Introduction
Periodontal disease is among the most common
pathological conditions of the oral cavity. It is a predominantly
polymicrobial infection and its main consequence is tooth loss,
accompanied by esthetic, phonetic and masticatory problems, with
significant psychosocial impairment. There is evidence indicating
that periodontal disease is a risk factor for cardiovascular
diseases (1), stroke (2), pulmonary infections (3) and low birth weight (4). This evidence renders periodontal
disease an important public health issue.
The inflammatory process in periodontal disease is
triggered and perpetuated by periodontal pathogens. These are
Gram-negative anaerobic bacteria, such as Porphyromonas
gingivalis (P. gingivalis), Aggregatibacter
actinomycetemcomitans (A. actinomycetemcomitans) and
Fusobacterium nucleatum (F. nucleatum) (5).
In periodontal disease, bacteria and the products
from microbial metabolism, such as butyric and propionic acids,
interact with the junctional epithelium, thus enabling the invasion
of the underlying connective tissue. This invasion activates an
immune response with the consequent production of pro-inflammatory
mediators [interleukin (IL)-1, prostaglandin E2 (PGE2)
and tumor necrosis factor (TNFα)] produced by T lymphocytes that
are present within the periodontal pouch, which promote the
differentiation and activation of osteoclasts (OCs), culminating in
alveolar bone resorption and consequent tooth loss (6).
The role of the immune system in differentiating and
activating OCs is of fundamental importance, particularly the
participation of the receptor activator of nuclear factor (NF)-κB
ligand (RANKL), which is present in osteoblasts and cells of the
bone marrow stroma and macrophage colony-stimulating factor
(M-CSF), which is produced by the bone marrow stroma, osteoblasts
and activated T lymphocytes. The co-stimulation of RANKL and M-CSF
is important in inducing the differentiation and activation of OCs,
and thus actively participates in the homeostasis of bone tissue
(7). Bone resorption induced by
OCs is one of the main causes of tooth loss in periodontal disease.
Accordingly, the modulation of OC formation and function is
suggested as one of the therapeutic targets in the prevention of
alveolar bone loss associated with periodontal disease (8).
The clinical procedure most commonly used for
treating periodontal disease and/or limiting the damage caused by
alveolar bone destruction is root planing and scaling in
association with the use of antimicrobial agents. However, studies
have reported resistance to antibiotics among periodontal pathogens
(9–11). Furthermore, despite proven
results, antiseptic substances based on chlorhexidine gluconate
present adverse effects that compromise their use for prolonged
periods of time. In this regard, the use of medicinal plants may be
a promising alternative.
Medicinal plants are potentially useful for
preventing and treating periodontal disease, given that an
inhibitory effect on the growth of periodontal pathogens has been
reported by some studies, from the use of phytotherapy in
vitro (12,13). Therefore, there is an enormous
social relevance in studying the effects of these plants on the
microbiota of periodontal pathogens and in relation to controlling
the activation of OCs, which would lead to preventive control with
greater accessibility for the population, at a lower cost.
Lippia alba (Mill.) N.E.Br. (L. alba)
is a plant in the family Verbenaceae that is popularly known
as bushy matgrass, bushy lippia and pitiona in English, and as
erva-cidreira, chá-de-tabuleiro, cidrila, alecrim-selvagem,
cidreira-brava, falsa-melissa, erva-cidreira-de-campo, salva,
salva-do-brasil, salva-limão, alecrim-do-campo, salva-brava and
sálvia in Portuguese (14). This
plant is considered to be the second most commonly used medicinal
plant in the state of Bahia, Brazil. It is used for sedation,
hypertension, flatulence and pain relief (15,16). It is also abundantly present in
the southern part of the United States (Florida), and in the
northern parts of Argentina, India (17) and Australia (18).
The first major objective of the present study was
to investigate whether essential oils from L. alba display
in vitro antibacterial activity against periodontal
pathogens, such as P. gingivalis, A.
actinomycetemcomitans and F. nucleatum, by determining
their minimum inhibitory concentration (MIC) and minimum
bactericidal concentration (MBC).
Essential oils from L. alba have been also
been shown to exert analgesic, anticonvulsant and anti-inflammatory
effects (19). These effects may
be mainly attributed to the decreased production of
pro-inflammatory cytokines, principally through the inhibition of
NF-κB activation. Furthermore, NF-κB plays a critical role in OC
biology by regulating the expression of a large number of
OC-specific genes through the RANKL/RANK signaling cascade
(20,21). On the basis of such evidence, the
second major objective of this study was to determine the effects
of these essential oils on OCs in terms of the induction of
programmed cell death (apoptosis).
Materials and methods
Plant samples
L. alba was cultivated from March to June
2010, in the Medicinal Plants Garden at the Federal University of
Reconcavo of Bahia, located in the municipality of Santo Antônio de
Jesus, Bahia, Brazil. Herborization and treatment were carried out
according to Mori et al (22). The botanical material collected
was deposited in the Herbarium of the State University of Feira de
Santana, where it was identified as L. alba (Mill.) N.E.Br.
(Verbenaceae) - HUEFS 167949, according to the Cronquist system
(23).
Extraction of essential oils
Steam hydrodistillation was used for the extraction
of the essential oils from the dry leaves and flowers. The chemical
composition of the essential oils was determined by gas
chromatography-mass spectrometry (GC/MS) in a Shimadzu GC-2010 gas
chromatograph coupled to a CG/MS-QP 2010 Shimadzu mass
spectrometer. (Shimadzu Corp., Kyoto, Japan) The components were
identified by comparing the obtained mass spectra with the library
of the equipment used, and by comparing the calculated Kovats
indices with those found in the literature (24) using a homologous series of
hydrocarbons.
Determination of antimicrobial activity
against periodontal pathogens
Antimicrobial susceptibility testing was performed
according to the CLSI broth macrodilution method M11-A5, with some
modifications (25). The
bacterial inoculum was prepared by suspending recent colonies of
A. actinomycetemcomitans (ATCC 43717), F. nucleatum
(ATCC 25586), P. gingivalis (ATCC 33277) and Bacteroides
fragilis (B. fragilis; ATCC 25285) in 5 ml of
brain-heart infusion (BHI) broth supplemented with hemin (5 μg/ml)
and menadione (1 μg/ml vitamin K), followed by incubation under
anaerobic conditions (90% N2 and 10% CO2) at
37°C for 48 h. After the incubation period, the inoculum was
adjusted to 1.5×108 colony-forming units (CFU)/ml. Stock
solutions of essential oils (50 mg/ml), sterilized by membrane
filtration (0.22 μm) were prepared using 10% Tween-80 as a solvent.
Increasing concentrations of the compounds (0.00625–3.2 mg/ml) were
used for the tests. MIC determination was carried out through
medium observation for turbidity and/or the presence of sediment.
Chlorhexidine gluconate 0.12% (Colgate® Periogard) was
used as the control. Experiments were carried out in triplicate.
Test tubes with no visible growth in the MIC determination step had
10 μl plated on blood agar supplemented with hemin (5 μg/ml) and
menadione (1 μg/ml vitamin K), and anaerobically incubated (90%
N2 and 10% CO2) at 37°C for 72 h to determine
the MBC.
Cell culture
Human primary OCs were obtained as previously
described by Matsuzaki et al (26), with minor modifications. Briefly,
peripheral blood (PB) was collected from healthy volunteers after
informed consent was obtained. PB mononuclear cells (PBMCs) were
obtained from diluted PB (1:2 in Hanks solution), separated by
Histopaque®-1077 (Sigma, St. Louis, MO, USA) and
subsequently grown in DMEM high glucose medium (EuroClone SpA,
Milan, Italy) in the presence of M-CSF (25 ng/ml), RANKL (30 ng/ml)
at 37°C in a 5% CO2 atmosphere for 14 days. To evaluate
osteoclastogenesis, staining for tartrate-resistant acid
phosphatase (TRAP) was carried out using the Acid Phosphatase
Leukocyte (TRAP) kit no. 386 (Sigma), according to the
manufacturer’s instructions.
Cytotoxicity assay
Mature OCs were plated in 96-well plates and
incubated for 3 days both in the presence of essential oils (5, 50
and 500 μg/ml) and the purified compounds, citral, carvone and
limonene (Sigma), at 5, 50 and 500 μg/ml. A 3% methanol/dimethyl
sulfoxide (DMSO) solution was used as a negative control and to
solubilize the compounds. The determination of cell viability was
carried out following MTT (thiazolyl blue) colorimetric assay. The
assay, based on the conversion of the yellow tetrazolium salt, MTT
(Sigma), to purple formazan crystals by metabolically active cells,
provides a quantitative determination of viable cells. Following 72
h of treatment (experiments performed in triplicate), 25 μl of MTT
were added to each well, and the plates were incubated for 2 h at
37°C. The medium was removed, and the MTT crystals were solubilized
with 50% dimethylformamide; the spectrophotometric absorbance of
each sample was then measured at 570 nm using a Sunrise™ Absorbance
Reader (Tecan Group Ltd., Männedorf, Switzerland).
Apoptosis (TUNEL assay)
At the end of treatment, the cells were rinsed twice
with phosphate-buffered saline (PBS) and fixed for 25 min in 4%
paraformaldehyde at room temperature. Apoptotic cells were detected
by the DeadEnd Colorimetric Apoptosis Detection System (Promega,
Madison, WI, USA) according to the manufacturer’s instructions.
Moreover, all cells were subjected to hematoxylin staining, showing
blue stained nuclei. The cells were mounted in glycerol/PBS 9:1 and
observed under a Leica microscope (Leica Microsystems GmbH,
Wetzlar, Germany). The measurement of apoptosis was calculated as a
percentage of apoptotic nuclei (dark brown nuclei) vs. total nuclei
of multinucleated TRAP-positive cells, evaluated from 3
experiments.
Immunocytochemistry
Immunocytochemical analysis was performed using an
ImmPRESS Universal Reagent kit (Vector Laboratories, Inc.,
Burlingame, CA, USA). The OCs were seeded in 4-well chamber slides,
fixed in cold 100% methanol and permeabilized with 0.2% (vol/vol)
Triton X-100 (Sigma), in Tris-buffered saline (TBS). The cells were
incubated in 0.3% H2O2, and the endogenous
peroxidase was blocked with ready-to-use (2.5%) normal horse serum
blocking solution (ImmPRESS reagent kit; Vector Laboratories,
Inc.). Following incubation with the primary antibodies (1:500
dilution), specific for the Fas receptor (C-20, rabbit anti-human)
and matrix metalloproteinase-9 (MMP-9; H-129, rabbit anti-human)
(Santa Cruz Biotechnology, Inc., Dallas, TX, USA) the cells were
incubated for 16 h at 4°C. After rinsing in TBS, the cells were
incubated for 30 min at room temperature with ImmPRESS reagent
(ImmPRESS reagent kit; Vector Laboratories, Inc.) and then
incubated with substrate/chromogen mix (ImmPACT™ DAB; Vector
Laboratories, Inc.). After washing, the cells were mounted in
glycerol/PBS 9:1 and observed under a Leica microscope (Leica
Microsystems GmbH).
Statistical analysis
The sample test for equal proportions without
continuity correction data was carried out, using the program R
version 2.10.1 (Copyright Foundation for Statistical Computing ISBN
3-900051-07-0). A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
Chromatographic profile of essential
oils
The chromatographic profile in Fig. 1 shows that the essential oils
derived from L. alba, citral, carvone and limonene are the
most concentrated components (Table
I). The essential oil extracted from the leaves of L.
alba contained 18.2% of citral (mixture of neral and geranial),
15.7% of limonene and 33.7% of carvone, whereas the oil extracted
from the flowers of L. alba contained 19.2% of citral, 16.7%
of limonene and 36.8% of carvone. The chemotype III of L.
alba was studied, according to the study of Hennebelle et
al (27).
 | Table IChemical composition of Lippia
alba leaves and flowers. |
Table I
Chemical composition of Lippia
alba leaves and flowers.
| Compound |
KIlit |
KIcalc | Leaves (%) | Flowers (%) |
|---|
| 1 | α-thujene | 930 | 931 | 0.2 | 0.6 |
| 2 | 1-Octen-3-ol | 979 | 977 | 0.4 | 0.7 |
| 3 |
6-Methyl-5-hepten-2-one | 985 | 985 | 1.8 | 1.0 |
| 4 | Myrcene | 990 | 992 | 8.3 | 7.8 |
| 5 | Limonene | 1029 | 1029 | 15.7 | 16.7 |
| 6 | (Z)-β-Ocimene | 1037 | 1040 | 0.2 | 0.2 |
| 7 | (E)-β-Ocimene | 1050 | 1051 | 0.5 | 0.2 |
| 8 | Linalool | 1096 | 1101 | 1.1 | 1.0 |
| 9 | Nerol | 1229 | 1231 | 2.2 | 0.6 |
| 10 | Neral | 1238 | 1246 | 11.7 | 11.6 |
| 11 | Carvone | 1243 | 1250 | 33.7 | 36.8 |
| 12 | Geraniol | 1252 | 1256 | 2.9 | 2.5 |
| 13 | Geranial | 1267 | 1272 | 6.5 | 7.6 |
| 14 |
E-Caryophyllene | 1419 | 1427 | 2.9 | 1.6 |
| 15 | Germacrene D | 1485 | 1488 | 3.4 | 2.7 |
| Total
identified | | | 91.4 | 91.6 |
Antimicrobial activity
Table II shows
the antimicrobial activity of the compounds against the periodontal
pathogens examined. The lowest MIC values were observed for P.
gingivalis in the essential oil extracted from L. alba
leaves (MIC 0.00625 mg/ml), when compared with the activity of
A. actinomycetemcomitans (MIC >3.2 mg/ml), F.
nucleatum (MIC 0.8 mg/ml) and B. fragilis (MIC 0.4
mg/ml). As regards the essential oil extracted from the flowers,
the lowest MIC was again observed for P. gingivalis (MIC
0.0125 mg/ml), when compared to the activity of A.
actinomycetemcomitans (MIC >3.2 mg/ml), B. fragilis
(MIC 1.6 mg/ml) and F. nucleatum (MIC 0.8 mg/ml).
 | Table IIMinimum inhibitory concentration
(MIC) and minimum bactericidal concentration (MBC) given in mg/ml
of the essential oil and methanolic extract of Lippia alba
against strict anaerobic microorganisms. |
Table II
Minimum inhibitory concentration
(MIC) and minimum bactericidal concentration (MBC) given in mg/ml
of the essential oil and methanolic extract of Lippia alba
against strict anaerobic microorganisms.
| Microorganisms |
|---|
|
|
|---|
| B.
fragilis | A.aa | P.
gingivalis | F.
nucleatum |
|---|
|
|
|
|
|
|---|
| Compounds | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
|---|
| Essential oil |
| Lippia alba
(leaves) | 0.4 | 0.4 | >3.2 | >3.2 | 0.00625 | 0.00625 | 0.8 | 1.6 |
| Lippia alba
(flowers) | 1.6 | 1.6 | >3.2 | >3.2 | 0.0125 | 0.0125 | 0.8 | 0.8 |
| Control |
| Clorexidin | 0.0075 | 0.0075 | 0.0075 | 0.0075 | 0.001875 | 0.001875 | 0.00375 | 0.00375 |
| Tween-80 10% | NO | NO | NO | NO | NO | NO | NO | NO |
| DMSO 50% | NO | NO | NO | NO | NO | NO | NO | NO |
Cytotoxicity
OCs were obtained from PB and cultured in complete
DMEM plus M-CSF and RANKL for 14 days. OC differentiation was
examined by TRAP staining (TRAP-positive cells) and by measuring
MMP-9 expression (data not shown). In order to determine whether
essential oils from L. alba retained any cytotoxic activity,
the OCs were treated with increasing amounts (0.5–500 μg/ml) of
essential oils (from the leaves and flowers) for up to 72 h, and
the viability of the cells was examined by MTT assay. The viability
of the OCs was 68% (±2.4) and 39% (±1.5) in those treated with 5
μg/ml of essential oil from L. alba flowers and leaves,
respectively. Furthermore, the cytotoxicity increased to 75% when
the OCs were incubated with the highest concentration (500 μg/ml).
Thus, we considered these essential oils as toxic to OCs.
Furthermore, it was confirmed that there was a cytotoxic effect on
PBMCs (data not shown). We also verified the effects of the L.
alba chemotype III major components, citral, carvone and
limonene in the same experimental conditions. Unlike the whole
extract, the isolated compounds did not cause any cytotoxic effects
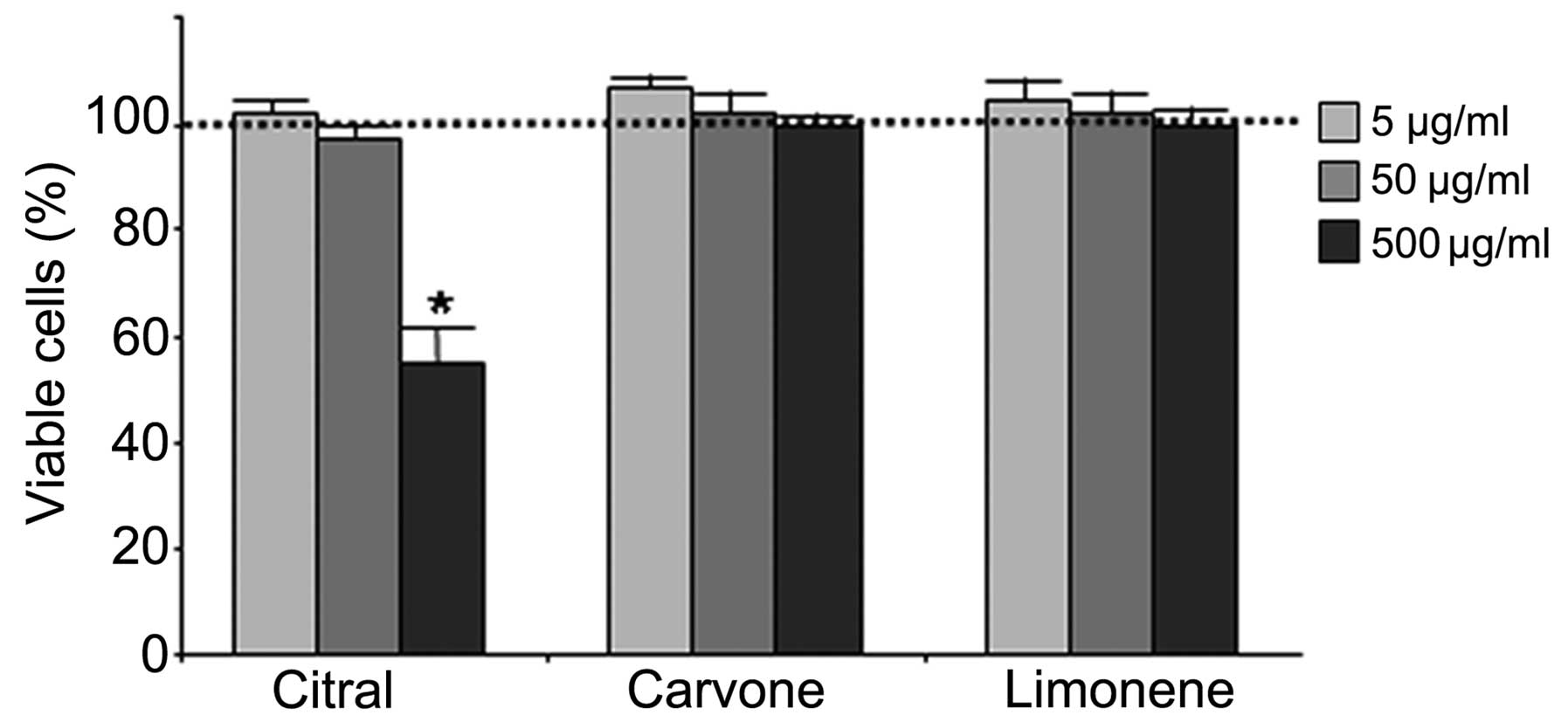
on the total cell population (Fig.
2). Only citral at 500 μg/ml was found to induce a significant
decrease in cell viability. Based on this evidence, further
experiments were performed with all 3 compounds from L. alba
at a maximum concentration of 50 μg/ml.
L. alba chemotype III components induce
apoptosis in OCs
TUNEL assay was performed on mature OCs after 72 h
of exposure to 5 and 50 μg/ml of the abovementioned compounds; the
results are shown in Fig. 3.
Citral and limonene induced a high level of apoptosis (almost 100%
of apoptotic nuclei) even at a low concentration (5 μg/ml). In the
presence of carvone, a significant increase in the number of
TUNEL-positive nuclei was observed, mainly at the highest
concentrations. These results were confirmed by immunocytochemistry
for FAS receptor, a well known apoptosis-related protein (28) whose expression increased (Fig. 3) in the OCs treated with the 3
components at all concentrations. OCs treated only with the vehicle
(methanol/DMSO) were used as control cells.
Discussion
Natural phytochemicals isolated form plants used in
traditional medicine are considered to be a good alternative to
synthetic chemicals (29–31). The exploration of botanicals used
in traditional medicine may lead to the development of novel
preventive or therapeutic strategies for oral health. The aim of
this study was to determine whether essential oils from L.
alba are useful as potential therapy against periodontal
disease. This plant is considered to be the second most commonly
used medicinal plant in the state of Bahia, Brazil, and it is
widely used in folk medicine in Brazil as for its anti-inflammatory
effects.
In this study, we demonstrated that the essential
oils extracted from the leaves and flowers of L. alba retain
an antimicrobial activity against the major Gram-negative
periodontal pathogens, such as P. gingivalis, B.
fragilis and F. nucleatum. Moreover, they showed no
activity against the A. actinomycetemcomitans (ATCC 43717)
strain, possibly as it has rapid growth in vitro. We
observed that this strain was the most resistant, with an MIC
>3.2 mg/ml.
As has already been reported (32), the use of an antiseptic mouthwash
containing essential oils, associated with scaling and root
planing, can significantly inhibit the levels of F.
nucleatum and P. gingivalis in supra- and subgingival
biofilms. As a consequence, antiseptic mouthwash containing
compounds from medicinal plants can complement this conventional
periodontal therapy.
Since the antimicrobial activity of medicinal plants
is due to the presence of potential bioactive compounds, in the
case of L. alba, the considerable effect against periodontal
pathogens may be explained by the presence of limonene, carvone and
citral. According to previous studies, each one of these compounds
retains the ability to inhibit Gram-negative bacteria proliferation
and has a proven anti-inflammatory effect in different cellular
models (33,34).
In order to verify the possible effects of essential
oils from L. albia on OC activity, the use of purified
compounds is mandatory, in consideration of the toxic effects of
essential oils of L. alba on OCs (>75%) and PBMCs (data
not shown). The lack of cytotoxicity of purified compounds prompted
us to examine their effects on the induction of OC apoptosis and
include in our experimental plan, the study of the potential
activity of these compounds on bone turnover, particularly on OC
behaviour. Moreover, it has been demonstrated that periodontal
inflammation not only stimulates osteoclastogenesis but also
interferes with the uncoupling of bone formation and resorption,
consistent with a pathological process (35). In this study, we verified that
different concentrations of limonene, carvone and citral did not
cause any cytotoxic effect on the total cell population and induced
programmed cell death in human primary OCs. As reported in our
study, a high percentage of TUNEL-positive OCs was observed in all
experimental conditions. When evaluating the effects of these
compounds on OCs, the apoptotic activity was confirmed by the
increasing levels of Fas receptor. Hence, we conclude that citral,
carvone and limonene are strong inducers of the apoptotic pathway
in primary human OCs.
In particular, the interest in citral is also
sustained by the patent application WO200419922A1 (36), relating to methods of freshening
breath and oral cleansing, together with obtaining bactericidal
effects. Interestingly, citral can be delivered as dentifrice,
chewing gum, confection, lozenge, mouthwash, mouth spray or edible
film containing an effective amount of the compound (37).
Therefore, our study supports the concept that
citral has great biotechnological potential for use in the
treatment of periodontal disease, as it is non-toxic, as shown by
MTT assay, has antibacterial activity against P. gingivalis
(38) and is able to induce
apoptosis in 100% of OCs even at low concentrations (5 μg/ml).
These results are in agreement with those of Chaouki et al
(39), who indicated that citral
is able to induce cell cycle arrest in the G2/M phase with the
subsequent induction of apoptosis. Citral is a β-substituted vinyl
aldehyde present in the leaves and fruits of many plants. It is
widely used in the food and cosmetics industries due to its flavor
and aroma, and it is classified as a safe chemical agent (40). In conclusion, with respect to
periodontal diseases, citral is a double-acting compound exhibiting
antibacterial activity and a strong induction of OC apoptosis.
In addition to citral, limonene and carvone were
also shown to be promising alternatives in the treatment of
periodontal disease. These chemicals are volatile monoterpenes that
contribute to flavors and aromas in food and pharmaceutical
preparations. In folk medicine, as well as in phytotherapy, they
are still used as therapeutic agents, for example as a carminative,
laxative or as a digestion aid and particularly against infections
of the respiratory tract. Limonene has been widely used clinically
for the relief of heartburn and has well-established
chemopreventive activity against many types of cancer (41). Carvone has also been associated
with chemopreventive activity, since it has been found to induce
the detoxifying enzyme glutathione S-transferase in several mouse
target tissues (42).
In conclusion, the use of natural products in the
treatment of diseases may lead to novel therapeutic strategies with
lower costs. The compounds that showed activity in this study may
be used for formulating a mouthwash, or may even be impregnated in
biodegradable chips for insertion into the gingival sulcus of
individuals affected by periodontal disease, following scaling and
root planing.
This study demonstrated that L. alba, widely
used in folk medicine in Brazil, has a potential biotechnological
application in drug formulation. In relation to dentistry,
epidemiological studies estimate that 90% of the population has
some form of periodontal disease (43,44). Thus, research on periodontitis
treatment, whether at the microbiological, genetic or immune level,
should always be considered relevant in the search for curative
and/or complementary therapeutic methods.
Acknowledgements
We thank Coordenação de Aperfeiçoamento de Pessoal
de Nível Superior (CAPES), Brazil, for the support and assistance
during this study.
References
|
1
|
Shoji M, Sato K, Yukitake H, Kondo Y, et
al: Por secretion system-dependent secretion and glycosylation of
Porphyromonas gingivalis hemin-binding protein 35. PLoS One.
6:e213722011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu T, Trevisan M, Genco RJ, Dorn JP,
Faikner KL and Sempos CT: Periodontal disease and risk of
cerebrovascular disease: the first national health and nutrition
examination survey and its follow-up study. Arch Intern Med.
160:2749–2755. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bansal M, Khatri M and Taneja V: Potential
role of periodontal infection in respiratory diseases - a review. J
Med Life. 6:244–248. 2013.PubMed/NCBI
|
|
4
|
Jacob PS and Nath S: Periodontitis among
poor rural Indian mothers increases the risk of low birth weight
babies: a hospital-based case control study. J Periodontal Implant
Sci. 44:85–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Darveau RP, Tanner A and Page RC: The
microbial challenge in periodontitis. Periodontol 2000. 14:12–32.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ishikawa I: Host responses in periodontal
diseases: a preview. Periodontol 2000. 43:9–13. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hasegawa S, Yonezawa T, Ahn JY, et al:
Honokiol inhibits osteoclast differentiation and function in vitro.
Biol Pharm Bull. 33:487–492. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Di Benedetto A, Gigante DI, Colucci S and
Grano M: Periodontal disease: linking the primary inflammation to
bone loss. Clin Dev Immunol. 2013:1–7. 2013. View Article : Google Scholar
|
|
9
|
Roberts MC: Antibiotic toxicity,
interactions and resistence development. Periodontol 2000.
28:280–297. 2002. View Article : Google Scholar
|
|
10
|
Ardila CM, Lòpez M and Guzmán IC: High
resistance against clindamycin, metronidazole and amoxicillin in
Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans
isolates of periodontal disease. Med Oral Patol Oral Cir Bucal.
15:e947–e951. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Japoni A, Vasin A, Noushadi S, Kiany F,
Japoni S and Alborzi A: Antibacterial susceptibility patterns of
Porphyromonas gingivalis isolated from chronic periodontitis
patients. Med Oral Patol Cir Bucal. 16:e1031–e1035. 2011.
View Article : Google Scholar
|
|
12
|
Iauk L, Lo Bue AM, Milazzo I, Rapisarda A
and Blandino G: Antibacterial activity of medicinal plant extracts
against periodontopathic bacteria. Phytother Res. 17:599–604. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee JH, Lee JS, Chung MS and Kim KH: In
vitro anti-adhesive activity of an acidic polysaccharide from Panax
ginseng on Porphyromonas gingivalis binding to erythrocytes. Planta
Med. 70:566–568. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lorenzi H and Matos FJA: Plantas
Medicinais no Brasil: Nativas e Exóticas. 2nd edition. Instituto
Plantarum; Nova Odessa, São Paulo: 2008
|
|
15
|
Rodrigues ACC and Guedes MLS: Utilização
de plantas medicinais no Povoado Sapucaia, Cruz das Almas-Bahia.
Rev Bras Pl Med. 8:1–7. 2006.(In Portuguese).
|
|
16
|
Pinto EPP, Amorozo MCM and Furlan A:
Conhecimento popular sobre plantas medicinais em comunidades rurais
de mata atlantica - Itacaré, BA, Brasil. Acta Bot Bras. 20:751–762.
2006.(In Portuguese). View Article : Google Scholar
|
|
17
|
Singh G, Rao GP, Kapoor PS and Singh OP:
Chemical constituents and antifungal activity of Lippia alba Mill.
leaf essential oil. JMAPS. 22:701–703. 2000.
|
|
18
|
Day MD and McAndrew TD: The biology and
host range of Falconia intermedia (Hemiptera: Miridae), a potential
biological control agent for Lantana camara (Verbenaceae) in
Australia. Biocontrol Sci Techn. 13:13–22. 2003. View Article : Google Scholar
|
|
19
|
Blanco MA, Colareda GA, van Baren C,
Bandoni AL, Ringuelet J and Consolini AE: Antispasmodic effects and
composition of the essential oils from two South American
chemotypes of Lippia alba. J Ethnopharmacol. 149:803–809. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Novack DV: Role of NF-κB in the skeleton.
Cell Res. 21:169–182. 2011. View Article : Google Scholar
|
|
21
|
Wittrant Y, Theoleyre S, Couillaud S,
Dunstan C, Heymann D and Rédini F: Relevance of an in vitro
osteoclastogenesis system to study receptor activator of NF-κB
ligand and osteoprotegerin biological activities. Exp Cell Res.
293:292–301. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mori SA, Mattos Silva LA, Lisboa G and
Coradin L: Manual de Manejo do Herbário Fanerogâmico. 2nd edition.
Centro de Pesquisas do Cacau; Ilhéus, Bahia, Brazil: 1989
|
|
23
|
Cronquist A: An integrated system of
classification of flowering plasts. The Evolution and
Classification of Flowering Plants. Columbia University Press; New
York: 1981
|
|
24
|
Adams RP: Identification of Essential Oil
Components by Gas Chromatography/Mass Spectroscopy. 4th edition.
Allured Pub Corp; Carol Stream, IL: 2007
|
|
25
|
CLSI - Clinical and Laboratory Standards
Institute. Methods for antimicrobial susceptibility testing of
anaerobic bacteria. Approved standard. 7th edition. M11-A5. CLSI -
Clinical and Laboratory Standards Institute; Wayne, PA, USA:
2007
|
|
26
|
Matsuzaki K, Katayama K, Takahashi Y, et
al: Human osteoclast-like cells are formed from peripheral blood
mononuclear cells in a coculture with SaOS-2 cells transfected with
the parathyroid hormone (PTH)/PTH-related protein receptor gene.
Endocrinology. 140:925–932. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hennebelle T, Sahpaz S, Joseph H and
Bailleul F: Ethnopharmacology of Lippia alba. J Ethnopharmacol.
116:211–222. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kovacic N, Grcevic D, Katavic V, Lukic IK
and Marusic A: Targeting Fas in osteoresorptive disorders. Expert
Opin Ther Targets. 14:1121–1134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fibach E, Prus E, Bianchi N, Zuccato C, et
al: Resveratrol: Antioxidant activity and induction of fetal
hemoglobin in erythroid cells from normal donors and β-thalassemia
patients. Int J Mol Med. 29:974–982. 2012.PubMed/NCBI
|
|
30
|
Ruimi N, Rwashdeh H, Wasser S, et al:
Daedalea gibbosa substances inhibit LPS-induced expression of iNOS
by suppression of NF-κB and MAPK activities in RAW 264.7 macrophage
cells. Int J Mol Med. 25:421–432. 2010.PubMed/NCBI
|
|
31
|
Lau FY, Chui CH, Gambari R, et al:
Antiproliferative and apoptosis-inducing activity of Brucea
javanica extract on human carcinoma cells. Int J Mol Med.
16:1157–1162. 2005.PubMed/NCBI
|
|
32
|
He JY, Qi GG, Huang WJ, et al: Short-term
microbiological effects of scaling and root planing and
essential-oils mouthwash in Chinese adults. J Zhejiang Univ Sci B.
14:416–425. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mizrahi B, Shapira L, Domb AJ and
Houri-Haddad Y: Citrus oil and MgCl2 as antibacterial
and anti-inflammatory agents. J Periodontol. 77:963–968. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Behan JM, Bradshaw DJ, Richards J and
Munroe MJ: Flavour compositions Process PI US patent 20060153959A1.
Filed July 13, 2006.
|
|
35
|
Belibasakis GN and Bostanci N: The
RANKL-OPG system in clinical periodontology. J Clin Periodontol.
39:239–248. 2012. View Article : Google Scholar
|
|
36
|
Broderick K, Greenberg M, Maxwell J and
Stawski B: Breath freshening and oral cleansing product using
citral US Patent 20040253190 A1. Filed August 26, 2003; issued
March 11, 2004.
|
|
37
|
Manafi A, Hashemlou A, Momeni P and
Moghimi HR: Enhancing drugs absorption through third-degree burn
wound eschar. Burns. 34:698–702. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Goyal R and Ananad MK: Antibacterial
effect of lemongrass oil on oral microoganisms: an in vitro study.
JPSI. 2:41–43. 2013.
|
|
39
|
Chaouki W, Leger DY, Liagre B, Beneytout
JL and Hmamouchi M: Citral inhibits cell proliferation and induces
apoptosis and cell cycle arrest in MCF-7 cells. Fundam Clin
Pharmacol. 23:549–556. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ress NB, Hailey JR, Maronpot RR, et al:
Toxicology and carcinogenesis studies of microencapsulated citral
in rats and mice. Toxicol Sci. 71:198–206. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sun J: D-Limonene: safety and clinical
applications. Altern Med Rev. 12:259–264. 2007.PubMed/NCBI
|
|
42
|
Zheng GQ, Kenney PM and Lam LK:
Anethofuran, carvone, and limonene: potential cancer
chemopreventive agents from dill weed oil and caraway oil. Planta
Med. 58:338–341. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Morris AJ, Steele J and White DA: The oral
cleanliness and periodontal health of UK adults in 1998. Br Dent J.
191:186–192. 2001.PubMed/NCBI
|
|
44
|
Borrell LN, Burt BA, Gillespie BW, Lynch J
and Neighbors H: Periodontitis in the United States: beyond black
and white. J Public Health Dent. 62:92–101. 2002. View Article : Google Scholar : PubMed/NCBI
|