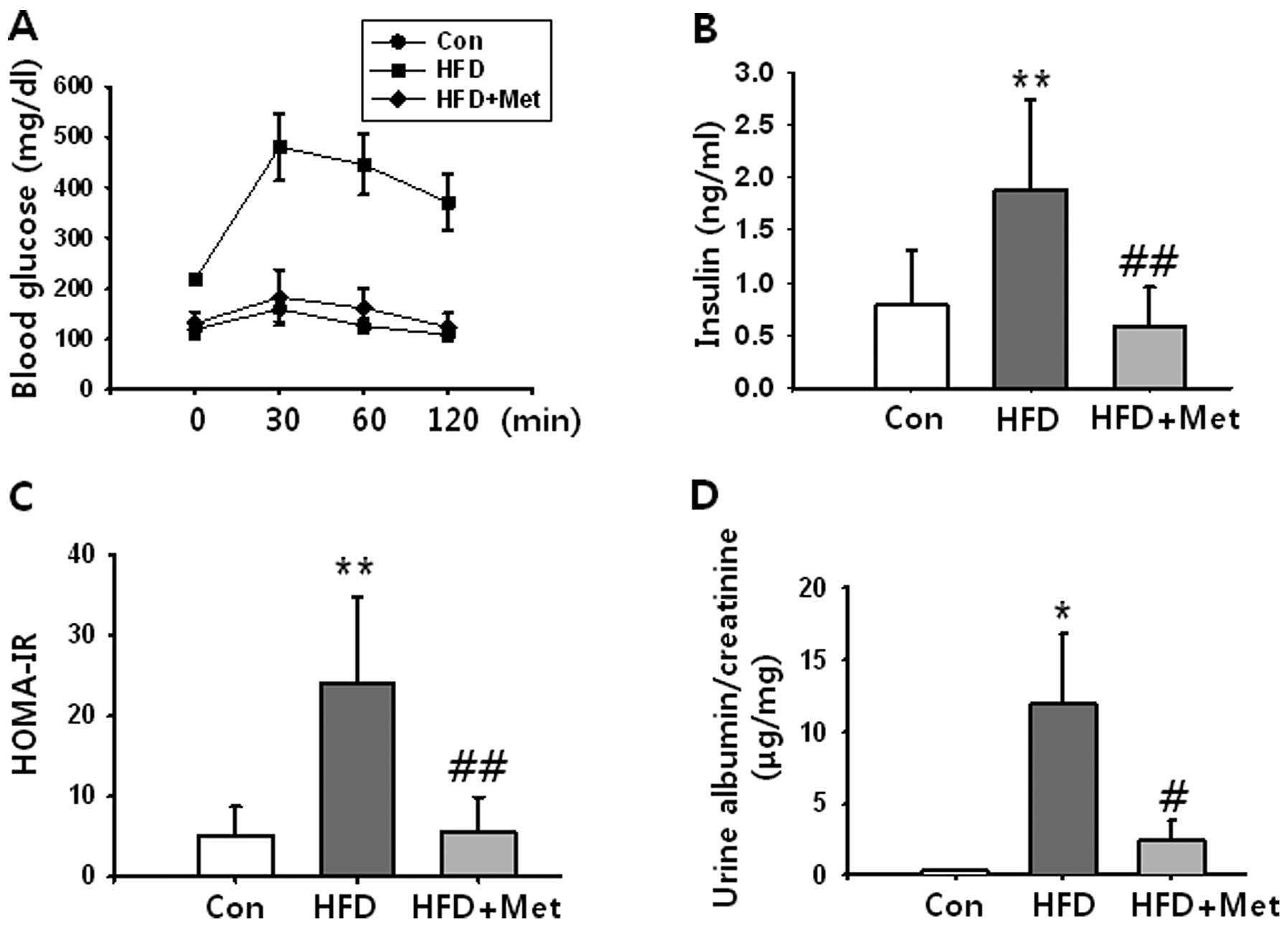
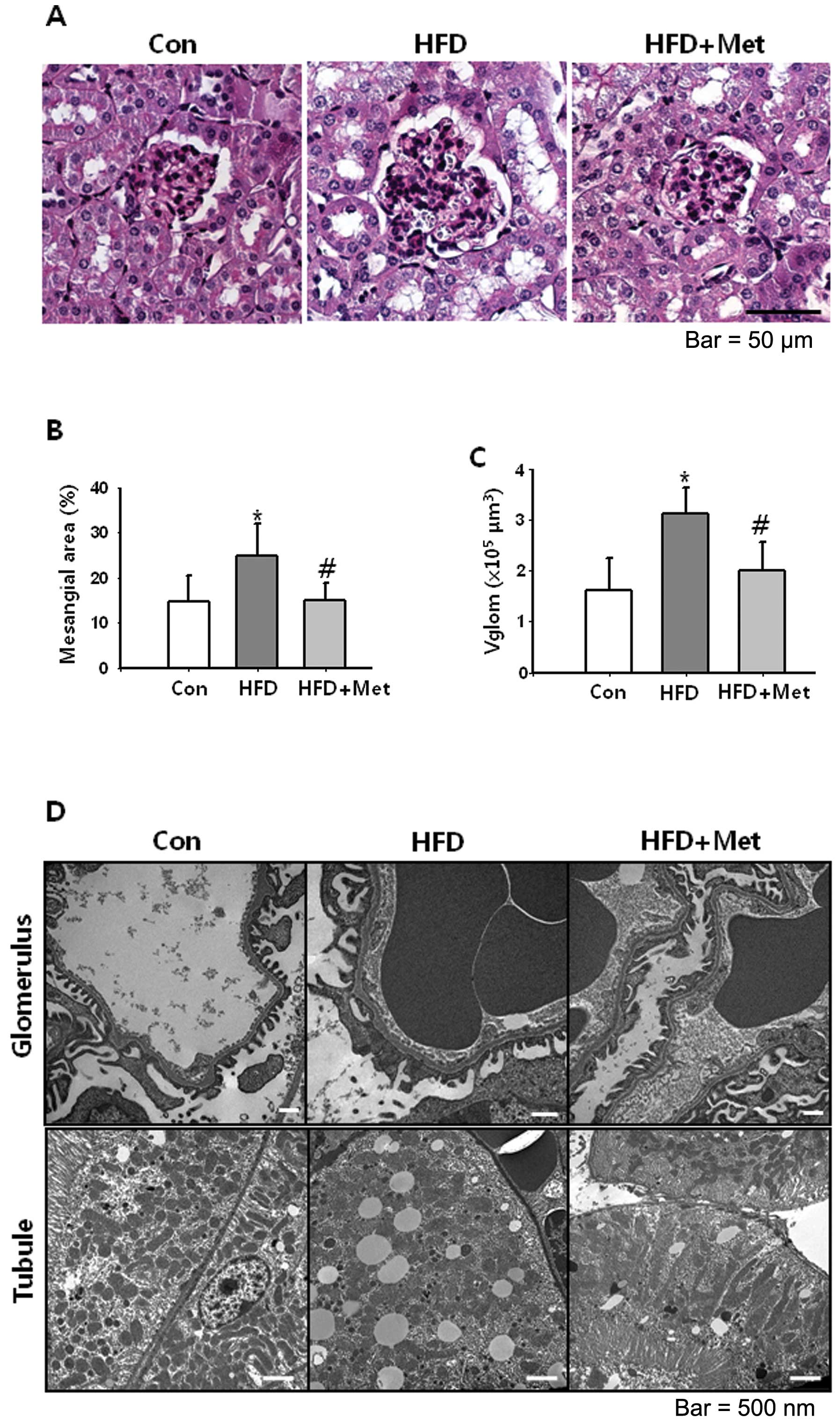
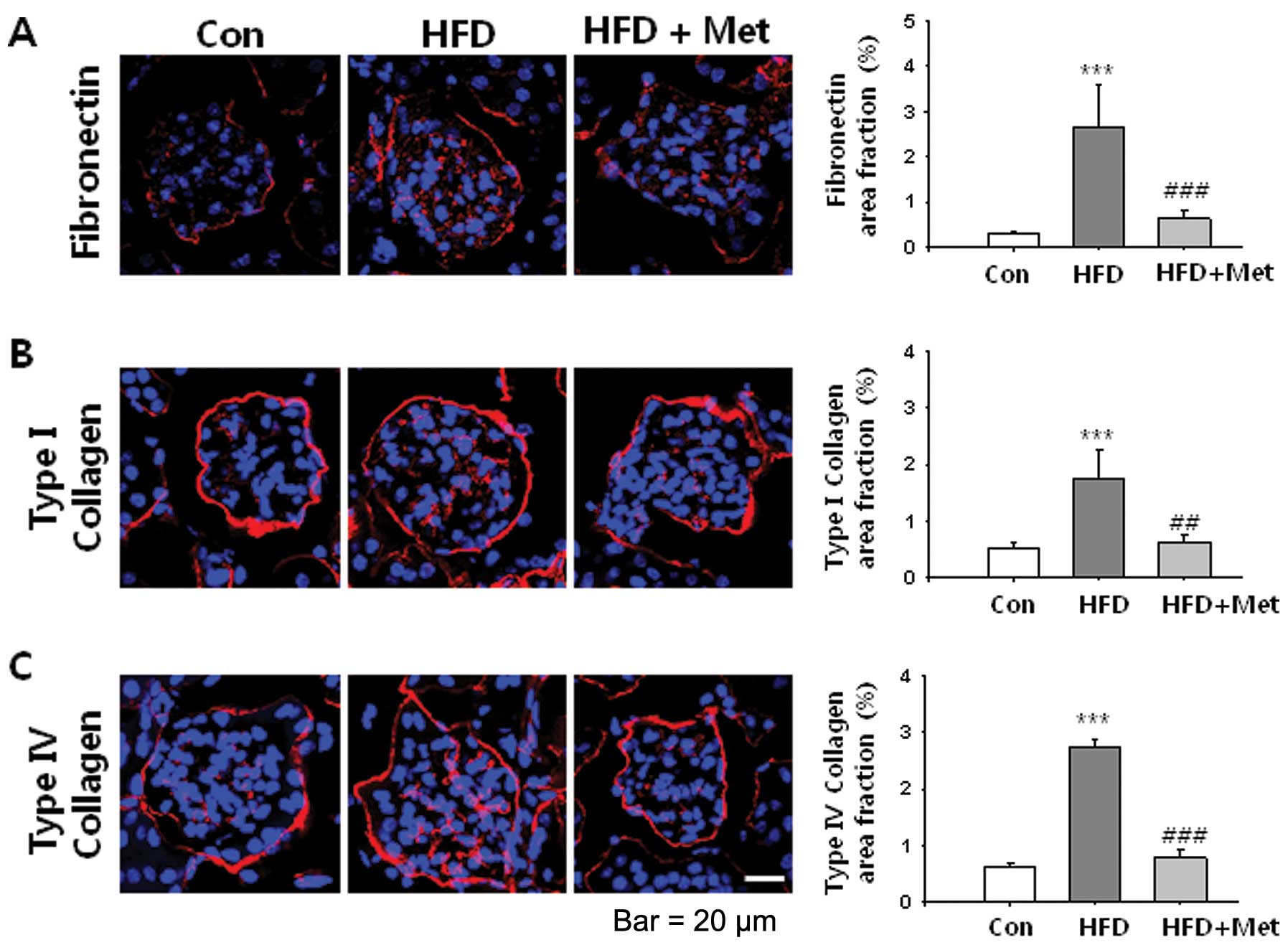
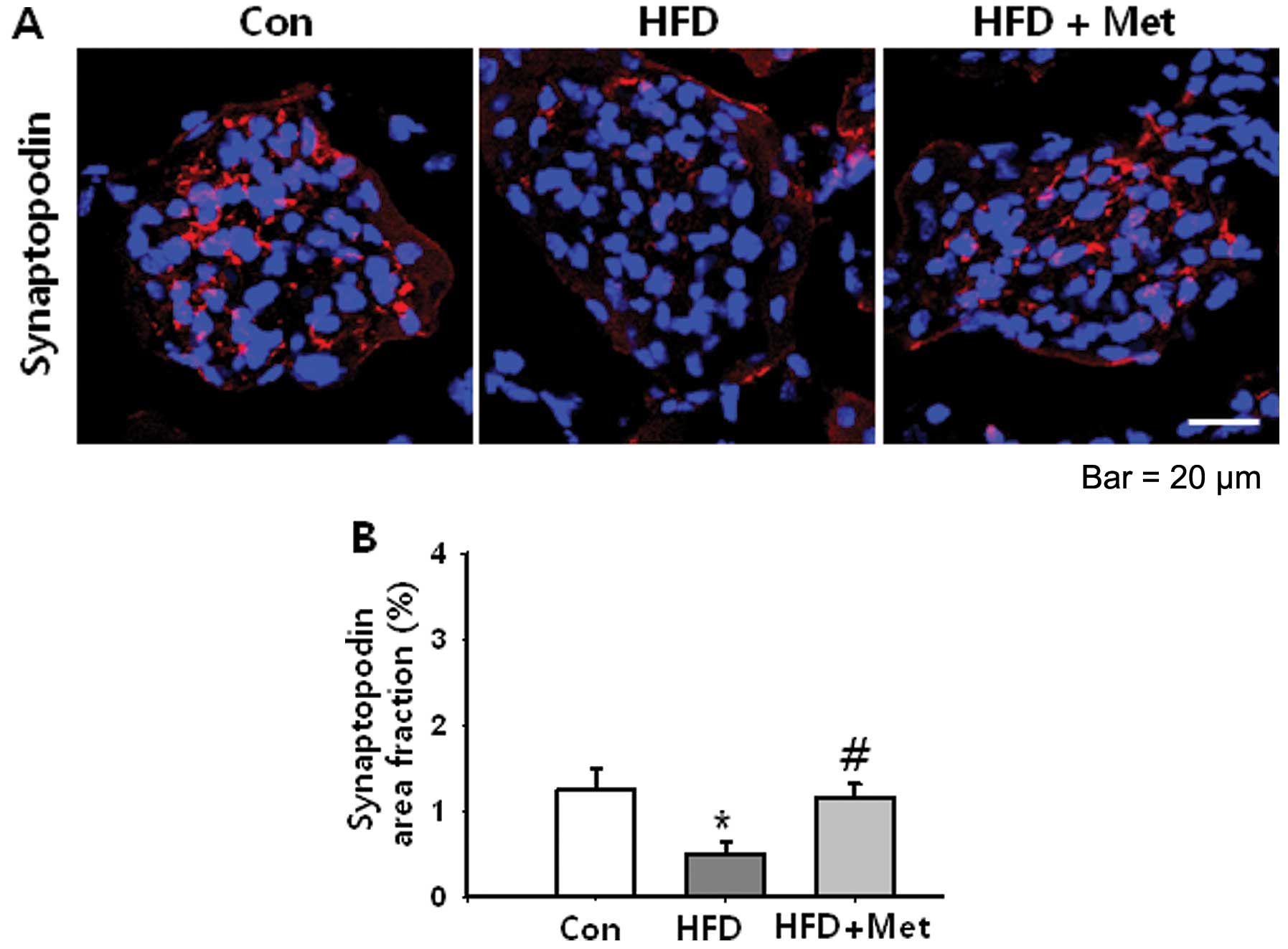
|
1
|
Bagby SP: Obesity-initiated metabolic
syndrome and the kidney: a recipe for chronic kidney disease? J Am
Soc Nephrol. 15:2775–2791. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen J, Muntner P, Hamm LL, et al: Insulin
resistance and risk of chronic kidney disease in nondiabetic US
adults. J Am Soc Nephrol. 14:469–477. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen J, Muntner P, Hamm LL, et al: The
metabolic syndrome and chronic kidney disease in U.S. adults. Ann
Intern Med. 140:167–174. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Agrawal V, Shah A, Rice C, Franklin BA and
McCullough PA: Impact of treating the metabolic syndrome on chronic
kidney disease. Nat Rev Nephrol. 5:520–528. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maury E and Brichard SM: Adipokine
dysregulation, adipose tissue inflammation and metabolic syndrome.
Mol Cell Endocrinol. 314:1–16. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang J, Yan H and Zhuang S: Inflammation
and oxidative stress in obesity-related glomerulopathy. Int J
Nephrol. 2012:6083972012.PubMed/NCBI
|
|
7
|
Towler MC and Hardie DG: AMP-activated
protein kinase in metabolic control and insulin signaling. Circ
Res. 100:328–341. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nathan DM, Buse JB, Davidson MB, et al:
Medical management of hyperglycemia in type 2 diabetes: a consensus
algorithm for the initiation and adjustment of therapy: a consensus
statement of the American Diabetes Association and the European
Association for the Study of Diabetes. Diabetes Care. 32:193–203.
2009. View Article : Google Scholar
|
|
9
|
Viollet B, Guigas B, Sanz Garcia N,
Leclerc J, Foretz M and Andreelli F: Cellular and molecular
mechanisms of metformin: an overview. Clin Sci (Lond). 122:253–270.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
El-Mir MY, Nogueira V, Fontaine E, Avéret
N, Rigoulet M and Leverve X: Dimethylbiguanide inhibits cell
respiration via an indirect effect targeted on the respiratory
chain complex I. J Biol Chem. 275:223–228. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Owen MR, Doran E and Halestrap AP:
Evidence that metformin exerts its anti-diabetic effects through
inhibition of complex 1 of the mitochondrial respiratory chain.
Biochem J. 348:607–614. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Salminen A, Hyttinen JM and Kaarniranta K:
AMP-activated protein kinase inhibits NF-κB signaling and
inflammation: impact on healthspan and lifespan. J Mol Med (Berl).
89:667–676. 2011.
|
|
13
|
Zhou G, Myers R, Li Y, et al: Role of
AMP-activated protein kinase in mechanism of metformin action. J
Clin Invest. 108:1167–1174. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Viollet B, Guigas B, Leclerc J, et al:
AMP-activated protein kinase in the regulation of hepatic energy
metabolism: from physiology to therapeutic perspectives. Acta
Physiol (Oxf). 196:81–98. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abu-Elheiga L, Matzuk MM, Abo-Hashema KA
and Wakil SJ: Continuous fatty acid oxidation and reduced fat
storage in mice lacking acetyl-CoA carboxylase 2. Science.
291:2613–2616. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Decleves AE, Mathew AV, Cunard R and
Sharma K: AMPK mediates the initiation of kidney disease induced by
a high-fat diet. J Am Soc Nephrol. 22:1846–1855. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kita Y, Takamura T, Misu H, et al:
Metformin prevents and reverses inflammation in a non-diabetic
mouse model of nonalcoholic steatohepatitis. PLoS One.
7:e430562012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lane PH, Steffes MW and Mauer SM:
Estimation of glomerular volume: a comparison of four methods.
Kidney Int. 41:1085–1089. 1992.PubMed/NCBI
|
|
19
|
Kim DH, Jung YJ, Lee AS, et al:
COMP-angiopoietin-1 decreases lipopolysaccharide-induced acute
kidney injury. Kidney Int. 76:1180–1191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kang KP, Kim DH, Jung YJ, et al:
Alpha-lipoic acid attenuates cisplatin-induced acute kidney injury
in mice by suppressing renal inflammation. Nephrol Dial Transplant.
24:3012–3020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kang KP, Park SK, Kim DH, et al: Luteolin
ameliorates cisplatin-induced acute kidney injury in mice by
regulation of p53-dependent renal tubular apoptosis. Nephrol Dial
Transplant. 26:814–822. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dengel DR, Goldberg AP, Mayuga RS, Kairis
GM and Weir MR: Insulin resistance, elevated glomerular filtration
fraction, and renal injury. Hypertension. 28:127–132. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Henegar JR, Bigler SA, Henegar LK, Tyagi
SC and Hall JE: Functional and structural changes in the kidney in
the early stages of obesity. J Am Soc Nephrol. 12:1211–1217.
2001.PubMed/NCBI
|
|
24
|
Wolf G, Hamann A, Han DC, et al: Leptin
stimulates proliferation and TGF-beta expression in renal
glomerular endothelial cells: potential role in glomerulosclerosis
[see comments]. Kidney Int. 56:860–872. 1999.PubMed/NCBI
|
|
25
|
Han DC, Isono M, Chen S, et al: Leptin
stimulates type I collagen production in db/db mesangial cells:
glucose uptake and TGF-beta type II receptor expression. Kidney
Int. 59:1315–1323. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chudek J, Adamczak M, Nieszporek T and
Wiecek A: The adipose tissue as an endocrine organ - a
nephrologists’ perspective. Contrib Nephrol. 151:70–90.
2006.PubMed/NCBI
|
|
27
|
Diez JJ and Iglesias P: The role of the
novel adipocyte-derived hormone adiponectin in human disease. Eur J
Endocrinol. 148:293–300. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sharma K, Ramachandrarao S, Qiu G, et al:
Adiponectin regulates albuminuria and podocyte function in mice. J
Clin Invest. 118:1645–1656. 2008.PubMed/NCBI
|
|
29
|
Ix JH and Sharma K: Mechanisms linking
obesity, chronic kidney disease, and fatty liver disease: the roles
of fetuin-A, adiponectin, and AMPK. J Am Soc Nephrol. 21:406–412.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Deen WM: What determines glomerular
capillary permeability? J Clin Invest. 114:1412–1414. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mundel P, Heid HW, Mundel TM, Kruger M,
Reiser J and Kriz W: Synaptopodin: an actin-associated protein in
telencephalic dendrites and renal podocytes. J Cell Biol.
139:193–204. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ghayur A, Liu L, Kolb M, et al:
Adenovirus-mediated gene transfer of TGF-beta1 to the renal
glomeruli leads to proteinuria. Am J Pathol. 180:940–951. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bobulescu IA: Renal lipid metabolism and
lipotoxicity. Curr Opin Nephrol Hypertens. 19:393–402. 2010.
View Article : Google Scholar
|
|
34
|
Bailey CJ and Turner RC: Metformin. N Engl
J Med. 334:574–579. 1996. View Article : Google Scholar : PubMed/NCBI
|