ectopic endometrial stromal cells than in
eutopic endometrial stromal cells (9), and the elevated expression of ICAM-1
is induced in endometrial stromal cells by pro-inflammatory
cytokines, such as IFN-γ (10).
These data suggest that crosstalk may occur between endometrial
stromal cells and leukocytes in the endometrium and peritoneal
fluid via ICAM-1 and its receptors, contributing to the
pathological processes of endometriosis. In addition to ICAM-1,
VCAM-1 is expressed in HESCs, but little is known about the
expression and role of VCAM-1 in endometriosis (11).
TNF-α, a pleiotropic pro-inflammatory cytokine, is
regarded as an important regulatory molecule in eliciting
inflammatory immune responses in endometriosis, and its abnormal
production may contribute to the pathophysiology of endometriosis,
including cell proliferation and adhesion (1,12).
The nuclear factor-κB (NF-κB) and activator protein-1 (AP-1)
transcription factors play key roles in immune and inflammatory
responses, and modulate cell proliferation, apoptosis, adhesion,
invasion and angiogenesis in several cell types, including
endometrial cells (13,14). The activation of NF-κB has been
implicated in the early development of endometriotic lesions in
vivo (13). In particular,
AP-1 and NF-κB are the principal transcription factors that
regulate the expression of adhesion molecules, such as ICAM-1 and
VCAM-1 (15).
Garlic (Allium sativum; A. Savitum) has long
been cultivated as a food and used as a home remedy in Asia. Garlic
derivatives have important pharmacological properties, such as
anti-inflammatory, anti-microbial, anti-thrombotic,
anti-hypertensive, anti-hyperlipidemic, anti-hyperglycemic, immune
system enhancement and anti-tumor properties (16). These findings indicate that garlic
extracts are clinically effective for the treatment of diverse
human diseases. Aged black garlic, intended as a healthy dietary
supplement, is a type of fermented garlic used as a food ingredient
in Korea, Thailand and Japan. Its unique qualities are the result
of the fermentation of whole garlic bulbs under high temperatures
for several months. Upon fermentation, regular garlic, which has a
strong flavor and pungent odor, turns into soft, sweet, odorless
and black garlic.
The aim of the present study was to investigate the
effects of a hexane extract of aged black garlic (HEABG) on the
cell proliferation and expression of ICAM-1 and VCAM-1 in
TNF-α-activated HESCs isolated from patients with
endometriosis.
Materials and methods
Plant materials
Garlic (A. sativum L.) specimens were
collected in January 2008 from Changnyeong, Korea. Aged black
garlic was manufactured by Newgreen Food Co. (Changnyeong, Korea)
by incubating raw garlic at 75°C and 70% relative humidity for 2
weeks. A voucher specimen (accession no. NBG-PRDR-11) was deposited
in the Plant Drug Research Laboratory of Pusan National University
(Miryang, Korea).
Preparation of HEABG from aged black
garlic
Aged black garlic extracts (500 ml) were purchased
directly from Newgreen Food Co. in January 2009. The aged black
garlic extracts were successively partitioned with hexane,
chloroform and n-butanol. The upper suspension layer was filtered
and evaporated under reduced pressure at 45°C and then lyophilized.
A yellow, oily residue of hexane extract (17.6 mg) was obtained.
The remaining aqueous layer was then sequentially partitioned with
chloroform and n-butanol to yield chloroform (348 mg) and n-butanol
fractions (11.53 g), respectively. The hexane fraction was used in
this study. Hexane, chloroform, ethyl acetate, n-butanol and
methanol were purchased from Fisher Scientific, Ltd. (Pittsburgh,
PA, USA).
Subjects
Eighteen women (aged 25–45 years; mean age,
35.44±5.99 years) with advanced endometriosis (III/IV) were
enrolled in this study (Table I).
These women, who were treated for dysmenorrhea, pelvic pain or
leiomyoma, were found to have endometriosis during laparoscopy, had
no endometrial hyperplasia or neoplasia, and had not received
anti-inflammatory nor any other hormonal treatment in the 3 months
prior to surgery (Table I). The
menstrual cycle phase of each patient enrolled in this study was
recorded (Table I). Ovarian
endometrioma specimens were obtained by performing laparoscopic
surgery (Table I). Ovarian
endometrioma capsules were removed from the normal ovarian cortex
for pathologic examination and further culture preparation.
Endometriotic biopsy samples were immediately placed in sterile
Hanks’ balanced salt solution (HBSS) containing 100 U/ml
penicillin, 100 mg/ml streptomycin and 0.25 mg/ml amphotericin and
maintained at 4°C. The diagnosis and staging of endometriosis in
all cases was performed according to the revised American Society
for Reproductive Medicine classification system (17) and confirmed by histopathological
analysis. Written informed consent was obtained from all of the
patients, and the study protocol was approved by the Institutional
Review Board at Pusan National University Hospital.
 | Table I.Clinical characteristics of patients,
sources of endometriotic tissue biopsy samples, laparoscopic
findings and clinical parameters. |
Table I.
Clinical characteristics of patients,
sources of endometriotic tissue biopsy samples, laparoscopic
findings and clinical parameters.
| Patient No. | Age (years) | Cell cycle
phase | ASRM stage of
endometriosis | Location of
endometriotic tissue | Clinical
parameters | Type of
experiment |
|---|
| 1 | 42 | Proliferative | IV | Both ovaries | Dysmenorrhea | Cytotoxicity,
proliferation |
| 2 | 38 | Secretory | IV | Both ovaries | Menorrhagia,
dysmenorrhea | Cytotoxicity,
qRT-PCR |
| 3 | 44 | Proliferative | IV | Right ovary | Presence of
leiomyoma, intermittent pelvic pain | Proliferation,
FACS |
| 4 | 35 | Proliferative | III | Left ovary | Presence of
leiomyoma | Western blot,
FACS |
| 5 | 38 | Secretory | IV | Right ovary | Dysmenorrhea,
pelvic pain | Proliferation,
ELISA |
| 6 | 45 | Proliferative | IV | Right ovary | Dysmenorrhea | Cytotoxicity,
FACS |
| 7 | 35 | Proliferative | III | Left ovary | Dysmenorrhea | qRT-PCR, western
blot |
| 8 | 31 | Secretory | IV | Right ovary | Pelvic pain | Western blot,
IF |
| 9 | 28 | Secretory | IV | Left ovary | Dysmenorrhea | Proliferation,
EMSA |
| 10 | 36 | Secretory | III | Left ovary | Pelvic pain,
presence of leiomyoma | qRT-PCR, IF,
FACS |
| 11 | 30 | Proliferative | IV | Both ovaries | Dysmenorrhea,
presence of leiomyoma | IF, western blot,
EMSA |
| 12 | 41 | Proliferative | IV | Both ovaries | Dysmenorrhea | FACS, EMSA |
| 13 | 31 | Proliferative | IV | Right ovary | Dysmenorrhea | IF, FACS |
| 14 | 42 | Secretory | IV | Right ovary | Dysmenorrhea,
pelvic pain | Western blot,
ELISA |
| 15 | 28 | Secretory | IV | Both ovaries | Intermittent pelvic
pain | FACS, IF,
proliferation |
| 16 | 25 | Proliferative | IV | Left ovary | Pelvic pain | FACS, ELISA |
| 17 | 38 | Proliferative | III | Right ovary | Dysmenorrhea | Western blot,
qRT-PCR |
| 18 | 31 | Proliferative | IV | Both ovaries | Dysmenorrhea | Western blot,
ELISA |
Cell culture system
Immediately after the tissues were transported to
the laboratory, cell culture was performed in Dulbecco’s modified
Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS).
Chemicals and culture media were purchased from Life Technologies
(Gaithersburg, MD, USA), and cytokines were purchased from Chemicon
International (Temecula, CA, USA). Culture flasks and dishes were
obtained from BD Biosciences Discovery Labware (Bedford, MA, USA).
Tissues were gently rinsed and washed in DMEM containing
antibiotics/antimycotics (300 IU/ml penicillin G, 300 μg/ml
streptomycin and 3 μg/ml fungizone). The tissues were then
treated with 0.25% collagenase type I in DMEM at 37°C for 30 min in
a shaking water bath. The cells were then passed through a
70-μm sieve (BD Biosciences Discovery Labware). Following
sedimentation, the supernatant was transferred to a new tube and
the cells were collected by centrifugation. The cells were
maintained and proliferated in DMEM supplemented with 10% FBS in a
humidified atmosphere of 5% CO2 at 37°C. The stromal
cell-rich supernatant was placed in a culture flask and cells were
allowed to adhere for 20 min, and then washed with the medium.
Adherent stromal cells were cultured in monolayers in flasks with
DMEM/F-12 (1:1) containing antibiotics/antimycotics, 5 μg/ml
insulin (Sigma, St. Louis, MO, USA) and 10% FBS. Culture purity was
determined morphologically after hematoxylin and eosin (H&E)
staining. Stromal cells were fusiform, but more round than
fibroblasts. Cells were also evaluated immunohistochemically using
antibodies to human cytokeratin, vimentin, von Willebrand factor
and CD45. The HESCs expressed vimentin, but not cytokeratin, von
Willebrand factor or CD45. As defined by these criteria, the purity
of the HESCs was >97%. After the second subculture, the HESCs
were cultured in DMEM/F-12 (1:1) containing
antibiotics/anti-mycotics and 10% FBS. Subsequently, the culture
medium was changed to serum-starved (5% FBS) DMEM/F-12 (1:1), and
the cells were incubated for an additional 24 h prior to
treatment.
Cell cytotoxicity assay
After the HESCs (4×103 cells/well) in
96-well plates were treated with various doses of HEABG (1, 10, 30,
50 or 70 μg/ml) for 48 h, cell viability was determined
using the colorimetric WST-1 conversion assay (Roche Diagnostics,
Mannheim, Germany). A total of 10 μl of WST-1 reagent was
added to each well, and the cells were incubated for 2 h in a
humidified incubator at 37°C under 5% CO2. Absorbance
was measured in a microplate reader (Tecan Group Ltd., Männedorf,
Switzerland) at 450 nm.
Cell proliferation assay
After the HESCs (4×103 cells/well) in
96-well plates were pre-treated with various doses of HEABG (30 or
50 μg/ml), 50 μM of an extracellular signal-regulated
kinase (ERK) inhibitor (PD98059; Tocris Bioscience, Bristol, UK) or
20 μM of a c-Jun N-terminal kinase (JNK) inhibitor
(SP600125; Tocris Bioscience) for 1 h, the cells were further
treated with 10 ng/ml of TNF-α for 48 h. Cell proliferation was
determined using the colorimetric WST-1 conversion assay as
described above.
Cell cycle analysis
Cell cycle analysis was performed using a FACSCanto
II with FACSDiva software (both from BD Biosciences, San Jose, CA,
USA). Cells were harvested by trypsin-EDTA treatment and washed
with phosphate-buffered saline (PBS) containing 1% FBS and 0.1%
NaN3, and fixed with 70% ethanol at 4°C for 20 min.
After washing 3 times with ice-cold PBS, the cells were suspended
in propidium iodide (PI)-solution (0.001% Triton X-100, 3.4% RNase
A, 0.005% PI in PBS). An electronic gate was set on the HESCs using
forward and side scatter characteristics. In all the experiments,
cells were gated on forward and side scatter to eliminate dead
cells and debris.
Flow cytometry
Immunophenotypic analysis of the cells was performed
using a FACSCanto II with FACSDiva software (both from BD
Biosciences). Cells were harvested by trypsin-EDTA treatment and
washed with PBS containing 1% FBS and 0.1% NaN3. For
flow cytometry, the cells were incubated with 2.4G2 (anti-Fc
receptor) hybridoma culture supernatant to block non-specific
antibody binding and then stained with fluorochrome-conjugated
monoclonal antibodies (mAbs). Phycoerythrin (PE)-anti-ICAM-1 (CD54
and MEM-111) and PE-anti-VCAM-1 (CD106 and STA) mAbs were purchased
from BioLegend Inc. (San Diego, CA, USA). Background fluorescence
was determined by measuring the fluorescent signal from cells
stained with fluorochrome-labeled isotype-matched non-reactive mAbs
for 30 min at 4°C. An electronic gate was set on the HESCs using
forward and side scatter characteristics. In all the experiments,
cells were gated on forward and side scatter to eliminate dead
cells and debris.
Immunofluorescence staining
For immunofluorescence analysis, the cells were
cultured on 12-mm glass coverslips (Paul Marienfeld GmbH & Co.
KG, Lauda-Königshofen, Germany) and fixed with cold 4%
paraformaldehyde in 0.1 M phosphate buffer (PB) for 10 min.
Subsequently, the fixative was removed by washing the coverslips 3
times for 5 min each with cold PBS followed by permeabilization
with 0.1% Triton X-100 in PBS for 5 min. The cells were washed
again with cold PBS and then incubated with 1% bovine serum albumin
(Sigma) for 60 min at room temperature. Excess solution was shaken
off and the cells were incubated for 16–18 h at 4°C with
PE-anti-ICAM-1 or PE-anti-VCAM-1 (both from BioLegend) mAbs diluted
1:100. Following incubation with the primary antibodies, the cells
were washed 3 times for 5 min each with cold PBS. The cells were
then rinsed in cold PBS and mounted on glass slides using
Vectashield® (Vector Laboratories, Burlingame, CA, USA).
Labeled cells were examined using an Olympus BX50 microscope
equipped with fluorescent epi-illumination. Photomicrographs were
acquired digitally at 1360×1024 pixel resolution with an Olympus
DP70 digital camera.
Preparation of cytosolic and nuclear cell
extracts
Cells were resuspended in 70 μl of buffer A
[10 mM HEPES (pH 7.9), 1.5 mM MgCl2, 10 mM KCl, 0.5 mM
DTT, 0.5 mM PMSF, protease inhibitor cocktail and phosphatase
inhibitor cocktail I and II (both from Sigma)] and incubated on
ice. After 15 min, 0.5% nonyl phenoxypolyethoxylethanol P (NP)-40
was added to lyse the cells, followed by vortexing for 10 sec.
Cytosolic cell extracts were obtained after centrifugation at 6,500
rpm for 60 sec at 4°C. Nuclei were resuspended in 50 ml of buffer C
[20 mM HEPES (pH 7.9), 1.5 mM MgCl2, 420 mM NaCl, 0.2 mM
EDTA, 25% v/v glycerol, 0.5 mM PMSF and protease inhibitor
cocktail] and incubated on ice for 20 min with gentle pipetting
every 5 min. Nuclear cell extracts were recovered after
centrifugation for 10 min at 12,000 rpm at 4°C. Protein
concentration was determined using the Bradford protein assay
reagent (Bio-Rad, Hercules, CA, USA).
Western blot analysis
Equal amounts of protein samples were heated for 10
min at 95°C in sample buffer and separated by performing sodium
dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on
12% SDS polyacrylamide gel, using a Mini-Protean III system
(Bio-Rad). Proteins were transferred onto a polyvinylidene
difluoride (PVDF) membrane using a semi-dry transfer apparatus
(both from Bio-Rad). The blotted PVDF membrane was blocked with 5%
skim milk in Tris-buffered saline (TBS) at room temperature for 2
h. The membrane was then incubated overnight at 4°C with
anti-ICAM-1 (sc-1511), anti-VCAM-1 (sc-1504), anti-NF-κB p65
(sc-8008), anti-IκBα (sc-203), anti-phospho (p)-IκBα (sc-7977-R)
(all from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA),
anti-p-ERK (p44/42 MAPK) (4370), anti-ERK (p44/42 MAPK) (9102),
anti-p-JNK (4668), anti-JNK (9258), anti-p-p38 MAPK (9215) and
anti-p38 MAPK mAbs (9212) (Cell Signaling Technology, Danvers, MA,
USA). These antibodies, as well as that for α-tubulin (MU121-UC;
BioGenex Laboratories, Inc., San Ramon, CA, USA) and β-actin (8226;
Abcam, Cambridge, UK), were incubated with the membranes at a
dilution of 1:1,000 in TBS containing 2% skim milk. After 3 washes
with TBS-T (TBS containing 0.1% Tween-20), the membrane was
incubated for 2 h at room temperature with the secondary antibody,
rabbit anti-goat IgG horseradish peroxidase (HRP) conjugate
(sc-2768), goat anti-mouse IgG HRP conjugate (sc-2031), goat
anti-rabbit IgG HRP conjugate (sc-2004) (all from Santa Cruz
Biotechnology, Inc.) and goat anti-rabbit IgG HRP (7074; Cell
Signaling Technology) diluted 1:2,000, and re-washed 3 times with
TBS-T. Immunoreactivity was detected using enhanced
chemiluminescence (ECL; SuperSignal West Pico Chemiluminescent
Substrate kit; Pierce, Rockford, IL, USA) according to the
manufacturer’s instructions. Images were acquired and the signals
were quantified using a Fujifilm LAS-3000 imaging system (Fujifilm,
Tokyo, Japan).
Quantitative reverse transcriptase-PCR
(qRT-PCR)
Total RNA was then isolated from the cells using
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions and treated with DNase (DNA-free kit;
Ambion, Austin, TX, USA). Total RNA (500 ng) was converted into
cDNA (M-MLV Reverse Transcrip tase; Promega, Madison, WI, USA).
qRT-PCR amplification was performed using the following qRT-PCR
primers: ICAM-1 sense, 5′-CCGTGAAT GTGCTCTCCC-3′ and antisense,
5′-ATTTCTTGATCTT CCGCTGG-3′ (GenBank accession no. NM_000201); and
VCAM-1 sense, 5′-CAGGTGGAGCTCTACTCATTCC-3′ and antisense,
5′-GAATAGTCTCCCCCTTAAGTAATTC-3′ (GenBank accession no. NM_001078).
For normalization, the GAPDH gene was amplified using the following
primers: GAPDH sense, 5′-GAAGATGGTGATGGGATTTC-3′ and antisense,
5′-GAAGGTGAAGGTCGGAGT-3′ (GenBank accession no. NM_002046). qRT-PCR
amplification was performed in a Rotor-Gene 6000 (Corbett Life
Science, Sydney, Australia) with QuantiTect®
SYBR®-Green PCR Master Mix (Qiagen, Hilden, Germany).
qRT-PCR amplification of ICAM-1, VCAM-1 and GAPDH genes were
carried out for 50 cycles at 94°C for 10 sec, 56°C for 15 sec and
72°C for 15 sec. Melting curve analysis was conducted for 30 sec at
55–99°C. Amplification products exhibited single, sharp peaks,
indicating that the primers amplified only one specific PCR
product. All the samples were amplified in triplicate. An
amplification reaction containing no template was used as a control
to establish non-specific background amplification. Data were
analyzed using the Rotor-Gene 6000 program (Corbett Life Science).
Gene expression levels were calculated using the formula, R0 = RCt
x (1 + E) - Ct and were normalized to GAPDH expression levels.
Enzyme-linked immunosorbent assay
(ELISA)
Cells cultured in 96-well plates
(4×103/well) in DMEM/F-12 medium in the presence of 5%
FBS were pre-treated with or without HEABG (50 μg/ml) for 1
h and stimulated with TNF-α (10 ng/ml) for 15 h, after which
supernatants were harvested. The concentrations of IL-6 in the
supernatants were measured using standard ELISA kits (R&D
Systems, Minneapolis, MN, USA). Diluted supernatant (1:100) and
HRP-conjugated anti-IL-6 antibodies were added separately into each
antibody-coated well for evaluation. Plate wells were covered with
a fresh plastic sealer and incubated at room temperature for 2 h
with gentle shaking. The solution was then discarded, and the wells
were washed 6 times with wash buffer. Chromogen solution
(tetramethylbenzidine) was added to the wells and incubated for 20
min at room temperature, followed by the addition of stop solution.
The amount of conjugate bound to each well was quantified
photometrically from these colored products by measuring the
optical density at 450 nm. A concentration of IL-6 was extrapolated
from a standard curve using recombinant IL-6. The sensitivity of
the ELISA assay of IL-6 was ≥0.7 pg/ml.
Electrophoretic mobility shift assay
(EMSA)
The cells were pre-treated with HEABG (50
μg/ml) for 1 h and then stimulated with TNF-α (10 ng/ml) for
30 min at 37°C prior to the extraction of nuclear protein. The
NF-κB oligonucleotide (5′-AGTTGAGGGGACTTTCCCAGGC-3′) and AP-1
oligonucleotide (5′-CGCTTGATGACTCAGCCGGAA-3′) (Bioneer, Inc.,
Seoul, Korea) were end-labeled with biotin. Nuclear protein
extracts (10 μg) were incubated with 20 fmol of
biotin-labeled NF-κB oligonucleotide and AP-1 oligonucleotide for
20 min prior to loading onto 4% (for NF-κB) and 6% (for AP-1)
non-denaturing polyacrylamide gels. Each gel was run at room
temperature in 0.3X TBE buffer at 100 V until the bromophenol blue
dye reached the bottom of the gel. When electrophoresis was
completed, the gel was blotted onto a Biodyne® B Nylon
Membrane (Pierce), and labeled oligonucleotides were detected using
the LightShift Chemiluminescent EMSA kit according to the
manufacturer’s instructions (Pierce). Images were acquired and the
signals were quantified using the LAS-3000 imaging system.
Statistical analysis
The results of the present study are expressed as
the means ± SD under all conditions and statistically analyzed
using a two-tailed Student’s t-test. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of HEABG on the viability of
HESCs
We first examined the cytotoxic effects of HEABG on
the HESCs by WST-1 assay. HEABG did not affect the viability of the
HESCs up to a concentration of 70 μg/ml (Fig. 1).
Effect of HEABG on the proliferation of
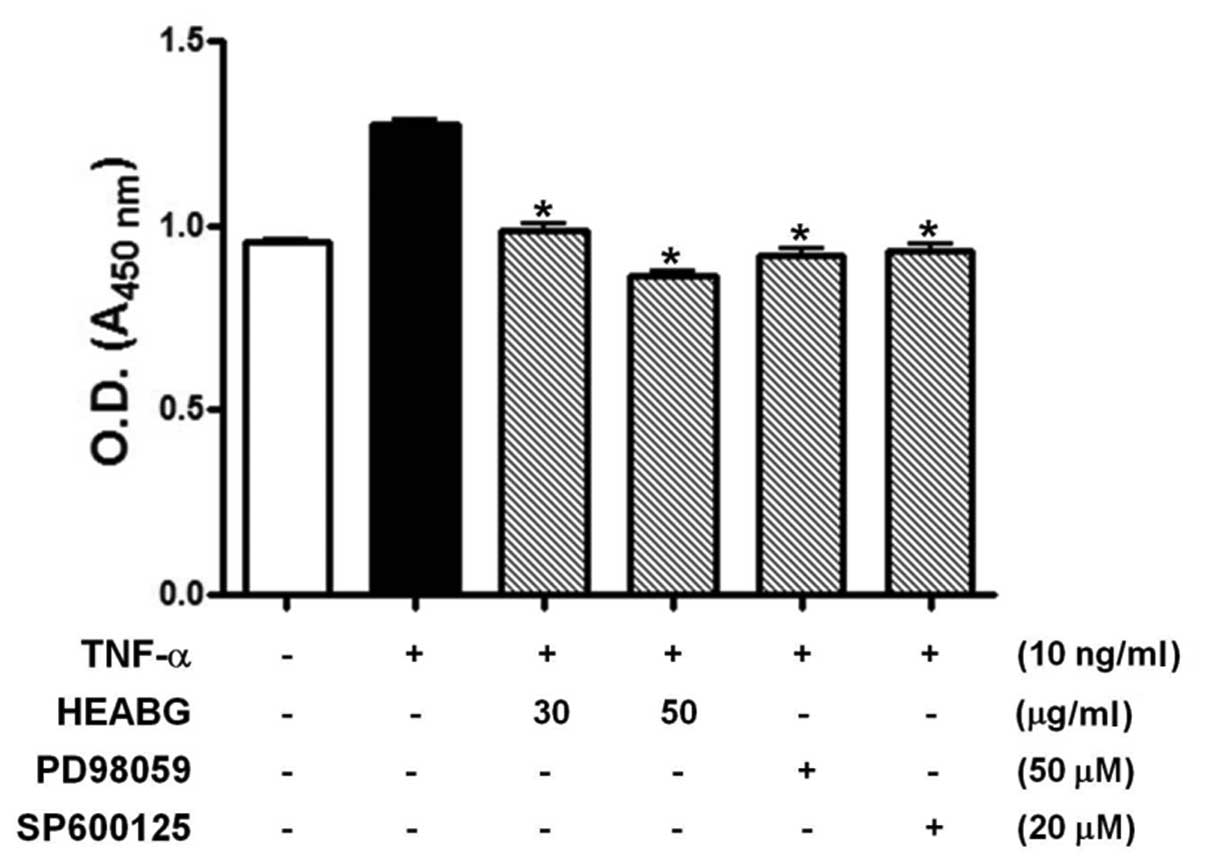
HESCs
Since enhanced endometrial cell proliferation is an
important pathological feature of human endometriosis, we evaluated
the effect of HEABG on the inhibition of TNF-α-stimulated HESC
proliferation by WST-1 assay. The stimulation of HESCs with TNF-α
(10 ng/ml) for 48 h significantly enhanced HESC proliferation
(Fig. 2). By contrast, HESCs
pre-treated with HEABG (30 and 50 μg/ml) exhibited a
significant reduction in cell proliferation induced by TNF-α,
indicating that HEABG used at non-toxic concentrations in HESCs
exhibits a significant inhibitory effect on the proliferation of
HESCs (Fig. 2). Furthermore, the
growth-promoting effect of TNF-α in HESCs was also completely
blocked by the treatment with either the ERK inhibitor, PD98059 (50
μM), or the JNK inhibitor, SP600125 (20 μM), for 1 h
prior to stimulation with TNF-α (10 ng/ml). This finding led us to
investigate the effect of HEABG on TNF-α-induced MAPK activation.
Treatment of the HESCs with TNF-α clearly induced the
phosphorylation of ERK, JNK and p38 MAPK. Notably, pre-treatment
with HEABG resulted in the inhibition of the TNF-α-induced ERK and
JNK phosphorylation, but not p38 MAPK phosphorylation (Fig. 3). Taken together, these results
indicate that the TNF-α-induced enhancement of the proliferation of
HESCs is mediated through the ERK/MAPK and JNK/MAPK signal
transduction pathways.
Effect of HEABG on the cell cycle in
HESCs
The effect of HEABG on the cell cycle of HESCs was
determined by flow cytometric analysis. Treatment of the HESCs with
TNF-α (10 ng/ml) for 15 h significantly reduced the proportion of
cells in the G0/G1 phase and increased the number of cells in the S
and G2/M phase (Fig. 4).
Pre-treatment with HEABG (30 and 50 μg/ml) markedly
suppressed these cell cycle alterations (Fig. 4). Furthermore, the stimulating
effects of TNF-α on cell cycle progression in HESCs were also
completely abolished by treatment with either the ERK inhibitor,
PD98059 (50 μM), or the JNK inhibitor, SP600125 (20
μM), for 1 h prior to stimulation with TNF-α (10 ng/ml),
indicating that the TNF-α-induced promotion of the cell cycle
progression of HESCs is mediated through the ERK/MAPK and JNK/MAPK
signal transduction pathways (Fig.
4).
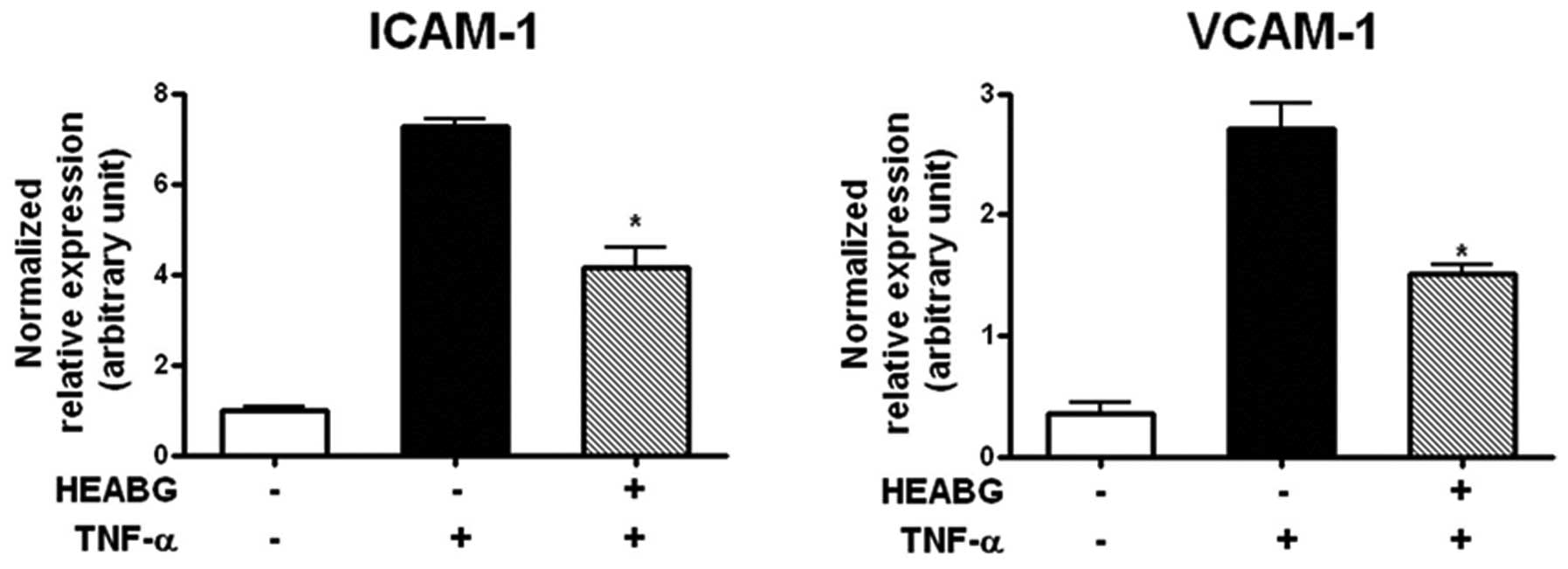
Suppression of ICAM-1 and VCAM-1
transcription by HEABG
To analyze the transcription of cell adhesion
molecules in HESCs, the mRNA expression of ICAM-1 and VCAM-1 was
evaluated by qRT-PCR analysis. Treatment of the HESCs with TNF-α
(10 ng/ml) for 15 h significantly increased the mRNA expression
levels of ICAM-1 and VCAM-1 (Fig.
5). Pre-treatment with HEABG (50 μg/ml) markedly
suppressed the TNF-α-induced ICAM-1 and VCAM-1 mRNA expression
(42.5 and 44.6% inhibition, respectively; P<0.005) (Fig. 5).
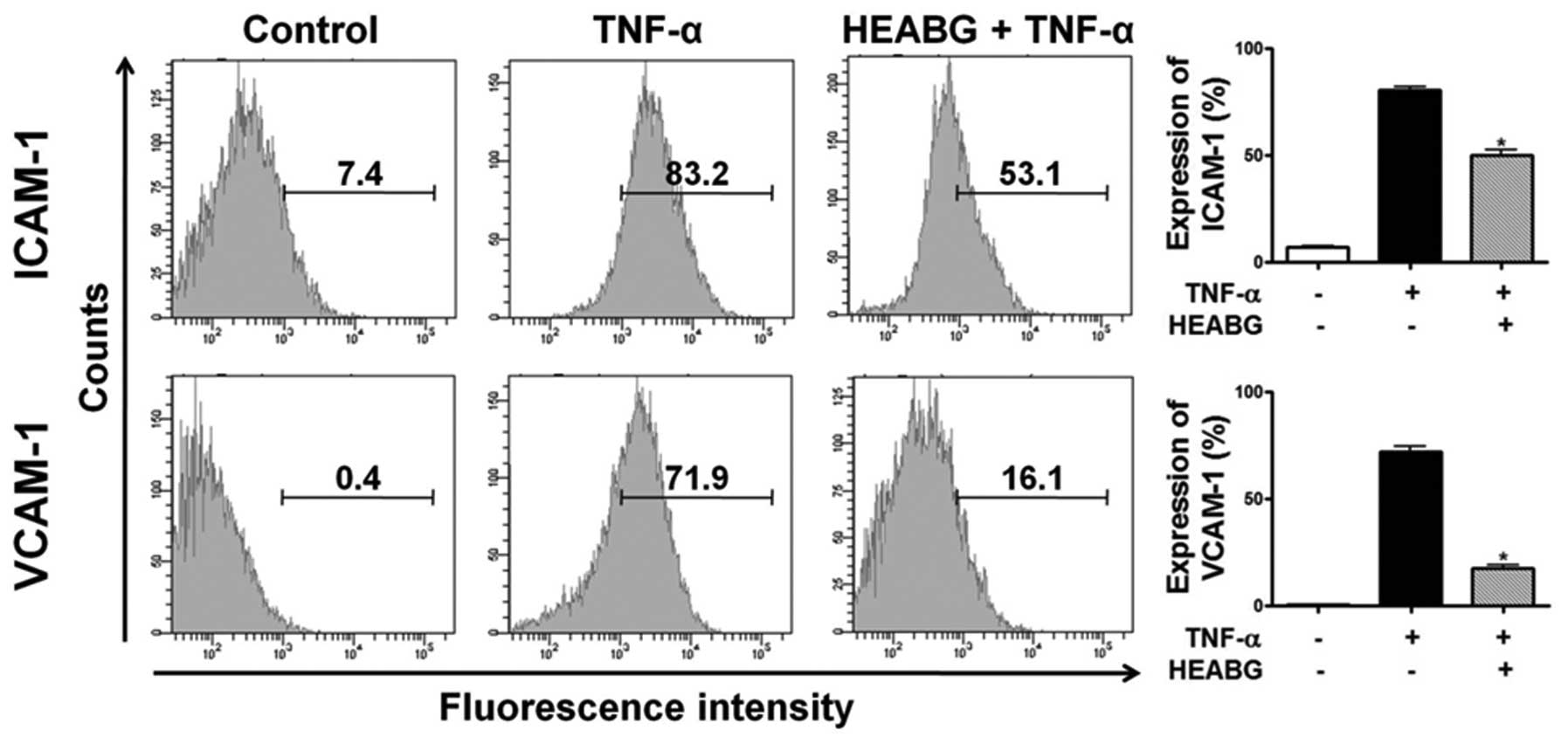
Inhibition of cell surface expression of
ICAM-1 and VCAM-1 by HEABG
To analyze cell adhesion molecule expression on
endometrial stromal cell surfaces, ICAM-1 and VCAM-1 expression was
evaluated by flow cytometry. Treatment of the HESCs with TNF-α (10
ng/ml) for 15 h markedly induced the cell surface expression of
ICAM-1 and VCAM-1 proteins (Fig.
6). Pre-treatment with HEABG (50 μg/ml) significantly
suppressed the TNF-α-induced ICAM-1 and VCAM-1 cell surface
expression (37.6 and 75.6% inhibition, respectively; P<0.001)
(Fig. 6).
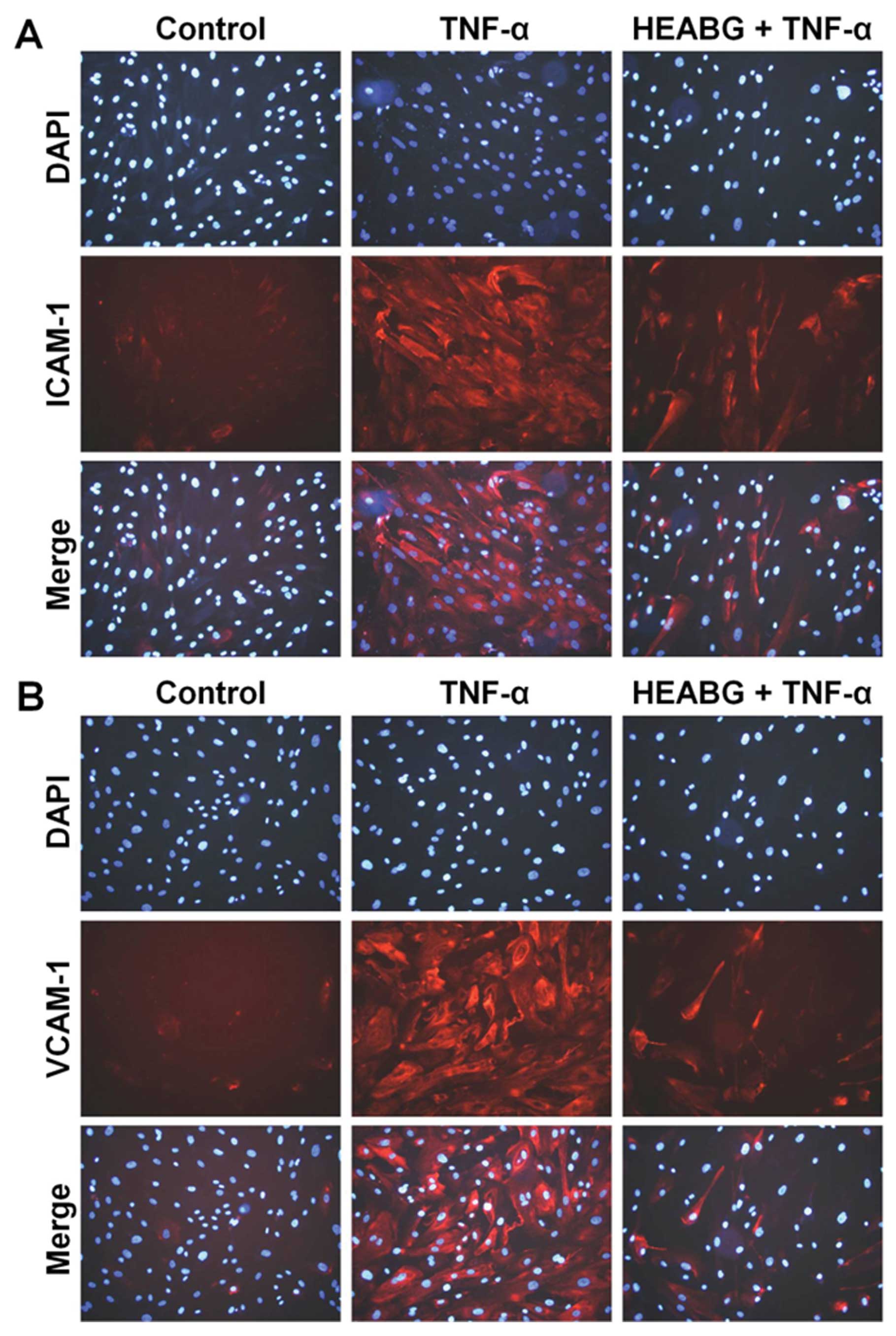
Suppression of total cellular ICAM-1 and
VCAM-1 protein expression by HEABG
The incubation of HESCs with TNF-α (10 ng/ml) for 15
h markedly increased the protein levels of total cellular ICAM-1
and VCAM-1, as revealed by immunofluorescence microscopic analysis
(Fig. 7). Pre-treatment with
HEABG (50 μg/ml) for 1 h significantly suppressed the total
cellular protein expression of ICAM-1 and VCAM-1 in HESCs induced
by TNF-α (Fig. 7).
Additionally, western blot analysis revealed a
single specific band correlating for ICAM-1 and VCAM-1 in the HESCs
treated with TNF-α (10 ng/ml) for 15 h (Fig. 8). Importantly, this effect was
effectively inhibited by pre-treatment with HEABG (50 μg/ml)
for 1 h (Fig. 8). The protein
expression of ICAM-1 and VCAM-1 in the TNF-α-activated HESCs
following treatment with HEABG, as measured by flow cytometric
analysis (Fig. 6),
immunofluorescence microscopic analysis (Fig. 7) and western blot analysis
(Fig. 8), was in accordance with
the transcription patterns of ICAM-1 and VCAM-1 shown by qRT-PCR
analysis (Fig. 5), clearly
indicating that both the cell surface and cytoplasmic protein, as
well as the mRNA expression of ICAM-1 and VCAM-1 in the HESCs
induced by TNF-α was definitely inhibited by HEABG.
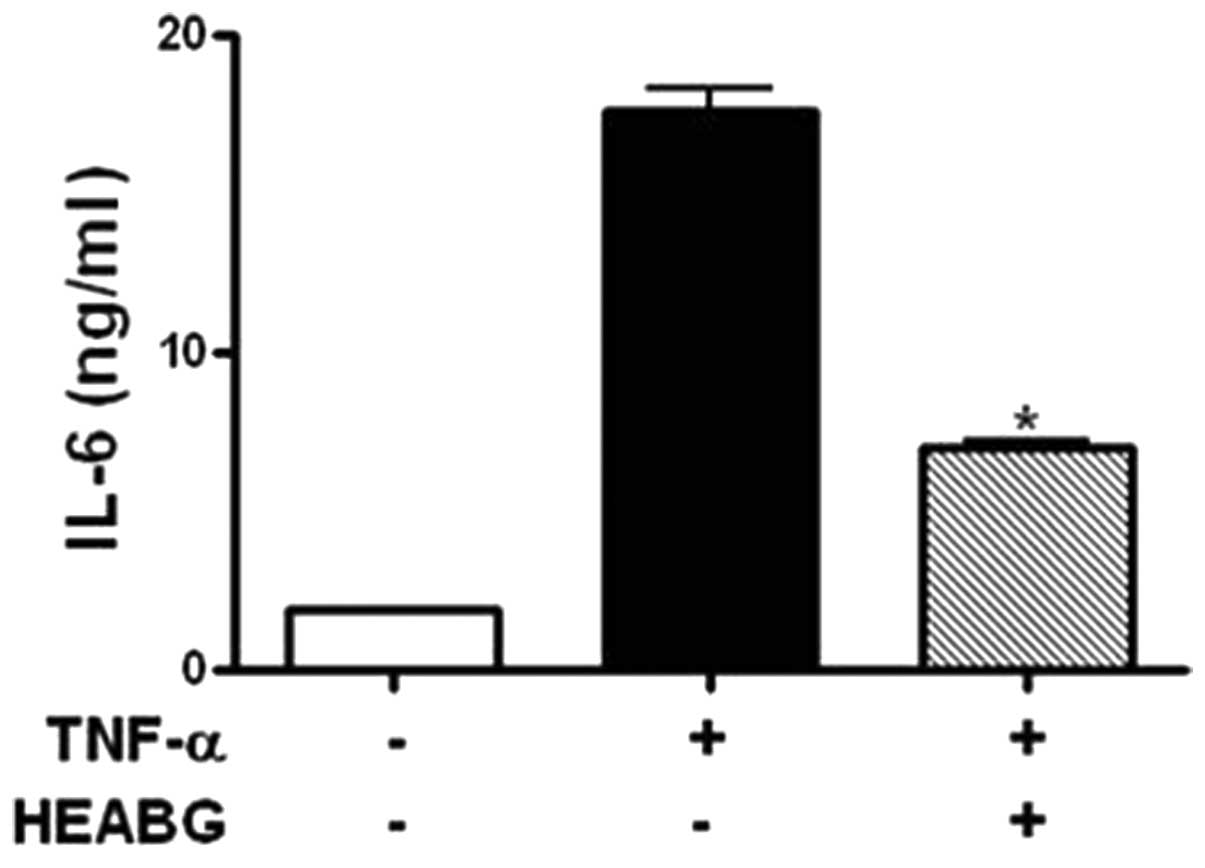
Inhibition of secretion of IL-6 by
HEABG
The levels of IL-6 secreted by HESCs were evaluated
by ELISA. Treatment of the HESCs with TNF-α (10 ng/ml) for 15 h
markedly induced the secretion of IL-6 (Fig. 9). HEABG (50 μg/ml) potently
suppressed the TNF-α-induced secretion of IL-6 from HESCs (60.0%
inhibition; P<0.01) (Fig.
9).
Inhibition of the TNF-α-induced
translocation of NF-κB and phosphorylation of IκBα by HEABG
To determine whether the activation of NF-κB by
TNF-α can be inhibited by HEABG, experiments were carried out to
measure the major components of the NF-κB complex. Western blot
analysis indicated that TNF-α induced the translocation of NF-κB
p65 from the cytosol to the nucleus (Fig. 10). Additionally, treatment of the
HESCs with TNF-α (10 ng/ml) for 1 h induced the phosphorylation and
degradation of the NF-κB inhibitor, IκBα, in the cytosol (Fig. 10). Pre-treatment with 50
μg/ml HEABG for 1 h blocked the TNF-α-induced nuclear
trans-location of NF-κB p65 from the cytosol to the nucleus and
significantly inhibited the TNF-α-induced phosphorylation and
degradation of IκBα (Fig. 10).
These results suggest that HEABG inhibits the translocation of
NF-κB from the cytosol to the nucleus in the TNF-α-activated HESCs
by the inhibition of IκBα degradation, which is due to the
inhibition of IκBα phosphorylation.
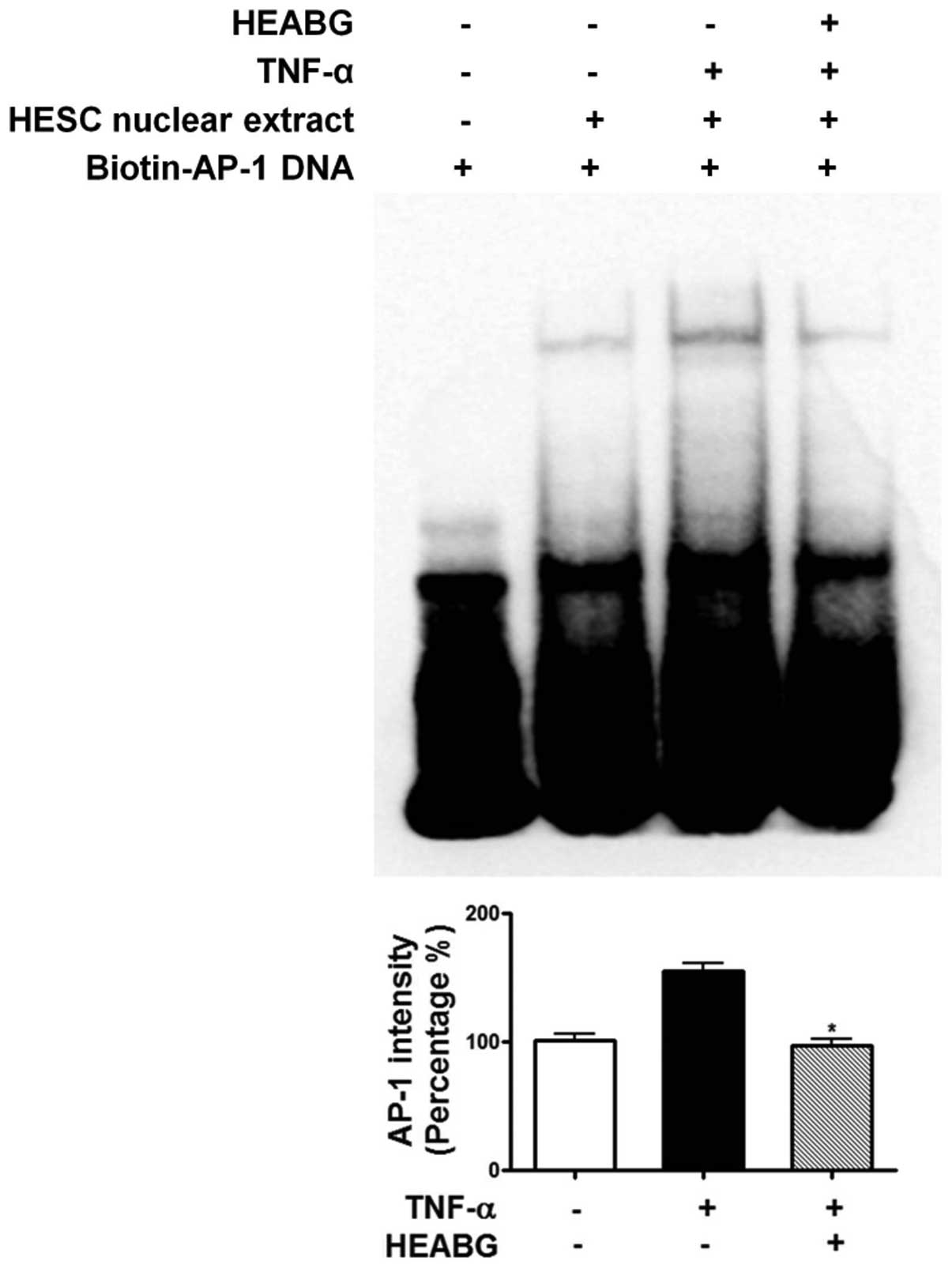
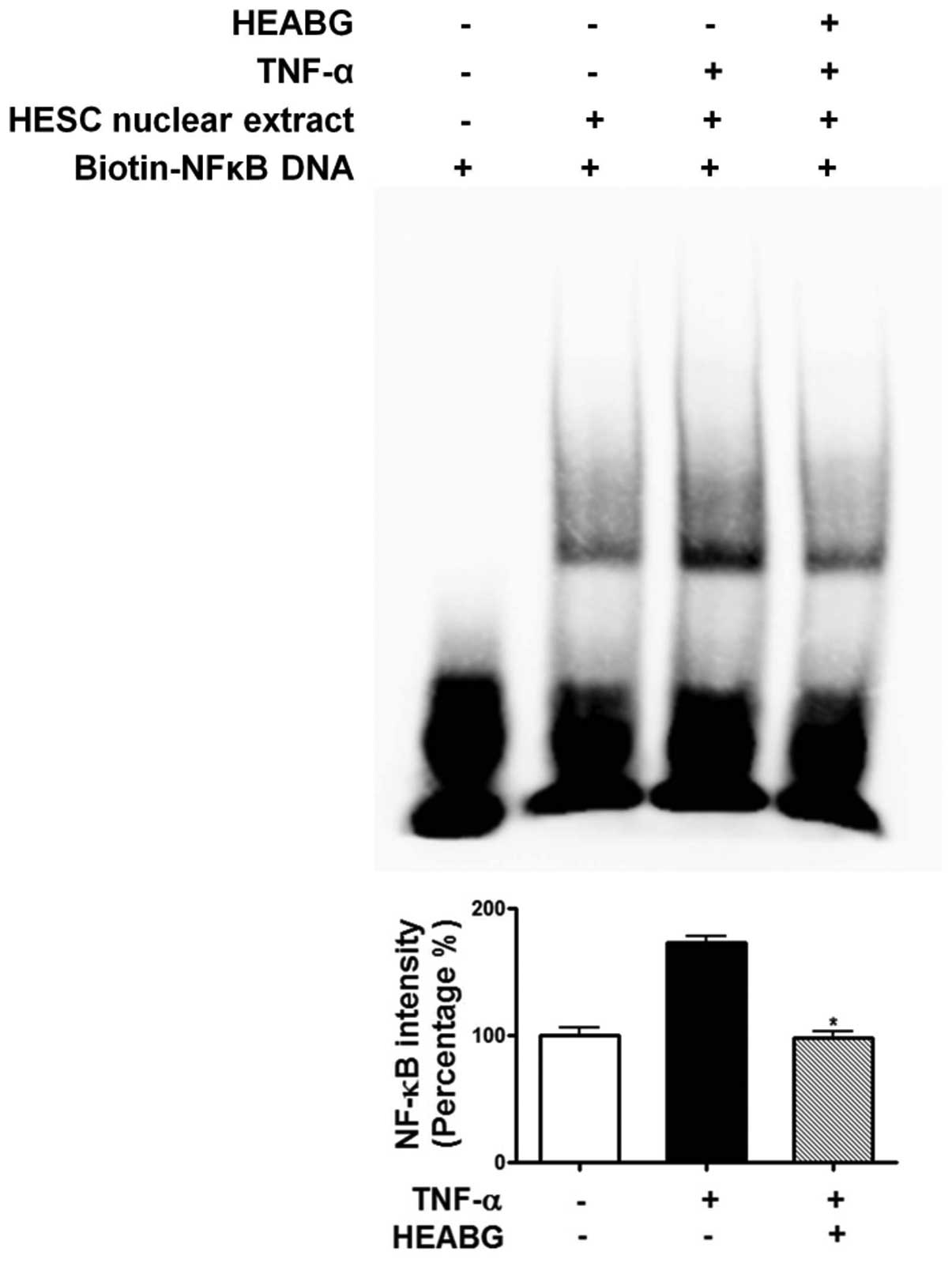
Attenuation of TNF-α-induced activation
of NF-κB and AP-1 by HEABG
To further investigate the mechanism responsible for
the inhibitory effect of HEABG on ICAM-1 and VCAM-1 expression, we
examined the effect of HEABG on NF-κB and AP-1 activation by EMSA.
Under control conditions, the nuclear expression of NF-κB (Fig. 11) and AP-1 (Fig. 12) in HESCs was barely detectable.
However, the stimulation of the HESCs with TNF-α (10 ng/ml) led to
the appearance of NF-κB (Fig.
11) and AP-1 (Fig. 12) as a
shifted band. Pre-treatment with HEABG (50 μg/ml)
significantly reduced the density of the NF-κB (Fig. 11) and AP-1 (Fig. 12) shifted band (46.7 and 38.5%
inhibition, P<0.01, respectively). These results indicate that
HEABG potently inhibits the activation of NF-κB and AP-1
transcription factors induced by TNF-α in HESCs.
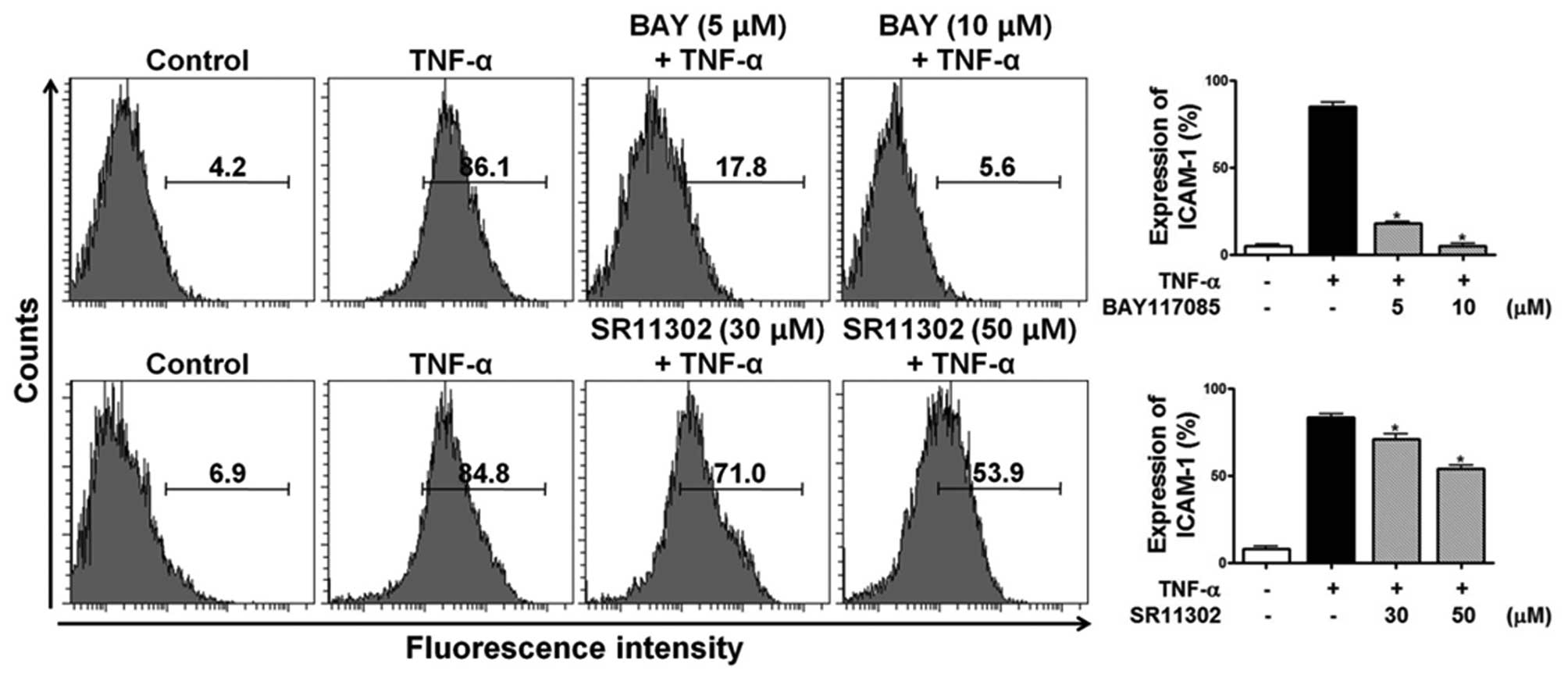
Effects of specific inhibitors of NF-κB
and AP-1 on TNF-α-induced ICAM-1 expression
To determine the role of NF-κB and AP-1 in
TNF-α-induced ICAM-1 expression in HESCs, the specific NF-κB
inhibitor, BAY 11-7085, and the specific AP-1 inhibitor, SR11302,
were used. HESCs were incubated with either BAY 11-7085 (5 and 10
μM) or SR11302 (30 and 50 μM) for 1 h prior to
stimulation with 10 ng/ml TNF-α for 15 h, and the cell surface
expression of ICAM-1 was analyzed by flow cytometric analysis. Of
note, ICAM-1 expression in the TNF-α-treated HESCs was completely
abolished by the NF-κB inhibitor and partially inhibited by the
AP-1 inhibitor in HESCs (Fig.
13). These data indicate that the effects of HEABG on the
TNF-α-induced ICAM-1 expression in HESCs are mediated through the
NF-κB signal transduction pathway and partially through the AP-1
pathway.
Discussion
The results of the present study clearly indicate
that the treatment of TNF-α-activated HESCs with HEABG suppresses
the expression of ICAM-1 and VCAM-1 at the mRNA and protein level,
as well as cell proliferation, cell cycle progression and the
secretion of IL-6. We demonstrated that TNF-α, a central
pro-inflammatory cytokine, markedly promotes the proliferation,
cell cycle progression and the expression of ICAM-1, VCAM-1 and
IL-6, induces the activation of the NF-κB and AP-1 transcription
factors, and activates ERK and JNK and ERK, subgroups of the MAPK
family in HESCs. These data suggest that TNF-α plays a crucial role
in the pathogenesis of endometriosis through the marked enhancement
of cell proliferation, as well as the expression of these critical
inflammatory mediators in HESCs, as shown in Fig. 14. It has been shown that the
abnormal production of TNF-α contributes to the pathophysiology of
endometriosis (18).
The immune system plays an important role in the
development of endometriosis (2,3).
Cell adhesion molecules, such as ICAM-1 and VCAM-1, play critical
roles in various inflammatory and immunological functions,
including cell-cell adhesion (4).
ICAM-1 and VCAM-1 are strongly expressed in the HESCs of patients
with endometriosis, suggesting that ICAM-1 and VCAM-1 play a role
in the pathophysiology of this disease (19,20). The pathophysiological importance
of ICAM-1 expression in HESCs has attracted much attention. In
particular, ICAM-1 expression may be associated with the defective
functions of natural killer (NK) cells in endometriosis (2,6,8).
In patients with endometriosis, the elevated expression of ICAM-1
in HESCs gives rise to the shedding of soluble ICAM-1 (sICAM-1)
from the cell surface to the peritoneal cavity, which subsequently
binds to ICAM-1 receptors on NK cells (6). This interferes with the cytotoxic
function of NK cells, and consequently, hinders them from killing
ectopic endometrial cells in the peritoneal cavity (2,6,21).
This mechanism appears to form the basis for HESCs to escape immune
surveillance, thus enabling the ultimate development of
endometriosis. This hypothesis is further supported by findings
that the endometrial release of sICAM-1 directly correlates with
the number and score of endometriotic implants (22), and that women with endometriosis
have elevated sICAM-1 levels in serum, demonstrating a correlation
between the peritoneal and endometrial sICAM-1 release levels and
the extent and severity of the disease (6,23).
These facts strongly underline the importance of the identification
of agents that inhibit the expression of ICAM-1 for the development
of effective inhibitors of the activity of HESCs. Thus, it is of
significance that HEABG was found to have an effective and potent
suppressive effect on the TNF-α-induced upregulation of ICAM-1 and
VCAM-1 expression in HESCs in the present study.
In agreement with the results of previous studies,
we also demonstrate that TNF-α stimulation promotes the activation
of NF-κB and AP-1, critical regulators of cell proliferation and
inflammatory responses in HESCs, suggesting that these
transcription factors are key factors in the pathogenesis of
endometriosis (3,14). The canonical pathway of NF-κB is
activated in endometriotic lesions, and modulation of NF-κB
activity and the consequent cell responses, including
proliferation, inflammation and adhesion are important in the
development of endometriosis (3,13,24,25). Thus, it is noteworthy that the
present study demonstrated that HEABG significantly inhibits the
TNF-α-induced activation of NF-κB and AP-1 in HESCs. In particular,
the involvement of the NF-κB and AP-1 pathways in the TNF-α-induced
ICAM-1 and VCAM-1 upregulation in HESCs was confirmed in this
study, using specific NF-κB and AP-1 inhibitors. Taken together,
these results suggest that HEABG exerts its inhibitory effect on
ICAM-1 and VCAM-1 expression via the modulation of the activation
and DNA binding activity of NF-κB and AP-1.
The critical roles of pro-inflammatory cytokines,
including IL-6 are well defined in the pathogenesis of
endometriosis (2,3). The activation of the NF-B and AP-1
transcription factors has been shown to play a crucial role in the
enhanced expression of several pro-inflammatory cytokines,
including IL-6 in HESCs (14).
These findings along with the results of the present study suggest
that HEABG blocks the TNF-α-induced IL-6 expression in HESCs
through the inhibition of the NF-κB and AP-1 transcription
factors.
Cell proliferation is a fundamental process in the
development of endometriotic lesions (2,3).
The MAPK cascade plays an important role in the signal transduction
pathways that contribute to the regulation of complex cellular
functions, including cell proliferation (26). There are at least 3 distinct and
parallel MAPK pathways characterized by ERK, JNK and p38 MAPK.
These MAPK signaling pathways are also present in endometrial
cells, and MAPKs play pathophysiological roles in the development
of endometriosis (14,27). It has been shown that MAPK
signaling pathways are abnormally activated in HESCs, and that MAPK
acts upon the regulation of HESC proliferation (28,29).
In agreement with previous studies, the present
study clearly demonstrates that TNF-α induces the expression of all
3 types of MAPKs in HESCs (14,27). Since we observed that HEABG
significantly inhibited the TNF-α-induced activation of ERK and JNK
in HESCs, we performed an experiment to define whether MAPKs are
involved in the regulation of cell proliferation in TNF-α-activated
HESCs using their specific inhibitors. Of note, it was found that
the inhibition of ERK and JNK hinders the proliferation and cell
cycle progression of HESCs, suggesting that the TNF-α-induced
activation of the ERK/MAPK and JNK/MAPK signaling pathways plays a
regulatory role in HESC proliferation. Moreover, HEABG markedly
suppressed ERK/MAPK and JNK/MAPK activation in HESCs. Thus, our
data indicate that HEABG effectively inhibits the TNF-α-induced
proliferation and cell cycle progression of HESCs by suppressing
ERK/MAPK and JNK/MAPK activation.
Taken together, our data clearly indicate that HEABG
may be effective in the prevention and treatment of endometriosis
in humans. Of particular importance, our results indicate that
HEABG, as a natural product, provides valuable insight into the
development of novel and beneficial therapeutic modalities for
combating endometriosis, since traditional hormone-based
therapeutic agents have serious adverse effects when used for the
long-term management of endometriosis.
Acknowledgements
This study was supported by the
Bio-Scientific Research Grant funded by the Pusan National
University (PNU, Bio-Scientific Research Grant)
(PNU-2008-101-208).
References
|
1.
|
Sampson JA: The development of the
implantation theory for the origin of peritoneal endometriosis. Am
J Obstet Gynecol. 40:549–557. 1940.
|
|
2.
|
Paul Dmowski W and Braun DP: Immunology of
endometriosis. Best Pract Res Clin Obstet Gynaecol. 18:245–263.
2004.
|
|
3.
|
Olovsson M: Immunological aspects of
endometriosis: an update. Am J Reprod Immunol. 66(Suppl 1):
101–104. 2011. View Article : Google Scholar
|
|
4.
|
Mousa SA: Cell adhesion molecules:
potential therapeutic and diagnostic implications. Mol Biotechnol.
38:33–40. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Wu TC: The role of vascular cell adhesion
molecule-1 in tumor immune evasion. Cancer Res. 67:6003–6006. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Somigliana E, Viganò P, Gaffuri B,
Guarneri D, Busacca M and Vignali M: Human endometrial stromal
cells as a source of soluble intercellular adhesion molecule
(ICAM)-1 molecules. Hum Reprod. 11:1190–1194. 1996.PubMed/NCBI
|
|
7.
|
Tabibzadeh SS and Poubouridis D:
Expression of leukocyte adhesion molecules in human endometrium. Am
J Clin Pathol. 93:183–189. 1990.PubMed/NCBI
|
|
8.
|
Viganó P, Pardi R, Magri B, Busacca M, Di
Blasio AM and Vignali M: Expression of intercellular adhesion
molecule-1 (ICAM-1) on cultured human endometrial stromal cells and
its role in the interaction with natural killers. Am J Reprod
Immunol. 32:139–145. 1994.PubMed/NCBI
|
|
9.
|
Viganò P, Gaffuri B, Somigliana E, Busacca
M, Di Blasio AM and Vignali M: Expression of intercellular adhesion
molecule (ICAM)-1 mRNA and protein is enhanced in endometriosis
versus endometrial stromal cells in culture. Mol Hum Reprod.
4:1150–1156. 1998.PubMed/NCBI
|
|
10.
|
Wu MH, Yang BC, Lee YC, Wu PL and Hsu CC:
The differential expression of intercellular adhesion molecule-1
and regulation by interferon-gamma during the pathogenesis of
endometriosis. Am J Reprod Immunol. 51:373–380. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Defrère S, Donnez J, Moulin P, Befahy P,
Gonzalez-Ramos R, Lousse JC and Van Langendonckt A: Expression of
intercellular adhesion molecule-1 and vascular cell adhesion
molecule-1 in human endometrial stromal and epithelial cells is
regulated by interferon-gamma but not iron. Gynecol Obstet Invest.
65:145–154. 2008.PubMed/NCBI
|
|
12.
|
Braun DP, Ding J and Dmowski WP:
Peritoneal fluid-mediated enhancement of eutopic and ectopic
endometrial cell proliferation is dependent on tumor necrosis
factor-alpha in women with endometriosis. Fertil Steril.
78:727–732. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
González-Ramos R, Van Langendonckt A,
Defrère S, Lousse JC, Colette S, Devoto L and Donnez J: Involvement
of the nuclear factor-κB pathway in the pathogenesis of
endometriosis. Fertil Steril. 94:1985–1994. 2010.
|
|
14.
|
Yamauchi N, Harada T, Taniguchi F, Yoshida
S, Iwabe T and Terakawa N: Tumor necrosis factor-alpha induced the
release of interleukin-6 from endometriotic stromal cells by the
nuclear factor-kappaB and mitogen-activated protein kinase
pathways. Fertil Steril. 82(Suppl 3): 1023–1028. 2004. View Article : Google Scholar
|
|
15.
|
Okamoto H, Cujec TP, Yamanaka H and
Kamatani N: Molecular aspects of rheumatoid arthritis: role of
transcription factors. FEBS J. 275:4463–4470. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Pittler MH and Ernst E: Clinical
effectiveness of garlic (Allium sativum). Mol Nutr Food Res.
51:1382–1385. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Revised American Society for Reproductive
Medicine classification of endometriosis: 1996. Fertil Steril.
67:817–821. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Pino M, Galleguillos C, Torres M, Sovino
H, Fuentes A, Boric MA and Johnson MC: Association between MMP1 and
MMP9 activities and ICAM-1 cleavage induced by tumor necrosis
factor in stromal cell cultures from eutopic endometria of women
with endometriosis. Reproduction. 138:837–847. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Prefumo F, Semino C, Melioli G and
Venturini PL: A defective expression of ICAM-1 (CD54) on secretory
endometrial cells is associated with endometriosis. Immunol Lett.
80:49–53. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Kyama CM, Overbergh L, Mihalyi A, Meuleman
C, Mwenda JM, Mathieu C and D’Hooghe TM: Endometrial and peritoneal
expression of aromatase, cytokines, and adhesion factors in women
with endometriosis. Fertil Steril. 89:301–310. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Sikora J, Mielczarek-Palacz A and
Kondera-Anasz Z: Role of natural killer cell activity in the
pathogenesis of endometriosis. Curr Med Chem. 18:200–208. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Viganò P, Somigliana E, Gaffuri B,
Santorsola R, Busacca M and Vignali M: Endometrial release of
soluble intercellular adhesion molecule 1 and endometriosis:
relationship to the extent of the disease. Obstet Gynecol.
95:115–118. 2000.PubMed/NCBI
|
|
23.
|
De Placido G, Alviggi C, Di Palma G,
Carravetta C, Matarese G, Landino G and Racioppi L: Serum
concentrations of soluble human leukocyte class I antigens and of
the soluble intercellular adhesion molecule-1 in endometriosis:
relationship with stage and non-pigmented peritoneal lesions. Hum
Reprod. 13:3206–3210. 1998.
|
|
24.
|
González-Ramos R, Van Langendonckt A,
Defrère S, Lousse JC, Mettlen M, Guillet A and Donnez J: Agents
blocking the nuclear factor-kappaB pathway are effective inhibitors
of endometriosis in an in vivo experimental model. Gynecol Obstet
Invest. 65:174–186. 2008.PubMed/NCBI
|
|
25.
|
Nasu K, Nishida M, Ueda T, Yuge A, Takai N
and Narahara H: Application of the nuclear factor-kappaB inhibitor
BAY 11-7085 for the treatment of endometriosis: an in vitro study.
Am J Physiol Endocrinol Metab. 293:E16–E23. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Zhang W and Liu HT: MAPK signal pathways
in the regulation of cell proliferation in mammalian cells. Cell
Res. 12:9–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Wu MH, Wang CA, Lin CC, Chen LC, Chang WC
and Tsai SJ: Distinct regulation of cyclooxygenase-2 by
interleukin-1beta in normal and endometriotic stromal cells. J Clin
Endocrinol Metab. 90:286–295. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Hirota Y, Osuga Y, Hirata T, Harada M,
Morimoto C, Yoshino O, Koga K, Yano T, Tsutsumi O and Taketani Y:
Activation of protease-activated receptor 2 stimulates
proliferation and inter-leukin (IL)-6 and IL-8 secretion of
endometriotic stromal cells. Hum Reprod. 20:3547–3553. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Yotova IY, Quan P, Leditznig N, Beer U,
Wenzl R and Tschugguel W: Abnormal activation of Ras/Raf/MAPK and
RhoA/ROCKII signalling pathways in eutopic endometrial stromal
cells of patients with endometriosis. Hum Reprod. 26:885–897. 2011.
View Article : Google Scholar : PubMed/NCBI
|