Introduction
Mammalian tooth development is regulated by
signaling cascades involving various genes (1–5).
In our previous studies, (6–19)
we reported the genes that were differentially expressed between
mouse mandibles on embryonic day (E)10.5 and E12.0 using a cDNA
subtraction method (6), and that
these genes are associated with tooth development. Thymosin beta 4,
X-linked (Tb4) was one of the genes highly expressed in the E12.0
mandible (6). Tb4 is closely
associated with the differentiation of dental epithelial cells
during tooth development (8,17).
Tb4-overexpressing transgenic mice were observed to have enamel
hypoplasia-like abnormal tooth development (20). These results suggest that Tb4
plays an important role in tooth development.
Tb4 consists of 43 amino acid residues, and is a
4.9-kDa actin-sequestering peptide (22). Tb4 plays a role in cell motility
by regulating the polymerization and depolymerization of actin
(23). We have previously
demonstrated that Tb4 is tightly associated with tooth
morphogenesis through runt-related transcription factor 2 (Runx2)
expression in the organ-cultured tooth germ following Tb4 knockdown
(17). The expression of
odontogenesis-related genes, such as Runx2, amelogenin, X-linked
(Amelx), ameloblastin (Ambn) and enamelin (Enam) was induced in
non-odontogenic human keratinocytes transfected with a Tb4
expression vector (19). Tb4 may
participate in tooth development through the regulation of Runx2
expression. In addition, Smart et al (24) previously reported that the mouse
epicardium pre-treated with Tb4 was induced to re-express Wt1, a
key embryonic epicardial gene, and that the tissue was converted
into cardiomyocytes. Taken together, these previous findings
suggest that Tb4 has the ability to induce gene expression.
RUNX2 is a key differentiation marker of osteoblasts
and regulates bone formation. The knockdown of type II/III RUNX2
expression has been shown to reduce the calcification of calvarial
cells (25). Additionally, RUNX2
is tightly involved in calcification during tooth formation
(26–28) and regulates the expression of
odontogenesis-related genes (9,17,19,29–31). RUNX2 expression is observed at
various stages in tooth development (32,33). Therefore, RUNX2 is considered to
play an important role in the development and calcification of the
tooth germ.
Various signaling pathways involving Smad, PI3K-Akt,
MAPK, Hedgehog, Wnt/β-catenin and so on have been reported to be
upstream of RUNX2 expression during bone formation (34,35). Some of these signaling pathways
are also associated with RUNX2 expression during tooth development
(21,36,37). Tb4 has been shown to promote MAPK
and Smad signaling to induce the formation of calcified materials
in human dental pulp cells (21).
Tb4 activates the JNK signaling pathway to increase the expression
of pro-inflammatory cytokines in cancer cells (38), and induces the upregulation of ERK
phosphorylation to increase the resistance of cancer cells to
paclitaxel (39). These studies
suggest that Tb4 activates signaling pathways upstream of RUNX2.
However, little is known about the role of Tb4-RUNX2 signaling in
the developing tooth germ.
In the present study, we therefore investigated
Tb4-RUNX2 signaling in the mouse dental epithelial cell line, mDE6.
Our results demonstrated that the Smad and PI3K-Akt pathways may be
involved in tooth development, and provide new information
concerning the signaling pathway from Tb4 to RUNX2 expression in
the mDE6 cells, which may help to understand the regulation of
tooth development and regeneration.
Materials and methods
Cell lines and cell culture
The mouse dental epithelial cell line, mDE6,
established from mouse tooth germ was kindly provided by Professor
Satoshi Fukumoto (Tohoku University, Sendai, Japan). The mDE6 cells
were cultured in DMEM/F12 medium supplemented with 10% fetal bovine
serum, 100 U/ml penicillin and 100 mg/ml streptomycin (all from
Life Technologies, Carlsbad, CA, USA) in a humidified atmosphere of
5% CO2 at 37°C, as previously described (17,18).
Induction of calcification in cell
culture
The mDE6 cells were seeded in Ø35 mm dishes and were
incubated in culture medium without antibiotics. At 48 h after
seeding, the induction of calcification began with the use of
calcified induction medium (CIM), which was culture medium
containing 50 μg/ml ascorbic acid and 10 mM
β-glycerophosphate. The protocol for the induction of calcification
was based on that of a previous study (19). The CIM was changed every 3 days.
After the induction of calcification for 21 days, some dishes were
fixed with 4% paraformaldehyde (PFA) in 0.01 M phosphate-buffered
saline (pH 7.2) and stained with 1% Alizarin red S (ALZ) or von
Kossa (Kossa) for histological evaluation to identify
calcification. The others were analyzed by reverse
transcription-quantitatvie polymerase chain reaction (RT-qPCR) or
by western blot analysis.
Semi-quantitative RT-PCR
RT-qPCR was performed as described in previous
studies (17,19). In brief, total RNA was isolated
from the mDE6 cells using the SV Total RNA Isolation system
(Promega, Madison, WI, USA), and was reverse transcribed using the
SuperScript® VILO™ cDNA Synthesis kit and master mix
(Life Technologies) according to the manufacturer's instructions.
The expression of target genes was analyzed using the Thermal
Cycler Dice® Real-Time system, with SYBR®
Premix Ex Taq™ II (both from Takara, Shiga, Japan). The primers
used are listed in Table I.
Glyceraldehyde-3-phosphate dehydrogenase (Gapdh) was used as an
endogenous reference gene for relative quantifications. The
relative expression level of each target gene was normalized using
the ΔΔCT comparative method based on the reference gene threshold
cycle (CT) values, as previously described (16,17,19).
 | Table IPrimers used in RT-qPCR. |
Table I
Primers used in RT-qPCR.
| Gene name | Accession no. | Primer
sequences |
|---|
| Gapdh | NM_001289726.1 | F: 5′-TGT GTC CGT
CGT GGA TCT GA-3′ |
| NM_008084.3 | R: 5′-TTG CTG TTG
AAG TCG CAG GAG-3′ |
| Tb4 | NM_021278.2 | F: 5′-CTG ACA AAC
CCG ATA TGG CTG A-3′ |
| | R: 5′-ACG ATT CGC
CAG CTT GCT TC-3′ |
| Runx2 | NM_001146038.2 | F: 5′-GGT TAA TCT
CTG CAG GTC ACT ACC A-3′ |
| NM_001271627.1 | R: 5′-ACG GTG TCA
CTG CGC TGA A-3′ |
| NM_009820.5 | |
| Amelx | NM_009666.4 | F: 5′-AGC ATC CCT
GAG CTT CAG ACA GA-3′ |
| | R: 5′-AAC CAG GGC
TTC CAG GAT GAG-3′ |
| Ambn | NM_009664.1 | F: 5'- CCT GGG AGC
ACA GTG AAT GTC-3' |
| | R: 5′-TCA AAC TAG
CCA TGC CAG GAG-3′ |
| Enam | NM_017468.3 | F: 5′-CCG AAT GCC
TGG ATT TAG CAG TA-3' |
| | R: 5'-GGG TTG CTG
CCA TCC ATT G-3′ |
Western blot analysis
Sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and western blot analysis were performed
as previously described (19).
Briefly, each sample of total protein (10 μg/lane) was
fractionated by 10 or 15% gel electrophoresis, and the proteins
were transferred onto a polyvinylidene difluoride membrane
(Bio-Rad, Hercules, CA, USA). The membrane was incubated with the
primary antibodies (Table II).
Bound antibodies were reacted with a 1:5,000 dilution of
HRP-conjugated secondary antibodies, and were visualized using the
ECL Prime Western Blotting Detection system (GE Healthcare, Little
Chalfont, UK). Emitted light was detected using the ImageQuant LAS
4000 (GE Healthcare), a cooled CCD-camera. In the semi-quantitative
analyses of the levels of protein expression, the intensity of the
bands was measured using the ImageQuant TL software (GE
Healthcare). GAPDH was used as an internal control protein. The
ratio of target protein/GAPDH based on the intensity of the bands
was calculated, as previously described (17,19). After the detection of a targeted
phosphorylated protein, the membrane was reprobed to detect the
targeted non-phosphorylated protein on the same membrane.
 | Table IIAntibody types and source. |
Table II
Antibody types and source.
| Target protein | Provider ID | Antibody | Dilution |
|---|
| GAPDH | SC20357 | Goat Polyclonal
IgG | 1:1000 |
| TB4 | SC67114 | Rabbit Polyclonal
IgG | 1:1000 |
| RUNX2 | AB76956 | Mouse Monoclonal
IgG2a | 1:1000 |
| Smad1/5/8 | SC6031 | Rabbit Polyclonal
IgG | 1:1000 |
| p-Smad1/5 | CST9516 | Rabbit Monoclonal
IgG | 1:2000 |
| ERK1 | BD610030 | Mouse Monoclonal
IgG1 | 1:8000 |
| p-ERK1/2 | BD612358 | Mouse Monoclonal
IgG1 | 1:4000 |
| Akt | CST4691 | Rabbit Monoclonal
IgG | 1:2000 |
| p-Akt | CST4060 | Rabbit Monoclonal
IgG | 1:2000 |
| β-catenin | CST9582 | Rabbit Monoclonal
IgG | 1:4000 |
Inhibition assays
LDN193189 [an inhibitor of the phosphorylated (p-)
Smad1/5/8 pathway] was obtained from AdooQ BioScience (Irvine, CA,
USA). Triciribine (a p-Akt pathway inhibitor) and dimethyl
sulfoxide (DMSO) were obtained from Wako Chemical Inc. (Osaka,
Japan). The mDE6 cells were cultured in DMEM/F12 medium with
LDN193189 or triciribine in Ø35 mm dishes for 48 h. The final
concentration of DMSO in the medium was 0.1% (v/v). The final
concentrations of the inhibitors were: 50 and 500 nM LDN193189 and
250 and 2,500 nM triciribine. The mDE6 cells were also cultured in
CIM with LDN193189 or triciribine for 10 days when the mDE6 cells
were fully confluent in Ø35 mm dishes. The CIM with the inhibitor
was changed every other day. The cells treated with DMSO alone were
used as the controls. All samples were analyzed by RT-qPCR, western
blot analysis or ALZ staining.
Transfection with siRNA against Tb4
The cells were seeded in Ø35 mm dishes in culture
medium without antibiotics. At 24 h after seeding, the cells were
treated with siRNAs according to the manufacturer’s instructions
using the Lipofectamine® RNAiMAX Transfection Reagent
(Life Technologies). Three siRNAs against Tb4 (siRNA-1, -2 and -3)
were designed and prepared. Their target sites were different.
siRNA (final concentration 10 nM) was transfected into the cells
with the aid of 4 μl of the RNAiMAX reagent. The cells were
incubated with the siRNA complex for 48 h. A universal negative
control siRNA (Sigma-Aldrich, St. Louis, MO, USA) was used as a
negative control. The transfected cells were analyzed by RT-qPCR
and western blot analysis.
Analysis of the effect of Tb4 inhibition
using siRNA on the calcification of mDE6 cells
At 48 h after siRNA transfection (as mentioned
above), the induction of calcification began with the use of CIM.
Transfection of the mDE6 cells with siRNA against Tb4 was
repetitively performed using Lipofectamine RNAiMAX every time the
CIM was changed. After the induction of calcification for 10 days,
the cells were fixed with 4% PFA and stained with ALZ.
Statistical analysis
All the experiments were independently performed at
least in triplicate. All values are presented as the means ± SD. A
one-way ANOVA with the Tukey-Kramer comparison test was used to
analyze the data obtained with by RT-qPCR and western blot
analysis. Differences resulting in P-values of <0.05 or 0.01
were considered to be statistically significant.
Results
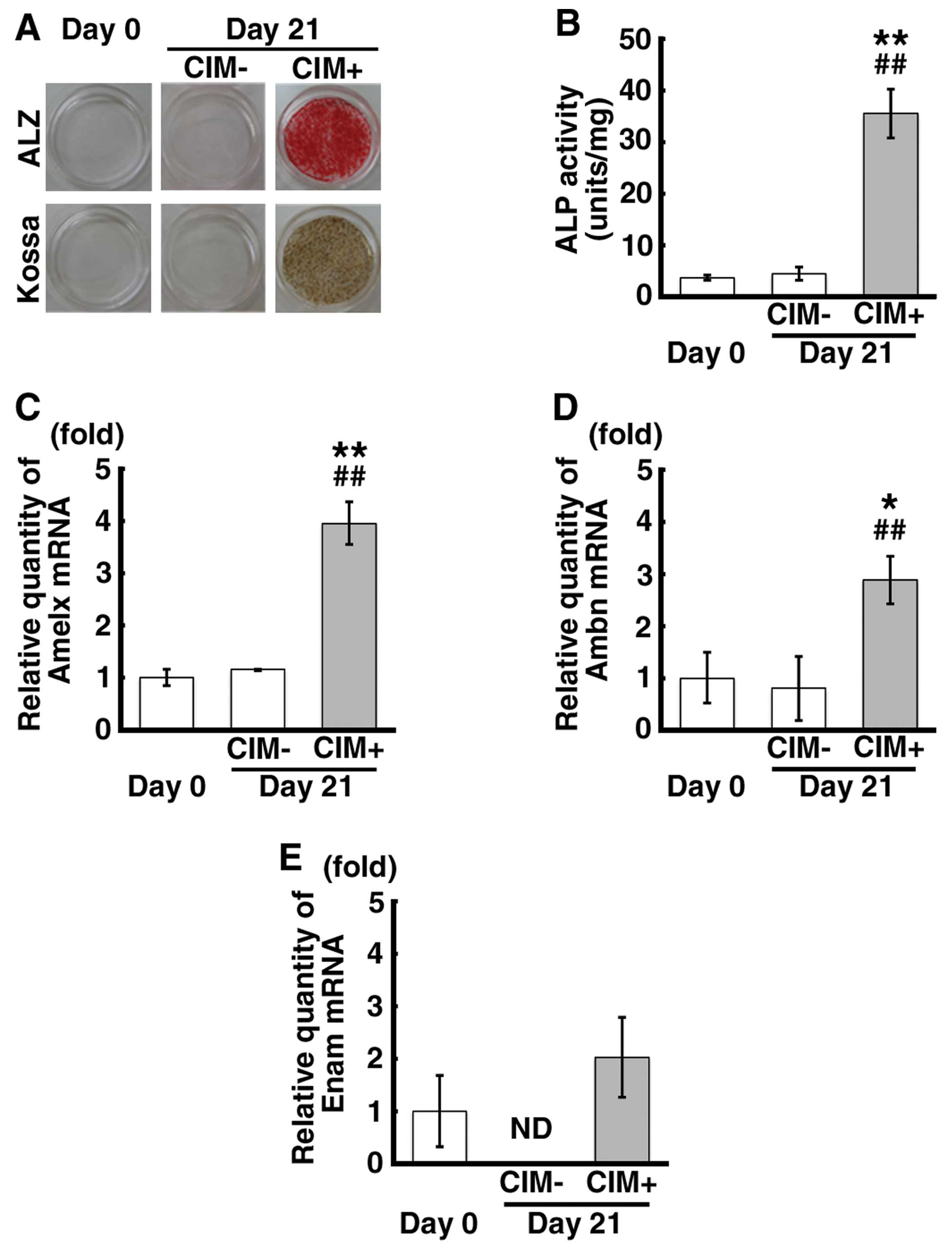
Calcification of the mDE6 cells
We wished to determine whether the formation of
calcified material was induced in mDE6 cells by the use of CIM, as
well as whether the activity of alkaline phosphatase (ALP) is
altered during calcification and whether the cells express
odontogenesis-related genes, such as Amelx, Ambn and Enam.
Calcification, as indicated by positive ALZ and Kossa staining, was
observed in the mDE6 cells cultured in CIM for 21 days (CIM+ cells)
(Fig. 1A), while no calcification
was noted in the cells cultured without CIM (CIM-cells). ALP
activity was significantly increased in the CIM+ cells (Fig. 1B). The mRNA expression levels of
Amelx and Ambn were significantly increased in the CIM+ cells
(Fig. 1C and D). Although there
were no significant differences observed in Enam mRNA expression
between the CIM+ cells and the controls (CIM-cells) and the cells
just before the induction of calcification (cells on day 0), the
Enam mRNA expression appeared to be increased in the CIM+ cells
(Fig. 1E). There were no marked
differences observed in the expression levels of these genes in the
CIM- cells compared with those observed on day 0 (Fig. 1C–E). These results indicate that
the mDE6 cells have the ability to express odontogenesis-related
genes and form calcified material, depending on the culture
conditions, and partially show the characteristics of odontogenic
epithelial cells in vivo.
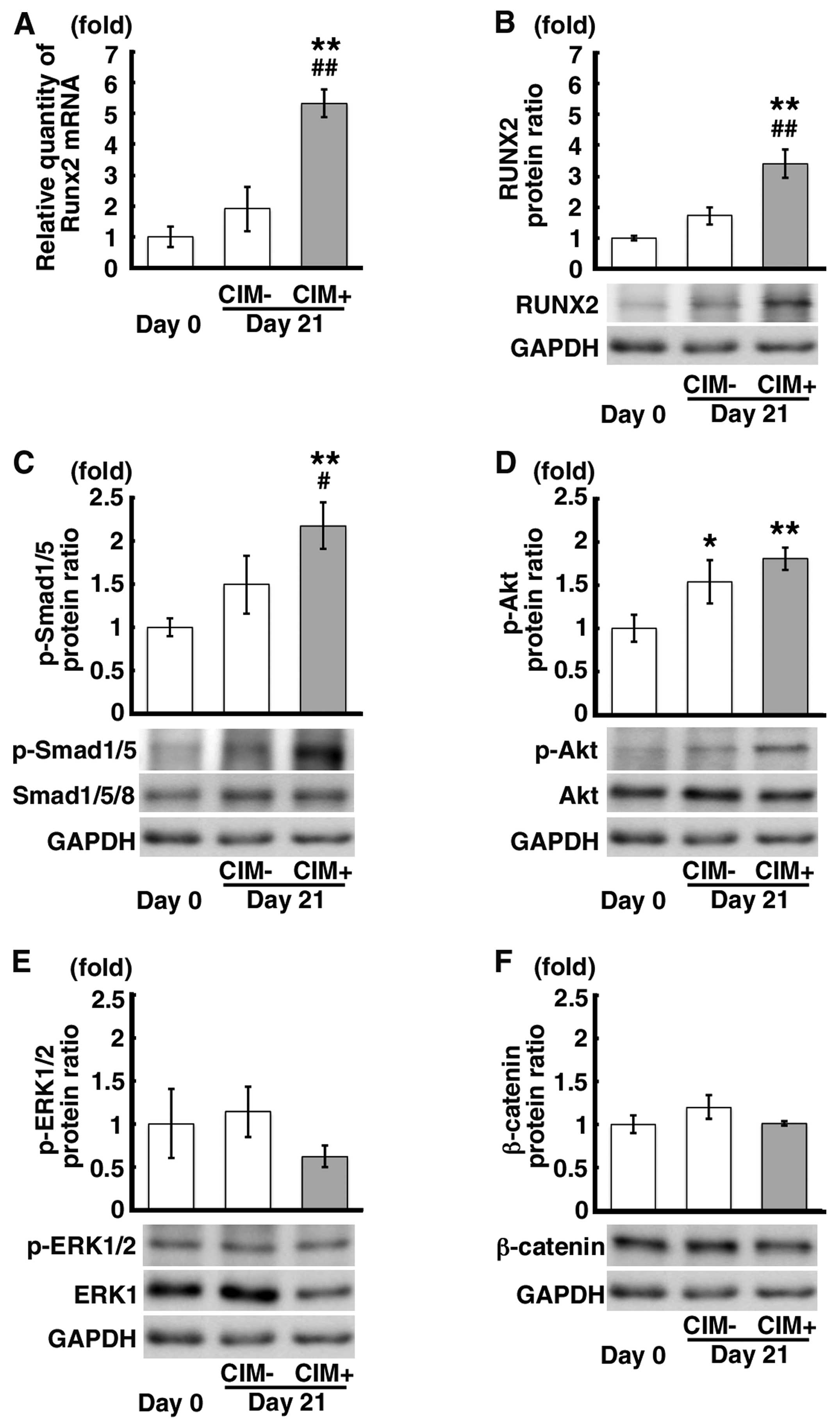
Signaling pathways upstream of Runx2
expression in the mDE6 cells cultured in CIM
In order to confirm which signaling pathway(s)
is/are involved in calcification as the upstream mediator of Runx2
expression, we examined the mRNA and protein expression levels of
Runx2 and the protein expression of p-Smad1/5, p-Akt, p-ERK1/2 and
β-catenin in the CIM+ cells in comparison to that observed in the
CIM-cells and the cells on day 0. The mRNA and protein expression
levels of Runx2 were significantly increased in the CIM+ cells in
comparison to those observed in the CIM- and the cells on day 0
(Fig. 2A and B). The p-Smad1/5
protein level was also increased in the CIM+ cells in comparison to
that observed in the CIM- cells and the cells on day 0 (Fig. 2C). Although the p-Akt protein
expression level was increased in the CIM+ cells compared to that
observed in the cells on day 0 (Fig.
2D), there were no significant differences in p-Akt protein
expression between the CIM+ cells and CIM- cells (Fig. 2D). Although there was a decrease
in the p-ERK1/2 protein expression in the CIM+ cells and no marked
changes were observed in β-catenin protein expression, there were
no significant differences observed in these levels between the
CIM+ cells and the controls (Fig. 2E
and F). These findings suggest that the Smad signaling pathway
is associated with RUNX2 expression in the mDE6 cells.
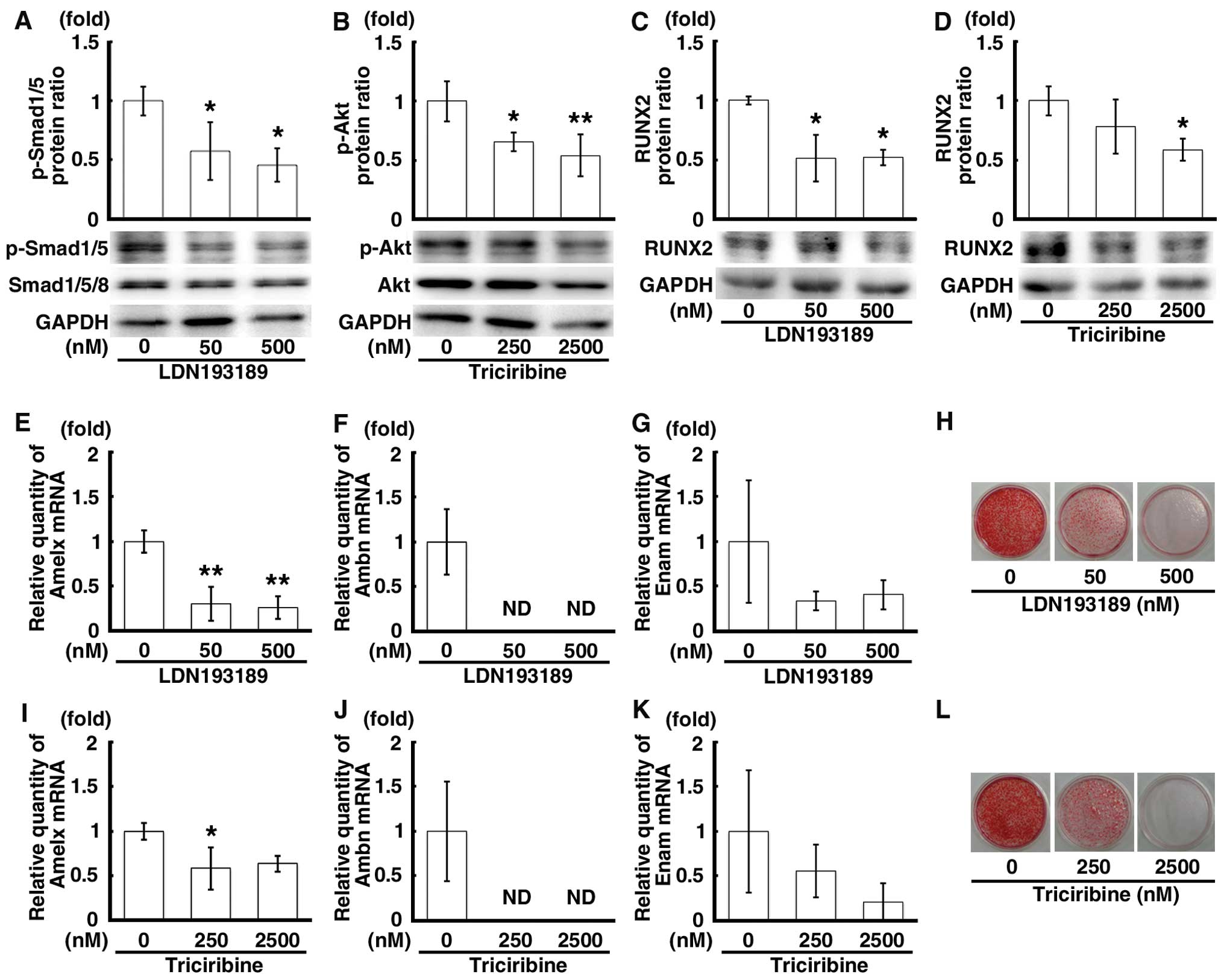
Inhibition of the Smad and PI3K-Akt
signaling pathways upstream of RUNX2 expression in mDE6 cells
By using 2 different inhibitors of the Smad
(LDN193189) and PI3K-Akt (triciribine) signaling pathways, we
examined the association between the Smad and PI3K-Akt signaling
pathways and the expression of RUNX2 in the mDE6 cells. These
agents prevent the phosphorylation of Smad1/5/8 and Akt,
respectively. Following treatment with the inhibitors for 48 h, the
protein expression levels of p-Smad1/5 and p-Akt were significantly
decreased in a concentration-dependent manner by LDN193189 or
triciribine (Fig. 3A and B). A
decrease in the protein expression level of RUNX2 was also observed
in the cells treated with LDN193189 or triciribine (Fig. 3C and D). The mRNA expression
levels of Amelx, Ambn and Enam were markedly decreased in the cells
treated with these inhibitors (Fig.
3E–G and I–K). Of note, the mRNA expression of Ambn was ‘not
detectable’ (Fig. 3F and J).
Moreover, when the mDE6 cells were cultured in CIM with LDN193189
or triciribine for 10 days, the formation of calcified material was
attenuated in a concentration-dependent manner in the treated cells
(Fig. 3H and L). Thus, the Smad
and PI3K-Akt pathways are necessary for the expression of
odontogenesis-related genes, including Runx2, as well as for the
calcification of the mDE6 cells.
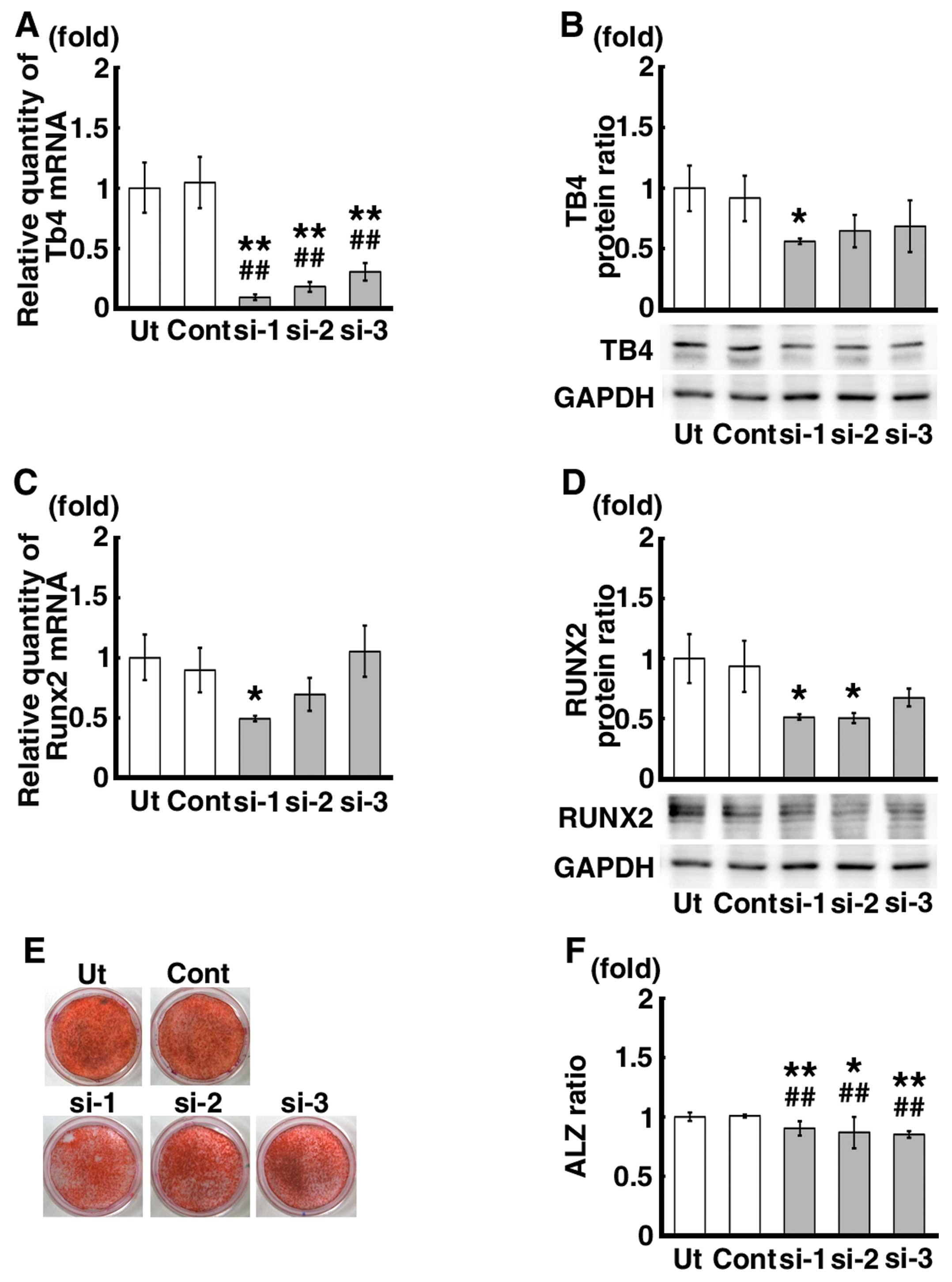
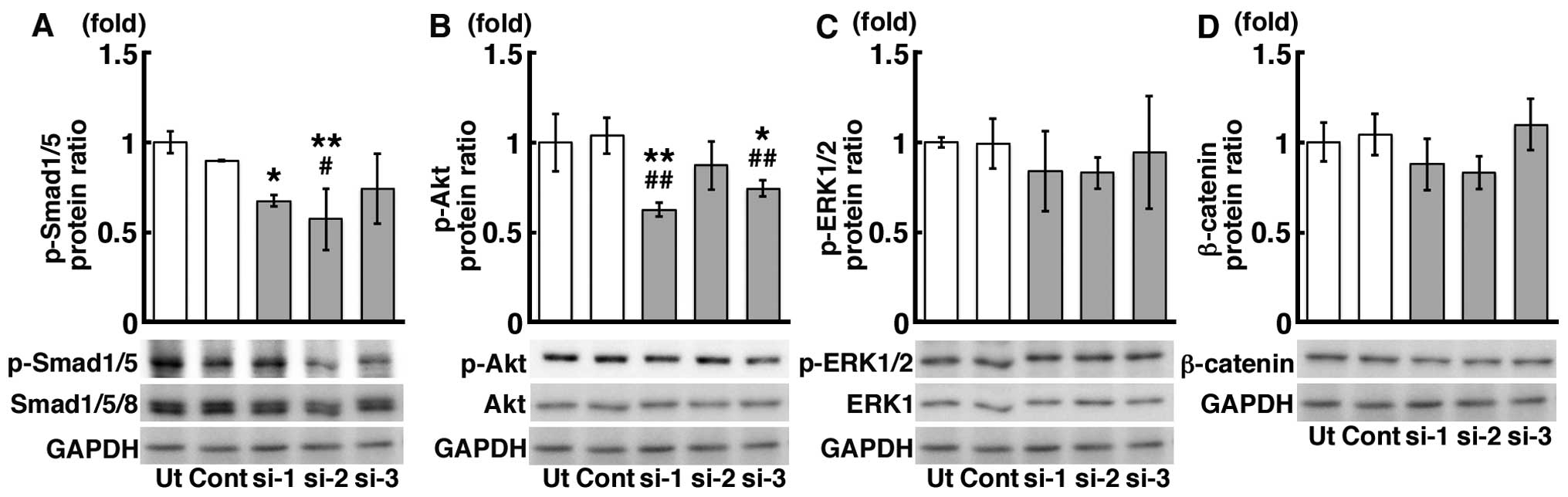
Effects of the siRNA-mediated Tb4
knockdown on RUNX2 expression and calcification of the mDE6
cells
Transfection of the cells with 3 different siRNAs
against Tb4 (siRNA-1, -2 and -3) significantly decreased the mRNA
expression levels of Tb4 by approximately 70–90% compared to those
in the untreated control mDE6 cells (Ut) and the mDE6 cells treated
with a universal negative control siRNA (Cont) (Fig. 4A). The protein expression level of
TB4 was also decreased by approximately 40–50% (Fig. 4B). The mRNA expression level of
Runx2, which has been suggested to be one of the downstream genes
of Tb4 (17,19), tended to decrease in the mDE6
cells treated with siRNA-1, and -2, not -3 (Fig. 4C). A significant decrease in the
Runx2 mRNA expression level was observed in the siRNA-1 treated
cells (Fig. 4C). The protein
expression level of RUNX2 was also significantly decreased in the
mDE6 cells treated with siRNA-1 and -2 (Fig. 4D), although no significant
differences in the RUNX2 protein expression level were noted
between the siRNA-3 treated cells and the controls (Fig. 4D). When the mDE6 cells cultured in
CIM were treated with siRNA-1, -2 or -3 for 10 days, the formation
of ALZ-positive calcification was slightly decreased (Fig. 4E). The ratio of the ALZ-positive
area to the total area (ALZ ratio) was significantly reduced in the
siRNA-treated cells (Fig.
4F).
Effects of siRNA against Tb4 on the
signaling pathways upstream of Runx2 expression in mDE6 cells
We analyzed the effects of siRNA against Tb4 on the
signaling pathways upstream of Runx2 expression in the mDE6 cells.
The protein expression levels of p-Smad1/5 and p-Akt were
significantly decreased in the Tb4-siRNA treated cells (Fig. 5A and B), although the degree of
decrease varied depending on the siRNA used (siRNA-1, -2 and -3).
No significant differences were observed in the expression levels
of p-ERK1/2 and β-catenin between the Tb4-siRNA treated cells and
the controls (Fig. 5C and D).
The data from our study indicate the putative
signaling pathways from Tb4 to Runx2 expression in the mDE6 cells.
The Smad and PI3K-Akt signaling pathways also appeared to play a
role in the Tb4-RUNX2 pathway in mDE6 cells (Fig. 6). However, little is known as to
whether upregulated Tb4 can induce the expression of
odontogenesis-related genes, such as Amelx, Ambn and Enam, without
the expression of Runx2, and of the association between Tb4 and
other signaling pathways upstream of Runx2 expression.
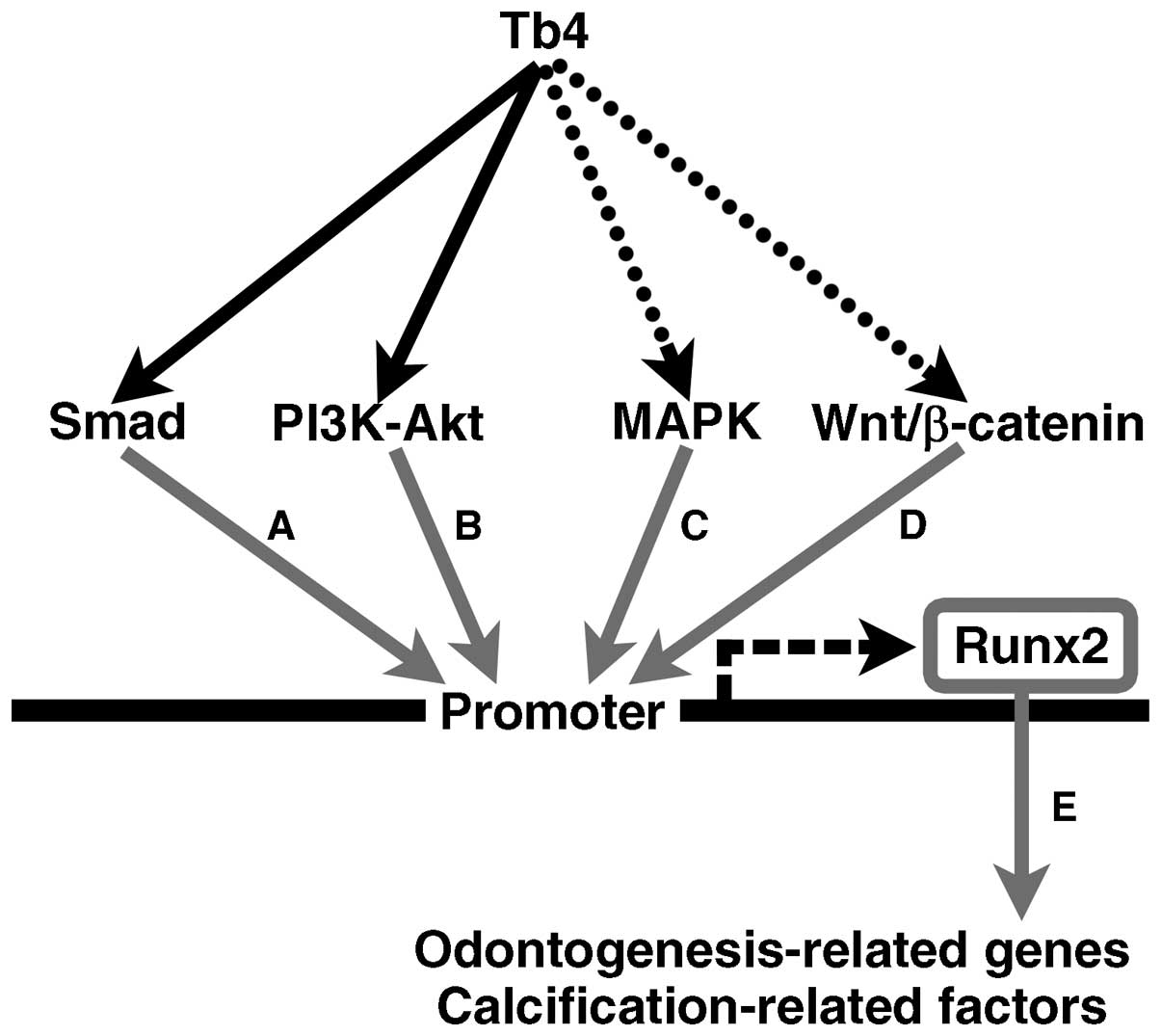 | Figure 6Schematic illustration of the
putative signaling pathways from thymosin beta 4 (Tb4) to
runt-related transcription factor 2 (RUNX2) in the mDE6 cells.
Several signaling pathways through Smad, PI3K-Akt, MAPK and/or
Wnt/β-catenin have been reported to be upstream of RUNX2
expression. The results of this study suggest that Smad and
PI3K-Akt may participate in Tb4-RUNX2 signaling pathway(s) in the
mDE6 cells. Tb4 may increase RUNX2 expression to induce the
expression of odontogenesis-related genes in the mDE6 cells. The
black arrows indicate putative Tb4-RUNX2 signaling pathways
revealed in this study, while the dotted arrows indicate possible
Tb4-RUNX2 signaling pathways that were not supported in this study.
The gray arrows indicate signaling pathways reported in previous
studies [(A) (34,42); (B) (34,43); (C) (34,44); (D) 34,45; (E) (9,17,19,29–31)]. RUNX2 can upregulate the
expression of downstream biological effectors, including
odontogenesis-related genes and calcification-related factors. |
Discussion
The tooth comprises hard matrices consisting of
enamel, dentin and cementum as the outer parts, and the dental pulp
soft tissue as the central part of the tooth. As a result of
sequential and reciprocal epithelial-mesenchymal interactions, the
dental epithelium differentiates into ameloblasts, which secrete
enamel matrix. In our previous studies, we suggested that Tb4 plays
an important role in the development of the tooth germ through
Runx2 expression. Tb4 appeared to be associated with the
differentiation of the dental epithelium (8,17,19). In the present study, we suggest
that Tb4 is associated with RUNX2 expression through the Smad and
PI3K-Akt signaling pathways, and with calcification through RUNX2
expression in mDE6 cells.
The present study, as well as our previous studies
suggested that Tb4 affects Runx2 expression in dental epithelial
cells and in the developing tooth germ (17,19). Although accumulating evidence
suggests that Runx2 is a key gene involved in tooth development,
and that it can lead to the expression of odontogenesis-related
genes, such as fibroblast growth factor 3 (Fgf3), Sonic hedgehog
(Shh), Amelx, Ambn, Enam, dentin matrix acidic phosphoprotein (Dmp)
and dentin sialophospho-protein (Dspp) (9,17,19,29–31), little has been reported on the
mechanism(s) underlying the signaling pathways from Tb4 to Runx2 in
dental epithelial cells. This prompted us to investigate this
signaling.
Tb4 is an actin-binding peptide known to regulate
the polymerization and depolymerization of actin (23), but does not contain the
DNA-binding, signal-sensing, or transactivation domains found in a
prototypical transcription factor (40). However, Tb4 appears to upregulate
a number of biological effectors, such as vascular endothelial
growth factor (VEGF), laminin-5 (23) and transforming growth factor
(TGF)β. Tb4 has been reported to translocate from the cytoplasm
into the nucleus (41).
Therefore, a transcription factor mediator activity for Tb4 has
been suggested. Thus, in this study, we examined which signaling
pathway(s) associated with RUNX2 expression is/are affected by Tb4
expression in dental epithelial cells.
In this study, we investigated the association
between Tb4 and Smad, Akt, ERK and/or β-catenin in dental
epithelial cells, as the signal pathways of these factors are
associated with Runx2 expression during bone differentiation
(42-45). In addition, the Smad, PI3K-Akt,
MAPK and Wnt/β-catenin signaling pathways are associated with tooth
development (21,36,46,47), but it is unclear as to whether
these pathways are mediated by Tb4 and affect Runx2 expression.
mDE6, a mouse dental epithelial cell line, was used in this study,
as we confirmed that mDE6 cells expressed some
odontogenesis-related genes and formed calcified material with
enamel formation following culture in CIM (Fig. 1). Following the induction of
calcification and treatment with inhibitors of the p-Smad1/5/8 and
p-Akt pathways, our results revealed that the p-Smad1/5 and p-Akt
signaling pathways were required for the induction of Runx2
expression and the expression of Amelx, Ambn and Enam. When either
of these pathways was inhibited, the calcification of the mDE6
cells was markedly suppressed (Fig.
3). These results are in accordance with those of the study by
Hu et al (42), which
indicated that the expression of Runx2 was significantly reduced by
LDN193189 (final concentration, 500 nM) in bone marrow stromal
cells. The activity of Smad1/5/8 is regulated by bone morphogenic
protein (BMP)-2 and -4, and affects tooth development (48). Takayama et al (49) also reported that enamel matrix
derivative stimulates Runx2 expression through the activation of
Smad1 in mouse myoblast cells. BMP-2 has previously been shown to
induce the expression of Amelx and Ambn in ameloblast-like cells
(50). The Smad signaling pathway
contributes to Runx2 and odontogenesis-related gene expression in
tooth development.
On the other hand, a study on the PI3K-Akt signaling
pathway reported that this pathway plays a role in the
differentiation and proliferation of odontogenic tumors (47). Although little is known about the
activity of the PI3K-Akt signaling pathway during tooth germ
differentiation, the present study revealed that the PI3K-Akt
signaling pathway plays an important role in the induction of the
expression of Runx2, Amelx, Ambn and Enam and in the calcification
of mDE6 cells. No apparent differences were observed following the
inhibition of the ERK and β-catenin pathways in our study (data not
shown). These results suggest that not only the Smad signaling
pathway, but also the PI3K-Akt signaling pathway, are important for
the induction of Runx2, Amelx, Ambn and Enam expression that occurs
during the calcification of mDE6 cells.
Moreover, the knockdown of Tb4 expression using
siRNA significantly reduced the Runx2 expression level and the
degree of calcification of the mDE6 cells (Fig. 4). The protein expression of
p-Smad1/5 and p-Akt was decreased in the mDE6 cells in which Tb4
was knocked down (Fig. 5).
Previous studies have reported that Akt is phosphorylated by Tb4
(51), and that BMP-2 and -4 are
also activated by Tb4 to induce Smad1/5/8 signaling (21). This suggests that Tb4 participates
in the calcium deposition through Runx2 expression during tooth
development, which is compatible with the findings of our previous
studies (17,19). However, despite the marked
decrease in Tb4 expression induced by siRNA against Tb4 in the mDE6
cells in the present study, the degree of restraint of Runx2
expression and calcification was minor (although significant)
compared to that observed in our previous study (19). This may have been caused by the
off-target effects of the methods used, the characteristics of the
specific cell line and so on. The mDE6 cells used in this study
were established from dental epithelial cells of mouse tooth germ,
and have some of the characteristics of ameloblasts. In our
previous study, non-odontogenic keratinocytes were transfected with
a Tb4 expression vector to enforce the expression of Tb4, which led
to the expression of some odontogenesis-related genes (19). Factors other than Tb4 may also be
associated with the Runx2 expression in the mDE6 cells. During the
induction of calcification, the CIM may directly upregulate Runx2
expression and calcification and/or may indirectly upregulate its
expression through interactions with other factors. The reason for
these differences is currently unknown. The interaction of other,
currently unknown, factors in mDE6 cells may affect Runx2
expression and calcification. However, the present study suggests
that Tb4 has the ability to upregulate the expression of downstream
biological effectors, including Amelx, Ambn and Enam in the mDE6
cells, as well as in the tooth germ (17) and non-odontogenic keratinocytes
(19).
In conclusion, the present study suggests that Smad
and PI3K-Akt signaling pathways play important roles in expression
of Runx2 induced by Tb4, which is an important factor involved in
the development of the tooth germ, in mDE6 cells (a mouse dental
epithelial cell line). These signaling pathways may be tightly
associated with the expression of odontogenesis-related genes, such
as Amelx, Ambn and Enam, and the formation of calcified material
through Runx2 expression. It is unknown whether the Smad pathway or
PI3K-Akt signaling pathway are the main pathways involved. Further
studies are required to identify the detailed signaling cascades
associated with tooth development. Although little is known of the
Tb4-mediated upregulation of odontogenesis-related gene expression
in the absence of Runx2 expression, and of the association between
Tb4 and the other signaling pathways upstream of Runx2 expression,
this study provides new information concerning the putative
signaling pathways from Tb4 to Runx2 expression in mDE6 cells,
which may be helpful in understanding the optimized regulation and
improving the success rate of tooth development and
regeneration.
Acknowledgments
The authors wish to acknowledge support by
Grants-in-Aid from the Ministry of Education, Culture, Sports,
Science and Technology of Japan, no. 20390466 (to Hidetaka Sakai),
no. 23659859 (to Tamotsu Kiyoshima) and no. 25861747 (to Hiroaki
Fujiwara).
References
|
1
|
Maas R and Bei M: The genetic control of
early tooth development. Crit Rev Oral Biol Med. 8:4–39. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thesleff I and Aberg T: Molecular
regulation of tooth development. Bone. 25:123–125. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jernvall J and Thesleff I: Reiterative
signaling and patterning during mammalian tooth morphogenesis. Mech
Dev. 92:19–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pispa J and Thesleff I: Mechanisms of
ectodermal organogenesis. Dev Biol. 262:195–205. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thesleff I: Epithelial-mesenchymal
signalling regulating tooth morphogenesis. J Cell Sci.
116:1647–1648. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamaza H, Matsuo K, Kiyoshima T, Shigemura
N, Kobayashi I, Wada H, Akamime A and Sakai H: Detection of
differentially expressed genes in the early developmental stage of
the mouse mandible. Int J Dev Biol. 45:675–680. 2001.PubMed/NCBI
|
|
7
|
Wada H, Kobayashi I, Yamaza H, Matsuo K,
Kiyoshima T, Akhtar M, Sakai T, Koyano K and Sakai H: In situ
expression of heat shock proteins, Hsc73, Hsj2 and Hsp86 in the
developing tooth germ of mouse lower first molar. Histochem J.
34:105–109. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Akhter M, Kobayashi I, Kiyoshima T, Matsuo
K, Yamaza H, Wada H, Honda JY, Ming X and Sakai H: Possible
functional involvement of thymosin beta 4 in developing tooth germ
of mouse lower first molar. Histochem Cell Biol. 124:207–213. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kobayashi I, Kiyoshima T, Wada H, Matsuo
K, Nonaka K, Honda JY, Koyano K and Sakai H: Type II/III
Runx2/Cbfa1 is required for tooth germ development. Bone.
38:836–844. 2006. View Article : Google Scholar
|
|
10
|
Xie M, Kobayashi I, Kiyoshima T, Yamaza H,
Honda JY, Takahashi K, Enoki N, Akamine A and Sakai H: Functional
implication of nucleolin in the mouse first molar development. J
Biol Chem. 282:23275–23283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Honda JY, Kobayashi I, Kiyoshima T, Yamaza
H, Xie M, Takahashi K, Enoki N, Nagata K, Nakashima A and Sakai H:
Glycolytic enzyme Pgk1 is strongly expressed in the developing
tooth germ of the mouse lower first molar. Histol Histopathol.
23:423–432. 2008.PubMed/NCBI
|
|
12
|
Xie M, Kobayashi I, Kiyoshima T, Nagata K,
Ookuma Y, Fujiwara H and Sakai H: In situ expression of ribosomal
protein L21 in developing tooth germ of the mouse lower first
molar. J Mol Histol. 40:361–367. 2009. View Article : Google Scholar
|
|
13
|
Akhter M, Kobayashi I, Kiyoshima T, Nagata
K, Wada H, Ookuma Y, Fujiwara H, Honda JY and Sakai H: In situ
expression of 15 kDa interferon alpha responsive gene in the
developing tooth germ of the mouse lower first molar. J Mol Histol.
41:185–191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takahashi KF, Kiyoshima T, Kobayashi I,
Xie M, Yamaza H, Fujiwara H, Ookuma Y, Nagata K, Wada H, Sakai T,
et al: Protogenin, a new member of the immunoglobulin superfamily,
is implicated in the development of the mouse lower first molar.
BMC Dev Biol. 10:115–130. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Honda JY, Kobayashi I, Kiyoshima T, Nagata
K, Wada H, Ookuma Y, Fujiwara H, Shiotsuka M, Takahashi I and Sakai
H: In situ expression of the mitochondrial ATPase6 gene in the
developing tooth germ of the mouse lower first molar. J Mol Histol.
42:83–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shiotsuka M, Wada H, Kiyoshima T, Nagata
K, Fujiwara H, Kihara M, Hasegawa K, Someya H, Takahashi I and
Sakai H: The expression and function of thymosin beta 10 in tooth
germ development. Int J Dev Biol. 57:873–883. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ookuma YF, Kiyoshima T, Kobayashi I,
Nagata K, Wada H, Fujiwara H, Yamaza H, Nonaka K and Sakai H:
Multiple functional involvement of thymosin beta-4 in tooth germ
development. Histochem Cell Biol. 139:355–370. 2013. View Article : Google Scholar
|
|
18
|
Kihara M, Kiyoshima T, Nagata K, Wada H,
Fujiwara H, Hasegawa K, Someya H, Takahashi I and Sakai H: Itm2a
expression in the developing mouse first lower molar, and the
subcellular localization of Itm2a in mouse dental epithelial cells.
PLoS One. 9:e1039282014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kiyoshima T, Fujiwara H, Nagata K, Wada H,
Ookuma YF, Shiotsuka M, Kihara M, Hasegawa K, Someya H and Sakai H:
Induction of dental epithelial cell differentiation marker gene
expression in non-odontogenic human keratinocytes by transfection
with thymosin beta 4. Stem Cell Res (Amst). 12:309–322. 2014.
View Article : Google Scholar
|
|
20
|
Cha HJ, Philp D, Lee SH, Moon HS, Kleinman
HK and Nakamura T: Over-expression of thymosin beta 4 promotes
abnormal tooth development and stimulation of hair growth. Int J
Dev Biol. 54:135–140. 2010. View Article : Google Scholar
|
|
21
|
Lee SI, Kim DS, Lee HJ, Cha HJ and Kim EC:
The role of thymosin beta 4 on odontogenic differentiation in human
dental pulp cells. PLoS One. 8:e619602013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hannappel E and Huff T: The thymosins.
Prothymosin alpha, parathymosin, and beta-thymosins: Structure and
function. Vitam Horm. 66:257–296. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Safer D, Elzinga M and Nachmias VT:
Thymosin beta 4 and Fx, an actin-sequestering peptide, are
indistinguishable. J Biol Chem. 266:4029–4032. 1991.PubMed/NCBI
|
|
24
|
Smart N, Bollini S, Dubé KN, Vieira JM,
Zhou B, Davidson S, Yellon D, Riegler J, Price AN, Lythgoe MF, et
al: De novo cardiomyocytes from within the activated adult heart
after injury. Nature. 474:640–644. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiao ZS, Hjelmeland AB and Quarles LD:
Selective deficiency of the ‘bone-related’ Runx2-II unexpectedly
preserves osteoblast-mediated skeletogenesis. J Biol Chem.
279:20307–20313. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stock M and Otto F: Control of RUNX2
isoform expression: The role of promoters and enhancers. J Cell
Biochem. 95:506–517. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Camilleri S and McDonald F: Runx2 and
dental development. Eur J Oral Sci. 114:361–373. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen S, Gluhak-Heinrich J, Wang YH, Wu YM,
Chuang HH, Chen L, Yuan GH, Dong J, Gay I and MacDougall M: Runx2,
osx, and dspp in tooth development. J Dent Res. 88:904–909. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aberg T, Wang XP, Kim JH, Yamashiro T, Bei
M, Rice R, Ryoo HM and Thesleff I: Runx2 mediates FGF signaling
from epithelium to mesenchyme during tooth morphogenesis. Dev Biol.
270:76–93. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dhamija S and Krebsbach PH: Role of Cbfa1
in ameloblastin gene transcription. J Biol Chem. 276:35159–35164.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee HK, Lee DS, Ryoo HM, Park JT, Park SJ,
Bae HS, Cho MI and Park JC: The odontogenic ameloblast-associated
protein (ODAM) cooperates with RUNX2 and modulates enamel
mineralization via regulation of MMP-20. J Cell Biochem.
111:755–767. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
D'Souza RN, Aberg T, Gaikwad J, Cavender
A, Owen M, Karsenty G and Thesleff I: Cbfa1 is required for
epithelial-mesenchymal interactions regulating tooth development in
mice. Development. 126:2911–2920. 1999.PubMed/NCBI
|
|
33
|
Ducy P, Zhang R, Geoffroy V, Ridall AL and
Karsenty G: Osf2/Cbfa1: A transcriptional activator of osteoblast
differentiation. Cell. 89:747–754. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Martin JW, Zielenska M, Stein GS, van
Wijnen AJ and Squire JA: The Role of RUNX2 in Osteosarcoma
Oncogenesis. Sarcoma. 2011:2827452011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim EJ, Cho SW, Shin JO, Lee MJ, Kim KS
and Jung HS: Ihh and Runx2/Runx3 signaling interact to coordinate
early chondrogenesis: A mouse model. PLoS One. 8:e552962013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu F, Chu EY, Watt B, Zhang Y, Gallant
NM, Andl T, Yang SH, Lu MM, Piccolo S, Schmidt-Ullrich R, et al:
Wnt/beta-catenin signaling directs multiple stages of tooth
morphogenesis. Dev Biol. 313:210–224. 2008. View Article : Google Scholar
|
|
37
|
Hardcastle Z, Mo R, Hui CC and Sharpe PT:
The Shh signalling pathway in tooth development: Defects in Gli2
and Gli3 mutants. Development. 125:2803–2811. 1998.PubMed/NCBI
|
|
38
|
Zhang Y, Feurino LW, Zhai Q, Wang H,
Fisher WE, Chen C, Yao Q and Li M: Thymosin Beta 4 is overexpressed
in human pancreatic cancer cells and stimulates proinflammatory
cytokine secretion and JNK activation. Cancer Biol Ther. 7:419–423.
2008. View Article : Google Scholar
|
|
39
|
Oh SY, Song JH, Gil JE, Kim JH, Yeom YI
and Moon EY: ERK activation by thymosin-beta-4 (TB4) overexpression
induces paclitaxel-resistance. Exp Cell Res. 312:1651–1657. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sosne G, Qiu P, Goldstein AL and Wheater
M: Biological activities of thymosin beta4 defined by active sites
in short peptide sequences. FASEB J. 24:2144–2151. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huff T, Rosorius O, Otto AM, Müller CS,
Ballweber E, Hannappel E and Mannherz HG: Nuclear localisation of
the G-actin sequestering peptide thymosin beta4. J Cell Sci.
117:5333–5341. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hu Y, Du Y, Jiang H and Jiang GS: Cerium
promotes bone marrow stromal cells migration and osteogenic
differentiation via Smad1/5/8 signaling pathway. Int J Clin Exp
Pathol. 7:5369–5378. 2014.PubMed/NCBI
|
|
43
|
Ma P, Gu B, Xiong W, Tan B, Geng W, Li J
and Liu H: Glimepiride promotes osteogenic differentiation in rat
osteoblasts via the PI3K/Akt/eNOS pathway in a high glucose
microenvironment. PLoS One. 9:e1122432014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hou X, Shen Y, Zhang C, Zhang L, Qin Y, Yu
Y, Wang L and Sun X: A specific oligodeoxynucleotide promotes the
differentiation of osteoblasts via ERK and p38 MAPK pathways. Int J
Mol Sci. 13:7902–7914. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Baron R and Rawadi G: Targeting the
Wnt/beta-catenin pathway to regulate bone formation in the adult
skeleton. Endocrinology. 148:2635–2643. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cho KW, Cai J, Kim HY, Hosoya A, Ohshima
H, Choi KY and Jung HS: ERK activation is involved in tooth
development via FGF10 signaling. J Exp Zoolog B Mol Dev Evol.
312:901–911. 2009. View Article : Google Scholar
|
|
47
|
Kumamoto H and Ooya K: Immunohistochemical
detection of phosphorylated Akt, PI3K, and PTEN in ameloblastic
tumors. Oral Dis. 13:461–467. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang G, Yuan G, Ye W, Cho KW and Chen Y:
An atypical canonical bone morphogenetic protein (BMP) signaling
pathway regulates Msh homeobox 1 (Msx1) expression during
odonto-genesis. J Biol Chem. 289:31492–31502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Takayama T, Suzuki N, Narukawa M, Tokunaga
T, Otsuka K and Ito K: Enamel matrix derivative stimulates core
binding factor alpha1/Runt-related transcription factor-2
expression via activation of Smad1 in C2C12 cells. J Periodontol.
76:244–249. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xu L, Takahashi R, Harada H and Taniguchi
A: Effect of BMP-2 on gene expression of enamel matrix proteins at
the dental epithelial cell line. Open Biotechnol J. 1:18–20. 2007.
View Article : Google Scholar
|
|
51
|
Bock-Marquette I, Saxena A, White MD,
Dimaio JM and Srivastava D: Thymosin beta4 activates
integrin-linked kinase and promotes cardiac cell migration,
survival and cardiac repair. Nature. 432:466–472. 2004. View Article : Google Scholar : PubMed/NCBI
|