Introduction
Hypertension is the most common risk factor for
congestive heart failure (1),
which can induce apoptotic changes in heart function and peripheral
vascular resistance (2). Evidence
indicates higher levels of activated cardiomyocyte apoptosis and
cardiac apoptosis in hypertensive models (3). Since the side-effects of western
drugs in the treatment of cardiac diseases cannot be avoided, the
investigation of natural products or dietary supplements which can
protect cardiac abnormalities and injuries is essential (4).
Apoptosis is a recognized mechanism for the
elimination of redundant cells, although it may also inhibit cell
proliferation. In fact, it has been suggested that apoptosis plays
a critical role in the pathogenesis of cardiac disorders (3,5,6).
An increase in the levels of the pro-apoptotic protein, angiotensin
II (Ang II), has been observed in hypertension, coronary artery
disease, left ventricular hypertrophy and heart failure and may
function as a regulator of apoptosis in cardiac tissues (7,8).
Both Fas-dependent and mitochondrial-dependent apoptotic pathways
are considered as major pathways which directly cause cardiac
apoptosis (9,10).
The ‘extrinsic’ Fas-dependent apoptotic pathway is
initiated by binding the Fas ligand to the Fas death receptor or by
binding tumor necrosis factor (TNF)-α to TNF receptor 1, which
results in the clustering of receptors, initiating an extrinsic
pathway (11). A previous study
reported that cardiac Fas receptor-dependent apoptotic pathways
were activated in obese rat hearts, which is the one of the
possible mechanisms behind cardiac abnormalities in obesity
(12). The ‘intrinsic’
mitochondrial-dependent apoptotic pathway is mediated by internal
factors, particularly in the mitochondria, the main site of action
for apoptosis-regulating proteins, exemplified by the Bcl-2 family,
such as Bax, Bad, t-Bid and Bak (11). Pro-apoptotic and anti-apoptotic
Bcl-2 family members can homodimerize or heterodimerize to each
other and appear to interact with and neutralize each other, so
that the relative balance of these effectors strongly influences
the release of cytochrome c. When cytochrome c is
released from the mitochondria into the cytosol, it is responsible
for activating caspase-9, which further activates caspase-3 and
executes the apoptotic program (13). In a recent study, we observed
cardiac mitochondrial-dependent but not Fas-dependent apoptosis in
hamsters fed a hyper-cholesterol diet, and demonstrated the
cardioprotective effects induced by the activation of the
insulin-like growth factor-I receptor (IGF-IR) survival pathway by
treatment with Li-Fu formula (4).
Previous studies have indicated that insulin-like
growth factor (IGF)-1 signaling contributes to the modulation of
survival responses in cardiomyocytes and that low IGF-I levels are
associated with a high risk of myocardial infarction and heart
failure (11,14). IGF-1 is the survival factor
through which IGF-1R activates the phosphatidylinositol-3 kinase
(PI3K)/protein kinase B (PKB) pathway, thus preventing myocyte
apoptosis (15). In particular,
activated PI3K enhances the levels of phosphorylated Akt (p-Akt)
(16), which in turn regulates
the activity of phosphorylated-Bad (p-Bad) and Bcl-2 to prevent the
apoptosis of cardiomyocytes (11).
The consumption of γ-aminobutyric acid
(GABA)-enriched fermented milk has been reported to depress
elevated blood pressure in spontaneously hypertensive rats (SHRs)
and mildly hypertensive humans (17–19). In a previous study, we
demonstrated that purple sweet potato (PSP) [Ipomoea batatas
(L.) Lam.] contains high levels of anthocyanin and can easily grow
in subtropical areas through fermentation by 3 lactic acid bacteria
(LAB), with high levels of GABA (20). However, the protective effects of
probiotic-fermented milk and its derivatives, such as GABA on
cardiac apoptosis have not been reported. This study was therefore
undertaken to examine the potential benefits of GABA-enriched PSP
yogurt (PSPY) on the cardiac apoptotic pathways in SHR hearts. We
demonstrate that PSPY attenuates cardiac apoptosis by activating
the PI3K/AKT survival signaling pathway.
Materials and methods
Bacterial strains and growth
conditions
In this study, the 3 experimental LAB strains used
were purchased from the Food Industry Research and Development
Institute of the Biological Resources Conservation and Research
Center: Lactobacillus acidophilus BCRC 14065; L.
delbrueckii subsp. lactis BCRC 12256; and L.
gasseri BCRC 14619. The stock culture was maintained at −80°C
in 20% glycerol prior to use. The bacteria were propagated twice in
Lactobacilli MRS broth (Difco, Sparks, MD, USA) containing 0.05%
L-cysteine overnight at 37°C before the experimental
procedures.
Preparation of PSPY
PSP was acquired from the Taiwan Agricultural
Research Institute and stored at 4°C after being cleaned with tap
water. To prepare the PSPY, the potatoes were first peeled, cut
into 1-cm-thick slices and steamed at 100°C for 20 min. The cooked
spuds were then homogenized and then 0.05% α-amylase, 10% skimmed
milk powder, 0.05% protease and 3% whey protein were added to the
mixture before pasteurization (121°C, 15 min). The 3 different LAB
strains were inoculated at a concentration of 109 CFU/ml
to the PSP milk and incubated at 37°C for 24 h until the fermented
PSPY was obtained. The final product was stored at 4°C in a
refrigerator for use in subsequent experiments.
Animals and experimental groups
Twenty-two male SHRs and 12 male Wistar Kyoto (WKY)
rats were purchased from BioLASCO Taiwan Co., Ltd, Taipei, Taiwan.
The animals (aged 6 weeks) were housed individually in a
temperature (20±2°C)- and humidity (55±5%)-controlled environment.
The rats were maintained on a 12 h dark-light cycle with lights on
from 8 a.m. to 8 p.m. The rats were allowed access to food [chow
pellets (MF-18; Oriental Yeast Co. Ltd., Tokyo, Japan)] and water
ad libitum. An acclimatization period of 1 week after
delivery by the supplier was allowed before the SHRs were randomly
divided into the following 4 groups: i) the SHR control group (rats
administered 2.5 ml distilled water); ii) the anti-hypertensive
captopril group [rats administered captopril, 15.6 mg/kg, body
weight (BW)/day]; iii) the 10% PSPY group (rats administered a
10-fold dilution of PSPY, 1.068 μg GABA/2.5 ml PSPY); and iv) the
100% PSPY group (rats administered undiluted PSPY, 10.68 μg
GABA/2.5 ml PSPY). Experimental feeding concentrations of PSPY were
calculated on the basis of GABA concentrations by the
high-performance liquid chromatography (HPLC) method. Captopril and
PSPY were orally administered daily to the rats until the rats were
sacrificed on the 8th week of the experimental period. WKY rats
were used as the negative control. The entire experimental
procedure was performed according to the NIH Guide for the Care and
Use of Laboratory Animals, and the protocol was approved by the
Institutional Animal Care and Use Committee of HungKuang
University, Taichung, Taiwan.
4′,6-diamidine-2-phenylindole
dihydrochloride (DAPI) staining and terminal deoxynucleotidyl
transferase-mediated deoxyuridine triphosphate (dUTP) nick
end-labeling (TUNEL)
After the hearts were excised, the hearts were
soaked in formalin, dehydrated through graded alcohols and embedded
in paraffin wax. From the heart tissues, the 3-μm-thick paraffin
sections were cut from the paraffin-embedded tissue blocks. The
sections were deparaffinized by immersing them in xylene (5 min,
thrice), rehydrated and incubated in phosphate-buffered saline
(PBS), pH 7.4, with 3% H2O2 to inactivate
endogenous peroxidase. The sections were then incubated with
proteinase K (20 μg/ml) for 30 min, washed in 0.1 M PBS, and
incubated with TUNEL reaction mixture for 60 min at 37°C using an
apoptosis detection kit (Roche Applied Science, Indianapolis, IN,
USA). After washing with PBS twice, the sections were stained with
DAPI (Sigma-Aldrich, St. Louis, MO, USA) for 5 min to detect cell
nuclei by UV light microscopic observations (blue). TUNEL-positive
nuclei (fragmented DNA) fluoresced bright green at 450–500 nm,
whereas DAPI-positive nuclei (intact DNA) fluoresced blue at 360
nm. The mean numbers of TUNEL-positive and DAPI-labeled cells were
counted for at least 3 separate fields from 2 slides excised from
the rat hearts in each group. All counts were performed by at least
2 independent individuals in a blinded manner.
Tissue extraction
The left ventricle was cut into 8 sections. One
section from the left ventricle was minced with scissors; then
lysis buffer (20 mM Tris, 2.0 mM EDTA, 50 mM 2-mercaptoethanol, 10%
glycerol, pH 7.4), a proteinase inhibitor cocktail tablet and
phosphatase inhibitor cocktail (Roche Diagnostics, Mannheim,
Germany) were added at a concentration of 100 mg tissue/ml buffer
and the mixture was homogenized on ice using a Model PT l0/35
Polytron homogenizer for 2 cycles of l0 sec each. The homogenate
was then placed on ice for 10 min and then centrifuged at 12,000 ×
g for 40 min. The supernatant was collected and stored at −70°C for
further western blot analysis.
Protein content
The protein content of the left ventricle extracts
was determined using the Bradford protein assay using the
protein-dye kit (Bio-Rad, Richmond, CA, USA). Commercially
available bovine serum albumin (Sigma-Aldrich) was used as a
standard. Changes in absorption were monitored at 595 nm.
Electrophoresis and western blot
analysis
The left ventricle extracts were prepared as
described above. Sodium dodecyl sulphate-polyacrylamide gel
electrophoresis was performed using 10% polyacrylamide gels. Equal
amounts (20 mg) of the samples were electrophoresed at 100 V for 3
h and equilibrated for 15 min in transfer buffer [25 mM Tris-HCl,
pH 8.3, containing 192 mM glycine and 20% (v/v) methanol].
Subseqently, the electrophoresed proteins were transferred onto
polyvinylidene difluoride (PVDF) membranes (0.45 μm pore size)
(Millipore, Bedford, MA, USA) using a Bio-Rad Scientific
Instruments Transphor Unit at 100 V with transfer buffer for 3 h.
The PVDF membranes were incubated at room temperature for 1 h in
blocking buffer containing 100 mM Tris-Base, 0.9% (w/v) NaCl, 0.1%
(v/v) Tween-20 (pH 7.4) and 5% non-fat milk. Monoclonal antibodies
against Akt (BD Pharmingen, San Jose, CA, USA), p-Akt (Cell
Signaling Technology, Inc., Danvers, MA, USA) and polyclonal
antibodies against Fas, Bid, t-Bid, Bcl-xL, PI3K, phoshorylated
PI3K (p-PI3K), Bak, Bax, caspase-3, α-tubulin (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), cleaved caspase-9, p-Bad
(Cell Signaling Technology, Inc.) and IGF-IR and
phosphorylated-IGF-IR (p-IGF-IR) (Abcam, Taipei, Taiwan) were
diluted in an antibody-binding buffer containing 100 mM Tris-Base,
pH 7.5, 0.9% (w/v) NaCl and 0.1% (v/v) Tween-20. The immunoblots
were washed 3 times in binding buffer for 10 min and then immersed
in secondary antibody solution containing goat anti-mouse IgG-HRP,
goat anti-rabbit IgG-HRP, or donkey anti-goat IgG-HRP (Santa Cruz
Biotechnology, Inc.) for 1 h and diluted 500-fold in binding
buffer. The filters were then washed 3 times (10 min each) in
blotting buffer. The immunoblotted proteins were visualized by
using enhanced chemiluminescence (ECL) Western Blotting Luminal
Reagent (Santa Cruz Biotechnology, Inc.) and quantified using a
Fujifilm LAS-3000 chemiluminescence detection system (Fujifilm,
Tokyo, Japan). Color was developed in a 20 ml mixture consisting of
7 mg nitro blue tetrazolium, 5 mg
5-bromo-4-chloro-3-indolyl-phosphate, 100 mM NaCl, and 5 mM
MgCl2 in 100 mM Tris-HCl, pH 9.5. The immunoblot with
antibody against α-tubulin, which was prepared with the same
procedure, was used as an internal control.
Statistical analysis
All statistical analyses were performed using SPSS
17.0 software (SPSS Inc., Chicago, IL, USA). Data were compared
between the animal groups using one-way analysis of variance
(ANOVA). Dunnett’s test was used to determine significant
differences. P-values <0.05 were considered to indicate
statistically significant differences. Significant differences are
indicated with symbols, as shown in the tables and figures.
Results
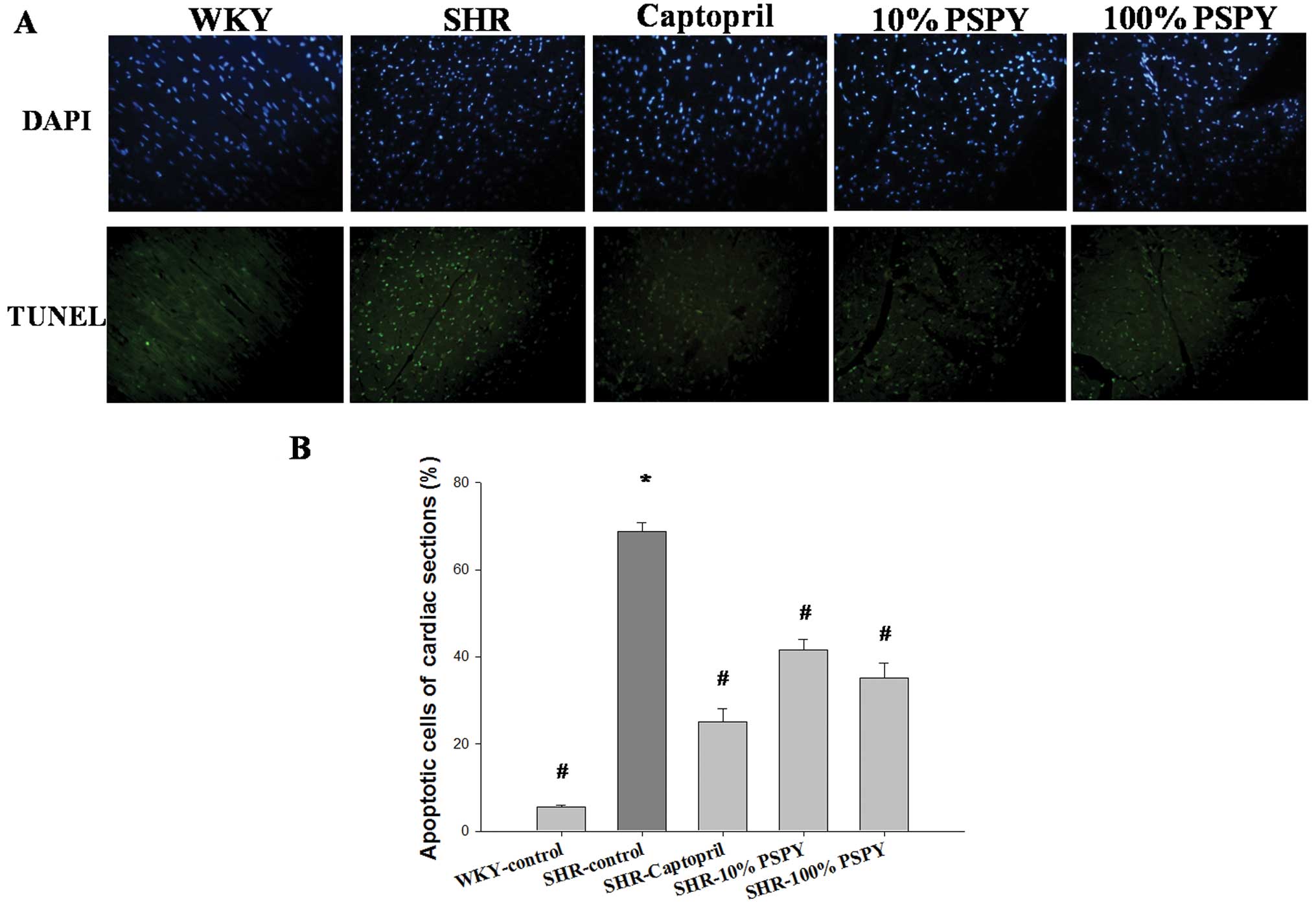
TUNEL-positive apoptotic cells in cardiac
tissues
We wished to determine the effects of PSPY on
hypertension-induced apoptosis in cardiac cells. We examined the
TUNEL-positive cardiac cells in the excised hearts from the WKY
rats, and from the SHRs in the control, captopril and PSPY (both
concentrations, 10 and 100%) groups by TUNEL assay. Following TUNEL
staining, an increased number of TUNEL-positive cardiac cells was
observed in the left ventricle in the SHR-control group compared
with the WKY group (Fig. 1A).
Notably, a significantly reduced number of TUNEL-positive cardiac
cells was found in the left ventricle of the SHR hearts in the
captopril, and 10 and 100% PSPY groups compared with the
SHR-control group (Fig. 1A). The
percentage of TUNEL-positive cardiac cells was calculated and the
quantified results are shown in Fig.
1B.
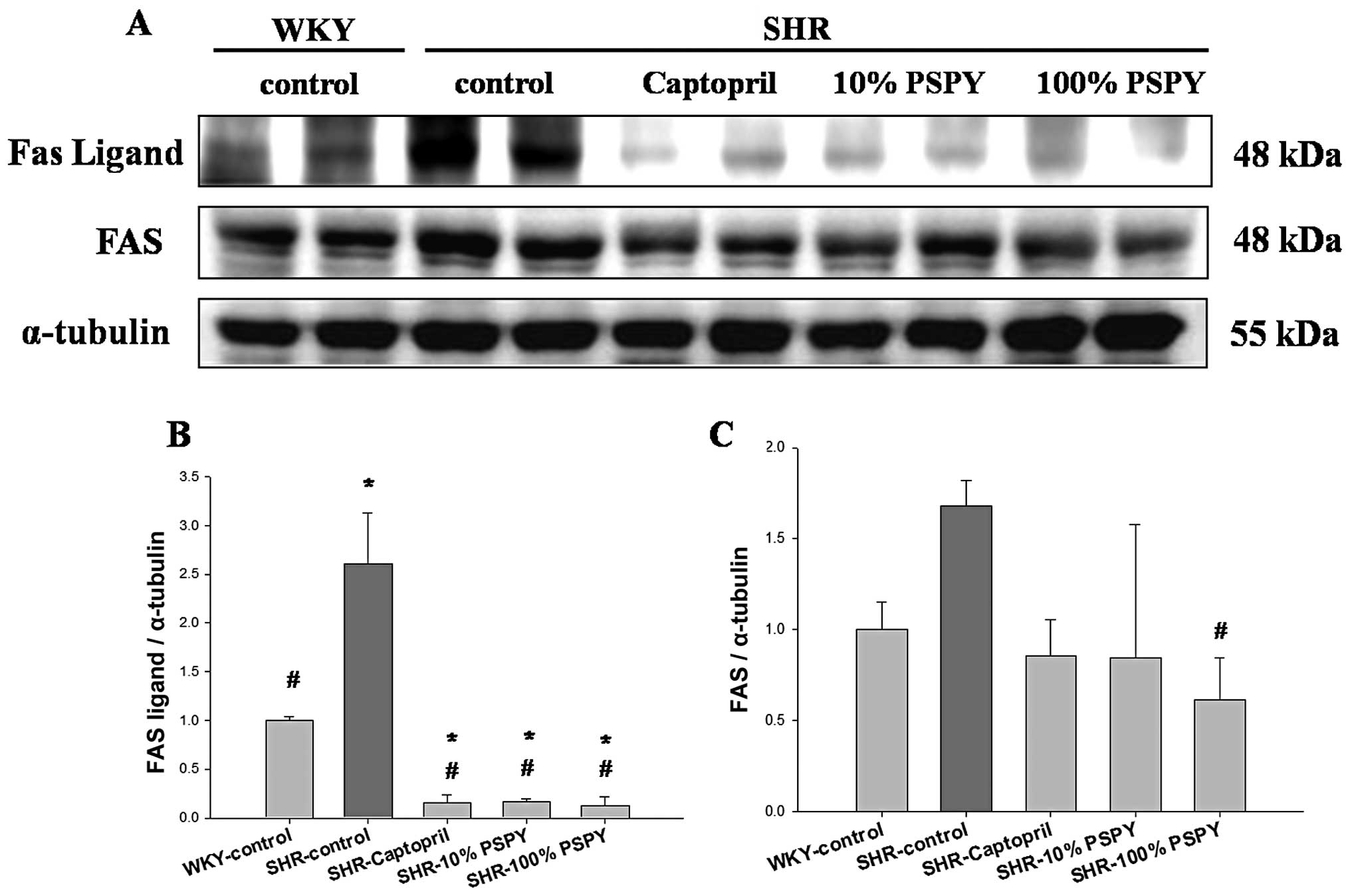
Changes in the levels of Fas death
receptor-related components in the hearts of SHRs fed different
concentrations of PSPY
We examined the variations in the levels of Fas
death receptor-associated proteins in the hearts of SHRs
administered captopril and various concentrations of PSPY by
western blot analysis (Fig. 2).
The protein products of Fas ligands and Fas extracted from the left
ventricles of the excised hearts in the SHR-control group were
significantly increased compared with the WKY group (Fig. 2). By contrast, significantly
decreased levels of protein products of Fas ligands were detected
in the SHR hearts from the captopril, and 10 and 100% PSPY groups
compared with the SHR-control group (P<0.05) (Fig. 2B). The levels of the protein
products of Fas were significantly decreased in the SHR-100% PSPY
group, compared with the SHR-control group (P<0.05) (Fig. 2C). The levels of protein products
of Fas were slightly decreased in the SHR hearts in the captopril
and 10% PSPY groups, and these levels did not differ significantly
from those in the SHR-control group (Fig. 2C). The ratios of the protein
products of Fas ligands and Fas relative to α-tubulin were
calculated and are shown in Figs. 2B
and C.
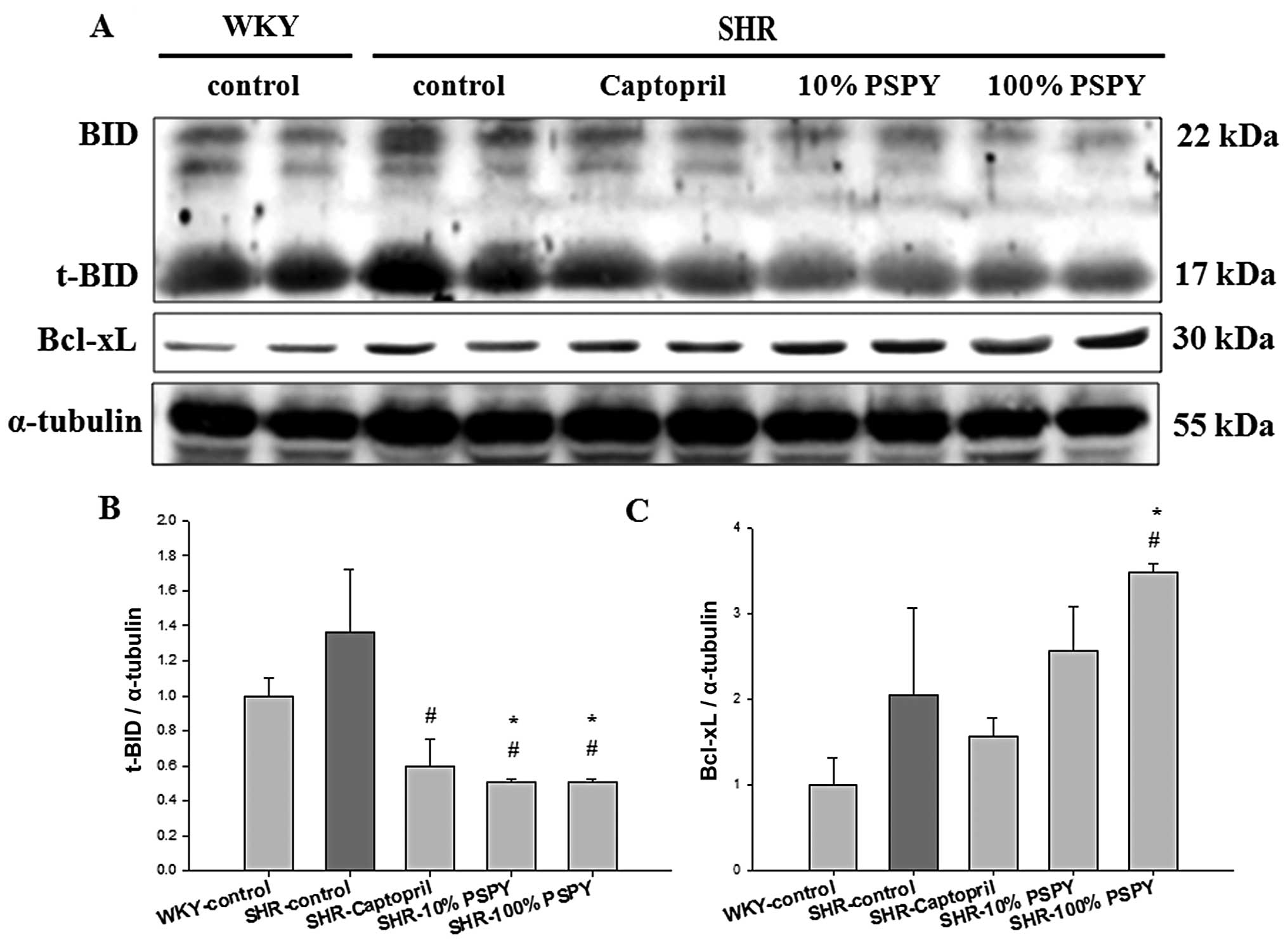
Changes in the levels of
mitochondrial-dependent apoptotic components in the hearts of SHRs
fed different concentrations of PSPY
We examined the variations in the levels of
mitochondrial-dependent apoptotic components in the cardiac tissues
of SHRs fed PSPY. The protein levels of Bcl-2 family members
(t-Bid, Bcl-xL, Bak, Bax and p-Bad) were examined by western blot
analysis (Figs. 3 and 4). Significantly decreased levels of the
protein products of t-Bid were detected in the left ventricles of
excised SHR hearts from the captopril, and 10 and 100% PSPY groups,
compared with the SHR-control group (P<0.05); however, the
SHR-control group showed no significant difference to the WKY group
(Fig. 3B). In addition, the
levels of anti-apoptotic proteins (Bcl-xL) were significantly
increased in the SHR hearts in the 100% group compared with the WKY
and SHR-control groups (Fig. 3C).
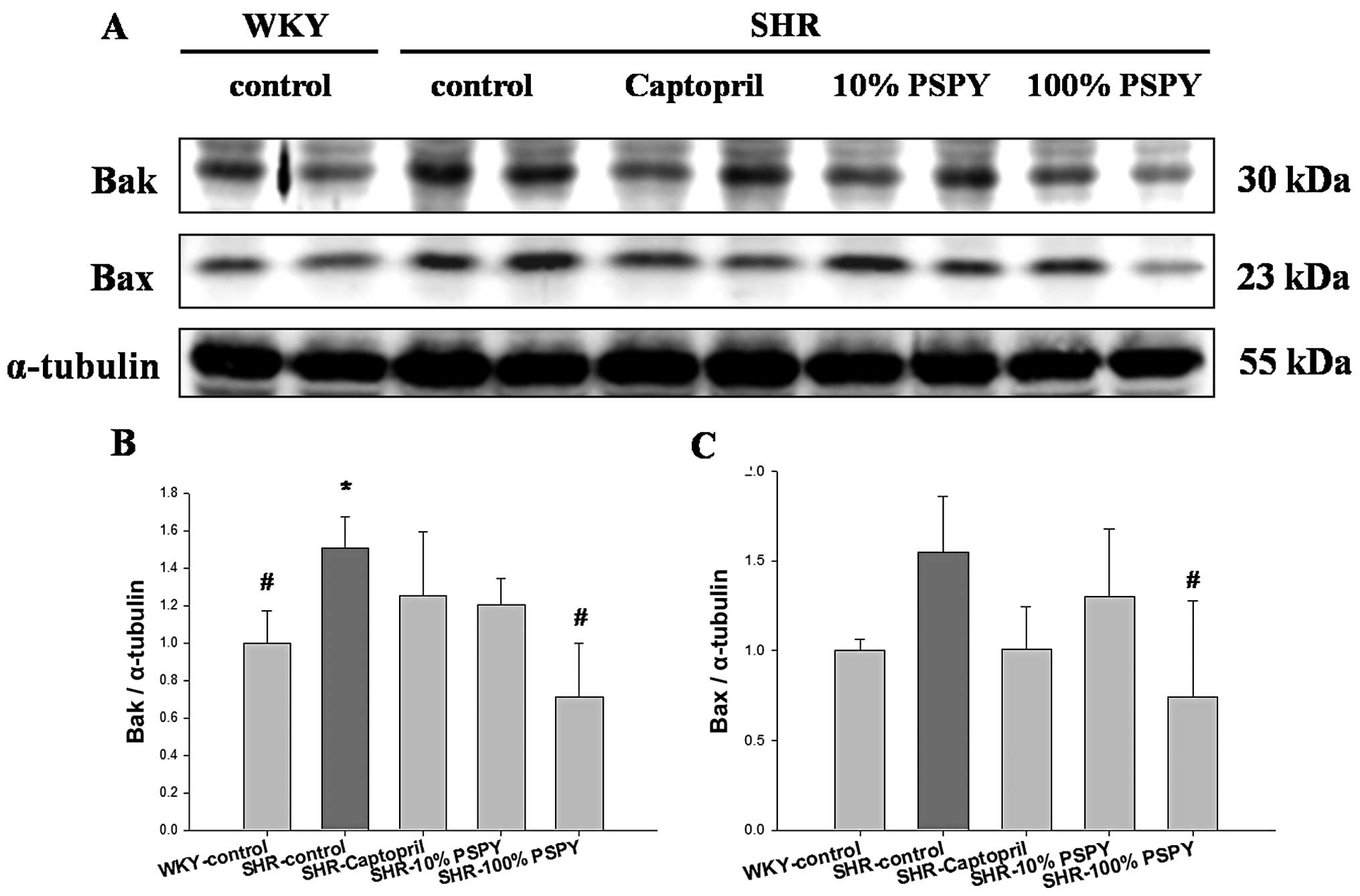
However, the levels of mitochondrial related pro-apoptotic proteins
(Bak and Bax) were significantly increased in the SHR-control
group, compared with the WKY group (P<0.05) (Fig. 4), whereas significantly decreased
levels of Bak and Bax protein were detected in the SHR hearts from
the 100% PSPY group, compared with the SHR-control group (Fig. 4B and C). Moreover, the protein
levels of activated caspase-9 did not differ significantly among
the groups (Fig. 5B). By
contrast, the levels of activated caspase-3 were significantly
increased in the SHR-control group compared with the WKY group
(P<0.05) (Fig. 5C), and were
significantly decreased in the SHR hearts from the 10 and 100% PSPY
groups compared with the SHR-control group (Fig. 5C).
Change in the levels of cardiac survival
signaling components in the hearts of SHRs fed different
concentrations of PSPY
In order to determine the effects of PSPY on cardiac
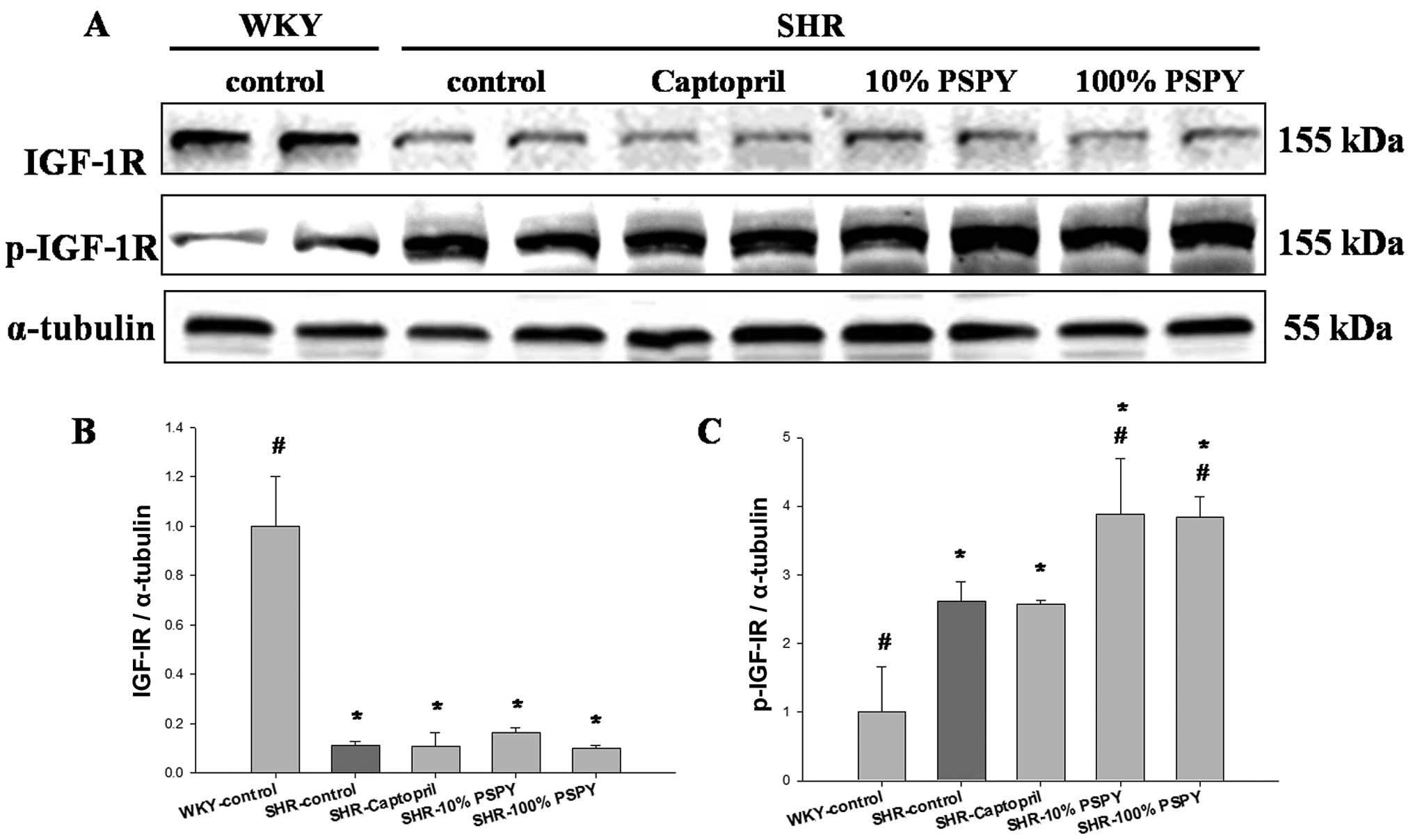
IGF-IR-dependent survival pathways, we examined the protein levels
of IGF-IR, p-IGF-IR and IGF-IR signaling components, including
PI3K, p-PI3K, AKT and p-AKT in the rat hearts. The protein levels
of IGF-IR were significantly decreased in the SHR hearts from the
SHR-control, captopril, and 10 and 100% PSPY groups compared with
the WKY group (P<0.05) (Fig.
6). However, the protein levels of p-IGF-1R were significantly
increased in the SHR hearts from the SHR-control group compared
with the WKY group. The SHR-10 and 100% PSPY groups showed even
higher levels of p-IGF-IR than the SHR-control group (P<0.05)
(Fig. 6C). Notably, we found
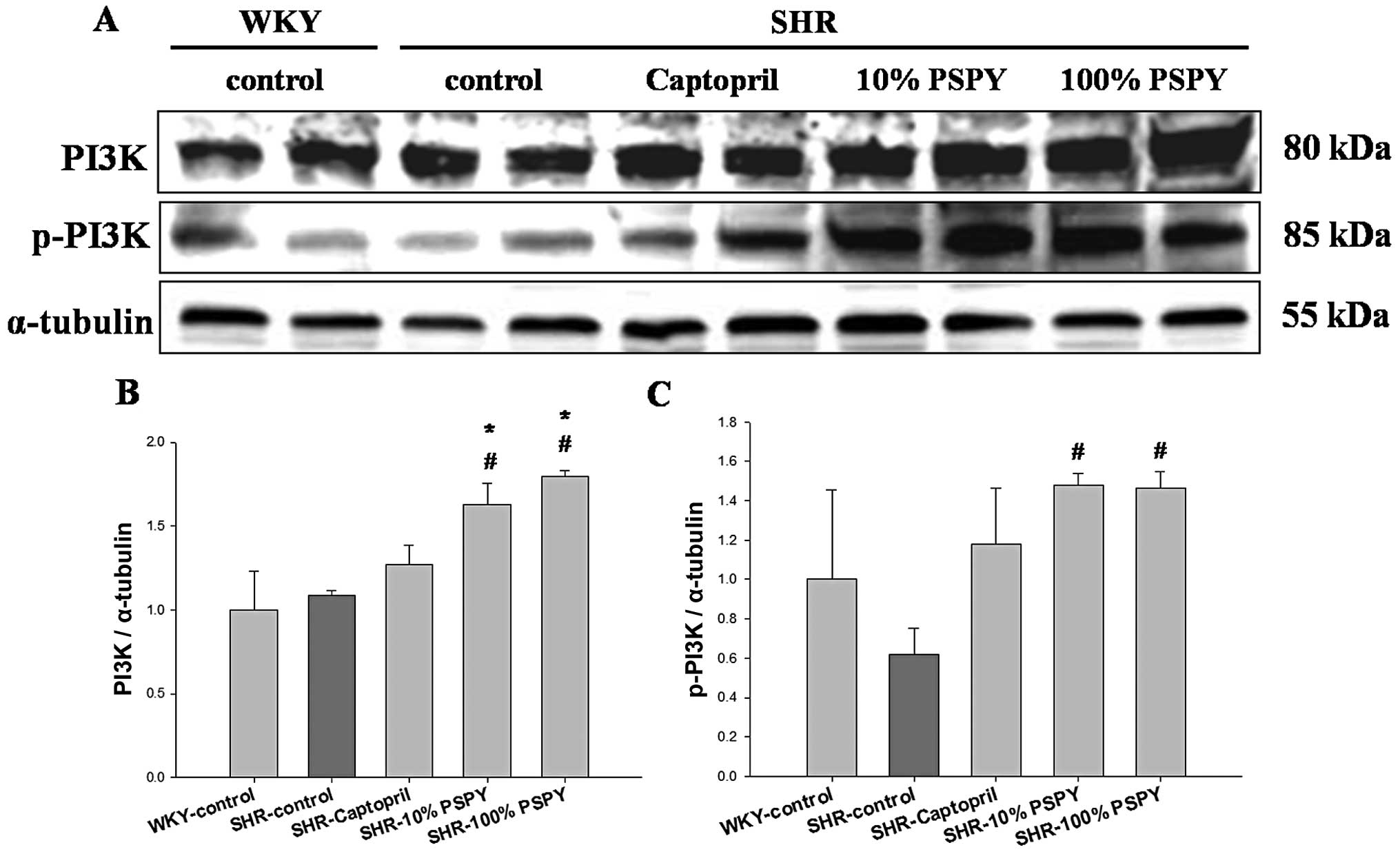
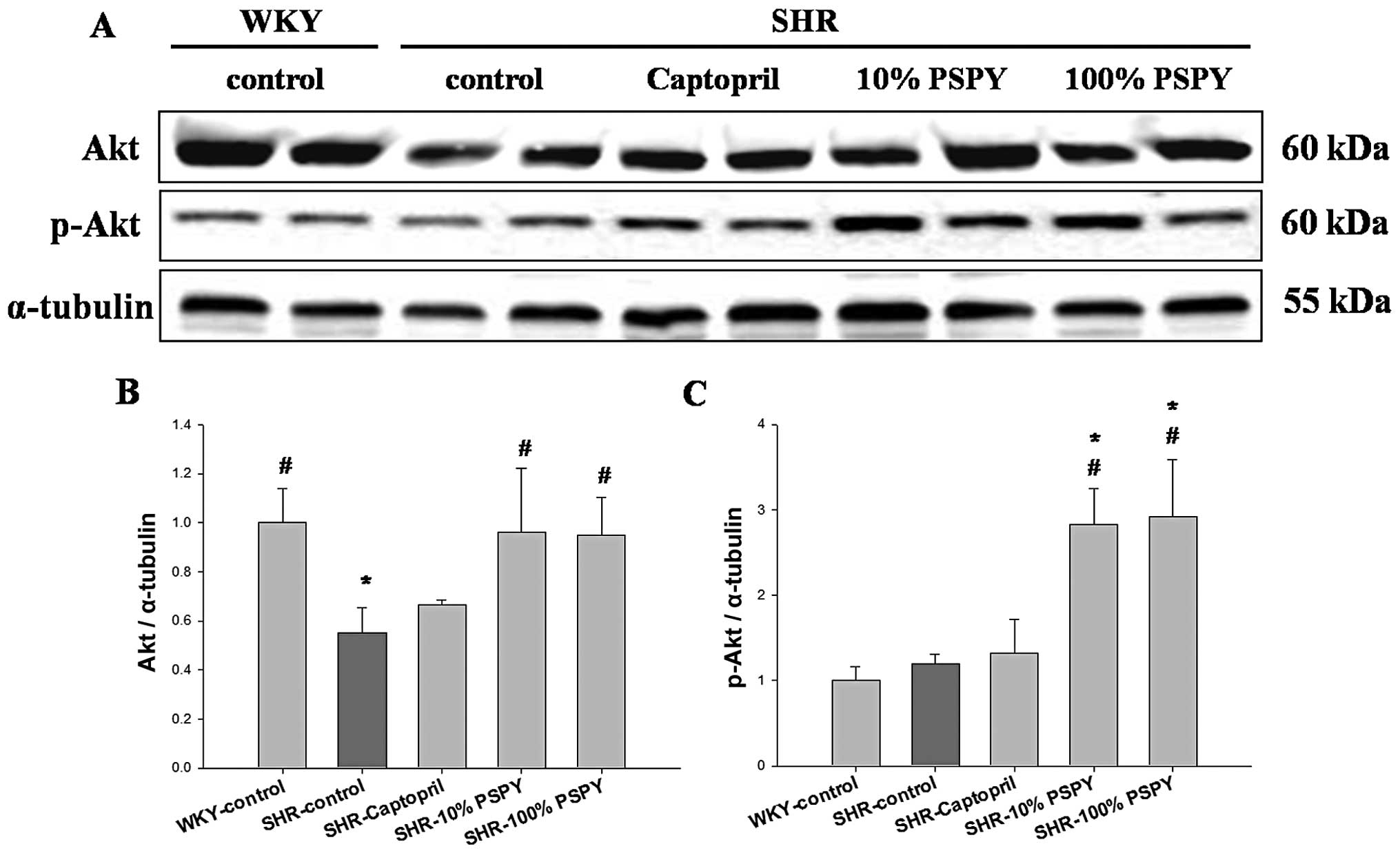
significantly increased levels of PI3K, p-PI3K, AKT and p-AKT in
the SHR hearts from the 10 and 100% PSPY groups compared with the
SHR-control group (P<0.05) (Figs.
7 and 8). By contrast, no
significant increase in the levels of cardiac PI3K, p-PI3K and
p-AKT was observed in the SHR hearts from the control and captopril
groups compared with the WKY group (Figs. 7 and 8).
Discussion
The major findings of the present study can be
summarized as follows: i) a higher number of TUNEL-positive
apoptotic cells was observed in the SHR-control group than in the
WKY group, whereas a significantly reduced number of TUNEL-positive
cells was observed in the 10 and 100% PSPY groups. ii) The levels
of Fas receptor- and mitochondrial-dependent apoptotic signaling
pathway components, such as Fas ligand, Fas, t-BID, Bak, Bax and
activated caspase-3 were significantly reduced and those of
anti-apoptotic proteins (Bcl-xL and p-Bad) were increased in the
left ventricle tissues in the SHR hearts from the 10 and 100% PSPY
groups compared with the SHR-control group. iii) A significant
decrease in the levels of IGF-IR downstream-associated components
was detected in the excised ventricle tissues from the SHR-control
group even with the increase in the compensatory activated form
(p-IGF-IR). However, the increased levels of p-IGF-IR significantly
increased IGF-IR signaling components, such as p-IGF-IR, p-PI3K and
p-AKT, as observed in the SHR hearts from rats fed 10 and 100%
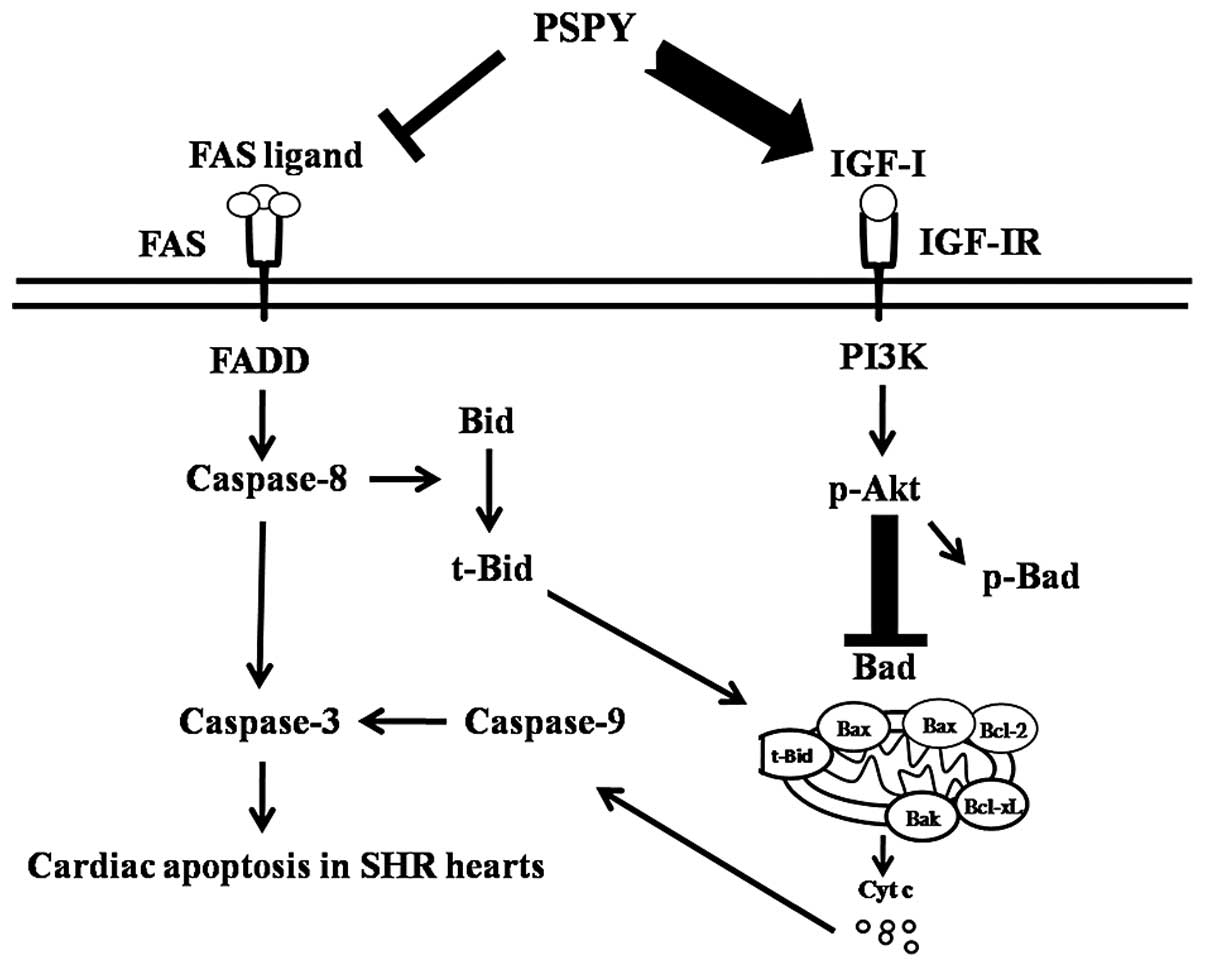
PSPY. After integrating our current findings into previously
proposed apoptotic theories, a hypothesized path diagram was
created (Fig. 9), which suggested
that cardiac Fas receptor-dependent and mitochondrial-dependent
pathways may be activated in hypertensive rats and may be
suppressed by the supplementation of PSPY. By contrast, the
compensatory IGF-IR pathway was activated, but the levels of the
downstream survival components were still decreased in the
hypertensive rats; these levels increased by the supplementation of
PSPY; PSPY completely restored the survival mechanisms. Our
findings demonstrate the novel therapeutic effects of PSPY in
hypertensive rat hearts; PSPY can be used to prevent apoptosis and
enhance survival. It seemed to be more effective than
captopril.
Chronic hypertension is a major risk factor for the
development of cardiovascular diseases (1). Hypertension induces pathological
cardiac hypertrophy secondary to the pressure overload, thereby
contributing to decreased cardiac function and increased cardiac
apoptosis (21,22). In a previous study, we
demonstrated that PSPY exerted anti-hypertrophic effects in the
hearts of SHRs by interfering with the IGF-II signaling pathway and
the IL-6-related-ERK5 pathway (23). In the present study, the results
of cardiomyocyte loss, as shown by TUNEL assay are consistent with
those of previous studies describing the gradual cardiac
decompensation in SHR hearts (24). Similar results have also been
reported in a recent study, where the authors observed that the
left ventricles of SHR hearts had more TUNEL-positive cardiac cells
than those in the WKY group (25).
The balance between cell death and survival is a
tightly controlled process, particularly in terminally
differentiated cells, such as cardiomyocytes (21). The Fas receptor-dependent
apoptotic pathway is mediated by the Fas ligand, the Fas receptor,
TNF-α, the TNF receptor, Fas-associating death domain-containing
protein (FADD) and the activation of caspase-8 (5,11).
In our findings, PSPY significantly prevented the activation of the
Fas receptor-dependent apoptotic pathways in SHR hearts, as
evidenced by the decrease in the levels of hypertension-upregulated
Fas ligand and Fas receptor after the oral administration of 10 and
100% PSPY. To our knowledge, the present study is the first to
illustrate that PSPY prevents the activation of cardiac Fas
receptor-dependent apoptotic pathways in hypertensive rats.
The mitochondrial-dependent apoptotic pathway is
tightly controlled by the Bcl-2 family. Pro-apoptotic and
anti-apoptotic members of the Bcl-2 family seem to interact with
and neutralize each other, so that the relative balance of these
effectors strongly influences cell fate (26). Shifting the balance of Bcl-2
family members toward pro-apoptotic members activates caspase-9,
which further activates caspase-3 and executes the apoptotic
program (13). In the present
study, PSPY prevented the activation of pro-apoptotic members of
the Bcl-2 family in SHR hearts, as evidenced by the decrease in the
levels of hypertension-upregulated components, t-Bid, Bak and Bax,
after the supplementation of PSPY in the 10 and 100% groups. The
supplementation of PSPY significantly increased the levels of
anti-apoptotic components due to the elevation of Bcl-xL and p-Bad
levels, and thus decreased activated caspase-3 levels in the
hypertensive rat hearts. Therefore, our results strongly suggest
that the oral administration of PSPY in SHRs may prevent the
activation of cardiac apoptotic pathways.
The cardiac survival pathway can be mediated by
IGF-I-related survival pathways, such as IGF-I, IGF-IR, p-PI3K and
p-Akt. Previous studies have indicated that increased Bcl-xL levels
in the mitochondria were observed in IGF-I pre-treated rats and
that cardiac-specific IGF-I overexpression has an anti-apoptotic
function; however, increased apoptosis followed by myocardial
infarction was observed in IGF-I-deficient mice (27,28). Consistent with previous findings,
our results demonstrated that a significant decrease in the levels
of IGF-IR pathway-associated components was detected in the
ventricles from the excised SHR hearts. By contrast, the oral
administration of 10 and 100% PSPY activated the compensatory
cardiac survival pathway in SHR hearts, evidenced by the increased
levels of p-IGF-IR, and restored the levels of PI3K, p-PI3K, Akt
and p-Akt. Similar results were also reported in a previous study,
demonstrating that the IGF-I/PI3K/Akt survival pathway was
activated in the SHR-exercise training groups compared with SHR and
WKY groups (25). These findings
suggest that PSPY attenuates cardiac apoptosis and facilitates the
activation of the compensatory IGFI/PI3K/Akt survival pathway.
The 2 concentrations (10 and 100%) of PSPY exerted a
significant effect on cardiac apoptosis and the IGF-I survival
pathway in our study, although there was no significant
concentration-response effect observed between the 10-fold
differences in PSPY concentrations. We speculate that the component
of PSPY may contain other elements apart from GABA, whose
biological function has yet been elucidated, regardless of the
original presumption that the cardiac anti-apoptotic effects came
from the presence of GABA. Various compounds of plants with
antioxidant properties have been shown to exert a number of
therapeutic effects in animal models of hypertension, which
supports the hypothesis that reactive oxygen species (ROS) are
involved in the progression of hypertension (29). In a previous study, we
demonstrated that the fermentation procedure could further elevate
anthocyanin levels and antioxidative activity significantly in PSPY
with multi-strain probiotics (20). Additionally, the supplementation
of probiotics may influence host intestinal microflora and
subsequently improve cardiovascular functions. Lam et al
(30) demonstrated that the oral
administration of the commercially available probiotic juice (L.
plantarum 299v) reduced myocardial infarct size in Dahl S rats.
Sobol et al (31)
considered that LAB and their metabolic products in particular may
positively affect the calcium signal in cells in the cardiovascular
system, which may result in increased contractile activity of blood
vessels and cardiac cells. Based on previous findings, we
hypothesized that anthocyanidin-enriched purple potato and the
probiotics in PSPY may also be the components that potentially
contribute to the activation of the PI3K/AKT survival pathway and
the attenuation of cardiac apoptosis, and account for the
insignificant difference between the 2 PSPY dosages.
In conclusion, hypertension is considered a major
risk factor for the development of heart failure. Our current
findings indicate that impaired cardiac IGF-I/PI3K/Akt survival and
Bcl-2 family anti-apoptotic pathways in hypertensive rats may
provide an important mechanism to explain the development of
hypertensive heart diseases. Additionally, the supplementation of
PSPY in the diet, found to be beneficial by enhancing cardiac
survival and activating anti-apoptotic pathways in hypertensive
hearts, may be considered as a potential novel therapeutic strategy
to prevent the development of apoptosis-related cardiac diseases in
hypertension. Further clinical experiments are required to clarify
the survival and apoptotic mechanisms responsible for the
beneficial effects of PSPY in human hypertensive hearts.
Acknowledgements
This study was supported in part by grants form the
Taiwan Department of Health Clinical Trial and Research Center of
Excellence (DOH102-TD-B-111-004), the Taiwan Department of Health
Cancer Research Center of Excellence (DOH102-TD-C-111-005) and the
National Science Council, Taiwan, R.O.C.
(NSC97-2313-B-241-004-MY3).
References
|
1
|
Tocci G, Sciarretta S and Volpe M:
Development of heart failure in recent hypertension trials. J
Hypertens. 26:1477–1486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Morillas P, de Andrade H, Castillo J,
Quiles J, Bertomeu-González V, Cordero A, Tarazón E, Roselló E,
Portolés M, Rivera M and Bertomeu-Martínez V: Inflammation and
apoptosis in hypertension. Relevance of the extent of target organ
damage. Rev Esp Cardiol. 65:819–825. 2012.PubMed/NCBI
|
|
3
|
Lee SD, Chu CH, Huang EJ, Lu MC, Liu JY,
Liu CJ, Hsu HH, Lin JA, Kuo WW and Huang CY: Roles of insulin-like
growth factor II in cardiomyoblast apoptosis and in hypertensive
rat heart with abdominal aorta ligation. Am J Physiol Endocrinol
Metab. 291:E306–E314. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuo WW, Hsu TC, Chain MH, Lai CH, Wang WH,
Tsai FJ, Tsai CH, Wu CH, Huang CY and Tzang BS: Attenuated cardiac
mitochondrial-dependent apoptotic effects by li-fu formula in
hamsters fed with a hypercholesterol diet. Evid Based Complement
Alternat Med. 2011:5303452011.PubMed/NCBI
|
|
5
|
Haunstetter A and Izumo S: Apoptosis:
basic mechanisms and implications for cardiovascular disease. Circ
Res. 82:1111–1129. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Narula J, Haider N, Arbustini E and
Chandrashekhar Y: Mechanisms of disease: apoptosis in heart failure
- seeing hope in death. Nat Clin Pract Cardiovasc Med. 3:681–688.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harrison DG, Cai H, Landmesser U and
Griendling KK: Interactions of angiotensin II with NAD(P)H oxidase,
oxidant stress and cardiovascular disease. J Renin Angiotensin
Aldosterone Syst. 4:51–61. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Filippatos G, Tilak M, Pinillos H and Uhal
BD: Regulation of apoptosis by angiotensin II in the heart and
lungs (Review). Int J Mol Med. 7:273–280. 2001.PubMed/NCBI
|
|
9
|
Fujio Y, Nguyen T, Wencker D, Kitsis RN
and Walsh K: Akt promotes survival of cardiomyocytes in vitro and
protects against ischemia-reperfusion injury in mouse heart.
Circulation. 101:660–667. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Athanasiou A, Clarke AB, Turner AE,
Kumaran NM, Vakilpour S, Smith PA, Bagiokou D, Bradshaw TD,
Westwell AD, Fang L, Lobo DN, Constantinescu CS, Calabrese V,
Loesch A, Alexander SP, Clothier RH, Kendall DA and Bates TE:
Cannabinoid receptor agonists are mitochondrial inhibitors: a
unified hypothesis of how cannabinoids modulate mitochondrial
function and induce cell death. Biochem Biophys Res Commun.
364:131–137. 2007. View Article : Google Scholar
|
|
11
|
Bishopric NH, Andreka P, Slepak T and
Webster KA: Molecular mechanisms of apoptosis in the cardiac
myocyte. Curr Opin Pharmacol. 1:141–150. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee SD, Tzang BS, Kuo WW, Lin YM, Yang AL,
Chen SH, Tsai FJ, Wu FL, Lu MC and Huang CY: Cardiac fas
receptor-dependent apoptotic pathway in obese Zucker rats. Obesity
(Silver Spring). 15:2407–2415. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brown GC and Borutaite V: Nitric oxide,
cytochrome c and mitochondria. Nitric oxide, cytochrome c and
mitochondria. Biochem Soc Symp. 66:17–25. 1999.
|
|
14
|
Ren J, Samson WK and Sowers JR:
Insulin-like growth factor I as a cardiac hormone: physiological
and pathophysiological implications in heart disease. J Mol Cell
Cardiol. 31:2049–2061. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vincent AM and Feldman EL: Control of cell
survival by IGF signaling pathways. Growth Horm IGF Res.
12:193–197. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Simoncini T, Hafezi-Moghadam A, Brazil DP,
Ley K, Chin WW and Liao JK: Interaction of oestrogen receptor with
the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature.
407:538–541. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu CF, Tung YT, Wu CL, Lee BH, Hsu WH and
Pan TM: Antihypertensive effects of Lactobacillus-fermented milk
orally administered to spontaneously hypertensive rats. J Agric
Food Chem. 59:4537–4543. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Inoue K, Shirai T, Ochiai H, Kasao M,
Hayakawa K, Kimura M and Sansawa H: Blood-pressure-lowering effect
of a novel fermented milk containing gamma-aminobutyric acid (GABA)
in mild hypertensives. Eur J Clin Nutr. 57:490–495. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Komatsuzaki N, Nakamura T, Kimura T and
Shima J: Characterization of glutamate decarboxylase from a high
gamma-aminobutyric acid (GABA)-producer, Lactobacillus
paracasei. Biosci Biotechnol Biochem. 72:278–285. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu TY, Tsai CC, Hwang YT and Chiu TH:
Effect of antioxidant activity and functional properties of
chingshey purple sweet potato fermented milk by Lactobacillus
acidophilus, L. delbrueckii subsp lactis, and L.
gasseri strains. J Food Sci. 77:M2–M8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fortuno MA, Ravassa S, Fortuno A, Zalba G
and Diez J: Cardiomyocyte apoptotic cell death in arterial
hypertension: mechanisms and potential management. Hypertension.
38:1406–1412. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Richey PA and Brown SP: Pathological
versus physiological left ventricular hypertrophy: a review. J
Sports Sci. 16:129–141. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin PP, Hsieh YM, Kuo WW, Lin CC, Tsai FJ,
Tsai CH, Huang CY and Tsai CC: Inhibition of cardiac hypertrophy by
probiotic-fermented purple sweet potato yogurt in spontaneously
hypertensive rat hearts. Int J Mol Med. 30:1365–1375.
2012.PubMed/NCBI
|
|
24
|
Díez J, Fortuño MA and Ravassa S:
Apoptosis in hypertensive heart disease. Curr Opin Cardiol.
13:317–325. 1998.
|
|
25
|
Huang CY, Yang AL, Lin YM, Wu FN, Lin JA,
Chan YS, Tsai FJ, Tsai CH, Kuo CH and Lee SD: Anti-apoptotic and
pro-survival effects of exercise training on hypertensive hearts. J
Appl Physiol. 112:883–891. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
McGowan BS, Ciccimaro EF, Chan TO and
Feldman AM: The balance between pro-apoptotic and anti-apoptotic
pathways in the failing myocardium. Cardiovasc Toxicol. 3:191–206.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Torella D, Rota M, Nurzynska D, Musso E,
Monsen A, Shiraishi I, Zias E, Walsh K, Rosenzweig A, Sussman MA,
Urbanek K, Nadal-Ginard B, Kajstura J, Anversa P and Leri A:
Cardiac stem cell and myocyte aging, heart failure, and
insulin-like growth factor-1 overexpression. Circ Res. 94:514–524.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Palmen M, Daemen MJ, Bronsaer R, Dassen
WR, Zandbergen HR, Kockx M, Smits JF, van der Zee R and Doevendans
PA: Cardiac remodeling after myocardial infarction is impaired in
IGF-1 deficient mice. Circ Res. 50:516–524. 2001.PubMed/NCBI
|
|
29
|
Wong CM, Bansal G, Pavlickova L, Marcocci
L and Suzuki YJ: Reactive oxygen species and antioxidants in
pulmonary hypertension. Antioxid Redox Signal. 18:1789–1796. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lam V, Su J, Koprowski S, Hsu A, Tweddell
JS, Rafiee P, Gross GJ, Salzman NH and Baker JE: Intestinal
microbiota determine severity of myocardial infarction in rats.
FASEB J. 26:1727–1735. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sobol KV, Belostotskaya GB and Nesterov
VP: The effect of probiotics and their metabolic products on
cardiovascular system cells in vitro. Dokl Biol Sci. 436:9–12.
2011. View Article : Google Scholar : PubMed/NCBI
|