Introduction
Non-alcoholic fatty liver disease (NAFLD) is a
complex health condition which is characterized by the excessive
accumulation of fat in the liver. It ranges from simple steatosis
to non-alcoholic steatohepatitis (NASH) and, potentially, cirrhosis
(1). With the increase in obesity
and associated metabolic syndrome, NAFLD has emerged as a public
health issue with a prevalence of 15–30% in Western populations
(2–5) and 6–25% in Asian populations
(6). Accumulating evidence
indicates that NAFLD is not only associated with liver-related
morbidity or mortality, but also with an increased risk of coronary
heart disease and other cardiovascular complications (7,8).
Therefore, NAFLD is a new challenge for medical researchers.
MicroRNAs (miRNAs or miRs) are non-coding RNAs ~22
nt in length, which are emerging as important regulators involved
in a number of normal homeostatic processes, including the
regulation of metabolic pathways, cellular stress, immune defense
and inflammation (9–11). Certain studies have revealed the
alteration of miRNA profiles in NAFLD (12–15). In the study by Li et al
(12), it was demonstrated that
miR-21 was downregulated in the livers of mice with diet-induced
NASH. This suggests that miR-21 is associated with NAFLD. However,
to the best of our knowledge, there is no report to date on the
role of miR-21 in NAFLD. In the present study, we measured the
serum levels of miR-21 in patients with NAFLD and also performed
in vitro experiments using a cellular model of NAFLD to
further investigate the effects of miR-21 on triglyceride (TG) and
cholesterol metabolism. Moreover, a novel target through which
miR-21 exerted its effects on NAFLD was identified.
Materials and methods
Ethics statement and patients
The study was approved by the Ethics Committee of
the Third Xiangya Hospital, Central South University, Changsha,
China and written informed consent was obtained from each patient
prior to participation. A total of 25 patients with NAFLD and 12
healthy controls were enrolled in the present study. The blood
samples were collected prior to any therapeutic procedure.
Approximately 5 ml of venous blood was collected from each
participant. The whole blood was centrifuged at 4,000 rpm for 10
min, then the supernatant serum was isolated and stored at −80°C
until analysis.
Cell culture
HepG2 cells were purchased from the American Type
Culture Collection (ATCC; Manassas, VA, USA) and cultured in
Dulbecco’s modified Eagle’s medium (DMEM; Gibco/Invitrogen Life
Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum (FBS; Gibco). The HepG2 cells were incubated at 37°C in 5%
CO2 humidity.
Transfection
The cells were seeded into a 6-well plate at a
density of ×105 cells/well. Transfection of cells with
the miR-NC, miR-21 mimic, miR-21 inhibitor, HMGCR 3′-UTR plasmid
and HMGCR overexpression plasmid was carried out using
Lipofectamine 2000 (Invitrogen Life Technologies) in accordance
with the manufacturer’s instructions, followed by fatty acid
treatment.
Fatty acid treatment
Palmitic acid (PA) and oleic acid (OA) were obtained
from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in isopropyl
alcohol at a stock concentration of 800 mM. PA and OA were added to
the culture medium at a concentration of 800 μM at a 1:2
molar ratio according to the method described in the study by
Cazanave et al (16). The
concentration of the vehicle, isopropyl alcohol, was 1% in the
final incubations.
Reverse transcription-quantitative
(real-time) PCR
Total RNA was extracted from 100 μl of serum
and cells using a miRNeasy Mini kit (Qiagen GmbH, Hilden, Germany)
in accordance with the manufacturer’s instructions. Total RNA (1
μg) was used as a template for reverse transcription using
the First Strand cDNA Synthesis kit (Fermentas, Vilnius,
Lithuania). Quantitative PCR was carried out using a SYBR-Green PCR
kit (Applied Biosystems, Foster City, CA, USA) in a 7900 Sequence
Detection System (Applied Biosystems). The mRNA expression level
was expressed relative to the internal control using the
comparative threshold cycle (Ct) method.
Western blot analysis
Mouse monoclonal antibodies against
3-hydroxy-3-methylglutaryl-co-enzyme A reductase (HMGCR; sc-271595)
and GAPDH (sc-166574) were purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). Proteins were extracted from the cells
using a total protein extraction kit (Applygen Technologies Inc.,
Beijing, China) according to the manufacturer’s instructions.
Protein (50 μg) was separated on 10% sodium dodecyl sulfate
polyacrylamide gel electrophoresis gel (SDS-PAGE) and transferred
onto a polyvinylidene fluoride membrane (EMD Millipore Corp.,
Billerica, MA, USA). The membrane was blocked with 5% dry milk in
Tris-buffered saline Tween-20 at room temperature for 1 h. After
washing 3 times with PBS, the membrane was incubated with primary
antibodies (HMGCR antibody, 1:800; GAPDH antibody, 1:2,000) at 37°C
for 2 h, then incubated with secondary antibody (horseradish
peroxidase-labeled anti-mouse antibody, 1:4,000; sc-358914) at 37°C
for 1 h. The signals were detected using an ECL detection kit
(Pierce Biotechnology, Inc., Rockford, IL, USA).
Luciferase reporter assay
The 3′-untranslated region (UTR) segment of HMGCR
and its mutant were amplified and inserted into the pGL3-control
luciferase reporter vector (Promega, Madison, WI, USA). The cells
were co-transfected with the miR-NC/miR-21 mimic and HMGCR 3′-UTR
using Lipofectamine 2000 reagent (Invitrogen Life Technologies)
according to the manufacturer’s instructions. Forty-eight hours
later, the cell lysates were prepared and luciferase activity was
measured using a luciferase assay kit (Promega).
Measurement of TG, free cholesterol (FC)
and total cholesterol (TC) levels
The levels of TG, FC and TC in the HepG2 cells were
determined using commercial TG, FC and TC assay kits from Solarbio
Inc. (Shanghai, China) following the manufacturer’s instructions.
Briefly, the cells were lysed in ice-cold cell lysis buffer and
centrifuged at 1,500 × g for 5 min. The supernant was used for the
determination of TG, FC and TC levels.
Statistical analysis
All data are presented as the means ± standard
deviation. A two-tailed Student’s t-test was used to analyze the
statistical difference between 2 groups with SPSS 19.0 statistical
software (SPSS, Inc., Chicago, IL, USA). A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of miR-21 and HMGCR in
patients with NAFLD
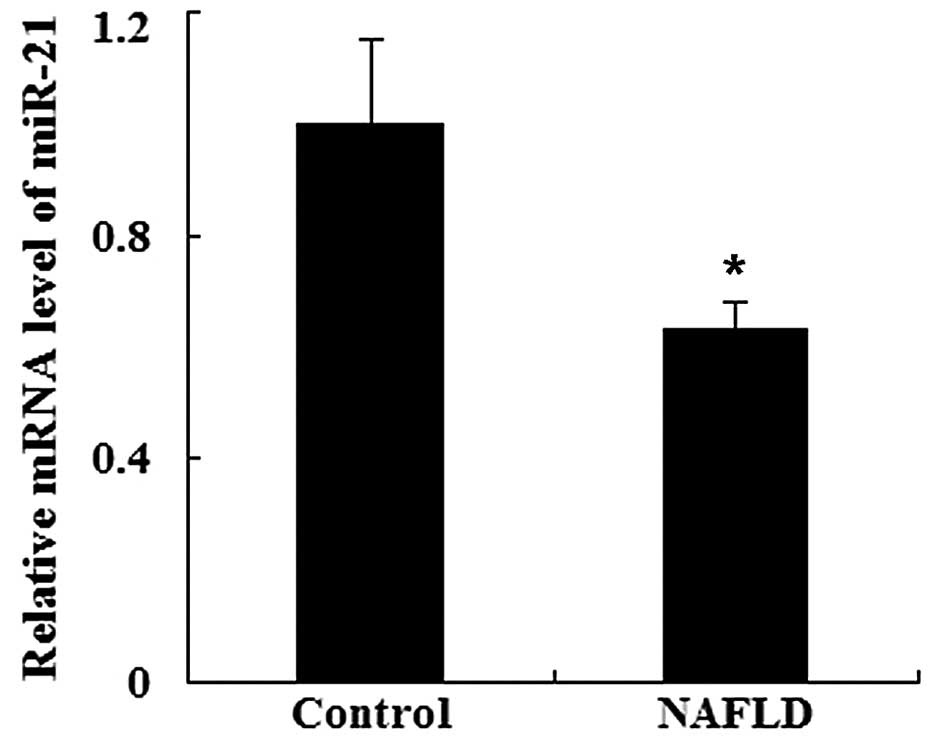
We investigated whether miR-21 is found in serum
from patients with NAFLD by RT-qPCR. As shown in Fig. 1, the serum levels of miR-21 were
significantly downregulated in the patients with NAFLD compared
with the healthy controls (P<0.05).
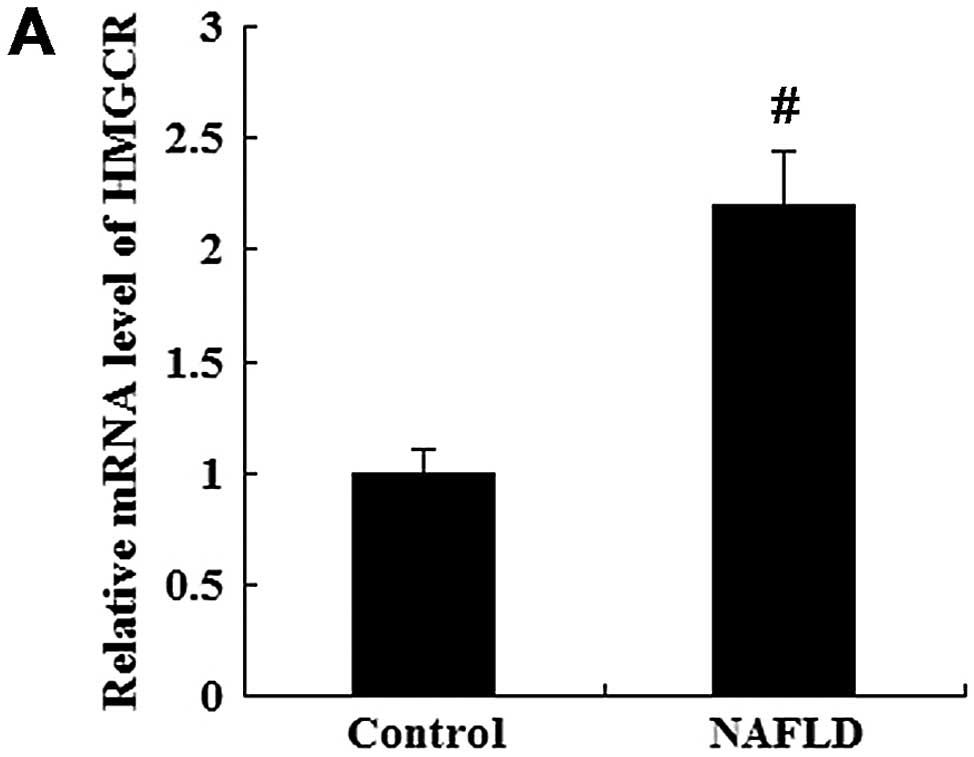
In addition, HMGCR mRNA and HMGCR protein levels in
serum were determined by RTqPCR and western blot analysis,
respectively. The mRNA level of HMGCR was significantly increased
in the patients with NAFLD compared with the healthy controls
(P<0.01; Fig. 2A). As shown in
Fig. 2B, the protein level of
HMGCR was also higher in the patients with NAFLD than in the
healthy controls (P<0.05).
Levels of TG, FC and TC in the in vitro
model of NAFLD
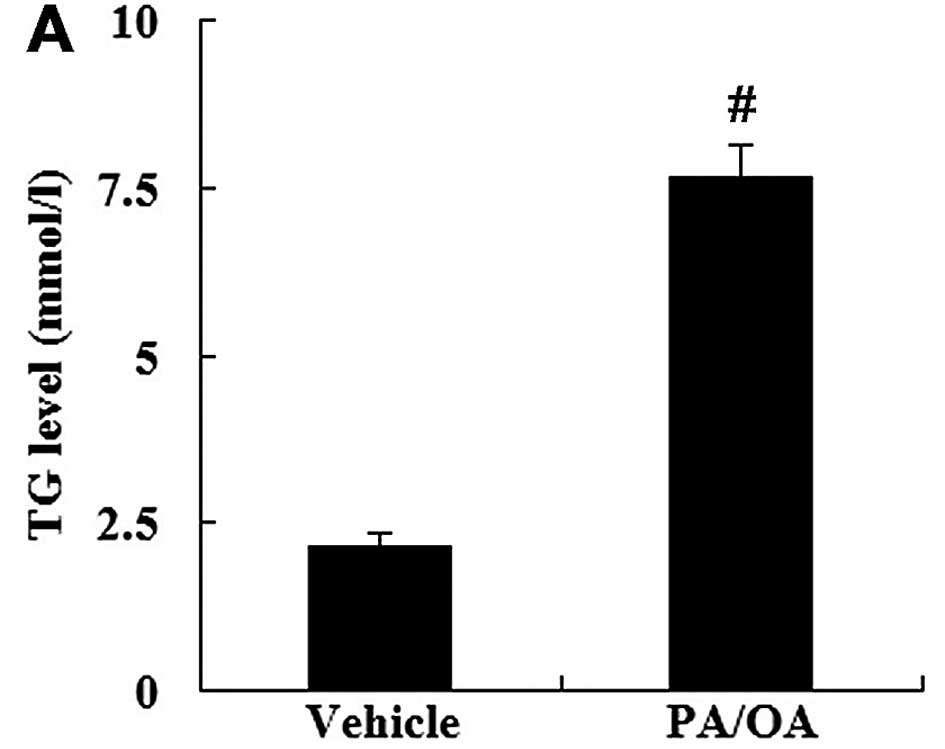
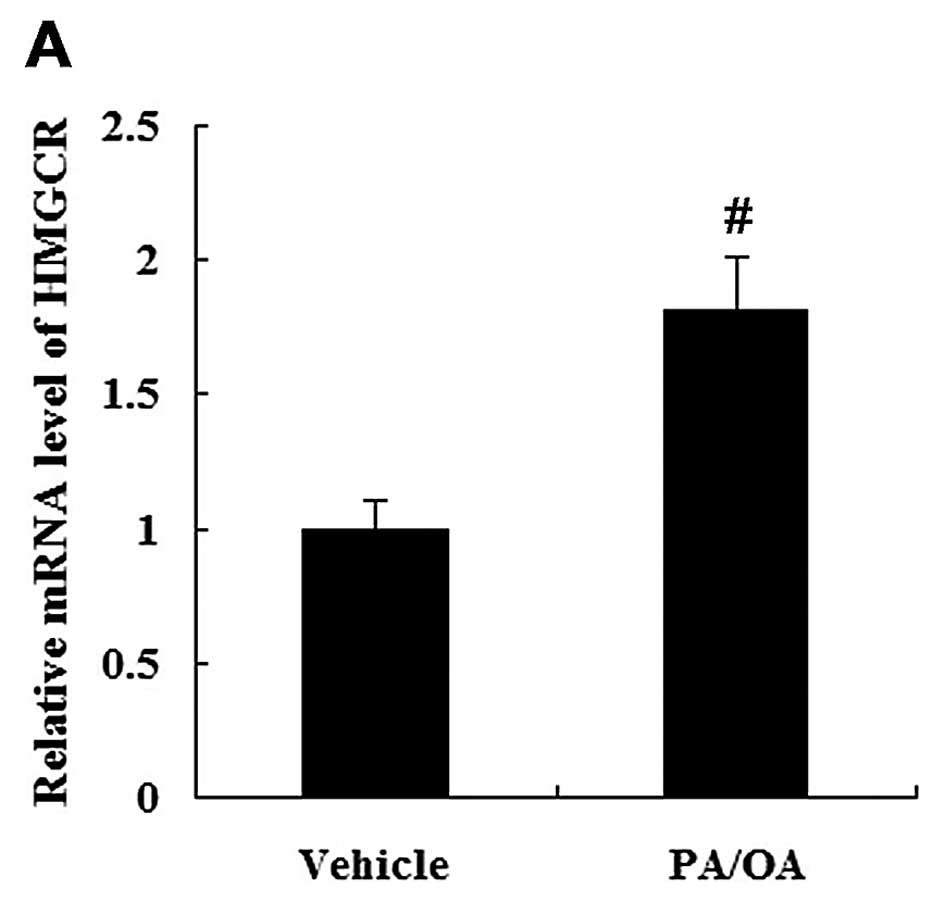
The HepG2 cells were treated with PA and OA to
establish the in vitro model of NAFLD. Compared with the
vehicle-treated cells, the level of TG in the PA/OA-treated cells
was significantly increased (P<0.01; Fig. 3A). The levels of FC and TC were
further examined. As shown in Fig. 3B
and C, the levels of FC and TC were significantly increased in
the PA/OA-treated cells compared with the vehicle-treated
cells.
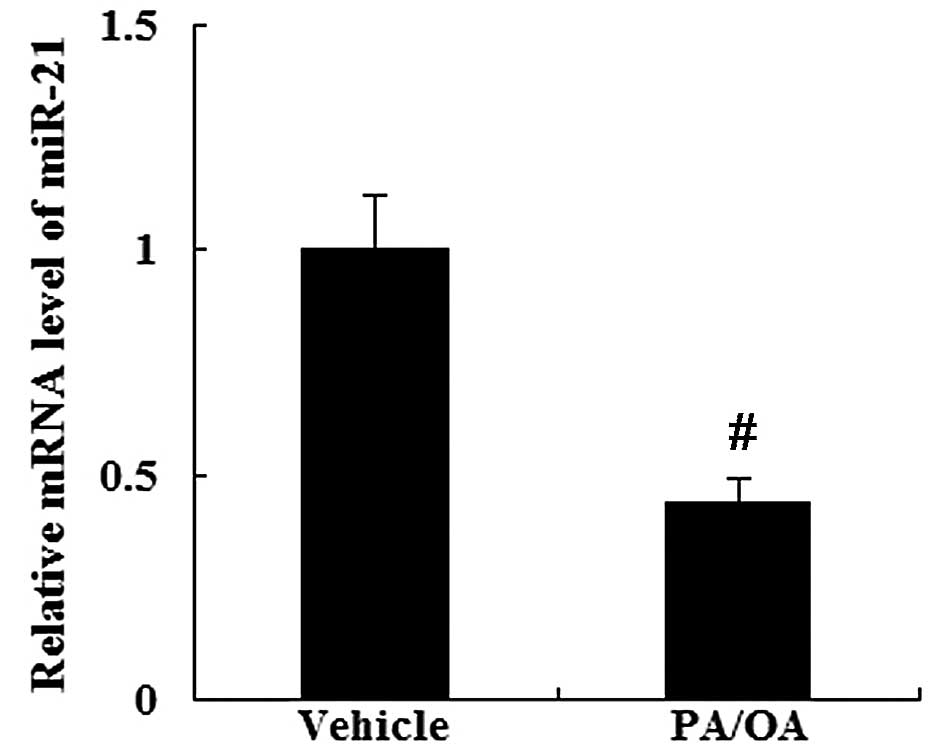
Expression of miR-21 and HMGCR in the in
vitro model of NAFLD
As demonstrated by RT-qPCR (Fig. 4), the PA/OA-treated HepG2 cells
showed a decreased expression of miR-21 (P<0.01). However, the
expression of HMGCR was increased in the PA/OA-treated HepG2 cells
at the mRNA and protein level compared with the vehicle-treated
HepG2 cells (Fig. 5).
miR-21 directly targets the HMGCR 3′-UTR
and regulates HMGCR expression
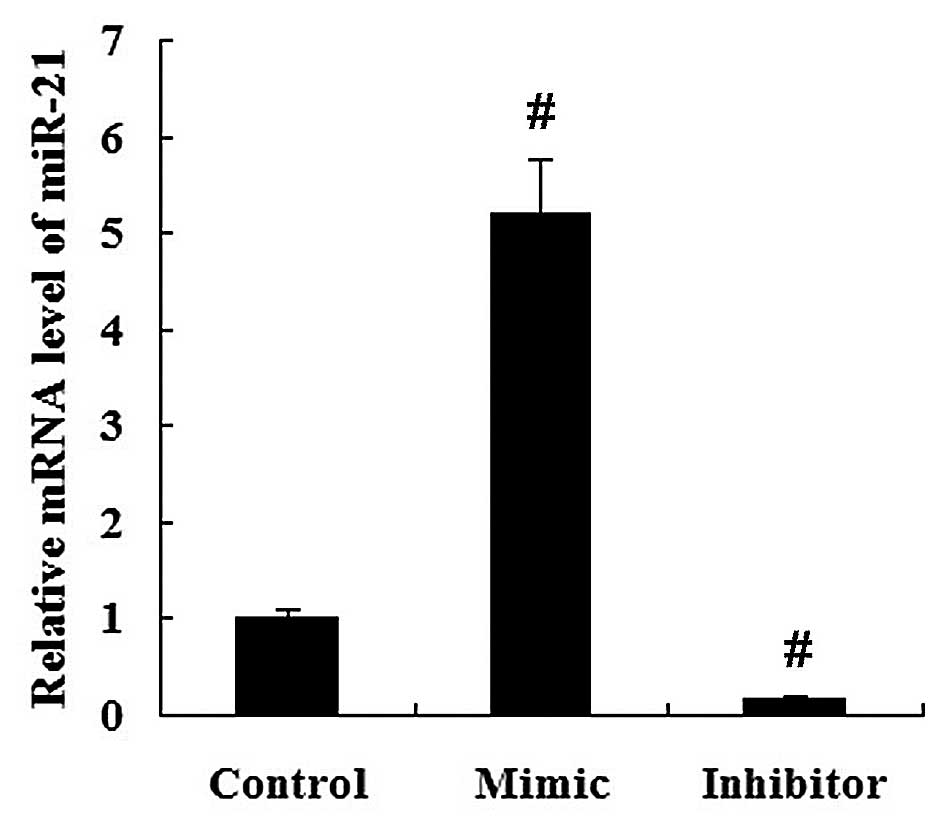
To investigate whether miR-21 regulates HMGCR
expression, miR-21 mimic and miR-21 inhibitor were transfected into
the HepG2 cells. It was demonstrated that the relative mRNA level
of miR-21 was significantly increased in the miR-21
mimic-transfected cells, but decreased in the miR-21
inhibitor-transfected cells compared with the miR-NC-transfected
cells (P<0.01; Fig. 6).
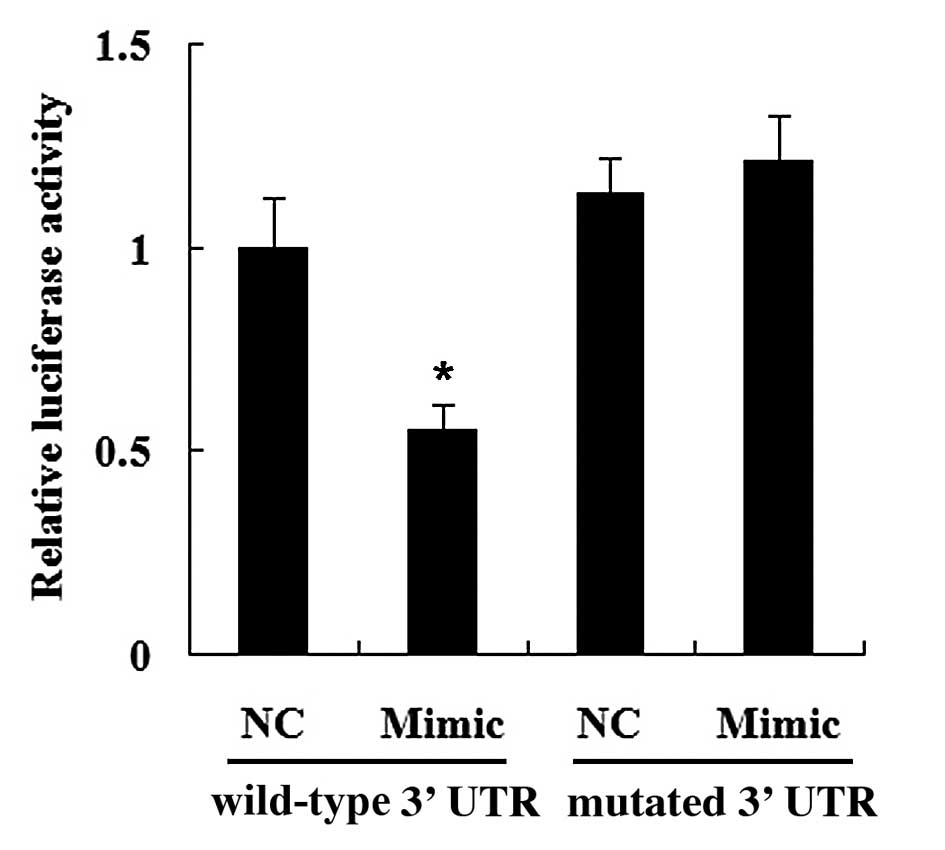
To determine whether HMGCR is a direct target of
miR-21, HMGCR wild-type 3′-UTR or HMGCR mutated 3′-UTR was cloned
into the luciferase reporter vector. As shown in Fig. 7, the luciferase activity was
significantly decreased in the cells co-transfected with the HMGCR
wild-type 3′-UTR and the miR-21 mimic (P<0.05). However,
transfection with the miR-21 mimic did not have any effect on the
luciferase activity of the HMGCR mutated 3′-UTR (P>0.05).
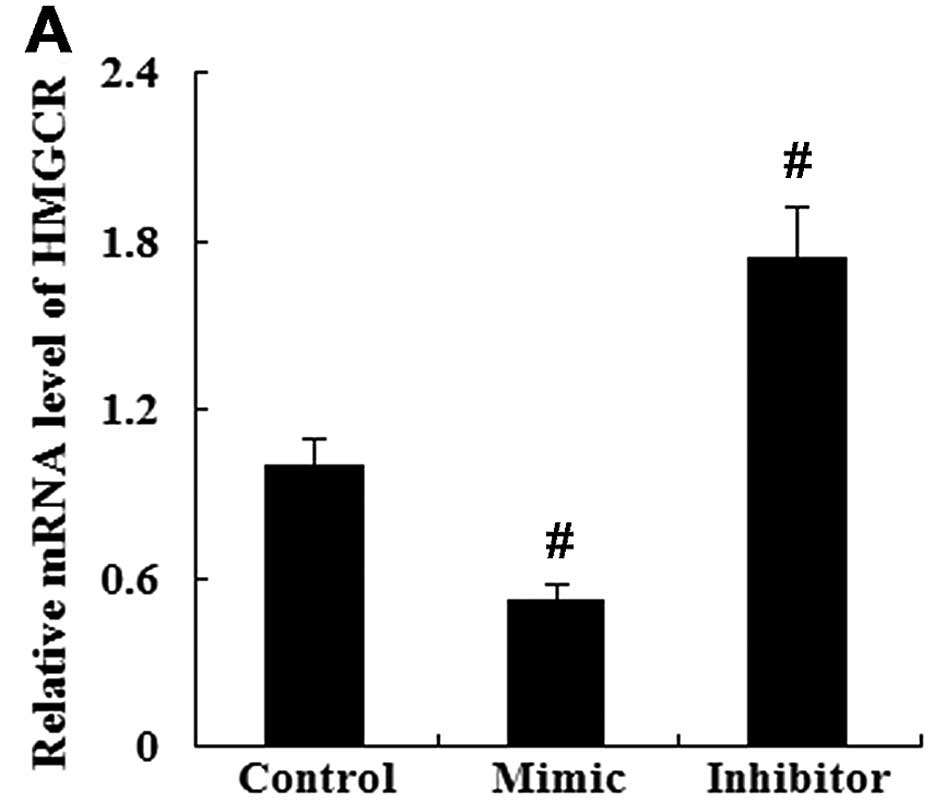
As assessed by RT-qPCR and western blot analysis,
the enhanced expression of miR-21 resulted in a significant
decrease in HMGCR mRNA and protein expression. However, the
suppression of miR-21 led to an increased HMGCR mRNA and protein
expression (Fig. 8).
Effect of miR-21 on the levels of TG, FC
and TC in the in vitro model of NAFLD
The miR-21 mimic was transfected into the
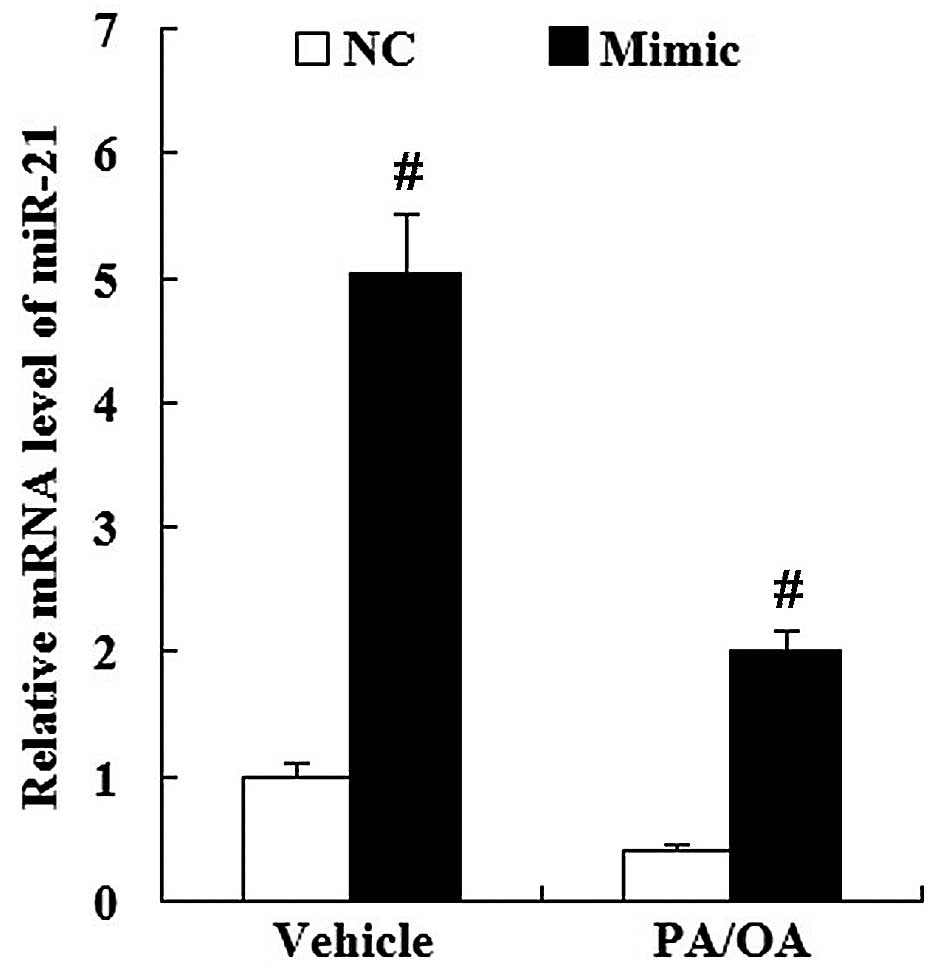
vehicle-treated or PA/OA-treated HepG2 cells. As shown in Fig. 9, the expression of miR-21 was
significantly increased in the miR-21-transfected cells compared
with the miR-NC-transfected cells for both the vehicle-treated and
PA/OA-treated HepG2 cells (P<0.01).
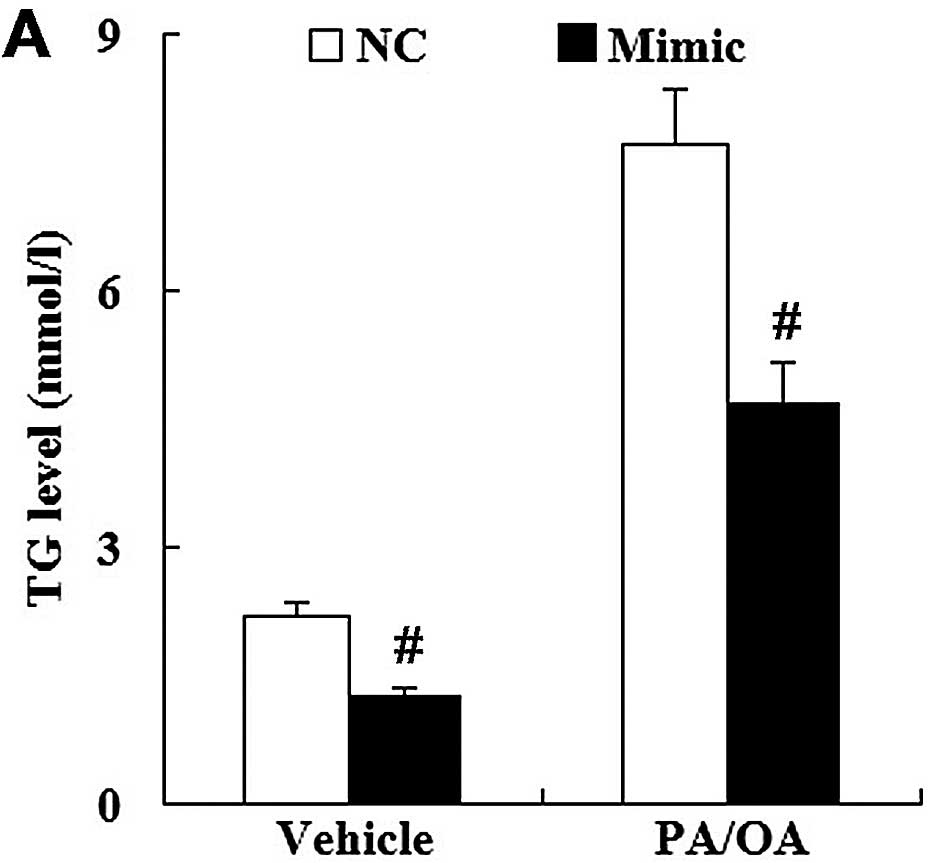
Using vehicle-treated HepG2 cells transfected with
the miR-NC as the control, it was demonstrated that transfection
with the miR-21 mimic significantly suppressed the level of TG in
the vehicle-treated HepG2 cells. In addition, the levels of FC and
TC were significantly decreased in the vehicle-treated HepG2 cells
transfected with the miR-21 mimic. As for the PA/OA-treated HepG2
cells, the levels of TG, FC and TC were also decreased in the cells
transfected with the miR-21 mimic compared with those transfected
with miR-NC (Fig. 10).
Effect of miR-21 on HMGCR expression in
the in vitro model of NAFLD
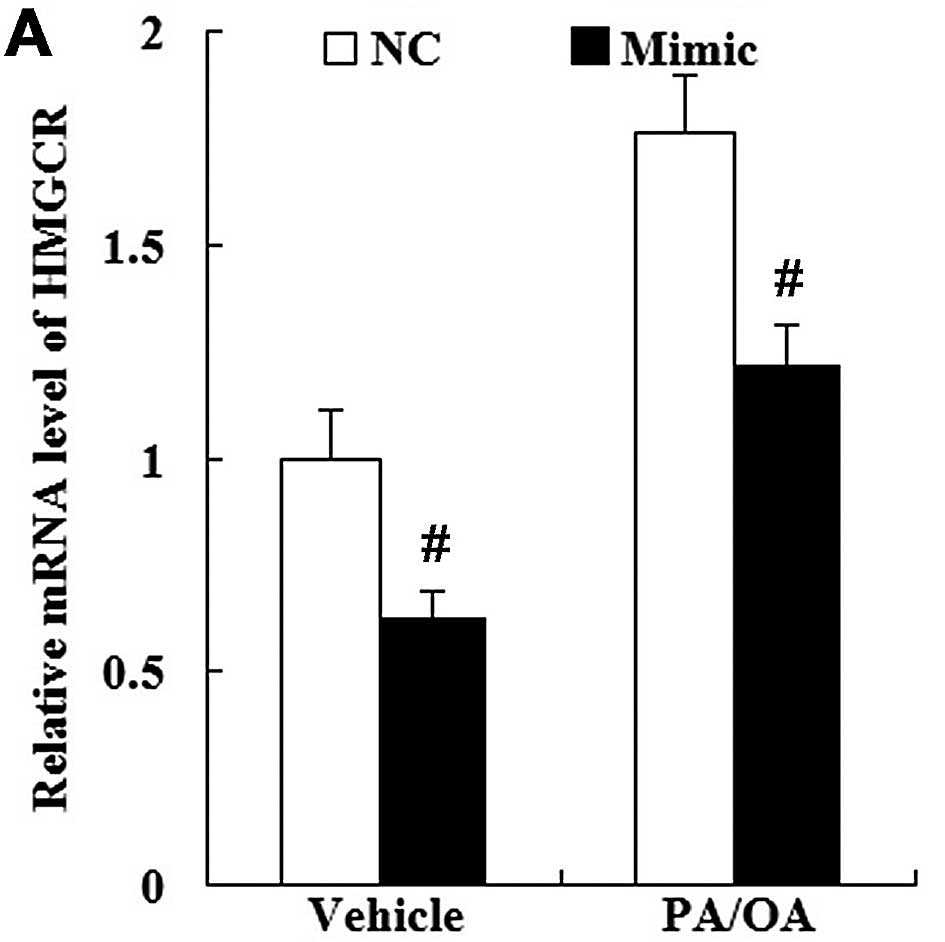
We then examined whether changes in miR-21
expression modulate HMGCR expression in PA/OA-treated HepG2 cells.
As shown in Fig. 11, the
enhanced expression of miR-21 not only downregulated HMGCR
expression in the vehicle-treated HepG2 cells, but also in the
PA/OA-treated HepG2 cells both at the mRNA and protein level
(Fig. 11).
HMGCR is involved in the effects of
miR-21 on the levels of TG, FC and TC in the in vitro model of
NAFLD
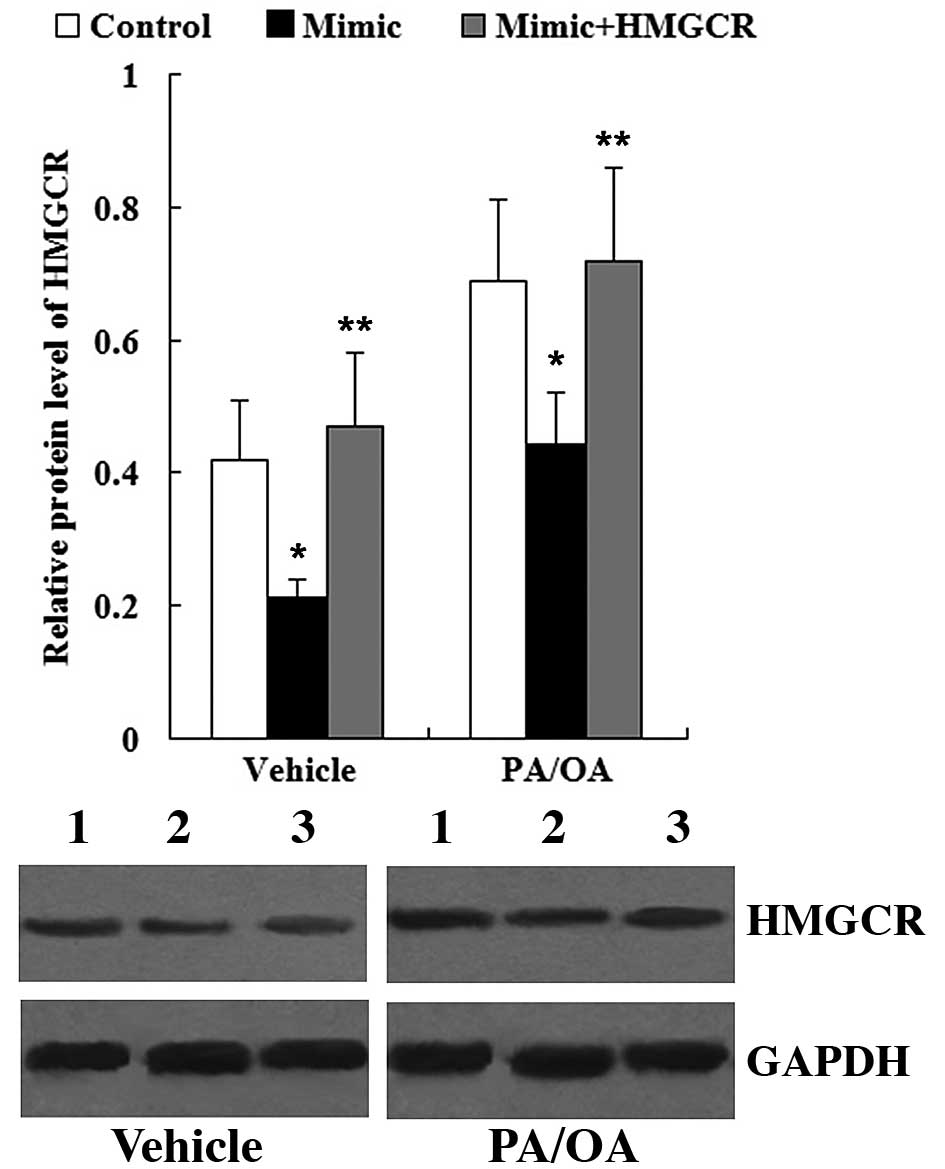
To determine whether HMGCR is involved in the
effects of miR-21 on the levels of TG, FC and TC in PA/OA-treated
HepG2 cells, the HMGCR overxpression plasmid was transfected into
the HepG2 cells. As shown in Fig.
12, the HMGCR protein level was significantly increased in the
cells transfected with the miR-21 mimic + HMGCR overexpression
plasmid compared with the cells transfected with the miR-21 mimic;
this was observed in both the vehicle-treated and PA/OA-treated
HepG2 cells.
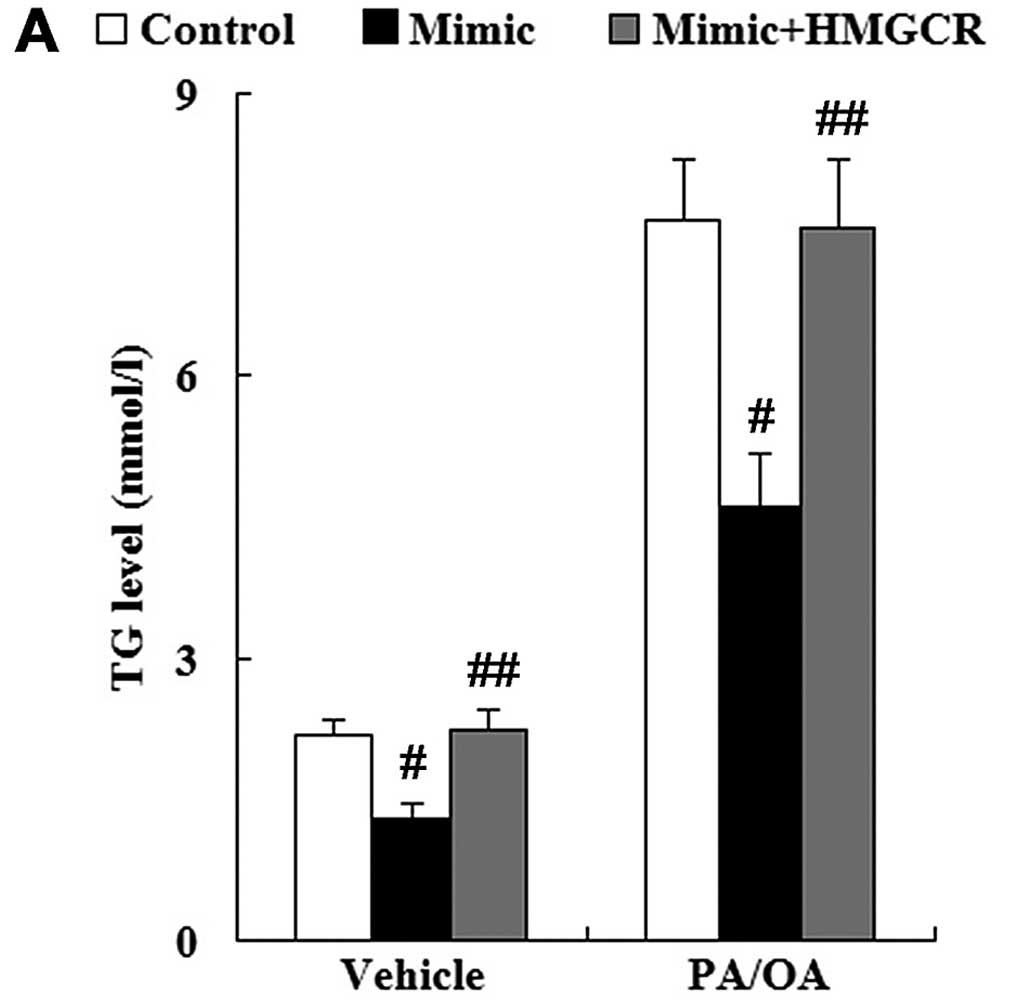
As shown in Fig.
13, compared with the cells transfected with the miR-21 mimic,
following transfection with the HMGCR overexpression plasmid, the
levels of TG, FC and TC were significantly increased in both the
vehicle-treated and PA/OA-treated HepG2 cells.
Discussion
Ahn et al (17) found that the expression of miR-21
was decreased in the livers from mice fed a high-fat diet and in
Hepa 1–6 cells treated with stearic acid (SA), and that miR-21
upregulation markedly blocked the SA-induced intracellular lipid
accumulation. In this study, we investigated miR-21 expression in
NAFLD both in vivo and in vitro. The results revealed
that the serum level of miR-21 was lower in patients with NAFLD.
Subsequently, in order to mimic the NAFLD condition in
vitro, HepG2 cells were treated with PA and OA. The
concentration of free fatty acids (FFA) used in the experiments was
similar to the fasting FFA plasma concentrations observed in human
NASH, ranging from 700 to 1,000 μM (18–21). This treatment mimics the FFA
lipotoxicity and steatosis which occurs during the course of the
disease (22). It was
demonstrated that the levels of TG were significantly increased in
the PA/OA-treated HepG2 cells; therefore, the in vitro model
of NAFLD was successfully established. Consistent with the results
from the in vivo experiments, the data revealed that miR-21
was also decreased in the in vitro model of NAFLD.
A previous study found that HMGCR was relatively
dephosphorylated in both phenotypes of NAFLD and thus in its active
form (23,24). Increased HMGCR expression is
associated with NAFLD (25) and
is related to FC levels and the severity of liver disease (26). In the present study, we examined
the expression levels of HMGCR in patients with NAFLD and in an
in vitro model of NAFLD. Both the in vivo and in
vitro results demonstrated that HMGCR expression was
upregulated in NAFLD.
Subsequently, we performed in vitro
experiments to examine the effects of miR-21 on TG and cholesterol
metabolism. It was demonstrated that miR-21 decreased the levels of
TG, FC and TC in the PA/OA-treated HepG2 cells; this suggests that
miR-21 regulates TG and cholesterol metabolism in NAFLD.
Cholesterol is mainly synthesized in the liver and
cholesterol homeostasis is maintained by HMGCR transcriptional
inhibition and enzyme modifications (27). HMGCR is the rate-limiting enzyme
in the hepatic cholesterol biosynthesis pathway (28). HMGCR converts
3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) to mevalonate and
is the major target of cholesterol-lowering drugs (29).
Using prediction software (http://www.microrna.org/), HMGCR was identified to be
a predicted target gene of miR-21. In the present study, to the
best of our knowledge, we demonstrate for the first time that HMGCR
is a direct target of miR-21 through luciferase reporter assay. In
addition, RT-qPCR and western blot analysis revealed that HMGCR
expression was altered following transfection with the miR-21 mimic
or miR-21 inhibitor. These observations suggest an effect of miR-21
on both HMGCR transcript degradation and protein translation.
HMGCR overexpression plasmid was also transfected
into vehicle-treated or PA/OA-treated HepG2 cells. It was found
that HMGCR reversed the reducing effects of miR-21 on TG, FC and TC
levels in NAFLD. Thus, these results suggest that the effects of
miR-21 on TG and cholesterol metabolism in NAFLD are mediated
through HMGCR.
Taken together, our data confirm that miR-21 is
downregulated in the serum of patients with NAFLD. miR-21 regulated
TG and cholesterol metabolism in our in vitro model of
NAFLD, and this effect was achieved through the inhibition of HMGCR
expression. The present study provides evidence that miR-21 may be
a useful biomarker for the diagnosis and treatment of NAFLD.
References
|
1
|
Targher G, Bertolini L, Padovani R, et al:
Prevalence of nonalcoholic fatty liver disease and its association
with cardiovascular disease among type 2 diabetic patients.
Diabetes Care. 30:1212–1218. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Clark JM and Diehl AM: Hepatic steatosis
and type 2 diabetes mellitus. Curr Diab Rep. 2:210–215. 2002.
View Article : Google Scholar
|
|
3
|
Bedogni G, Miglioli L, Masutti F,
Tiribelli C, Marchesini G and Bellentani S: Prevalence of and risk
factors for nonalcoholic fatty liver disease: the Dionysos
nutrition and liver study. Hepatology. 42:44–52. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Browning JD, Szczepaniak LS, Dobbins R, et
al: Prevalence of hepatic steatosis in an urban population in the
United States: impact of ethnicity. Hepatology. 40:1387–1395. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vernon G, Baranova A and Younossi ZM:
Systematic review: the epidemiology and natural history of
non-alcoholic fatty liver disease and non-alcoholic steatohepatitis
in adults. Aliment Pharmacol Ther. 34:274–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fan JG: An introduction of strategies for
the management of nonalcoholic fatty liver disease (NAFLD)
recommended by Asia Pacific Working Party on NAFLD. Zhonghua Gan
Zang Bing Za Zhi. 15:552–553. 2007.In Chinese. PubMed/NCBI
|
|
7
|
Ballestri S, Lonardo A, Bonapace S, Byrne
CD, Loria P and Targher G: Risk of cardiovascular, cardiac and
arrhythmic complications in patients with non-alcoholic fatty liver
disease. World J Gastroenterol. 20:1724–1745. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oni ET, Agatston AS, Blaha MJ, et al: A
systematic review: burden and severity of subclinical
cardiovascular disease among those with nonalcoholic fatty liver;
should we care? Atherosclerosis. 230:258–267. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nelson KM and Weiss GJ: MicroRNAs and
cancer: past, present, and potential future. Mol Cancer Ther.
7:3655–3660. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu P, Guo M and Hay BA: MicroRNAs and the
regulation of cell death. Trends Genet. 20:617–624. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Karp X and Ambros V: Encountering
microRNAs in cell fate signaling. Science. 310:1288–1289. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li S, Chen X, Zhang H, et al: Differential
expression of microRNAs in mouse liver under aberrant energy
metabolic status. J Lipid Res. 50:1756–1765. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cheung O, Puri P, Eicken C, et al:
Nonalcoholic steatohepatitis is associated with altered hepatic
microRNA expression. Hepatology. 48:1810–1820. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin X, Ye YF, Chen SH, Yu CH, Liu J and Li
YM: MicroRNA expression pattern in different stages of nonalcoholic
fatty liver disease. Dig Liver Dis. 41:289–297. 2009. View Article : Google Scholar
|
|
15
|
Pogribny IP, Starlard-Davenport A,
Tryndyak VP, Han T, Ross SA, Rusyn I and Beland FA: Difference in
expression of hepatic microRNAs miR-29c, miR-34a, miR-155, and
miR-200b is associated with strain-specific susceptibility to
dietary nonalcoholic steatohepatitis in mice. Lab Invest.
90:1437–1346. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cazanave SC, Mott JL, Elmi NA, et al:
JNK1-dependent PUMA expression contributes to hepatocyte
lipoapoptosis. J Biol Chem. 284:26591–26602. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ahn J, Lee H, Jung CH and Ha T: Lycopene
inhibits hepatic steatosis via microRNA-21-induced downregulation
of fatty acid-binding protein 7 in mice fed a high-fat diet. Mol
Nutr Food Res. 56:1665–1674. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nehra V, Angulo P, Buchman AL and Lindor
KD: Nutritional and metabolic considerations in the etiology of
nonalcoholic steatohepatitis. Dig Dis Sci. 46:2347–2352. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Richieri GV and Kleinfeld AM: Unbound free
fatty acid levels in human serum. J Lipid Res. 36:229–240.
1995.PubMed/NCBI
|
|
20
|
Sanyal AJ, Campbell-Sargent C, Mirshahi F,
et al: Nonalcoholic steatohepatitis: association of insulin
resistance and mitochondrial abnormalities. Gastroenterology.
120:1183–1192. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Belfort R, Harrison SA, Brown K, et al: A
placebo-controlled trial of pioglitazone in subjects with
nonalcoholic steatohepatitis. N Engl J Med. 355:2297–2307. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yao HR, Liu J, Plumeri D, et al:
Lipotoxicity in HepG2 cells triggered by free fatty acids. Am J
Transl Res. 3:284–291. 2011.PubMed/NCBI
|
|
23
|
Beg ZH, Stonik JA and Brewer HB Jr:
Phosphorylation of hepatic 3-hydroxy-3-methylglutaryl coenzyme A
reductase and modulation of its enzymic activity by
calcium-activated and phospholipid-dependent protein kinase. J Biol
Chem. 260:1682–1687. 1985.PubMed/NCBI
|
|
24
|
Beg ZH, Stonik JA and Brewer HB Jr:
Modulation of the enzymic activity of 3-hydroxy-3-methylglutaryl
coenzyme A reductase by multiple kinase systems involving
reversible phosphorylation: a review. Metabolism. 36:900–917. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Caballero F, Fernández A, De Lacy AM,
Fernández-Checa JC, Caballería J and García-Ruiz C: Enhanced free
cholesterol, SREBP-2 and StAR expression in human NASH. J Hepatol.
50:789–796. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Min HK, Kapoor A, Fuchs M, et al:
Increased hepatic synthesis and dysregulation of cholesterol
metabolism is associated with the severity of nonalcoholic fatty
liver disease. Cell Metab. 15:665–674. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
DeBose-Boyd RA: Feedback regulation of
cholesterol synthesis: sterolaccelerated ubiquitination and
degradation of HMG CoA reductase. Cell Res. 18:609–621. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Trapani L, Segatto M, Simeoni V, et al:
Short- and long-term regulation of 3-hydroxy 3-methylglutaryl
coenzyme A reductase by a 4-methylcoumarin. Biochimie.
93:1165–1171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maron DJ, Fazio S and Linton MF: Current
perspectives on statins. Circulation. 101:207–213. 2000. View Article : Google Scholar : PubMed/NCBI
|