Introduction
Although there is much controversy over the use of
methylprednisolone (MP), it is currently one of the main drugs used
in the treatment of acute spinal cord injury (SCI). Following
traumatic spinal cord injury, an ischemic and low oxygen enviroment
exists in both primary and secondary lesions (1,2).
It is well known under low oxygen conditions, hypoxia-inducible
factor-1α (HIF-1α) will not undergo proteosomal degradation and
plays a key role in a series of reactions associated with protoming
the survival of cells and helping them adapt to low oxygen
(3). A previous study
demonstrated that between conditions of anoxia to 20%
O2, the highest proliferation of mouse neural stem cells
was observed at 2–3% oxygen (4).
Certain studies have shown that hypoxic conditions increase the
formation and proliferation of neural progenitor cells (NPCs) and
the proportion of neurons (5,6).
The study by Schröter et al (7), as well as our previous study,
demonstrated that corticosteroids inhibit the proliferation of NPCs
in vitro and following SCI (8). However, dexamethasone has been shown
to have an inhibitory effect on HIF-1α in T cells (9). To the best of our knowledge, the
effects of MP on HIF-1α in NPCs have not been reported to date.
Certain studies have demonstrated the regulatory function of HIF-1α
on the proliferation and differentiation of several stem cell types
through Notch signaling components under low oxygen conditions
(10,11). However, inhibiting Notch signaling
leads to NPC differentiation, including an increased proportion of
neurons in the rat brain (12).
The effects of MP on the proliferation and
differentiation of rat spinal cord-derived NPCs, as well as the
mechanisms involved remain to be elucidated. Thus, in this study,
we treated NPCs cultured under both normal oxygen (normoxic) and
low oxygen (hypoxic) conditions with or without MP to determine
whether any changes occur in HIF-1α expression and Notch signaling,
as well as to elucidate the possible effects of MP on the
proliferation and differentiation of NPCs in vitro.
Materials and methods
Ethics approval
All procedures involving animals were approved by
the ‘Committee for the Care and Use of Laboratory Animals’ of Sun
Yat-sen University College of Medicine, Guangzhou, China.
Cell culture and characterization by
immunocytochemistry
Spinal cords were isolated from neonatal (1-day-old)
Sprague-Dawley rats under a microscope and digested by repeated
trituration with fire-polished Pasteur pipettes into single cells,
according to a previously described method for spinal cord stem
cells (13,14) The cells were incubated and
passaged in medium, as described in our previous study (8) The 2nd generation of neurospheres was
used in all the experiments. The antibodies used in this study were
as follows: primary antibodies to nestin (mouse anti-rat, 1:400;
Millipore Corp., Billerica, MA, USA), microtubule-associated
protein 2 (MAP2; rabbit anti-rat, 1:100; Sigma-Aldrich, St. Louis,
MO, USA) and glial fibrillary acidic protein (GFAP; rabbit
anti-rat, 1:500), as well as anti-oligodendrocyte-specific protein
antibody (Oligo; rabbit anti-rat; 1:100) (both from Abcam,
Cambridge, MA, USA); and the secondary antibodies, FITC goat
anti-mouse or Cy3 goat anti-rabbit (1:200; Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA). The fluorochrome Hoechst
33342 (Cell Signaling Technology, Danvers, MA, USA) was used to
stain the nuclei. Low oxygen conditions were generated by aerating
the cell incubator chamber (Galaxy 48R; New Brunswick, Hamburg,
Germany) with CO2 and N2. The modulator can
adjust the O2 concentration to target the oxygen level
to 3%, CO2 to 5% and N2 92%.
Immunocytochemistry was performed as described in our previous
study (8). Images were captured
using an inverted fluorescence microscope (Carl Zeiss, Inc.,
Oberkochen, Germany).
Cell quantitative analysis and CCK-8
assay
The 2nd generation of NPCs was split into to 2 main
groups (with 20% O2 for the control, with 3%
O2 for the experimental group). Each group was divided
into 3 subgroups, each treated with a different concentration of MP
(0 μg/ml as the control, or 3 and 10 μg/ml). The cells were plated
into 6-well plates (BD Falcon/BD Biosciences, Franklin Lakes, NJ,
USA) at a density of 5×104 cells/ml (3 ml cell
suspension per well). Every 24 h all samples were removed from the
cell incubator and digested into a single cell suspension and were
randomly and repeatedly counted by 2 individuals using a cell
counting chamber for 7 days. A density of 1.5×104
cells/ml was planted into a 96-well flat bottom plate (0.2 ml per
well). Each sample was divided into 3 wells for repetition. Three
neonatal rats were used as biological replicates. In total, 10
plates (half with 20% O2, and the other with 3%
O2) were planted. Every 24 h CCK-8 solution (Dojindo
Laboratories, Kumamoto, Japan) was added to 2 plates (one in 20%
O2, and the other in 3% O2), followed by
incubation at 37°C for 4 h and subsequent testing using a
microplate reader (Varioskan; Thermo Fisher Scientific, Waltham,
MA, USA). Each well was detected at an excitation light length of
450 and 630 nm. The final optical density (OD) value was made
firstly by all values at 450 nm OD value, substracting the 630 nm
OD value, then substracting the OD value of the blank well (culture
medium only).
Quantitative reverse transcription
PCR
The NPCs were incubated for 24 h. The 2 main groups
(3 or 20% O2) were divided into 2 subgroups, each
treated with a different concentration of MP (0 or 10 μg/ml). Total
RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA,
USA) according to the manufacturer’s instructions and the RNA
concentration was determined using a NanoDrop spectrophotometer
(from Thermo Fisher Scientific). cDNA was reverse transcribed using
PrmieScript RT Master mix (Takara Bio, Dalian, China) with 200 ng
RNA per sample. Quantitative PCR (qPCR) was performed on a
real-time PCR instrument (light cycler 480; Roche, Mannheim,
Germany), using SYBR Premix Ex TaqTM II (Tli RNaseH
Plus; Takara code no. DRR820A). All data were normalized to the
housekeeping gene, β-actin (ACTIN). The primers used in the
real-time PCR amplification were as follows: β-actin forward,
5′-GGAGATTACTGCCCTGGCTCCTA-3′ and reverse,
5′-GACTCATCGTACTCCTGCTTGCTG-3; HIF-1α forward,
5′-CCAGATTCAAGATCAGCCAGCA-3′ and reverse,
5′-GCTGTCCACATCAAAGCAGTACTCA-3′; VEGF forward,
5′-TGGACCCTGGCTTTACTGCTG-3′ and reverse,
5′-GGCAATAGCTGCGCTGGTAGA-3′; phosphoglycerate kinase (PGK1)
forward, 5′-TCCATGGTGGGTGTGAATCTG-3′ and reverse,
5′-CAGCTGGATCTTGTCTGCAACTTTA-3′; and Hes1 forward,
5′-CAACACGACACCGGACAAAC-3′ and reverse, 5′-TTGGAATGCCGGGAGCTATC-3′.
All data were normalized to the housekeeping gene, β-actin
(ACTIN).
Western blot analysis of extracts
The cells were placed into 6-well plates at 3 ml per
well (cell density, 50×104 cells/ml). The 2 main groups
(treated with 10 μM DAPT or without DAPT), were divided into 4
subgroups (cultured in 20% O2 and treated with 0 or 10
μg/ml MP for 48 h; or cultured in 3% O2 and treated with
0 or 10 μg/ml MP for 48 h). Cell lysis buffer was then added (50 mM
Tris pH 7.4, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate,
0.1% SDS and 1 mM PMSF) containing protease inhibitor cocktail
tablets (Roche). Protein concentrations were measured using the BCA
protein assay in a spectrophotometer (Multiskan; Thermo Fisher
Scientific) at 562 nm. In each lane, 20 μg protein sample was
serperated on a 10% SDS-polyacrylamide gel (SDS-PAGE) then
transferred onto a PVDF membrane (Millipore Corp.). The membrane
was then incubated with primary antibody with 5% BSA overnight at
4°C. The primary antibodies were as follows: anti-β-actin (1:1,000;
Cell Signaling Technology); anti-HIF-1α (1:500; Novus Biologicals,
Ltd., Cambridge, UK); anti-Hes1 (1:1,000; Abcam); anti-Notch 1
intracelluar domain (NICD) (cleaved Notch 1) (1:1,000; Cell
Signaling Technology). DAPT (an NICD blocker; Tocris, Ballwin, MO,
USA) was diluented into 10 μM per well. The blots were incubated
for 1 h with horseradish peroxidase secondary antibodies (Millipore
Corp.). Immunoreactive protein bands were detected and visualized
in a Gel imaging box (G:BOX Chemi; Sygene Technologies Corp., Palos
Heights, IL, USA) with ECL substrate (Pierce Biotechnology, Inc.,
Rockford, IL, USA).
Fluorescence-activated cell sorting
(FACS)
Cell grouping and treatment were the same as for
western blot analysis. Following 3 days in culture with 5% fetal
bovine serum (FBS), the cells were tenderly blown into single cells
in 0.05% trypsin using a Pasteur pipette and then treated with
fixation and permeabilization reagents (Invitrogen). Followng
incubation with primary antibody to nestin (1:500; Sigma-Aldrich),
GFAP (1:500; Abcam) or MAP2 (1:500; Sigma-Aldrich), the cells were
centrifuged and rinsed with PBS twice. FITC (1:1,000; Jackson
Immunoresearch Laboratories) was used as the secondary antibody.
FACS was performed in a flow cytometer (BD FACSCalibur; BD
Biosciences).
Statistical analysis
One-way ANOVA followed by least significant
difference (LSD) multiple comparisons t-tests were used to
determine statistical significance. P values were derived from at
least 3 independent experiments. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
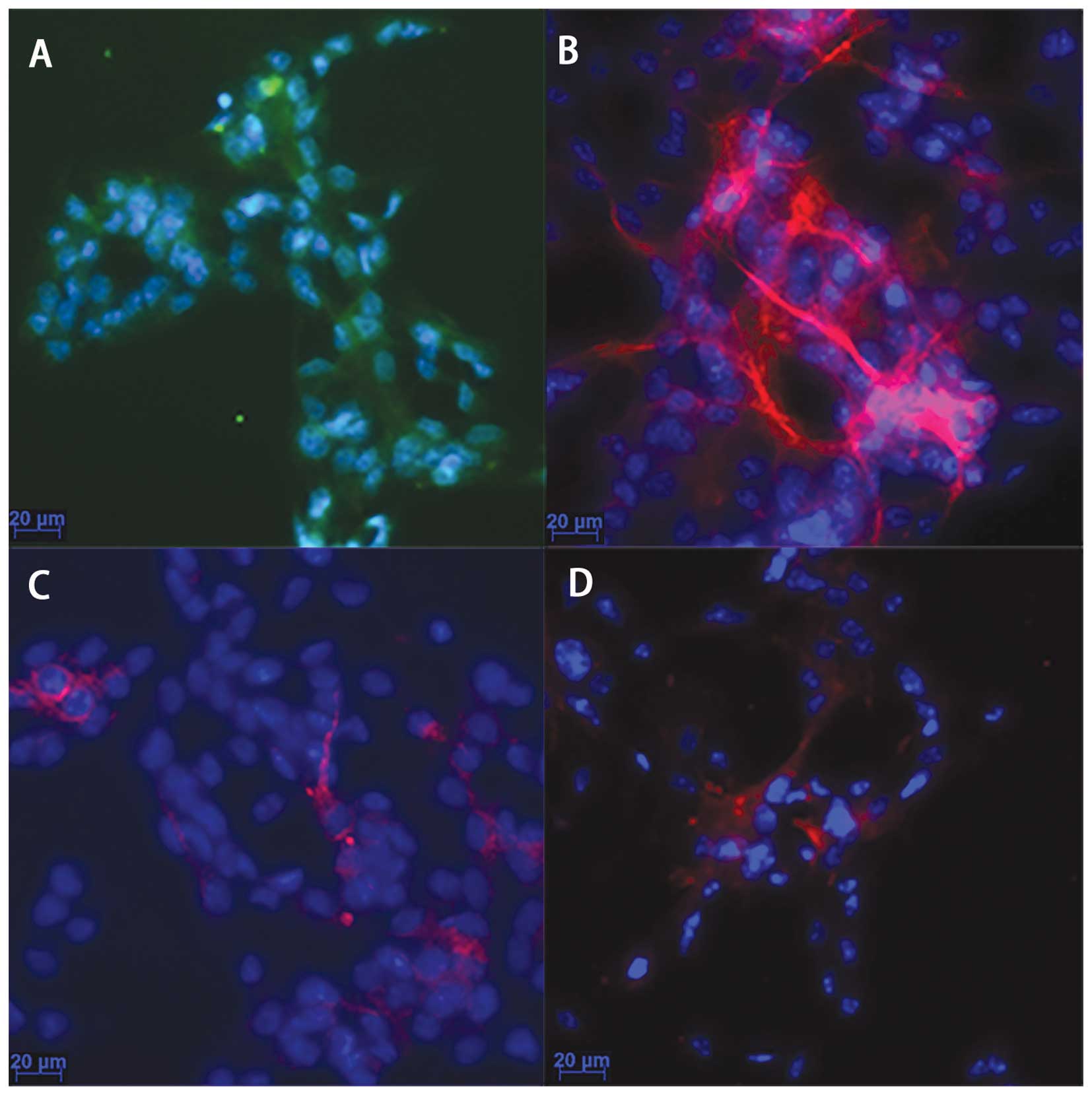
Cell culture and characterization
As observed under a light microscope, the cells had
a round shape, bright plasma and formed neurospheres. Following the
addition of 5% FBS and normal culture for 3 days, different
cellular processes were observed. The results from
immunocytochemistry (Fig. 1)
revealed that the majority of the cultured cells were
nestin-positive (Fig. 1A), a
marker of NPCs; the GFAP-positive cells were abundant (Fig. 1B), indicating that the majority of
the NPCs had differentiated into astrocytes; the neurons
(MAP2-positive cells; Fig. 1C)
and oligodendrocytes (Oligo4-positive cells; Fig. 1D) were a minority. These results
demonstrated that the cultured cells were NPCs and were able to
differentiate into other types of neural cells.
A low oxygen enviroment promotes NPC
proliferation, but MP inhibits NPC proliferation even under low
oxygen conditions
Cell quantitative analysis and CCK-8 assay indicated
that the NPCs not treated with MP (0 μg/ml) proliferated more
rapidly when cultured in 3% O2 than in 20% O2
following treatment with MP (P<0.05) (Fig. 2A and B). The proliferation of the
cells cultured in either 20 or 3% O2 was markedly
inhibited following treatment with MP from day 2 of culture
(P<0.05) (Fig. 2A). The
increase in the concentration of MP from 3 to 10 μg/ml did not
enhance the inhibitory effect (Fig.
2A). As shown by CCK-8 assay, the inhibitory effect on cell
proliferation was observed in the cells cultured in 20%
O2 from day 2 (P<0.05), and in the cells cultured in
3% O2 on day 3 (P<0.05) (Fig. 2B). The absolute value of the
decrease in proliferation was greater in the cells cultured in low
oxygen conditions than in those cultured in normal oxygen
conditions.
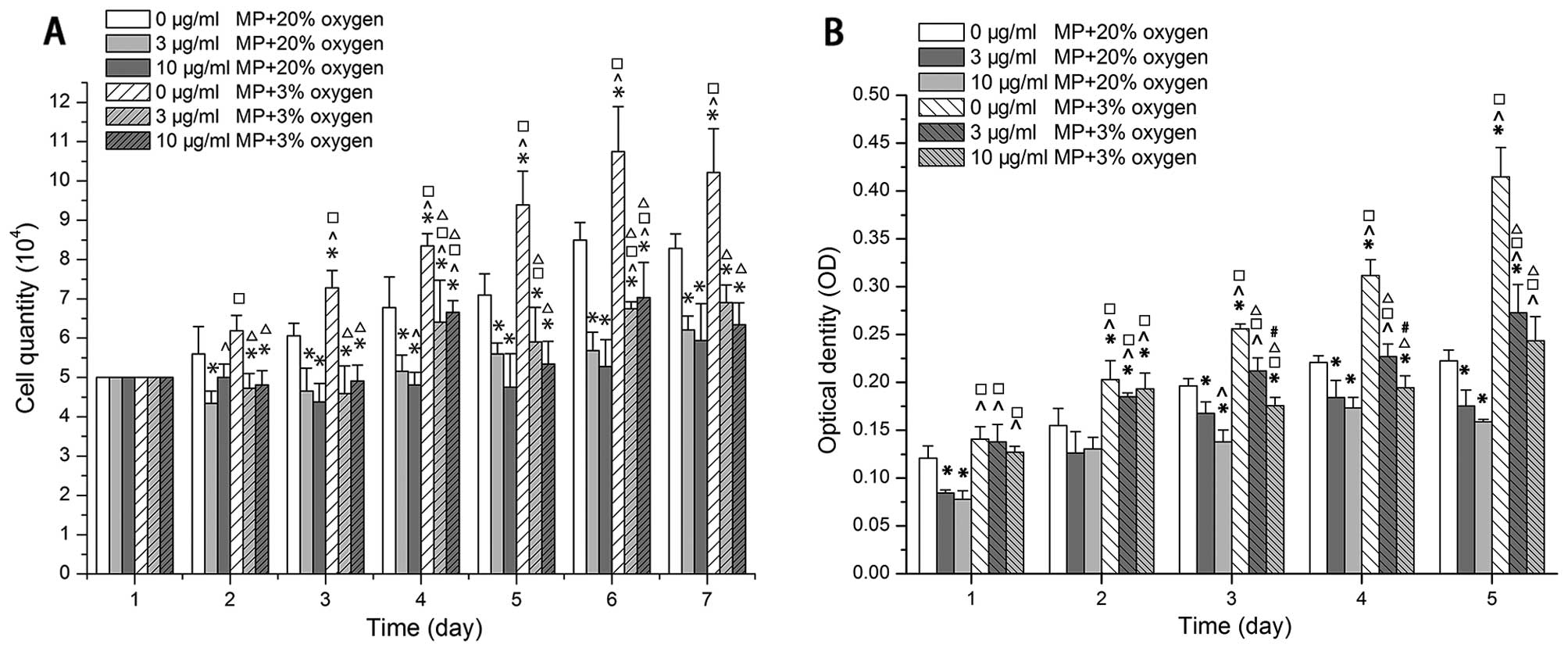 | Figure 2Low oxygen promoted proliferation,
but methylprednisolone (MP) inhibited proliferation under both low
(3% 02) and normal (20% 02) oxygen
conditions. Increasing the dose of MP (from 3 to 10 μg/ml) did not
enhance the inhibitory effect. (A) Results of cell quantitative
analysis (x104). Data are expressed as the means ± SD,
n=3. (B) Results of CCK-8 assay optical density (OD). Following the
addition of MP, the OD value of the cells cultured in in low oxygen
(3% O2) was still higher than that of the cells cultured
in normoxic conditions (20% O2). The OD values formed
from the data at 450 minus 630 nm after the cell medium OD value
substracted as a blank. Data are expressed as the means ± SD, n=3.
*P<0.05, (0 μg/ml MP + 20% oxygen) vs. (3 μg/ml MP +
20% oxygen), (10 μg/ml MP + 20% oxygen), (0 μg/ml MP + 3% oxygen),
(3 μg/ml MP + 3% oxygen), (10 μg/ml MP + 3% oxygen);
^P<0.05, (3 μg/ml MP + 20% oxygen) vs. (10 μg/ml MP +
20% oxygen), (0 μg/ml MP + 3% oxygen), (3 μg/ml MP + 3% oxygen),
(10 μg/ml MP + 3% oxygen); □P<0.05, (10 μg/ml MP +
20% oxygen) vs. (0 μg/ml MP +3% oxygen), (3 μg/ml MP + 3% oxygen),
(10 μg/ml MP + 3% oxygen); ΔP<0.05, (0 μg/ml MP + 3%
oxygen) vs. (3 μg/ml MP + 3% oxygen), (10 μg/ml MP + 3% oxygen);
#P<0.05, (3 μg/ml MP + 30% oxygen) vs. (10 μg/ml MP +
3% oxygen). |
MP not only inhibits the gene and protein
expression of HIF-1α and its downstream genes, VEGF and PGK1, but
also that of Hes1 in the Notch signaling pathway in NPCs in
vitro
To examine the effects of MP on the HIF-1α-VEGF
signaling pathway in NPCs, HIF-1α and its downstream genes, VEGF
and PGK1, were investigated in the cells cultured in 20 and 3%
O2. Unlike in non-stem cells, the HIF-1α protein did not
undergo proteosomal degradation in the NPCs cultured in 20%
O2 (Fig. 3A). Low
oxygen induced the expression of the HIF-1α gene (Fig. 3C) and protein (Fig. 3A and B) in the cells cultured in
3% O2 compared to those cultured in 20% O2
and not treated with MP (0 μg/ml MP). However, following treatment
with 10 μg/ml MP, HIF-1α expression was inhibited in the cells
cultured in both 20 and 3% O2, although the decrease was
more significant in the cells cultured in a low oxygen enviroment
(Fig. 3B and C). VEGF and PGK1
mRNA expression (in the cells treated with 0 μg/ml MP) increased in
the cells cultured in 3% O2 by approximately 2-fold
compared to those cultured in 20% O2; however, following
treatment with MP (10 μg/ml) their expression was suppressed only
in the cells cultured in 3% O2 but not in those cultured
in normoxic conditions (Fig. 3D and
E). Although VEGF expression in the cells cultured in a low
oxygen enviroment was still higher than that in the cells culrured
in a normoxic enviroment and treated with 10 μg/ml MP, the absolute
decrease in its expression was more obvious in the cells cultured
in a low oxygen enviroment (Fig.
3D). Hes1 expression also increased by approximately 1.9-fold
in the cells cultured in 3% O2 and not treated with MP
(0 μg/ml). MP also inhibited the expression of Hes1 in the cells
cultured in 3 and 20% O2, which suggests that MP affects
the Notch signaling pathway (Fig.
3F).
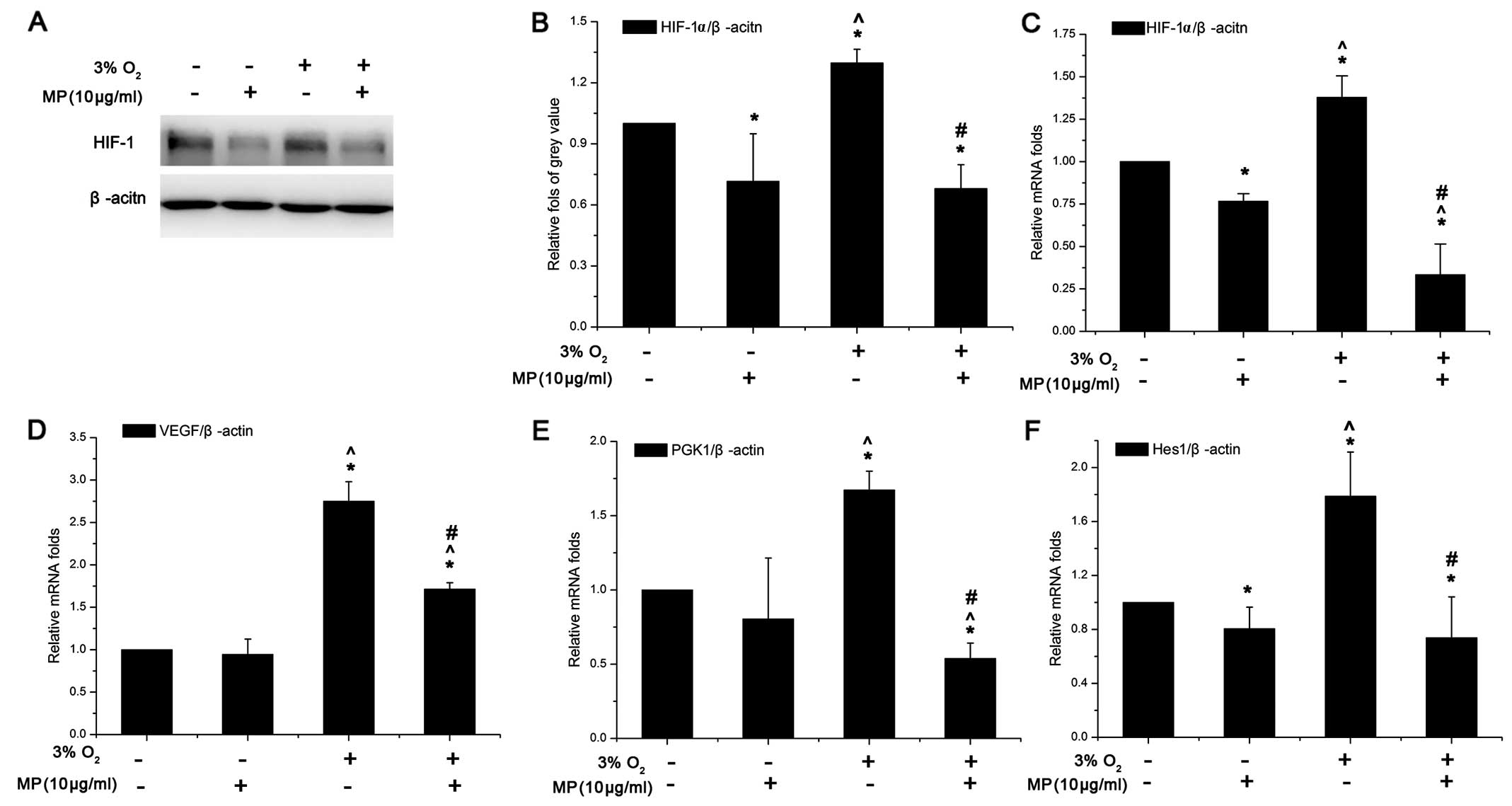 | Figure 3(A) Western blot analysis of
hypoxia-inducible factor-1α (HIF-1α) (100 kDa). (B) The relative
fold of protein HIF-1α compared to β-actin was increased in the
cells cultured in 3% O2 + (0 μg/ml) methylprednisolone
(MP). Both under normoxic and low oxygen conditions, HIF-1α protein
expression was suppressed. Data are expressed as the means ± SD,
n=3. (C-F) Results of quantitative PCR. The relative folds of
target mRNA compared to housekeeping mRNA β-actin (ACTIN) were
calculated form a Cp value. Data are expressed as the means ± SD,
n=3. (C) HIF-1α increased by approximately 1.5-fold in low oxygen
(3% O2) compared to normoxic conditions (20%
O2), and its gene expression was suppressed
(approximately 0.75-fold in normoxic and approximately 0.4-fold in
low oxygen conditions) following treatment with MP (10 μg/ml). The
suppressive effects were more prominent in a low oxygen than in the
normoxic environment (^P<0.05 between the group of
20% O2 + 0 μg/ml MP and 3% O2 + 0 μg/ml MP).
(D) VEGF expression increased by approximately 2.3-fold in low
oxygen (3% O2), when added MP (10 μg/ml) the inhibition
effect only worked in low oxygen. In low oxygen the VEGF still
expressed higher than in in the normoxic conditions even following
treatment with MP. (E) PGK1 expression also increased by
approximately 1.7-fold in low oxygen when no MP was added. MP was
more effective in decreasing PGK1 expression compared to VEGF. (F)
Hes1 expression also increased by approximately 1.9-fold in the
cells cultured in 3% O2 + (0 μg/ml) MP. MP also
inhibited the expression of Hes1 under both oxygen conditions,
which suggests that MP affects the Notch signaling pathway.
*P<0.05, (20% O2) normoxia + (0 μg/ml) MP
group vs. (20% O2) normoxia + (10 μg/ml) MP group, (3%
O2) low oxygen + (0 μg/ml) MP group and (3%
O2) low oxygen + (10 μg/ml) MP group;
^P<0.05, (20% O2) normoxia + (10 μg/ml) MP
group vs. (3% O2) low oxygen + (0 μg/ml) MP group and
(3% O2) low oxygen + (10 μg/ml) MP group;
#P<0.05, (3% O2) low oxygen + (0 μg/ml) MP
group vs. (3% O2) low oxygen + (10 μg/ml) MP group. |
MP only suppresses the expression of the
Notch signaling pathway downstream protein, Hes1, but not that of
upstream NICD
As shwon by our results (Fig. 3F), MP can affect the Notch
signaling pathway. Low oxygen conditions can also help maintain the
undifferentiated state of NPCs by regulating the Notch signal
pathway (11). Therefore, we
examined the protein levels of the Notch pathway upstream protein,
NICD, and the downstream protein, Hes1, in the NPCs following
treatment with or without MP under low oxygen and normoxic
conditions. The results from western blot analysis revealed that:
i) NICD expression was increased (without MP) in the cells cultured
in 3% O2 compared to those cultured in 20%
O2. MP (10 μg/ml) did not have an inhibitory effect on
NICD expression in the cells cultured in 3 and 20% O2.
That is, MP did not affect the Notch signaling pathway by altering
NICD expression (Fig. 4A and B).
ii) In the cells cultured in 20 and 3% O2 without DAPT,
Hes1 expression increased more significantly in the cells cultured
in low oxygen conditions (P<0.05). MP (10 μg/ml) suppressed the
protein expression of Hes1 (Fig. 4A
and C) in the Notch signaling pathway and more prominent
inhibitory effects were observed in the cells cultured in a low
oxygen enviroment (P<0.05); the expression of Hes1 showed the
same tendency as HIF-1α (Fig. 3A and
C). iii) After blocking the expression of NICD with 10 μM DAPT
(Fig. 4A), Hes1 expression was
not elevated under low oxygen conditions, but remained at levels
similar to those observed under normoxic conditions (P>0.05);
however, Hes1 expression still decreased following treatment with
MP (10 μg/ml) (Fig. 4C). These
results suggest that MP affects the differentiation of NPCs by
regulating Hes1 expression without influencing NICD expression.
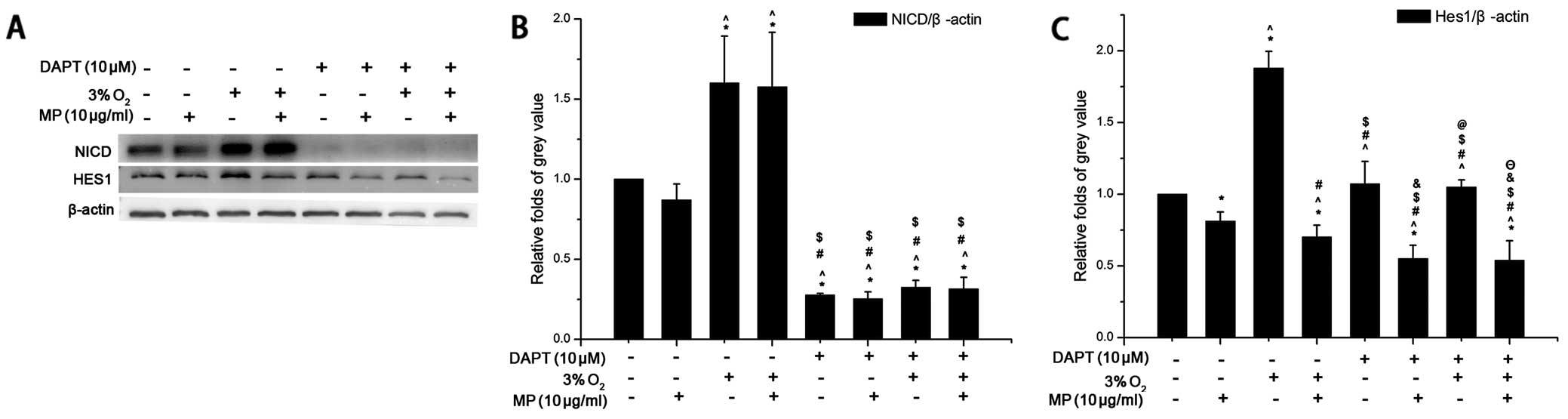 | Figure 4Western blot analysis was carried out
with the cell lysis buffer of neural progenitor cells (NPCs)
following treatment. In response to methylprednisolone (MP)
treatment, low oxygen decreased the expression of the Notch
signaling pathway, downstream protein, Hes1, but not that of
upstream Notch-1 intracelluar domain (NICD). After blocking NICD
with an inhibitor (DAPT) MP still inhibited the expression of Hes1.
Three independent experiments were performed. The data of target
protein compared to internal reference protein are expressed as the
means ± SD, n=3. Low oxygen (3% O2), normoxia (20%
O2) and MP was added at a concentration of 10 μg/ml, the
NICD inhibitor, DAPT, was used at a concentration of 10 μM. (A)
β-actin was an internal reference protein (approximately 45 kDa),
Hes1 (approximately 30 kDa), NICD (approximately 110 kDa). (B) The
expression of NICD was elevated under low oxygen conditions
compared to normoxic conditions following treatment of the cells
with 0 μg/ml MP (*P<0.05). MP did not have an
inhibitory effect on NICD following treatment of the cells with 10
μg/ml MP under both normoxic and low oxygen conditions. After the
addition of DAPT (10 μM), NICD expression was significantly
inhibited and MP did not affect the expression of NICD. (C) The
relative fold of Hes1 compared to β-actin was increased in the
cells cultured in low oxygen without MP (*P<0.05),
but MP was more effective in a low oxygen environment when applied
to the NPCs (^P<0.05). Following treatment with DAPT
(10 μM) Hes1 expression did not increase in low oxygen compared to
normoxia (&P>0.05), but Hes1 expression was still
inhibited by MP (10 μg/ml)(&P<0.05;
ΘP<0.05). *P<0.05, (20% O2)
normoxia + (0 μg/ml) MP group + 0 μM DAPT vs. the other 6 groups
apart from the control group; ^P<0.05, (20%
O2) normoxia + (10 μg/ml) MP group + 0 μM DAPT vs. the
other 6 groups apart from the control group; #P<0.05,
(3% O2) low oxygen + (0 μg/ml) MP group + 0 μM DAPT vs.
the other 6 groups apart from the control group;
$P<0.05 vs. (3% O2) low oxygen + (10
μg/ml) MP group + 0 μM DAPT vs. the other 6 groups apart from the
control group; &P<0.05, (20% O2)
normoxia + (0 μg/ml) MP group + 10 μM DAPT vs. the other 6 groups
apart from the control group; @P<0.05, (20%
O2) normoxia + (10 μg/ml) MP group + 10 μM DAPT vs. the
other 6 groups apart from the control group; ΘP<0.05,
(3% O2) low oxygen + (0 μg/ml) MP group + 10 μM DAPT vs.
the other 6 groups apart from the control group. |
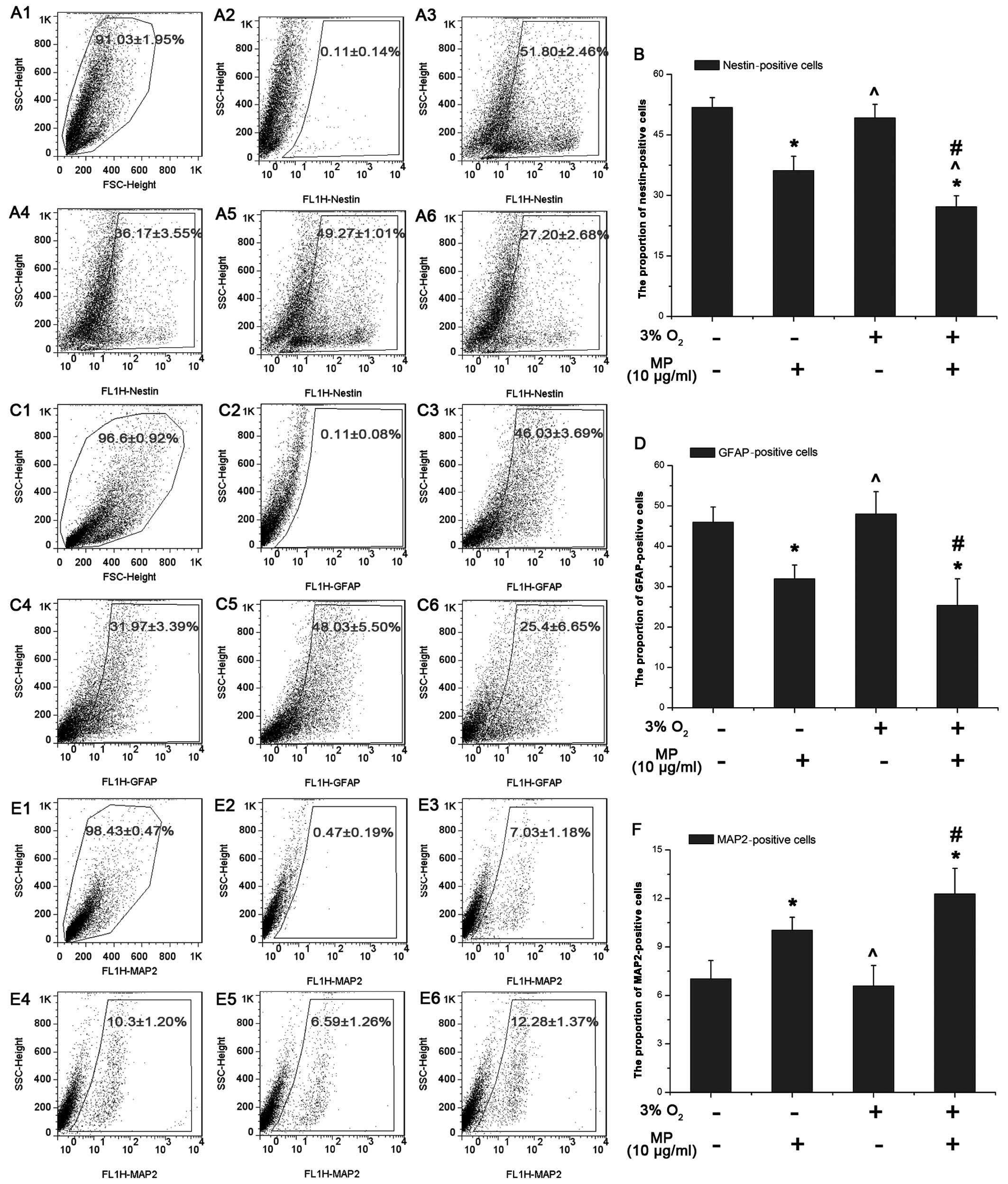
MP decreases the proportion of
nestin-positive NPCs more significantly in a low oxygen enviroment
and affects NPC differentiation with a decrease in the number of
astrocytes, and a slight increase in the number of neurons
As shown by our results from western blot analysis,
MP decreased the expression of Hes1, which indicated that MP can
affect NPC differentiation. Thus, we examined the proportion of
nestin-, GFAP- and MAP2-positive NPCs following 3 days of culture
with 5% FBS by FACS, as previously described (15). The proportion of nestin-positive
NPCs (0 μg/ml MP) cultured in normoxic conditions (51.8±2.46%;
Fig. 5A-3) was almost the same as
that of the NPCs cultured in a low oxygen enviroment (49.27±1.01%)
(P>0.05; Fig. 5A-5). Similar
results were observed for the GFAP- and MAP2-positive cells (not
treated with MP) (P>0.05; Fig. 5D
and F). Following the addition of MP (10 μg/ml), the number of
nestin-positive cells decreased more prominently under low oxygen
conditions (3% O2) (27.20±2.68%; Fig. 5A-6) than under normal oxygen
conditions (20% O2) (36.17±3.55%; Fig. 5A-4). These results suggested that
MP induced the differentiation of the NPCs into non-stem like
cells. Following treatment with MP (10 μg/ml) the number of
GFAP-positive cells decreased under both normoxic (31.97±3.39%)
(Fig. 5C-4) and hypoxic
conditions (25.40±6.65%; Fig.
5C-6) with a similar tendency as the nestin-positive cells
(Fig. 5B). This suggested that MP
suppressed the differentiation of NPCs into astrocytes (Fig. 5D). On the contrary, more NPCs
differentiated into neurons, as the proportion of MAP2-positive
cells was elevated following treatment with MP (10 μg/ml) both
under normoxic (10.30±1.20%; Fig.
5E-4) and hypoxic conditions (12.28±1.37%; Fig. 5E-6).
Discussion
In this study, we explored the effects of MP on NPC
proliferation and differentiation under both low oxygen and
normoxic conditions in vitro. Our results revealed that a
low oxygen enviroment promoted the proliferation of NPCs; however,
MP inhibited NPC proliferation, reduced the percentage of stem-like
cells and affected cell differentiation under both normoxic and
hypoxic conditions. MP suppressed the expression of HIF-1α and its
downstream target genes, VEGF and PGK1, in a low oxygen enviroment.
Furthermore, the Notch signaling pathway downstream proteins, Hes1,
which play an important role in the regulation of NPC
differentiation, were downregulated following treatment with
MP.
Our previous study and the study by Schröter et
al showed that MP exerted inhibitory effects on the
proliferation of endogenous NPCs in vitro and in vivo
following SCI (7,8); however, the mechanisms involved
remain unclear. A number of studies have demonstrated that a low
oxygen enviroment promotes the proliferation of neural stem cells
in vitro and in vivo (5–7).
In a previous study, Zhao et al indicated that HIF-1α is
critical in this process, as when HIF-1α expression was knocked
down, the proliferation of embryonic mesencephalon-derived neural
stem cell was suppressed (16).
It has also been demonstrated that dexamethasone attenuates HIF-1
activity in a GR-dependent manner and suppresses VEGF expression in
HepG2 cells (17). However, to
the best of our knowledge, no study to date has focused on the
effects of MP on spinal cord-derived NPCs in a low oxygen
enviroment. In this study, we first demonstrated that in a low
oxygen enviroment, MP had an inhibitory effect on the proliferation
of rat spinal cord-derived NPCs and decreased the expression of
HIF-1α in the NPCs at the mRNA and protein level.
In their study, Murata et al indicated that
Hes1 directly promotes the proliferation of embryonic carcinoma
cells (18). Blocking Hes1
expression has been shown to suppress human neural stem cell
proliferation in vitro by stimulating the of expression of
Cyclin-dependent Cdk kinase inhibitor (19). In addition, the activation of the
Notch receptor promotes the survival of fetal neural stem cells by
inducing the expression of Hes3 (20). Dexamethasone has been shown to
reduce the expression of Hes1 in cochlear cells following exposure
to noise (21). In this study, we
demonstrated that MP reduced the expression of Hes1 under both
normoxic and hypoxic conditions and inhibited the proliferation of
spinal cord-derived NPCs.
The Notch signaling pathway plays a very important
role in regulating the differentiation of NPCs (10,22). In mouse neural stem cells, the
activation of Notch signaling promotes the differentiation of
astrocytes, but inhibits the differentiation of oligodendrocytes
and neurons (23). It has also
been demonstrated that Notch can prevent the degradation of nestin
protein in MHP36 neural stem cells (24). Blocking Hes1 in human neural stem
cells in vitro stimulates the differentiation of GABAergic
neurons (19). Recent evidence
also indicates novel roles for HIF-1α in stem cell differentiation
through the modulation of Notch signaling pathways (25). A previous study demonstrated that
HIF-1α was vital to maintain the activation of the Notch-1 pathway
and maintain MDB stem cell viability and proliferation (26). Low oxgen levels in embryonic NPCs
enhances Notch signaling through direct HIF-1α binding to activated
Notch-1 (NICD), resulting in the increased stabilization of NICD
and the transcription of Notch-1 target genes (11). Our results demonstrated that Hes1
expression levels were almost the same as those of HIF-1α in the
cells cultured in 20 and 3% O2 without DAPT treatment.
Previous studies have shown that dexamethasone inhibits HIF-1α
protein expression in Th and HepG2 cells (9,17).
These data suggest that glucocorticoids suppress the expression of
Notch through the inhibition of HIF-1α. Dexamethasone can also
decrease the expression of Hes1 (21), which suggests taht glucocorticoids
can also directly restrain the Notch signaling pathway. Our study
demonstrated MP did not affect NICD, but inhibited HIF-1α and the
Notch signaling pathway downstream protein, Hes1, in spinal
cord-derived cells. The effects was more notable in a low oxygen
enviroment. After blocking the expression of NICD with DAPT, MP
still inhibited the expression of Hes1. Those results demonstrate a
possible mechanism through MP affects NPCs in a low oxygen
enviroment. Our results from FACS analysis suggested that MP
affected the differentiation of spinal cord-derived NPCs by
suppressing HIF-1α and Hes1, which led to a decrease in the
proportion of progenitor cells and the percentage of astrocytes,
and a slight increase in the number of neurons.
In conclusion, our results confirm that MP
significantly inhibits the proliferation of NPC and affects their
differentiation, altering the proportion astrocytes in a low oxygen
enviroment in vitro. Our results from PCR and western blot
analysis provide insight into the molecular mechanisms responsible
for the inhibition of proliferation and the effects on
differentiation mediated by MP and suggestthat the downregulation
of HIF-1α and Hes1 play a vital role in this process.
Acknowledgements
This study was supported by grants from the Health
Public Scientific Research Fund of China (201002018) and the
Program of National Natural Science Foundation of China (81000529)
and the Guangdong Science and Technology Program (2011B031800019)
and the National Natural Foundation of China-Guangdong Province
Combined Foundation (U1301223).
References
|
1
|
Hausmann ON: Post-traumatic inflammation
following spinal cord injury. Spinal Cord. 41:369–378. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jones TB, McDaniel EE and Popovich PG:
Inflammatory-mediated injury and repair in the traumatically
injured spinal cord. Curr Pharm Des. 11:1223–1236. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Majmundar AJ, Wong WJ and Simon MC:
Hypoxia-inducible factors and the response to hypoxic stress. Mol
Cell. 40:294–309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Horie N, So K, Moriya T, et al: Effects of
oxygen concentration on the proliferation and differentiation of
mouse neural stem cells in vitro. Cell Mol Neurobiol. 28:833–845.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu LL, Wu LY, Yew DT and Fan M: Effects
of hypoxia on the proliferation and differentiation of NSCs. Mol
Neurobiol. 31:231–242. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen X, Tian Y, Yao L, Zhang J and Liu Y:
Hypoxia stimulates proliferation of rat neural stem cells with
influence on the expression of cyclin D1 and c-Jun N-terminal
protein kinase signaling pathway in vitro. Neuroscience.
165:705–714. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schröter A, Lustenberger RM, Obermair FJ
and Thallmair M: High-dose corticosteroids after spinal cord injury
reduce neural progenitor cell proliferation. Neuroscience.
161:753–763. 2009.PubMed/NCBI
|
|
8
|
Li SY, Wang P, Tang Y, Huang L, Wu YF and
Shen HY: Analysis of methylprednisolone-induced inhibition on the
proliferation of neural progenitor cells in vitro by gene
expression profiling. Neurosci Lett. 526:154–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gaber T, Schellmann S, Erekul KB, et al:
Macrophage migration inhibitory factor counterregulates
dexamethasone-mediated suppression of hypoxia-inducible factor-1
alpha function and differentially influences human CD4+
T cell proliferation under hypoxia. J Immunol. 186:764–774. 2011.
View Article : Google Scholar
|
|
10
|
Alexson TO, Hitoshi S, Coles BL, Bernstein
A and van der Kooy D: Notch signaling is required to maintain all
neural stem cell populations-irrespective of spatial or temporal
niche. Dev Neurosci. 28:34–48. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gustafsson MV, Zheng X, Pereira T, et al:
Hypoxia requires notch signaling to maintain the undifferentiated
cell state. Dev Cell. 9:617–628. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oya S, Yoshikawa G, Takai K, et al:
Attenuation of Notch signaling promotes the differentiation of
neural progenitors into neurons in the hippocampal CA1 region after
ischemic injury. Neuroscience. 158:683–692. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shihabuddin LS, Ray J and Gage FH: FGF-2
is sufficient to isolate progenitors found in the adult mammalian
spinal cord. Exp Neurol. 148:577–586. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shihabuddin LS, Horner PJ, Ray J and Gage
FH: Adult spinal cord stem cells generate neurons after
transplantation in the adult dentate gyrus. J Neurosci.
20:8727–8735. 2000.PubMed/NCBI
|
|
15
|
Sergent-Tanguy S, Chagneau C, Neveu I and
Naveilhan P: Fluorescent activated cell sorting (FACS): a rapid and
reliable method to estimate the number of neurons in a mixed
population. J Neurosci Methods. 129:73–79. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao T, Zhang CP, Liu ZH, et al:
Hypoxia-driven proliferation of embryonic neural stem/progenitor
cells-role of hypoxia-inducible transcription factor-1alpha. FEBS
J. 275:1824–1834. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wagner AE, Huck G, Stiehl DP, Jelkmann W
and Hellwig-Burgel T: Dexamethasone impairs hypoxia-inducible
factor-1 function. Biochem Biophys Res Commun. 372:336–340. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Murata K, Hattori M, Hirai N, et al: Hes1
directly controls cell proliferation through the transcriptional
repression of p27Kip1. Mol Cell Biol. 25:4262–4271. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kabos P, Kabosova A and Neuman T: Blocking
HES1 expression initiates GABAergic differentiation and induces the
expression of p21(CIP1/WAF1) in human neural stem cells. J Biol
Chem. 277:8763–8766. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Androutsellis-Theotokis A, Leker RR,
Soldner F, et al: Notch signalling regulates stem cell numbers in
vitro and in vivo. Nature. 442:823–826. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang B, Liu Y, Chi F, Zhang Y, Yang M and
Zhu X: Dexamethasone suppresses cochlear Hes1 expression after
noise exposure. Acta Otolaryngol. 133:233–238. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mizutani K, Yoon K, Dang L, Tokunaga A and
Gaiano N: Differential Notch signalling distinguishes neural stem
cells from intermediate progenitors. Nature. 449:351–355. 2007.
View Article : Google Scholar
|
|
23
|
Grandbarbe L, Bouissac J, Rand M, Hrabé de
Angelis M, Artavanis-Tsakonas S and Mohier E: Delta-Notch signaling
controls the generation of neurons/glia from neural stem cells in a
stepwise process. Development. 130:1391–1402. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mellodew K, Suhr R, Uwanogho DA, et al:
Nestin expression is lost in a neural stem cell line through a
mechanism involving the proteasome and Notch signalling. Brain Res.
151:13–23. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cunningham LA, Candelario K and Li L:
Roles for HIF-1alpha in neural stem cell function and the
regenerative response to stroke. Behav Brain Res. 227:410–417.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pistollato F, Rampazzo E, Persano L, et
al: Interaction of HIF1α and Notch signaling regulates
medulloblastoma precursor proliferation and fate. Stem Cells.
28:1918–1929. 2010.
|