Introduction
In 2006, Sonoyama et al isolated a new
population of mesenchymal stem cells from the root apical papilla
of human tooth, which were designated as stem cells from apical
papilla (SCAP) (1). SCAP
expressed mesenchymal stem cell phenotypes, including STRO-1 and
CD146, and a specific surface marker, i.e., CD24 (2–5).
SCAP are considered the source of odontoblasts, which are
responsible for the development of root dentin (3–7).
In a minipig model, tooth root development was ceased when the
apical papilla was surgically removed, whereas root development was
completed when the apical papilla was conserved (6). SCAP have a significantly higher
proliferation rate and mineralization capacity than dental pulp
stem cells (DPSCs) (3,7). Findings of previous studies
demonstrated that the proliferation ratio of SCAP was approximately
2 times greater than that of DPSCs in vitro (3,5).
Additionally, both DPSCs and SCAP presented an initiative potential
for mineralization and osteo/odontogenic gene expression, including
alkaline phosphatase (ALP), osteocalcin (OCN), bone sialoprotein
(BSP) and dentin sialophosphoprotein (DSPP) (3). Furthermore, the mineralization ratio
of SCAP was significantly higher than that of DPSCs with
osteo/odontogenic stimuli, which resulted in increased amounts of
mineralized deposits in SCAP (3,5,7).
As a novel seed cell for dental regeneration, SCAP are believed to
have broad prospects. However, the genes that regulate the
odontogenic differentiation of SCAP remain unclear.
Bone morphogenetic protein 2 (BMP2) is a key
gene for modulating odontogenic differentiation in tooth
development (8–10). It is involved in the regulation of
odontogenic differentiation of dental pulp cells, and in the
control of the mineralization processes of the dentin matrix
(11). Co-cultured with
recombinant human BMP2 (rhBMP2) medium, the odontogenic
differentiation of DPSCs was promoted, resulting in an increased
gene expression of DSPP in vitro, and an enhanced reparative
dentin formation on amputated pulp (12). Yang et al constructed human
BMP2 gene-transfected DPSCs (DPSCs/BMP2), and reported that
the osteo/odontogenic differentiation genes, including ALP, OCN,
collagen type I (Col I), BSP, DSPP and dentin matrix protein 1
(DMP1), were significantly enhanced at different time points,
compared with DPSCs (13–15). Furthermore, rhBMP2-treated DPSCs
presented significantly reduced proliferation compared with the
control (16). However, the
effects of BMP2 on the odontogenic differentiation of SCAP remain
to be elucidated.
In this study, BMP2 gene was transfected into
SCAP by lentiviral-mediated transfection to construct SCAP/BMP2.
The proliferation capacity of SCAP/BMP2 was analyzed by CCK-8. The
odontogenic differentiation potential of SCAP/BMP2 was evaluated by
quantitative polymerase chain reaction (qPCR), ALP staining and
alizarin red (AR) staining. We hypothesized that SCAP/BMP2 have
greater odontogenic differentiation potential as compared to
SCAP/Vector, when cultured without osteo/odontogenic stimuli. The
results of the present study suggested the effects of the
BMP2 gene on the odontogenic differentiation of SCAP.
Materials and methods
Isolation and culture of SCAP
The experiments were performed with the approval of
the Ethics Committee of Guanghua School and Hospital of
Stomatology, Sun Yat-sen University (Guangzhou, China). Human
impacted wisdom teeth with immature roots were collected from 16-
to 18-year-old patients at the Department of Oral Maxillofacial
Surgery of the Guanghua Hospital of Stomatology. The extracted
third molars were carefully rinsed by phosphate-buffered saline
(PBS) with 100 U/ml penicillin and 100 mg/ml streptomycin, and then
temporarily maintained in Hanks’ solution. The root apical papillae
were gently separated from teeth roots, minced and digested by 3
mg/ml type I collagenase (Worthington Biochemical Co., Freehold,
NJ, USA) and 4 mg/ml dispase (Gibco Life Technologies, Beijing,
China) for 1 h at 37°C. Single-cell suspension was obtained by
passing through a 70 μm strainer (BD Biosciences, Bedford, MA,
USA). These isolated SCAP with 5×104/well density were
seeded in 6-well plates (Corning Life Sciences, Oneonta, NY, USA)
containing α-MEM (Invitrogen, Hong Kong, China) supplemented with
10% FBS (Gibco Life Technologies Australia Pty Ltd., Mulgrave
Victoria, Australia), 100 U/ml penicillin-G and 100 mg/ml
streptomycin, and then cultured with 5% CO2 at 37°C.
Differentiation stimulation
The multipotential of SCAP was confirmed by
osteogenic and adipogenic differentiation induction. Briefly, SCAP
were cultured with OriCell™ Human Mesenchymal Stem Cell Osteogenic
Differentiation Medium and OriCell Human Mesenchymal Stem Cell
Adipogenic Differentiation Medium (both from Cyagen Biosciences
Inc., Guangzhou, China) for 32 and 8 days, respectively. AR
solution and Oil Red O reagent kit (Jiancheng Bioengineering
Institute, Nanjing, China) were used to visualize the mineralized
nodules and lipid droplets, respectively.
Flow cytometry (FCM) analysis
The phenotypes of the passage 2 SCAP were evaluated
by FCM for the expression of STRO-1/Alexa Fluor 647-APC (BioLegend,
San Diego, CA, USA), CD146/PE, CD24/FITC and CD45/FITC (all from BD
Pharmingen, San Diego, CA, USA).
Construction of recombinant lentivirus
plasmid
Human BMP2 gene primers were designed by
Oligo 7.0 software (Molecular Biology Insights, Inc., Plymouth, MN,
USA) according to the NCBI GenBank no. KC294426.1. PCR primers were
designed as follows: forward, GCCGAATTCA TGGTGGCCGGGACCCGCTG
(the underlined is tje EcoRI site) and reverse,
GCCGGATCCCTAGCGACACCCACAAC CCTC
(the underlined is the BamHI site). For PCR amplification,
the primers were processed with a pre-denaturation step (95°C for 3
min), followed by 30 cycles of denaturation (95°C for 15 sec),
annealing (55°C for 30 sec), and extension (72°C for 1 min), then
stored at 72°C for 7 min prior to termination of the amplification
action at 4°C for 10 min. The amplified primers were subsequently
treated with EcoRI and BamHI, and the fragment
containing human BMP2 gene was combined into the lentivirus
vector pCDH-CMV-MCS-EF1-copGFP (pCDH; System Biosciences, Mountain
View, CA, USA) to construct the recombinant plasmid, i.e.,
pCDH-BMP2.
Lentiviral-mediated gene transfection of
SCAP
A volume of 5 ml 293FT cell (System Biosciences)
suspensions with 2.4×105 cells/ml density was bred on a
10 cm dish 2 days prior to transfection, and was cultured by DMEM
(Invitrogen) with 10% FBS. The recombinant plasmid pCDH-BMP2,
packaging plasmid psPAX.2 and envelope plasmid pMD2.G (the latter
two from Cyagen) were co-transfected into 293FT cells by
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) (17,18). The supernatant of 293FT cells was
assembled after 48 h, centrifuged at 1,000 × g 37°C for 10 min, and
filtered by 0.2 μm syringe filter (Millipore Corp., Bedford, MA,
USA). To obtain an optimum multiplicity of infection (MOI), SCAP
were infected with pCDH-BMP2 plasmid at different MOI values (5,
10, 20, 50 and 70) to obtain SCAP/BMP2. Green fluorescent protein
(GFP) fluoresce of SCAP/BMP2 was assessed under a fluorescence
microscope (Carl Zeiss Microimaging GmbH, Gottingen, Germany), and
the transfection efficiency was evaluated by the GFP expression
proportion of the cells. SCAP/Vector was constructed by infecting
SCAP with blank vector and served as the control. The BMP2
gene expression of SCAP/Vector and SCAP/BMP2 was assessed by qPCR
and western blot analysis 4 days after transfection. SCAP/Vector
and SCAP/BMP2 were bred in 25 cm2 culture flasks
containing α-MEM with 10% FBS, and cultured with 5% CO2
at 37°C. The culture medium was changed at 48-h intervals.
Cell proliferation
SCAP/Vector and SCAP/BMP2 were seeded in 96-well
plates with a density of 2×103 cells/well and were
cultured in α-MEM with 10% FBS. The cell proliferation rate was
analyzed using a cell counting kit-8 (CCK-8; Dojindo, Tokyo, Japan)
on the 1st, 2nd, 4th and 8th day following gene transfection.
qPCR analysis
The total RNA of the SCAP/Vector and SCAP/BMP2 was
extracted using TRIzol (Invitrogen) on the 1st, 4th, 8th, and 16th
day following gene transfection. The RNA quantity was assessed by a
spectrophotometer (Model 400-MR; Varian Inc., Palo Alto, CA, USA).
For each sample, 2 μg RNA was utilized to synthesize cDNA by
RevertAid First Strand cDNA Synthesis kit (Thermo Fisher Scientific
Inc., Beijing, China). Real-time reaction was performed by iQ
SYBR-Green Supermix and regulated by spectrofluorimetric thermal
iCycler iQ5 (both from Bio-Rad, Hercules, CA, USA). The BMP2 and
odontogenic differentiation genes, including ALP,
OCN, DSPP and DMP1, were assessed by qPCR.
Each sample was assessed 3 times. Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) was selected as the housekeeping gene
(Table I). The gene-specific
primers were amplified with a denaturation step (95°C for 3 min);
followed by 39 cycles of denaturation (95°C for 10 sec), annealing
(55°C for 10 sec), and extension (72°C for 30 sec).
 | Table IPrimer sequences used in qPCR. |
Table I
Primer sequences used in qPCR.
| Gene | Sense | Antisense | Size (bp) |
|---|
| BMP2 |
CACTGTGCGCAGCTTCC |
CCTCCGTGGGGATAGAACTT | 107 |
| ALP |
CTATCCTGGCTCCGTGCTC |
GCTGGCAGTGGTCAGATGTT | 100 |
| OCN |
CTCACACTCCTCGCCCTATT |
TTGGACACAAAGGCTGCAC | 107 |
| DSPP |
GCCACTTTCAGTCTTCAAAGAGA |
GCCCAAATGCAAAAATATGTAA | 130 |
| DMP1 |
AAAATTCTTTGTGAACTACGGAGG |
GAGCACAGGATAATCCCCAA | 94 |
| GAPDH |
AAGGTGAAGGTCGGAGTCAA |
AATGAAGGGGTCATTGATGG | 108 |
Western blot analysis
Western blot analysis was performed as described in
a previous study (19). Briefly,
the total proteins were calculated by a Bio-Rad Blue protein assay
(Bio-Rad Laboratories, Richmond, CA, USA). Protein was
distinguished with rabbit polyclonal antibody against human BMP2
(1:500 dilution; Abcam, Cambridge, UK) and human GAPDH (1:5,000
dilution).
ALP and AR staining
SCAP/Vector and SCAP/BMP2 were cultured in 6-well
plates at the initial density of 5×104 cells/well, in
α-MEM containing 10% FBS. After 16 days incubation, an ALP staining
kit (Jiancheng Bioengineering Institute) was applied to demarcate
ALP granules. Microscopic images were captured at a magnification
of ×100, ×200, ×400 and ×800, respectively. The AR staining
solution was used to demarcate mineralized deposits. AR staining
images were captured at a magnification of ×0 and ×200.
Statistical analysis
Data of cell proliferation and odontogenic
differentiation gene expression were expressed as the mean ±
standard deviation. A two-way ANOVA test was used to analyze the
differences between SCAP/Vector and SCAP/BMP2 (SPSS,Inc., Chicago,
IL, USA). The significance was set as P<0.05.
Results
Multipotential and phenotypic
characteristics of SCAP
The apical papillae were kidney-shaped (Fig. 1A). Isolated cells from apical
papilla presented a spindle appearance with extending cytoplasmic
processes (Fig. 1B). After 32
days osteogenic induction of SCAP, a mass of mineralized deposits
formed and presented positive Alizarin Red staining (Fig. 1C). After 8 days adipogenic
induction, a few lipid droplets formed and showed positive Oil Red
O staining (Fig. 1D). The
representative FCM results confirmed that the freshly isolated SCAP
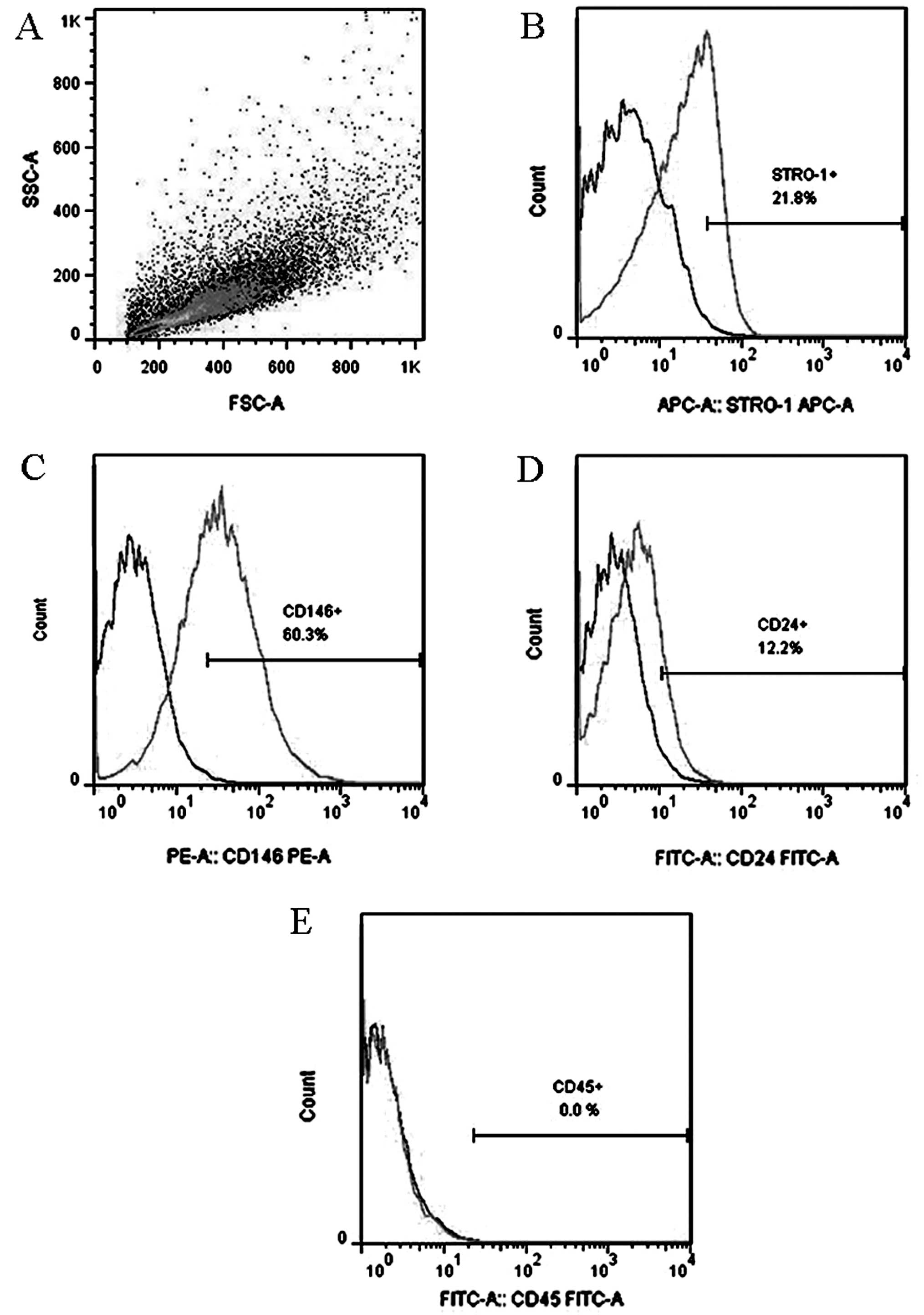
had a typical FSC/SSC character (Fig.
2A) as reported previously (3). The isolated cells expressed
mesenchymal stem cell (MSC) phenotypes, including STRO-1 and CD146,
and a specific phenotype CD24 (Fig.
2B–D). The expression of CD45, a hematopoietic stem cell
character, was negative (Fig.
2E).
Transfection efficiency
When the MOI value increased from 5 to 70, the
transfection efficiency was enhanced from 2% to >90% 48 h after
transfection (Fig. 3A and B).
When the MOI value was 70, the SCAP/Vector and SCAP/BMP2 presented
a high GFP expression proportion 4 days following gene
transfection. However, the morphology of the transfected stem cells
was slightly slimmer (Fig. 3C) as
compared to SCAP. The BMP2 gene expression was higher in
SCAP/BMP2 (6.23±0.04) than in SCAP/Vector (1.00±0.12) (P<0.01) 4
days after transfection (Fig.
3D). The western blot analysis confirmed that BMP2 expression
in SCAP/BMP2 was significantly increased compared with SCAP/Vector
(Fig. 3E).
Characterization of cell
proliferation
No significant difference of cell proliferation was
found between SCAP/Vector and SCAP/BMP2 (Fig. 4). The average optical density (OD)
of the two groups of cells generally increased from 0.2 to 0.8
during the observation period.
Odontogenic differentiation gene
expression
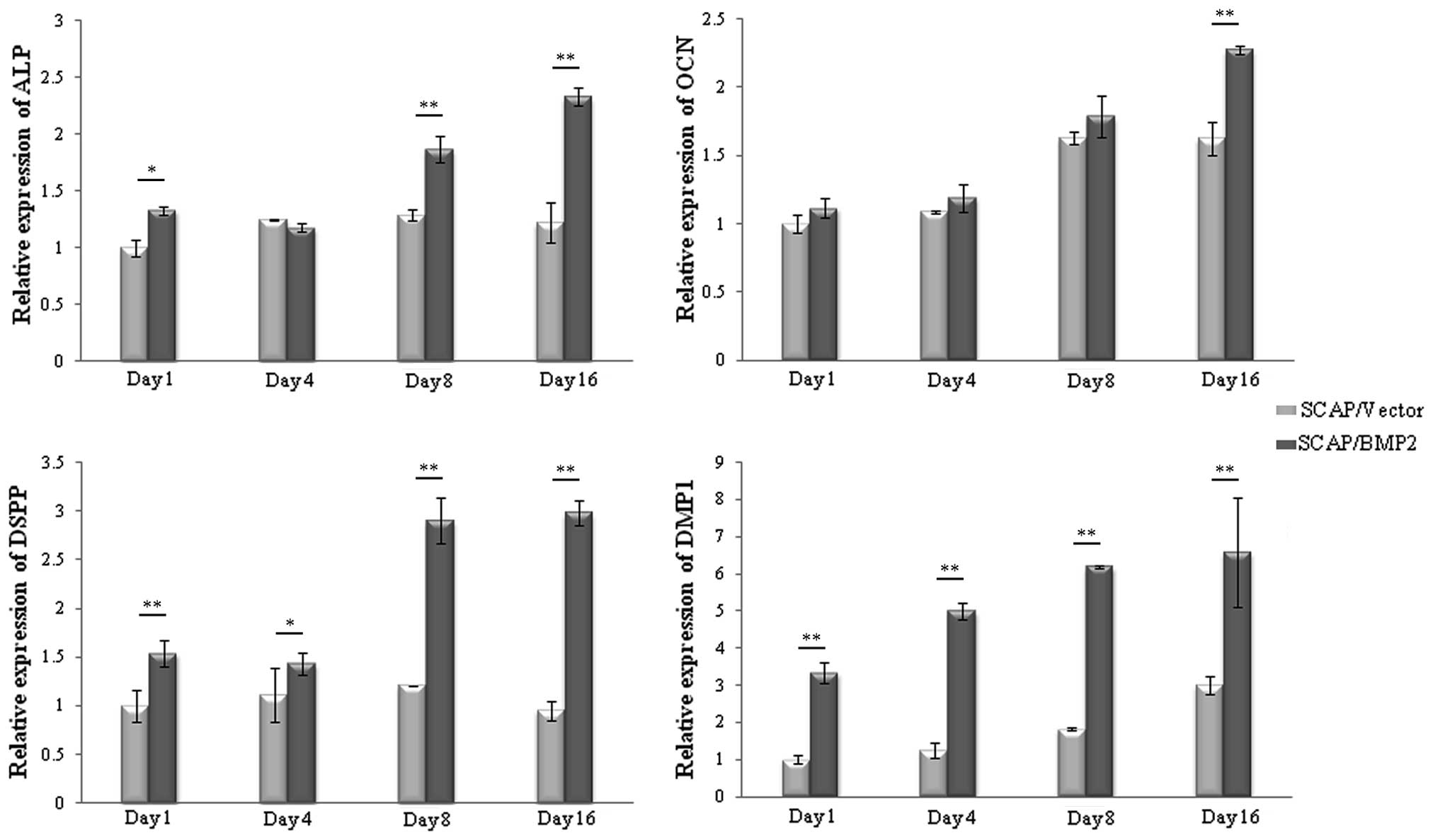
The relative peak expression of ALP in SCAP/Vector
was on the 8th day after transfection, while for SCAP/BMP2 the
expresion was on the 16th day. The ALP expression in SCAP/BMP2 was
significantly enhanced on the 1st day (P<0.05), 8th day
(P<0.01) and 16th day (P<0.01) compared with SCAP/Vector
(Fig. 5). The relative peak
expression of OCN in SCAP/Vector was on the 8th day, while in
SCAP/BMP2 it was on the 16th day. The OCN expression in SCAP/BMP2
was significantly upregulated on the 16th day after gene
transfection (P<0.01) compared with SCAP/Vector. The relative
peak expression of DSPP in SCAP/Vector was on the 8th day, while in
SCAP/BMP2 it was on the 16th day. The DSPP expression in SCAP/BMP2
was significantly upregulated on the 1st (P<0.01), 4th
(P<0.05), 8th (P<0.01) and 16th (P<0.01) day compared with
SCAP/Vector. The relative peak expression of DMP1 in SCAP/Vector
and SCAP/BMP2 was on the 16th day. The DMP1 expression in SCAP/BMP2
was significantly upregulated at the four time points (P<0.01)
compared with SCAP/Vector.
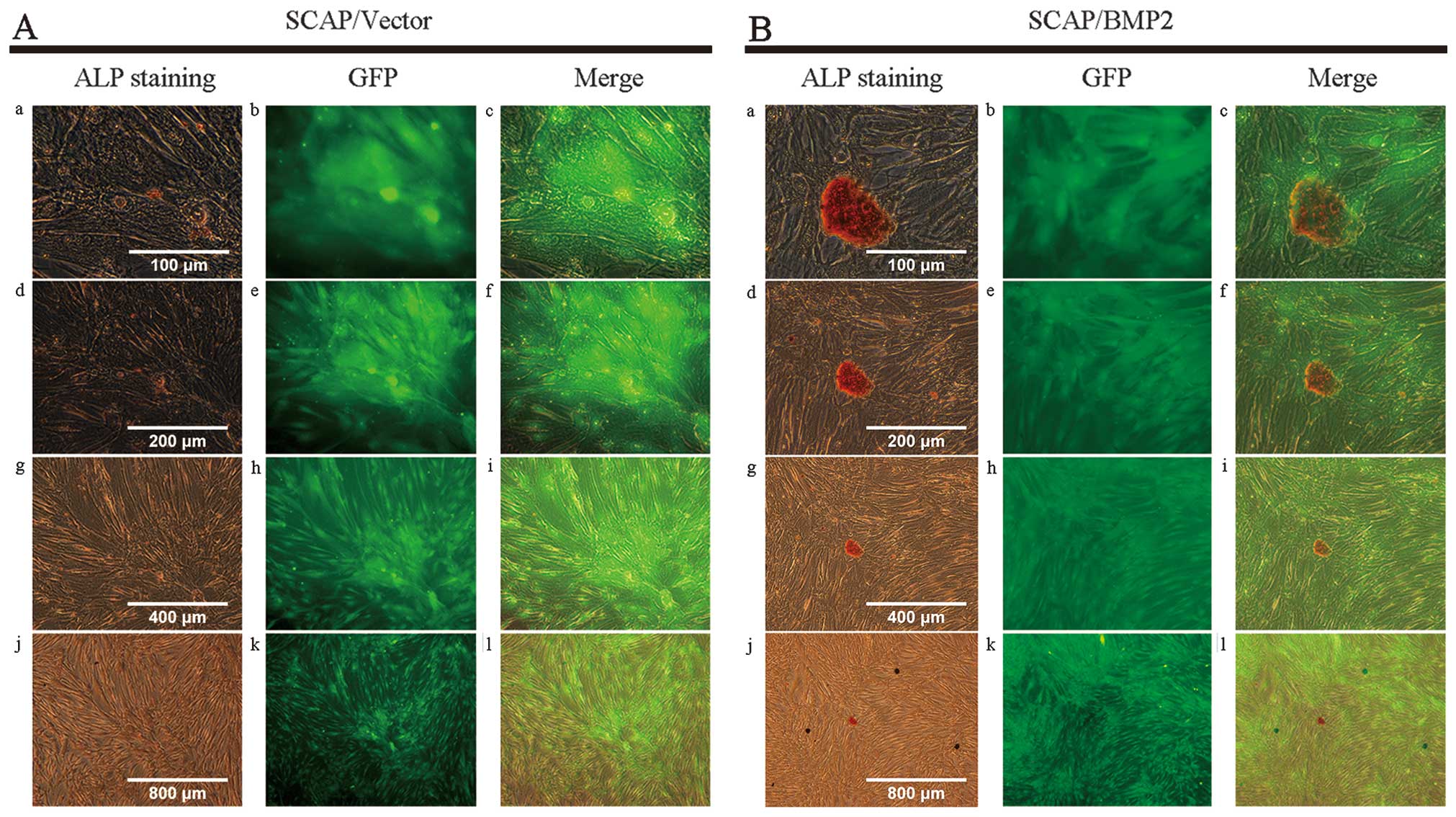
ALP staining
A positive ALP expression, defined as golden
staining granules and strips, was observed in both the SCAP/Vector
and SCAP/BMP2 (Fig. 6). The
quantity and brightness of ALP staining in SCAP/BMP2 was greater
than that of SCAP/Vector. ALP expression was observed in
intracellular as well as extracullar mineralized deposits.
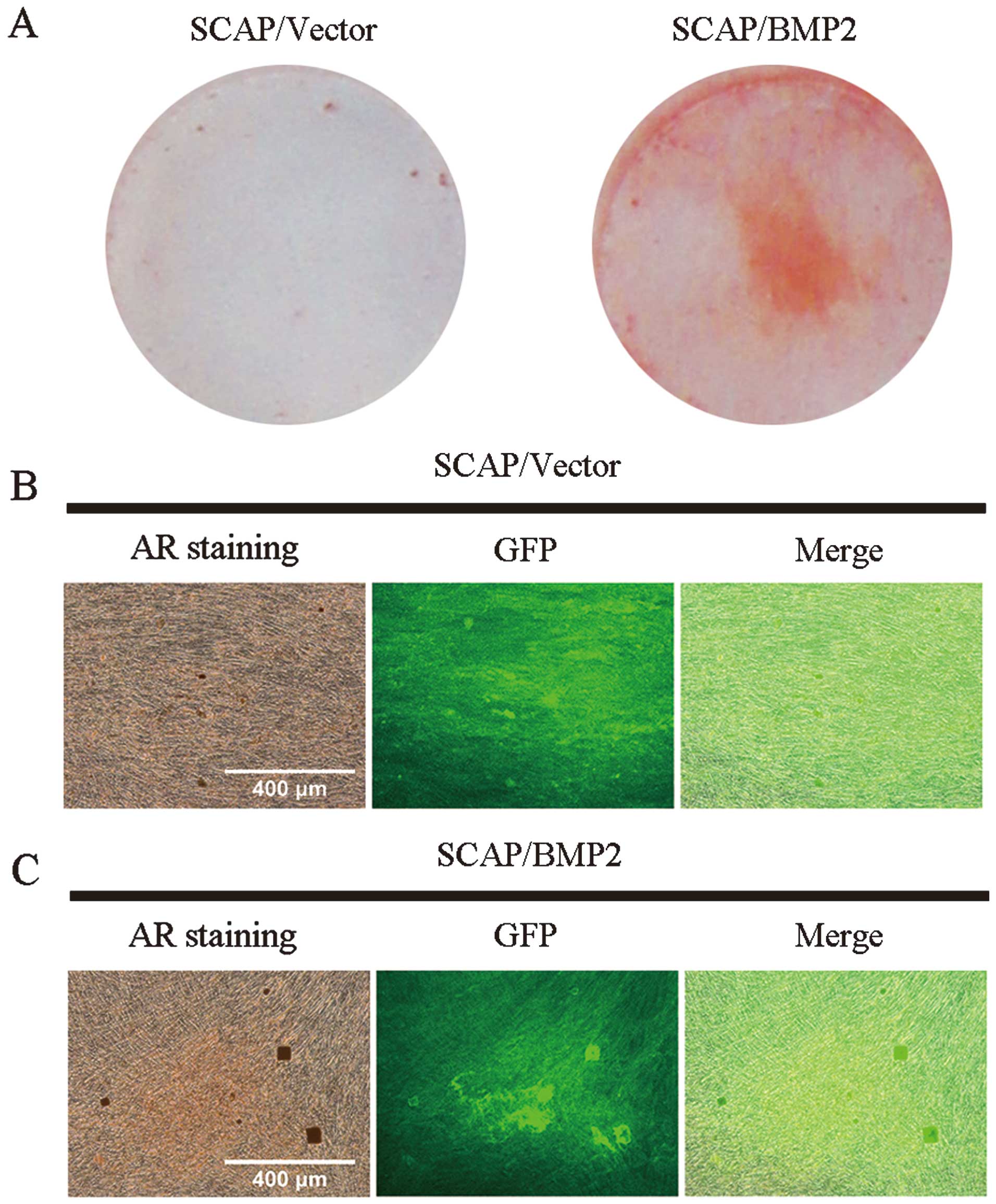
AR staining
The SCAP/BMP2 demonstrated significantly stronger AR
staining compared with SCAP/Vector on visual inspection (Fig. 7). More and larger mineralization
deposits were observed in SCAP/BMP2 compared with SCAP/Vector under
a microscope. For SCAP/BMP2, an intensive GFP expression was found
in the mineralized deposits.
Discussion
Dental papilla is commonly regarded as the source
for tooth formation and ultimately converts to dentin and dental
pulp tissue (1). SCAP, as a novel
population of multipotential stem cells isolated from the dental
papilla, have the capacity to differentiate into osteoblasts,
odontoblast-like cells and adipocytes in vitro (1–7).
Sonoyama et al induced the osteo/odontogenic differentiation
of SCAP by using basic culture medium (α-MEM and 15% FBS) plus 10
nmol/l dexamethasone, 10 mmol/l β-glycerophosphate, 50 μg/ml
ascorbate phosphate, and 10 nmol/l 1,25 dihydroxyvitamin D3
(1,5). SCAP formed mineralized deposits
progressively after 4–8 weeks of induction. In the present study,
the osteogenic differentiation of SCAP was induced using Human
Mesenchymal Stem Cell Osteogenic Differentiation Medium which
contains a similar formula to that described by Sonoyama et
al. Mineralized deposits were formed following induction for 32
days. Sonoyama et al induced the adiogenic differentiation
of SCAP by using the basic culture medium plus 0.5 mmol/l
hydrocortisone, 60 mmol/l indomethacin, 10 mg/ml insulin and 0.5
mmol/l isobutylmethylxanthine (1). Results of those studies showed that
the adipogenic differentiation capacity of SCAP was not as
qualitative as bone marrow mesenchymal stem cells, and few lipid
droplets were formed after 3 weeks. In the present study, lipid
droplet formation of SCAP was induced with Human Mesenchymal Stem
Cell Adipogenic Differentiation Medium for 8 days. The results of
the present study confirmed that SCAP have the capacity for
osteogenic and adipogenic differentiation.
SCAP were found to express MSC phenotypes, including
STRO-1, CD146, CD24, CD105, CD73, CD90, CD29, CD44 and CD49
(4). STRO-1, a marker that is
recognized as a trypsin insensitive epitope of perivascular cells,
has been used to isolate MSC populations from dental pulps and
apical papilla (4,13). STRO-1 selected DPSCs and SCAP
showed an enhanced ability of osteo/odontogenic differentiation.
Findings of previous studies have shown that the expression of
STRO-1 among SCAP is usually >18%. Additionally, the STRO-1
expression of passage 1 SCAP is up to 20–30% (4,7).
CD146 is one of the most employed key markers to characterize
perivascular multipotent stem cells in connective tissues. The
range of CD146 among SCAP is 47.3–84.8% (3). In the present study, the
representative expression of STRO-1 of passage 2 SCAP was 21.8%,
while the expression of CD146 was 60.3%. These results are
consistent with those of previous studies. CD24 is considered a
specific marker for SCAP, which is undetectable in other MSCs,
including DPSCs (1). The CD24
expression of SCAP ranges from 3.2 to 15.3% (4), and its expression decreases with an
upregulated expression of ALP during the odontogenic
differentiation of SCAP (1). In
the present study, the CD24 expression of passage 2 SCAP was 12.2%,
which is consistent with that identified in previous studies. The
FCM results confirmed that the phenotypes of the cells isolated
from the apical papilla in this study are characteristic of
SCAP.
Stem cells cultured with dexamethasone have been
confirmed to form more mineralized deposits in vitro
(20). Stem cells cultured with
L-ascorbic acid-2-phosphate have also been reported to result in an
upregulated cell proliferation and osteogenic differentiation
(20–22). Culturing cells with mineralization
stimuli may partly obscure the positive effects, which may due to
BMP2 gene transfection. Therefore, the cell-culturing media
without external supplements of dexamethasone or any other
mineralization stimuli were specifically selected for the present
in vitro study.
In tooth development, BMP2 was found to express
during the whole process of differentiation and maturation of
odontoblasts (8,9). BMP2 is crucial in the regulation of
odontoblast differentiation and dentin formation. BMP2 gene
transfection has been considered an effective strategy in the
improvement of the odontogenic differentiation potential of DPSCs
in vitro (13,14,16), leading to a significantly
increased gene expression of ALP, OCN, COL I,
BSP, DSP and DMP1. In the present study, the
odontogenic differentiation mRNA expression, including ALP, OCN,
DSPP and DMP1, was also significantly upregulated in SCAP/BMP2
compared with SCAP/Vector. Although in this study, BMP2 expression
was only tested on the 4th day following gene transfection in,
other studies have proven that lentivirus transfection can mediate
a stable expression of target genes in cells (23–25).
ALP, which was detected during the process of cell
mineralization, is considered an early marker of osteo/odontogenic
differentiation of stem cells derived from dental tissues (2,3,11,13,26–30). Results of the present study have
shown that SCAP/BMP2 had significantly upregulated ALP expressions
on the 1st, 8th and 16th day compared with SCAP/Vector, which was
similar to that in DPSCs/BMP2 (13). Additionally, ALP and AR staining
demonstrated that SCAP/BMP2 formed more ALP granules and
mineralized deposits compared with SCAP/Vector. OCN is a marker of
the late stages of osteo/odontogenic differentiation, which
regulates the mineral phase of bone and dentin (3). In a previous study it was found that
DPSCs/BMP2 had a significantly upregulated expression of OCN on the
8th and 16th day after transfection (13), while in the present study
SCAP/BMP2 showed a significantly upregulated expression of OCN on
the 16th day. DSPP is a significant part of the dentin
non-collagenous proteins, which play a key role in the dentin
mineralization process (3,26).
It is usually synthesized by terminally differentiated odontoblasts
during the secretory phase, and is regarded as a late-stage marker
of odontogenic differentiation. In the present study, qPCR showed
that lentiviral-mediated BMP2 gene transfection
significantly enhanced the expression of DSPP gene at the four
time-points, which was similar to a previous report (13). DMP1 is an acidic phosphorylated
extracellular matrix protein (12,31). Although DMP1 is not exclusively
expressed in odontogenic differentiation, it has been considered an
important marker for odontoblasts (26,32). DMP1 has dual functions, as a
transcription factor that targets the nucleus and as an
extracellular matrix protein that initiates mineralization
(29). In the present study, DMP1
expression in SCAP/BMP2 was significantly improved, which was also
consistent with a previous report (13). These results provided evidence
that the specific transfection of the BMP2 gene enhanced the
odontogenic differentiation of SCAP.
In summary, human dental papilla stem cells were
successfully transfected by BMP2 gene lentiviral plasmid.
Under the circumstances without mineralization stimuli, SCAP/BMP2
expressed more odontogenic differentiation genes, revealed more ALP
granules, and formed more mineralization deposits than SCAP/Vector.
These results are consistent with our hypothesis. Transfection of a
homologous BMP2 gene may therefore be an effective strategy
to improve the tissue engineering applications of SCAP.
Acknowledgements
We would like to thank Dr Chenfei Zhang (Clinical
Associate Professor in Endodontics, The University of Hong Kong)
for his sincere aid in the starting period of this study. We would
also like to thank all the staff and postgraduate students of the
Guanghua School of Stomatology, Sun Yat-sen University, who
assisted in the clinical sample collection and laboratory analysis.
This study was supported by the Guangdong Medical Science Research
Fund (B2012142), Fundamental Research Funds for Sun Yat-sen
University (12YKPY68) and National Natural Science Found of China
(81170932).
References
|
1
|
Sonoyama W, Liu Y, Fang D, et al:
Mesenchymal stem cell-mediated functional tooth regeneration in
swine. PLoS One. 1:e792006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu J, Huang GT, He W, et al: Basic
fibroblast growth factor enhances stemness of human stem cells from
the apical papilla. J Endod. 38:614–622. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bakopoulou A, Leyhausen G, Volk J, et al:
Comparative analysis of in vitro osteo/odontogenic differentiation
potential of human dental pulp stem cells (DPSCs) and stem cells
from the apical papilla (SCAP). Arch Oral Biol. 56:709–721. 2011.
View Article : Google Scholar
|
|
4
|
Bakopoulou A, Leyhausen G, Volk J, Koidis
P and Geurtsen W: Comparative characterization of (neg)/CD146(pos)
and STRO-1 (neg)/CD146(pos) apical papilla stem cells enriched with
flow cytometry. Arch Oral Biol. 58:1556–1568. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sonoyama W, Liu Y, Yamaza T, et al:
Characterization of the apical papilla and its residing stem cells
from human immature permanent teeth: a pilot study. J Endod.
34:166–171. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang GT, Sonoyama W, Liu Y, Liu H, Wang S
and Shi S: The hidden treasure in apical papilla: the potential
role in pulp/dentin regeneration and bioroot engineering. J Endod.
34:645–651. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang GT, Gronthos S and Shi S:
Mesenchymal stem cells derived from dental tissues vs. those from
other sources: their biology and role in regenerative medicine. J
Dent Res. 88:792–806. 2009.PubMed/NCBI
|
|
8
|
Yang W, Harris MA, Cui Y, Mishina Y,
Harris SE and Gluhak-Heinrich J: Bmp2 is required for odontoblast
differentiation and pulp vasculogenesis. J Dent Res. 91:58–64.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Li L, Zheng Y, et al: BMP activity
is required for tooth development from the lamina to bud stage. J
Dent Res. 91:690–695. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rakian A, Yang WC, Gluhak-Heinrich J, et
al: Bone morphogenetic protein-2 gene controls tooth root
development in coordination with formation of the periodontium. Int
J Oral Sci. 5:75–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saito T, Ogawa M, Hata Y and Bessho K:
Acceleration effect of human recombinant bone morphogenetic
protein-2 on differentiation of human pulp cells into odontoblasts.
J Endod. 30:205–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iohara K, Nakashima M, Ito M, Ishikawa M,
Nakasima A and Akamine A: Dentin regeneration by dental pulp stem
cell therapy with recombinant human bone morphogenetic protein 2. J
Dent Res. 83:590–595. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang X, van der Kraan PM, van den Dolder
J, et al: STRO-1 selected rat dental pulp stem cells transfected
with adenoviral-mediated human bone morphogenetic protein 2 gene
show enhanced odontogenic differentiation. Tissue Eng.
13:2803–2812. 2007. View Article : Google Scholar
|
|
14
|
Yang X, Walboomers XF, van den Dolder J,
et al: Non-viral bone morphogenetic protein 2 transfection of rat
dental pulp stem cells using calcium phosphate nanoparticles as
carriers. Tissue Eng Part A. 14:71–81. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang X, van der Kraan PM, Bian Z, Fan M,
Walboomers XF and Jansen JA: Mineralized tissue formation by
BMP2-transfected pulp stem cells. J Dent Res. 88:1020–1025. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang X, Walboomers XF, van den Beucken JJ,
Bian Z, Fan M and Jansen JA: Hard tissue formation of
STRO-1-selected rat dental pulp stem cells in vivo. Tissue Eng Part
A. 15:367–375. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lahmy R, Soleimani M, Sanati MH, Behmanesh
M, Kouhkan F and Mobarra N: Pancreatic islet differentiation of
human embryonic stem cells by microRNA overexpression. J Tissue Eng
Regen Med. 30: View Article : Google Scholar : 2013.PubMed/NCBI
|
|
18
|
Hwang SY, Foley J, Numaga-Tomita T,
Petranka JG, Bird GS and Putney JW Jr: Deletion of Orai1 alters
expression of multiple genes during osteoclast and osteoblast
maturation. Cell Calcium. 52:488–500. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wei X, Wu L, Ling J, et al: Differentially
expressed protein profile of human dental pulp cells in the early
process of odontoblast-like differentiation in vitro. J Endod.
34:1077–1084. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Langenbach F and Handschel J: Effects of
dexamethasone, ascorbic acid and β-glycerophosphate on the
osteogenic differentiation of stem cells in vitro. Stem Cell Res
Ther. 4:1172013.
|
|
21
|
Hitomi K, Torii Y and Tsukagoshi N:
Increase in the activity of alkaline phosphatase by L-ascorbic acid
2-phosphate in a human osteoblast cell line, HuO-3N1. J Nutr Sci
Vitaminol (Tokyo). 38:535–544. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shima N, Kimoto M, Yamaguchi M and
Yamagami S: Increased proliferation and replicative lifespan of
isolated human corneal endothelial cells with L-ascorbic acid
2-phosphate. Invest Ophthalmol Vis Sci. 52:8711–8717. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choi KS, Ahn SY, Kim TS, et al:
Characterization and biodistribution of human mesenchymal stem
cells transduced with lentiviral-mediated BMP2. Arch Pharm Res.
34:599–606. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kutner RH, Zhang XY and Reiser J:
Production, concentration and titration of pseudotyped HIV-1-based
lentiviral vectors. Nat Protoc. 4:495–505. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reiser J: Production and concentration of
pseudotyped HIV-1-based gene transfer vectors. Gene Ther.
7:910–913. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li R, Peng L, Ren L, Tan H and Ye L:
Hepatocyte growth factor exerts promoting functions on murine
dental papilla cells. J Endod. 35:382–388. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Choi MH, Noh WC, Park JW, Lee JM and Suh
JY: Gene expression pattern during osteogenic differentiation of
human periodontal ligament cells in vitro. J Periodontal Implant
Sci. 41:167–175. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu J, Deng Z, Shi J, et al:
Differentiation of dental pulp stem cells into regular-shaped
dentin-pulp complex induced by tooth germ cell conditioned medium.
Tissue Eng. 12:3097–3105. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang C, Ren L, Peng L, Xu P, Dong G and Ye
L: Effect of Wnt6 on human dental papilla cells in vitro. J Endod.
36:238–243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen K, Xiong H, Huang Y and Liu C:
Comparative analysis of in vitro periodontal characteristics of
stem cells from apical papilla (SCAP) and periodontal ligament stem
cells (PDLSCs). Arch Oral Biol. 58:997–1006. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yue J, Wu B, Gao J, et al: DMP1 is a
target of let-7 in dental pulp cells. Int J Mol Med. 30:295–301.
2012.PubMed/NCBI
|
|
32
|
Feng JQ, Huang H, Lu Y, et al: The Dentin
matrix protein 1 (Dmp1) is specifically expressed in mineralized,
but not soft, tissues during development. J Dent Res. 82:776–780.
2003. View Article : Google Scholar : PubMed/NCBI
|