Introduction
Cardiovascular diseases, in particular coronary
artery disease (CAD), remain leading causes of mortality in
developed countries (1). Coronary
chronic total occlusion (CTO) affects up to 18.4% of all CAD
patients and significantly influences cardiac function and outcomes
of treatments, such as angioplasty and surgical bypass (2). Coronary collateral circulation (CCC)
provides a natural bypass that supplies heart, potentially with
therapeutic potential to improve myocardial viability in CAD
patients (1,3). Survivin, a unique member of the
inhibitor of apoptosis proteins (IAPs) family that exhibits cell
cycle-regulated expression peaking at mitosis, has been
demonstrated to impact cardiomyocyte replication and apoptosis,
providing a potentially promising myocardial regeneration target
(4). The role of survivin in
peripheral blood mononuclear cells (PBMCs), cells whose gene
expression profiles have been linked to CAD severity (5), remains unknown.
The role of survivin in angiogenesis is well
documented, particularly in tumor cells (6). It has been demonstrated that
survivin is minimally expressed in non-proliferating endothelial
cells, but upregulated in newly formed blood vessels (7). Survivin, however, also plays a role
in collateral vessel formation, as demonstrated by increasing
microvessel density in survivin-positive tumors (8). In CAD patients, survivin influences
cardiac function by controlling total cardiomyocyte numbers through
its impact of collateral vessel formation and angiogenesis
(4). Furthermore, Zwerts et
al (9) reported that
regulation of endothelial cell survival and maintenance of vascular
integrity by survivin are crucial for normal embryonic
angiogenesis, cardiogenesis and neurogenesis, demonstrating the
importance of survivin in vascularization and
revascularization.
In CTO patients, the role of CCC has been widely
disputed; however, modern study has generally indicated that
well-developed CCC is indicative of severe stenosis (10). When cardiac events occur, such as
acute myocardial infarction, the presence of a well-developed CCC
can mediate the detrimental effects of ischemia on heart tissues,
thus preserving left ventricular function, reducing overall infarct
size, preventing left ventricular aneurysm and increasing survival
(10). Notably, collateral blood
flow is often reduced after successful CTO recanalization, as
antegrade blood flow is re-established and resistance is increased
in collateral vessels (10).
Thus, collateral vessel formation may be observed as a marker of
stenosis and prognosis in CAD patients.
Altered survivin expression may impact collateral
vessel formation, as indicated by Conway et al (11) who showed that survivin was
uniquely expressed by microvessels in the peri-infarct and infarct
regions 2 days after permanent artery occlusion. Furthermore, using
a mouse model with heterozygous deficiency of middle cerebral of
the survivin gene (survivin+/‒ mice), no alterations in
infarct size were apparent (11).
As the microRNA signature of PBMCs, including survivin, has been
linked to CAD (5), it is likely
that these cells also play a role in collateral formation.
Furthermore, rising levels of vascular endothelial growth factor
(VEGF), an angiogenic and vasoprotective molecule modulated
primarily by inflammatory mediators, may also impact collateral
formation in CAD patients, and intercellular adhesion molecule-1
(ICAM-1) may impact collateral formation and CAD onset (12,13), although the relationship between
these molecules and survivin in PBMCs is unknown. Assessment of
survivin levels as well as other molecules in PBMCs may thus be
linked with collateral formation.
While the role of survivin in angiogenesis is well
documented, much less is known about the distinct role survivin
plays in collateral formation during coronary CTO. The present
study examined the clinical relationship between PBMC survivin
expression and coronary collateral formation in humans and the PBMC
signatures associated with collateral formation. Correlations of
survivin, VEGF and ICAM-1 expression were also examined in
peripheral blood samples from human patients, and these
correlations were confirmed in a rat model of hind limb ischemia.
These experiments provided a basis for assessment of collateral
formation based on PBMC survivin levels, potentially useful in
revascularization therapies for CTO and CAD.
Materials and methods
Study design
A total of 46 coronary CTO patients (mean age
60.1±8.5, male 54.3%) (CTO group) and 18 patients with normal
coronary artery vascularity (mean age 58.0±10.0, male 55.6%)
(control group) were included in a prospective study between June
2006 and February 2007 at the Department of Cardiology of the the
First Affiliated Hospital of Shantou University Medical College
(China). In addition, an animal model was established using 36 male
Sprague-Dawley rats aged 4–5 months and weighing 250–300 g. The
rats were randomly divided into four equal groups of 9 rats each,
including the normal control, sham operation, operation and
granulocyte macrophage colony-stimulating factor (GM-CSF) treatment
groups. The study protocol was approved by the Ethics Committee of
Shantou University Medical College. Procedures involving
experimental animals were performed in accordance with protocols
approved by Institutional Guidelines for Care and Use of Laboratory
Animals of Shantou University Medical College, in compliance with
the Law of the People’s Republic of China on the Protection of
Wildlife (2004). The patients provided written informed consent for
participation.
Patients
Included patients in the coronary CTO group (n=46;
mean age 60.1±8.5, male 54.3%) exhibited i) angiographic studies
showing coronary CTO occlusion (>99% occluded) for >2 weeks
in any 3 major coronary artery branches, consistent with the
diagnostic guidelines of Teeuwen et al (14); ii) measurable collateral flow
according to Rentrop classification prior to reopening of the
artery, as previously described (15). Patients that presented with other
serious illness that potentially affected testing, such as acute
myocardial infarction, cancer, pulmonary heart disease and severe
renal insufficiency were excluded. Control subjects experienced
chest pain and were suspected to have coronary heart disease,
although normal coronary artery flow was confirmed by angiography
for the patients included in the control group. The study protocol
was approved by the First Affiliated Hospital of Shantou University
Medical College, Shantou, and all the participants provided written
informed consent.
Sample collection
Angiography was performed using the Judkins
technique via the femoral approach with 6F guiding catheters as
previously described (16). The
patients received a bolus of heparin (50 IU/kg), aspirin (100
mg/day), and clopidogrel (75 mg/day) for ≥3 day when percutaneous
coronary intervention (PCI) was performed. Approximately 10 ml of
peripheral blood was drawn with moderate suction in a 10 sec period
using the femoral approach, and angioplasty was continued.
Angiographic grading
Angiograms were assessed independently by two
blinded investigators, and in case of disagreement, a consensus was
obtained (through consultation). According to the Rentrop
classification (15), the
angiographically visible diameter of collateral connections was
graded as: C0, no opacification; C1, filling of artery side
branches by collateral vessels without visualization of the
epicardial segments; C2, partial filling of epicardial segments by
collateral vessels; and C3, complete filling of epicardial segments
by collateral vessels. Rentrop C1 was regarded as poor collateral
circulation, and Rentrop C2 and C3 were regarded as good collateral
circulation.
PBMC isolation
PBMCs were separated by standard density gradient
centrifugation using the Ficoll-Paque method (Biochrom, Berlin,
Germany), according to the manufacturer’s instructions. PBMCs
(3×106 cells/ml) were then cultured in buffered
RPMI-1640 supplemented with 10% (v/v) heat-inactivated fetal bovine
serum (both from Sigma, St. Louis, MO, USA).
Flow cytometry
PBMCs were washed once with phosphate-buffered
saline (PBS) solution, fixed in 4% paraformaldehyde at room
temperature for 40 min, and incubated in 0.2% Triton X-100 (Sangon
Corp., Shanghai, China) for 10 min and 5% calf serum at 4°C for 10
min. PBMCs were then incubated in PBS containing survivin (1:1,000;
Novus Biologicals, Inc., Littleton, CO, USA), CD4 (1:100), CD8
(1:100), CD44 (1:100) (all from Boster Biotechnology Co., Wuhan,
China), VEGF (1:200), and ICAM-1 (1:200; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) antibody overnight at 4°C. After washing,
the PBMCs were incubated in appropriate fluorescein isothiocyanate
(FITC) (survivin) and/or Cy3 (CD4, CD8, CD44, VEGF and
ICAM-1)-conjugated secondary antibodies. Flow cytometric analysis
was conducted using a Coulter Epics XL flow cytometer (Beckman
Coulter, Brea, CA, USA), and the percentage of positive cells was
calculated.
Immunocytochemistry
Survivin expression in PBMCs was detected by
immunocytochemical staining using an Ultravision Detection System
(Lab Vision Corporation, Newmarket Suffolk, UK), as previously
described (17). Briefly, after
blocking endogenous peroxidase activity, PBMCs were incubated for 1
h at room temperature with rabbit polyclonal antibody to survivin
(1:1,000; Novus Biologicals). After washing, the cells were
incubated for 1 h at room temperature with biotinylated goat
anti-rabbit IgG (1:10,000; Santa Cruz Biotechnology, Inc., Brea,
CA, USA). Nuclei were counter-stained with hematoxylin. Three
replicates were performed for each sample.
RT-PCR detection of survivin mRNA
expression in PBMCs
Total RNA was isolated from PBMCs using TRIzol
reagent (British Biocell International, Cardiff, UK). RNA purity
was determined using absorbance at 260 and 280 nm (A260/280), and
RNA integrity was verified by 1.5% agarose gel electrophoresis
(Invitrogen, Grand Island, NY, USA). Total RNA was
reverse-transcribed into cDNA using RevertAid 7MM-MuLV
Reverse Transcriptase and oligo(dT) 18 primer (both from Thermo
Scientific, Waltham, MA, USA). Approximately 436 bp of the human
survivin region and 612 bp of the β-actin region were generated by
RT-PCR using total RNA (2 μg). Primer sequences for survivin
were: forward, 5′-ATG GGT GCC CCG ACG TTG-3′ and reverse, 5′-AGA
GGC CTC AAT CCA TGG-3′. Primer sequences for β-actin were: forward,
5′-CGC TGC GCT GGT CGT CGA CA-3′ and reverse, 5′-GTC ACG CAC GAT
TTC CCG CT-3′. Reactions involved initial denaturation at 95°C for
3 min followed by 35 cycles at 94°C for 1 min, annealing at 61°C
for 1 min, and extension at 72°C for 1 min and an additional cycle
at 72°C for 10 min. β-actin was used as an internal control. RT-PCR
products were subjected to 1.5% agarose gel electrophoresis, and
the abundance of each mRNA was normalized to β-actin using Image
Lab (Bio-Rad, Hercules, CA, USA). Relative mRNA expression was
considered to be the optical density (OD) of survivin by the OD of
β-actin (ODsurvivin/ODβ-actin).
Western blot analysis
Western blot analysis was performed as previously
described (17). Total protein
was isolated from PBMCs using TRIzol Reagent (British Biocell
International). Proteins were quantified by a Bradford assay. Total
protein (50 μg) was separated on 12% sodium dodecyl
sulfate-polyacrylamide gel (SDS-PAGE) gels, and electro-transferred
to NC membranes (Millipore, Bedford, MA, USA). After non-specific
binding sites were blocked with 5% skim milk for 1 h, the membranes
were then incubated in a mixture of Tris-buffered saline and
Tween-20 (TBS-T) containing survivin (1:1,000), CD4 (1:100), CD8
(1:100), CD44 (1:100), VEGF (1:200) and ICAM-1 (1:200) antibodies
for 2 h at room temperature. After washing, the membrane was
incubated in TBS-T containing the appropriate secondary anti-IgG
antibodies (1:5,000; Santa Cruz Biotechnology, Inc.) at room
temperature for 1 h. The target protein was detected using western
blotting luminol reagent (Santa Cruz Biotechnology, Inc.),
according to the manufacturer’s instructions. Results were
visualized by autoradiography using X-Omat autoradiography film
(Kodak, Rochester, NY, USA). For protein loading normalization, a
similar procedure was performed using a monoclonal antibody against
tubulin (1:1,000; Beyotime Institute of Biotechnology, Hangzhou,
China) as an internal standard. Three replicates were performed for
each sample.
Rat model of hind limb ischemia
Using four groups of 9 rats each (normal control,
sham operation, operation and GM-CSF treatment groups) a rat model
of hind limb ischemia was constructed, as previously described
(18). For operation and GM-CSF
treatment groups, the right femoral artery of each rat was ligated
directly distal to the inguinal ligament to achieve hind limb
ischemia on the right side. Sham operations were performed
similarly, but without femoral artery ligation.
The day after surgery, the mice in the treatment
group were administered rHuGM-CSF (10 μg/kg/day; Xiamen
Amoytop Biotech Co., Xiamen, China) in 0.9% normal saline solution
by subcutaneous daily for 5 days. The control, sham operation, and
operation groups were administered the same volume of 0.9% normal
saline solution. At day 28 after the surgery, the rats were
sacrificed by overdose of injected anesthetic drugs (phenobarbital
sodium), blood samples were taken by cardiac puncture from each
specimen, and postmortem angiography was performed. PBMCs were
isolated from blood samples, and the number of cells positive for
survivin, CD4, CD8, CD44, VEGF and ICAM-1 were detected by flow
cytometry.
Postmortem angiography
Postmortem angiography was performed as previously
described (19). Briefly,
peripheral vascular tissues were fully expanded to better reveal
collateral vessels and then perfused through the descending aorta
with PBS containing adenosine (1 g/l) (Bio Basic Inc., Amherst, NY,
USA) as a vasodilator for 3 min at physiological pressure.
Vasculature was fixed with 2% paraformaldehyde in PBS for 5 min,
flushed with PBS for 2 min, and infused with 76% meglumine
diatrizoate and 10% gelatin (1:1) in PBS as a contrast reagent. The
contrast reagent was solidified by immersing whole specimens in ice
for 5 min. Angiograms were taken at two different angles, resulting
in stereoscopic images that were used in 3-dimensional (3D)
collateral growth analysis.
Statistical analysis
Data were analyzed using SPSS version 13.0 for
Windows (SPSS Inc., Chicago, IL, USA). Data were presented as the
means ± standard deviations (SD). Differences between groups were
analyzed by one-way ANOVA and Student-Newman-Keuls (SNK)
Games-Howell tests. P<0.05 was considered statistically
significant (P<0.05).
Results
Baseline demographic and clinical
characteristics of experimental and control patients
Of the total 46 patients, no collateral flow was
observed in 12 patients (C0), poor collateral circulation was
observed in 18 patients (C1), and good collateral circulation was
observed in 16 patients (11 C2 patients and 5 C3 patients). No
significant differences in baseline demographic or clinical
characteristics, including age, gender, cardiovascular risk
factors, median duration of occlusion were observed among the
coronary CTO and control group patients (P>0.05) (Table I). Notably, all the coronary CTO
patients were currently using vasoactive and lipid-lowering drugs,
although none of the patients in the control group were on similar
medication. Heparin was administered immediately prior to
angiography in the coronary CTO and control patients.
 | Table IBaseline clinical and demographic
characteristics for CTO patients and normal controls. |
Table I
Baseline clinical and demographic
characteristics for CTO patients and normal controls.
| Variables | Normal
control
(n=18) | Rentrop
classificationa
| Total patients
|
|---|
C0
(n=12) | C1
(n=18) | C2 + C3
(n=16) | (C0 – C3)
(n=46) |
|---|
| Age (year) | 58.0±10.0 | 58.8±8.6 | 61.5±9.4 | 64.1±8.5 | 60.1±8.5 |
| Gender (M:F) | 10/8 | 7/5 | 9/9 | 9/7 | 25/21 |
| LAD | 0 | 54.3±38.2 | 84.6±19.5 | 88.9±23.5 | 76.8±28.4 |
| LCX | 0 | 24.6±36.4 | 48.6±36.1 | 52.6±40.1 | 46.2±38.1 |
| RCA | 0 | 22.2±32.4 | 43.6±34.7 | 45.9±45.9 | 40.5±38.3 |
| TC (mmol/l) | 4.96±0.11 | 4.77±0.72 | 4.65±0.76 | 4.96±0.71 | 4.80±0.78 |
| TG (mmol/l) | 1.57±0.10 | 1.74±0.91 | 1.89±0.81 | 2.06±0.71 | 1.92±0.86 |
| LDL (mmol/l) | 2.86±0.12 | 2.50±0.60 | 2.26±0.64 | 2.36±0.55 | 2.38±0.72 |
| HDL (mmol/l) | 1.39±0.10 | 1.45±0.19 | 1.40±0.22 | 1.43±0.27 | 1.43±0.33 |
Survivin expression in human PBMCs during
coronary collateral formation
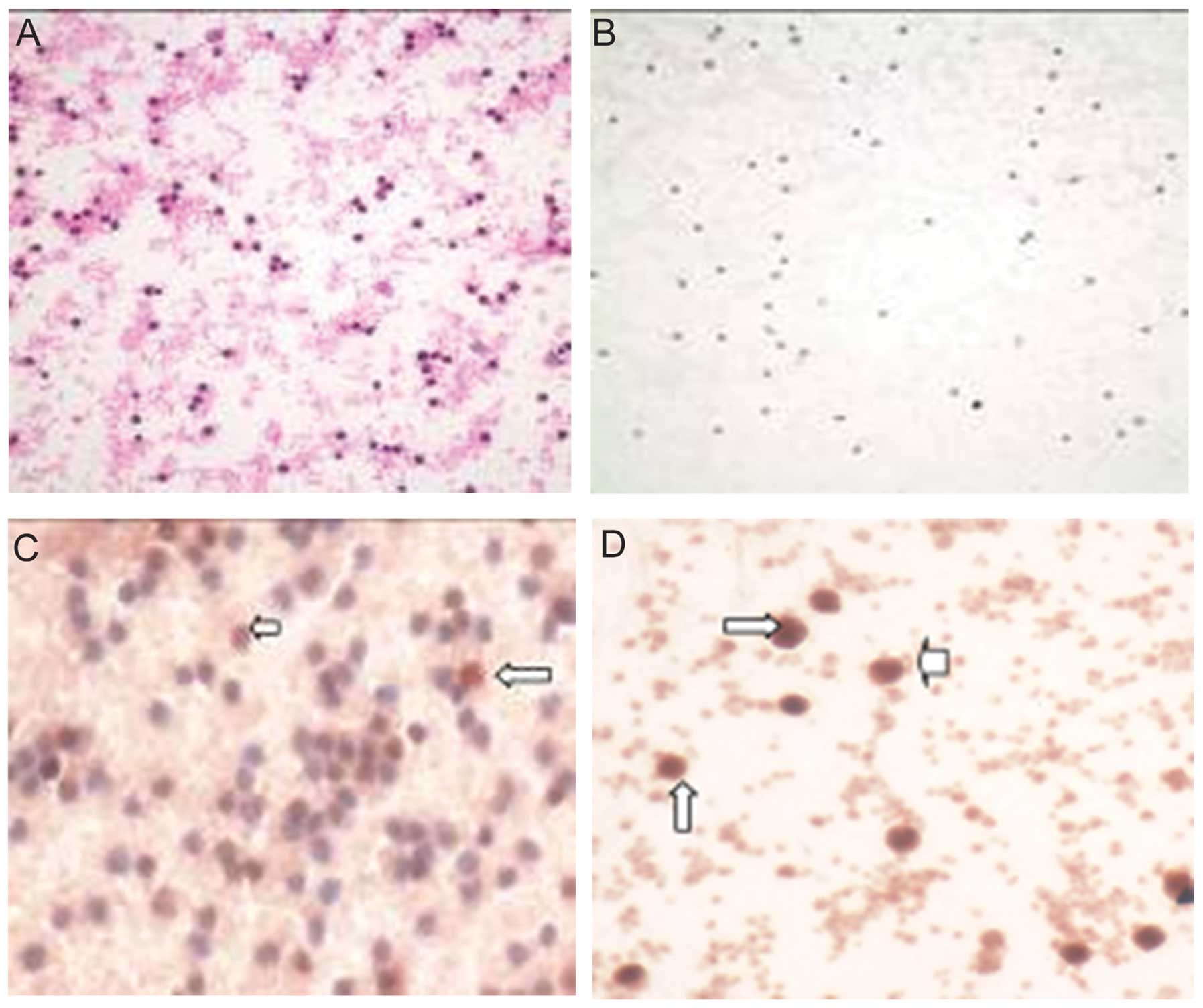
Immunocytochemical staining indicated that
survivin-positive PBMCs were significantly upregulated in patients
with good collateral formation, including plasma and nuclear
regions (Fig. 1). Similarly,
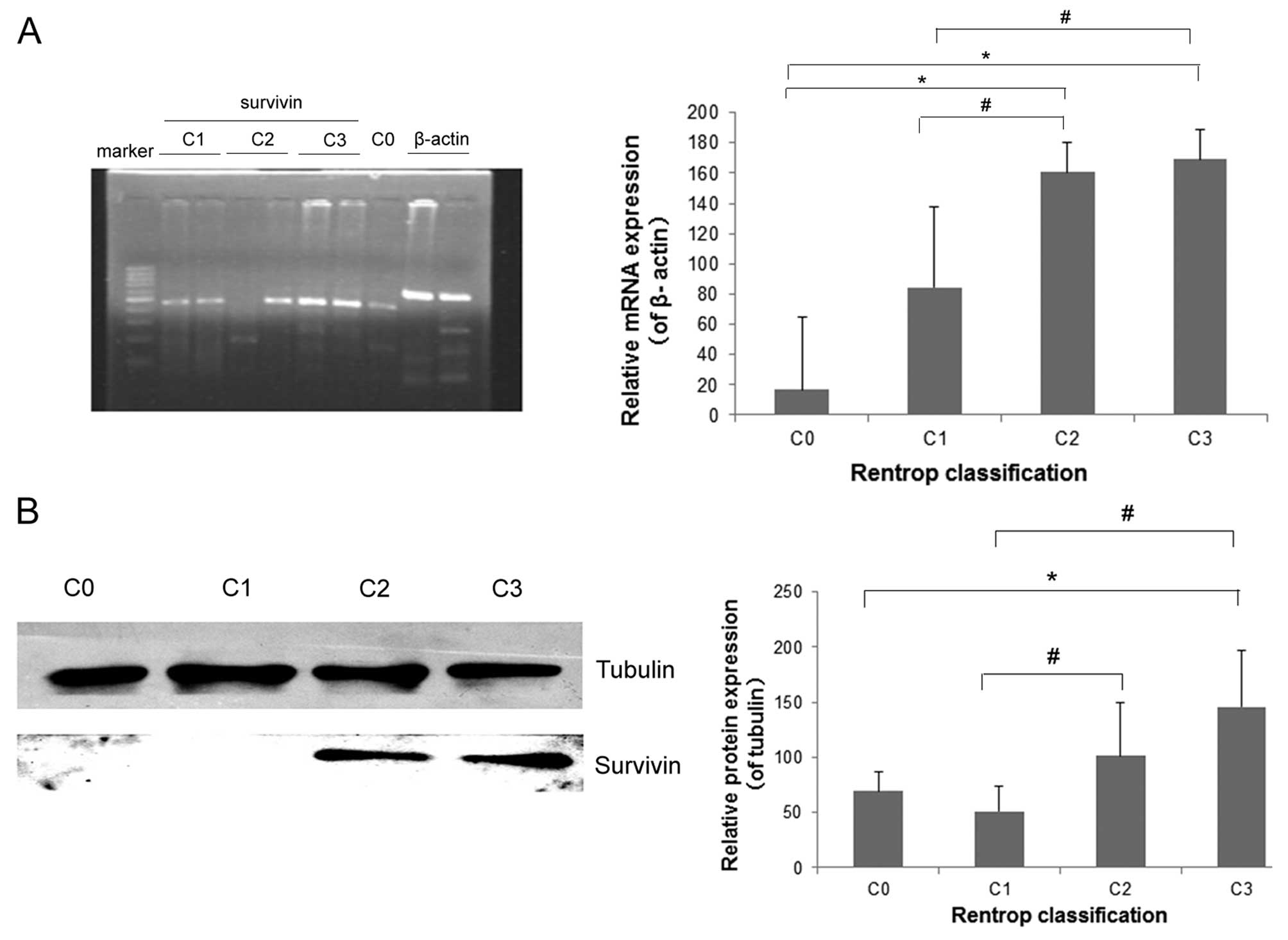
RT-PCR and western blotting indicated that the mRNA and protein
expression of survivin in PBMCs was significantly higher in
patients with good collateral circulation (C2 + C3) than that in
patients with no collateral flow (C0) (P<0.05), although no
significant differences were observed between C2 and C3 patients
(P>0.05) (Fig. 2).
CD4, CD8, CD44, VEGF and ICAM-1 protein
expression in human PBMCs during coronary collateral formation
Western blot analysis revealed that CD8 and CD44
expression in PBMCs was significantly higher in patients with good
collateral formation (C3 + C2) than in patients with poor
collateral formation (C1), no collateral formation (C0), and normal
controls (all P<0.05) (Fig.
3). CD4 and VEGF expression were also higher in patients with
good collateral circulation than in those with no collateral flow
(C0) and normal control patients (all P<0.05). However, this
result was non-significant between patients with good collateral
circulation (C3 + C2) and poor collateral circulation (C1)
(P>0.05). ICAM-1 expression was higher in patients with
collateral formation than that in normal control patients (all
P<0.05). However, this result was non-significant among good
collateral (C3 + C2), poor collateral (C1), and no collateral (C0)
groups (P>0.05).
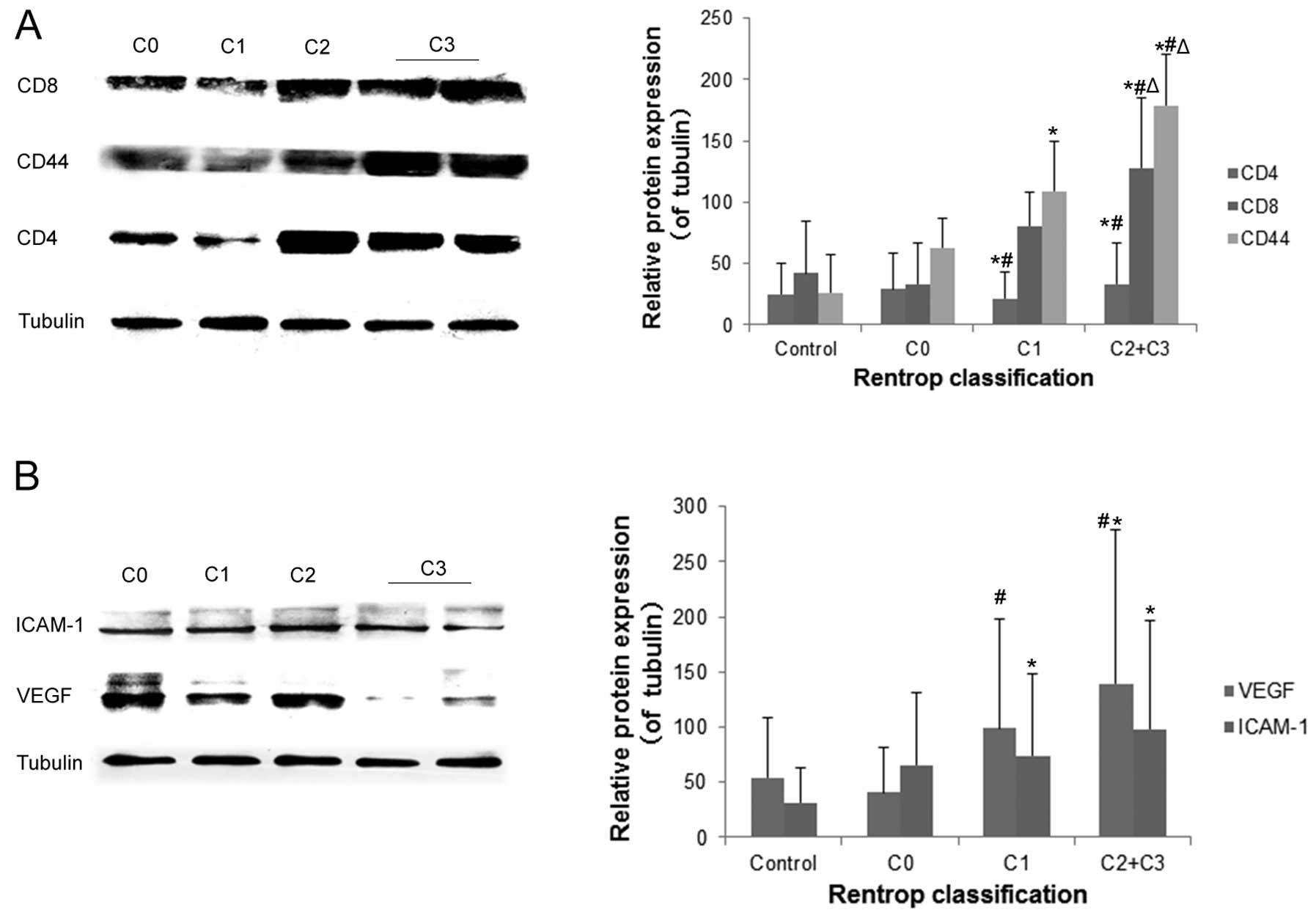 | Figure 3CD4, CD8, CD44, vascular endothelial
growth factor (VEGF) and intercellular adhesion molecule-1 (ICAM-1)
expression in human peripheral blood mononuclear cells (PBMCs)
during coronary collateral formation. CD4, CD8, CD44, VEGF and
ICAM-1 expression was determined by western blotting. Tubulin was
used as an internal control. (A) CD4, CD8 and CD44 protein
expression and (B) VEGF and ICAM-1 protein expression. The data are
the means ± SD of three independent experiments.
*P<0.05, C3 + C2, C1, C0 vs. normal control;
#P<0.05, C3 + C2, C1 vs. C0; △P<0.05,
C3 + C2 vs. C1. |
Flow cytometric analysis of survivin
single- and double-positives in human PBMCs
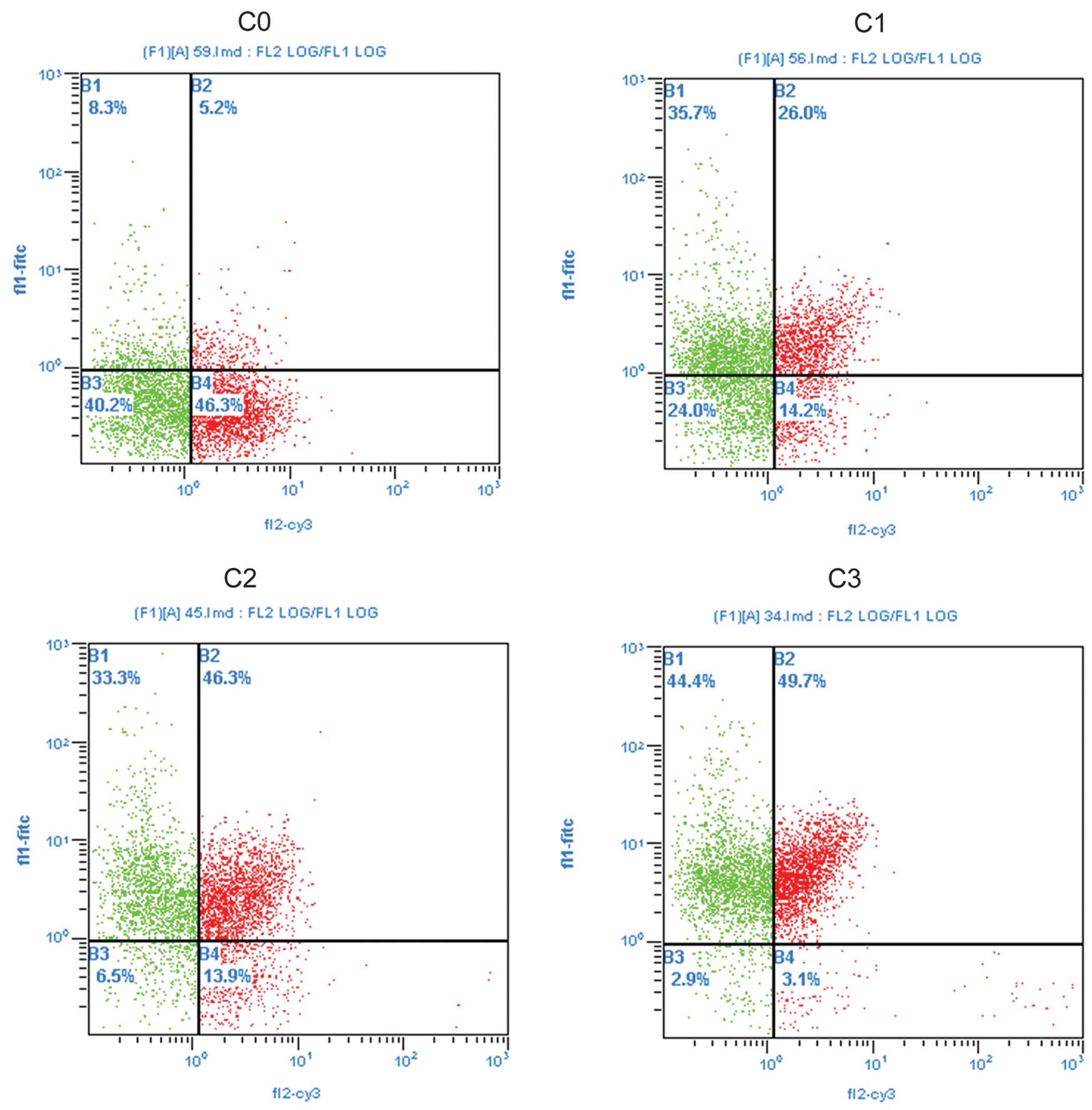
Flow cytometric analysis revealed that the
percentages of survivin and CD44 single-positive PMBCs, survivin
and CD8 double-positive (survivin + CD8), survivin and VEGF
double-positive (survivin + VEGF), and survivin and ICAM-1
double-positive (survivin + ICAM-1) PBMCs were significantly higher
in patients with good collateral formation than those in the normal
controls and those with no collateral formation (C0) (all
P<0.05) (Fig. 4 and Table II). Percentages of survivin + CD8
and survivin + VEGF double-positive PBMCs were significantly higher
in patients with good collateral formation than those in the normal
control and with no collateral formation (C0) (all P<0.05).
 | Table IIFlow cytometric analysis of survivin
and CD44 single-positive, and survivin and CD4, CD8, VEGF and
ICAM-1 double-positive in human PBMCs during coronary collateral
formation. |
Table II
Flow cytometric analysis of survivin
and CD44 single-positive, and survivin and CD4, CD8, VEGF and
ICAM-1 double-positive in human PBMCs during coronary collateral
formation.
| Rentrop
classification | Survivin % | Sur + CD4 % | Sur + CD8 % | CD44 % | Sur + VEGF % | Sur + ICAM-1 % |
|---|
| Normal control | 6.2±2.5 | 56.1±6.2 | 5.1±2.2 | 10.6±4.1 | 9.0±3.9 | 4.4±2.1 |
| C0 | 21.6±10.8a | 38.4±6.8a | 4.4±0.9 | 17.5±5.7 | 19.5±4.6a | 5.0±1.4 |
| C1 | 19.7±15.9 | 37.2±8.6a | 3.8±1.5 | 31.1±8.3a,b | 34.7±8.6a,b | 3.0±1.1b |
| C2 ± C3 | 45.0±16.2a–c | 31.9±7.4a | 36.5±5.1a–c | 65.4±8.2a–c | 54.6±6.2a–c | 21.4±5.4a–c |
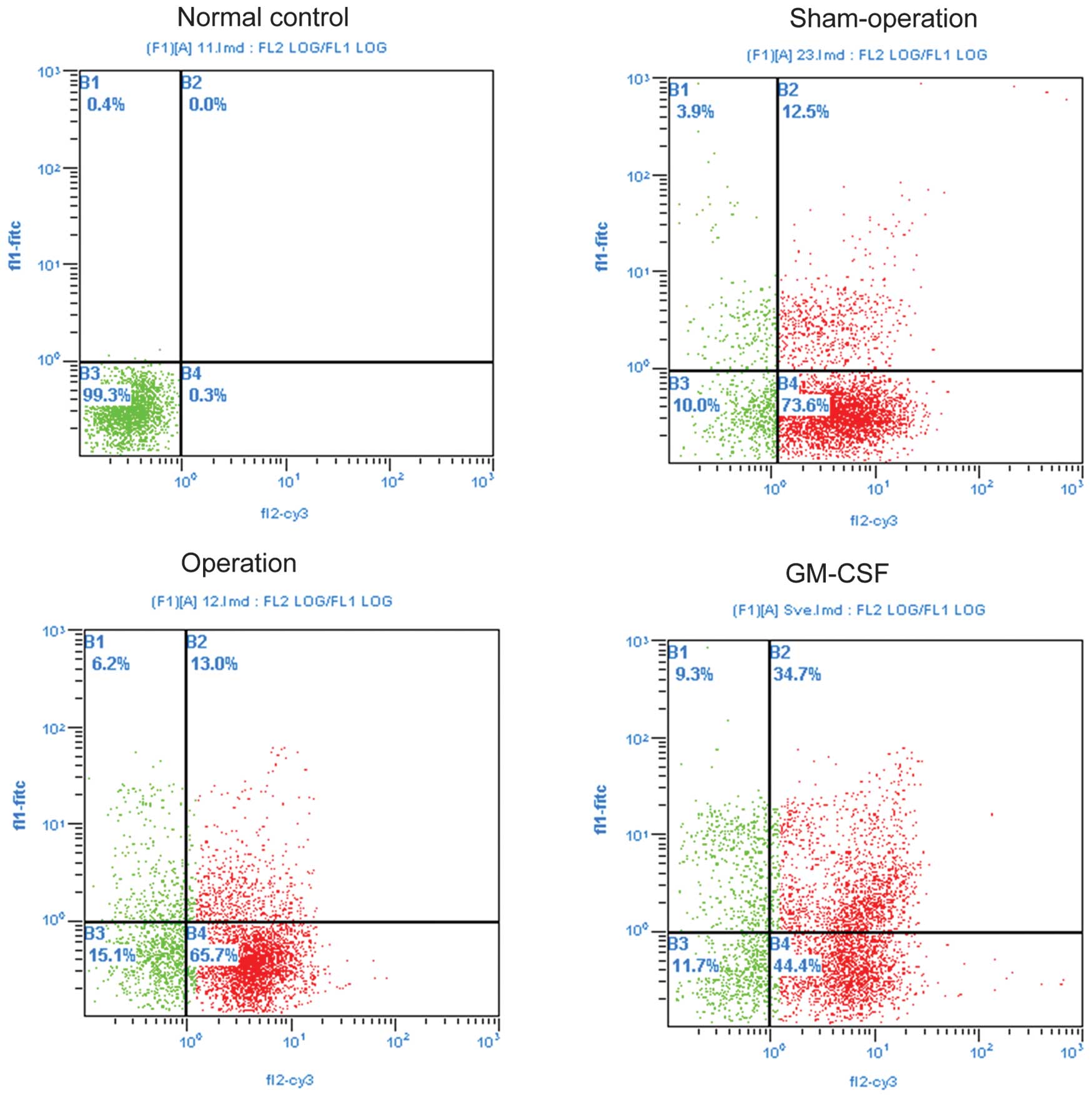
Postmortem angiography in rat models and
PBMC survivin expression correlation
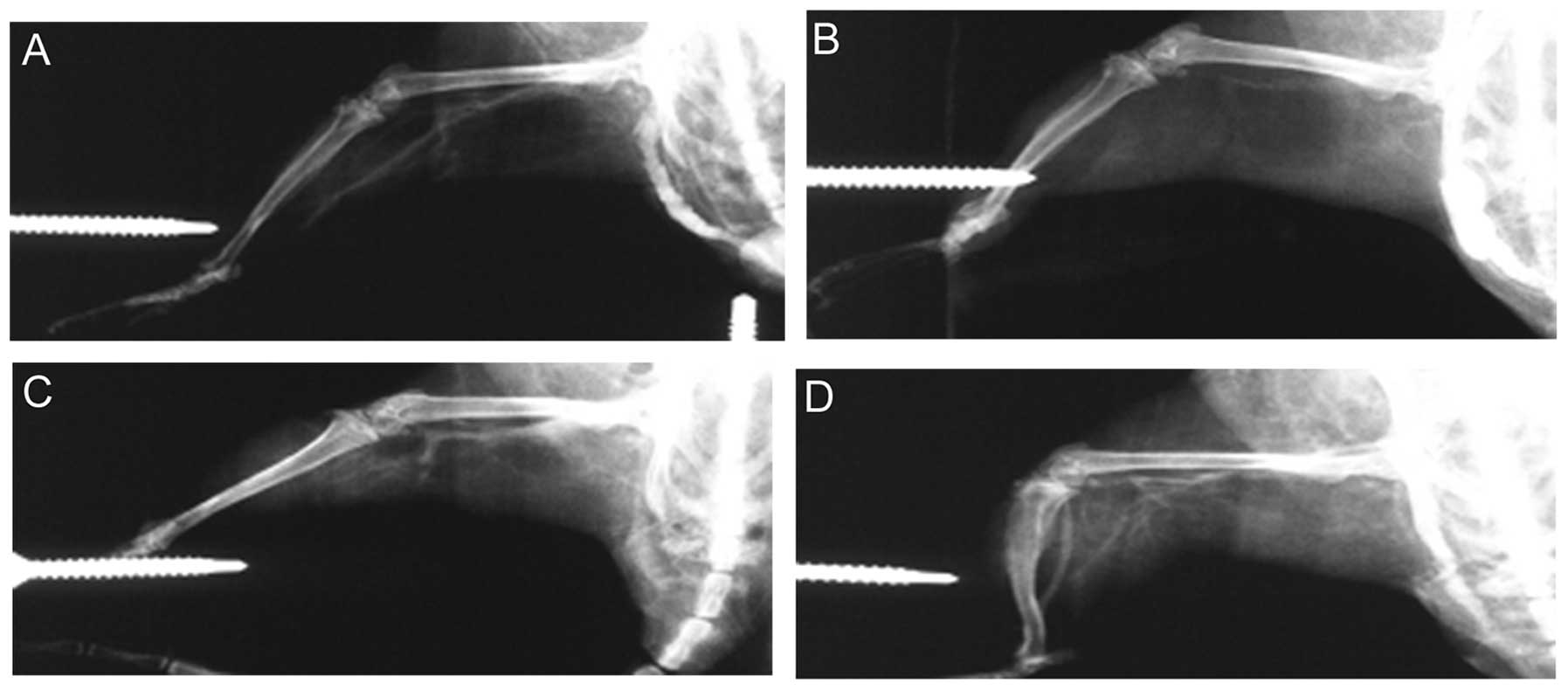
In rat models, macroscopic collateral arteries were
visible after right femoral artery ligation (operation and GM-CSF
treatment groups) (Fig. 6).
Percentages of survivin and CD44 single-positive PMBCs, and
survivin + CD8, + VEGF, and + ICAM-1 double-positive PBMCs were
observed significantly higher in the GM-CSF treatment group as
compared to those observed in the remaining groups (all P<0.05).
Percentages of survivin single-positive PMBCs, and survivin and
CD8, VEGF and ICAM-1 double-positive PBMCs were significantly
higher in the operation group than those in the sham-operation and
normal control groups (all P<0.05) (Fig. 5 and Table III). Furthermore, in specimens
with higher survivin levels, significantly larger and more visible
collateral vessels were consistently observed.
 | Table IIIFlow cytometric analysis for survivin
and CD44 single-positive, and survivin and CD4, CD8, VEGF and
ICAM-1 double-positive in rat model of hind limb ischemia. |
Table III
Flow cytometric analysis for survivin
and CD44 single-positive, and survivin and CD4, CD8, VEGF and
ICAM-1 double-positive in rat model of hind limb ischemia.
| Group | Survivin % | Sur + CD4 % | Sur + CD8 % | CD44 % | Sur + VEGF % | Sur + ICAM-1 % |
|---|
| Normal control | 6.3±2.3 | 55.2±6.4 | 5.3±2.1 | 10.0±4.2 | 7.8±3.7 | 4.8±2.2 |
| Sham-operation | 7.1±2.9 | 48.4±7.1 | 4.8±1.2 | 11.5±5.6 | 10.7±2.3 | 4.8±1.3 |
| Operation | 16.9±6.1a,b | 35.8±7.7a,b | 5.9±5.3 | 30.1±7.5a,b | 25.1±7.5a,b | 3.3±1.1 |
| GM-CSF | 40.5±13.1a–c | 42.5±7.4a | 33.7±7.4a–c | 50.5±7.0a–c | 52.0±9.0a–c | 20.5±5.8a–c |
Discussion
Increased survivin expression in PBMCs was
associated with greater coronary collateral formation in CTO
patients. Moreover, the flow cytometric analysis revealed that the
percentage of survivin-positive PBMCs was significantly higher in
patients with good collateral formation than that in patients with
poor collateral formation. These findings were confirmed in a rat
model of hind limb ischemia, indicating that GM-CSF treatment after
femoral artery ligation is capable of significantly promoting
collateral formation. Notably, survivin, CD4, CD44 single-positive
PBMCs as well as survivin + CD8, + VEGF, and + ICAM-1
double-positive PBMCs were increased, consistent with findings in
samples from human cells. While survivin levels have previously
been associated with CAD (4),
this novel finding demonstrates that the survivin expression
signature of PBMCs may increase collateral formation, potentially
providing a valuable prognostic indicator in CAD patients,
particularly those with coronary CTO. Furthermore, these
observations are potentially useful in developing revascularization
strategies.
The role of CD4+, CD8+ and
CD44+ T lymphocytes in arteriogenesis and collateral
development is well documented. Accumulating evidence has indicated
that survivin is expressed in normal adult cells, particularly in
primitive hematopoietic cells, T lymphocytes, polymorphonuclear
neutrophils, and vascular endothelial cells, where it is important
in cell proliferation, regulation, and survival (20). CD4+ T cells control the
arteriogenic response to acute hind limb ischemia, in part by
recruiting macrophages to the site of active collateral artery
formation and triggering the development of collateral arteries
through arteriogenic cytokine synthesis (21). During collateral formation,
CD8+ T cells contribute to the early phases of
collateral development. Following femoral artery ligation,
CD8+ T cells infiltrate the site of collateral vessel
growth and recruit CD4+ mononuclear cells by expressing
IL-16 (21). Notably, CD44
deficiency has been reported to impede arteriogenesis (22), and increased CD44 expression on
isolated monocytes is increased in patients with good
collateralization (23).
Confirming these findings, Arslan et al (24) reported a significant association
between increased circulating CD14+ + CD16‒
monocyte levels and good coronary collateral development. Those
findings are consistent with those of the present findings showing
that CD4-, CD8- and CD44-positive T cells play a crucial role in
collateral formation.
Expression of ICAM-1 and/or VEGF is critical to the
growth of coronary collaterals (24,25). As shear stress upregulates the
expression of endothelial cell adhesion receptors such as ICAM-1
and vascular cell adhesion molecule-1 (26,27), monocyte adhesion to shear
stress-activated endothelium is important in arteriogenesis and in
collateral artery growth (28).
Hoefer et al (29)
demonstrated that ICAM-1-mediated monocyte adhesion via
ICAM-1/Mac-1 interaction to the endothelium of collateral arteries
is essential in these processes by treating in vivo subjects
with monoclonal antibodies against ICAM-1, which totally abolished
the stimulatory effect of MCP-1 on collateral artery growth
(30). Similarly, Rivard et
al (31) reported that a
reduced VEGF expression in diabetic mice resulted in impairment of
new blood vessel formation, although cytokine supplementation using
intramuscular adeno-VEGF gene transfer restored neovascularization
(25). Consistent with the
present study, ICAM-1 and VEGF are essential in many
vascularization processes, including collateral formation. In
particular, the present study demonstrates that expression of VEGF
and ICAM-1 was elevated in patients with collateral formation.
Furthermore, PBMCs in patients with elevated collateral evidenced
distinctly higher rates of survivin single-positive as well as
survivin + VEGF and + ICAM-1 double-positive cells, indicating that
the mechanism of coronary collateral formation may be, in part,
associated with high survivin, VEGF, ICAM-1 expression in
PBMCs.
However, it has been shown that VEGF exerts
anti-apoptotic effects during angiogenesis that may be mediated by
the induced survivin expression in endothelial cells (31). Although these findings seem to
limit the practical implementation of survivin upregulation, the
importance of survivin during vascularization processes, including
collateral formation is confirmed. However, the clinical
relationships between compounds, such as VEGF and survivin, in
human PMBCs merits further investigation prior to development of
clinical strategies. It is important to consider also that, this
study may be limited by the relatively small study group and use of
a hind limb ischemia rat model as compared to the coronary
collateral formation model due to the insufficiently small size of
the rat coronary artery for contrast-based angiography.
Furthermore, the rat hind limb model was selected for comparison
with human coronary collateral formation as it has been reported
that the femoral artery ligation rat model is simple and accurate
for use in angiographic studies, whereas coronary artery ligation
in rats is extremely difficult and less accurate (32,33). Thus, the present model has been
demonstrated to produce low animal mortality and high success rates
in numerous investigations of coronary CTO in the literature
(32,33). Despite the use of this established
model, any error occurring may be due to the discrepancies between
the hind limb ischemia and coronary collateral rat models, which
should be considered in the analysis of these results and design of
future clinical studies.
In conclusion, in PMBCs from clinical coronary CTO
patients and a rat model of hind limb ischemia, an elevated
survivin expression in PBMCs, particularly of survivin and CD8,
VEGF, ICAM-1 double-positive PBMCs were associated with good
collateral formation. Thus, assessment of survivin level in the
peripheral blood may provide a viable clinical alternative for CAD
assessment. Furthermore, there may be potential applications of
these findings in revascularization strategies for treating CAD
patients that may undergo ischemic events due to disease or bypass
surgery.
References
|
1
|
Seiler C: The human coronary collateral
circulation. Heart. 89:1352–1357. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fefer P, Knudtson ML, Cheema AN, et al:
Current perspectives on coronary chronic total occlusions: the
Canadian Multicenter Chronic Total Occlusions Registry. J Am Coll
Cardiol. 59:991–997. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Petronio AS, Baglini R, Limbruno U, et al:
Coronary collateral circulation behaviour and myocardial viability
in chronic total occlusion treated with coronary angioplasty. Eur
Heart J. 19:1681–1687. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Levkau B, Schäfers M, Wohlschlaeger J, et
al: Survivin determines cardiac function by controlling total
cardiomyocyte number. Circulation. 117:1583–1593. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hoekstra M, van der Lans CA, Halvorsen B,
et al: The peripheral blood mononuclear cell microRNA signature of
coronary artery disease. Biochem Biophys Res Commun. 394:792–797.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhen HN, Zhang X, Hu PZ, et al: Survivin
expression and its relation with proliferation, apoptosis, and
angiogenesis in brain gliomas. Cancer. 104:2775–2783. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
O’Connor DS, Schechner JS, Adida C, et al:
Control of apoptosis during angiogenesis by survivin expression in
endothelial cells. Am J Pathol. 156:393–398. 2000. View Article : Google Scholar
|
|
8
|
Lee GH, Joo YE, Koh YS, et al: Expression
of survivin in gastric cancer and its relationship with tumor
angiogenesis. Eur J Gastroenterol Hepatol. 18:957–963. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zwerts F, Lupu F, De Vriese A, et al: Lack
of endothelial cell survivin causes embryonic defects in
angiogenesis, cardiogenesis, and neural tube closure. Blood.
109:4742–4752. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Waksman R, Saito S, Galassi AR and
Tomasello SD: Collateral circulation in CTO. Chronic Total
Occlusions: A Guide to Recanalization. Waksman R and Saito S: John
Wiley and Sons; Oxford:
|
|
11
|
Conway EM, Zwerts F, Van Eygen V, et al:
Survivin-dependent angiogenesis in ischemic brain: molecular
mechanisms of hypoxia-induced up-regulation. Am J Pathol.
163:935–946. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cotton JM, Mathur A, Hong Y, Brown AS,
Martin JF and Erusalimsky JD: Acute rise of circulating vascular
endothelial growth factor-A in patients with coronary artery
disease following cardiothoracic surgery. Eur Heart J. 23:953–959.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Labarrere CA, Nelson DR, Miller SJ, et al:
Value of serum-soluble intercellular adhesion molecule-1 for the
noninvasive risk assessment of transplant coronary artery disease,
posttransplant ischemic events, and cardiac graft failure.
Circulation. 102:1549–1555. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Teeuwen K, Adriaenssens T, Van den Branden
BJ, et al: A randomized multicenter comparison of hybrid
sirolimus-eluting stents with bioresorbable polymer versus
everolimus-eluting stents with durable polymer in total coronary
occlusion: rationale and design of the Primary Stenting of Occluded
Native Coronary Arteries IV study. Trials. 13:2402012. View Article : Google Scholar
|
|
15
|
Rentrop KP, Cohen M, Blanke H and Phillips
RA: Changes in collateral channel filling immediately after
controlled coronary artery occlusion by an angioplasty balloon in
human subjects. J Am Coll Cardiol. 5:587–592. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kato M, Shiode N, Yamagata T, Matsuura H
and Kajiyama G: Coronary segmental responses to acetylcholine and
bradykinin in patients with atherosclerotic risk factors. Am J
Cardiol. 80:751–755. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu YG, Zhou SH, Li YG, et al: The
mechanism underlying vascular smooth muscle cell apoptosis induced
by atorvastatin may be mainly associated with down-regulation of
survivin expression. Cardiovasc Drugs Ther. 21:145–153. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee CW, Stabile E, Kinnaird T, et al:
Temporal patterns of gene expression after acute hindlimb ischemia
in mice: insights into the genomic program for collateral vessel
development. J Am Coll Cardiol. 43:474–482. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu J, deMuinck ED, Zhuang Z, et al:
Endothelial nitric oxide synthase is critical for ischemic
remodeling, mural cell recruitment, and blood flow reserve. Proc
Natl Acad Sci USA. 102:10999–11004. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fukuda S and Pelus LM: Survivin, a cancer
target with an emerging role in normal adult tissues. Mol Cancer
Ther. 5:1087–1098. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stabile E, Burnett MS, Watkins C, et al:
Impaired arteriogenic response to acute hindlimb ischemia in
CD4-knockout mice. Circulation. 108:205–210. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stabile E, Kinnaird T, la Sala A, et al:
CD8+ T lymphocytes regulate the arteriogenic response to
ischemia by infiltrating the site of collateral vessel development
and recruiting CD4+ mononuclear cells through the
expression of interleukin-16. Circulation. 113:118–124. 2006.
View Article : Google Scholar
|
|
23
|
van Royen N, Voskuil M, Hoefer I, et al:
CD44 regulates arteriogenesis in mice and is differentially
expressed in patients with poor and good collateralization.
Circulation. 109:1647–1652. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arslan U, Kocaoğlu I, Falay MY, Balci M,
Duyuler S and Korkmaz A: The association between different monocyte
subsets and coronary collateral development. Coron Artery Dis.
23:16–21. 2012. View Article : Google Scholar
|
|
25
|
Zentilin L, Tafuro S, Zacchigna S, et al:
Bone marrow mononuclear cells are recruited to the sites of
VEGF-induced neovascularization but are not incorporated into the
newly formed vessels. Blood. 107:3546–3554. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hong KH, Ryu J and Han KH: Monocyte
chemoattractant protein-1-induced angiogenesis is mediated by
vascular endothelial growth factor-A. Blood. 105:1405–1407. 2005.
View Article : Google Scholar
|
|
27
|
Gimbrone MA Jr, Nagel T and Topper JN:
Biomechanical activation: an emerging paradigm in endothelial
adhesion biology. J Clin Invest. 99:1809–1813. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Toyota E, Warltier DC, Brock T, et al:
Vascular endothelial growth factor is required for coronary
collateral growth in the rat. Circulation. 112:2108–2113. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hoefer IE, van Royen N, Rectenwald JE, et
al: Arteriogenesis proceeds via ICAM-1/Mac-1-mediated mechanisms.
Circ Res. 94:1179–1185. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Scholz D, Ito W, Fleming I, et al:
Ultrastructure and molecular histology of rabbit hind-limb
collateral artery growth (arteriogenesis). Virchows Arch.
436:257–270. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rivard A, Silver M, Chen D, et al: Rescue
of diabetes-related impairment of angiogenesis by intramuscular
gene therapy with adeno-VEGF. Am J Pathol. 154:355–363. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Heil M, Ziegelhoeffer T, Pipp F, et al:
Blood monocyte concentration is critical for enhancement of
collateral artery growth. Am J Physiol Heart Circ Physiol.
283:H2411–H2419. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Heil M, Ziegelhoeffer T, Wagner S, et al:
Collateral artery growth (arteriogenesis) after experimental
arterial occlusion is impaired in mice lacking CC-chemokine
receptor-2. Circ Res. 94:671–677. 2004. View Article : Google Scholar : PubMed/NCBI
|