Introduction
Hyperglycemia is not only one of the main clinical
characteristics of diabetes mellitus (DM), but is also a risk
factor for the development of cardiovascular complications
associated with chronic diabetes, such as diabetic cardiomyopathy
(1,2). Multiple factors have been reported
to contribute to hyperglycemia-induced cardiac damage, such as
oxidative stress (3–6), mitochondrial dysfunction (5–8),
apoptosis (5–9) and activation of several signaling
molecules, including mitogen-activated protein kinase (MAPK)
(3,5,10,11), leptin (6,12)
and p53 (7,9). Another signaling molecule involved
in hyperglycemia-induced cardiomyocyte damage may be nuclear
factor-κB (NF-κB). NF-κB is a dimeric transcription factor that
regulates numerous genes associated with stress responses,
including oxidative stress, apoptosis and inflammation. The NF-κB
family is composed of 5 subunits, Rel A (p65), Rel B, c-Rel, NF-κB1
(p50) and NF-κB2 (p52), each of which may form hemo- or
heterodimers. Among these, the nuclear translocation of the p65
subunit is a key step in the activation of NF-κB (13). NF-κB is known to be activated by
p38 MAPK (a member of the MAPK family) in cardiomyocytes (14–16). Accumulating evidence indicates
that NF-κB plays a significant role in cardiac damage induced by
various stimuli (14–16). Guo et al demonstrated the
involvement of the NF-κB pathway in doxorubicin-induced cardiac
cytotoxicity, resulting in a decrease in cell viability (14,15). The NF-κB pathway has also been
implicated in G-protein-coupled receptor angonist-elicited
cardiomyocyte hypertrophy (16).
Recently, the roles of NF-κB in hyperglycemia-induced cardiac
insults and inflammation have attracted attention. As shown in a
previous study, in mice with streptozotocin-induced DM, a marked
increase in cardiac hypertrophy, fibrosis and inflammation was
observed with a corresponding upregulation of reactive oxygen
species (ROS) generation, interleukin (IL)-1β, IL-6, tumor necrosis
factor (TNF)-α, cellular adhesion molecules and phosphorylated
(p)-p38 MAPK expression, and NF-κB activation (17), suggesting that the NF-κB pathway
participates in hyperglycemia-induced myocardial damage and
inflammation. However, the authors did not observe the effects of
the inhibition of NF-κB activation on cardiac injury and
inflammation induced by hyperglycemia. Thus, it is important to
further clarify the role of the inhibition of NF-κB activation in
the protective effects against hyperglycemia-induced cardiac
insults and inflammation.
In a recent study, we demonstrated that exogenous
hydrogen sulfide (H2S) protects H9c2 cardiac cells
against high glucose (HG)-induced injury, including cytotoxicity,
apoptosis, oxidative stress and mitochondrial damage, by inhibiting
the activation of the p38 MAPK, extracellular signal-regulated
protein kinase 1/2 (ERK1/2) (5)
and leptin pathways (18).
H2S, previously regarded as a poisonous gas, has been
recognized as the third endogenous gasotransmitter signaling
molecule alongside nitric oxide (NO) and carbon monoxide (19,20). H2S exerts its
cytoprotective effects in various models of cardiac injury. In
isoproterenol-treated rat hearts, treatment with H2S has
been shown to attenuate myocardial necrosis and rescue contractile
activity (21). In an in
vivo model of mice subjected to ischemia/reperfusion (I/R),
pre-treatment with Na2S markedly reduced the extent of
myocardial injury, oxidative stress and apoptosis (22). Recently, it was also demonstrated
that exogenous H2S protects H9c2 cardiac cells against
chemical hypoxia-induced injury by inhibiting oxidative stress and
enhancing heat shock protein 90 (HSP90) expression (23,24). Of note, since lower circulating
H2S levels have been measured in animal models of
diabetes (25–27) and patients with type 2 DM
(26,28), the role of H2S in
hyperglycemia-induced cardiac insults has attracted considerable
attention. Peake et al reported that the exogenous
administration of H2S (Na2S) ameliorates
myocardial I/R injury in db/db mice (4). Furthermore, H2S has been
shown to exert protective effects against myocardial I/R-induced
injury in diabetic rats (29). Of
note, although H2S has been shown to exert
cytoprotective and anti-inflammatory effects by inhibiting the
NF-κB pathway in doxorubicin-treated cardiac cells (15) or cobalt chloride
(CoCl2, a hypoxia mimetic agent)-treated human skin
keratinocytes (HaCaT cells) (30), whether exogenous H2S
protects cardiomyocytes against HG-induced injury and inflammation
by inhibiting NF-κB activation remains unclear. To investigate this
hypothesis, in the current study, H9c2 cardiac cells were exposed
to 35 mM glucose (HG) to establish a model of HG-induced cardiac
injury and inflammation. We then examined the following: i) the
effects of exogenous H2S on the HG-induced increase in
NF-κB activation; ii) the roles of NF-κB activation in HG-induced
cardiac injury and inflammation; iii) the roles of the inhibition
of NF-κB activation in the protective effects of exogenous
H2S against HG-induced injury and inflammation; and iv)
the interaction between NF-κB and IL-1β in H9c2 cardiac cells.
Materials and methods
Materials
Sodium hydrogen sulfide (NaHS), was obtained from
Sigma Chemical Co., (St. Louis, MO, USA) protected from sunlight
and stored at 2–4°C. Pyrrolidine dithiocarbamate (PDTC),
2′7′-dichlorodihydrofluorescein diacetate (DCFH-DA),
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzimidazol-carbocyanine
iodide (JC-1) and Hoechst 33258 were purchased from Sigma-Aldrich
(Sigma Chemicals Co.) and stored at −20°C. IL-1 receptor antagonist
(IL-1Ra) was purchased from ProSpec (Ness-Ziona, Israel). The
enzyme-linked immunosorbent assay (ELISA) kits for IL-1β, IL-6 and
TNF-α were purchased from Wuhan Boster Biological Technology, Ltd.
(Wuhan, China). The Cell Counting kit-8 (CCK-8) was purchased from
Dojindo Laboratories (Kumamoto, Japan). Fetal bovine serum (FBS)
and DMEM were obtained from Gibco-BRL (Grand Island, NY, USA).
Anti-p-NF-κB p65 antibody, anti-total (t)-NF-κB p65 antibody and
anti-cleaved caspase-3 antibody were procured from Cell Signaling
Technology, Inc. (Boston, MA, USA); horseradish peroxidase
(HRP)-conjugated secondary antibody and the BCA protein assay kit
were obtained from KangChen Bio-tech, Inc. (Shanghai, China).
Enhanced chemiluminescence (ECL) solution was purchased from
Nanjing KeyGen Biotech Co. Ltd., (Nanjing, China). Lysis buffer was
purchased from the Beyotime Institute of Biotechnology (Shanghai,
China) and Urea sodium dodecyl sulphate-polyacrylamide gel
electrophoresis (SDS-PAGE) was purchased from Hangzhou Fude
Chemical Co., Ltd. (Hangzhou, China).
Cell culture and treatment
The H9c2 cardiac cells, a rat cardiac myoblast cell
line, were supplied by the Sun Yat-sen University Experimental
Animal Center (Guangzhou, Guangdong, China). The cells were
cultured in DMEM medium supplemented with 10% fetal bovine serum at
37°C under an atmosphere of 5% CO2 and 95% air. For the
treatment procedure, the cells were cultured in DMEM (5.5 mM
glucose) medium for 12 h piror to the administration of 35 mM
glucose (final concentration) for 24 h. The glucose concentration
of the control group was 5.5 mM. To explore the protective effects
of H2S against HG (35 mM glucose)-induced injury, the
cells were treated with 400 μM NaHS (a well-known H2S
donor) for 30 min prior to exposure to 35 mM glucose for 24 h. To
further determine whether the protective effects of H2S
are associated with the inhibition of the acvitation of the
NF-κB/IL-1 pathway, the H9c2 cells were treated with 100 μM PDTC (a
specific inhibitor of NF-κB) prior to exposure to 35 mM glucose for
24 h or co-treated with 20 ng/ml IL-1Ra (an antagonist of IL-1) and
35 mM glucose for 24 h.
Cell viability assay
The H9c2 cells were seeded in 96-well plates at a
concentration of 1×104 cells/ml and incubated at 37°C.
CCK-8 assay was employed to assess the viability of the H9c2
cardiac cells. After the indicated treatments, 10 μl CCK-8 solution
at a 1/10 dilution was added to each well and then the plate was
incubated for 2 h in an incubator. Absorbance at 450 nm was assayed
using a microplate reader (Molecular Devices, Sunnyvale, CA, USA).
The means of the optical density (OD) of 3 wells in the indicated
groups were used to calculate the percentage of cell viability
according to the following formula: cell viability (%) =
(ODtreatment group/ODcontrol group) ×100%.
The experiment was repeated 5 times.
Hoechst 33258 nuclear staining for the
determination of apoptosis
Apoptotic cell death was observed by Hoechst 33258
staining followed by photofluorography. In brief, the H9c2 cells
were plated in 35-mm dishes at a density of 1×106
cells/well. After the indicated treatments, the cells were fixed
with 4% paraformaldehyde in 0.1 mol/l phosphate-buffered saline
(PBS, pH 7.4) for 10 min. The slides were then washed 5 times with
PBS. After staining with 5 mg/ml Hoechst 33258 for 15 min, the H9c2
cells were washed 5 times with PBS, and the cells were then
visualized under a fluorescence microscope (BX50-FLA; Olympus,
Tokyo, Japan). Viable H9c2 cells displayed a uniform blue
fluorescence throughout the nucleus and a normal nuclear size.
However, apoptotic H9c2 cells showed condensed, distorted or
fractured nuclei. The experiment was carried out 3 times.
Examination of intracellular ROS
generation
Intracellular ROS generation was determined by the
oxidative conversion of the cell-permeable oxidation of DCFH-DA to
fluorescent DCF. The H9c2 cells were cultured on a slide with DMEM.
After the different treatments, the slides were washed 3 times with
PBS. DCFH-DA solution (10 μM) in serum-free medium was added to the
slides, and the cells were then incubated at 37°C for a further 30
min in an incubator. The slides were washed 5 times with PBS, and
DCF fluorescence was measured over the entire field of vision by
using a fluorescence microscope connected to an imaging system
(BX50-FLA; Olympus). The mean fluorescence intensity (MFI) from 5
random fields was measured using ImageJ 1.47i software and the MFI
was used as an index of the amount of ROS. The experiment was
carried out 5 times.
Measurement of mitochondrial membrane
potential (MMP)
MMP was assessed using a fluorescent dye, JC-1, a
cell-permeable cationic dye that preferentially enters the
mitochondria based on the highly negative MMP. The depolarization
of MMP results in the loss of MMP from the mitochondria and a
decrease in red/green fluorescence. The cells were cultured on a
slide with DMEM. After the indicated treatments, the slides were
washed 3 times with PBS. The cells were incubated with 1 mg/l JC-1
at 37°C for 30 min in an incubator, washed briefly with PBS 3 times
and air dried again. Fluorescence was then measured over the whole
field of vision using a fluorescence microscope connected to an
imaging system (BX50-FLA, Olympus). The MFI of JC-1 from 5 random
fields was analyzed using ImageJ 1.47i software, and the MFI was
taken as an index of the levels of MMP. The experiment was carried
out 5 times.
Western blot analysis
After the indicated treatments, the H9c2 cells were
harvested and lysed with cell lysis solution at 4°C for 30 min.
Total protein was quantified using the BCA protein assay kit.
Loading buffer was added to cytosolic extracts, followed by boiling
for 5 min, and the same amounts of supernatant from each sample
were fractionated by 10% SDS-PAGE, and then the total proteins were
transferred into polyvinylidene difluoride (PVDF) membranes. The
membranes were blocked in 5% fat-free milk for 60 min in fresh
blocking buffer [0.1% Tween-20 in Tris-buffered saline (TBS-T)] at
room temperature, and incubated with either anti-t-NF-κB p65
(1:1,000 dilution), anti-p-NF-κB p65 (1:1,000 dilution), or
anti-cleaved caspase-3 (1:1,000 dilution) in freshly prepared TBS-T
with 3% free-fat milk overnight with gentle agitation at 4°C. The
membranes were washed for 15 min with TBS-T and incubated with
HRP-conjugated goat anti-rabbit secondary antibody (1:2,500
dilution; KangChen Bio-tech, Inc.) in TBS-T with 3% fat-free milk
for 1.5 h at room temperature. The membranes were then washed 3
times with TBS-T for 15 min. The immunoreactive signals were
visualized by ECL detection. In order to quantify protein
expression, the X-ray films were scanned and analyzed using ImageJ
1.47i software. The experiment was carried out 3 times.
ELISA for the detection of IL-1β, IL-6
and TNF-α in the culture supernatant
The H9c2 cells were plated in 96-well plates. After
the indicated treatments, the levels of IL-1β, IL-6 and TNF-α in
the culture medium were determined by ELISA according to the
manufacturer’s instructions (Wuhan Boster Biological Technology,
Ltd.). The experiment was performed at least 5 times.
Statistical analysis
All data are presented as the means ± SEM.
Differences between groups were analyzed by one-way analysis of
variance (ANOVA) using SPSS version 13.0 software (SPSS, Inc.,
Chicago, IL, USA), followed by the least significant difference
(LSD) post hoc comparison test. A value of p<0.05 was considered
to indicate a statistically significant difference.
Results
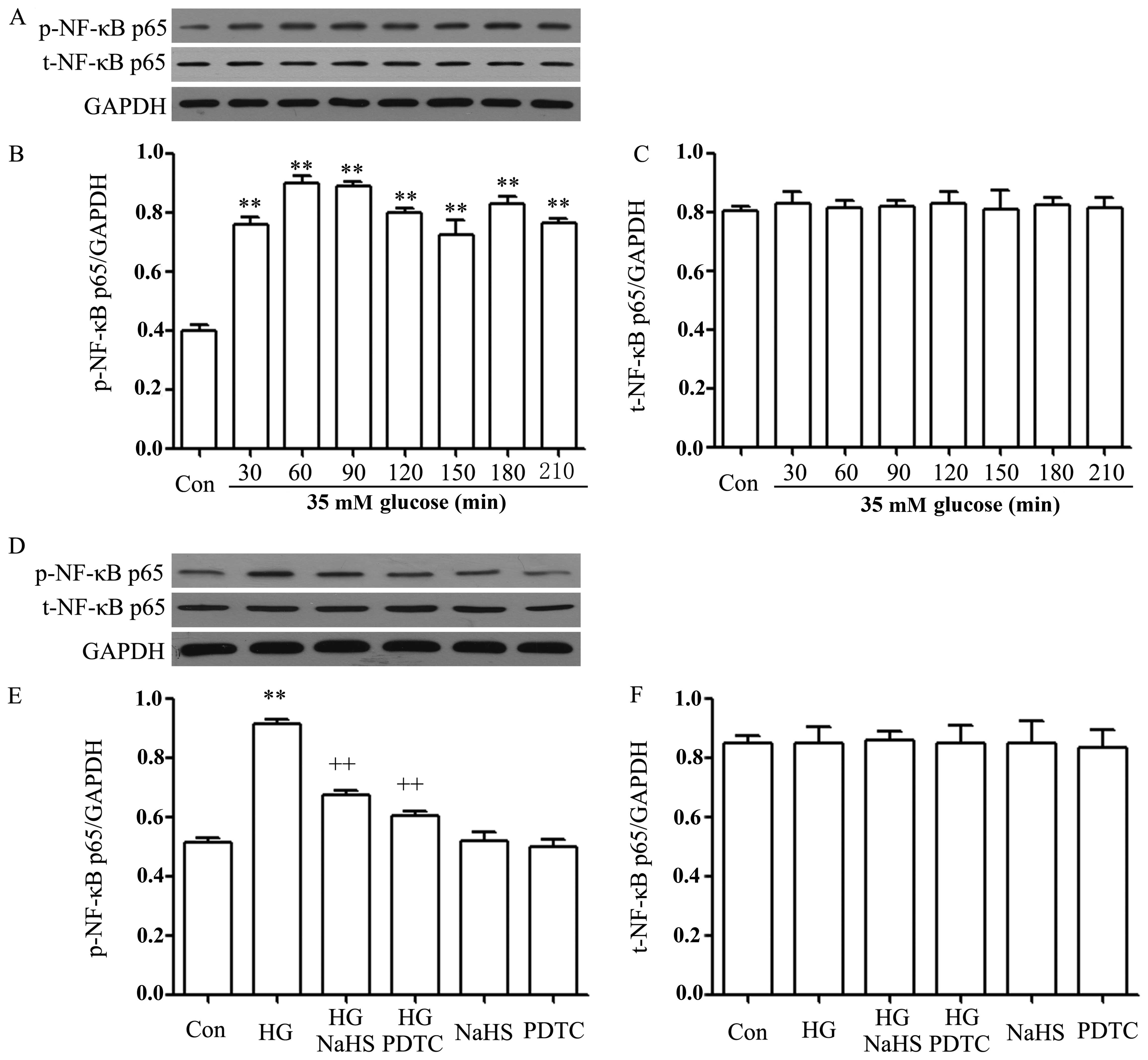
NaHS and NF-κB inhibitor ameliorate the
HG-induced increase in the expression of p-NF-κB p65 in H9c2
cardiac cells
To explore the effects of HG (35 mM glucose) on the
activation of the NF-κB pathway in H9c2 cells, a time-response
experiment to determine the expression level of NF-κB was
performed. As shown in Fig. 1A–C,
after the cells were exposured to HG for 30, 60, 90, 120, 150, 180
and 210 min, the expression levels of the p-NF-κB p65 subunit were
significantly enhanced, reaching a peak at 60 and 90 min. However,
the expression levels of the t-NF-κB p65 subunit remained unaltered
following exposure of the cells to HG for the indicated periods of
time. Based on these data, the p-NF-κB expression level was
determined at 90 min following exposure to HG in the following
experiments.
Of note, the increased expression of p-NF-κB p65 was
reduced by treatment of the cells with 400 μM NaHS (a donor of
H2S) for 30 min prior to exposure to HG for 90 min
(Fig. 1D–F). Similarly, treatment
of the cells with 100 μM PDTC, an inhibitor of NF-κB, for 30 min
prior to exposure to HG also attenuated the increased expression of
p-NF-κB p65 (Fig. 1D and E). The
basal expression levels of p-NF-κB p65 (control) were not altered
by the separate treatment with 400 μM NaHS or 100 μM PDTC (Fig. 1D and F).
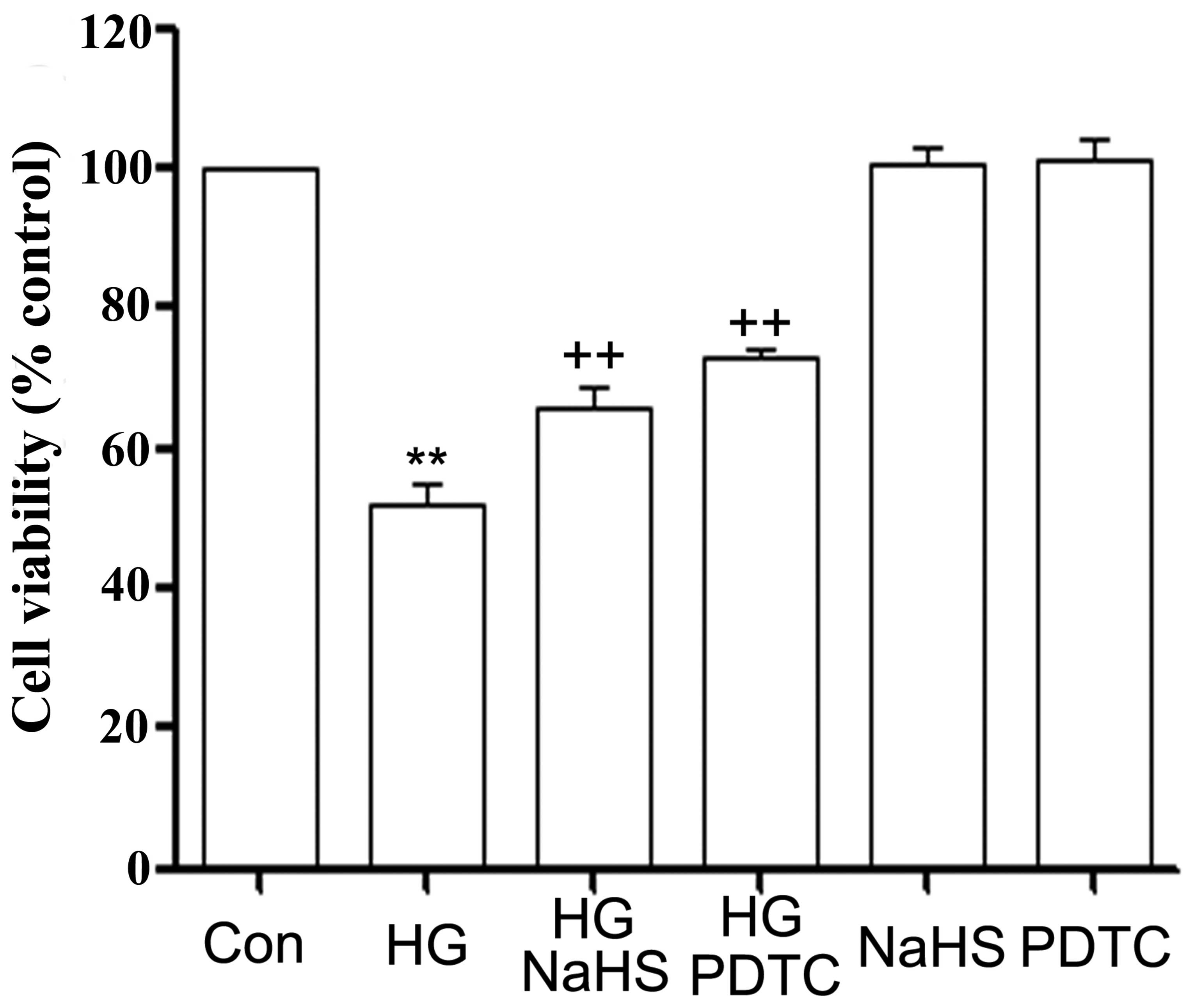
Role of inhibition of NF-κB activation in
the protective effects of H2S against HG-induced
cytotoxicity in H9c2 cardiac cells
In agreement with our previous studies (5,18),
treatment of the cells with 400 μM NaHS for 30 min prior to
exposure to HG for 24 h markedly decreased HG-induced cytotoxicity,
as indicated by an increase in cell viability (Fig. 2). Since we demonstrated that the
expression level of p-NF-κB p65 was increased by HG treatment
(Fig. 1), we then wished to
confirm the role of NF-κB activation in the HG-induced
cytotoxicity. As shown in Fig. 2,
treatment of the H9c2 cardiac cells with 100 μM PDTC (an inhibitor
of NF-κB) for 30 min prior to exposure to 35 mM glucose markedly
blocked the HG-induced cytotoxicity, leading to an increase in cell
viability, indicating the involvement of NF-κB activation in
HG-induced cytotoxicity.
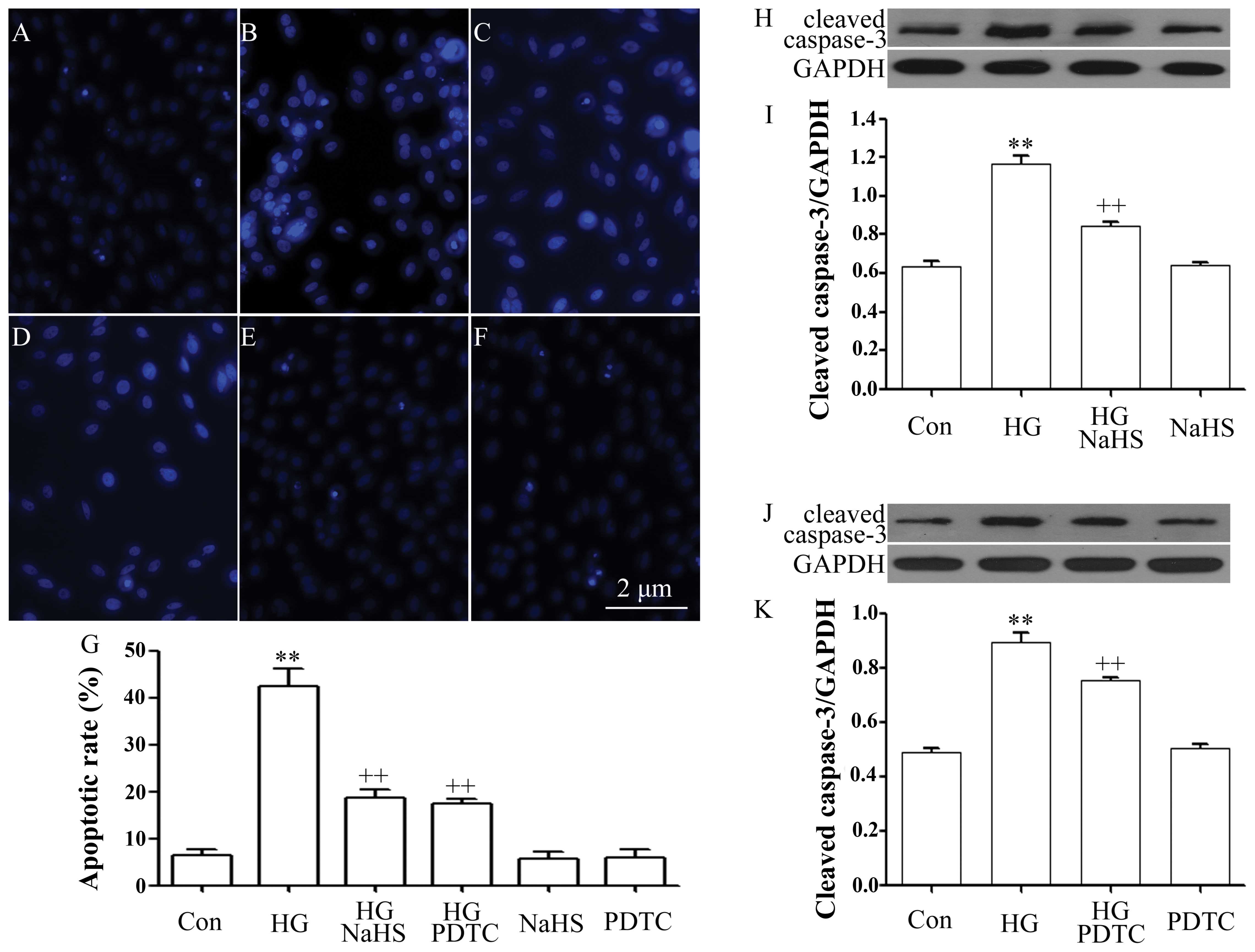
Role of inhibition of NF-κB activation in
the protective effects of H2S against HG-induced
apoptosis in H9c2 cells
As illustrated in Fig.
3, exposure of the cells to 35 mM glucose for 24 h induced
significant apoptosis, as evidenced by an increase in the number of
apoptotic cells (Fig. 3B and G)
and in the expression of cleaved caspase-3 (Fig. 3H and J). However, the increased
number of apoptotic cells and the cleaved caspase-3 expression
level were markedly diminished by treatment with 400 μM NaHS for 30
min prior to exposure to HG (Fig. 3C
and G–I). Similarly, treatment of the cells with 100 μM PDTC
for 30 min prior to exposure to HG also considerably blocked
HG-induced apoptosis, leading to a decrease in the number of
apoptotic cells and the expression level of cleaved caspase-3
(Fig. 3D, G, J and K). Alone,
NaHS or PDTC did not significantly alter the percentage of
apoptotic cells and the basal expression level of cleaved
caspase-3. These results reveal that the inhibition of the
activation of NF-κB plays a role in the protective effects of
exogenous H2S against HG-induced apoptosis.
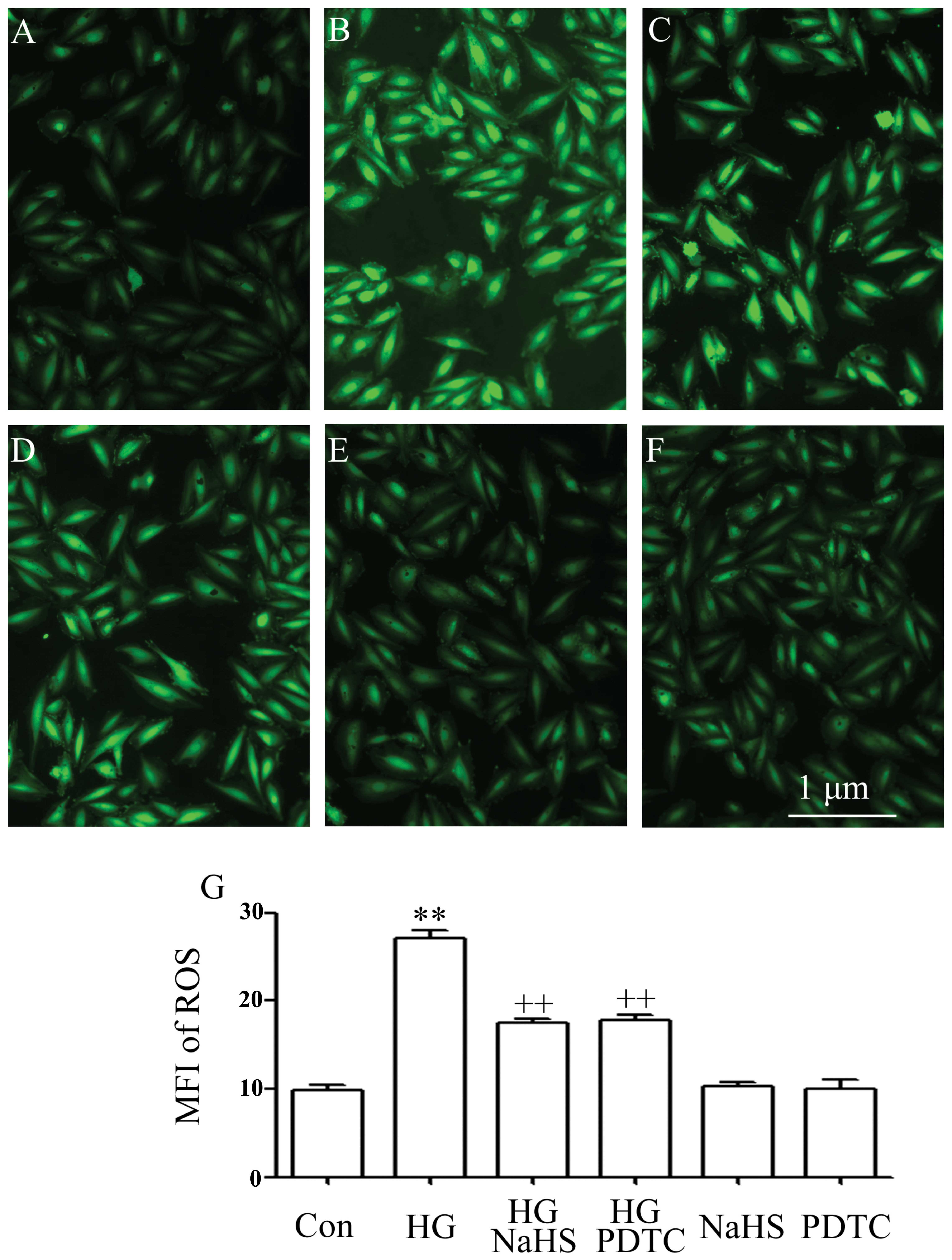
Role of inhibition of NF-κB activation in
the inhibitory effects of H2S on the HG-induced
generation of ROS in H9c2 cardiac cells
Consistent with our previous studies (5,6,10,18), exposure of the cells to 35 mM
glucose for 24 h markedly increased the intracellular generation of
ROS (Fig. 4B). The elevated ROS
generation was reduced by treatment of the cells with 400 μM NaHS
for 30 min prior to exposure to HG (Fig. 4C). To determine whether the
activation of NF-κB plays a role in HG-induced oxidative stress,
the H9c2 cells were treated with 100 μM PDTC for 30 min prior to
exposure to HG. It was shown that pre-treatment with PDTC markedly
reduced the increased intracellular ROS generation induced by HG
(Fig. 4D), indicating the
contribution of the activation of the NF-κB pathway to HG-induced
oxidative stress in H9c2 cells.
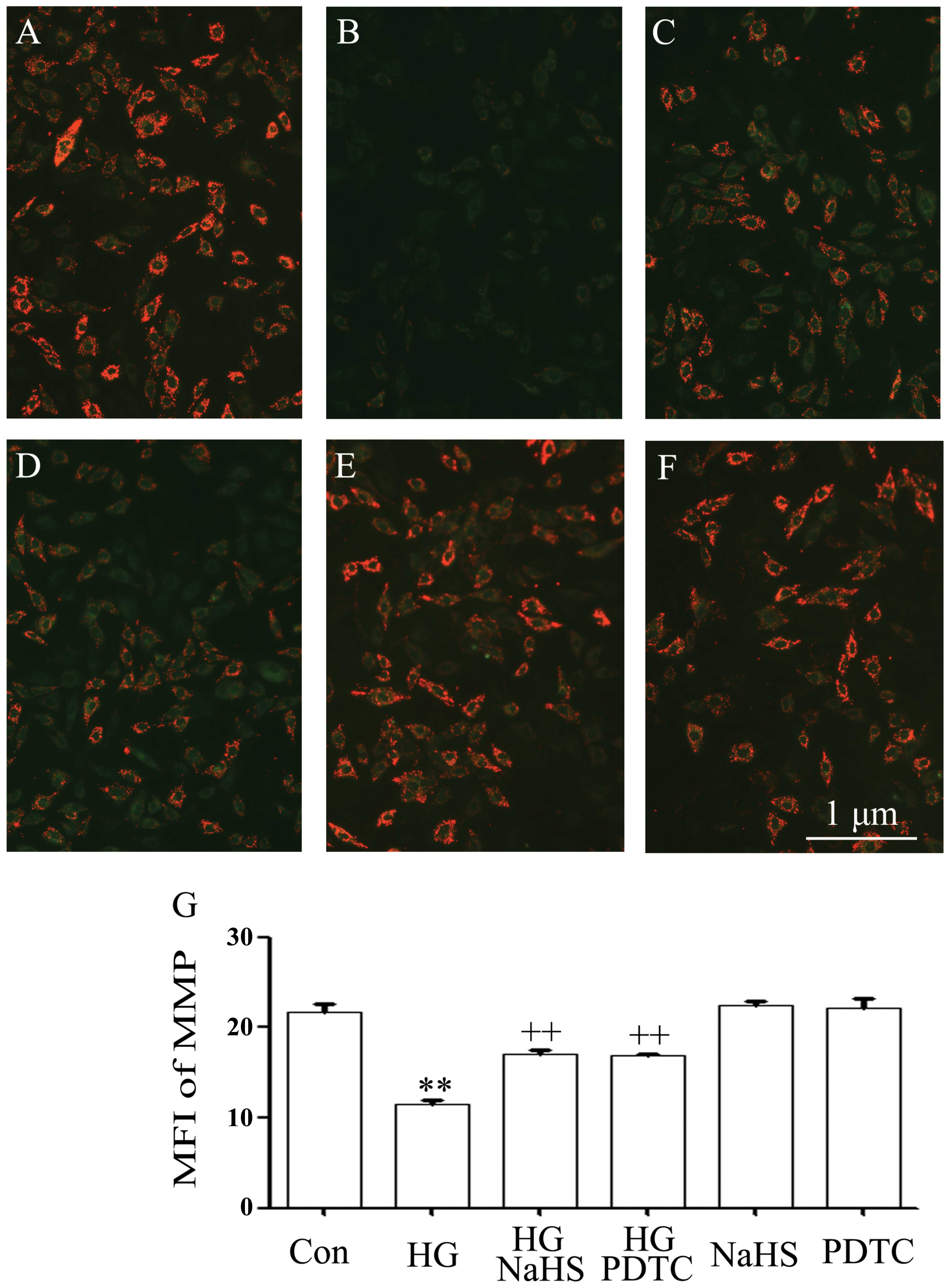
Role of the inhibition of NF-κB
activation in the protective effects of H2S against the
HG-induced dissipation of MMP in H9c2 cardiac cells
It has been reported that HG induces mitochondrial
damage in cardiomyocytes (5,6,10,18). As illustrated in Fig. 5B and G, exposure of the cells to
35 mM glucose for 24 h induced the marked dissipation of MMP. The
dissipation of MMP was blocked by pre-treatment with 400 μM NaHS
for 30 min prior to exposure to HG (Fig. 5C and G). Additionally, treatment
of the H9c2 cells with 100 μM PDTC for 30 min prior to exposure to
HG for 24 h also markedly reduced the dissipation of MMP,
suggesting that the activation of NF-κB participates in the
HG-induced loss of MMP in H9c2 cells.
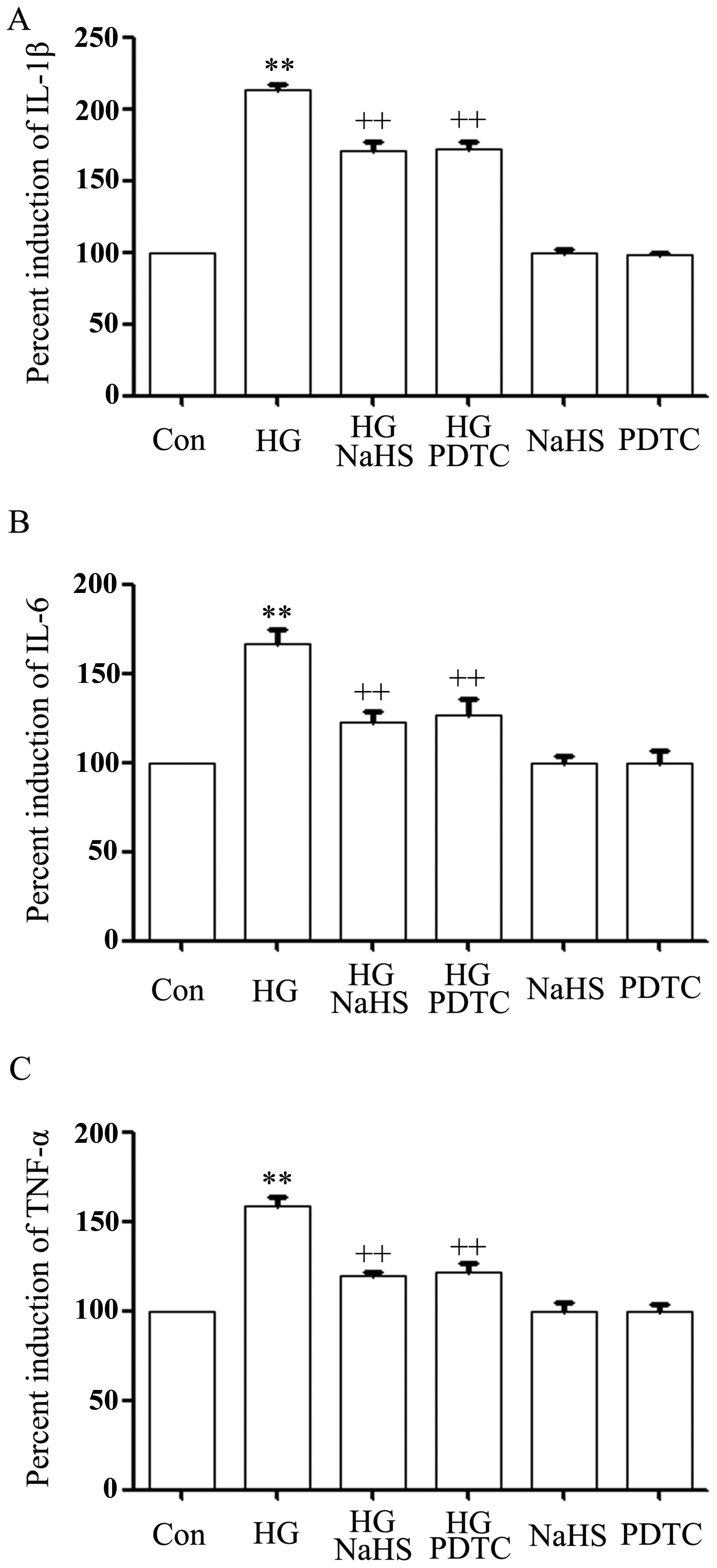
NaHS and NF-κB inhibitor reduce the
HG-induced production of pro-inflammatory cytokines in H9c2 cardiac
cells
As indicated in Fig.
6, after the cells were treated with 35 mM glucose (HG) for 24
h, the levels of IL-1β (Fig. 6A),
IL-6 (Fig. 6B) and TNF-α
(Fig. 6C) were markedly enhanced,
compared with the control group (p<0.01). However, the increased
levels of IL-1β, IL-6 and TNF-α induced by HG were markedly
attenuated by treatment with 400 μM NaHS for 30 min prior to
exposure to HG for 24 h, revealing the inhibitory effects of
exogenous H2S on the production of pro-inflammatory
cytokines, including IL-1β, IL-6 and TNF-α induced by HG.
Similarly, treatment of the cells with 100 μM PDTC for 30 min prior
to exposure to HG also alleviated the increased production of
IL-1β, IL-6 and TNF-α (Fig.
6A–C), suggesting that the activation of NF-κB contributes to
the HG-induced inflammatory response in H9c2 cardiac cells.
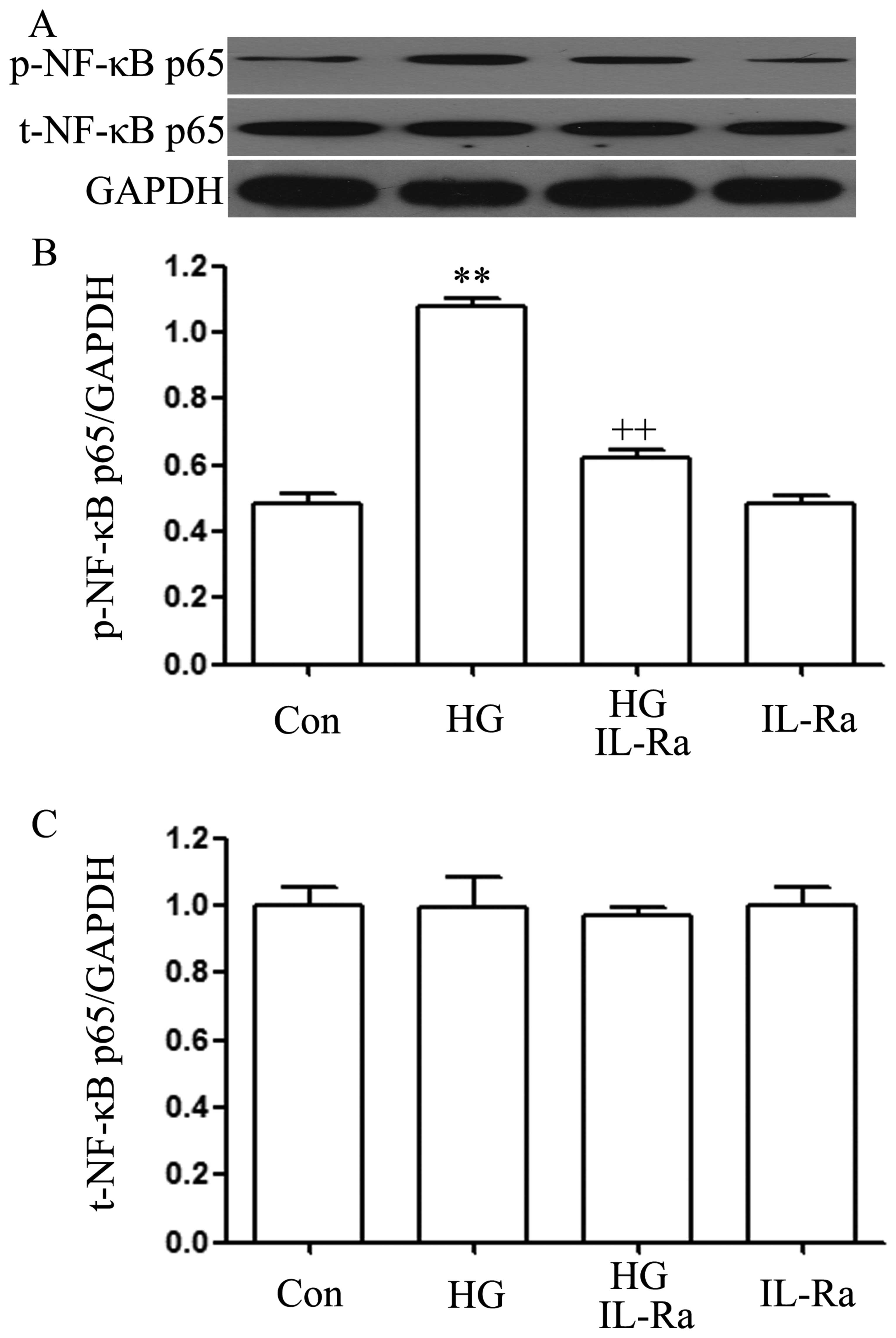
IL-1Ra suppresses the HG-induced
upregulation of p-NF-κB p65 expression in H9c2 cardiac cells
Since the above results (Fig. 1) revealed that exposure to HG
upregulated the expression of the p-NF-κB p65 subunit and that the
activation of NF-κB was involved in the production of IL-1β induced
by HG (Fig. 6), and a recent
study reported that IL-1Ra reduces hyperglycemia and tissue
inflammation in type 2 diabetic GK rats (31), we then wished to explore whether
IL-1β contributes to the activation of NF-κB by HG. As shown in
Fig. 7, exposure of the H9c2
cells to 35 mM glucose for 90 min significantly enhanced the
expression level of p-NF-κB p65. However, co-treatment of the cells
with 20 ng/ml IL-1Ra and 35 mM glucose for 90 min significantly
attenuated the HG-induced increase in p-NF-κB p65 expression
(Fig. 7A and B). Alone, IL-1Ra
did not affect the basal expression of p-NF-κB in the H9c2 cells
(Fig. 7A and C).
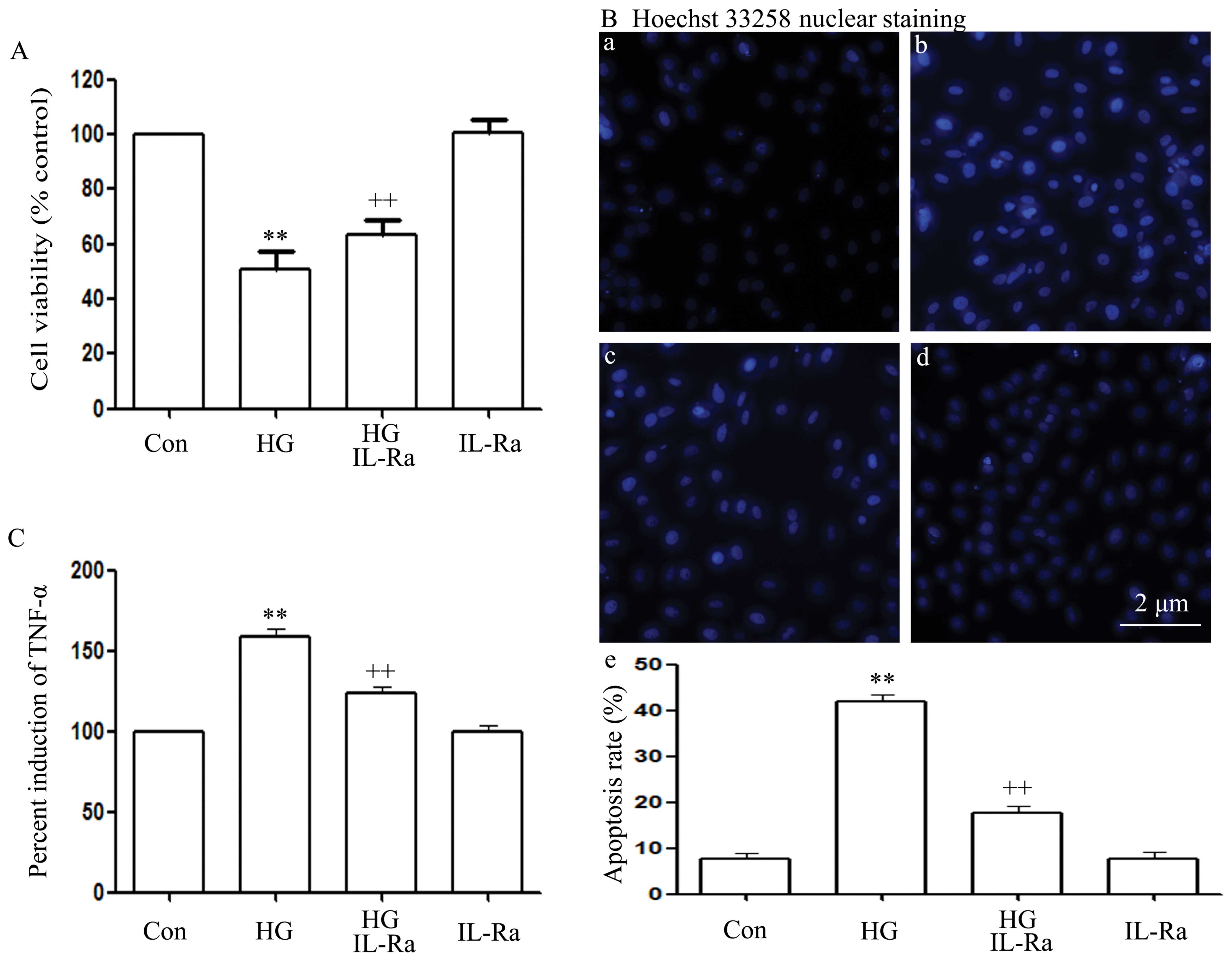
IL-1Ra attenuates HG-induced
cytotoxicity, apoptosis and TNF-α production in H9c2 cardiac
cells
As shown in Fig.
8, co-treatment of the cells with 20 ng/ml IL-1Ra and 35 mM
glucose for 24 h markedly attenuated the HG-induced cytotoxicity,
apoptosis and TNF-α production, as evidenced by an increase in cell
viability (Fig. 8A), as well as
by a decrease in the number of apoptotic cells (Fig. 8B, panels b and e) and TNF-α
production (Fig. 8C). Alone,
IL-1Ra did not affect cell viability, the number of apoptotic cells
and the basal production of TNF-α in the H9c2 cells (Fig. 8A–C).
Discussion
To the best of our knowledge, the present study
demonstrates for the first time that exogenous H2S
protects H9c2 cardiac cells against HG-induced injury and
inflammation by inhibiting the activation of NF-κB and IL-1β and
that the positive interaction between NF-κB and IL-1β contributes
to the HG-induced injury and inflammation. Several lines of
evidence support our conclusion: i) exogenous H2S
attenuated the HG-induced increase in p-NF-κB p65 expression, and
IL-1β production; ii) exogenous H2S inhibited HG-induced
cardiomyocyte injury and inflammation, as evidenced by an increase
in cell viability, and a decrease in the number of apoptotic cells
and cleaved caspase-3 expression, as well as a decrease in ROS
production and the dissipation of MMP and the production of IL-1β,
IL-6 and TNF-α; iii) similar to the inhibitory effects of exogenous
H2S, pre-treatment of the H9c2 cells with PDTC (an
inhibitor of NF-κB) also ameliorated the HG-induced injury and
inflammation; and iv) co-treatment of the H9c2 cells with IL-Ra (an
inhibitor of IL-1β receptor) and HG alleviated the cytotoxicity,
apoptosis and upregulation of p-NF-κB expression induced by HG.
NF-κB is a key transcription factor that regulates
inflammatory processes (32).
Previous studies have demonstrated that NF-κB is involved in
cellular damage and inflammation induced by various stimuli,
including chemical hypoxia (30),
doxorubicin (14,15) and lipopolysaccharide (LPS)
(33). NF-κB may participate in
some of the downstream effects of NADH oxide on cardiac hypertrophy
and it regulates the expression of inflammatory genes, such as
TNF-α and IL-6 (34,35). Recently, the pathophysiological
roles of the NF-κB pathway in diatetic complications, such as
diabetic cardiomyopathy, have attracted considerable attention
(17,31,36). In diabetic rats, NF-κB is
activated (17,31,36). However, the roles of the NF-κB
pathway in HG-induced cardiac injury and inflammation remain poorly
understood. In order to explore this, in this study, we first
observed the effects of HG on the activation of NF-κB in H9c2
cardiac cells. In agreement with the results of previous studies
(17,31,36), it was shown that exposure to HG
significantly induced an increase in p-NF-κB expression, suggesting
the activation of the NF-κB pathway by HG. Secondly, we
investigated the roles of the NF-κB activation in HG-induced
injury. Our results demonstrated that pre-treatment of the H9c2
cardiac cells with PDTC (an inhibitor of NF-κB) markedly suppressed
HG-induced multiple injury, including cytotoxicity, apoptosis,
oxidative stress and mitochondrial damage, as characterized by an
increase in cell viability, as well as a decrease in the number of
apoptotic cells, cleaved caspase-3 expression, ROS generation and
the dissipation of MMP. These findings suggest that NF-κB
activation is involved in HG-induced multiple damage in H9c2 cells.
Since our (30), as well as other
previous studies (37) have shown
that ROS generation induces the activation of NF-κB in other cell
types, the combination of the results of this study with those of
other studies (30,38) suggests that there may be a
crosstalk between the ROS and NF-κB pathway in HG-treated H9c2
cells. To confirm this hypothesis, further studies are required.
Moreover, since data from diabetic patients (39,40) and diabetic animals (17,31,36) have demonstrated that hyperglycemia
is associated with tissue inflammation, in this study, we examined
the effects of NF-κB activation on pro-inflammatory factors.
Similar to the results of previous studies (17,31,36,39,40), this study demonstrated that
exposure to HG induced significant cardiac inflammatory responses,
as indicated by the increased production of IL-1β, IL-6 and TNF-α.
However, the increased production of IL-1β, IL-6 and TNF-α was
reduced by PDTC, suggesting the involvement of NF-κB activation in
the HG-induced production of pro-inflammatory factors. These data
provide clear evidence that the activation of the NF-κB pathway
contributes to HG-induced injury and inflammation in H9c2 cardiac
cells, which confirms and adds to the results of previous studies
demonstrating the roles of NF-κB activation in
hyperglycemia-induced cardiac damage and inflammation (41).
Another major finding of this study relates to a
positive interaction between NF-κB and IL-1β which is critical for
the induction of cardiomyocyte injury and inflammation induced by
HG. This consideration is based on the results of a previous study
demonstrating an interaction between NF-κB and IL-1β in aged murine
lungs in response to endotoxic stress (42). IL-1 has been shown to play
critical roles in the pathogenesis of heart disease (31,43). IL-1 binds to the transmembrane
IL-1 type receptor (IL-1R) and initiates a downstream signal. Its
natural antagonist, IL-1Ra, competitively interacts with the same
receptor to prevent IL-1 signaling (44). Ehses et al demonstrated
that IL-Ra reduces hyperglycemia and tissue inflammation in type 2
diabetic rats (31). Similarly,
we observed the inhibitory effects of IL-1 Ra on the HG-induced
cardiac cytotoxicity, apoptosis and inflammation. In addition, this
study confirmed the contribution of NF-κB activation to the
HG-induced cytotoxicity, apoptosis, oxidative stress, loss of MMP
and inflammation. Therefore, we speculate that there may be an
interaction between NF-κB and IL-1β in the induction of injury and
inflammation in HG-exposed H9c2 cardiac cells. Significantly, our
data demonstrate that PDTC inhibits the HG-induced production of
IL-1β and that IL-1Ra attenuates the increased expression of
p-NF-κB p65 subunit expression, which clearly confirms our
hypothesis. These results provide novel mechanistic evidence for
the roles of both NF-κB and IL-1β in the HG-induced cardiac injury
and inflammation.
Another important finding of the present study
relates to the roles of the inhibition of the NF-κB and IL-1β
pathways in the cardioprotective effects of exogenous
H2S against HG-induced multiple cardiac injury and
inflammation. Accumulating evidence indicates that H2S
exerts cardioprotective effects (4,5,15,18,21–24). H2S therapy offers
protection against several models of myocardial injury in the
setting of type 1 (25,29,45) or type 2 (4) diabetes by attenuating apoptosis and
oxidative stress. Recently, we demonstrated that exogenous
H2S alleviates cytotoxicity, apoptosis, oxidative stress
and the loss of MMP by inhibiting the p38 MAPK, ERK1/2 and leptin
pathways in HG-treated H9c2 cardiac cells (5,18).
Since we have reported that exogenous H2S suppresses the
p38 MAPK/NF-κB-mediated inflammatory response and cytotoxicity in
doxorubicin-treated H9c2 cardiac cells (15), this study further explored whether
H2S protects the cells against HG-induced injury and
inflammation by inhibiting the NF-κB and IL-1β pathways. Our
findings demonstrated that exogenous H2S markedly
reduced NF-κB activation and IL-1β production. In addition, similar
to the inhibitory effects of both PDTC and IL-1Ra as indicated
above, pre-treatment with H2S markedly attenuated the
HG-induced cardiac injury and inflammation. These results suggest
that the inhibition of the NF-κB and IL-1β pathways may be a
critical mechanism responsible for the protective effects of
exogenous H2S against HG-induced cardiac injury and
inflammation. Similarily, several recent studies have indicated
that exogenous H2S reduces the chemical hypoxia-induced
(30) or doxorubicin-induced
(15) cell injury and
inflammation through the inhibition of NF-κB and IL-1β. These
studies (15,30) support our results.
In conclusion, the present study provides novel
evidence that a positive interaction between the NF-κB and IL-1β
pathways contributes to the HG-induced cardiac injury and
inflammation and that exogenous H2S protects H9c2
cardiac cells against HG-induced injury and inflammation through
the inhibition of the NF-κB and IL-1β pathways. These findings may
aid in the development of novel therapeutic approaches for the
prevention and treatment of hyperglycemia-related injury and
inflammation.
Acknowledgements
The present study was supported by grants from the
Guangdong Medical Research Foundation (no. A2012172), Science and
Techonology Planning Project of Guangdong Province, China (nos.
2012B031800358, 2012B031800365 and 2010B08071044) and the Guangdong
Natural Science Foundation (S2011010004381).
References
|
1
|
Brownlee M: Biochemistry and molecular
cell biology of diabetic complications. Nature. 414:813–820. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ren J and Davidoff AJ: Diabetes rapidly
induces contractile dysfunctions in isolated ventricular myocytes.
Am J Physiol. 272:H148–H158. 1997.PubMed/NCBI
|
|
3
|
Soetikno V, Sari FR, Sukumaran V, et al:
Curcumin prevents diabetic cardiomyopathy in streptozotocin-induced
diabetic rats: possible involvement of PKC-MAPK signaling pathway.
Eur J Pharm Sci. 47:604–614. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peake BF, Nicholson CK, Lambert JP, et al:
Hydrogen sulfide preconditions the db/db diabetic mouse heart
against ischemia-reperfusion injury by activating Nrf2 signaling in
an Erk-dependent manner. Am J Physiol Heart Circ Physiol.
304:H1215–H1224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu W, Wu W, Chen J, et al: Exogenous
hydrogen sulfide protects H9c2 cardiac cells against high
glucose-induced injury by inhibiting the activities of the p38 MAPK
and ERK1/2 pathways. Int J Mol Med. 32:917–925. 2013.PubMed/NCBI
|
|
6
|
Chen J, Mo H, Guo R, et al: Inhibition of
the leptin-induced activation of the p38 MAPK pathway contributes
to the protective effects of naringin against high glucose-induced
injury in H9c2 cardiac cells. Int J Mol Med. 33:605–612.
2014.PubMed/NCBI
|
|
7
|
Huang H, Wu K, You Q, et al: Naringin
inhibits high glucose-induced cardiomyocyte apoptosis by
attenuating mitochondrial dysfunction and modulating the activation
of the p38 signaling pathway. Int J Mol Med. 32:396–402.
2013.PubMed/NCBI
|
|
8
|
Cai L, Li W, Wang G, et al:
Hyperglycemia-induced apoptosis in mouse myocardium: mitochondrial
cytochrome C-mediated caspase-3 activation pathway. Diabetes.
51:1938–1948. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fiordaliso F, Leri A, Cesselli D, et al:
Hyperglycemia activates p53 and p53-regulated genes leading to
myocyte cell death. Diabetes. 50:2363–2375. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen J, Guo R, Yan H, et al: Naringin
inhibits ROS-activated MAPK pathway in high glucose-induced
injuries in H9c2 cardiac cells. Basic Clin Pharmacol Toxicol.
114:293–304. 2014. View Article : Google Scholar
|
|
11
|
Yan J, Young ME, Cui L, et al: Increased
glucose uptake and oxidation in mouse hearts prevent high fatty
acid oxidation but cause cardiac dysfunction in diet-induced
obesity. Circulation. 119:2818–2828. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Majumdar P, Chen S, George B, et al:
Leptin and endothelin-1 mediated increased extracellular matrix
protein production and cardiomyocyte hypertrophy in diabetic heart
disease. Diabetes Metab Res Rev. 25:452–463. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baldwin AS Jr: The NF-kappa B and I kappa
B proteins: new discoveries and insights. Annu Rev Immunol.
14:649–683. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo RM, Xu WM, Lin JC, et al: Activation
of the p38 MAPK/NF-κB pathway contributes to doxorubicin-induced
inflammation and cytotoxicity in H9c2 cardiac cells. Mol Med Rep.
8:603–608. 2013.PubMed/NCBI
|
|
15
|
Guo R, Wu K, Chen J, et al: Exogenous
hydrogen sulfide protects against doxorubicin-induced inflammation
and cytotoxicity by inhibiting p38MAPK/NFκB pathway in H9c2 cardiac
cells. Cell Physiol Biochem. 32:1668–1680. 2013.
|
|
16
|
Hirotani S, Otsu K, Nishida K, et al:
Involvement of nuclear factor-kappaB and apoptosis
signal-regulating kinase 1 in G-protein-coupled receptor
agonist-induced cardiomyocyte hypertrophy. Circulation.
105:509–515. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thandavarayan RA, Giridharan VV, Sari FR,
et al: Depletion of 14-3-3 protein exacerbates cardiac oxidative
stress, inflammation and remodeling process via modulation of
MAPK/NF-κB signaling pathways after streptozotocin-induced diabetes
mellitus. Cell Physiol Biochem. 28:911–922. 2011. View Article : Google Scholar
|
|
18
|
Zhuang XD, Hu X, Long M, et al: Exogenous
hydrogen sulfide alleviates high glucose-induced cardiotoxicity via
inhibition of leptin signaling in H9c2 cells. Mol Cell Biochem.
391:147–155. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Łowicka E and Bełtowski J: Hydrogen
sulfide (H2S) - the third gas of interest for
pharmacologists. Pharmacol Rep. 59:4–24. 2007.
|
|
20
|
Moore PK, Bhatia M and Moochhala S:
Hydrogen sulfide: from the smell of the past to the mediator of the
future? Trends Pharmacol Sci. 24:609–611. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Geng B, Chang L, Pan C, et al: Endogenous
hydrogen sulfide regulation of myocardial injury induced by
isoproterenol. Biochem Biophys Res Commun. 318:756–763. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Calvert JW, Jha S, Gundewar S, Elrod JW,
et al: Hydrogen sulfide mediates cardioprotection through Nrf2
signaling. Circ Res. 105:365–374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen SL, Yang CT, Yang ZL, et al: Hydrogen
sulphide protects H9c2 cells against chemical hypoxia-induced
injury. Clin Exp Pharmacol Physiol. 37:316–321. 2010. View Article : Google Scholar
|
|
24
|
Yang Z, Yang C, Xiao L, et al: Novel
insights into the role of HSP90 in cytoprotection of H2S
against chemical hypoxia-induced injury in H9c2 cardiac myocytes.
Int J Mol Med. 28:397–403. 2011.PubMed/NCBI
|
|
25
|
Suzuki K, Olah G, Modis K, et al: Hydrogen
sulfide replacement therapy protects the vascular endothelium in
hyperglycemia by preserving mitochondrial function. Proc Natl Acad
Sci USA. 108:13829–13834. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jain SK, Bull R, Rains JL, et al: Low
levels of hydrogen sulfide in the blood of diabetes patients and
streptozotocin-treated rats causes vascular inflammation? Antioxid
Redox Signal. 12:1333–1337. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ahmad FU, Sattar MA, Rathore HA, et al:
Exogenous hydrogen sulfide (H2S) reduces blood pressure
and prevents the progression of diabetic nephropathy in
spontaneously hypertensive rats. Ren Fail. 34:203–210. 2012.
|
|
28
|
Whiteman M, Gooding KM, Whatmore JL, et
al: Adiposity is a major determinant of plasma levels of the novel
vasodilator hydrogen sulphide. Diabetologia. 53:1722–1726. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao Y, Yao X, Zhang Y, et al: The
protective role of hydrogen sulfide in myocardial
ischemia-reperfusion-induced injury in diabetic rats. Int J
Cardiol. 152:177–183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang C, Yang Z, Zhang M, et al: Hydrogen
sulfide protects against chemical hypoxia-induced cytotoxicity and
inflammation in HaCaT cells through inhibition of ROS/NF-κB/COX-2
pathway. PLoS One. 6:e219712011. View Article : Google Scholar
|
|
31
|
Ehses JA, Lacraz G, Giroix MH, et al: IL-1
antagonism reduces hyperglycemia and tissue inflammation in the
type 2 diabetic GK rat. Proc Natl Acad Sci USA. 106:13998–14003.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Barnes PJ and Karin M: Nuclear
factor-kappaB: a pivotal transcription factor in chronic
inflammatory diseases. N Engl J Med. 336:1066–1071. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hu LF, Wong PT, Moore PK and Bian JS:
Hydrogen sulfide attenuates lipopolysaccharide-induced inflammation
by inhibition of p38 mitogen-activated protein kinase in microglia.
J Neurochem. 100:1121–1128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Satoh M, Ogita H, Takeshita K, et al:
Requirement of Rac1 in the development of cardiac hypertrophy. Proc
Natl Acad Sci USA. 103:7432–7437. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu SF, Ye X and Malik AB: Inhibition of
NF-kappaB activation by pyrrolidine dithiocarbamate prevents in
vivo expression of proinflammatory genes. Circulation.
100:1330–1337. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kowluru RA and Kanwar M: Effects of
curcumin on retinal oxidative stress and inflammation in diabetes.
Nutr Metab (Lond). 4:82007. View Article : Google Scholar
|
|
37
|
Cichon MA and Radisky DC: ROS-induced
epithelial-mesenchymal transition in mammary epithelial cells is
mediated by NF-κB-dependent activation of Snail. Oncotarget.
5:2827–2838. 2014.PubMed/NCBI
|
|
38
|
Goldin A, Beckman JA, Schmidt AM and
Creager MA: Advanced glycation end products: sparking the
development of diabetic vascular injury. Circulation. 114:597–605.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Varo N, Vicent D, Libby P, et al: Elevated
plasma levels of the atherogenic mediator soluble CD40 ligand in
diabetic patients: a novel target of thiazolidinediones.
Circulation. 107:2664–2669. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Aronson D, Bartha P, Zinder O, et al:
Association between fasting glucose and C-reactive protein in
middle-aged subjects. Diabet Med. 21:39–44. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kuo WW, Wang WJ, Tsai CY, et al: Diallyl
trisufide (DATS) suppresses high glucose-induced cardiomyocyte
apoptosis by inhibiting JNK/NFκB signaling via attenuating ROS
generation. Int J Cardiol. 168:270–280. 2013. View Article : Google Scholar
|
|
42
|
Chang CK and LoCicero J 3rd: Overexpressed
nuclear factor kappaB correlates with enhanced expression of
interleukin-1beta and inducible nitric oxide synthase in aged
murine lungs to endotoxic stress. Ann Thorac Surg. 77:1222–1227.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bujak M and Frangogiannis NG: The role of
IL-1 in the pathogenesis of heart disease. Arch Immunol Ther Exp
(Warsz). 57:165–176. 2009. View Article : Google Scholar
|
|
44
|
Dinarello CA, Cannon JG, Mancilla J, et
al: Interleukin-6 as an endogenous pyrogen: induction of
prostaglandin E2 in brain but not in peripheral blood mononuclear
cells. Brain Res. 562:199–206. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
El-Seweidy MM, Sadik NA and Shaker OG:
Role of sulfurous mineral water and sodium hydrosulfide as potent
inhibitors of fibrosis in the heart of diabetic rats. Arch Biochem
Biophys. 506:48–57. 2011. View Article : Google Scholar
|