Introduction
The liver sinusoid is regarded as a unique capillary
differing from others (1), and
the liver sinusoidal endothelial cells (LSECs) have a typical
phenotype, well integrated into the special needs of the liver.
LSECs can form a fenestrated barrier between blood and hepatocytes,
which permits greater oxygenation for hepatocytes and promotes the
more efficient clearance of drugs, perhaps also of chylomicron
remnants (2,3). Additionally, the association between
LSECs and hepatocytes may be critical for the recovery of
hepatocytes from toxic injury (4,5).
Generally, the ability of normal cells to
proliferate in vitro is tightly controlled. Cells have a
finite lifespan, experiencing replicative senescence and eventual
death after a certain number of cell divisions (6–8).
However, increasing evidence indicates that some types of rodent
cells, such as 3T3 fibroblasts, mouse epidermal cells and rat
epithelial cells are capable of spontaneous immortalization in
vitro (9–12). These immortalized cells have
emerged from replicative senescence, have lost contact inhibition
and have piled up on top of each other to form foci (13). It is believed that genetic
instability plays a crucial role in spontaneous immortalization,
including alterations in chromosomes and mutations in genes, such
as p53 (14–16). However, the molecular mechanisms
involved remain obscure.
In the present study, we successfully isolated,
purified and cultured LSECs. After a prolonged culture, these LSECs
gradually experienced senescence and post-senescence and eventually
became immortalized. We further performed a detailed
characteristics analysis for these immortalized LSECs. The results
indicated that although some distinctive phenotypes were
maintained, these immortalized LSECs obtained certain novel
biological characteristics which rendered them different from early
passage cells.
Materials and methods
Preparation of LSECs
The present study was approved by the Ethics
Committee of Central South University, Changsha, China. After
Kunming white mice (n=6; Central South University Animal Studies)
were sacrificed by cervical dislocation, the whole liver was
completely resected and repeatedly washed with phosphate-buffered
saline (PBS; Gibco, Carlsbad, CA, USA). In order to avoid any
potential contamination by large vessel and biliary endothelial
cells, identifiable vascular structures were excised from the liver
specimens. The remaining liver tissue was sectioned into
5-mm3 cubes, and then transferred to a dish containing
2.0 U/ml of dispase and 1X penicillin-phytomycin (Sigma, St. Louis,
MO, USA) and incubated at 4°C for 24 h. After terminating the
digestion with 10% fetal bovine serum (FBS; Gibco) in MCDB 131
medium (Sigma), the liver cubes were mechanically disaggregated in
MCDB 131 medium with a flat instrument to release the endothelial
cells. The cell suspension was transferred to a 15-ml conical tube
and centrifuged at 600 × g for 10 min. Following centrifugation,
the supernatant was discarded and the pellet was resuspended in
appropriate volumes of MCDB 131 medium. The cell suspension was
then pipetted onto a density gradient of 35% Percoll (Sigma) and
centrifuged at 12,000 × g, 4°C for 15 min. Following
centrifugation, the band which was located on the red cell band of
the gradient was transferred very carefully to a 15-ml conical tube
containing PBS. After mixing gently, the sample was centrifuged at
600 × g, 4°C for 10 min and the pellet was resuspended in MCDB 131
medium. Following centrifugation at 100 × g for 5 min, the pellet
was suspended in the liver endothelial cell culture medium and
plated on 6-well tissue culture dishes pre-coated with fibronectin
(Sigma). Non-adherent cells or debris were removed by washing steps
after 5 h of culture at 37°C in 5% CO2 in a humidified
incubator. The adherent cells were further washed with complete
endothelial cell selective medium and cultured in the same medium.
The endothelial cell selective medium contained 40% MCDB 131, 40%
endothelial cell growth medium (EGM)-2 (Lonza, Basel, Switzerland),
10% FBS and 10% endothelial cell conditioned medium (EC-CM, see
below). The medium was also supplemented with the following growth
factors: 1% L-glutamine (Gibco), 10 ng/ml vascular endothelial
growth factor (VEGF; Invitrogen, Carlsbad, CA, USA), 10 ng/ml basic
fibroblast growth factor (bFGF; Invitrogen) and 1 ng/ml
dexamethasone (Sigma).
Preparation of EC-CM
The preparation of the EC-CM was as follows: The
mouse bone marrow endothelial cell line (a gift from Professor Qiru
Wang, Central South University, China) was cultured in Iscove’s
modified Dulbecco’s medium (IMDM) with 10% FBS until 80% confluent.
The medium was replaced with 5 ml IMDM without serum in each 100-mm
plate to collect the conditioned medium. Following incubation for
24 h, the culture medium was collected. The collected conditioned
medium was centrifuged at 740 × g for 20 min. The supernatant was
then filtered with a 0.22-μm filter and stored at −20°C
until use. EC-CM was used within a week after being thawed.
Flow cytometry
All staining was performed according to the
manufacturer’s instructions, using 106 cells and the
recommended amount of antibodies, followed by incubation for 30 min
at room temperature. Briefly, the cells were incubated separately
with the primary antibody rat anti-mouse CD31 (ab56299, 1:50;
Abcam, Burlingame, CA, USA) for 30 min. After washing 3 times with
PBS, the cells were incubated with the secondary antibody goat
anti-rat Alexa Flour 488 (A-11006, 1:1,000; Invitrogen). For the
negative controls, the cells were incubated only with the secondary
antibody. The expression level of CD31 was determined using a
FACScan flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA).
Immunoperoxidase and immunofluorescence
staining
The LSECs were seeded in fibronectin-coated
coverslips. The cells were fixed in 4% paraformaldehyde for 15 min.
After washing 3 times with PBS, the cells were incubated for 1 h at
room temperature in PBS containing 5% BSA (Sigma) to block
non-specific binding. For determining the expression of von
Willebrand Factor (vWF), the cells were premeabilized by incubation
with 0.5% Triton X-100 (Sigma) in PBS for 10 min at room
temperature and then incubated with mouse anti-mouse vWF antibody
(555849, 1:200; BD Biosciences) followed by incubation with
avidin-biotin complex (EastCoast Bio, Birmingham, UK) for 60 min at
room temperature. The peroxidase reaction was developed with 0.3
mg/ml 3-3′-diaminobenzidine (EastCoast Bio) in buffer containing
0.05% hydrogen peroxide (EastCoast Bio) for 5–10 min at room
temperature. Images were obtained under a phase contrast microscope
(Nikon, Tokyo, Japan). For determining the expression of other CD
genes, the cells were incubated with primary antibodies [rat
anti-mouse CD31 (ab56299), rat anti-mouse CD105 (ab188488), mouse
anti-mouse CD90 (ab226), goat anti-mouse CK19 and goat anti-mouse
desmin (ab80503); Abcam] at a 1:50 concentration followed by
incubation with corresponding secondary antibodies of goat anti-rat
Alexa Flour 488 (A-11006, 1:1,000; Invitrogen), rat anti-mouse
Ig-FITC (RMG101, 1:250; Invitrogen) and bovine anti-goat Ig-FITC
(1:250; Invitrogen) for 30 min at room temperature. The nuclei were
counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Sigma),
and the slides were mounted with vector-shield, covered and sealed
with nail polish. Images were acquired under an inverted
fluorescent microscope (Nikon).
Uptake of DiI-acetylated-low density
lipoprotein (DiI-Ac- LDL)
An incorporation analysis of DiI-Ac-LDL was
performed. The cell monolayer was incubated with 10 μg/ml of
DiI-Ac-LDL (Biomedical Technologies Inc., Stoughton, MA, USA) in
medium for 4 h at 37°C, washed 3 times and examined for the uptake
of DiI-Ac-LDL under an inverted fluorescence microscope
(Nikon).
Capillary tube formation assay
The cells (2×104) suspended in 200
μl EGM-2 medium supplemented with 50 ng/ml VEGF and 1% FBS
were plated onto 50 μl of Matrigel (BD Biosciences) in a
96-well plate. Endothelial progenitor cells (EPCs) were used as a
positive control, which were derived from the cord blood as
previously described (40). The
degree of tube formation was observed and photographed every 2 h
under an inverted phase contrast microscope (Nikon).
Scanning electron microscopy
The cells were fixed in 2.5% glutaraldehyde in 0.1 M
cacodylate buffer (Sigma) pH 7.4 for 6 h. After rinsing with
ddH20, the specimens were post-fixed with 1%
OsO4 (Sigma) in ddH20 for 1 h, then rinsed
with ddH20 prior to dehydration in a series of graded
ethanol solutions and subsequently embedded in a mixture of
Epon-Araldite. Thin sections were cut using a diamond knife mounted
on an LKB Ultratome and stained with aqueous uranylacetate. The
specimens were examined under a JEOL 1200 EX electron microscope
(Jeol, Tokyo, Japan).
Senescence-associated β-galactosidase
(SA-β-gal) assay
Positive SA-β-gal staining has been reported to
reflect the replicative senescence of cells, but not in quiescent
or terminally differentiated cells, and the stained cells are
representative of the enzymatic activity (17). SA-β-galactosidase staining was
performed as previously described (16). Briefly, the cells were fixed with
0.5% glutaraldehyde (pH 7.2). After washing with PBS (pH 7.2)
supplemented with 1 mM MgCl2, the cells were stained in
X-gal solution [1 mg/ml X-gal, 0.12 mM K3Fe
(CN)6, 0.12 mM K4Fe (CN)6, 1 mM
MgCl2] in PBS at pH 6.0 overnight at 37°C. The senescent
(stained) and non-senescent (unstained) cells were viewed under a
microscope (Nikon).
Growth characteristics of immortalized
LSECs
The density of the immortalized LSEC suspension at
passages 20 and 21 was adjusted to 3×103 cells/ml and
plated on 24-well tissue culture dishes. Every other day, the cells
were digested and enumerated. Cell growth kinetic curves were
generated and the doubling-time was cacluated using the following
formula: T = 0.693 (T2 − T1)/ln (N2 − N1) where T2−T1 is the
difference in the value of time of twice successive detections; and
N2−N1 is the difference in the value of cell numbers of twice
successive detections.
Western blot analysis
Cell extracts were prepared using
radio-immunoprecipitation assay lysis buffer (Sigma) containing 1
mM phenylmethanesulfonyl fluoride (Sigma). Proteins (50 μg)
in the cell extracts were separated on a 10% SDS-PAGE gradient
(Invitrogen) and transferred onto PVDF membranes (Millipore,
Billerica, MA, USA) in accordance to the manufacturer’s
instructions (Invitrogen). The membranes, which had been blocked
with 5% skim milk, were incubated with mouse anti-p53 antibody
(sc-374087, 1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA)
and mouse anti-β-actin antibody (A1978, 1:3,000; Sigma), followed
by incubation with horseradish peroxidase-conjugated goat
anti-mouse IgG secondary antibody (ab175740, 1:10,000; Abcam).
After washing with PBS-T solution 4 times, the immunoreactive bands
were detected by electrochemiluminescence (Sigma) by exposing the
blots.
Cytogenetics analysis
The cells were harvested and G-banded chromosome
preparations were made using standard methods (18). The harvested cells were fixed in a
methanol:acetic acid (3:1) solution. The chromosomes were stained
with 4% Giemsa solution in Gurr buffer and the number and G-C band
of the chromosomes in metaphase from each cell line were
determined.
Soft agar assay
To measure cell anchorage independence, the cells
(1×104) were plated in 6-well soft agar dishes (0.5%
bottom and 0.33% top agar, respectively) for 3 weeks. HPG-2 cells
(human hepatocellular carcinoma cell line; Cell Bank of Central
South University, Changsha, China) were used as positive controls.
The ability for anchorage-independent growth shown by colony
formation was analyzed after 20 days. Colonies containing >10
viable cells were scored as positive.
Tumorigenesis in SCID mice
The immortalized LSECs were assayed for the ability
to form tumors in SCID mice. Ten million viable cells were injected
into the lower limb muscles of 6- to 8-week old SCID mice. Control
mice were injected with HPG-2 cells. The animals were observed at
regular intervals for tumor development for a period of 6 months.
The mouse muscle tissue sections were stained with hematoxylin and
eosin (H&E).
Results
Isolation, culture and phenotype
characteristics of LSECs
After the plating of the cells isolated from the
mouse livers on fibronectin-coated culture dishes, small adherent
clusters of cells were observed within 24 h. With the prolonged
culture, these clusters slowly became enlarged until they formed a
confluent monolayer. Replating of the cells after digestion
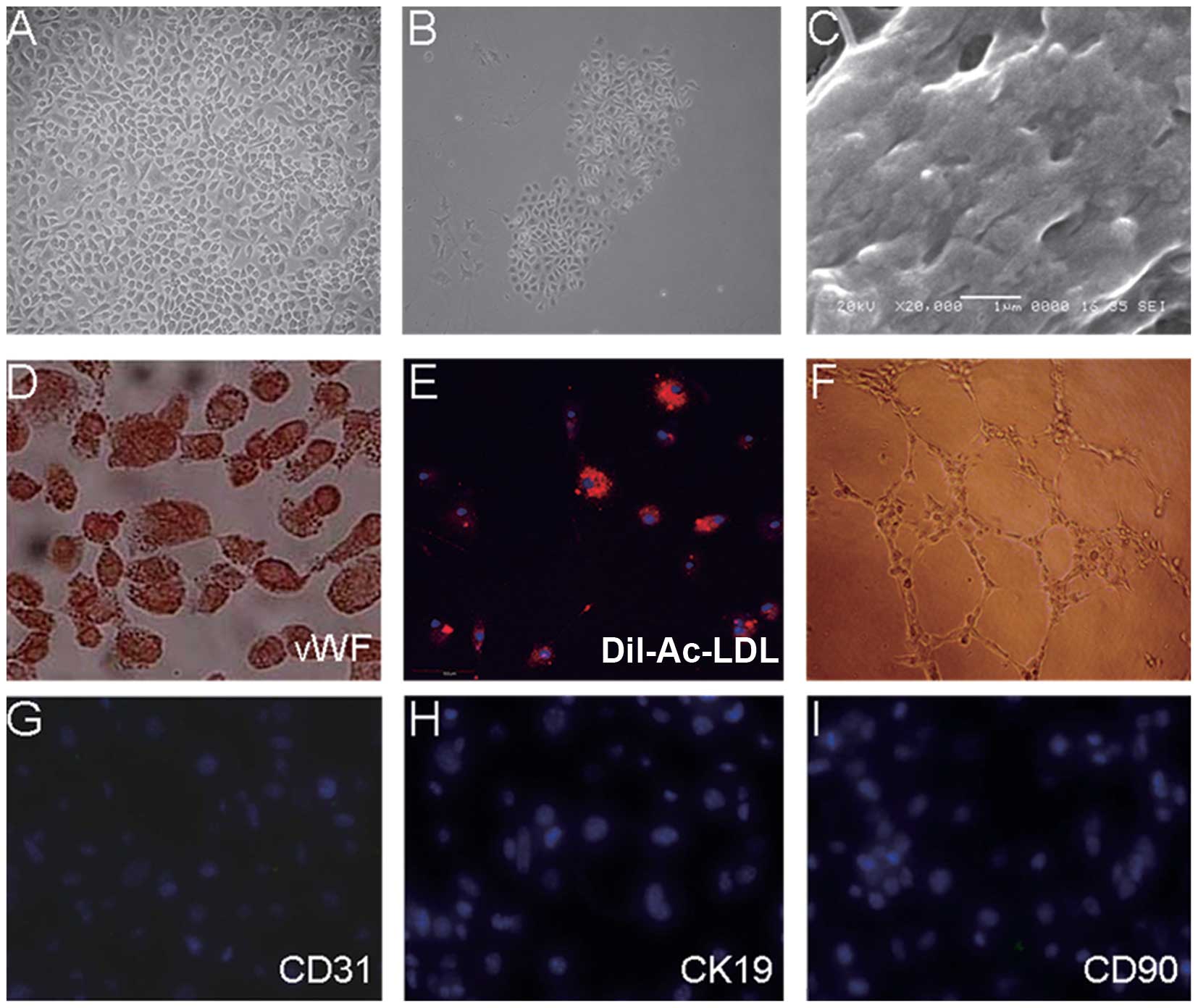
revealed clonal growth and a typical cobblestone morphology, a
characteristic of endothelial cells (Fig. 1A); the colonies were picked up,
pooled and expanded for several weeks, and these culture showed a
very homogenous morphology and strictly grew as a monolayer
(Fig. 1B). The contaminating
non-endothelial-like cells were successfully eliminated by the use
of endothelial-selective medium and contrast-digestion after the
second passage in serial culture.
At present, the only gold standard defining LSECs is
the presence of fenestration with a diameter of 100–150 nm. We
examined the monolayer of the cultured LSECs by transmission
electron microscopy. As shown in Fig.
1C, these cells had typical transcellular fenestrations.
The most commonly used characteristics to identify
endothelial cells include the expression of vWF and CD31, the
uptake of acetylated low-density lipoprotein (Ac-LDL) and the
formation of a capillary-like structure in vitro (19,20). The monolayers of the LSECs showed
positive staining for vWF expression (Fig. 1D) and the uptake of Dil-Ac-LDL
following 4 h of exposure to Dil-Ac-LDL (Fig. 1E). In addition, the LSECs formed
typical capillary-like structures in Matrigel after 14 h of culture
(Fig. 1F), and the expression of
CD31, a marker of vascular endothelial cells, was not detected
(Fig. 1G). To examine whether
these LSECs were contaminated by other liver cells, we further
examined the expression of CK19 (a marker of hepatocytes) and
CD105, CD90 and desmin (markers of mesenchymal fibroblast-like
cells which reside in perisinusoidal, portal and around the
centrilobular vein; desmin is also a classical stellate cell
maker). As shown in Fig. 1H and
I, the expression of CK19/CD90/CD105/desmin was not detected in
the monolayer of LSECs (CD105 and desmin; data not shown).
Spontaneous immortalization of LSECs
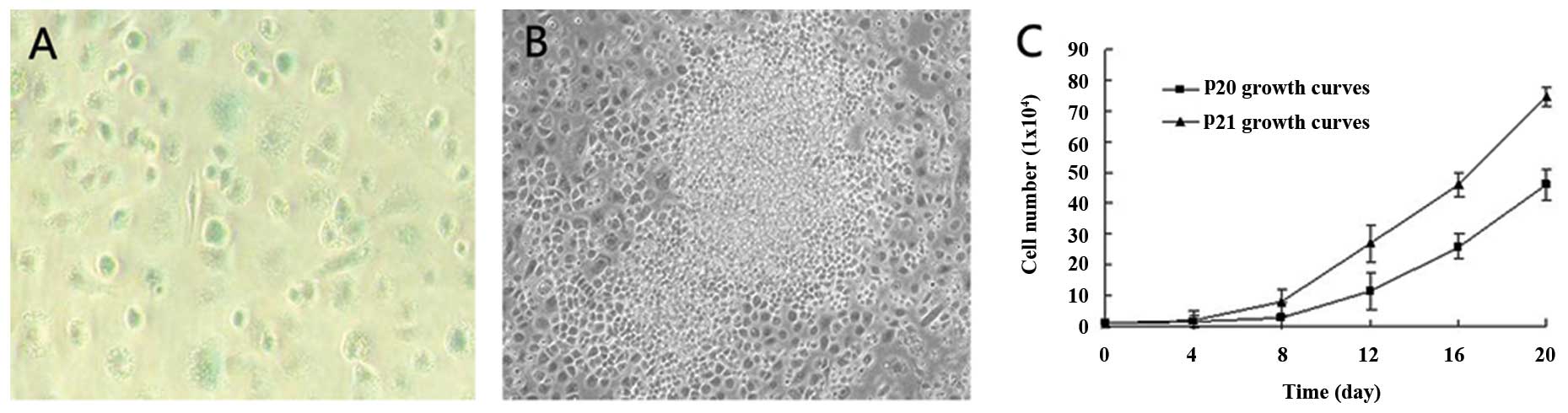
By passage 8, the majority of the LSECs appeared
senescent as judged by the presence of numerous LSECs which
exhibited a large and flattened morphology and SA-β-Gal activity
(Fig. 2A). Following extended
culture, a small population of round strong refractive
proliferating cells emerged from the senescent cultures, and these
cells showed clonal and multilayer growth (Fig. 2B), and their proliferation rate
increased with the increase in the number of passages. As shown in
Fig. 2C, at passages 20 and 21,
these cells continued vigorous growth. The doubling time of the
cells at passages 20 was 4.01 days, while the doubling time of the
cells at passage 21 was 3.50 days. Thus, the cells at passage 21
displayed a higher proliferation potential than those at passage
20. All these characteristics were consistent with those of
immortalized cells (13,14).
Biological characteristics of the
immortalized LSECs
To clarify whether the immortalized LSECs had
different properties compared to primary LSECs, we examined the
characteristics of the immortalized cells. As shown in Fig. 3A, the immortalized LSECs
maintained typical transcellular fenestrations, as well as the
expression of vWF and the uptake of DiI-Ac-LDL; these
characteristics were consistent with those of the primary cells.
However, as shown in Fig. 3B, the
immortalized LSECs lost the ability to form capillary-like
structures, but obtained the expression of CD31 and desmin
(Fig. 3C and D).
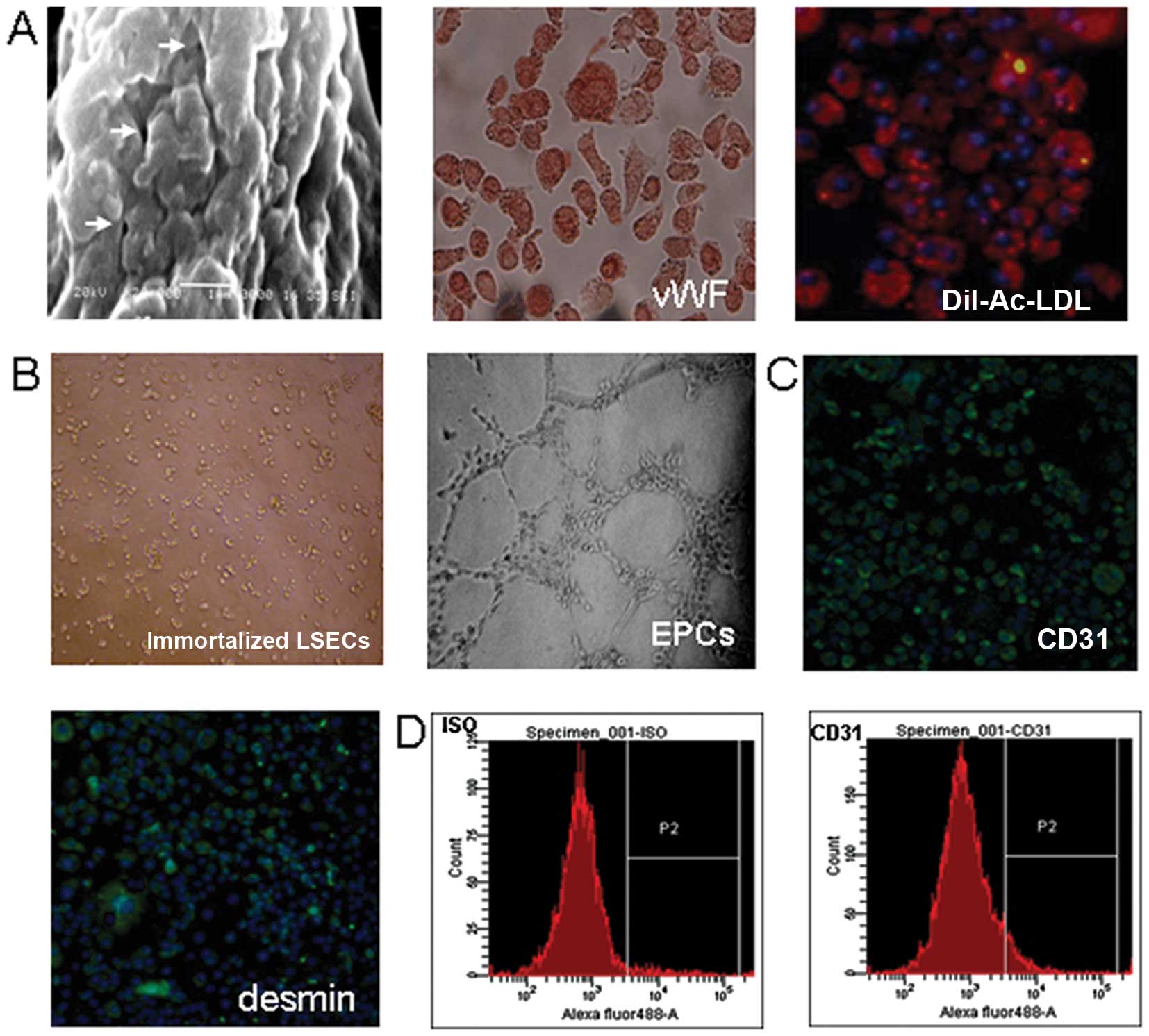 | Figure 3Biological characteristics of the
immortalized liver sinusoidal endothelial cells (LSECs). (A) These
immortalized LSECs maintained typical fenestration structures
(indicated by arrows) on the cell surface (scale bar, 1 μm),
the expression of von Willebrand factor (vWF, red; magnification,
x200) and the uptake of DiI-acetylated-low density lipoprotein
(DiI-Ac-LDL, red; magnification, x200). (B) The immortalized LSECs
did not show migration and sprouting after 14 h of incubation,
while the endothelial progenitor cells (EPCs) derived from cord
blood [as previously described (40)], as a positive control, formed
typical capillary-like structures after only 4-h incubation in
Matrigel. (C) The immortalized LSECs expressed CD31 and desmin
(antibody, green; DAPI, blue; magnification, x200). (D) FACS
quantitative analysis showed that the positive percentage of CD31
was 84.1% in the immortalized sinusoidal endothelial cells at
passage 15. |
Analysis of p53 protein, karyotype and
transformed phenotype
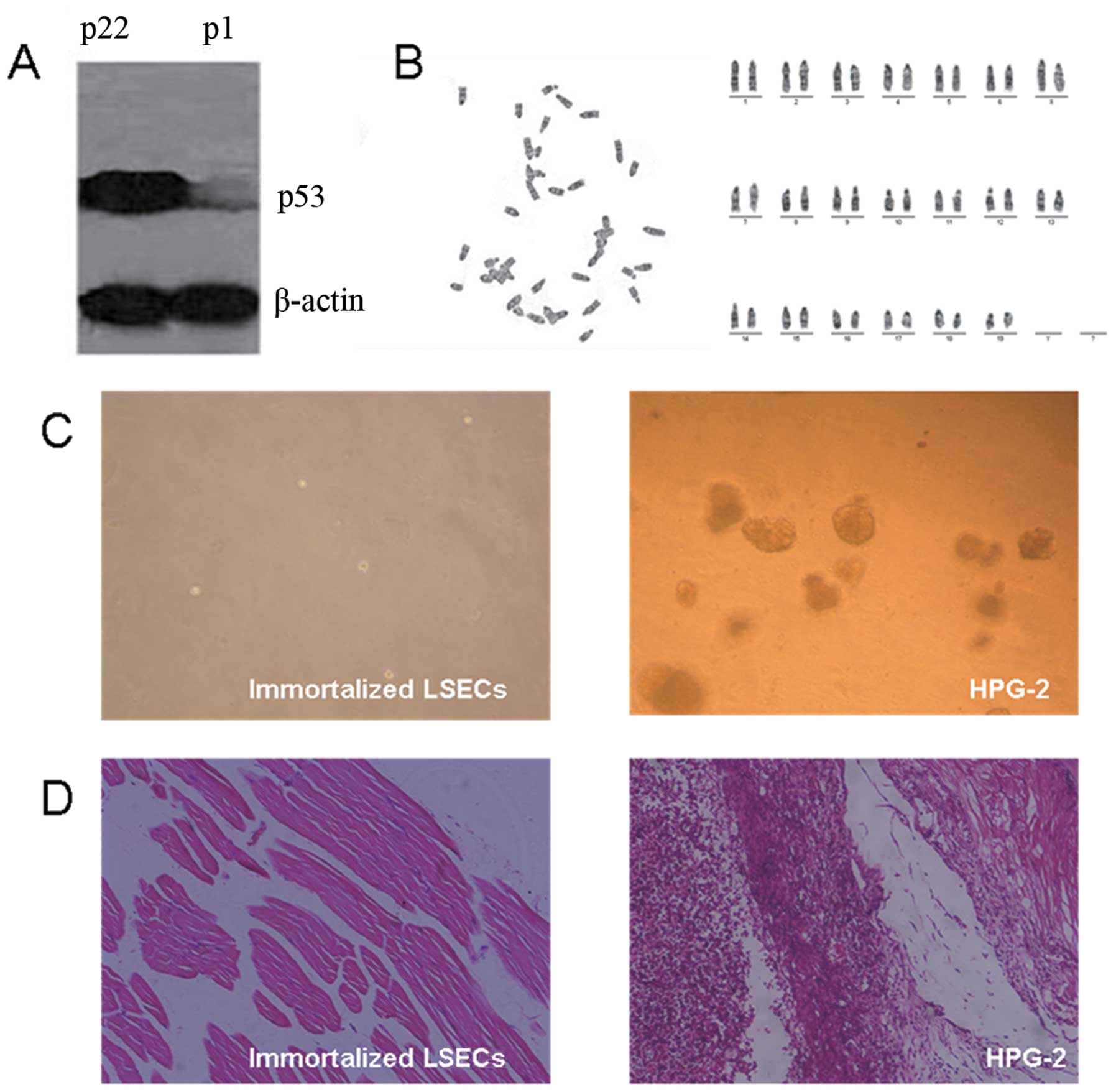
We then examined p53 protein expression and
karyotype in the immortalized LSECs, which are both potentially
important factors of immortalization. Compared with the primary
LSECs, the protein expression level of p53 in the immortalized
LSECs was significantly upregulated (Fig. 4A). However, the immortalized LSECs
showed a normal karyotype (Fig.
4B).
Given that these immortalized LSECs showed some
characteristics of transformed cells, such as growth
characteristics, immunophenotypes and an extended lifespan, we
futher analyzed their tumorigenic potential. As shown in Fig. 4C, no colony formation was observed
in the immortalized LSECs in comparison to the HPG-2 cells, which
served as a positive control. Therefore, the immortalized LSECs
were not able to grow in an anchorage-independent manner,
indicating no tumorigenic potential and a high differentiation
status. This notion was further supported by the fact that all the
SCID mice implanted with the immortalized LSECs did not develop any
tumors within 6 months; by contrast, the SCID mice implanted with
the HPG-2 cells developed tumors within 3 weeks (Fig. 4D).
Discussion
LSECs are a valuable tool for the study of the
physiology and pathophysiology of the liver. Several methods can be
used to isolate LSECs, such as the perfusion of an intact organ
with an enzymatic cocktail and the mechanical disruption of the
organ followed by enzymatic digestion. The cell suspension is then
fractionated by differential centrifugation techniques, such as
counter flow elutriation or density gradient centrifugation
(21–24). In the present study, we isolated
LSECs from mouse liver tissue by a combination of mechanical
disruption and density gradient centrifugation. Subsequently, these
isolated LSECs were further purified based on their different
adhesive abilities from other contaminating cells, as well as by
the use of endothelial cell selective medium. The endothelial cell
selective medium contained EGM-2, MCDB-131, EC-CM and some growth
factors, which stimulate the proliferation of LSECs and suppress
the growth of Kupffer cells, stellate cells and mesenchymal
fibroblast-like cells. A previous study demonstrated that AcSDKP in
a <3 kDa fraction of EC-CM had a significant inhibitory effect
on the growth of mesenchymal fibroblast-like cells (25). In the process of LSEC culture,
mesenchymal fibroblast-like cells were the major contaminating
cells, and the addition of EC-CM can efficiently inhibit the growth
of mesenchymal stem cells and promote the proliferation of LSECs
(26). This method was easy to
operate and required no special equipment. Following purification,
we finally obtained the homogeneous cultures of LSECs.
In order to confirm that these cells were purified
LSECs, we analyzed their structural characteristics,
immunophenotypes and other biological characteristics. Under a
scanning electron microscope, these cells exhibited a typical
fenestration structure on the cell surface. Fenestration is
generally considered to be the only gold standard of LSECs, making
them clearly distinguishable from all other types of liver cells
(27,28). Their endothelial origin was
further confirmed by the expression of vWF, the uptake of
DiI-Ac-LDL and the formation of capillary-like structures in
Matrigel. These characteristics can be used as markers to
effectively distinguish LSECs from other types of cells in the
liver, including stellar cells and Kupffer cells. During adherent
culture, stellate cells exhibit a fibroblast-like morphology. In
addition, stellate cells often contain fat and express desmin, but
do not express vWF and cannot uptake DiI-Ac-LDL, characteristics
which are different from LSECs. Kupffer cells are a type of
macrophages in the liver, and they always have many pseudopodia and
microvilli, and hence exhibit an irregular morphology. Furthermore,
these cells we obtained did not express CD31 (a marker of
endothelial progenitor cells and vascular endothelial cells), CK19
(a marker of liver epithelial cells) and CD90, CD105 and desmin
(markers of mesenchymal fibroblast-like cells). Hence, we concluded
that these cells were purified LSECs which were not contaminated by
any other cells in the liver.
It has been demonstrated that immortalized cell
lines can generally be established by several methods, such as
transgenic technology, adding chemical carcinogen and using γ-ray
irradiation (29,30). However, the probability of
spontaneous immortalization is extremely low (31). In the present study, LSECs at
passage 8 experienced senescence as judged by the phenomenon that
numerous LSECs exhibited a large and flattened morphology, and
showed SA-β-Gal activity. During extended culture, a small
population of round strong refractive proliferating cells emerged
from the senescent cultures, and showed clonal and multilayer
growth. All these characteristics were consistent with those of the
spontaneously immortalized cells reported by other studies
(13,14). In the present study, these
immortalized LSECs showed some altered characteristics which
differed from the early passage LSECs. Although the typical
fenestration structure, the expression of vWF and the uptake of
DiI-Ac-LDL were maintained in the immortalized LSECs, they lost the
ability to form capillary-like structures, and obtained some
immunophenotypes, such as the expression of CD31 and desmin.
Similar findings have also been reported in other types of cells
during spontaneous immortalization (32).
Although the molecular mechanisms of spontaneous
immortalization remain unclear, increasing evidence indicates that
uncontrolled cellular growth by the activation of oncogenes, the
inhibition of cancer suppressor genes and the upregulated activity
of telomerase may play crucial roles in the process of
immortalization (33,34). It has been reported that a
mutation in anti-oncogene p53 leads to its inactivation which may
contribute to the spontaneous immortalization of breast epithelial
cells during in vitro culture (33), and that mutant p53 is only a
facilitator of ‘classical’ immortalization in cells with shortened
telomeres (34). However, in the
present study, p53 in the immortalized LSECs was not inactivated
but upregulated. Furthermore, these immortalized LSECs did not show
any chromosomal changes or tumorigenic potential in vitro
and in vivo. Similar findings have also been reported in the
establishment of a spontaneously immortalized bovine mammary
epithelial cell line (35). Based
on these results, we speculated that a ‘non-classical’
immortalization may exist, consistent with previous studies
demonstrating that the spontaneous immortalization of mouse
myogenic cells can occur without the loss of p53 (36) or karyotype abnormality (35).
Previous studies (37,38) have demonstrated that environmental
conditions may be relevant to spontaneous immortalization. In the
present study, we used endothelial selective condition medium
containing EGM-2, MCDB131 and EC-CM derived from immortalized mouse
bone marrow endothelial cell lines. It has been reported that mouse
bone marrow endothelial cells secrete many types of cytokines, such
as granulocyte-macrophage colony-stimulating factor (GM-CSF),
interleukin (IL)-6, IL-11 and IL-13 to promote the proliferation of
hematopoietic cells (39). Hence,
we speculated that the culture conditions for LSECs and long-term
subculture may contribute to the immortalization of LSECs.
In conclusion, in this study, we successfully
established a spontaneously immortalized mouse LSEC line. These
immortalized LSECs maintained some distinctive phenotypes of early
passage LSECs, but also obtained certain novel biological
characteristics.
Acknowledgments
The present study was supported by a Project
supported by the National Basic Research Program (973 Program) of
China (grant no. 2007CB947901); a Project supported by the National
High Technology Research and Development Program (863 Program) of
China (grant no. 2011AA020113); a Project supported by the Natural
Science Foundation of China (grant no. 30771053); and a Project
supported by the Natural Science Foundation of Hunan Province,
China (grant no. 08JJ3075); Fundamental Research Funds for the
Central Universities of Central South University (2012zzts030). We
would also like to thank Professor Qiru Wang for her critical
reading of this manuscript.
References
|
1
|
Wisse E: An electron microscopic study of
the fenestrated endothelial lining of rat liver sinusoids. J
Ultrastruct Res. 31:125–150. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Braet F, Riches J, Geerts W, Jahn KA,
Wisse E and Frederik P: Three-dimensional organization of fenestrae
labyrinths in liver sinusoidal endothelial cells. Liver Int.
29:603–613. 2009. View Article : Google Scholar
|
|
3
|
Elvevold K, Smedsrod B and Martinez I: The
liver sinusoidal endothelial cell: a cell type of controversial and
confusing identity. Am J Physiol Gastrointest Liver Physiol.
294:G391–G400. 2008. View Article : Google Scholar
|
|
4
|
Wisse E, De Zanger RB, Charels K, Van Der
Smissen P and McCuskey RS: The liver sieve: considerations
concerning the structure and function of endothelial fenestrae, the
sinusoidal wall and the space of Disse. Hepatology. 5:683–692.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
LeCouter J, Moritz DR, Li B, et al:
Angiogenesis-independent endothelial protection of liver: role of
VEGFR-1. Science. 299:890–893. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ratsch SB, Gao Q, Srinivasan S, Wazer DE
and Band V: Multiple genetic changes are required for efficient
immortalization of different subtypes of normal human mammary
epithelial cells. Radiat Res. 155:143–150. 2001. View Article : Google Scholar
|
|
7
|
Yaswen P and Stampfer MR: Molecular
changes accompanying senescence and immortalization of cultured
human mammary epithelial cells. Int J Biochem Cell Biol.
34:1382–1394. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsutsui T, Fujino T, Kodama S, Tainsky MA,
Boyd J and Barrett JC: Aflatoxin B1-induced immortalization of
cultured skin fibroblasts from a patient with Li-Fraumeni syndrome.
Carcinogenesis. 16:25–34. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Todaro GJ and Green H: Quantitative
studies of the growth of mouse embryo cells in culture and their
development into established lines. J Cell Biol. 17:299–313. 1963.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yuspa SH, Poirier MC, Harness JR, Olsom DR
and Steinert PM: Specific quantification of mouse and human keratin
proteins by radioimmunoassay. Biochem J. 187:281–284.
1980.PubMed/NCBI
|
|
11
|
Heimann R and Rice RH: Rat esophageal and
epidermal keratinocytes: intrinsic differences in culture and
derivation of continuous lines. J Cell Physiol. 117:362–367. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Phillips MA and Rice RH: Convergent
differentiation in cultured rat cells from nonkeratinized
epithelia: keratinocyte character and intrinsic differences. J Cell
Biol. 97:686–691. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Davis T and Kipling D: Telomeres and
telomerase biology in vertebrates: progress towards a non-human
model for replicative senescence and ageing. Biogerontology.
6:371–385. 2005. View Article : Google Scholar
|
|
14
|
Christman SA, Kong BW, Landry MM, Kim H
and Foster DN: Contributions of differential p53 expression in the
spontaneous immortalization of a chicken embryo fibroblast cell
line. BMC Cell Biol. 7(27): 2006
|
|
15
|
Dimri G, Band H and Band V: Mammary
epithelial cell transformation: insights from cell culture and
mouse models. Breast Cancer Res. 7:171–179. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oh HY, Jin X, Kim JG, et al:
Characteristics of primary and immortalized fibroblast cells
derived from the miniature and domestic pigs. BMC Cell Biol. 8(20):
2007
|
|
17
|
Itahana K, Campisi J and Dimri GP: Methods
to detect biomarkers of cellular senescence: the
senescence-associated beta-galactosidase assay. Methods Mol Biol.
371:21–31. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gustashaw KM: Chromosome stains. The AGT
Cytogenetics Laboratory Manual. Barch MJ, Knutsen T and Spurbeck
JL: Lippincott-Raven Press; Philadelphia, New York: pp. 269–280.
1997
|
|
19
|
Daneker GW, Lund SA, Caughman SW, et al:
Culture and characterization of sinusoidal endothelial cells
isolated from human liver. In Vitro Cell Dev Biol Anim. 34:370–377.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Karrar A, Broome U, Uzunel M, Qureshi AR
and Sumitran-Holgersson S: Human liver sinusoidal endothelial cells
induce apoptosis in activated T cells: a role in tolerance
induction. Gut. 56:243–252. 2007. View Article : Google Scholar
|
|
21
|
Knolle PA and Limmer A: Neighborhood
politics: the immunoregulatory function of organ-resident liver
endothelial cells. Trends Immunol. 22:432–437. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
DeLeve LD, Wang X, McCuskey MK and
McCuskey RS: Rat liver endothelial cells isolated by anti-CD31
immunomagnetic separation lack fenestrae and sieve plates. Am J
Physiol Gastrointest Liver Physiol. 291:G1187–G1189. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Karaa A, Kamoun WS and Clemens MG:
Oxidative stress disrupts nitric oxide synthase activation in liver
endothelial cells. Free Radic Biol Med. 39:1320–1331. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Knolle PA, Germann T, Treichel U, et al:
Endotoxin down-regulates T cell activation by antigen-presenting
liver sinusoidal endothelial cells. J Immunol. 162:1401–1407.
1999.PubMed/NCBI
|
|
25
|
Huang WQ and Wang QR: Bone marrow
endothelial cells secrete thymosin beta4 and AcSDKP. Exp Hematol.
29:12–18. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang QR, Wang BH, Huang YH, Dai G, Li WM
and Yan Q: Purification and growth of endothelial progenitor cells
from murine bone marrow mononuclear cells. J Cell Biochem.
103:21–29. 2008. View Article : Google Scholar
|
|
27
|
DeLeve LD, Wang X, Hu L, McCuskey MK and
McCuskey RS: Rat liver sinusoidal endothelial cell phenotype is
maintained by paracrine and autocrine regulation. Am J Physiol
Gastrointest Liver Physiol. 287:G757–G763. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Braet F and Wisse E: Structural and
functional aspects of liver sinusoidal endothelial cell fenestrae:
a review. Comp Hepatol. 1(1): 2002
|
|
29
|
Namba M, Nishitani K, Fukushima F, Kimoto
T and Yuasa Y: Multi-step neoplastic transformation of normal human
fibroblasts by Co-60 gamma rays and Ha-ras oncogenes. Mutat Res.
199:415–423. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chapman S, Liu X, Meyers C, Schlegel R and
McBride AA: Human keratinocytes are efficiently immortalized by a
Rho kinase inhibitor. J Clin Invest. 120:2619–2626. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Katakura Y, Alam S and Shirahata S:
Immortalization by gene transfection. Methods Cell Biol. 57:69–91.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Masamune A, Satoh M, Kikuta K, Suzuki N
and Shimosegawa T: Establishment and characterization of a rat
pancreatic stellate cell line by spontaneous immortalization. World
J Gastroenterol. 9:2751–2758. 2003.PubMed/NCBI
|
|
33
|
Shay JW, Tomlinson G, Piatyszek MA and
Gollahon LS: Spontaneous in vitro immortalization of breast
epithelial cells from a patient with Li-Fraumeni syndrome. Mol Cell
Biol. 15:425–432. 1995.PubMed/NCBI
|
|
34
|
Elmore LW, Turner KC, Gollahon LS, Landon
MR, Jackson-Cook CK and Holt SE: Telomerase protects cancer-prone
human cells from chromosomal instability and spontaneous
immortalization. Cancer Biol Ther. 1:391–397. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao C, Meng L, Hu H, et al: Spontaneously
immortalised bovine mammary epithelial cells exhibit a distinct
gene expression pattern from the breast cancer cells. BMC Cell
Biol. 11(82): 2010
|
|
36
|
Nowak JA, Malowitz J, Girgenrath M, et al:
Immortalization of mouse myogenic cells can occur without loss of
p16INK4a, p19ARF, or p53 and is accelerated
by inactivation of Bax. BMC Cell Biol. 5(1): 2004
|
|
37
|
Gillio-Meina C, Swan CL, Crellin NK,
Stocco DM and Chedrese PJ: Generation of stable cell lines by
spontaneous immortalization of primary cultures of porcine
granulosa cells. Mol Reprod Dev. 57:366–374. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Denhardt DT, Edwards DR, McLeod M, Norton
G, Parfett CL and Zimmer M: Spontaneous immortalization of mouse
embryo cells: strain differences and changes in gene expression
with particular reference to retroviral gag-pol genes. Exp Cell
Res. 192:128–136. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li WM, Huang YH, Jiang DZ and Wang QR: The
effects of cytokines produced by bone marrow endothelial cells on
the regulation of hematopoiesis. Sheng Li Xue Bao. 52:45–49.
2000.(In Chinese).
|
|
40
|
Duan HX, Cheng LM, Wang J, Hu LS and Lu
GX: Angiogenic potential difference between two types of
endothelial progenitor cells from human umbilical cord blood. Cell
Biol Int. 30:1018–1027. 2006. View Article : Google Scholar : PubMed/NCBI
|