Introduction
Epithelial ovarian cancer is the most common and
fatal gynecological malignancy, and is associated with a 5-year
survival rate of approximately 30%. The standard therapeutic
strategy comprises optimal cytoreductive surgery followed by
cisplatin-based systemic chemotherapy. Although the initial results
of this debulking followed by chemotherapy are usually good, the
majority patients relapse in <5 years due to chemoresistance
(1–5). Several mechanisms have been reported
to account for drug resistance (6), among which cancer stem cells have
been widely studied in different tumor models over the past decade
(4,6,7).
According to this model, a pool of cancer stem
cells, which are capable of both self-renewal and differentiation,
is the initiating contributor towards tumor pathogenesis, drug
resistance and recurrence (8–11).
Cancer stem cells are highly resistant to chemotherapy due to their
stem cell properties, mainly their quiescence and the expression of
drug membrane transporters. Therefore, cancer stem cells can
survive the therapy regimen and regenerate tumors, thus leading to
relapse. The development of novel drug candidates and therapeutic
strategies against ovarian cancer should therefore address the need
to combat both differentiated and stem cell populations.
Previous studies have demonstrated the feasibility
of isolating, enriching and propagating cancer stem-like cells
(CSLCs), highly expressing stemness marker genes [such as aldehyde
dehydrogenase (ALDH1), ALDH2, ATP-binding cassette,
sub-family G (WHITE), member 2 (ABCG2), chemokine (C-X-C
motif) receptor 4 (CXCR4), myeloid differentiation primary
response 88 (MyD88) and lin-28 homolog A (C. elegans)
(LIN28)] and being able to develop xenograft tumors with
high efficiency from ovarian cancer cells (11–13). More importantly, CSLCs have been
found not only in primary tumor samples, but also in immortalized
cell lines and long-term culture cancer cells (14–17).
In our previous studies employing a serum-free
suspension culturing system, we successfully enriched CSLCs from
ovarian cancer cell lines in vitro (18–21). These CSLCs formed non-adherent
spheroids and displayed remarkable stem cell properties, with
higher drug resistance and tumorigenic efficiency than their
differentiated counterparts. This system provides us with a
valuable investigating system with which to screen novel drug
candidates against human ovarian cancer cells (hOVCCs) and
CSLCs.
In the present study, we aimed to investigate the
anticancer effects of a bioactive fungal metabolite, namely
terrein, against both hOVCCs and CSLCs. Terrein
(4,5-dihydroxy-3-[(E)-1′-propenyl]-2-cyclopenten-l-one,
C8H10O3) was first isolated from
Aspergillus terreus Thom in 1935 (22), and has since been tested in
several applications across different fields, including the fields
of medicine, cosmetology and agriculture (23–29). However, the biological function of
terrein in targeting human diseases has not been extensively
investigated. Studies have demonstrated the strong
anti-proliferative effects of terrein on skin equivalents through
the induction of G2/M cell cycle arrest (27,30), suggesting its potential as a
valuable candidate for treating hyper-proliferative skin diseases.
However, the antitumor activity of terrein remains to be
investigated.
In our previous studies, we successfully isolated
the (+)-terrein from the fermentation broth of the marine
sponge-derived fungus, Aspergillus terreus strain PF26, with
high production efficiency and high quality (31,32). In the present study, we aimed to
investigate the anticancer effects of terrein isolated in this
manner on a human epithelial ovarian cancer model.
Materials and methods
Terrein preparation and cell culture
Terrein was separated from the fermentation broth of
Aspergillus terreus strain PF-26 (31,32), and subjected to identification and
quantification by high-performance liquid chromatography (HPLC) as
previously described (31). The
human ovarian epithelial cancer cell line, SKOV3, was purchased
from the Shanghai Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). Adherent SKOV3 cells were cultured in regular
culture plates at 37°C, in a humidified environment containing 5%
CO2 with the McCoy’s 5A medium (Sigma-Aldrich, St.
Louis, MO, USA), supplemented with 10% fetal bovine serum (FBS;
HyClone, Logan, UT, USA), 100 units/ml penicillin and 100 μg/ml
streptomycin.
Primary human epithelial ovarian tumor cells were
isolated from the tumors of 3 patients classified as stage III,
grade 2–3 serous adenocarcinoma according to the International
Federation of Gynecology and Obstetrics (FIGO) classification. The
study was approved by the Institutional Review Board at Shanghai
Jiaotong University (Shanghai, China). The cells were cultured in
the McCoy’s 5A medium (Sigma-Aldrich), supplemented with 10% fetal
bovine serum (FBS; HyClone), 100 units/ml penicillin and 100 μg/ml
streptomycin. Cisplatin was purchased from Sigma-Aldrich, dissolved
in DMSO and added to the culture medium where indicated.
Enrichment of CSLCs
Ovarian CSLCs were enriched as previously described
(21). Upon reaching 80%
confluence, the SKOV3 cells were dissociated by 0.25% Trypsin-EDTA
(Life Technologies, Carlsbad, CA, USA) for 1–2 min at 37°C. Single
cells were suspended in DMEM/F12 medium supplemented with 10 ng/ml
basic fibroblast growth factor (bFGF; Invitrogen, Carlsbad, CA,
USA) and 10% knockout serum (Gibco, Grand Island, NY, USA) in low
attachment plates. The medium was renewed every 2 days following
centrifugation at 800 rpm for 5 min to remove the dead cell debris.
After 7 days, spheroid-shaped CSLCs were selected for further
treatment or analysis.
Cell viability assay
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl
tetrazolium bromide (MTT) assay was employed to measure cell
viability. Approximately 1×104 cells were seeded per
well in a 96-well plate and allowed to attach overnight. The
culture medium was replaced with fresh medium containing terrein
(or PBS as mock treatment). Following 48 or 72 h of treatment, cell
viability was determined by MTT assay. The cells were incubated
with 10 μl MTT (5 mg/ml; Sigma-Aldrich) added to the medium for 4 h
at 37°C. The medium was then removed and the converted dye was
solubilized with 150 μl DMSO (dimethyl sulfoxide; Sigma-Aldrich).
Absorbance at 490 nm was measured on a microplate reader (Bio-Rad,
Hercules, CA, USA). Each assay was repeated in at least 6-wells and
each experiment was independently repeated 3 times.
Transwell assay
The migratory ability of the cells was determined by
Transwell assay as previously described (21). Briefly, the cells were seeded into
the top chamber of 8.0 μm Transwell invasion chambers (Corning
Inc., Corning, NY, USA) in serum-free medium. Complete medium was
added to the lower chambers. The cells were allowed to migrate for
24 h before being fixed in methanol and visualized by crystal
violet staining. The cells which did not migrate to the lower
chamber were removed by scraping. At least 3 random microscope
fields of view were observed.
Cell cycle analysis
Cell cycle analysis was performed as previously
described (21). The SKOV3 cells
were seeded in 6-cm dishes at a density of 3×105
cells/dish and allowed to attach and proliferate for 24 h. After
being treated with 15 mg/l terrein for 24 h, the cells were
harvested by trypsin digestion, before being washed with PBS and
fixed with 70% cold-ethanol for 24 h at −20°C. The cells were
treated with RNase A for 30 min at 37°C, and then stained with
propidium iodide (PI) for 30 min at 4°C. The cells were analyzed
using a Cytomics™ FC500 flow cytometer (Beckman Coulter, Brea, CA,
USA). Data were analyzed using Beckman Coulter CXP software.
Analysis of cell apoptosis
Following incubation with or without 15 mg/l terrein
for 48 h, the SKOV3 cells were harvested and analyzed as previously
described (33). Cell apoptosis
assay was performed using the Annexin V-FITC Apoptosis Detection
kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) according to
the manufacturer’s instructions. The cells were analyzed using a
Cytomics FC500 flow cytometer (Beckman Coulter).
Western blot analysis
The cells were washed in cold PBS, and lysed in
lysis buffer [50 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1%
sodium deoxycholate, 0.1% SDS, 1 mM phenylmethylsulfonyl fluoride,
20 μg/ml aprotinin and 25 μg/ml leupeptin] for 30 min on ice.
Protein was quantified by a standard BCA assay (Pierce
Biotechnology, Rockford, IL, USA). Total protein (50 μg) was
loaded, separated by SDS-PAGE gel, transferred onto PVDF membranes
and incubated with specific antibodies overnight at 4°C. The
antibodies used in the present study were rabbit monoclonal
antibodies against LIN28 (1:50,000; Epitomics, Burlingame, CA,
USA), against Cdc2 (1:10,000; Epitomics), against cyclin B1
(1:5000; Epitomics) and rabbit polyclonal antibodies against GAPDH
(1:2,000; HangZhou HuaAn Biotechnology Co., Ltd, Hangzhou, China).
This was followed by incubation with HRP (horseradish
peroxidase)-conjugated anti-rabbit IgG secondary antibodies
(1:1,000; Beyotime Institute of Biotechnology, Shanghai, China) for
1 h at room temperature. Immunoblot signals were visualized using
SuperSignal West Pico Chemiluminescent Substrate (Pierce
Biotechnology).
Quantitative PCR
RNA extraction and quantitative PCR were performed
as previously described (33).
The cells were harvested after being rinsed with PBS; RNA was
extracted using TRIzol reagent (Life Technologies) according to the
manufacturer’s instructions. DNase I (Fermentas, Hanover, MD, USA)
was used to exclude genomic DNA contamination. cDNA was synthesized
with random primers by a ReverTra Aca-α kit (Toyobo Co., Ltd.,
Osaka,. Japan). The expression of ALDH1, ALDH2,
ABCG2, CXCR4 and MyD88 was evaluated by
quantitative PCR. Quantitative PCR was performed with SYBR-Green
real-time PCR Master Mix Plus (Toyobo) using Mastercycler ep
realplex (Eppendorf, Hamburg, Germany). The primers used in the
present study are listed in Table
I.
 | Table IPrimers used for quantitative
PCR. |
Table I
Primers used for quantitative
PCR.
| Primer | Sequence
(5′→3′) |
|---|
| 18s rRNA-F |
CGGCGACGACCCATTCGAAC |
| 18s rRNA-R |
GAATCGAACCCTGATTCCCCGTC |
| LIN28-F |
AGTGGCCTGGATAGGGAAGT |
| LIN28-R |
CTTGGCTCCATGAATCTGGT |
| ALDH1-F |
TGTTAGCTGATGCCGACTTG |
| ALDH1-R |
TTCTTAGCCCGCTCAACACT |
| ALDH2-F |
TTCAACCAGGGCCAGTGCTGCTGT |
| ALDH2-R |
CCCCTCTTGCTTCCCCGTGTTGAT |
| ABCG2-F |
TGAGCCTTTGGTTAAGACCG |
| ABCG2-R |
TGGTGTTTCCTTGTGACACTG |
| CXCR4-F |
GGTGGTCTATGTTGGCGTCT |
| CXCR4-R |
TGGAGTGTGACAGCTTGGAG |
| MyD88-F |
GCACATGGGCACATACAGAC |
| MyD88-R |
TAGCTGTTCCTGGGAGCTGT |
Results
Anti-proliferative effects of terrein on
human epithelial ovarian cancer cells
We extracted and purified terrein from the
fermentation broth of the marine sponge-derived fungus,
Aspergillus terreus strain PF26, with high production
efficiency and high quality as previously described (31,32). The structure of terrein is
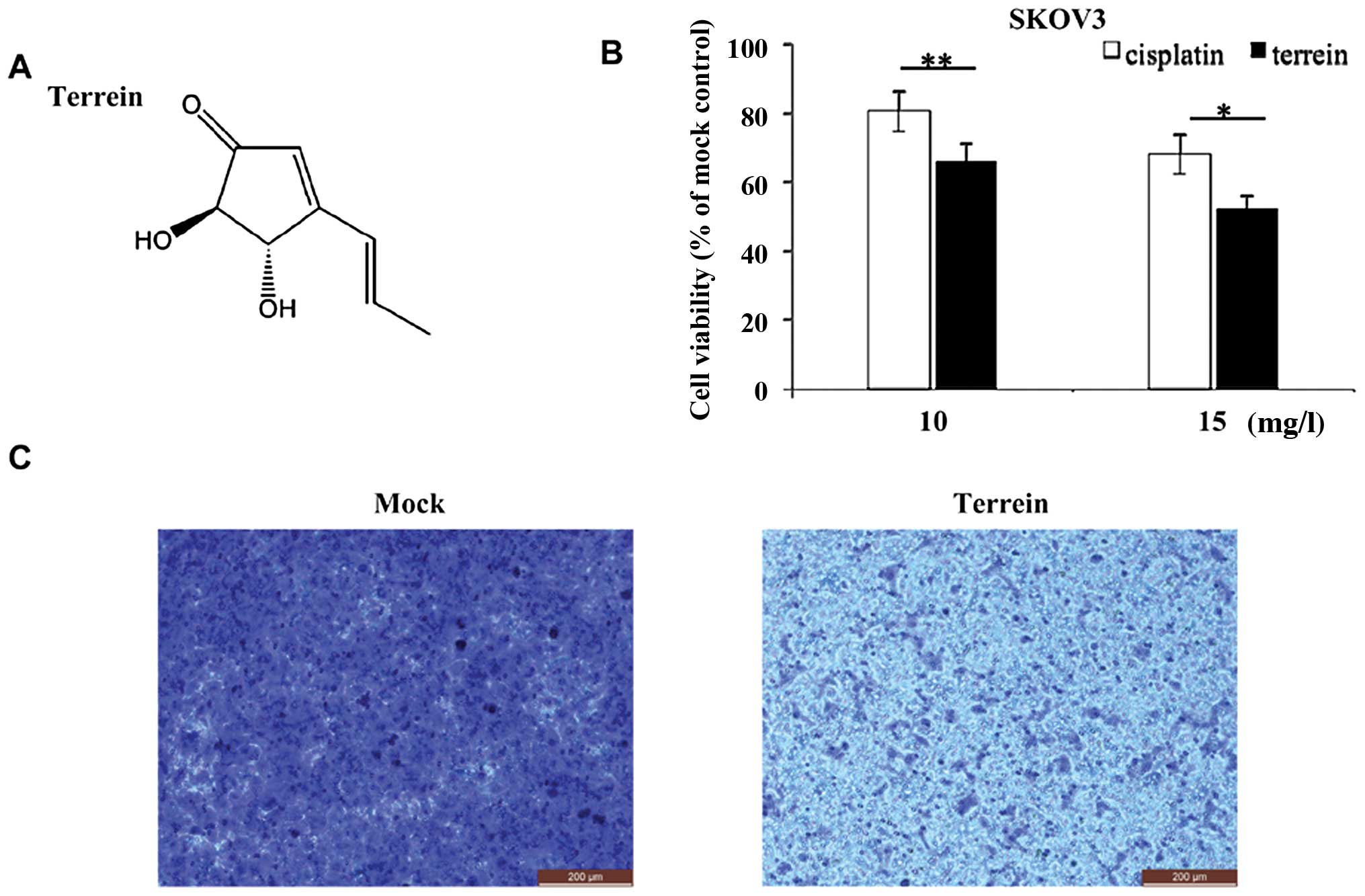
illustrated in Fig. 1A. To
determine whether terrein exerts anticancer effects against human
epithelial ovarian cancer, we first evaluated its
anti-proliferative effects on a cisplatin-resistant human
epithelial ovarian cancer cell line (SKOV3).
As shown in Fig.
1B, cisplatin, used as a clinical reference, displayed moderate
anticancer activity against the SKOV3 cells. By contrast, treatment
with terrein led to a more effective suppression of cell
proliferation: following 48 h of incubation with 15 mg/l terrein,
only ~50 viable SKOV3 cells remained. This suggests that terrein
effectively inhibits the proliferation of immortalized ovarian
cancer cells.
To investigate the effects of terrein on cell
migration, we performed a Transwell migration assay and compared
the migration of the cells treated either with PBS (mock treatment)
or incubated with terrein for 24 h. After 24 h, the number of cells
that had migrated to the bottom of the wells was significantly
reduced following treatment with terrein (Fig. 1C), indicating that terrein
attenuated the migration of SKOV3 cells. These data indicate that
terrein exerts anticancer effects on ovarian cancer cells
(SKOV3).
Terrein induces G2/M phase
cell cycle arrest
To better characterize the anticancer activity of
terrein and investigate the mechanisms underlying its
anti-proliferative effects, we examined its effects on apoptosis
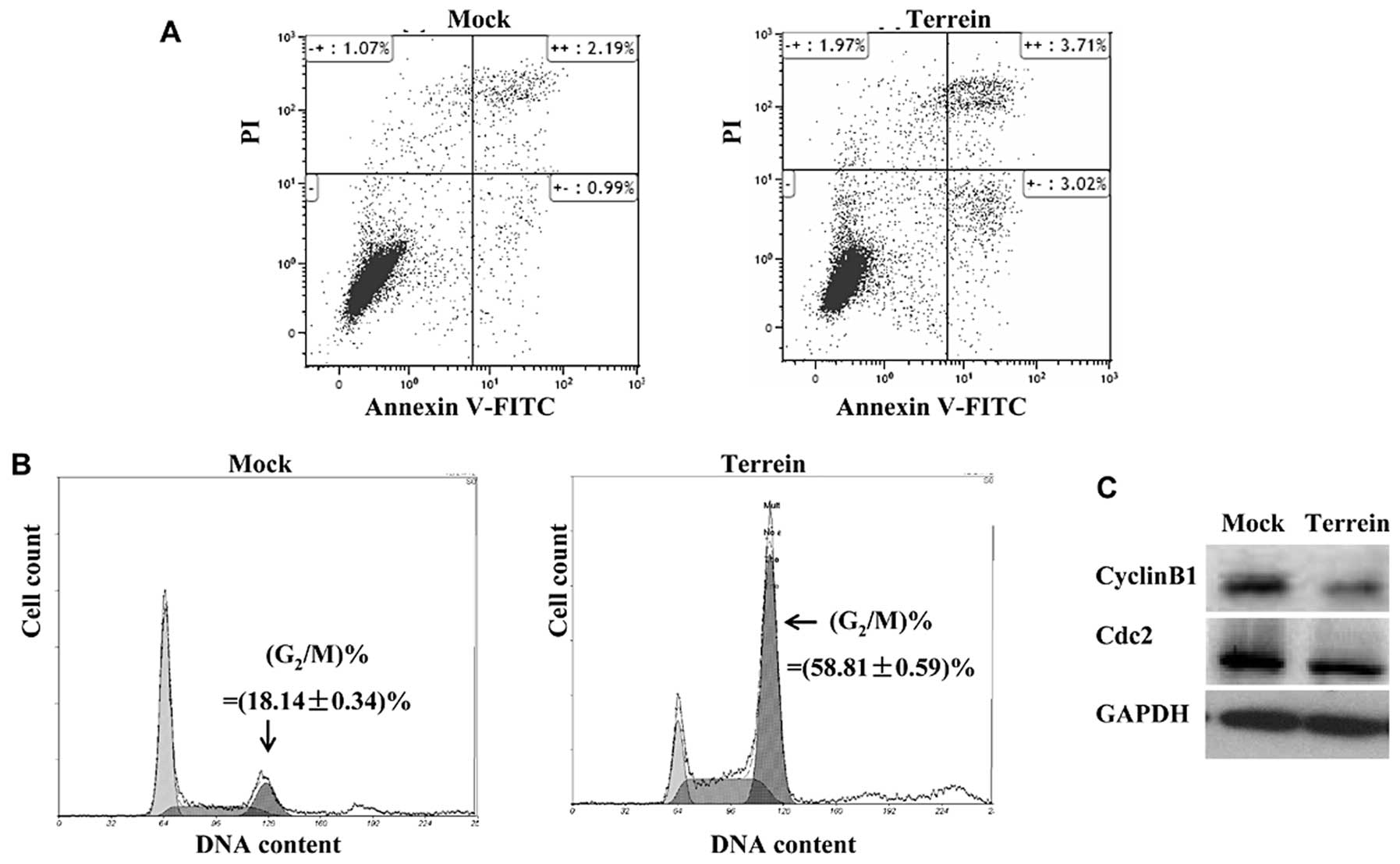
and the cell cycle. We used SKOV3 cells, which were harvested after
48 h of incubation with 15 mg/l terrein. Flow cytometric analysis
was performed using Annexin V-FITC to label the apoptotic cells and
PI to stain the necrotic cells. As shown in Fig. 2A, terrein moderately induced cell
apoptosis: the percentage of early apoptotic cells increased from
0.99 to 3.02% [indicated by Annexin V-FITC(+)/PI(-) labeling], and
the late apoptotic percentage slightly increased from 2.19 to 3.71%
[indicated by Annexin V-FITC(+)/PI(+) labeling].
We then analyzed the cell cycle distribution by
staining the cells with PI. As illustrated in Fig. 2B, 24 h of treatment with 15 mg/l
terrein led to a significant increase (from ~18.14 to ~58.81%) in
the number of SKOV3 cells at the G2/M phase of the cell
cycle. To better understand the ability of terrein to induce cell
cycle arrest, we examined the expression of cyclin B1 and Cdc2,
both of which play essential roles in the control of the cell cycle
at the G2/M phase (34,35). Protein detection by western blot
analysis revealed a notable depletion in the levels of both cyclin
B1 and Cdc2 (Fig. 2C), suggesting
that terrein modified the expression of the cell cycle regulators
and consequently induced cell cycle arrest.
Terrein suppresses the expression of
LIN28 in ovarian cancer cells
The RNA-binding protein, LIN28, has been reported to
bind to and promote the translation of certain mRNAs encoding cell
cycle regulators, including cyclin B1 (36–40), and thereby coordinates the cell
cycle at multiple checkpoints. More importantly, LIN28 has been
shown to contribute towards the malignancy of ovarian cancer and is
thus believed to be a potential target for ovarian cancer therapy
(41,42). It also should be noted that LIN28
is used to define stemness in several tissue lineages and, when
highly expressed, is associated with the stemness properties of
ovarian cancer stem cells (43–45).
To gain insight into the anticancer mechanisms of
terrein, we investigated whether it affects the expression of LIN28
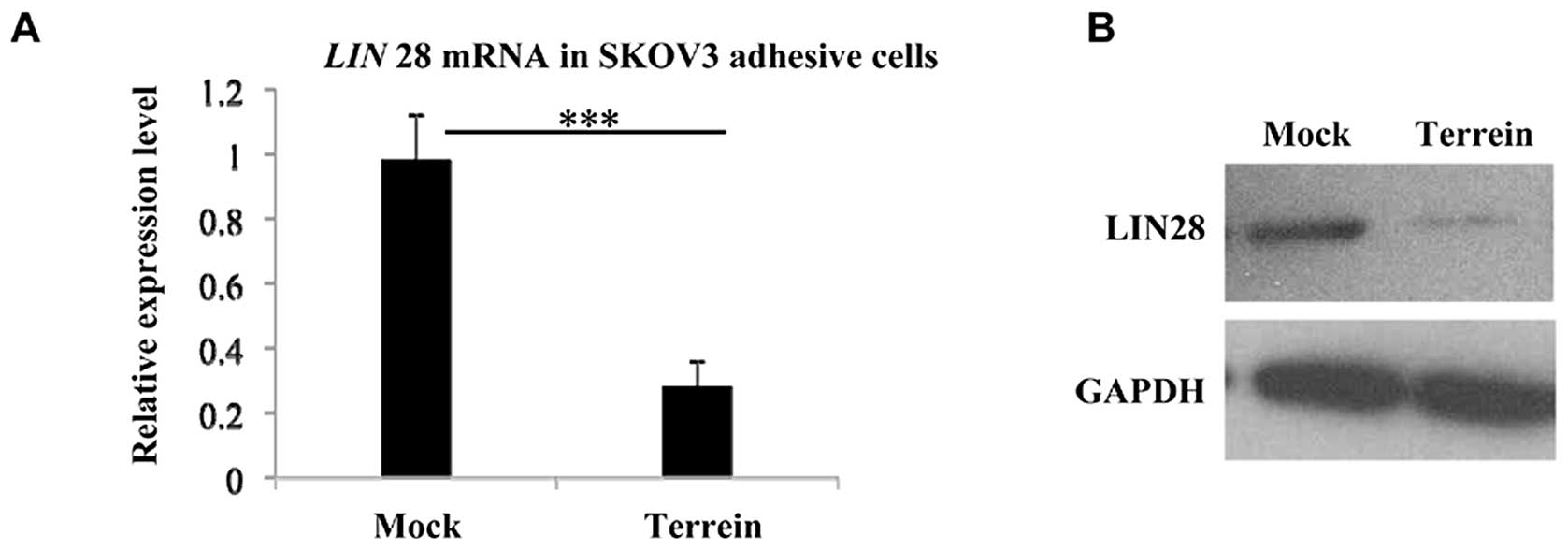
in SKOV3 cells. Firstly, we assessed LIN28 expression at the
transcriptional level by quantitative PCR, and observed a clear
decrease following incubation of the cells with terrein (Fig. 3A). Using western blot analysis, we
then examined the suppressive effects of terrein on LIN28 at the
translational level. As shown in Fig.
3B, the level of LIN28 protein was significantly depleted in
the SKOV3 cells which had been incubated with terrein for 48 h.
In order to further confirm the anti-proliferative
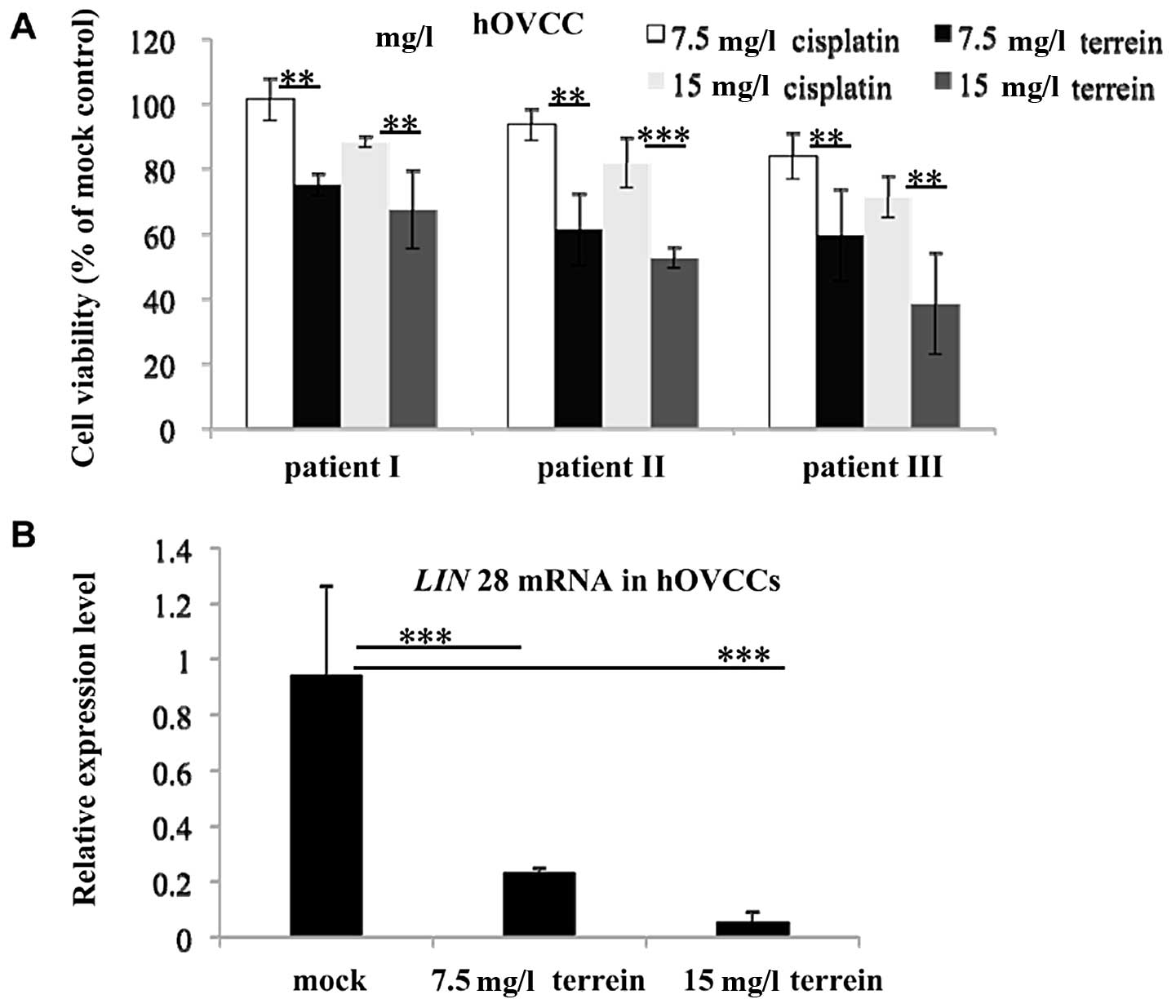
effects of terrein on epithelial ovarian cancer cells, we examined
the anticancer effects of terrein on primary hOVCCs isolated from
the tumors of 3 patients, classified as stage III, grade 2–3 serous
adenocarcinoma. MTT assay revealed that treatment with terrein led
to an marked reduction in cell viability (Fig. 4A). Although the anti-proliferative
effects were not uniform on the different samples, the efficiency
of terrein was consistently higher than that of cisplatin. In
accordance with the results obtained with the SKOV3 cells, terrein
also downregulated the expression of LIN28 in the hOVCCs
(Fig. 4B). These results indicate
that terrein exerts anti-proliferative effects and suppresses the
expression of LIN28 in both immortalized cells and primary
hOVCCs.
Terrein effectively inhibits the survival
of CSLCs
Since terrein downregulated the expression of LIN28
in the hOVCCs, we wished to determine whether it would have the
same effects on ovarian cancer stem cells, in which LIN28 has been
reported to be an essential regulator (38–45).
We have previously reported the feasibility of
enriching and propagating human ovarian CSLCs by culturing SKOV3
spheroid cells under serum-free conditions (18–21). Employing this serum-free culture
system, we examined the anticancer effects of terrein on CSLCs. As
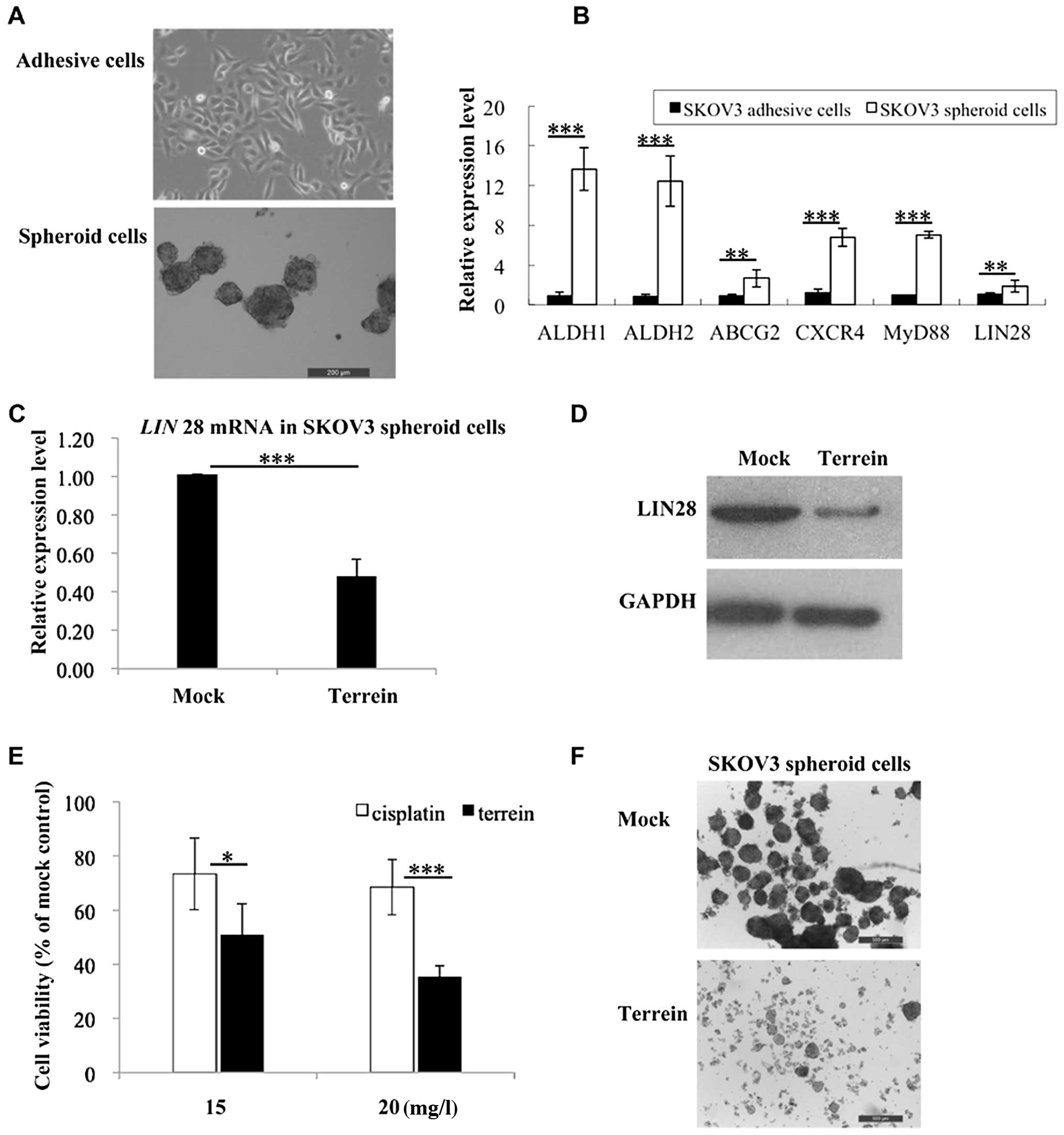
shown in Fig. 5A, the enriched
CSLCs grew as spheroid-shaped cells and could be easily
distinguished from the original differentiated SKOV3 adhesive
cells. To further confirm the stem cell characteristics of the
CSCLs, we assessed the expression levels of several cancer stem
cell markers including, ALDH1, ALDH2, ABCG2,
CXCR4, MyD88 and LIN28. Quantitative PCR
revealed that the expression of these markers was noticeably
increased in the spheroid-shaped cells (Fig. 5B), thus, validating their cancer
stem cell-like properties.
As shown in Fig.
5B, the enriched CSLCs displayed higher expression levels of
LIN28 compared to the original SKOV3 cells. Of note, treatment with
terrein significantly reduced the expression of LIN28 in the CSLCs
at both the transcriptional and translational levels (Fig. 5C and D). Encouraged by this
result, we examined the anti-proliferative effects of terrein on
the CSCLs (Fig. 5E). The result
showeds that cisplatin exhibited only a moderate effect in spheroid
cells; however, treatment with terrein significantly depleted the
cell survival of the SKOV3-derived CSLCs (Fig. 5E); incubation with 15 mg/l terrein
for 72 h reduced cell viability by ~50%. Fig. 5F shows the morphological changes
of SKOV3 spheroid-shaped cells following treatment with terrein:
the spheroids were dispersed into single cells; treatment for a
longer duration led to cell death.
Taken together, the results from our study
demonstrate that terrein suppresses the expression of LIN28 and
exerts anti-proliferative effects against ovarian CSLCs.
Discussion
Over the past decade, there is increasing evidence
supporting the cancer stem cell theory, and cancer stem cells have
been successfully separated from both primary tumor samples and
immortalized cell lines (6,7,11).
The separated cancer stem cells have been shown to survive in an
in vitro serum-free culture system, forming spheroids and
exhibiting stem-like properties (11,12). This in vitro system
provides a valuable model for the screening and determining the
underlying mechanisms of action of drugs against human cancers,
particularly those resistant to drugs.
Epithelial ovarian cancer is the most lethal
gynecological malignancy, which easily develops drug resistance,
thus causing relapse (1–5). The cancer stem cell theory has
significant therapeutic implications and provides a mechanistic
explanation for ovarian cancer carcinogenesis and development.
Based on this theory, novel drugs targeting cancer stem cells are
urgently required; however, little is known at present (46).
In this study, we demonstrate that one small
compound, namely terrein, effectively exerts anticancer effects in
both differentiated and stem-like ovarian cancer cells. Terrein is
a fungal metabolite which has already been shown to have valuable
bioactivity in the fields of medicine, cosmetology and agriculture
without any cytotoxic effects (22–30). In the present study, we separated
terrein from the fermentation broth of sponge-derived fungus with
high efficiency and purity, showing it to be an economic and
environmentally-friendly drug candidate (31). Our results revealed that terrein
effectively inhibited the proliferation of both immortalized
ovarian cancer cells and primary hOVCCs. Our investigation into the
underlying mechanisms revealed that terrein induced G2/M
phase cell cycle arrest by suppressing the expression of
G2/M cell cycle-related proteins (cyclin B1 and Cdc2).
More importantly, we identified that an important target of terrain
is LIN28, an evolutionarily conserved protein which plays a
critical role in embryonic development (38). LIN28 has also been reported to be
an essential oncogene in ovarian cancer, contributing to the
etiology and progression (41–43). Recently, LIN28 has gained
increasing attention due to its biological roles in cancer stem
cells, and is believed to be an important marker gene for cancer
stem cells (43–45).
Consequently, we sought to investigate whether
terrein has the ability to target CSLCs in our in vitro
experimental system. We found that terrein effectively suppressed
the expression of LIN28 and significantly reduced the viability of
CSLCs, suggesting that terrein also possesses the ability to target
cancer stem cells.
Thus, it can be concluded that terrein, an easily
produced small molecule, may prove to be a promising drug candidate
for use in the treatment of human epithelial ovarian cancer with a
novel mechanism of action targeting both differentiated and
stem-like ovarian cancer cells.
Acknowledgements
The present study was supported by grants from the
Shanghai Jiaotong University (YG2011ms13), the Shanghai Municipal
Health and Family Planning Commission (20134Y128), the Shanghai
Jiaotong University School of Medicine (13XJ10067), the National
Natural Science Foundation of China (No. 81401216) and the Shanghai
Committee of Science and Technology (14YF1408200).
References
|
1
|
Schwartz PE: Current diagnosis and
treatment modalities for ovarian cancer. Cancer Treat Res.
107:99–118. 2002.PubMed/NCBI
|
|
2
|
Cho KR and Shih IeM: Ovarian cancer. Annu
Rev Pathol. 4:287–313. 2009. View Article : Google Scholar
|
|
3
|
Sudo T: Molecular-targeted therapies for
ovarian cancer: prospects for the future. Int J Clin Oncol.
17:424–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Foster R, Buckanovich RJ and Rueda BR:
Ovarian cancer stem cells: working towards the root of stemness.
Cancer Lett. 338:147–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
6
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar
|
|
7
|
Dalerba P, Cho RW and Clarke MF: Cancer
stem cells: models and concepts. Annu Rev Med. 58:267–284. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nguyen LV, Vanner R, Dirks P and Eaves CJ:
Cancer stem cells: an evolving concept. Nat Rev Cancer. 12:133–143.
2012.PubMed/NCBI
|
|
9
|
Chen J, Li Y, Yu TS, McKay RM, Burns DK,
Kernie SG and Parada LF: A restricted cell population propagates
glioblastoma growth after chemotherapy. Nature. 488:522–526. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Driessens G, Beck B, Caauwe A, Simons BD
and Blanpain C: Defining the mode of tumour growth by clonal
analysis. Nature. 488:527–530. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bapat SA, Mali AM, Koppikar CB and Kurrey
NK: Stem and progenitor-like cells contribute to the aggressive
behavior of human epithelial ovarian cancer. Cancer Res.
65:3025–3029. 2005.PubMed/NCBI
|
|
12
|
Szotek PP, Pieretti-Vanmarcke R, Masiakos
PT, Dinulescu DM, Connolly D, Foster R, Dombkowski D, et al:
Ovarian cancer side population defines cells with stem cell-like
characteristics and Mullerian Inhibiting Substance responsiveness.
Proc Natl Acad Sci USA. 103:11154–11159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang S, Balch C, Chan MW, Lai HC, Matei
D, Schilder JM, Yan PS, et al: Identification and characterization
of ovarian cancer-initiating cells from primary human tumors.
Cancer Res. 68:4311–4320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kondo T, Setoguchi T and Taga T:
Persistence of a small subpopulation of cancer stem-like cells in
the C6 glioma cell line. Proc Natl Acad Sci USA. 101:781–786. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Patrawala L, Calhoun T,
Schneider-Broussard R, Zhou J, Claypool K and Tang DG: Side
population is enriched in tumorigenic, stem-like cancer cells,
whereas ABCG2+ and ABCG2− cancer cells are
similarly tumorigenic. Cancer Res. 65:6207–6219. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Calcagno AM, Fostel JM, To KK, Salcido CD,
Martin SE, Chewning KJ, Wu CP, et al: Single-step
doxorubicin-selected cancer cells overexpress the ABCG2 drug
transporter through epigenetic changes. Br J Cancer. 98:1515–1524.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schmandt RE, Broaddus R, Lu KH, Shvartsman
H, Thornton A, Malpica A, Sun C, et al: Expression of c-ABL, c-KIT,
and platelet-derived growth factor receptor-β in ovarian serous
carcinoma and normal ovarian surface epithelium. Cancer.
98:758–764. 2003.
|
|
18
|
Ma L, Lai D, Liu T, Cheng W and Guo L:
Cancer stem-like cells can be isolated with drug selection in human
ovarian cancer cell line SKOV3. Acta Biochim Biophys Sin.
42:593–602. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lai D, Wang F, Chen Y, Wang C, Liu S, Lu
B, Ge X, et al: Human ovarian cancer stem-like cells can be
efficiently killed by γδ T lymphocytes. Cancer Immunol Immunother.
61:979–989. 2012.PubMed/NCBI
|
|
20
|
Liu T, Cheng W, Lai D, Huang Y and Guo L:
Characterization of primary ovarian cancer cells in different
culture systems. Oncol Rep. 23:1277–1284. 2010.PubMed/NCBI
|
|
21
|
Luo X, Dong Z, Chen Y, Yang L and Lai D:
Enrichment of ovarian cancer stem-like cells is associated with
epithelial to mesenchymal transition through an miRNA-activated AKT
pathway. Cell Prolif. 46:436–446. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Raistrick H and Smith G: Studies in the
biochemistry of micro- organisms: the metabolic products of
Aspergillus terreus Thom. A new mould metabolic
product-terrein. Biochem J. 29:606–611. 1935.PubMed/NCBI
|
|
23
|
Ghisalberti EL, Narbey MJ and Rowland CY:
Metabolites of Aspergillus terreus antagonistic towards the
take-all fungus. J Nat Prod. 53:520–522. 1990.
|
|
24
|
Arakawa M, Someno T, Kawada M and Ikeda D:
A new terrein glucoside, a novel inhibitor of angiogenin secretion
in tumor angiogenesis. J Antibiot (Tokyo). 61:442–448. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park SH, Kim DS, Kim WG, Ryoo IJ, Lee DH,
Huh CH, Youn SW, Yoo ID and Park KC: Terrein: a new melanogenesis
inhibitor and its mechanism. Cell Mol Life Sci. 61:2878–2885. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Phattanawasin P, Pojchanakom K, Sotanaphun
U, Piyapolrungroj N and Zungsontiporn S: Weed growth inhibitors
from Aspergillus fischeri TISTR 3272. Nat Prod Res.
21:1286–1291. 2007.PubMed/NCBI
|
|
27
|
Kim DS, Cho HJ, Lee HK, Lee WH, Park ES,
Youn SW and Park KC: Terrein, a fungal metabolite, inhibits the
epidermal proliferation of skin equivalents. J Dermatol Sci.
46:65–68. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee JC, Yu MK, Lee R, Lee YH, Jeon JG, Lee
MH, Jhee EC, Yoo ID and Yi HK: Terrein reduces pulpal inflammation
in human dental pulp cells. J Endod. 34:433–437. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee YH, Lee NH, Bhattarai G, Oh YT, Yu MK,
Yoo ID, Jhee EC and Yi HK: Enhancement of osteoblast
biocompatibility on titanium surface with Terrein treatment.
Cell Biochem Funct. 28:678–685. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim DS, Lee HK, Park SH, Lee S, Ryoo IJ,
Kim WG, Yoo ID, et al: Terrein inhibits keratinocyte proliferation
via ERK inactivation and G2/M cell cycle arrest. Exp
Dermatol. 17:312–317. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu B, Yin Y, Zhang F, Li Z and Wang L:
Operating conditions optimization for (+)-terrein production in a
stirred bioreactor by Aspergillus terreus strain PF-26 from
marine sponge Phakellia fusca. Bioprocess Biosyst Eng.
35:1651–1655. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yin Y, Gao Q, Zhang F and Li Z: Medium
optimization for the high yield production of single (+)-terrein by
Aspergillus terreus strain PF26 derived from marine sponge
Phakellia fusca. Process Biochem. 47:887–891. 2012.
View Article : Google Scholar
|
|
33
|
Chen YF, Dong Z, Xia Y, Tang J, Peng L,
Wang S and Lai D: Nucleoside analog inhibits microRNA-214 through
targeting heat-shock factor 1 in human epithelial ovarian cancer.
Cancer Sci. 104:1683–1689. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cicenas J and Valius M: The CDK inhibitors
in cancer research and therapy. J Cancer Res Clin Oncol.
137:1409–1418. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Collins I and Garrett MD: Targeting the
cell division cycle in cancer: CDK and cell cycle checkpoint kinase
inhibitors. Curr Opin Pharmacol. 5:366–373. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hafner M, Max KE, Bandaru P, Morozov P,
Gerstberger S, Brown M, Molina H and Tuschl T: Identification of
mRNAs bound and regulated by human LIN28 proteins and molecular
requirements for RNA recognition. RNA. 19:613–626. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li N, Zhong X, Lin X, Guo J, Zou L, Tanyi
JL, Shao Z, et al: Lin-28 homologue A (LIN28A) promotes cell cycle
progression via regulation of cyclin-dependent kinase 2 (CDK2),
cyclin D1 (CCND1), and cell division cycle 25 homolog A (CDC25A)
expression in cancer. J Biol Chem. 287:17386–17397. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Moss EG, Lee RC and Ambros V: The cold
shock domain protein LIN-28 controls developmental timing in C.
elegans and is regulated by the lin-4 RNA. Cell.
88:637–646. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shyh-Chang N and Daley GQ: Lin28: primal
regulator of growth and metabolism in stem cells. Cell Stem Cell.
12:395–406. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu B, Zhang K and Huang Y: Lin28 modulates
cell growth and associates with a subset of cell cycle regulator
mRNAs in mouse embryonic stem cells. RNA. 15:357–361. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Viswanathan SR, Powers JT, Einhorn W,
Hoshida Y, Ng TL, Toffanin S, O’Sullivan M, et al: Lin28 promotes
transformation and is associated with advanced human malignancies.
Nat Genet. 41:843–848. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lu L, Katsaros D, Shaverdashvili K, Qian
B, Wu Y, de la Longrais IA, Preti M, et al: Pluripotent factor
lin-28 and its homologue lin-28b in epithelial ovarian cancer and
their associations with disease outcomes and expression of let-7a
and IGF-II. Eur J Cancer. 45:2212–2218. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Peng S, Maihle NJ and Huang Y:
Pluripotency factors Lin28 and Oct4 identify a sub-population of
stem cell-like cells in ovarian cancer. Oncogene. 29:2153–2159.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang X, Lin X, Zhong X, Kaur S, Li N,
Liang S, Lassus H, et al: Double-negative feedback loop between
reprogramming factor LIN28 and microRNA let-7 regulates aldehyde
dehydrogenase 1-positive cancer stem cells. Cancer Res.
70:9463–9472. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ma W, Ma J, Xu J, Qiao C, Branscum A,
Cardenas A, Baron AT, et al: Lin28 regulates BMP4 and functions
with Oct4 to affect ovarian tumor microenvironment. Cell Cycle.
12:88–97. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sanchez-Garcia I, Vicente-Duenas C and
Cobaleda C: The theoretical basis of cancer-stem-cell-based
therapeutics of cancer: can it be put into practice? Bioessays.
29:1269–1280. 2007. View Article : Google Scholar : PubMed/NCBI
|