Introduction
Traumatic brain injury (TBI) is a major cause of
mortality and morbidity globally (1,2),
and continues to be an important public health issue worldwide
(3,4). Appropriate treatment is vital for
patient survival and the restoration of nervous function following
TBI. The concept of enhancing endogenous neurogenesis and replacing
lost neurons following TBI is now becoming a reality (4). It is widely accepted that new
neurons are generated in the adult mammalian brain throughout life.
In the adult central nervous system (CNS), active neurogenesis
occurs in 2 discrete ‘neurogenic’ regions: the subgranular zone
(SGZ) of the dentate gyrus (DG) in the hippocampus and the
subventricular zone (SVZ) of the lateral ventricles in the
forebrain (5,6). Neurogenesis in these regions
maintains relatively quiescent states in a stable microenvironment.
However, studies have indicated that neurogenesis can be induced by
TBI (7–9) and increased levels of cell
proliferation have been observed in the DG and SVZ soon after TBI
(7,10,11). Traumatic insult to the brain
results in neural tissue injury through cell-death processes,
including apoptosis or programmed cell death. Therefore, the
processes of promoting the proliferation of neural stem cells and
the differentiation of newly generated neurons may play a role in
the enhancement of endogenous neurogenesis to replace injured
neural cells following TBI.
The Wnt/β-catenin signaling pathway is important for
neurogenesis in the developing nervous system (12,13), as well as in adults, which
consists of an active process encompassing proliferation,
migration, differentiation and synaptogenesis (14). Thus, increased β-catenin signaling
following TBI (15) may
contribute to repair deterioration and may promote the recovery of
neural function. In this study, we wished to explore the mechanisms
behind adult neurogenesis induced by Wnt/β-catenin signaling
following TBI. We aimed to determine whether survivin, the
downstream target gene of the Wnt/β-catenin signaling pathway
(16,17), plays a role in the enhancement of
neurogenesis following TBI.
Survivin, a member of the inhibitor of apoptosis
(IAP) gene family (18), is
expressed in the G2/M phase of the cell cycle in a cycle-regulated
manner. Survivin is also a regulator of mitosis, and is localized
to mitotic spindle microtubules (19) in a specific and saturable reaction
that is regulated by microtubule dynamics (20), to promote mitosis and cell
proliferation. Evidence indicates that disrupting the
survivin-microtubule interaction results in the loss of the
anti-apoptotic function of survivin (19). Survivin plays a role in
maintaining cell viability at mitosis, potentially also controlling
apoptosis, thus regulating cell division. Unlike other IAP
proteins, survivin plays a dual role as an apoptotic inhibitor and
as a chromosomal passenger protein (CPP) (21). In addition, survivin binds to and
inhibits the activity of caspase-3, caspase-7 and caspase-9
(22–25), which are involved in cell death
during mitosis. Most importantly, survivin is crucial for normal
embryonic neurogenesis (26,27). It is noteworthy that survivin is
ubiquitously expressed during embryonic development, and diffusely
expressed in the developing nervous system (28), but only minimally expressed in
adult tissues; in contrast, it is widely expressed in human
malignant tumors. Furthermore, studies have indicated that survivin
also plays a role in certain physiological processes, as well as in
pathological conditions, such as TBI (29). The expression of survivin may
attenuate DNA cleavage, in a cell-specific manner, following TBI
(30). These findings prompted us
to investigate the correlation between the expression of survivin
and adult neurogenesis following TBI. In this study, we
investigated the expression of survivin at certain time points
following TBI and detected variations in the expression of survivin
in several types of existing nerve cell in the DG of the
hippocampus using a mouse model of TBI induced by lateral fluid
percussion.
Materials and methods
A total of 146 adult male C57BL/6 mice (10 weeks of
age; 25–30 g) obtained from the Academy of Military Medical
Sciences, China, were housed in cages under a 12-h light-dark cycle
with a regular food and water supply. All experimental procedures
were approved by the China Small Animal Protection Association
(CSAPA). For the study, the mice were randomly divided into a TBI
group and a sham-operated group.
Lateral fluid percussion model of
TBI
Each mouse was anesthetized by an intraperitoneal
injection of 10% chloral hydrate (4 μl/g), placed in a stereotactic
apparatus and the dorsal scalp was exposed through a midline cut
under sterile conditions. A 3 mm craniotomy was made on the right
parietal bone, between the lambda and bregma suture and 1 mm
lateral to the sagittal suture. A Luer lock fitting was cemented to
the skull as previously described (31). The TBI group was subjected to
lateral fluid percussion injury of 202±2 kPa using a pre-calibrated
fluid percussion injury device. The sham-operated group was
subjected to identical surgery without lateral fluid percussion.
Following injury, the Luer lock fitting was removed, the wound was
sutured and the mice were returned to their cages.
Quantitative polymerase chain
reaction
Animals with TBI were sacrificed at designated times
(12 h, 1, 2, 5, 7 and 14 days post TBI) (each time point, n=6). The
sham-operated animals were sacrificed on day 3 post surgery (n=6).
Hippocampal tissues from the ipsilateral hemispheres were rapidly
excised and ‘snap-frozen’ with liquid nitrogen. The primers used
for PCR were as follows: survivin forward, 5′-TACCGCATCGCCA
CCTTC-3′ and reverse, 5′-CCAAATCAGGCTC GTTCTCG-3′; and GAPDH
forward, 5′-AGGTCGGTGTGAACGGA TTTG-3′ and reverse,
5′-TGTAGACCATGTAGTTGA GGTCA-3′. Total RNA was isolated from the
samples using TRIzol reagent (Cat# GMRS-001, GenePharma, Shanghai,
China). cDNA synthesis was performed using 1 mg of total RNA with
the mRNA Selective PCR Kit (AMV) version 1.1 (Takara Biotechnology,
Dalian, China) according to the manufacturer’s instructions.
Quantitative PCR was performed using the SYBR® Premix Ex
Taq™ (TaKaRa) in combination with 0.5 μl primers in the MX3000P™
Real-Time PCR Instrument (Stratagene, La Jolla, CA, USA). All PCR
reactions were performed using standard PCR conditions: stage 1:
95°C for 3 min (1 cycle); stage 2: 95°C for 12 sec, followed by
62°C for 40 sec; stage 3: from 62–95°C, followed by 0.2°C for 2 sec
(1 cycle). The controls were consistently found to be negative for
survivin. PCR products were resolved on a 1.5% agarose gel in
Tris-acetate buffer and visualized by ethidium bromide staining
under UV illumination.
Western blot analysis
To further detect the protein expression of survivin
in the hippocampus, the animals in the 2 groups were sacrificed at
12 h, 1, 2, 5, 7 and 14 days following TBI or sham operation (each
time point, n=6) and ipsilateral hippocampal tissues were
immediately ‘snap-frozen’ in liquid nitrogen. Each hippocampus was
homogenized in ice-cold homogenizing buffer with a sonicator.
Homogenized tissues were centrifuged at 12,000 rpm for 10 min at
4°C. Following electrophoretic separation on a 10% SDS-PAGE gel,
the resolved proteins were electrophoretically transferred onto a
PVDF membrane (Millipore, Billerica, MA, USA). The membranes were
incubated in blocking buffer for 2 h at room temperature and probed
with an anti-survivin antibody (1:1,000 dilution; Abcam, Cambridge,
MA, USA) and an antibody against β-actin (1:1,000 dilution; Cell
Signaling Technology, Inc., Danvers, MA, USA) overnight at 4°C. The
goat anti-rabbit secondary antibody conjugated to horseradish
peroxidase (HRP) were then applied for 2 h at room temperature.
Bound antibodies were detected by an enhanced chemiluminescence
assay (SuperSignal™ West Pico Chemiluminescent Substrates, Pierce
Biotechnology, Inc., Rockford, IL, USA).
5′-Bromo-2′-deoxyuridine (BrdU)
administration
The animals received intraperitoneal injections of
BrdU (Sigma-Aldrich, St. Louis, MO, USA; 100 mg/kg; dissolved in
saline to a final concentration of 20 mg/ml). Injections were
administered twice daily on days 1 and 2, followed by a single
injection on day 3 following brain injury. The animals were
sacrificed 2 h after the final BrdU injection. The BrdU-labeled
newborn neurons were counted within 3 days after administration
(from day 1 to 3 post TBI).
Tissue preparation and immunofluorescence
staining
For histological evaluation, the animals were
perfused with cold phosphate-buffered saline (PBS) followed by 4%
paraformaldehyde solution under deep anesthesia. The brains were
removed, kept in 4% paraformaldehyde for 24 h after fixation and
immersed in 30% sucrose for 24–48 h at 4°C. The frozen brains were
cut coronally in 35-μm-thick slices on a cryostat throughout the
rostrocaudal extent of the hippocampus and the slices were
collected in 6-well plates filled with PBS. In order to assess the
number of positive cells, 1-in-5 series of sections from each
animal were processed for immunostaining as previously described by
Wojtowicz and Kee (32). Briefly,
to eliminate non-specific background, the sections were incubated
in PBS blocking solution containing 0.3% Triton X-100, 2% goat
serum in 0.1 M PBS for 60 min at 37°C prior to staining. The
sections were then incubated with BrdU/doublecortin (DCX)/glial
fibrillary protein (GFAP)/NeuN and survivin primary antibody
diluted in blocking solution at 4°C overnight on a shaker. The
primary antibodies used in this study were rat anti-BrdU (1:50
dilutions, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA),
mouse anti-DCX (1:100 dilution; Santa Cruz Biotechnology), mouse
anti-GFAP (1:200 dilution; Millipore), mouse anti-NeuN (1:100
dilution; Millipore) and rabbit anti-survivin (1:400 dilution; Cell
Signaling Technology). Finally, the sections were incubated with
fluorochrome-conjugated secondary antibody in the dark, diluted in
0.1 M PBS (at pH 7.4) with 0.3% Triton X solution for 1 h at 37°C.
The fluorescent secondary antibodies were Alexa Fluor 555 goat
anti-rabbit IgG (1:1000 dilutions, Cell Signaling Technology),
Alexa Fluor 488 goat anti-mouse IgG (1:1000 dilutions, Cell
Signaling Technology) and Alexa Fluor 488 goat anti-rat IgG
(1:1,000 dilutions, Cell Signaling Technology). The slices for the
negative control of survivin staining were subjected to identical
staining without primary antibody. Fluorescence images were
acquired using an inverted Olympus fluorescence microscope and an
image capture system.
Quantification for immunofluorescence
results
All analyses were accomplished with stereological
counting methods. A systematic random sampling of 1-in-5 series of
coronal sections was prepared from each animal and processed for
immunofluorescence staining. The hippocampus (bregma −1.3 to −3.1
mm) was selected to prepare the sections. The entire SGZ of the DG
was assessed and each survivin-labeled cell was examined to assess
the co-labeling of survivin with cell-type-specific markers. Every
positive cell in the SGZ of the DG was counted using a ×40
objective lens. The percentage of double-labeled cells was
calculated as the number of cells that were stained with both
survivin and a given cell-type-specific marker against the total
number of survivin-positive cells. Four animals in each group with
10 sections per brain were examined. The total number counted in
the sections per brain was multiplied by 5 to estimate the total
number of positive cells in the SGZ of the hippocampus.
Statistical analysis
All the data are presented as the means ± SEM. The
following statistical analyses were performed using SPSS (version
17.0; SPSS, Inc., Chicago, IL, USA): one-way analysis of variance
(ANOVA) with a post hoc Bonferroni test was used to compare the
level of survivin mRNA and protein collected at different time
points following TBI. A Student’s t test was used to analyze the
number of survivin (+), BrdU (+), DCX (+), BrdU/survivin (+) and
DCX/survivin (+) cells between the sham-operated group and the TBI
group. A p-value <0.05 was considered to indicate a
statistically significant difference.
Results
Increased expression of survivin in
hippocampus following TBI
To investigate the role of survivin in adult
neurogenesis following TBI, we initially examined changes in
survivin expression in the ipsilateral hippocampus at the mRNA and
protein level. Quantitative PCR analysis revealed that survivin
expression was present 12 h following TBI and was sustained until
day 5, with a peak on day 1 and then declined to the level of the
sham-operated group on day 14. The results from quantitative PCR on
day 1 in the TBI group revealed a 2-fold increase in expression as
compared with the sham-operated group (Fig. 1). These results are in accordance
with those from a previous study on rat brains (33). Furthermore, in our study,
quantitative western blot analysis was performed to observe the
protein expression of survivin following TBI compared with the
sham-operated group at each time point; the results revealed that
the upregulation of survivin began on day 1 and lasted until day 7,
peaking on day 2 (Fig. 2). To
further identify the expression of survivin, we performed
immunofluorescence staining on day 3 post injury, when survivin
protein was steadily expressed following TBI. Compared with the
sham-operated group, the number of survivin (+) cells in the SGZ of
the DG was significantly increased on day 3 following TBI
(185±11.69 vs. 1207±63.26, n=4, p<0.001; Fig. 3A, D and G). These results strongly
suggested that survivin expression was stimulated by brain injury
and expressed in a time-dependant manner, with a highest increase
2–5 days following TBI in the hippocampus.
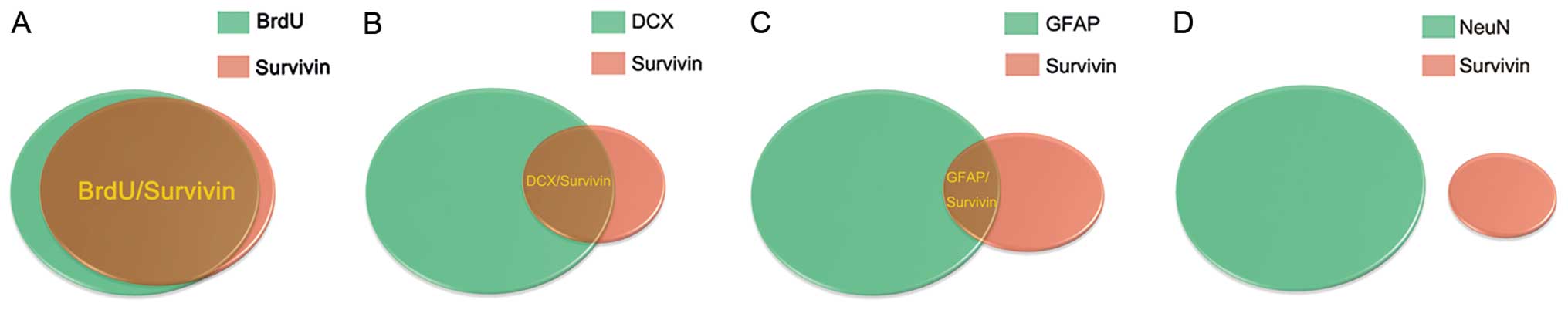
Survivin is expressed in neural stem
cells, immature neurons and in a minority of astrocytes, but not in
mature neurons in the adult DG of the hippocampus following
TBI
We utilized a double staining procedure using
BrdU/DCX/GFAP/NeuN and survivin to determine which cell types
express high levels of survivin following TBI. We initially found
that survivin was expressed by cells in the SGZ of the DG. This
suggested that the cells of survivin immunoreactivity in the SGZ
may be stem cells and immature neurons. Accordingly, we first
observed the BrdU/survivin co-labeled cells in the SGZ of the DG
(93.82±3%, n=4, Figs. 3C and
7A), suggesting that survivin
protein is expressed in the majority of BrdU (+) cells following
TBI. We also first found DCX/survivin co-labeled cells in the SGZ
of the DG (61.28±4%, n=4, Figs.
4C and 7B), indicating that
survivin protein is expressed in DCX (+)cells following TBI. The
results from immunofluorescence double staining clearly
demonstrated that increased levels of survivin expression following
TBI were mainly found in stem cells and immature neurons in the SGZ
of the DG. In addition, as shown in Figs. 5 and 6, survivin protein expression was
observed in a minority of GFAP (+) cells, but not in NeuN (+)
cells, indicating that increased survivin expression is partly
observed in astrocytes and not in mature neurons soon after TBI.
The model of cell count statistics is presented in Fig. 7.
We found that survivin protein was expressed in the
majority of BrdU (+) and DCX (+) cells and in the minority of GFAP
(+) cells, but not in NeuN (+) cells post injury. This strongly
suggests that increased survivin expression is mainly observed in
neural stem cells and immature neurons.
Increased expression of survivin
correlates with the proliferation of neural stem cells in the DG of
the hippocampus soon after TBI
Quantitative western blot analysis revealed that
survivin was steadily expressed on day 3 following TBI. To confirm
the correlation between survivin expression and the proliferation
of neural stem cell after injury, we quantified the number of
survivin (+) and BrdU (+) cells on day 3 following TBI by
immunofluorescence staining. When comparing the extent of cell
proliferation between the sham-operated group and the TBI group,
the number of BrdU (+) cells in the DG was significantly higher
than that in the TBI group (196±13.5 vs. 1306±28.36, n=4,
p<0.001; Fig. 3B, E and G).
Compared with the sham-operated group, the number of survivin (+)
cells increased in the DG of the hippocampus in the TBI group
(185±11.69 vs. 1207±63.26, n=4, p<0.001; Figs. 3A, D and G). Quantitative analysis
revealed a high percentage of BrdU/survivin co-labeled cells in the
TBI group (93.82±3%, n=4; Figs.
3C and 7A) and the
sham-operated group (96.42±1%, n=4; Fig. 3F). These results strongly suggest
that the increased expression of survivin promotes the
proliferation of neural stem cells in the DG of the hippocampus
soon after TBI.
Significant increase in the expression of
survivin in DCX (+) immature neurons in the DG of the hippocampus
soon after TBI
As described above, survivin promotes hippocampal
neurogenesis following TBI. To determine whether survivin is
involved in mediating the process of differerentiation in
hippocampal neurogenesis following injury, we first measured the
expression of survivin in DCX (+) cells in the SGZ of the DG. The
immunofluorescence double staining revealed that the number of
survivin (+) cells and DCX-positive cells was increased in the TBI
group compared with the sham-operated group (55±8.21 vs. 646±30.03,
n=4, p<0.001; Fig. 4C, F and
G). A significant percentage of survivin (+) cells also
expressed DCX (61.28±4%, n=4; Figs.
4C and 7B) indicating that
the expression of survivin was increased in immature neurons in the
DG of the hippocampus. This suggests that survivin plays a role in
the survival of immature neurons in the DG of the hippocampus soon
after TBI and promotes the differentiation of these immature
neurons into granular neurons.
Discussion
It is well known that adult neurogenesis exists in
the CNS. New neurons are continuously generated from neural
stem/progenitor cells in the SGZ of the DG throughout adulthood
(34). Previous studies have
demonstrated the correlation between the Wnt/β-catenin signaling
pathway and adult neurogenesis. In the adult brain, stabilized
β-catenin modulates the proliferation of neural stem cells, induces
newly generated neuronal cell migration into relevant regions and
then mediates their differentiation into mature neurons and
ultimately plays a role in synaptogenesis (14). The disruption of signaling is
associated with several pathological diseases, such as Alzheimer’s
disease (AD) (35–37), schizophrenia (38) and autism (39).
Survivin, as the downstream target gene of the
Wnt/β-catenin signaling pathway (16,17), plays a critical role in protecting
neuronal cells from apoptosis and promoting the proliferation of
neurogenic progenitor cells within the developing CNS. When
survivin expression is disrupted in the developing brain, embryos
survive until birth, but the majority of the brain is hypoplastic.
On a cellular level, this phenotype occurs as a result of massive
neuronal apoptosis. Survivin has been linked to the inhibition of
cell apoptosis in developing tissues, but the exact molecular
mechanisms involved remain unclear. Studies have shown that the
activity of caspase-3 and caspase-9 in the survivin-null brains is
increased, supporting the role of survivin in inhibiting caspase-3
and caspase-9 activity, thus preventing apoptosis, through either
indirect or direct mechanisms (26). Survivin plays a role in mitosis as
a CPP (40,41). In this capacity, it recruits
aurora B kinase to the CPP complex and ensures the proper alignment
of chromosomes before cell division (42). The majority of
survivin−/− embryos have grossly enlarged nuclei with
abnormal morphology. As this phenotype progresses, the cells cease
to complete mitosis, with the decreasing number of normal cells
replaced by a small number of giant cells with large and
morphologically unusual nuclei. Overall, survivin is an essential
factor during the development of the mammalian nervous system.
Survivin is prominently expressed in the neurogenic
regions of the embryonic CNS and its expression is maintained by a
subpopulation of neural progenitor cells post-natally in the 2 key
sites of adult neurogenesis, the SVZ and the SGZ (43). In our study, we investigated the
correlation between survivin expression and adult neurogenesis
following TBI in the DG of the hippocampus. Our results revealed
that the expression of survivin was increased in the mouse
hippocampus in a time-dependent manner and this change in
expression was associated with the proliferation of stem cells
early following TBI. Johnson et al detected changes in
survivin expression after injury in the rat brain (30), suggesting the correlation between
survivin and neurogenesis induced by TBI. To further demonstrate
the role of survivin expression in neurogenesis following TBI, we
utilized a double staining procedure of BrdU/DCX/GFAP/NeuN and
survivin to observe cell types with an increased expressio of
survivin following TBI. According to previous studies reporting the
PCNA/survivin co-labeled cells (33), we first observed the BrdU/survivin
co-labeled cells in the SGZ of the DG, suggesting that survivin is
expressed in neural stem cells. The results also revealed that
survivin is expressed in immature neurons and in a minority of
astrocytes, but not in mature neurons in the adult DG of the
hippocampus following TBI. Through the use of immunofluorescence
staining, newly generated cells labeled with BrdU were detected and
the majority of BrdU-labeled cells merged with survivin (+) cells
in the DG. The number of BrdU (+) and survivin (+) cells increased
in the TBI group versus the sham-operated group. These results
strongly suggest that TBI-induced neurogenesis may be associated
with the upregulation of survivin expression and that survivin
plays a role in promoting the proliferation of neural stem cell
following injury. Moreover, the double staining of survivin and DCX
revealed that a significant percentage of DCX (+) cells also
expressed survivin in the TBI group compared with the sham-
operated group, which indicated that the expression of survivin was
increased in immature neurons in the DG of the hippocampus sooon
after TBI. We consider that the expression of survivin is critical
to the proliferation of neural stem cells and to the survival of
existing cells in CNS development, as well as to the functional
maintenance of the adult brain. Furthermore, survivin may play a
role in the repair and restoration of neurons in certain
pathological processes, such as TBI.
Injury-induced neurogenesis is a compelling
potential contributor to recovery post-injury (7,8,44).
Progenitor cells in the SGZ are activated following TBI, although
it remains unclear whether this activation results in stable and
productive neurogenesis (8). In
addition, evidence for long-lasting hippocampal neurogenesis after
traumatic cortical injury has been accumulating (10). Even in adult animals, it is clear
that progenitors are activated and neurogenesis increases following
injury, whereby new neurons are found in the outer layers of the DG
(45,46).
The majority of the evidence to support these
findings is based on the increased dividing cells labeled by BrdU
when the proliferation of stem cells/progenitors is produced
following brain injury. The DCX-expressing cells within the DG
re-emerge and are the likely contributors to stable neurogenesis
(47). DCX, a marker of
developing, immature neurons, has been found in the adult rostal
migratory stream and in the DG of the hippocampus in adult rats
during the early differentiation stage of adult neurogenesis
(48). DCX (+) cells have also
been shown to be present near and among the glial scars following
brain injury (49). Similarly,
our study showed an increase in the number of BrdU (+) and DCX (+)
cells in the SGZ, also suggesting that the proliferation of stem
cells/progenitors was induced by injury. On the contrary, Rola
et al found that DCX (+) and BrdU (+) cells were reduced in
the ipsilateral SGZ following brain injury (50). This discrepancy may be caused by
the different experimental methods and animal conditions, i.e., the
model established, degree of injury, housing conditions and
technical considerations. However, it is important to note that
BrdU is not a marker of the S phase of the cell cycle; it is a
marker of DNA synthesis. Therefore, it also labels cells undergoing
DNA repair, aborting cell cycle re-entry, as a prelude to apoptosis
(51). However, the majority of
BrdU (+) cells were proliferating cells. Barha et al showed
a high percentage of BrdU (+) cells co-expressing DCX after TBI
(89.00%±3.42) (52) and a
co-localization of BrdU and DCX represents stem cells soon after
injury, suggesting that the majority of the BrdU (+) cells were the
proliferating stem cells in the SGZ.
Yu et al observed that DCX-expressing neural
progenitors are required for injury-induced remodeling to occur in
a highly proliferative environment, as evidenced by BrdU pulsing
(47) and we demonstrated the
increased expression of survivin in DCX (+) cells, suggesting the
crucial role of survivin in neurogenesis induced by injury.
However, there was a discrepancy as regards the time point in DCX
increasing expression after injury between our 2 research groups.
The potential factors that caused this discrepancy were
experimental methods and animal conditions. Furthermore, Yu et
al also demonstrated the essential role of nestin-expressing
early neural progenitors in TBI-induced hippocampal neurogenesis
(47). We consider that survivin
is expressed in the nestin-expressing progenitors and may be
involved in neurogenesis completed by the activation of
nestin-expressing progenitors. Further studies are required to
investigate the mechanisms of action of the survivin gene in
injury-induced adult neurogenesis, which is dependent on the
participation of different phenotypes of stem cells/progenitors,
i.e., BrdU (+), DCX (+) and nestin (+) cells. The necessary
alteration of survivin gene expression, i.e., downregulation with
siRNA or the development of survivin transgenic mice, may aid in
providing further insight into its role in neurogenesis.
Strong evidence is accumulating for a role of
survivin in certain pathological processes, such as
neurodegenerative diseases (3)
and brain ischemia (29,53–56). Based on these data, we
hypothesized that survivin, a downstream target gene of the
Wnt/β-catenin signaling pathway, plays a role in repairing
deterioration and promoting recovery of neural function in neuronal
degeneration diseases and various brain injuries involving ischemia
and trauma. However, the mechanisms of TBI require further
investigatation. Our study only elucidated the correlation between
survivin expression and neurogenesis following TBI and initially
explored the function of survivin expression. We found that
survivin plays a role in the proliferation and differentiation of
neural cells following injury. Further investigation is required to
further elucidate its role in neurogenesis, in order to provide
basic methods of treatment for brain injury and recovery of
dysfunction.
Acknowledgements
We thank Linchun Huan, Wangmiao Zhao and Zhen Zhang
for their helpful comments on the manuscript. This study was
supported by grants from the Science and Technology Foundation of
Tianjin (no. 10JCYBJC25700) and the Health-System Science
Foundation of Binhai New Area in Tianjin (no. 2011BHKL002).
References
|
1
|
Narayan RK, Michel ME, Ansell B, et al:
Clinical trials in head injury. J Neurotrauma. 19:503–557. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pickard JD and Czosnyka M: Management of
raised intracranial pressure. J Neurol Neurosurg Psychiatry.
56:845–858. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baratchi S, Kanwar RK and Kanwar JR:
Survivin: a target from brain cancer to neurodegenerative disease.
Crit Rev Biochem Mol Biol. 45:535–554. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Richardson RM, Singh A, Sun D, Fillmore
HL, Dietrich DW III and Bullock MR: Stem cell biology in traumatic
brain injury: effects of injury and strategies for repair. J
Neurosurg. 112:1125–1138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alvarez-Buylla A and Lim DA: For the long
run: maintaining germinal niches in the adult brain. Neuron.
41:683–686. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lie DC, Song H, Colamarino SA, Ming GL and
Gage FH: Neurogenesis in the adult brain: new strategies for
central nervous system diseases. Annu Rev Pharmacol Toxicol.
44:399–421. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chirumamilla S, Sun D, Bullock MR and
Colello RJ: Traumatic brain injury induced cell proliferation in
the adult mammalian central nervous system. J Neurotrauma.
19:693–703. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Richardson RM, Sun D and Bullock MR:
Neurogenesis after traumatic brain injury. Neurosurg Clin N Am.
18:169–181. 2007. View Article : Google Scholar
|
|
9
|
Zhao WM, Huan LC, Zhao Y, et al:
Endogenous adult neurogenesis and cognitive function recovery
following traumatic brain injury in the rat hippocampus. Neural
Regen Res. 5:645–650. 2010.
|
|
10
|
Dash PK, Mach SA and Moore AN: Enhanced
neurogenesis in the rodent hippocampus following traumatic brain
injury. J Neurosci Res. 63:313–319. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun D, Colello RJ, Daugherty WP, Kwon TH,
McGinn MJ, Harvey HB and Bullock MR: Cell proliferation and
neuronal differentiation in the dentate gyrus in juvenile and adult
rats following traumatic brain injury. J Neurotrauma. 22:95–105.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gulacsi AA and Anderson SA:
Beta-catenin-mediated Wnt signaling regulates neurogenesis in the
ventral telencephalon. Nat Neurosci. 11:1383–1391. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zechner D, Fujita Y, Hulsken J, et al:
beta-Catenin signals regulate cell growth and the balance between
progenitor cell expansion and differentiation in the nervous
system. Dev Biol. 258:406–418. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang L, Yang X, Yang S and Zhang J: The
Wnt/beta-catenin signaling pathway in the adult neurogenesis. Euro
J Neurosci. 33:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
White BD, Nathe RJ, Maris DO, Nguyen NK,
Goodson JM, Moon RT and Horner PJ: Beta-catenin signaling increases
in proliferating NG2+ progenitors and astrocytes during
post-traumatic gliogenesis in the adult brain. Stem Cells.
28:297–307. 2010.PubMed/NCBI
|
|
16
|
Zhang T, Otevrel T, Gao Z, Ehrlich SM,
Fields JZ and Boman BM: Evidence that APC regulates survivin
expression: a possible mechanism contributing to the stem cell
origin of colon cancer. Cancer Res. 61:8664–8667. 2001.
|
|
17
|
Zhu H, Zhang G, Wang Y, et al: Inhibition
of ErbB2 by Herceptin reduces survivin expression via the
ErbB2-beta-catenin/TCF4-survivin pathway in ErbB2-overexpressed
breast cancer cells. Cancer Sci. 101:1156–1162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deveraux QL and Reed JC: IAP family
proteins-suppressors of apoptosis. Genes Dev. 13:239–252. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li F, Ambrosini G, Chu EY, Plescia J,
Tognin S, Marchisio PC and Altieri DC: Control of apoptosis and
mitotic spindle checkpoint by survivin. Nature. 396:580–584. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hirokawa N: Microtubule organization and
dynamics dependent on microtubule-associated proteins. Curr Opin
Cell Biol. 6:74–81. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Altieri DC: Survivin, versatile modulation
of cell division and apoptosis in cancer. Oncogene. 22:8581–8589.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kobayashi K, Hatano M, Otaki M, Ogasawara
T and Tokuhisa T: Expression of a murine homologue of the inhibitor
of apoptosis protein is related to cell proliferation. Proc Natl
Acad Sci USA. 96:1457–1462. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
O’Connor DS, Schechner JS, Adida C, et al:
Control of apoptosis during angiogenesis by survivin expression in
endothelial cells. Am J Pathol. 156:393–398. 2000.
|
|
24
|
Shin S, Sung BJ, Cho YS, et al: An
anti-apoptotic protein human survivin is a direct inhibitor of
caspase-3 and -7. Biochemistry. 40:1117–1123. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tamm I, Wang Y, Sausville E, Scudiero DA,
Vigna N, Oltersdorf T and Reed JC: IAP-family protein survivin
inhibits caspase activity and apoptosis induced by Fas (CD95), Bax,
caspases and anticancer drugs. Cancer Res. 58:5315–5320.
1998.PubMed/NCBI
|
|
26
|
Jiang Y, de Bruin A, Caldas H, et al:
Essential role for survivin in early brain development. J Neurosci.
25:6962–6970. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zwerts F, Lupu F, De Vriese A, et al: Lack
of endothelial cell survivin causes embryonic defects in
angiogenesis, cardiogenesis, and neural tube closure. Blood.
109:4742–4752. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Adida C, Crotty PL, McGrath J, Berrebi D,
Diebold J and Altieri DC: Developmentally regulated expression of
the novel cancer anti-apoptosis gene survivin in human, and mouse
differentiation. Am J Pathol. 152:43–49. 1998.
|
|
29
|
Li F and Brattain MG: Role of the Survivin
gene in pathophysiology. Am J Pathol. 169:1–11. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Johnson EA, Svetlov SI, Wang KK, Hayes RL
and Pineda JA: Cell-specific DNA fragmentation may be attenuated by
a survivin-dependent mechanism after traumatic brain injury in
rats. Exp Brain Res. 167:17–26. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thompson HJ, Lifshitz J, Marklund N, Grady
MS, Graham DI, Hovda DA and McIntosh TK: Lateral fluid percussion
brain injury: a 15-year review and evaluation. J Neurotrauma.
22:42–75. 2005.PubMed/NCBI
|
|
32
|
Wojtowicz JM and Kee N: BrdU assay for
neurogenesis in rodents. Nat Protoc. 1:1399–1405. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Johnson EA, Svetlov SI, Pike BR, et al:
Cell-specific upregulation of survivin after experimental traumatic
brain injury in rats. J Neurotrauma. 21:1183–1195. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ray J, Peterson DA, Schinstine M and Gage
FH: Proliferation, differentiation, and long-term culture of
primary hippocampal neurons. Proc Natl Acad Sci USA. 90:3602–3606.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
De Ferrari GV and Inestrosa NC: Wnt
signaling function in Alzheimer’s disease. Brain Res Brain Res Rev.
33:1–12. 2000.
|
|
36
|
De Ferrari GV, Papassotiropoulos A,
Biechele T, et al: Common genetic variation within the low-density
lipoprotein receptor-related protein 6 and late-onset Alzheimer’s
disease. Proc Natl Acad Sci USA. 104:9434–9439. 2007.PubMed/NCBI
|
|
37
|
Inestrosa NC, Alvarez A, Godoy J, Reyes A
and De Ferrari GV: Acetylcholinesterase-amyloid-beta-peptide
interaction and Wnt signaling involvement in Abeta neurotoxicity.
Acta Neurol Scand Suppl. 176:53–59. 2000. View Article : Google Scholar
|
|
38
|
Lovestone S, Killick R, Di Forti M and
Murray R: Schizophrenia as a GSK-3 dysregulation disorder. Trends
Neurosci. 30:142–149. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
De Ferrari GV and Moon RT: The ups and
downs of Wnt signaling in prevalent neurological disorders.
Oncogene. 25:7545–7553. 2006.PubMed/NCBI
|
|
40
|
Wheatley SP, Carvalho A, Vagnarelli P and
Earnshaw WC: INCENP is required for proper targeting of Survivin to
the centromeres and the anaphase spindle during mitosis. Curr Biol.
11:886–890. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Skoufias DA, Mollinari C, Lacroix FB and
Margolis RL: Human survivin is a kinetochore-associated passenger
protein. J Cell Biol. 151:1575–1582. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen J, Jin S, Tahir SK, et al: Survivin
enhances Aurora-B kinase activity and localizes Aurora-B in human
cells. J Biol Chem. 278:486–490. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Coremans V, Ahmed T, Balschun D, et al:
Impaired neurogenesis, learning and memory and low seizure
threshold associated with loss of neural precursor cell survivin.
BMC Neurosci. 11:22010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kernie SG and Parent JM: Forebrain
neurogenesis after focal Ischemic and traumatic brain injury.
Neurobiol Dis. 37:267–274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ramaswamy S, Goings GE, Soderstrom KE,
Szele FG and Kozlowski DA: Cellular proliferation and migration
following a controlled cortical impact in the mouse. Brain Res.
1053:38–53. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Urrea C, Castellanos DA, Sagen J, Tsoulfas
P, Bramlett HM and Dietrich WD: Widespread cellular proliferation
and focal neurogenesis after traumatic brain injury in the rat.
Restor Neurol Neurosci. 25:65–76. 2007.PubMed/NCBI
|
|
47
|
Yu TS, Zhang G, Liebl DJ and Kernie SG:
Traumatic brain injury-induced hippocampal neurogenesis requires
activation of early nestin-expressing progenitors. J Neurosci.
28:12901–12912. 2008. View Article : Google Scholar
|
|
48
|
Brown JP, Couillard-Despres S, Cooper-Kuhn
CM, Winkler J, Aigner L and Kuhn HG: Transient expression of
doublecortin during adult neurogenesis. J Comp Neurol. 467:1–10.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Itoh T, Satou T, Nishida S, Hashimoto S
and Ito H: Immature and mature neurons coexist among glial scars
after rat traumatic brain injury. Neurol Res. 29:734–742. 2007.
View Article : Google Scholar
|
|
50
|
Rola R, Mizumatsu S, Otsuka S, Morhardt
DR, Noble-Haeusslein LJ, et al: Alterations in hippocampal
neurogenesis following traumatic brain injury in mice. Exp Neurol.
202:189–199. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Taupin P: BrdU immunohistochemistry for
studying adult neurogenesis: paradigms, pitfalls, limitations, and
validation. Brain Res Rev. 53:198–214. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Barha CK, Ishrat T, Epp JR, Galea LA and
Stein DG: Progesterone treatment normalizes the levels of cell
proliferation and cell death in the dentate gyrus of the
hippocampus after traumatic brain injury. Exp Neurol. 231:72–81.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Conway EM, Zwerts F, Van Eygen V, DeVriese
A, Nagai N, Luo W and Collen D: Survivin-dependent angiogenesis in
ischemic brain: molecular mechanisms of hypoxia-induced
up-regulation. Am J Pathol. 163:935–946. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lu N, Li XJ, Li CZ, Li DL, Cui PF, Hou YN
and Wang YL: Effect of hypoxic preconditioning on the learning and
memory ability and expressions of survivin and HSP-70 proteins in
rats with focal cerebral ischemia/reperfusion injury. Nan Fang Yi
Ke Da Xue Xue Bao. 27:1856–1859. 2007.(Article in Chinese).
|
|
55
|
Okazaki T, Magaki T, Takeda M, et al:
Intravenous administration of bone marrow stromal cells increases
survivin and Bcl-2 protein expression and improves sensorimotor
function following ischemia in rats. Neurosci Lett. 430:109–114.
2008. View Article : Google Scholar
|
|
56
|
Zhang Y, Park TS and Gidday JM: Hypoxic
preconditioning protects human brain endothelium from ischemic
apoptosis by Akt-dependent survivin activation. Am J Physiol Heart
Circ Physiol. 292:H2573–H2581. 2007. View Article : Google Scholar
|