Introduction
Autoimmune diseases comprise a variety of different
malignancies, including at least two distinct forms of inflammatory
bowel disease (IBD), namely Crohn’s disease (CD) and ulcerative
colitis (UC) (1). In North
America, UC has a prevalence of 150–300 cases per 100,000
inhabitants, while the incidence is between 2.5 to 20 cases per
100,000 inhabitants each year (2). Even though environmental factors in
combination with genetic predisposition trigger the disease
(3), the detailed
pathophysiological mechanisms remain unclear.
The levels of interleukin (IL)-6 in sera positively
correlate with disease severity in IBD (4). IL-6 is mainly secreted from lamina
propria mononuclear cells and T cells in patients with CD (5,6).
As previously demonstrated, in an acquired immunity-dependent T
cell transfer colitis mouse model, IL-6 receptor (IL-6R) monoclonal
antibodies (mAbs) prevented the development of colitis (7). The neutralizing IL-6R mAb,
tocilizumab, represents a promising drug for CD (8). Importantly, IL-6 trans-signaling via
IL-6/soluble IL-6 receptor (sIL-6R) complexes, but not classic
signaling via IL-6/membrane bound IL-6 receptor (IL-6R) complexes
has been shown to prevent the apoptosis of T cells and promote
tissue damage (9). IL-6
trans-signaling also promotes the development of spontaneous
ileitis in SAMP1/Yit mice (10).
On the other hand, IL-6 stimulates the survival and proliferation
of intestinal epithelial cells. The abrogation of regenerative
pathways in the intestine may explain why Il-6-deficient
mice present with a widespread damage of the colonic mucosa in a
non-T cell-dependent innate immunity-dependent azoxymethane
(AOM)-dextran sodium sulfate (DSS)-induced colitis model (11), which is accompanied with decreased
tumor incidence (11). However,
an earlier study reported reduced colitis and mortality after DSS
administration in Il-6-deficient mice compared to wild-type
(wt) mice (12).
Il-6r-deficient mice are expected to exhibit
a similar phenotype as Il-6-deficient mice, since IL-6
solely signals via the IL-6R and glycoprotein 130 (gp130). This
view has been challenged with the identification of ciliary
neurotrophic factor (CNTF) as an additional low-affinity ligand of
the IL-6R/gp130/LIFR complex (13), and p28 (IL-30) as a ligand of the
IL-6R/gp130 complex (14,15). It is interesting to note that, in
contrast to Il-6-deficient mice, Il-6r-deficient mice
do not exhibit a delay in skin wound closure (16), pointing to additional roles of
IL-6 or the IL-6R apart from the IL-6/(s)IL-6R classic and
trans-signaling.
In the present study, we analyzed IL-6 mAb-treated
and Il-6r-deficient mice in a model of DSS-induced colitis.
DSS causes the destruction of intestinal epithelial cells
accompanied by subsequent intestinal inflammation and regeneration
(17,18).
In agreement with a previous report (12) using Il-6r-deficient mice,
our results demonstrated that neutralizing IL-6 mAbs slightly
improved DSS-induced colitis. Il-6r-deficient mice and mice
with tissue-specific deletion of the Il-6r in the myeloid
cell lineage (LysMCre) with acute and chronic DSS-induced colitis
were, however, indistinguishable from wt mice.
Materials and methods
Ethics statement
All mouse experiments were performed according to
the requirements of Landesamt für Natur, Umwelt und
Verbraucherschutz Nordrhein-Westfalen (LANUV NRW) with the
following approval number: 84-02.04.2011.A146.
Animals
Specific pathogen-free
Il-6rfl/fl and Cre-recombinase-expressing
mice, as well as their offspring were obtained from the animal
facility of the University of Düsseldorf (Düsseldorf, Germany). All
mice had a C57BL/6 background. The mice were fed a standard
laboratory diet and were provided with autoclaved tap water ad
libitum. They were kept in an air-conditioned room with a
controlled temperature (20–24°C), humidity (45–65%) and day/night
cycle (12 h light, 12 h dark). The mice were allowed to acclimatize
for 1 week prior to entering the study. Each group in our model of
DSS-induced colitis contained 4–16 animals. The animals were
monitored daily for changes in behaviour and body weight loss.
Animals presenting severe signs of suffering (weight loss of
>20%, prostration and tremors) were euthanized.
DSS-induced colitis
Acute DSS colitis was induced by the administration
of 1.5–3% DSS in the drinking water for 5 days followed by 5 days
of autoclaved tap water. The DSS-containing water was changed on
day 3. On day 10, the mice were anesthetized using xylazin (10
mg/kg body weight) and ketamin (100 mg/kg body weight). The mice
were coloscopied (endoscopy system AIDA control; Storz, Tuttlingen,
Germany) and images of the colon were acquired. Subsequently, the
mice were dissected (ventral), blood samples were acquired by
cardiac puncture and the colon was prepared in one section, washed
in PBS and continued to be processed as described in the sections
‘Immunohistochemistry’ and ‘Colon organ culture’.
Determination of clinical score
The clinical score was assessed without taking the
genotype of the mice into consideration and determined taking into
account the weight loss compared to day 0, stool consistency and
blood in the stool, as previously described (19). No weight loss or up to 5% weight
loss was registered as 0 points, weight loss of >5% up to 10% as
1 point, >10% up to 15% 2 points, >15% up to 20% 3 points,
and >20% 4 points. For stool consistency, 0 points were assigned
for well-formed pellets, 1 point for pasty and semi-formed stools
and 2 points for liquid stools. To assess bleeding, 0 points were
assigned for no blood by using the haemoccult test (HemoCare, Care
Diagnostica Laborreagenzien GmbH, Voerde, Germany), 1 point for a
positive haemoccult test and 2 points for visible bleeding.
Administration of neutralizing IL-6
mAbs
Male Il-6rfl/fl mice were
intraperitoneally injected with 250 μg rat IL-6 mAbs (MP5-20F3) 1 h
prior to replacing the tap-water with 1.5% DSS-containing
autoclaved tap water (day 0). The injection was repeated on days 2,
4, 6 and 8.
Generation and genetic analysis of
transgenic mice
The Il-6rfl/fl mice were
crossed with mice expressing Cre-recombinase under the control of
the lysozyme M (LysM) promoter (20) or a human cytomegalovirus (CMV)
minimal promoter, as previously described (21). Progeny
LysMCre+/−/Il-6rfl/+ mice were
further bred with
LysMCre−/−/Il-6rfl/fl mice. The
resulting offspring was kept, breeding
LysMCre+/−/Il-6rfl/fl with
LysMCre−/−/Il-6rfl/fl mice. The
littermates were used for the experiments. Human
CMVCre+/−/Il-6r−/+
mice were bred with Il-6rfl/fl mice. From
the resulting offspring, the Il-6rfl/fl or
Il-6r−/− mice were
used for the experiments.
DNA from the tail clippings was isolated using the
DirectPCR-Tail kit with proteinase K (Peqlab Biotechnologie GmbH,
Erlangen, Germany) following the instructions of the
manufacturer.
Primers for the wild-type Il-6r allele
(5′-GGTCACGGG CACTCCTTGGATAGGTACC-3′ and 5′-CCCAGTGAGCT
CCACCATCAAA-3′), the floxed Il-6r allele (5′-GGTCACGG
GCACTCCTTGGATAGGTACC-3′ and 5′-CCCAGTGAGC TCCACCATCAAA-3′), the
excised Il-6r allele (5′-GGGTAG GCCCTGCTACCATGAAG-3′ and
5′-CCCAGTGAGCTCC ACCATCAAA-3′) and human CMVCre
(5′-ACGACCAAGT GACAGCAATG-3′ and 5′-TCGACCAGTTTAGTTACCC-3′) were
used in PCR analysis. LysMCre mice were genotyped as
described in the JAX mouse database (The Jackson Laboratory, Bar
Harbor, ME, USA).
Preparation of cells
Spleen and femoral bones were isolated from the
LysMCre+/−/Il-6rfl/fl and
Il-6rfl/fl mice. Spleen and bone marrow
cells were passed through a nylon mesh into a 50-ml tube containing
10 ml PBS. The cell suspension was centrifuged at 290 × g for 5 min
at room temperature. The pellet was resuspended with erythrocyte
lysing buffer (150 mM NH4Cl, 10 mM KHCO3 and
100 nM EDTA), incubated for 5 min at room temperature and
centrifuged again at 290 × g for 5 min. These steps were repeated
until the supernatant lost its red colour completely. The pellet
was finally resuspended in 100 μl flow cytometry buffer [1% (w/v)
BSA in PBS].
Flow cytometry
To analyze the cell-surface expression of IL-6R,
cells (prepared as described in ‘Preparation of cells’) were
incubated in 100 μl flow cytometry buffer containing 0.5 μl
CD16/CD32 mAbs (Mouse BD Fc Block, 2.4G2; BD Pharmigen, San Jose,
CA, USA) for 5 min on ice. Diluted IL-6R-PE mAbs (1 μl) (D7715A7;
BioLegend, San Diego, CA, USA) and 3 μl diluted CD11b-APC mAbs
(M1/70; BD Pharmigen) were added and the samples were incubated on
ice for a further 30 min in the dark. After a single washing step
in the flow cytometry buffer, the cells were resuspended in the
flow cytometry buffer and were analyzed by flow cytometry
(FACSCanto II and FACSDiva software; BD Biosciences, Heidelberg,
Germany).
Chronic colitis
Chronic colitis was induced in the
LysMCre+/−/Il-6rfl/fl mice and
their littermate controls. Colitis was induced as described above.
DSS (1.5%) was administered in the drinking water for 5 days,
followed by 7 days of autoclaved tap water. After the first cycle,
1.0% DSS was used for the second cycle and performed like the
first; for the third cycle, we used 1.25% DSS and for the final
cycle 1.5% DSS. On days 47 and 48, the mice were anesthetized using
xylazin (10 mg/kg body weight) and ketamin (100 mg/kg body weight).
The subsequent procedure was performed as described in ‘DSS-induced
colitis’.
Serum preparation
Serum was generated by allowing blood to clot at
room temperature for 30 min and subsequent centrifugation at 2500 ×
g for 40 min.
FITC-dextran measurement
Intestinal permeability was assessed by the
administration of non-metabolizable FITC-dextran 4000 (TdB
Consultancy, Uppsala, Sweden) (0.6 g/kg body weight) by gavage 4 h
before sacrifice. Whole blood was obtained by cardiac puncture.
Dilutions of FITC-dextran 4000 in the DMEM high-glucose culture
medium (Life Technologies-Gibco, Darmstadt, Germany) were used as a
standard curve and absorption of 50 μl serum diluted in DMEM
high-glucose culture medium was measured in duplicate on the Tecan
Infinite 200 Pro fluorometer (excitation wavelength, 488 nm;
emission wavelength, 519 nm; software Tecan i-control; Tecan
Deutschland GmbH, Crailsheim, Germany). The concentration of
FITC-dextran 4000 was determined and standard errors were
calculated.
Colon organ culture
A segment of the distal colon was removed, cut
longitudinally and washed in PBS. A segment of approximately 0.5
cm2 was incubated in a 24-well plate in DMEM
high-glucose culture medium supplemented with penicillin (60 mg/l;
Life Technologies-Gibco) and streptomycin (100 mg/l; Gibco) at 37°C
with 5% CO2 in a water-saturated atmosphere for 24 h.
The cells were removed from the medium and were centrifuged at
20,000 × g for 2 min. The supernatants were stored at −20°C before
proceeding with ELISA procedure for quantification.
ELISA quantification of IL-6 and
IL-6R
ELISA for IL-6 (mouse interleukin-6 DuoSet; R&D
Systems, Minneapolis, MN, USA) and IL-6R (mouse interleukin-6 sR
DuoSet; R&D Systems) was performed following the manufacturer’s
instructions. The peroxidase reaction was terminated by the
addition of 50 μl 1.8 N H2SO4. The absorbance
was determined using the Tecan Infinite 200 Pro fluorometer
(absorption wavelength, 450 nm; software Tecan i-control).
Immunohistochemistry
The remaining stool was removed from the colon by
flushing with PBS. The colon was opened longitudinally, washed with
PBS, cut in the middle longitudinally and one part was formed as a
swiss-roll, as previously described (22). The colonic swiss-roll was
incubated overnight in 4% (w/v) paraformaldehyde (PFA) in PBS. The
liquid was replaced with pure PBS and the sections were incubated
for another night. Tissues were dehydrated using
Tissue-Tek® VIP™ 5Jr (Sakura Finetek Germany GmbH,
Staufen, Germany) in an ascending ethanol series followed by
UltraClear (J.T. Baker Inc., Phillipsburg, NJ, USA) and paraffin.
The samples were embedded in paraffin and dissected into 3-μm-thick
slices using a microtome (Leica RM 2135). The sections were mounted
on microscope slides, deparaffinized and rehydrated using a
descending xylene and ethanol series. Tissue sections were stained
using Mayer’s hematoxylin solution (Merck, Darmstadt, Germany) and
differentiated in pure ethanol containing 2 g HCl per 100 ml. The
tissue sections were washed and further stained using a 1.5%
aqueous eosin Y solution (Formafix Global Technologies,
Schleswig-Holstein, Germany). The stained sections were dehydrated
using an ascending ethanol and xylene series, embedded with a cover
slip and subsequently analyzed under a microscope (fluorescence
lifetime imaging microscope BZ-9000; Keyence Corp., Osaka,
Japan).
RNA and cDNA
RNA was extracted from the tissues using the PeqGOLD
Total RNA kit S-Line (Peqlab Biotechnologie GMBH, Erlangen,
Germany) following the manufacturer’s instructions. RNA (5 μg) was
used for cDNA synthesis using RevertAid reverse transcriptase
(Thermo Fisher Scientific, Waltham, MA, USA) and oligo(dT)
primers.
qPCR
Using SYBR-Green PCR Master Mix (Applied Biosystems,
Carlsbad, CA, USA), the qPCR reaction was performed in triplicate
using 25 ng of the cDNA as a template. The fluorescence detection
and measurements were taken using the Applied Biosystems thermal
cycler. The relative expression levels of transforming growth
factor (Tgf)-β, Il-1, tumor necrosis factor
(Tnf)-α, Kc and Vα14 for the DSS-challenged
LysMCre+/−/Il-6rfl/fl mice and
their littermate controls, as well as for the unchallenged
LysMCre+/−/Il-6rfl/fl mice, was
calculated after normalization with their unchallenged littermate
controls. Primers were used as previously described (23). Gapdh was used as a
housekeeping control. The resulting values were then averaged and
plotted as a bar plot. Standard errors are presented in Table I.
 | Table IDifferences in the expression levels
of relevant markers between the
LysMCre+/−/Il-6rfl/fl mice and
their littermate controls. |
Table I
Differences in the expression levels
of relevant markers between the
LysMCre+/−/Il-6rfl/fl mice and
their littermate controls.
|
LysM−/−/Il-6rfl/fl
control |
LysM+/−/Il-6rfl/fl
control |
LysM−/−/Il-6rfl/fl
DSS |
LysM+/−/Il-6rfl/fl
DSS |
|---|
| Tgf-β | 1±0.40 | 0.78±0.25 | 3.40±0.97 | 2.73±1.65 |
| Il-1 | 1±0.84 | 1.67±0.34 | 15.74±14.18 | 12.80±10.11 |
| Tnf-α | 1±1.14 | 2.51±0.43 | 2.02±0.73 | 2.42±1.52 |
| Kc | 1±0.46 | 1.25±0.27 | 0.77±0.17 | 1.73±0.78 |
| Vα14 | 1±0.43 | 3.22±1.47 | 1.32±0.66 | 1.74±0.35 |
Statistical analysis
Data are expressed as the mean values ± standard
deviation. Statistical analysis was performed using a Student’s
t-test test and a p-value <0.05 was considered statistically
significant.
Results
Neutralizing IL-6 mAbs slightly, although
significantly attenuate DSS-induced colitis
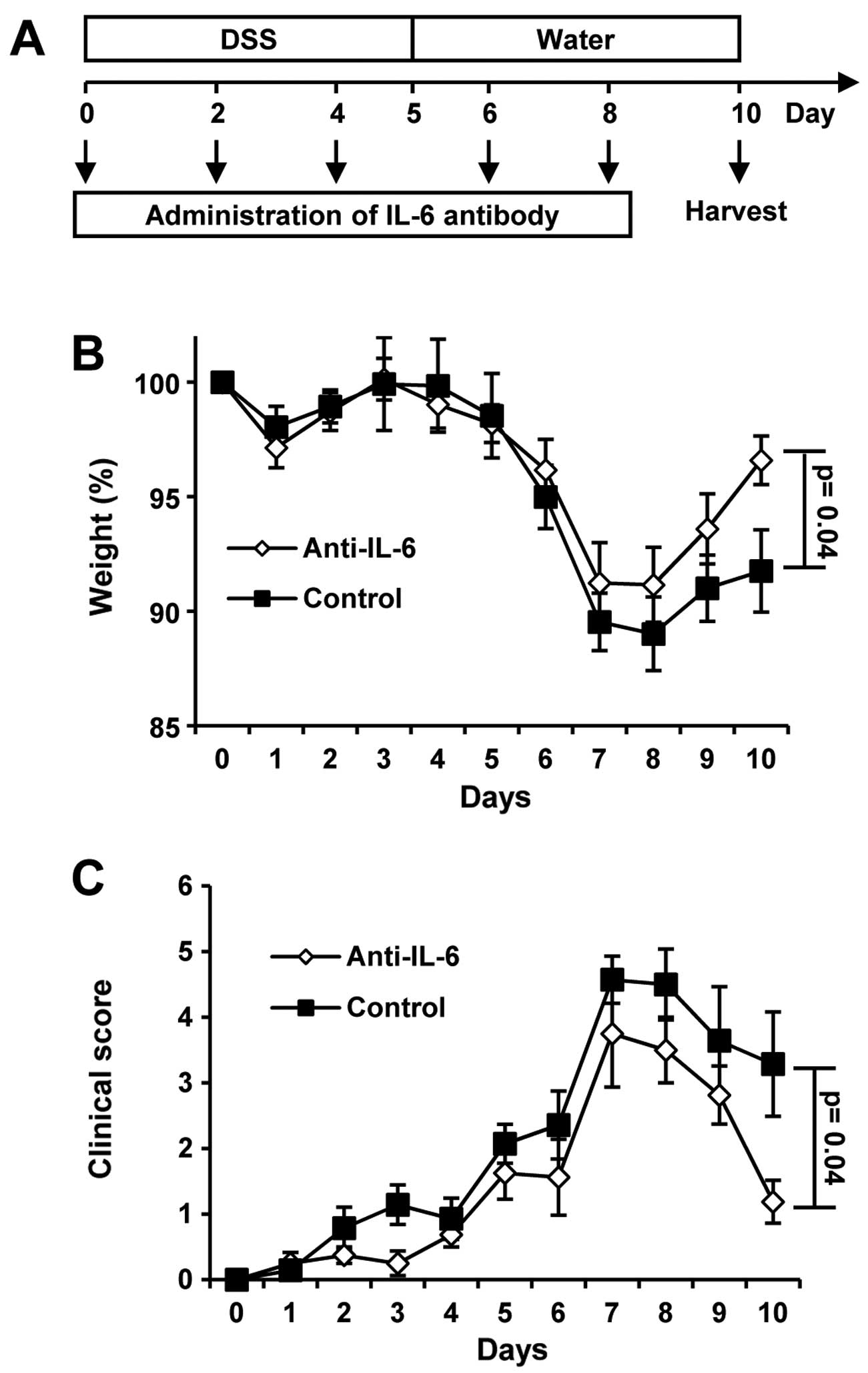
For DSS-induced colitis, male mice were administered
with 1.5% DSS in their drinking water, followed by 5 days of
drinking water without DSS. One group received neutralizing IL-6
mAbs every other day (Fig.
1A).
We observed no weight loss in the mice until days 4
to 5. Maximal weight loss was monitored between days 6 to 7. After
day 8, the mice began to gain weight during the recovery phase.
Significant differences between the 2 groups were apparent only on
day 10 (Fig. 1B). Statistically
significant differences in the clinical scores between the 2 groups
were also only detected on day 10 (Fig. 1C). Of note, the neutralization of
IL-6 by mAbs promoted weight gain and improved the clinical score
on day 10 as compared to the untreated wt mice. The body weight on
day 10 was 92% for the control mice and 97% for the IL-6
mAb-treated mice. The clinical score was 3 for the control group
and 1 for the IL-6 mAb-treated group. In agreement with the results
presented in the study by Naito et al (12), our results revealed that IL-6
plays a detrimental role in DSS-induced colitis.
Generation of global and tissue-specific
IL-6R-deficient mice
Conditional Il-6rfl/fl mice
have been used in previous studies (16). In this study,
Il-6rfl/fl mice were crossed with
CMVCre and LysMCre transgenic mice, as previously
described (20,21). Germline recombination of the
Il-6r allele was achieved using CMVCre. The deletion
of the Il-6r allele in the myeloid cell lineage, including
monocytes, mature macrophages and granulocytes, was accomplished
after the crossing of Il-6rfl/fl with
LysMCre mice. Germline recombination was verified by PCR
analysis of tail DNA from
Il-6r−/− mice
(Fig. 2A). Unspecific
recombination for
LysMCre+/−/Il-6rfl/fl mice was
excluded by PCR analysis of the colon and tail samples (Fig. 2B).
LysMCre+/−/Il-6rfl/fl mice have
been previously described and present with approximately 60%
reduced serum sIL-6R levels (16). In this study, our
LysMCre+/−/Il-6rfl/fl mice had
approximately 30% reduced serum levels of sIL-6R (Fig. 2C), even though our
LysMCre+/−/Il-6rfl/fl mice
showed a good recombination of the Il-6r allele. sIL-6R was
not detectable in the
Il-6r−/− mice
(Fig. 2D).
Subsequently, we analyzed the IL-6R cell surface
expression on monocytes of
LysMCre+/−/Il-6rfl/fl mice by
flow cytometry. Bone marrow cells and splenocytes were stained for
IL-6R and the monocyte marker, CD11b. Only 5% of the CD11b-positive
bone marrow cells from the
LysMCre+/−/Il-6rfl/fl mice, but
approximately 35% of their littermate controls expressed the IL-6R
(Fig. 2E). Only 20% of the
CD11b-positive splenocytes from the
LysMCre+/−/Il-6rfl/fl mice, but
approximately 72% of their littermate controls expressed the IL-6R
(Fig. 2F). Thus, it can be
concluded from these experiments that the recombination of the
IL-6R allele in the 2 transgenic mouse strains,
Il-6r−/− and
LysMCre+/−/Il-6rfl/fl, was
successful.
Il-6r−/− mice show no
difference in susceptibility to acute DSS-induced colitis compared
to Il-6rfl/fl control mice
After proving the successful recombination for the
Il-6r allele, we created a mouse model of acute DSS-induced
colitis using female Il-6rfl/fl and
Il-6r−/− mice
(Fig. 3A). There was no weight
loss in the mice until day 4. On day 5, the weight began to drop
below 100% of the weight at the beginning of the experiment. The
greatest weight loss occurred from day 5 to day 7. On day 8, the
weight curves reached their minimum in both groups and began to
rise again until the end of the experiment. No significant
difference in weight loss was observed between the
Il-6rfl/fl and
Il-6r−/− mice during
the entire experimental period (Fig.
3B). The clinical score began to markedly increase on day 4,
reaching maximum values for the
Il-6r−/− mice on day
7 and for the Il-6rfl/fl mice on day 8;
thereafter, the clinical score began to decrease until the end of
the experiment. Again, no statistically significant differences
were observed (Fig. 3C).
LysMCre+/−/Il-6rfl/fl mice show no difference
in susceptibility to acute DSS-induced colitis compared to
Il-6rfl/fl control mice
We then analyzed the tissue-specific knockout of the
Il-6r in mice with DSS-induced colitis, in order to exclude
the possibility that the global knock out showed no phenotype, as
the knock out in the myeloid cell lineage may compensate for the
effects of the knock out in other cell types. No significant
differences in weight loss or clinical scores between the male
LysMCre+/−/Il-6rfl/fl mice and
their littermate controls were observed (Fig. 4A and B). In the present sudy, we
used 2.5% DSS in drinking water. Therefore, many mice lost too much
weight and had to be excluded from the experiment. For the
LysMCre+/−/Il-6rfl/fl mice, the
mortality rate was 3 out of 9 compared to 7 out of 11 for their
littermate controls. This indicates that, under severe conditions
of DSS-induced colitis, the knock out of Il-6r in
neutrophils and monocytes may be beneficial to the survival of
mice. When we used 1.5% DSS in the drinking water, this difference
was not observed for the first cycle of chronic DSS-induced colitis
(Fig. 6), reflecting the
situation in a milder form of acute DSS-induced colitis.
We also performed coloscopy, immunohistochemistry,
FITC-dextran measurements, ELISA for IL-6 and qPCR for various
cytokines and other factors involved in colitis. In brief, we
compared the experimental data for the
LysMCre+/−/Il-6rfl/fl mice, as
well as their littermate controls with the unchallenged mice. As
expected from the weight loss curves and clinical scores, we
observed no difference in these parameters between the
LysMCre+/−/Il-6rfl/fl mice and
their littermate controls. We compared the endoscopy of the colon
of the challenged and unchallenged
LysMCre+/−/Il-6rfl/fl and their
littermate controls on day 10 (Fig.
4C). The unchallenged colon shows a normal colonic mucosa. The
surface is glistening and smooth, and blood vessels are clearly
visible. The challenged colon lost its glistening surface and blood
vessels are hard to see or not visible. From a distance, one can
see a deep red color, indicating bleeding or inflammation (Fig. 4C).
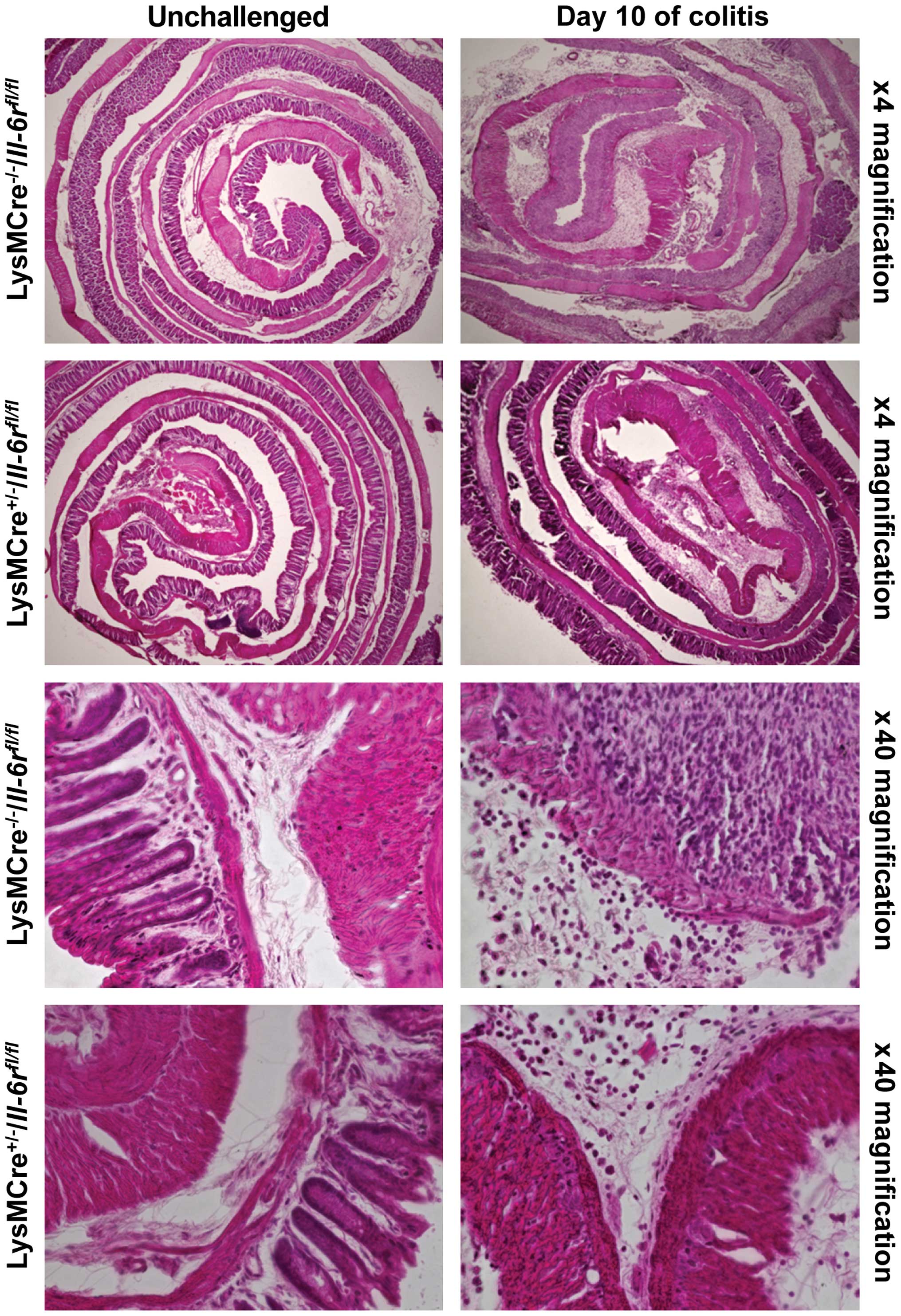
Furthermore, we performed hematoxylin and eosin
(H&E) staining. The unchallenged mice had normal crypt
architecture and no infiltration of immune cells into the mucosa.
The DSS-treated mice from day 10 showed a complete loss of rectal
crypt structure and a massive infiltration of immune cells
(Fig. 5). Again, no differences
between the knockout and wt mice were observed.
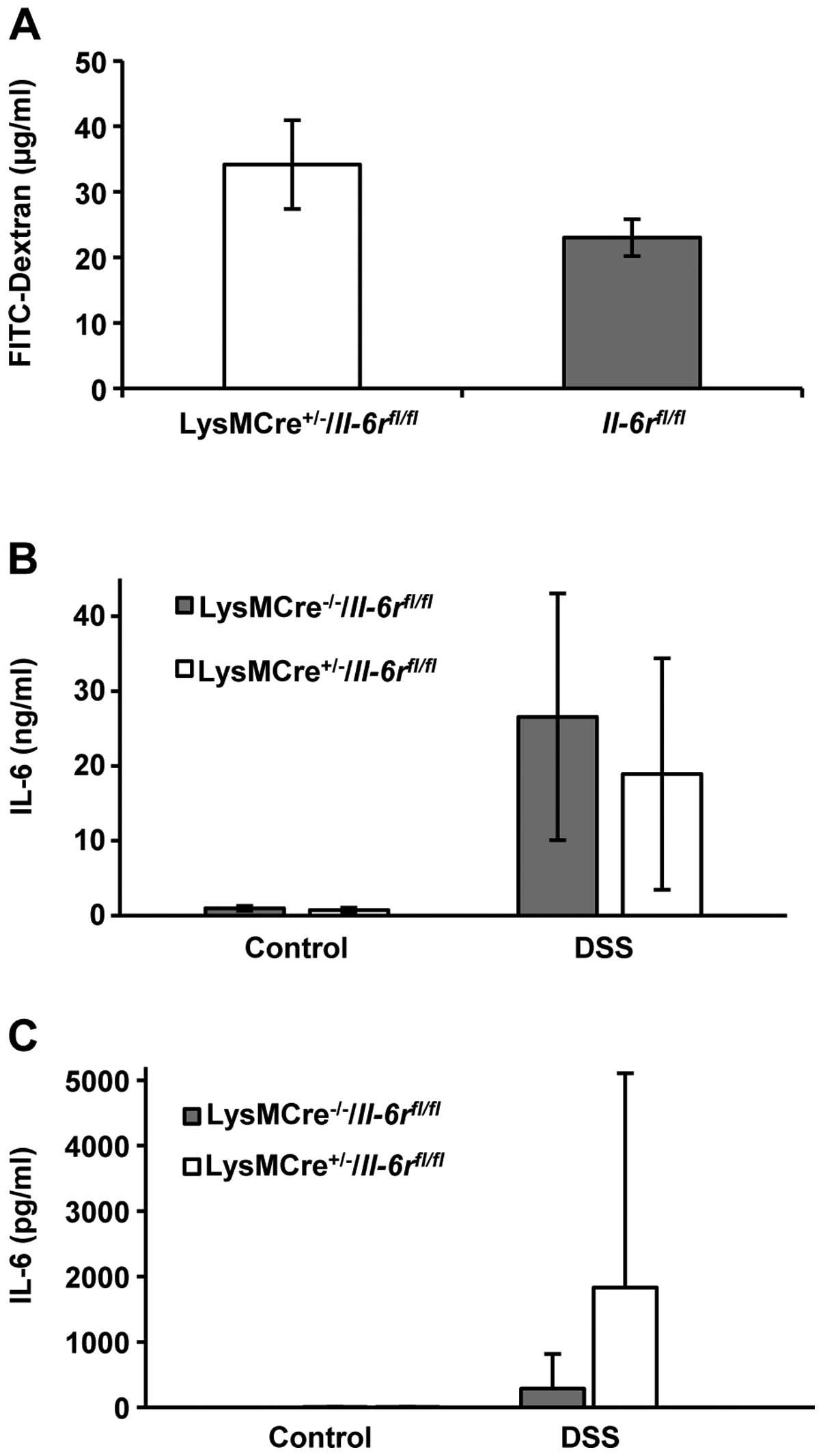
To analyze the permeability of the colon, we
administered FITC-dextran by gavage 4 h prior to sacrifice on day
10. FITC-dextran cannot permeate the intact colon epithelium. If
the barrier function is compromised, FITC-dextran will enter the
bloodstream and can be quantified in mouse serum. We detected
higher, although not significantly, FITC-dextran levels in the
serum of LysMCre+/−/Il-6rfl/fl
mice compared with their littermate controls (Fig. 6A).
On day 10, colon cultures were prepared. No
differences in IL-6 secretion from the colon cultures of the
LysMCre+/−/Il-6rfl/fl mice and
their littermate controls were observed (Fig. 6B). The serum levels of IL-6 were
increased in the
LysMCre+/−/Il-6rfl/fl mice
compared to their littermate controls (Fig. 6C). The difference was not
statistically significant.
Finally, using qPCR, we analyzed the expression of
Tgf-β, Il-1, Tnf-α, Kc and Vα14
in the LysMCre+/−/Il-6rfl/fl
mice and their littermate controls relative to the unchallenged
littermate controls (Table I). We
found that the expression of the anti-inflammatory cytokine,
Tgf-β, which is known to play a beneficial role in
DSS-induced colitis (24), was
slightly upregulated when the mice were challenged with DSS;
however, we did not observe a significant difference between the
LysMCre+/−/Il-6rfl/fl mice and
their littermate controls. The pro-inflammatory cytokine,
Il-1, was upregulated in both genotypes, and Tnf-α
and Kc expression was not significantly changed. As recently
investigated, iNKT cells in the colon can ameliorate DSS-induced
colitis (23). Therefore, we
screened for differences in the amount of Vα14, a marker of
iNKT cells, but did not observe any significant differences. Thus,
we concluded from these experiments that the course of acute
DSS-induced colitis was comparable in the
LysMCre+/−/Il-6rfl/fl and
control mice.
LysMCre+/−/Il-6rfl/fl mice show no
differences in susceptibility to chronic DSS-induced colitis
compared to Il-6rfl/fl control mice
In order to monitor long-term effects, we created a
mouse model of chronic DSS-induced colitis with the
LysMCre+/−/Il-6rfl/fl mice and
their littermate controls. Briefly, 4 cycles were conducted for 5
days each with DSS in their water followed by 7 days with normal
water. DSS concentrations were set to 1.5% for the first cycle,
1.0% for the second cycle, 1.25% for the third cycle and 1.5% for
the fourth cycle. The mice were sacrificed after 6 or 7 days with
normal water of the final cycle as indicated (Fig. 7A).
We observed no significant differences in weight
loss curves and clinical scores between the
LysMCre+/−/Il-6rfl/fl mice and
their littermate controls (Fig. 7B
and C).
Discussion
IBD affects a great number of individuals in
developed and developing countries (2). However, the exact pathophysiological
mechanisms underlying the malignancy remain elusive. One important
factor is IL-6, which has been shown to be upregulated in colitis
(25–28). Even though this suggests that IL-6
plays a negative role in colitis, animal models of colitis induced
by DSS (17) have shown
contradictory results (11,12). Using female mice and high DSS
concentrations in their drinking water, the mortality rate of mice
has been shown to be reduced in Il-6-deficient mice
(12). In contrast to this, male
mice deficient in Il-6 in a DSS-induced colitis using lower
concentrations of DSS, developed more severe colitis due to the
increased death rate of intestinal epithelial cells (11). One could speculate that the amount
of DSS administered is important for the switch in the
pro-inflammatory role of IL-6 to its regenerative role. It would
also be possible that the gender of the used mice determines the
difference in the role of IL-6. In this study, therefore, we
decided to use male Il-6+/+ mice, and
administer low doses of DSS and to neutralize IL-6 with a
neutralizing IL-6 antibody. This was done to mimic the latter
experiment (11). Surprisingly,
mice deficient in Il-6 showed significantly reduced weight
loss and a lower clinical score than the control animals. Although
IL-6 has positive effects on regeneration (16), its role in DSS-induced colitis was
shown to be detrimental.
We further showed that although the IL-6R is the
only known receptor for IL-6, the
LysMCre+/−/Il-6rfl/fl and
Il-6r−/− mice and
their littermate controls had the same susceptibility to
DSS-induced colitis. A difference in the mortality rate was
observed between the genotypes only in the severe form of
DSS-induced colitis.
We proved that the knock out of the Il-6r is
almost complete in monocytes from
LysMCre+/−/Il-6rfl/fl mice.
This was shown by flow cytometric analysis of the IL-6R in
splenocytes and bone marrow cells. Even though some cells were
positively stained for IL-6R and CD11b, this is more likely due to
the gating of cells and not to IL-6R expression. Surprisingly, we
found that although it has been published that approximately 2/3 of
sIL-6R in serum is produced by neutrophils and monocytes (16), the level of sIL-6R in the serum of
LysMCre+/−/Il-6rfl/fl was only
reduced by approximately 1/3 in the present study. This is likely
not due to an incomplete recombination in monocytes, as the flow
cytometric data revealed that the recombination was almost perfect.
However, we cannot exclude the fact that, for unknown reasons, the
recombination was less successful in neutrophils than in
monocytes.
We also generated
Il-6r−/− mice. Our
data revealed that the IL-6R does not play any role in DSS-induced
colitis. This finding is consistent with data on skin wound
healing, where it only plays a minor role compared to IL-6
(16).
There are two possible explanations for the
difference observed between
Il-6r−/− and
Il-6−/− mice: either
the other ligands of IL-6R play a role or IL-6 can also bind to a
yet unknown receptor. It is well known that the IL-27 subunit p28
(IL-30) binds to the membrane-bound and sIL-6R, inducing signal
transduction via a gp130 homodimer (14,15). However, p28 has been excluded as
the important factor contributing to the different results obtained
from Il-6−/− and
Il-6r−/− mice in
wound healing (16). Another
low-affinity ligand for the IL-6R is the widely expressed, CNTF
(13,29,30). It reduces the production of
pro-inflammatory cytokines (31)
and exerts protective effects against experimental autoimmune
encephalomyelitis (EAE), a mouse model of multiple sclerosis
(32,33). Due to its low affinity, CNTF is
unlikely to play a role as the IL-6R ligand that influences the
susceptibility to DSS-induced colitis in mice.
At this point of understanding, it would be
reasonable to breed double-deficient mice, which express neither
IL-6 nor IL-6R
(Il-6−/−/Il-6r−/−),
and challenge them in comparison with
Il-6−/−/Il-6r+/+,
Il-6+/+/Il-6r−/−
and
Il-6+/+/Il-6r+/+
mice in a model of DSS-induced colitis. CNTF and p28 may play a
beneficial role in DSS-induced colitis, as well as another unknown
ligand for the IL-6R. The double-deficient mice should then lack
the detrimental IL-6 signal and the beneficial alternate IL-6R
ligand signal and, therefore, show normal susceptibility to
DSS-induced colitis. Alternatively, if the double-deficient mice
are as susceptible to DSS-induced colitis as
Il-6−/−/Il-6r+/+
mice, this would suggest that IL-6 has another unknown receptor,
which mediates the detrimental IL-6 signal in colitis, while IL-6R
plays no role.
Acknowledgements
We wish to acknowledge Dr Philipp Lang from the
Department of Gastroenterology, Hepatology and Infectious Diseases,
Düsseldorf, Germany for providing us with the
Il-6rfl/fl mice, as well as the staff of
the animal facility at the University of Düsseldorf. The present
study was funded by a grant awarded to J.S. by the Deutsche
Forschungsgemeinschaft (SCHE 907/2-1), Bonn, Germany.
References
|
1
|
Podolsky DK: Inflammatory bowel disease. N
Engl J Med. 347:417–429. 2002. View Article : Google Scholar
|
|
2
|
Shapira Y, Agmon-Levin N and Shoenfeld Y:
Defining and analyzing geoepidemiology and human autoimmunity. J
Autoimmun. 34:J168–J177. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Danese S, Sans M and Fiocchi C:
Inflammatory bowel disease: the role of environmental factors.
Autoimmun Rev. 3:394–400. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mudter J and Neurath MF: Il-6 signaling in
inflammatory bowel disease: pathophysiological role and clinical
relevance. Inflamm Bowel Dis. 13:1016–1023. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reinecker HC, Steffen M, Witthoeft T,
Pflueger I, Schreiber S, MacDermott RP and Raedler A: Enhanced
secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by
isolated lamina propria mononuclear cells from patients with
ulcerative colitis and Crohn’s disease. Clin Exp Immunol.
94:174–181. 1993.PubMed/NCBI
|
|
6
|
Mudter J and Neurath MF: Apoptosis of T
cells and the control of inflammatory bowel disease: therapeutic
implications. Gut. 56:293–303. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamamoto M, Yoshizaki K, Kishimoto T and
Ito H: IL-6 is required for the development of Th1 cell-mediated
murine colitis. J Immunol. 164:4878–4882. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ito H: Novel therapy for Crohn’s disease
targeting IL-6 signalling. Expert Opin Ther Targets. 8:287–294.
2004.
|
|
9
|
Atreya R, Mudter J, Finotto S, Müllberg J,
Jostock T, Wirtz S, Schütz M, Bartsch B, Holtmann M, Becker C,
Strand D, Czaja J, Schlaak JF, Lehr HA, Autschbach F, Schürmann G,
Nishimoto N, Yoshizaki K, Ito H, Kishimoto T, Galle PR, Rose-John S
and Neurath MF: Blockade of interleukin 6 trans signaling
suppresses T-cell resistance against apoptosis in chronic
intestinal inflammation: evidence in crohn disease and experimental
colitis in vivo. Nat Med. 6:583–588. 2000. View Article : Google Scholar
|
|
10
|
Mitsuyama K, Matsumoto S, Rose-John S,
Suzuki A, Hara T, Tomiyasu N, Handa K, Tsuruta O, Funabashi H,
Scheller J, Toyonaga A and Sata M: STAT3 activation via interleukin
6 trans-signalling contributes to ileitis in SAMP1/Yit mice. Gut.
55:1263–1269. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Grivennikov S, Karin E, Terzic J, Mucida
D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H,
Eckmann L and Karin M: IL-6 and Stat3 are required for survival of
intestinal epithelial cells and development of colitis-associated
cancer. Cancer Cell. 15:103–113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Naito Y, Takagi T, Uchiyama K, Kuroda M,
Kokura S, Ichikawa H, Yanagisawa R, Inoue K, Takano H, Satoh M,
Yoshida N, Okanoue T and Yoshikawa T: Reduced intestinal
inflammation induced by dextran sodium sulfate in
interleukin-6-deficient mice. Int J Mol Med. 14:191–196.
2004.PubMed/NCBI
|
|
13
|
Schuster B, Kovaleva M, Sun Y, Regenhard
P, Matthews V, Grötzinger J, Rose-John S and Kallen KJ: Signaling
of human ciliary neurotrophic factor (CNTF) revisited. The
interleukin-6 receptor can serve as an α-receptor for CTNF. J Biol
Chem. 278:9528–9535. 2003.PubMed/NCBI
|
|
14
|
Garbers C, Spudy B, Aparicio-Siegmund S,
Waetzig GH, Sommer J, Holscher C, Rose-John S, Grötzinger J,
Lorenzen I and Scheller J: An interleukin-6 receptor-dependent
molecular switch mediates signal transduction of the IL-27 cytokine
subunit p28 (IL-30) via a gp130 protein receptor homodimer. J Biol
Chem. 288:4346–4354. 2013. View Article : Google Scholar
|
|
15
|
Crabé S, Guay-Giroux A, Tormo AJ, Duluc D,
Lissilaa R, Guilhot F, Mavoungou-Bigouagou U, Lefouili F, Cognet I,
Ferlin W, Elson G, Jeannin P and Gauchat JF: The IL-27 p28 subunit
binds cytokine-like factor 1 to form a cytokine regulating NK and T
cell activities requiring IL-6R for signaling. J Immunol.
183:7692–7702. 2009.PubMed/NCBI
|
|
16
|
McFarland-Mancini MM, Funk HM, Paluch AM,
Zhou M, Giridhar PV, Mercer CA, Kozma SC and Drew AF: Differences
in wound healing in mice with deficiency of IL-6 versus IL-6
receptor. J Immunol. 184:7219–7228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wirtz S, Neufert C, Weigmann B and Neurath
MF: Chemically induced mouse models of intestinal inflammation. Nat
Protoc. 2:541–546. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Perse M and Cerar A: Dextran sodium
sulphate colitis mouse model: traps and tricks. J Biomed
Biotechnol. 2012:7186172012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Siegmund B, Fantuzzi G, Rieder F,
Gamboni-Robertson F, Lehr HA, Hartmann G, Dinarello CA, Endres S
and Eigler A: Neutralization of interleukin-18 reduces severity in
murine colitis and intestinal IFN-γ and TNF-α production. Am J
Physiol Regul Integr Comp Physiol. 281:R1264–R1273. 2001.PubMed/NCBI
|
|
20
|
Clausen BE, Burkhardt C, Reith W,
Renkawitz R and Forster I: Conditional gene targeting in
macrophages and granulocytes using LysMcre mice. Transgenic Res.
8:265–277. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schwenk F, Baron U and Rajewsky K: A
cre-transgenic mouse strain for the ubiquitous deletion of
loxP-flanked gene segments including deletion in germ cells.
Nucleic Acids Res. 23:5080–5081. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moolenbeek C and Ruitenberg EJ: The ‘Swiss
roll’: a simple technique for histological studies of the rodent
intestine. Lab Anim. 15:57–59. 1981.
|
|
23
|
Montbarbon M, Pichavant M, Langlois A,
Erdual E, Maggiotto F, Neut C, Mallevaey T, Dharancy S, Dubuquoy L,
Trottein F, Cortot A, Desreumaux P, Gosset P and Bertin B: Colonic
inflammation in mice is improved by cigarette smoke through iNKT
cells recruitment. PLoS One. 8:e622082013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rani R, Smulian AG, Greaves DR, Hogan SP
and Herbert DR: TGF-β limits IL-33 production and promotes the
resolution of colitis through regulation of macrophage function.
Eur J Immunol. 41:2000–2009. 2011.
|
|
25
|
Raab Y, Hallgren R and Gerdin B: Enhanced
intestinal synthesis of interleukin-6 is related to the disease
severity and activity in ulcerative colitis. Digestion. 55:44–49.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schürmann G, Betzler M, Post S, Herfarth C
and Meuer S: Soluble interleukin-2 receptor, interleukin-6 and
interleukin-1 beta in patients with Crohn’s disease and ulcerative
colitis: preoperative levels and postoperative changes of serum
concentrations. Digestion. 51:51–59. 1992.PubMed/NCBI
|
|
27
|
Wang S, Zhou T, Zhai JP, Wang LH and Chen
J: Effects of Modified Sanhuang Decoction enema on serum tumor
necrosis factor-α and colonic mucosa interleukin-1β, interleukin-6
levels in ulcerative colitis rats. Chin J Integr Med. Oct
14–2014.(Epub ahead of print).
|
|
28
|
Lee MJ, Lee JK, Choi JW, Lee CS, Sim JH,
Cho CH, Lee KH, Cho IH, Chung MH, Kim HR and Ye SK: Interleukin-6
induces S100A9 expression in colonic epithelial cells through STAT3
activation in experimental ulcerative colitis. PLoS One.
7:e388012012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nesbitt JE, Fuentes NL and Fuller GM:
Ciliary neurotrophic factor regulates fibrinogen gene expression in
hepatocytes by binding to the interleukin-6 receptor. Biochem
Biophys Res Commun. 190:544–550. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ohta K, Hara H, Hayashi K, Itoh N, Ohi T
and Ohta M: Tissue expression of rat ciliary neurotrophic factor
(CNTF) mRNA and production of the recombinant CNTF. Biochem Mol
Biol Int. 35:283–290. 1995.PubMed/NCBI
|
|
31
|
Shapiro L, Panayotatos N, Meydani SN, Wu D
and Dinarello CA: Ciliary neurotrophic factor combined with soluble
receptor inhibits synthesis of proinflammatory cytokines and
prostaglandin-E2 in vitro. Exp Cell Res. 215:51–56. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Linker RA, Maurer M, Gaupp S, Martini R,
Holtmann B, Giess R, Rieckmann P, Lassmann H, Toyka KV, Sendtner M
and Gold R: CNTF is a major protective factor in demyelinating CNS
disease: a neurotrophic cytokine as modulator in neuroinflammation.
Nat Med. 8:620–624. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kuhlmann T, Remington L, Cognet I,
Bourbonniere L, Zehntner S, Guilhot F, Herman A, Guay-Giroux A,
Antel JP, Owens T and Gauchat JF: Continued administration of
ciliary neurotrophic factor protects mice from inflammatory
pathology in experimental autoimmune encephalomyelitis. Am J
Pathol. 169:584–598. 2006. View Article : Google Scholar
|