Introduction
Oxidative stress, a normal phenomenon in the body,
is caused by an imbalance between the production of reactive oxygen
species (ROS) and the protective action of the antioxidant system
that is responsible for their neutralization and removal. Under
normal conditions, the physiologically important intracellular
levels of ROS are maintained at low levels by various enzyme
systems participating in redox homeostasis (1,2).
However, disturbances in the normal redox state of cells and/or a
concomitant decline in the antioxidant scavenging capacity can
cause toxic effects through the production of peroxides and free
radicals that damage all the components of the cell, including
proteins, lipids and nucleic acids. Therefore, oxidative stress can
cause disruptions in the normal mechanisms involved in cellular
signaling pathways. Additionally, the elevated production of ROS
increases oxidative stress and causes a pathological response
leading to cellular dysfunction, and eventually, to apoptotic cell
death (3–5).
As ROS formation occurs naturally, mammalian cells
have developed several adaptive mechanisms to limit ROS formation
or to detoxify ROS. These mechanisms employ antioxidant enzymes or
antioxidant compounds. The nuclear factor erythroid-2-related
factor 2 (Nrf2), a basic leucine zipper-transcription factor, is a
master cellular sensor for oxidative stress and represents the
primary response to changes in the cellular redox state (6–8).
Nrf2 acts as the regulator of antioxidant-related genes such as
heme oxygenase 1 (HO-1) and nicotinamide adenine dinucleotide
phosphate: quinone oxidoreductase 1 (NQO1) through binding to the
antioxidant response element (ARE), a cis-acting enhancer
present in the promoter region of a large and distinct set of
target genes, which aims to restore redox homeostasis. However,
under pathological conditions and periods of chronic inflammation,
Nrf2 activity and expression of its cytoprotective target genes can
be significantly decreased, often contributing to the progression
of disease (6,9). Therefore, pharmacological activation
of Nrf2 has been shown to be critical for the protection of cells
under conditions of oxidative stress.
Numerous traditional medicines have been considered
as potential therapeutic candidates for the management of
intracellular oxidative balance due to their decreased
cytotoxicities and potent pharmacological features. Among them,
Schisandra chinensis Baill. fruits (Schisandrae fructus) as
one of the most well-known herbal medicines has been extensively
used in Asia, including Korea, China, Japan and Russia (10,11). Schisandrae fructus is often used
to increase physical working capacity and it produces a
stress-protective effect. Previous studies suggest that the major
bioactive constituents of Schisandrae fructus are the essential oil
(12–14) and lignans belonging to the
dibenzocy-clooctadiene type (15,16), and this has been intensively
studied from the pharmacological and phytochemical perspectives.
Previously, an essential oil purified from Schisandrae fructus,
whose main chemical components are monoterpenes, sesquiterpenes and
aromatic compounds (17), has
been shown to have various pharmacological potentials, such as
antioxidant, antimicrobial, and antiseptic effects (13,18–20). However, the seed sections of S.
chinensis (Schisandrae semen) have been disregarded and unused.
Additionally, to the best of our knowledge, no studies have
documented the protective action of Schisandrae semen essential oil
(SSeo), an essential oil purified from Schisandrae fructus, against
oxidative stress. Therefore, the aim of the present study was to
examine the ability of SSeo to protect cells from hydrogen peroxide
(H2O2)-induced cell damage and to elucidate
the mechanism underlying these protective effects using a C2C12
murine skeletal-muscle cell line.
Materials and methods
Preparation of SSeo
S. chinensis Baillon seeds were collected
around Mungyeong City (Gyeongbuk, Korea) and were completely dried
at 180°C in a furnace (Daihan Scientific Co., Seoul, Korea). The
dried seeds were pulverized and lyophilized in a programmable
freeze dryer (Freezone 1, Labconco Co., Kansas City, MO, USA).
Lyophilized materials were extracted with 100% ethanol at room
temperature for 24 h, filtered, and were subsequently concentrated
using a rotary vacuum evaporator (Buchi Rotavapor R-144, BÜCHI
Labortechnik, Flawil, Switzerland). Finally, the SSeo was isolated
by hydrodistillation using a Clevenger-type apparatus for 3 h
according to the method in a previous study (21). The oil was stored in a
refrigerator at 4°C to protect it from light and degeneration. The
yield of the oil based on the dried weight of the Schizandrae semen
was 0.66%.
Cell culture and treatment
The C2C12 myoblast cell line was obtained from the
American Type Culture Collection (Manassa, VA, USA) and was
maintained in Dulbecco’s modified Eagle’s medium (Gibco-BRL,
Gaithersburg, MD, USA) supplemented with 10% heat-inactivated fetal
bovine serum (Gibco-BRL), 100 U/ml penicillin G, 100 μg/ml
streptomycin and 0.25 μg/ml amphotericin fungizone at 37°C
in a humid atmosphere of 5% CO2 in air. SSeo was
dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis,
MO, USA) as a stock solution at a 100 mg/ml concentration, and the
stock solution was diluted with medium to the desired concentration
prior to use.
Cell viability assay and morphological
imaging
C2C12 cells were seeded in 6-well plates at a
density of 1×105 cells per well. After a 24-h
incubation, the cells were treated with various concentrations of
the SSeo in the absence or presence of H2O2
and/or zinc protoporphyrin IX (ZnPP; Sigma-Aldrich) for the
indicated times. The medium was removed, and the cells were
incubated with 0.5 mg/ml of
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma-Aldrich) solution for 2 h. The supernatant was discarded and
the formazan blue, which was formed in the cells, was dissolved in
DMSO. The optical density was measured at 540 nm with a microplate
reader (Dynatech Laboratories, Chantilly, VA, USA) and growth
inhibition was assessed as the percentage viability in which the
vehicle-treated cells were considered as 100% viable. Morphological
changes were monitored by obtaining photomicrographs under an
inverted phase contrast microscope (Carl Zeiss, Oberkochen,
Germany).
Flow cytometric detection of
apoptosis
The cells were trypsinized and washed with
phosphate-buffered saline (PBS) and resuspended in 100 μl of
binding buffer containing annexin-V-fluorescein isothiocyanate and
propidium iodide (PI) (BD Sciences, Heidelberg, Germany) for 15 min
at room temperature in the dark, as according to the manufacturer’s
instructions. The cells were immediately analyzed by flow cytometry
(FACS Calibur, Becton Dickinson, San Jose, CA, USA). The
percentages of apoptotic cells (annexin-V+ cells) are
presented as the mean ± standard deviation (SD), as described
previously (22).
Morphological observation of nuclear
change
The cells were washed with PBS and fixed with 3.7%
paraformaldehyde in PBS for 10 min at room temperature. The fixed
cells were washed with PBS and were stained with
4,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich) solution for 10
min at room temperature. The cells were analyzed using a
fluorescence microscope (Carl Zeiss).
Measurement of intracellular ROS
The measurement of ROS was performed using the ROS
sensitive 2′,7′,-dichlorofluores-cein-diacetate (H2DCFDA, Molecular
Probes, Eugene, OR, USA). Briefly, the cells were incubated with 10
μM H2DCFDA for 30 min at room temperature in the dark.
Spectrofluorimetry analysis using a microplate reader was performed
to quantitate the intracellular ROS using excitation and emission
wavelengths at 488 and 525 nm, respectively.
Comet assay (single-cell gel
electrophoresis)
The cell suspension was mixed with 0.5% low melting
agarose (LMA) at 37°C, and the mixture was spread on a
fully-frosted microscopic slide precoated with 1% normal melting
agarose. After the solidification of the agarose, the slide was
covered with 0.5% LMA and was subsequently immersed in a lysis
solution [2.5 M NaCl, 100 mM Na-EDTA, 10 mM Tris, 1% Triton X-100,
and 10% DMSO (pH 10)] for 1 h at 4°C. The slides were placed in a
gel electrophoresis apparatus containing 300 mM NaOH and 10 mM
Na-EDTA (pH 13) for 40 min to allow for DNA unwinding and the
expression of alkali-labile damage, and subsequently an electrical
field was applied (300 mA, 25 V) for 20 min at 4°C to draw the
negatively-charged DNA toward the anode. After electrophoresis, the
slides were washed three times for 5 min at 4°C in a neutralizing
buffer [0.4 M Tris, (pH 7.5)], followed by staining with 20
μg/ml PI (Sigma-Aldrich). The slides were examined under a
fluorescence microscope (Carl Zeiss).
Protein extraction and western blot
analysis
The cells were collected by trypsin-EDTA and lysed
with lysis buffer [20 mM sucrose, 1 mM EDTA, 20 μM Tris-Cl
(pH 7.2), 1 mM dithiothreitol, 10 mM KCl, 1.5 mM MgCl2,
5 μg/ml pepstatin A, 10 μg/ml leupeptin and 2
μg/ml aprotinin] containing protease inhibitors for 30 min
at 4°C. The mixtures were centrifuged (10,000 × g) for 10 min at
4°C and the supernatants were collected as whole-cell extracts. The
protein content was determined using the Bio-Rad protein assay
reagent (Bio-Rad, Hercules, CA, USA) and bovine serum albumin as a
standard, as according to the manufacturer’s instructions, to
determine the protein concentrations. Following normalization, an
equal amount of protein was subjected to electrophoresis on sodium
dodecyl sulfate-polyacrylamide gels and was subsequently
transferred to nitrocellulose membranes (Schleicher & Schuell,
Keene, NH, USA) by electroblotting. The blots were blocked with 5%
skimmed milk for 1 h at room temperature. The blots were incubated
overnight with primary antibodies, followed by horseradish
peroxidase-conjugated donkey anti-rabbit and sheep anti-mouse
immunoglobulin for 1 h. The immunoreactive bands were revealed by
enhanced chemiluminescence (ECL) with a commercially available ECL
kit (Amersham, Arlington Heights, IL, USA). Anti-Nrf2 (SC-13032),
anti-NQO1 (SC-16464), anti-TrxR1 (SC-28321) and anti-actin
(SC-1616) antibidoes were purchased from Santa Cruz Biotechnology,
Inc., (Santa Cruz, CA, USA). Antibodies against HO-1 (CS #5061S)
and p-γH2A.X (CS #9718S) were obtained from Cell Signaling
Technology (Danvers, MA, USA).
RNA isolation and reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated using the TRIzol reagent
according to the manufacturer’s instructions, and 2 μg of
RNA was used for cDNA synthesis using M-MLV reverse transcriptase
(Promega, Madison, WI, USA). RT-generated cDNA encoding the
HO-1 gene was amplified by PCR using a specific primer
(forward, 5′-CGA CAG CAT GTC CCA GGA TT-3′ and reverse, 5′-CTG GGT
TCT GCT TGT TTC GC-3′). The amplified cDNA products were separated
by 1% agarose gel electrophoresis and stained with ethidium bromide
(Sigma-Aldrich). In a parallel experiment,
glyceral-dehyde-3-phosphate dehydrogenase (forward, 5′-CGG AGT CAA
CGG ATT TGG TCG TAT-3′ and reverse, 5′-AGC CTT CTC CAT GGT GAA
GAC-3′) was used as an internal control.
Statistical analysis
Data are presented as the means ± SD. Statistical
significance was determined using an analysis of variance followed
by a Student’s t-test. A value of P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of SSeo on the cell viability in
C2C12 cells
The effect of increasing concentrations of SSeo was
first evaluated on the viability of 2C12 cells. As shown in
Fig. 1, treatment of the cell
cultures with 10–80 μg/ml of SSeo for 24 h had no effect on
cell viability. However, 100 μg/ml induced a significant
reduction in the number of cells after 24 h, suggesting that SSeo
may be toxic to the cells at this concentration. Therefore, 80
μg/ml SSeo was chosen as the optimal dose for studying the
cytoprotective effect of SSeo against
H2O2-induced cell damage.
Effects of SSeo on
H2O2-induced C2C12 cell growth
inhibition
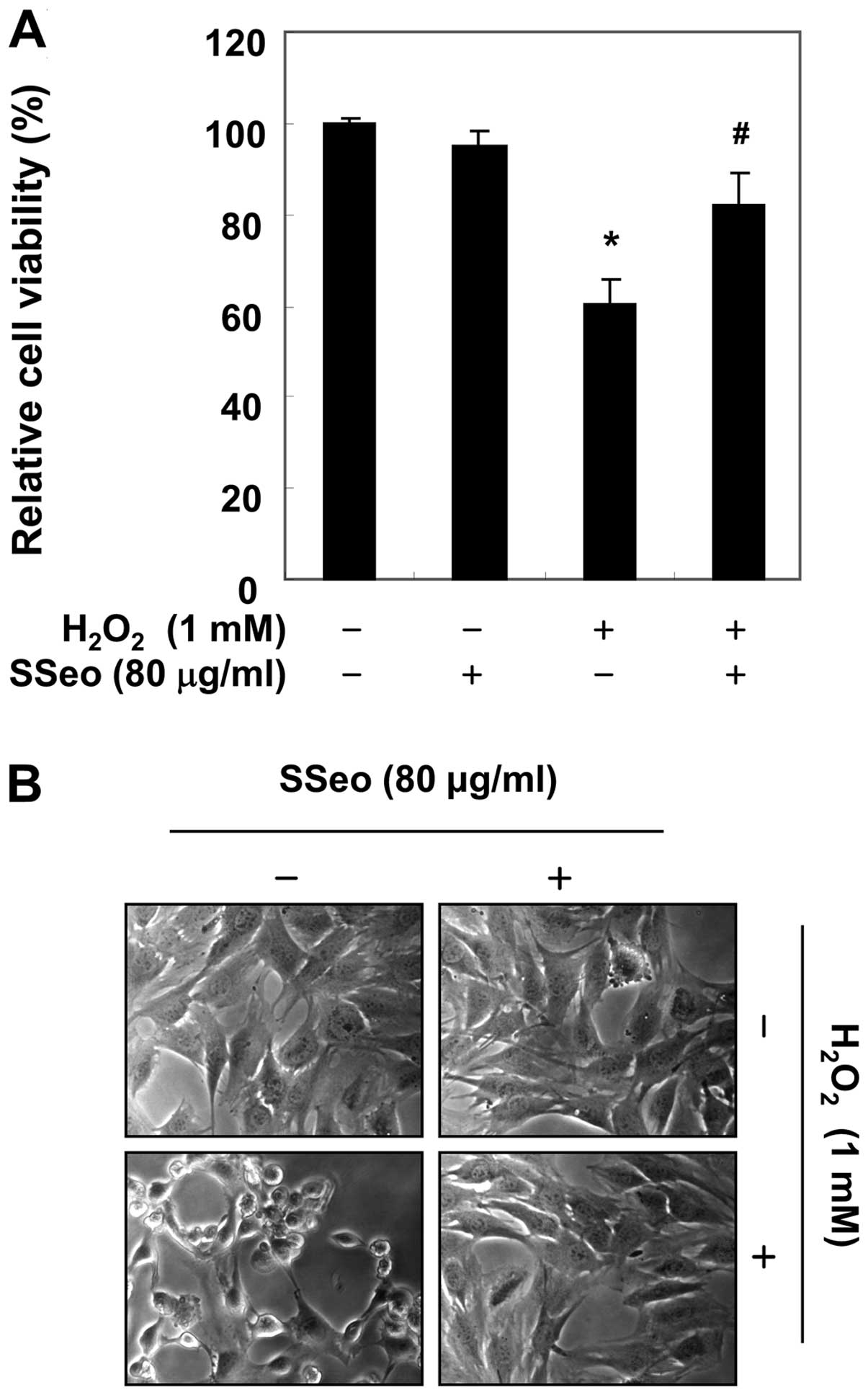
Subsequently, the potential protective effect of
SSeo was evaluated against oxidative stress-induced cytotoxicity
(Fig. 2A). The cell viability was
significantly reduced to 60.2% in 1 mM
H2O2-treated cells in the absence of SSeo;
however, this was increased to 82.2% in
H2O2-treated cells that were pretreated with
80 μg/ml SSeo. In addition, H2O2
stimulation significantly induced morphological changes, including
extensive cytosolic vacuolization and the presence of irregular
cell-membrane buds, which were effectively attenuated by SSeo
pretreatment (Fig. 2B).
Inhibition of
H2O2-induced apoptosis and ROS generation by
SSeo in C2C12 cells
To evaluate the potential effect of SSeo on
H2O2-apoptosis, the proportion of apoptotic
cells was further determined by staining the cells with annexin V
and PI. Flow cytometry analysis showed that the proportion of
apoptotic cells increased significantly from 3.1% in the untreated
control to 29.7% in the cells treated with 1 mM
H2O2; however, the enhanced ratio of
apoptosis was significantly alleviated by pre-incubation with SSeo
(Fig. 3A). Furthermore, DAPI
staining revealed nuclei with chromatin condensation and the
formation of apoptotic bodies, characteristic morphological changes
of apoptosis, in cells cultured with 1 mM
H2O2. By contrast, extremely few apoptotic
cells were observed in the control culture, and pretreatment of the
cells with SSeo significantly abrogated these apoptotic
characteristics (Fig. 3B). The
scavenging effect of SSeo against
H2O2-induced ROS was examined using the
H2DCFDA assay. These results indicated that the ROS levels were
increased in H2O2-treated cells compared to
non-treated cells, whereas SSeo decreased the ROS generation in
H2O2-treated cells (Fig. 3C). As a positive control, an ROS
scavenger of 10 mM N-acetyl-L-cysteine also markedly attenuated
H2O2-induced ROS generation, indicating that
SSeo scavenged H2O2-induced ROS.
 | Figure 3Protection of
H2O2-induced apoptosis and ROS generation by
SSeo in C2C12 cells. (A) Cells were pretreated with 80 μg/ml
SSeo for 1 h and were subsequently incubated with and without 1 mM
H2O2 for 6 h. To quantify the degree of
apoptosis, cells were stained with annexin-V-FITC and PI for flow
cytometry analysis. (B) The cells grown under the same conditions
as (A) were sampled, fixed and stained with DAPI solution. The
stained nuclei were observed under a fluorescent microscope
(original magnification, ×400). (C) Cells were pretreated with 80
μg/ml SSeo or 10 mM NAC for 1 h and were subsequently
stimulated with and without 1 mM H2O2 for 6
h. The fluorescence intensity of H2DCFDA taken up by the treated
cells was measured by spectrofluorimetry. Data are presented as
mean ± standard deviation of three independent experiments
(*P<0.05 compared to control group;
#P<0.05 compared to
H2O2-treated group). ROS, reactive oxygen
species; SSeo, Schisandrae semen essential oil; FITC, fluorescein
isothiocyanate; PI, propidium iodide; DAPI,
4,6-diamidino-2-phenylindole; NAC, N-acetyl-L-cysteine; H2DCFDA,
2′,7′,-dichlorofluorescein-diacetate. |
Reduction of
H2O2-mediated DNA damage by SSeo in C2C12
cells
As oxidative stress-induced damage to DNA produces
lesions that are responsible for the loss of cell viability,
H2O2-mediated damage to C2C12 cell DNA was
detected using the alkaline comet assay and western blot analysis.
As assessed using the comet assay, a longer comet tail moment (DNA
migration) occurred with an increase in
H2O2-treated cells (Fig. 4A), and the untreated control cells
only showed typical representative nuclei. In addition, the levels
of phosphorylation of nuclear histone H2A.X (Ser139) (p-γH2A.X), a
sensitive marker for DNA double-strand break (DSB) formation
(23), increased in the
H2O2-treated cells, as shown by western
blotting (Fig. 4B). However,
pretreatment with SSeo resulted in a significant decrease in comet
tails and in the expression of p-γH2A.X. The results suggest that
SSeo has a protective property against DNA damage induced by
H2O2 treatment.
Induction of HO-1 expression by SSeo in
C2C12 cells
As HO-1 is an important component of the cellular
defense against oxidative stress (7,24),
whether non-cytotoxic concentrations of SSeo induced HO-1 protein
expression was accessed in association with its antioxidant
activity. Treatment of C2C12 cells with SSeo induced a
concentration- and time-dependent enhancement in HO-1 protein
expression (Fig. 5A and B).
Additionally, the increased HO-1 expression correlated with
HO-1 mRNA levels in a concentration- and time-dependent
manner in SSeo-treated cells (Fig. 5C
and D), suggesting that the increased HO-1 expression at the
transcriptional level contributed to the enhanced expression of
HO-1 protein.
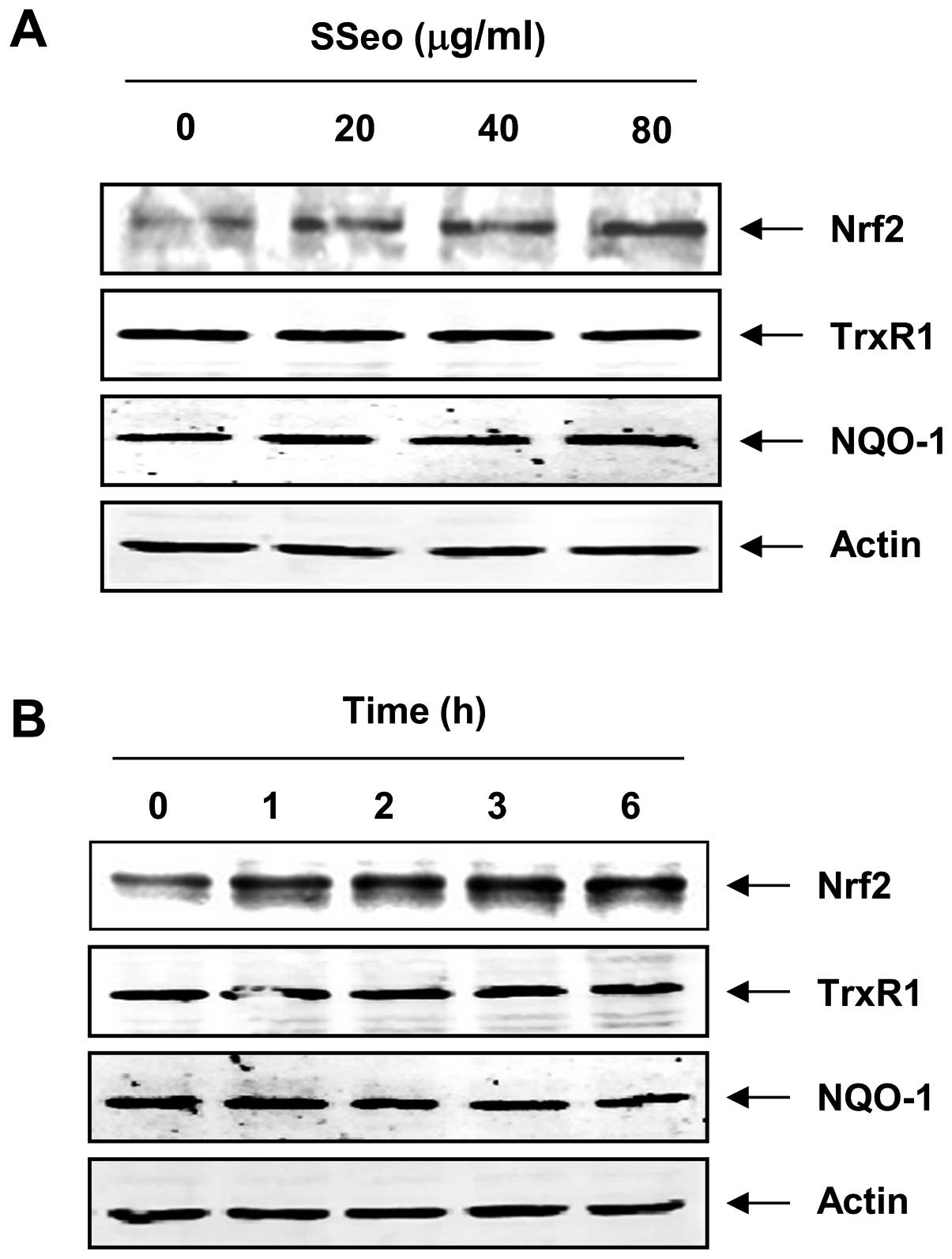
Effect of SSeo on the levels of Nrf2 in
C2C12 cells
As several studies have reported that Nrf2 is an
important transcription factor for the regulation of a number of
antioxidants and phase II detoxifying enzymes, including HO-1
(6,8,9),
whether SSeo was able to induce the expression of Nrf2 in C2C12
cells was further examined. Following exposure to SSeo, the C2C12
cells showed a gradual increase in Nrf2 levels in a concentration-
and time-dependent manner, which was strongly correlated with the
increase in HO-1 expression (Fig.
6). However, the levels of other Nrf2 target genes, such as
antioxidant enzyme thioredoxin reductase-1 (TrxR1) and
NQO1, remained unchanged in SSeo-treated C2C12 cells.
Involvement of HO-1 in C2C12 cell damage
induced by H2O2 treatment
To identify whether the HO-1 protein is involved in
the protective effect of SSeo on oxidative stress induced in
H2O2-treated C2C12 cells, a selective
inhibitor of HO-1, ZnPP, was applied in the present study. As shown
in Fig. 7A, ZnPP significantly
reversed the inhibition of ROS generation by SSeo in
H2O2-stimulated C2C12 cells. In addition, the
results of the MTT assay showed that the protective effect of SSeo
on H2O2-induced cytotoxicity was blocked by
the addition of ZnPP (Fig. 7B),
suggesting that the cytoprotective effect of SSeo is partly
mediated through HO-1 induction.
Discussion
Under normal conditions, the cells of aerobic
organisms contain safe levels of ROS, which are counterbalanced by
biochemical antioxidants. However, excess ROS generation and/or
antioxidant depletion under pathological conditions lead to
oxidative stress, along with direct or indirect ROS-mediated damage
to nucleic acids, proteins, and lipids. Although the cell and
tissue defense systems against ROS consist of various antioxidant
enzymes and antioxidants, these defense systems are overpowered in
the face of high levels of ROS. Accumulating evidence indicates
that ROS-provoked oxidative stress can kill cells via necrosis
and/or apoptosis through a variety of overlapping signaling
pathways and cascades (1,6,9,25).
Apoptosis, or a programmed cell death, is a complex process
characterized by cell shrinkage, chromatin condensation,
internucleosomal DNA fragmentation and the formation of apoptotic
bodies. Furthermore, the addition of exogenous
H2O2 is sufficient to trigger the apoptotic
process, with different concentrations required depending on the
cell type under investigation (26). Thus, the inhibition of oxidative
stress is theoretically an expeditious method for the management of
disorders associated with ROS-induced cell injury and apoptosis
(6,8,9).
For this reason, investigators are searching for natural
antioxidants that have safe and effective pharmacological activity
with low cytotoxicity for the prevention of oxidative
stress-mediated cellular damage. In the present study, as a part of
the screening program for therapeutic anti-oxidative agents from
traditional medicine resources, whether SSeo, an essential oil
purified from Schisandrae fructus, has protective effects against
oxidative stress-induced cytotoxicity was investigated.
Consequently, H2O2 was selected as an
oxidative inducer of ROS in a C2C12 myoblast model due to its high
diffusion capacity and rapid membrane permeability. Furthermore,
the mechanisms by which H2O2 induces
alterations in cellular components and regulates cell fate are well
understood (25,27), which makes this ROS particularly
of interest for study. The present study showed that C2C12 cells
exposed to H2O2 exhibited significantly
decreased cell viability and increased apoptosis; however, SSeo
markedly increased cell viability by inhibiting
H2O2-induced apoptosis and reduced ROS
generation generated by H2O2 treatment in
C2C12 cells (Figs. 2 and 3). In addition,
H2O2 treatment increased the expression of
p-γH2A.X, a sensitive marker for DNA DSBs (23), and increased the tail length of
DNA in the comet assay, whereas each event was mitigated in C2C12
cells by treatment with SSeo prior to H2O2
exposure (Fig. 4). The results
suggest that SSeo prevented ROS-mediated DNA damage and apoptosis
via the attenuation of oxidative stress.
Among the various antioxidant enzymes, the
protective functions of HO-1, an inducible isoform of the first and
rate-limiting enzyme of heme degradation, against oxidative stress
have recently been demonstrated (7,24).
Transcriptional regulation of the HO-1 gene is linked to the
transcription factor Nrf2, which plays a crucial role in cellular
defense (6,8,9).
Nrf2 is ubiquitously expressed at low levels in the cytoplasm under
normal physiological conditions and is constantly bound to the
repressor protein Kelch-like ECH-associated protein-1 (Keap1), a
special molecular ‘sensor’ for changes in intracellular
homeostasis. In response to oxidative stress, Nrf2 is released from
Keap1 and transmits the stress signal to the nucleus for the
activation of a distinct set of genes encoding phase II detoxifying
enzymes, as well as several stress-responsive proteins, including
HO-1 (6,7,24,28). Therefore, the present study
further determined the potential role of the Nrf2/HO-1 pathway in
H2O2-induced C2C12 cell damage and
SSeo-mediated cytoprotection. Evidence for the induction of HO-1 by
SSeo has been demonstrated and it was shown that SSeo-induced HO-1
protein expression occurred in a concentration- and time-dependent
manner, with a concomitant increase in Nrf2 expression, but not
TrxR1 or NQO-1 expression (Fig.
6). Therefore, exogenous induction of HO-1 by SSeo was
further confirmed and was useful in
H2O2-induced oxidative damage of C2C12 cells.
The data indicated that the inhibition of HO-1 function
through using an HO-1 inhibitor, ZnPP, effectively reduced the
protective effect of SSeo against
H2O2-induced ROS generation, as well as
cytoprotection (Fig. 7). The
present results clearly demonstrate that HO-1 induction by
SSeo is responsible for protecting C2C12 cells against
H2O2-induced oxidative stress, and also
suggest that SSeo-induced cytoprotection of C2C12 cells against
oxidative stress is critically dependent on the activation of the
Nrf2/HO-1 pathway.
In conclusion, the present study identified SSeo as
an antioxidant with the ability to scavenge intracellular ROS in
C2C12 myoblasts. In addition, SSeo prevented cell damage resulting
from H2O2 exposure and increased C2C12 cell
survival by boosting HO-1 induction for ROS detoxification.
Although further investigation to elucidate the detailed mechanism
by which SSeo mitigates apoptosis associated with the Nrf2/HO-1
signaling pathway is required, these data suggest that SSeo may
find utility as a therapeutic agent for the management of
ROS-linked clinical conditions and disorders.
Acknowledgments
The present study was supported by the R&D
program of MOTIE/KIAT (grant no. 10040391; Development of
Functional Food Materials and Device for Prevention of
Aging-associated Muscle Function Decrease). We thank the Aging
Tissue Bank for providing research materials.
References
|
1
|
Zhang Y, Du Y, Le W, Wang K, Kieffer N and
Zhang J: Redox control of the survival of healthy and diseased
cells. Antioxid Redox Signal. 15:2867–2908. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Piantadosi CA: Carbon monoxide, reactive
oxygen signaling, and oxidative stress. Free Radic Biol Med.
45:562–569. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ray PD, Huang BW and Tsuji Y: Reactive
oxygen species (ROS) homeostasis and redox regulation in cellular
signaling. Cell Signal. 24:981–990. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ott M, Gogvadze V, Orrenius S and
Zhivotovsky B: Mitochondria, oxidative stress and cell death.
Apoptosis. 12:913–922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Valko M, Leibfritz D, Moncol J, Cronin MT,
Mazur M and Telser J: Free radicals and antioxidants in normal
physiological functions and human disease. Int J Biochem Cell Biol.
39:44–84. 2007. View Article : Google Scholar
|
|
6
|
Chapple SJ, Siow RC and Mann GE: Crosstalk
between Nrf2 and the proteasome: therapeutic potential of Nrf2
inducers in vascular disease and aging. Int J Biochem Cell Biol.
44:1315–1320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alam J and Cook JL: Transcriptional
regulation of the heme oxygenase-1 gene via the stress response
element pathway. Curr Pharm Des. 9:2499–2511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jeong WS, Jun M and Kong AN: Nrf2: a
potential molecular target for cancer che-moprevention by natural
compounds. Antioxid Redox Signal. 8:99–106. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen XL and Kunsch C: Induction of
cytoprotective genes through Nrf2/antioxidant response element
pathway: a new therapeutic approach for the treatment of
inflammatory diseases. Curr Pharm Des. 10:879–891. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Panossian A and Wikman G: Pharmacology of
Schisandra chinensis Bail.: an overview of Russian research and
uses in medicine. J Ethnopharmacol. 118:183–212. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu Y and Chen DF: Analysis of Schisandra
chinensis and Schisandra sphenanthera. J Chromatogr A.
1216:1980–1990. 2009. View Article : Google Scholar
|
|
12
|
Ma CH, Liu TT, Yang L, Zu YG, Chen X,
Zhang L, Zhang Y and Zhao C: Ionic liquid-based microwave-assisted
extraction of essential oil and biphenyl cyclooctene lignans from
Schisandra chinensis Baill fruits. J Chromatogr A. 1218:8573–8580.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma CH, Yang L, Zu YG and Liu TT:
Optimization of conditions of solvent-free microwave extraction and
study on antioxidant capacity of essential oil from Schisandra
chinensis (Turcz) Baill. Food Chem. 134:2532–2539. 2012. View Article : Google Scholar
|
|
14
|
Ikeya Y, Taguchi H, Yosioka I and
Kobayashi H: The constituents of Schizandra chinensis Baill. I.
Isolation and structure determination of five new lignans, gomisin
A, B, C, F and G, and the absolute structure of schizandrin. Chem
Pharm Bull (Tokyo). 27:1383–1394. 1979. View Article : Google Scholar
|
|
15
|
Lee YW, Voyksner RD, Pack TW, Cook CE,
Fang QC and Ito Y: Application of countercurrent
chromatography/thermospray mass spectrometry for the identification
of bioactive lignans from plant natural products. Anal Chem.
62:244–248. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Opletal L, Sovová H and Bártlová M:
Dibenzo[a,c]cyclooctadiene lignans of the genus Schisandra:
importance, isolation and determination. J Chromatogr B Analyt
Technol Biomed Life Sci. 812:357–371. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang L, Chen Y, Song Y, Chen Y and Liu X:
GC-MS of volatile components of Schisandra chinensis obtained by
supercritical fluid and conventional extraction. J Sep Sci.
31:3238–3245. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu CJ, Zhang SQ, Zhang JS, Liang Q and Li
DS: Chemical composition and antioxidant activity of essential oil
from berries of Schisandra chinensis (Turcz) Baill. Nat Prod Res.
26:2199–2203. 2012. View Article : Google Scholar
|
|
19
|
Chen X, Zhang Y, Zu Y and Yang L: Chemical
composition and antioxidant activity of the essential oil of
Schisandra chinensis fruits. Nat Prod Res. 26:842–849. 2012.
View Article : Google Scholar
|
|
20
|
Song L, Ding JY, Tang C and Yin CH:
Compositions and biological activities of essential oils of Kadsura
longepedunculata and Schisandra sphenanthera. Am J Chin Med.
35:353–364. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Minaiyan M, Ghannadi AR, Afsharipour M and
Mahzouni P: Effects of extract and essential oil of Rosmarinus
officinalis L. on TNBS-induced colitis in rats. Res Pharm Sci.
6:13–21. 2011.PubMed/NCBI
|
|
22
|
Lee SJ, Hwang SO, Noh EJ, Kim DU, Nam M,
Kim JH, Nam JH and Hoe KL: Transactivation of bad by
vorinostat-induced acetylated p53 enhances doxorubicin-induced
cytotoxicity in cervical cancer cells. Exp Mol Med. 46:e762014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xie H, Wise SS, Holmes AL, Xu B, Wakeman
TP, Pelsue SC, Singh NP and Wise JP Sr: Carcinogenic lead chromate
induces DNA double-strand breaks in human lung cells. Mutat Res.
586:160–172. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee DS, Li B, Kim KS, Jeong GS, Kim EC and
Kim YC: Butein protects human dental pulp cells from hydrogen
peroxide-induced oxidative toxicity via Nrf2 pathway-dependent heme
oxygenase-1 expressions. Toxicol In Vitro. 27:874–881. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hampton MB and Orrenius S: Redox
regulation of apoptotic cell death. Biofactors. 8:1–5. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chandra J, Samali A and Orrenius S:
Triggering and modulation of apoptosis by oxidative stress. Free
Radic Biol Med. 29:323–333. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nguyen TT, Park HJ, Kim JY, Kim HE, Lee H,
Yoon J and Lee C: Microbial inactivation by cupric ion in
combination with H2O2: role of reactive
oxidants. Environ Sci Technol. 47:13661–13667. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee EK, Kim JA, Park SJ, Kim JK, Heo K,
Yang KM and Son TG: Low-dose radiation activates Nrf1/2 through
reactive species and the Ca2+/ERK1/2 signaling pathway
in human skin fibroblast cells. BMB Rep. 46:258–263. 2013.
View Article : Google Scholar : PubMed/NCBI
|