Introduction
Apelin peptide, the endogenous ligand of apelin
receptor (APJ), was first purified by Tatemoto et al
(1) from bovine stomach extracts
by means of the reverse pharmacology approach. The N-terminal amino
acid sequence of the apelin precursor peptide is a signal peptide,
while the C-terminal fragments can bind with high affinity to APJ
and exert biological activities. Currently, preproapelin contains
several proteolytic cleavage sites by changing the formation of
C-terminal peptides, such as apelin-36, apelin-13 and apelin-12.
However, pyroglutamylated apelin-13 (Pyr-AP13) has been identified
as a potentially important mediator in the vascular and cardiac
actions of the human heart (2).
According to our knowledge, both apelin and angiotensin II (Ang II)
are physiological substrates of human angiotensin-converting
enzyme-related carboxypeptidase (ACE2). APJ shows high sequence
similarity with the Ang II type-1 receptor, but Ang II is not the
endogenous ligand of APJ. Regardless of the multiple common
characteristics between the apelin-APJ and Ang II-AT1 systems, the
apelin-APJ signaling pathway appears to have a number of opposing
physiological and pathophysiological roles to the renin-angiotensin
system (3). For instance, Ang II
levels increase, whereas apelin levels decrease in heart failure.
According to a previous study, enhanced green fluorescent protein
was used to tag the C-terminal end of apelin (4) and was stably expressed in Chinese
hamster ovarian (CHO) cells. The results of that study indicated
that APJ may have different configurational states, based on which
to maintain its stability through binding of various apelin
fragments to the apelin receptor.
Previous studies, performed on animals and humans,
have indicated that apelin exerts definite protective effects on
various cardiovascular diseases, including hypertension, cardiac
hypertrophy, heart failure and myocardial ischemia-reperfusion
injury (5). It can attenuate
peripheral vascular resistance and regulate blood pressure by
relaxing blood vessels, inhibiting arteriosteogenesis, improving
vascular function and adjusting water-salt balance (6–8).
Additionally, it can improve heart function by enhancing myocardial
contractility, limiting oxidative stress and inhibiting hypertrophy
(9) and the apoptosis of
cardiomyocytes (10). A recent
study which enrolled 232 hypertensive patients and 76 healthy
controls reported that lower plasma apelin levels were observed in
the hypertensive patients compared with the control group (265±127
vs. 330±159 pg/ml; P<0.001), and the lower plasma apelin levels
were independently related to left ventricular systolic and
diastolic function impairment (11). In the present study, we focused
our attention on the exogenous infusion of Pyr-AP13 in hypertensive
rats with heart failure, and the fascinating perspectives provided
by the development of a novel specific biological target for the
treatment and control of progressive cardiac dysfunction.
Materials and methods
Animals and reagents
The experimental protocol was approved by the Animal
Care and Use Committee of Xuzhou Medical College, Xuzhou, China.
Six-week-old male Sprague-Dawley (SD) rats were provided by the
Animal Department, Xuzhou Medical College. The rats were housed in
a climate-controlled room. Sterile water and standard chow diet
were available ad libitum. Rabbit polyclonal anti-APJ
antibody, anti-total-Akt (t-Akt), anti-phosphorylation-Akt (p-Akt),
anti-total-extracellular signal-regulated kinase 1/2 (t-ERK1/2)
antibody and anti-phosphorylated-ERK1/2 (p-ERK1/2) antibody were
purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). All
other reagent-grade chemicals were purchased from the Sigma-Aldrich
Chemical Co. (St. Louis, MO, USA).
Creation of hypertensive rats
Male SD rats (200–220 g) were subjected to
hypertension as previously described (12). Briefly, under 10% chloral hydrate
(300 mg/kg, intraperitoneally) anesthesia, the left renal artery
was dissected from the surrounding tissues. Subsequently, a
U-shaped silver clip with an internal diameter of 0.2 mm was placed
around the left renal artery to constrict partially. Sham-operated
rats underwent a similar procedure without the clipping of the left
renal artery. The left flank incision was closed using catgut and
silk suture materials and the systolic blood pressure (SBP) of the
animals was measured weekly with a tail-cuff. Four weeks after
clipping, the animals were considered hypertensive if the SBP was
higher than 160 mmHg (BP-2000 system; Visitech Systems, Apex, NC,
USA), as previously described (13).
Echocardiography
The animals were assessed via a 10S phased array
probe (11.5 MHz) and a Vivid 7 Dimension system (GE Healthcare
Ultrasound, Horten, Norway). Seventeen weeks after the surgery, all
the rats were anesthetized with 10% chloral hydrate for the
non-invasive examination. The parameters measured were the
following: the left ventricular internal dimension at end diastole
(LVIDd), interventricular septal thickness at end diastole (IVSd),
left ventricular posterior wall thickness at end diastole (LVPWd),
left ventricular fractional shortening (FS%) and ejection fraction
(EF%). All parameters were measured 5 times by the same observer in
a blinded manner, with the final result being the average of the
5.
Histological determination of
fibrosis
Fresh heart ventricles from the sham-operated rats
(n=8) and the H-HF rats (n=8) were fixed using 4% paraformaldehyde,
dehydrated with alcohol, embedded in paraffin and cut into
5-mm-thick slices using a rotary microtome (RM2016; Leica
Microsystems, Wetzlar, Germany). The slices were then stained with
hematoxylin and eosin or Masson’s trichrome, and observed at x200
magnification using an inverted microscope (IX71; Olympus, Tokyo,
Japan).The area of cardiac and perivascular fibrosis was scored in
a blinded manner in 6 randomly selected areas per sample and
analyzed using Adobe ImageReady 7.0.1 software (Adobe Systems, San
Jose, CA, USA).
Apelin bolus and hemodynamic
responses
The left jugular vein of one set of animals (16
sham-operated rats and 16 H-HF rats), was treated with a continuous
intravenous infusion of either 5% glucose injection (GS) alone or
5% GS containing Pyr-AP13 (Bachem, Bubendorf, Switzerland) in
incremental doses as intravenous bolus injections of 0.1, 1 and 10
μg at 10-min intervals using a polyethylene catheter. The control
solution was administered in a dose, time-matched design. One end
of the second polyethylene catheter was introduced into the left
ventricle (LV) retrograde through the right jugular artery. The
other end was affixed to the RM 6240 multi-channel physiological
signal system (Chengdu Instruments, Chengdu, China) via a pressure
transducer. After the above intervention, the study did not begin
until the hemodynamics had returned to a steady state. The record
of hemodynamic responses was continued for 60 min in each group,
commencing 10 min prior to the bolus administration as the baseline
hemodynamic measurement and an additional 30 min beyond the final
bolus. Measurements were digitally integrated in 10-min recording
periods, from which an average value was calculated and the data
were recorded at preset intervals throughout the study.
The parameters measured were the following: the
heart rate (HR), mean left ventricular systolic pressure (mLVSP),
mean left ventricular diastolic pressure (mLVDP) and maximum rate
of LV pressure (± dP/dtmax). All measurements were made with the
animals under full anesthesia and the body temperature maintained
at 36–37°C using a ST-1 homeothermic blanket system (Chengdu
Instruments).
Cardiomyocyte isolation
The animals were divided into 5 groups: i) the
sham-operated group (n=8); ii) H-HF (n=8); iii) 0.1 μg: H-HF with
infusion of 0.1 μg dose of Pyr-AP13 (n=8) or 5% GS (n=8) at 0 min
and sacrificed at 10 min; iv) 1 μg: H-HF with infusion of 1 μg dose
of Pyr-AP13 (n=8) or 5% GS (n=8) in incremental doses of 0.1 μg and
1 μg at 10-min intervals, and sacrificed at 20 min; and v) 10 μg:
H-HF with infusion of 10 μg dose of Pyr-AP13 (n=8) or 5% GS (n=8)
in incremental doses of 0.1 μg, 1 μg and 10 μg at 10-min intervals,
and sacrificed at 30 min (Fig.
1).
A Langendorff isolated perfused heart assay was
performed as previously described (14). The heart was rapidly cannulated
via the aorta attached to a Langendorff perfusion apparatus and
perfused with KH solution [119 mM NaCl, 4.7 mM KCl, 1.2 mM
KH2PO4, 0.94 mM
MgSO4·7H2O, 25 mM NaHCO3 and 11.5
mM glucose·H2O (pH=7.4)] containing 1 mM Ca2+
at 37°C for 5 min in a retrograde manner. Subsequently, this
solution was changed to a low-Ca2+ solution (120 mM
NaCl, 5.4 mM KCl, 5 mM MgSO4·7H2O, 5 mM
sodium pyruvate, 20 mM glucose·H2O, 20 mM taurine, 10 mM
HEPES, 5 mM NTA and 12 mM Ca2+) followed by perfusion
for 5 min. Finally, the perfusate buffer was substituted with 1
mg/ml collagenase buffer (low-Ca2+ solution containing
50 μmol/l Ca2+, but without NTA) for 10 min. The
temperature remained at 37°C throughout the perfusion. The heart
ventricles were cut into small sections and incubated with
collagenase buffer in a thermostat water bath tank for 3 min at
35°C. The cardiomyocytes were collected by centrifuging with 400
rpm at room temperature for 1 min using a centrifuge (Avanti J-E;
Beckman Coulter, Fullerton, CA, USA).
Determination of cyclic adenosine
3′,5′-monophosphate (cAMP) concentration
The cardiomyocytes were washed 3 times in cold PBS.
The concentration was regulated to 1x106 cells/ml using
cell lysis buffer. After 3 cycles of freezing (-20°C)/thawing, the
supernatant was obtained through centrifuging at 600 x g for 10 min
at 4°C using a microfuge (Microfuge 22R; Beckman Coulter) and the
cAMP concentration was measured using a Sunrise ELISA reader (Tecan
Group Ltd., Männedorf, Switzerland). Based on the manufacturer’s
instructions provided with the ELISA kit, the absorbance was read
at 450 nm. The intensity of the color was inversely proportional to
the concentration of cAMP in the sample.
Western blot analysis
The membrane and cytosolic proteins were extracted
from the isolated cardiomyocytes. The protein concentration was
determined using bovine serum albumin (BSA) as the standard using a
UV-2450 spectrophotometer (Shimadzu, Kyoto, Japan). Proteins
subjected to heat denaturation were separated by 10% SDS-PAGE and
transferred onto nitrocellulose membranes. Subsequently, the
membranes were blocked and incubated with a primary antibody,
followed by incubation with the alkaline phosphatase-conjugated
secondary antibody with BCIP/NBT chromogenic substrate. All images
were captured and analyzed using the Gene Genius system (Syngene,
Cambridge, UK).
Statistical analysis
All data are presented as means ± standard
deviation. SPSS 13.0 software was used for the data analysis. The
Student’s t-test was applied for the statistical significance
between the sham-operated group and the H-HF rats. A repeated
measure ANOVA with a two-way model was used to calculate the time
and treatment differences between the Pyr-AP13-treated groups and
the control (untreated) group. A value of P<0.05 was considered
to indicate a statistically signficant difference. Where
significant differences were identified by ANOVA, priori Fisher’s
protected least square difference (LSD) tests were used to
calculate the time points which differed significantly from the
time-matched control after the start of the intravenous
infusion.
Results
Echocardiography images
Seventeen weeks after surgery, a marked increase was
observed in the IVSd, LVIDd and LVPWd in the H-HF rats compared
with the sham-operated group (P<0.05). Nevertheless, a
significant decrease was noted in the FS% and EF% in the H-HF rats
compared with the sham-operated group (P<0.05; Table I).
 | Table IEchocardiographical assessment of left
ventricular (LV) function at 17 weeks after clipping. |
Table I
Echocardiographical assessment of left
ventricular (LV) function at 17 weeks after clipping.
| Group | N | IVSd (mm) | LVIDd (mm) | LVPWd (mm) | FS (%) | EF (%) |
|---|
| Sham group | 32 | 2.1±0.05 | 7.2±0.5 | 1.6±0.06 | 36.8±2.7 | 72.9±2.0 |
| H-HF group | 80 | 2.7±0.07a | 10.3±0.9a | 2.3±0.11a | 24.4±2.2a | 55.8±2.3a |
Histological determination of cardiac and
perivascular fibrosis
Significant cardiac and perivascular fibrosis was
observed in the H-HF rats compared with the sham-operated group
(P<0.05; Fig. 2). During the
chronic form of heart failure, hemodynamic overload led to
cardiomyocyte hypertrophy and apoptosis, extracellular matrix
alterations, as well as mesenchymal fibrotic and inflammatory
processes. These events were the basis of ‘myocardial remodeling’.
Elastin degradation and collagen deposition were mainly involved in
medial and intimal calcifications during remodeling of the arterial
wall in hypertension. The medial layer was thickened due to the
proliferation, differentiation and hypertrophy of the vascular
smooth muscle cells. All these pathological modifications were
linked to ventricular contractile deficiency and caused severe
damage to heart function.
Assessment of Pyr-AP13 on cardiac
hemodynamics
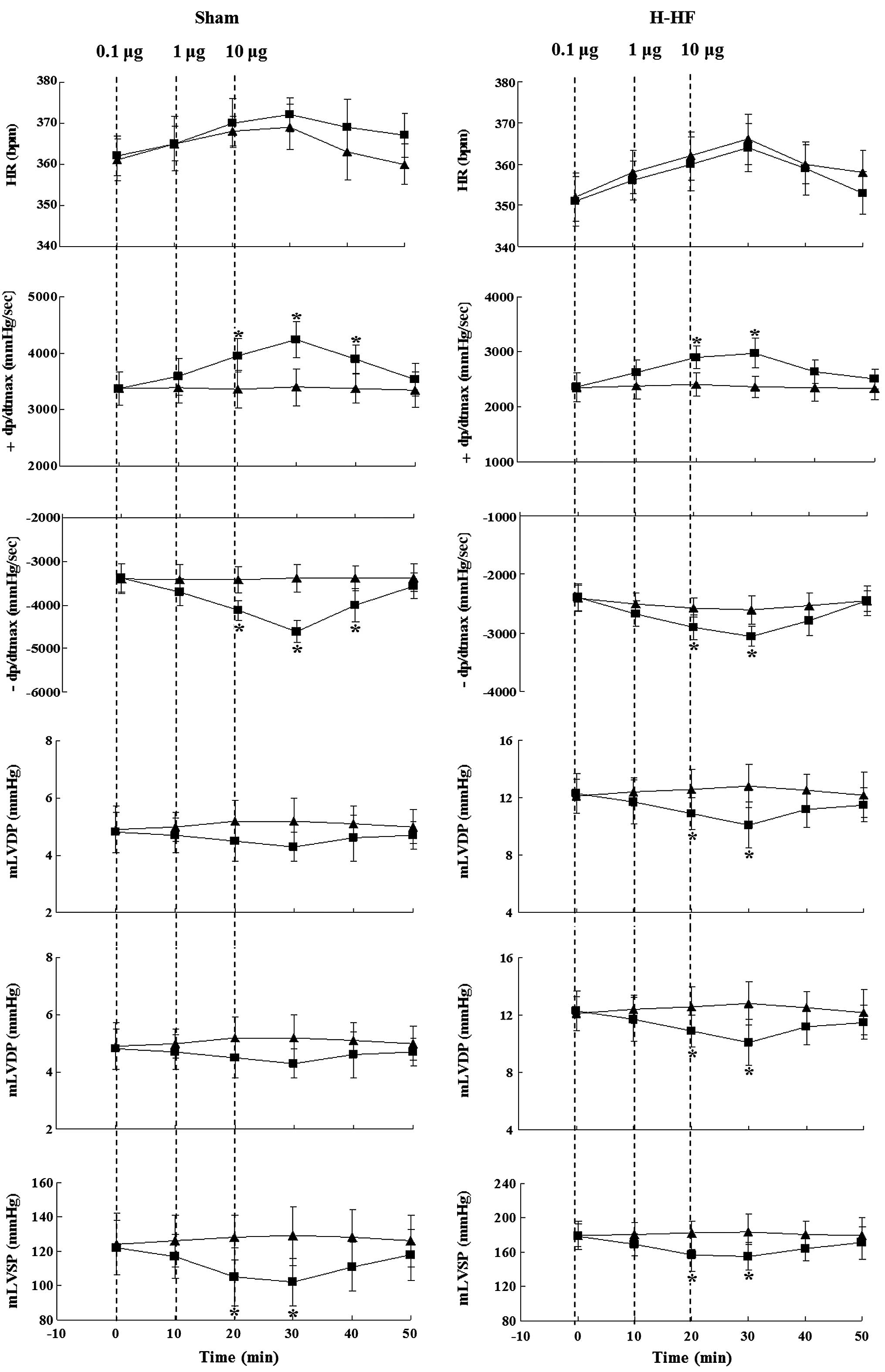
Lower doses of Pyr-AP13 (0.1 μg) had no significant
effect on the hemodynamic parameters (P>0.05). The HR of the
rats in both the sham-operated group and the H-HF rats did not
differ significantly with the Pyr-AP13 infusion (P>0.05). A
significant decrease was observed in the mLVSP in the sham-operated
group and the H-HF rats following the infusion of Pyr-AP13 (1 and
10 μg) (P<0.05). For the highest increase/decrease in the ±
dP/dtmax, a significant increase was observed after the infusion of
Pyr-AP13 (1 and 10 μg) (P<0.05). Pyr-AP13 (1 and 10 μg) also
improved the mLVDP (P<0.05) in the H-HF rats following
treatment, although it had no effect on the sham-operated group
(P>0.05). After an additional 30 min after the final infusion,
the above-mentioned changes gradually returned to the state before
intervention (Fig. 3 and Table II).
 | Table IIHemodynamic changes after 10 min of
Pyr-AP13 infusion (10 μg) in the sham-operated rats and H-HF
rats. |
Table II
Hemodynamic changes after 10 min of
Pyr-AP13 infusion (10 μg) in the sham-operated rats and H-HF
rats.
| Sham-operated
rats | H-HF rats |
|---|
|
|
|
|---|
| Parameter | 5% GS (n=8) | Pyr-AP13 (n=8) | 5% GS (n=8) | Pyr-AP13 (n=8) |
|---|
| HR (bpm) | 369.2±5.5 | 372.6±4.2 | 366.4±6.1 | 364.5±5.8 |
| mLVSP (mmHg) | 129.8±17.4 | 102.2±14.6a | 184.6±20.5 | 155.7±16.3a |
| mLVDP (mmHg) | 5.2±0.8 | 4.3±0.5 | 12.8±0.8 | 10.1±0.9a |
| + dP/dtmax
(mmHg/sec) | 3400.3±324.9 | 4247.5±325.6a | 2359.1±185.2 | 2977.5±266.4a |
| − dP/dtmax
(mmHg/sec) | −3391.8±317.1 | −4609.0±213.5a | −2601.3±241.6 | −3064.4±166.7a |
cAMP level in isolated left ventricular
myocytes
In the present study, decreased cAMP levels were
documented in the isolated left ventricular myocytes (Fig. 4) of the H-HF rats (P<0.05;
Fig. 5A). Compared with the 5% GS
group, no statistically significant difference was noted in the
level of cAMP after the infusion of 0.1 μg Pyr-AP13 (P>0.05).
Nevertheless, an upregulation in the cAMP level was observed in the
H-HF rats following treatment with 1 and 10 μg Pyr-AP13 (P<0.05)
compared with 5% GS control (P<0.05; Fig. 5B).
Expression of APJ protein in isolated
left ventricular myocytes
The expression of APJ receptor in the cellular
membrane (APJ-CM) in the H-HF rats was significantly lower than
that of the sham-operated group (P<0.05; Fig. 6). After the infusion of 0.1 μg
Pyr-AP13 in the H-HF rats, no significant effect was noted in the
expression of APJ-CM (P>0.05). When the dose of Pyr-AP13 was
increased to 1 and 10 μg, APJ-CM expression was significantly
higher compared with the 5% GS control (P<0.05). As regards the
the expression of APJ receptor in the cytoplasm (APJ-CP), its
expression in the H-HF rats was higher compared with the
sham-operated group (P<0.05). Compared with the 5% GS group,
APJ-CP expression in the H-HF rats showed significant a decrease
after the infusion of 1 and 10 μg Pyr-AP13 (P<0.05; Fig. 7).
Expression of p-Akt and p-ERK1/2 protein
in isolated left ventricular myocytes
Compared with the sham-operated group, a significant
increase was observed in the expression of p-Akt and p-ERK1/2 in
the H-HF rats (P<0.05; Fig.
6). Compared with the 5% GS control, our results indicated that
the expression of p-ERK1/2 was decreased, but the activity of p-Akt
was stimulated after the infusion of 1 and 10 μg Pyr-AP13
(P<0.05). The bolus of 0.1 μg Pyr-AP13 had no effect on the
expression of p-Akt and p-ERK1/2 (P>0.05; Fig. 7).
Discussion
It is now well established that hypertension is a
progressive cardiovascular syndrome arising from complex and
interrelated etiologies. The progression of hypertension is
associated with functional and structural cardiac and vascular
abnormalities, which result significant damage to target organs and
even death (15). Patients will
eventually develop myocardial fibrosis-based cardiac dilatation and
heart failure in the presence of consistent hypertension, which are
the most severe complications of hypertension (16). In the present study, rat models of
hypertension were induced using the two-kidney and one clip method.
Based on that, heart function and the ventricular structure were
determined using echocardiography after 17 weeks. The results
revealed that IVSd, LVIDd and LVPWd in the H-HF rats were higher
compared with the sham-operated group. The FS% and EF% in the group
with heart failure were lower compared with the sham-operated
group. These results indicated that increased myocardial stiffness
and reduced compliance of the myocardium were induced with the
elevation of blood pressure and the development myocardial
compensatory hypertrophy, which may gradually lead to systolic and
diastolic dysfunction.
The apelin-APJ signaling pathway emerges as an
important mediator of cardiovascular control. In a previous study,
after the placement of a left ventricular assist device (LVAD) in
11 patients with left ventricular dysfunction, the tissue protein
levels of apelin and the expression levels of the APJ receptor were
increased (17). A recent study
(18) indicated that decreased
left ventricular end-diastolic diameter (LVEDD) and FS% in rats
with heart failure were significantly ameliorated by Pyr-AP13.
Pyr-AP13 effectively inhibited the vascular lesion formation and
suppressed the expression of inflammation factors, such as tumor
necrosis factor-α (TNF-α) and interleukin-1β protein (IL-1β).
Another study (19) reported that
acute Pyr-AP13 administration increased vasodilatation and the
cardiac index and lowered mean arterial pressure and peripheral
vascular resistance in patients with heart failure and healthy
control subjects. Apelin treatment with an apelin receptor agonist
has also been shown to exert protective effects against ischaemia
reperfusion injury in rodents (20). These findings suggest that apelin
infusion may be of great benefit to these hemodynamic effects of
vasodilatation, increased cardiac output, reduced preload and
potential inotropy.
In the present study, no hemodynamic changes were
observed in the sham-operated group and the H-HF rats after the
exogenous infusion of 0.1 μg Pyr-AP13 for 10 min. Nevertheless,
mLVSP in both groups (sham-operated group and H-HF rats) and mLVDP
in the H-HF rats decreased significantly and ± dP/dtmax in both
groups increased significantly after the infusion of 1 and 10 μg
Pyr-AP13, demonstrating that Pyr-AP13 increased myocardial
contractility, improved diastolic function and reduced peripheral
resistance.
It seems that in addition to the above-mentioned
negative haemodynamic effects on pre and after-load, some
pathological modifications induced by the apelin-APJ system may be
involved in the deterioration of cardiac functionality. The orphan
seven transmembrane receptor APJ composed of 380 amino acids, is a
G-protein-coupled receptor (GPCR) (21). Our data indicated that APJ
expression in the cellular membrane showed a marked decrease in the
H-HF rats compared with the sham-operated group rats. After the
exogenous infusion of 1 and 10 μg Pyr-AP13, a significant increase
in APJ expression in the cellular membrane was observed, while APJ
expression in the cytoplasm was significantly lower compared with
the 5% GS control level. As mentioned above, we suggested that APJ
expression was downregulated in the rats with heart failure through
removal from the cell surface. Thus, agonist-activated receptors
are delivered back to the plasma membrane or facilitate receptors
transferred into lysosomes for degradation (22). Endocytosis of APJ receptor played
a key role in receptor downregulation. However, a more accurate
understanding of the molecular mechanisms underlying the
disproportion yet to be obtained.
The critical intracellular second messenger, cAMP,
regulates numerous growth and developmental processes in the
myocardial cell, including discrete compartments to maintain
homeostasis and simultaneously control diverse cellular functions
(23). Compared with
sham-operated group, the cAMP level in the H-HF rats was decreased.
Notably, a significantly higher level of cAMP was observed in the
H-HF rats treated with Pyr-AP13 (1 and 10 μg). However, the
increased expression of p-ERK1/2 in the H-HF rats was reduced after
the infusion of Pyr-AP13 (1 and 10 μg). Based on these results, we
speculated that Pyr-AP13 plays a crucial role in the regulation of
cellular responses as follows: a complex forms after Pyr-AP13
combined with cellular membrane APJ receptor, which may activate
the phosphorylation of ERK1/2 regulated by cAMP.
Crosstalk between the phosphoinositide 3-kinase
(PI3K)/ Akt and MEK/ERK cascades is a central regulator of cell
metabolism, proliferation and survival (24). Akt and ERK1/2 are pro-survival
protein kinases, which confer powerful cardioprotection upon being
activated, specifically at the time of myocardial reperfusion as a
viable target for cardioprotection in patients with acute
myocardial infarction (AMI) (25). In the present study, Akt and
ERK1/2 were activated in the H-HF rats. After the infusion of
Pyr-AP13 in the H-HF rats, we observed that Akt activation
coordinated with ERK inactivation. Therefore, we suggest that the
regulation of the crosstalk between the Akt and ERK1/2 cascades may
have a protective effect on hypertension with heart failure.
Finally, several mechanisms have been found to play
key roles in the limitation of the potential benefit from the
exogenous administration of apelin in left ventricular failure. We
have summarized these as follows: i) both elastin fragment
generation and the deposition of collagen fibers have been linked
to remodeling of the arterial wall in hypertension. These changes
contribute to the reduction of the elasticity of the vessel
associated with extracellular matrix composition and vascular cell
phenotype variation (26). ii)
The chronic form of heart failure and progressive cardiac
dysfunction is constituted by myocardial remodeling. This is a
complex pathological process of cardiomyocyte hypertrophy and
apoptosis, extracellular matrix alterations, mesenchymal fibrotic
and inflammatory processes in response to chronic haemodynamic
overload (27). Hence, these
effects give rise to a vicious circle between the deterioration of
cardiac functionality and myocardial remodeling. iii) Numerous
experimental studies have shown that the membrane receptor plays a
vital role in translating extracellular signals into intracellular
messages and extensively regulates a variety of physiological and
pathophysiological processes (28). As previously demonstrated, in H-HF
rats, APJ receptor activated by the exogenous infusion of Pyr-AP13,
was partially recycled from the cytoplasm back to the plasma
membrane. However, downregulation of APJ receptors dominated by
lysosomal degradation resulted in a lower combination between
Pyr-AP13 and membrane APJ, which limited the effect of Pyr-AP13.
APJ in response to activation by an agonist, can be recycled to the
plasma membrane as in a fully active state. Plasma apelin level in
rats with heart failure decreased, which was conducive to the lower
expression of APJ receptors (29).
In conclusion, the downregulated expression of APJ
protein in cardiomyocytes with heart failure, without the
compensatory upregulation of apelin, may limit the positive
inotropic actions of apelin. Our findings suggest that the
hypertensive remodeling of the heart during heart failure is
accompanied with the endogenous downregulation of APJ receptor,
which results in benefits from the exogenous administration of
apelin. However, we hypothesized that restoring the expression
levels of the APJ receptor by gene transfection may create a
favorable environment for the initiation and propagation of the
apelin-APJ signaling pathway. Further studies are required to
confirm whether it can improve inotropy and depress peripheral
resistance in a fully active state.
Acknowledgements
This study was supported by grants from the Xuzhou
Municipal Bureau of Science and Technology (no. XZZD1020) and the
Xuzhou Municipal Health Bureau (no. XWJ2011030).
References
|
1
|
Tatemoto K, Hosoya M, Habata Y, et al:
Isolation and characterization of a novel endogenous peptide ligand
for the human APJ receptor. Biochem Biophys Res Commun.
251:471–476. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maguire JJ, Kleinz MJ, Pitkin SL, et al:
[Pyr1] apelin-13 identified as the predominant apelin
isoform in the human heart: vasoactive mechanisms and inotropic
action in disease. Hypertension. 54:598–604. 2009.
|
|
3
|
Chun HJ, Ali ZA, Kojima Y, et al: Apelin
signaling antagonizes Ang II effects in mouse models of
atherosclerosis. J Clin Invest. 118:3343–3354. 2008.PubMed/NCBI
|
|
4
|
Iturrioz X, El Messari S, De Mota N, et
al: Functional dissociation between apelin receptor signaling and
endocytosis: implications for the effects of apelin on arterial
blood pressure. Arch Mal Coeur Vaiss. 100:704–708. 2007.PubMed/NCBI
|
|
5
|
Rastaldo R, Cappello S, Folino A, et al:
Effect of apelin-apelin receptor system in postischaemic myocardial
protection: a pharmacological postconditioning tool? Antioxid Redox
Signal. 14:909–922. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iturrioz X, Alvear-Perez R, De Mota N, et
al: Identification and pharmacological properties of E339-3D6, the
first nonpeptidic apelin receptor agonist. FASEB J. 24:1506–1517.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shan PF, Lu Y, Cui RR, et al: Apelin
attenuates the osteoblastic differentiation of vascular smooth
muscle cells. PLoS One. 6:e179382011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roberts EM, Newson MJ, Pope GR, et al:
Abnormal fluid homeostasis in apelin receptor knockout mice. J
Endocrinol. 202:453–462. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Z, Yu B and Tao GZ: Apelin protects
against cardiomyocyte apoptosis induced by glucose deprivation.
Chin Med J. 122:2360–2365. 2009.PubMed/NCBI
|
|
10
|
Foussal C, Lairez O, Calise D, et al:
Activation of catalase by apelin prevents oxidative stress-linked
cardiac hypertrophy. FEBS Lett. 584:2363–2370. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Przewlocka-Kosmala M, Kotwica T, Mysiak A,
et al: Reduced circulating apelin in essential hypertension and its
association with cardiac dysfunction. J Hypertens. 29:971–979.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khalili A, Khosravi MB and Nekooeian AA:
The effects of aqueous extract of vaccinium arctostaphylos leaves
on blood pressure in renal hypertensive rats. Iran Red Crescent Med
J. 13:123–127. 2011.PubMed/NCBI
|
|
13
|
Zhou ZY, Yang ZH, Wang XH, et al:
Increased expression of insulin-like growth factor-binding
protein-3 is implicated in erectile dysfunction in two-kidney
one-clip hypertensive rats after propranolol treatment. Asian J
Androl. 13:851–855. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lewis CJ, Gong H, Brown MJ, et al:
Overexpression of β1-adrenoceptors in adult rat ventricular
myocytes enhances CGP 12177A cardiostimulation: implications for
‘putative’ β4-adrenoceptor pharmacology. Br J Pharmacol.
141:813–824. 2004.
|
|
15
|
Glasser SP, Judd S, Basile J, et al:
Prehypertension, racial prevalence and its association with risk
factors: analysis of the REasons for Geographic and Racial
Differences in Stroke (REGARDS) study. Am J Hypertens. 24:194–199.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shahbaz AU, Sun Y, Bhattacharya SK, et al:
Fibrosis in hypertensive heart disease: molecular pathways and
cardioprotective strategies. J Hypertens. 28(Suppl 1): S25–S32.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen MM, Ashley EA, Deng DX, et al: Novel
role for the potent endogenous inotrope apelin in human cardiac
dysfunction. Circulation. 108:1432–1439. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koguchi W, Kobayashi N, Takeshima H, et
al: Cardioprotective effect of apelin-13 on cardiac performance and
remodeling in end-stage heart failure. Circ J. 76:137–144. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Japp AG, Cruden NL, Barnes G, et al: Acute
cardiovascular effects of apelin in humans: potential role in
patients with chronic heart failure. Circulation. 121:1818–1827.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Simpkin JC, Yellon DM, Davidson SM, et al:
Apelin-13 and apelin-36 exhibit direct cardioprotective activity
against ischemia-reperfusion injury. Basic Res Cardiol.
102:518–528. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Langelaan DN, Reddy T, Banks AW, et al:
Structural features of the apelin receptor N-terminal tail and
first transmembrane segment implicated in ligand binding and
receptor trafficking. Biochim Biophys Acta. 1828.1471–1483.
2013.PubMed/NCBI
|
|
22
|
Dang VC and Christie MJ: Mechanisms of
rapid opioid receptor desensitization, resensitization and
tolerance in brain neurons. Br J Pharmacol. 165:1704–1716. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoshizawa T, Sakurai T, Kamiyoshi A, et
al: Novel regulation of cardiac metabolism and homeostasis by the
adrenomedullin-receptor activity-modifying protein 2 system.
Hypertension. 61:341–351. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shaul YD and Seger R: The MEK/ERK cascade:
from signaling specificity to diverse functions. Biochim Biophys
Acta. 1773:1213–1226. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hausenloy DJ and Yellon DM: New directions
for protecting the heart against ischaemia-reperfusion injury:
targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway.
Cardiovasc Res. 61:448–460. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rattazzi M, Bertacco E, Puato M, et al:
Hypertension and vascular calcification: a vicious cycle? J
Hypertens. 30:1885–1893. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Distefano G and Sciacca P: Molecular
pathogenesis of myocardial remodeling and new potential therapeutic
targets in chronic heart failure. Ital J Pediatr. 38:412012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moser E, Kargl J, Whistler JL, et al: G
protein-coupled receptor-associated sorting protein 1 regulates the
postendocytic sorting of seven-transmembrane-spanning G
protein-coupled receptors. Pharmacology. 86:22–29. 2010. View Article : Google Scholar
|
|
29
|
Pang H and Zhang P: Detection of the level
of apelin during remodeling in hypertensive heart failure rats.
Chin J Clinicians. 6:64–67. 2012.
|