Introduction
Magnesium (Mg) and its alloys are susceptible to
dissolution in aqueous solutions due to their extremely low
corrosion potential, particularly in alloys containing chloride ion
electrolytes (1). For this
reason, Mg alloys have attracted considerable attention as
potential implant materials (2–8).
The interest in Mg alloys has been primarily motivated by their
biocompatibility, biodegradability and desirable mechanical
properties. For instance, the tensile strength and elastic modulus
of Mg alloys are closer to those of bone, as compared with the
commonly used steel and titanium alloys (2–8). A
previous in vivo study suggested that Mg alloy implantation
in animal models promoted new bone formation and biocompatibility
(2). Therefore, Mg and Mg alloys
may be used as biodegradable materials for orthopedic implants.
The Mg alloys that have been investigated as implant
materials are mostly commercial alloys designed for the
transportation industry (8). Most
of these commercial Mg alloys contain aluminium (Al) and rare earth
metals. However, Al is neurotoxic (9), whereas severe hepatotoxicity has
been detected following the administration of rare earth metals
(10). Therefore, the exploration
of novel non-toxic or low-toxicity Mg alloy systems has become a
research highlight.
In this study, a medical Mg alloy, designated as
Mg-Nd-Zn-Zr (JDBM), was further evaluated for its cytocompatibility
in vitro. This alloy contains a Mg matrix and approximately
3% rare earth metals (11).
Preliminary results revealed that JDBM possessed favorable in
vitro biocompatibility to rabbit chondrocytes (12). In this study, rabbit chondrocytes
were further used as an in vitro model to evaluate the
effects of JDBM, brushite
(CaHPO4·2H2O)-coated JDBM
(C-JDBM), AZ31, WE43, pure Mg and Ti alloys (TC4). The effects of
the Mg and Mg alloys on the adhesion, viability, proliferation and
apoptosis of chondrocytes were investigated. The glycosaminoglycan
(GAG) and collagen II (Col II) content, as well as the mRNA
expression of Col II and aggrecan were also investigated.
Materials and methods
Sample and extract preparation
JDBM was previously developed for biomaterial
applications by our group (11).
C-JDBM, the commercial Mg alloys, WE43 and AZ31, pure Mg (Shanxi
Yanbixin Magnesium Co., Ltd., Shanxi, China), and the Ti alloy, TC4
(Daiyuan, Shanghai, China), were analyzed for comparison. The
detailed preparation of these materials has been described in our
previous study (11).
Disk samples, 10 mm in diameter and 2.0 mm in
height, were obtained by electrode discharge machining from the
extruded JDBM, WE43, AZ31 and TC4 rods, as well as from high-purity
Mg ingots (99.99%). All samples were ground using SiC paper of up
to 1,200 grit and polished with 1 µm diamond abrasive paste,
followed by a series of ultrasonic cleaning in acetone, ethanol and
distilled water. The details of the
CaHPO4·2H2O treatment of the JDBM
disc, including the fabrication of the coating and the evaluation
of its properties are described in another study of ours (13). Prior to the in vitro
cytocompatibility experiments, the samples were sterilized with
ethylene oxide for 24 h.
Sample extracts were prepared according to ISO
10993. The disk samples were immersed in 1.7584 ml Dulbecco’s
modified Eagle’s medium with F12 (DMEM/F12) supplemented with 10%
fetal bovine serum (FBS) (both from Gibco-BRL, Carlsbad, CA, USA),
with a surface area-to-extraction medium volume ratio of 1.25
cm2/ml. The samples were then incubated in a humidified
atmosphere with 95% humidity and 5% CO2 at 37°C for 72
h. The supernatant was collected, and the obtained extracts were
refrigerated at 4°C and used within 3 days.
Chondrocyte harvest and culture
Chondrocytes were isolated from aseptically
harvested articular cartilage from the knee joints of adult New
Zealand rabbits weighing 2.0–2.5 kg. The cartilage samples were
rinsed in sterile phosphate-buffered saline (PBS) (pH 7.4;
Gibco-BRL) containing penicillin and streptomycin (100 U/ml and 100
µg/ml, respectively) (HuaBei, Shijiazhuang, China). The
samples were then cut into 1–2 mm3 fragments, placed in
a spinner flask containing 0.25% trypsin-EDTA (Gibco-BRL) at 37°C
for 30 min, and rinsed thrice in sterile PBS. The slices as
prepared samples were subsequently digested with 0.2% Col II
(Sigma, St. Louis, MO, USA) in sterile PBS at 37°C for a further
12–16 h. The chondrocytes were then harvested, counted and seeded
onto 25 cm2 culture flasks at a cell density of
2×104/cm2 in DMEM/F12 with 10% FBS
(Gibco-BRL). The cells were then cultured in an incubator (Thermo
8000; Thermo Fisher Scientific, Inc., Shanghai, China) at 37°C with
95% humidity and 5% CO2 for 7–10 days. The cell culture
medium was replenished every 3 days. The cells were passaged when
they reached 80–90% confluence. Cells at passage 2 were used for
further experiments.
Cell adhesion assay
Cell suspensions of 1.5 ml each were seeded into
24-well plates containing the JDBM, C-JDBM, AZ31, WE43, pure Mg and
TC4 disc samples, at a cell density of 1×105 cells/ml.
The culture medium was replenished daily. The cultures were
incubated in a humidified atmosphere (95%) with 5% CO2
at 37°C. The samples were collected after 1 and 3 days of
incubation and washed thrice with PBS (pH 7.4; Gibco-BRL) to remove
the non-adherent cells. The cells were then fixed in 2.5%
glutaraldehyde (Boster, Wuhan, China) solution at room temperature
for 2 h followed by rinsing thrice with PBS. Following gradient
ethanol dehydration (50, 60, 70, 80, 90 and 100%; for 10 min
gradient ethanol dehydration), the samples were dried in a
hexamethyldisilazane solution (Wuhan Boster Biological Technology,
Ltd.). The samples were subsequently sputter-coated with gold. The
surfaces of the cell-adhered experimental samples were observed by
scanning electron microscopy (SEM; FEI Quanta 250; FEI Co.,
Hillsboro, OR, USA). Three parallel samples were used for each
experimental condition.
Another set of 6 parallel samples were treated
according to the method described above. However, these samples
were fixed in 4% paraformaldehyde solution (Wuhan Boster Biological
Technology, Ltd.) at room temperature for 30 min and rinsed thrice
with PBS (pH 7.4; Gibco-BRL), followed by staining with a
4′,6-diamidino-2-phenylindole (DAPI) solution (5 µg/ml)
(Beyotime Institute of Biotechnology, Jiangsu, China) for 10 min.
The samples were again rinsed thrice with PBS, for 5 min for each
wash. The surfaces of the cell-adhered experimental samples were
observed and recorded using an inverted phase contrast microscope
(Olympus IX70; Olympus Corp., Tokyo, Japan).
Indirect cell cytotoxicity and
proliferation assay
The cells cultured in DMEM/F12 alone were used as
the negative controls, whereas those cultured in DMEM/F12 medium
with 10% DMSO (Beyotime Institute of Biotechnology) were used as
the positive controls. The cells were incubated in 96-well cell
culture plates at 5×103 cells per 100 µl medium
in each well for 24 h to allow attachment. The culture medium was
then replaced with 100 µl of the extraction medium with the
respective treatments. The 96-well cell culture plates were then
observed under an optical microscope (CSW-30B; Shenzhen Coosway
Optical Technology Co., Ltd, Shenzhen, China) on days 1, 3 and 5
following incubation. The culture medium was replenished every 2
days. Six parallel wells were established for each tested sample. A
total of 20 µl
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
(Sigma) solution (5 mg/ml in PBS) was added to each well. The
samples were then placed in a cell incubator for 4 h. Subsequently,
100 µl of the formazan solubilization solution [10% SDS in
0.01 MHCl (Sigma)] were added to each well and the plates were
again incubated overnight in a cell incubator with a humidified
atmosphere (95%) and 5% CO2 at 37°C. The
spectrophotometric absorbance of the samples at 570 nm was measured
using a microplate reader (Bio-Rad 680; Bio-Rad, Hercules, CA,
USA).
Another set of 6 parallel 96-well cell culture
plates were used to observe and record the cell morphology. The
test condition was the same the as one described above, and the
medium was replaced with 100 µl extraction medium. Following
incubation for 1, 3 and 5 days, the 96-well cell culture plates
were collected and rinsed thrice with PBS (pH 7.4; Gibco-BRL), and
then stained with Alcian blue and DAPI solution (5 µg/ml)
(both from Beyotime Institute of Biotechnology). The cell
morphologies of the samples in the 96-well cell culture plates were
observed and recorded using an inverted phase contrast microscope
(Olympus IX70; Olympus Corp.). Three parallel wells were
established for each stain.
Cell apoptosis assay
The chondrocytes were analyzed as follows: 7
treatment groups were set, namely, a negative control (DMEM/F12
medium), a JDBM, C-JDBM, AZ31, WE43 pure Mg and TC4 group. Cell
suspensions (10 ml) were seeded into 75 cm2 culture
flasks at a cell density of 1×105 cells/ml and incubated
for 24 h to allow attachment. The medium was then replaced with 10
ml extraction medium or DMEM/F12 medium. The culture media were
replaced after 2 days with 10 ml. Three parallel culture flasks
were established for each treatment condition. Following incubation
of the cells in a cell incubator for 5 days, the culture flasks
were observed under an optical microscope. Chondrocyte apoptosis
was assessed using the Alexa Fluor® 488 Annexin V/Dead
Cell Apoptosis kit (Invitrogen Life Technologies, Carlsbad, CA,
USA) with Alexa Fluor® 488 Annexin V and propidium
iodide (PI) (Beyotime Institute of Biotechnology) for flow
cytometry (Navios; Beckman Coulter, Inc., Brea, CA, USA). The assay
was performed according to the manufacturer’s instructions.
GAG quantification assay
The quantification of the GAG content was conducted
by a modification of the dimethylmethylene blue method (Beyotime
Institute of Biotechnology) (14). The chondrocytes were treated as
follows: the cells were seeded in 6-well cell culture plates at a
density of 1×105 cells/well and incubated for 24 h to
allow attachment. The medium was changed with 3 ml of extraction
medium every 2 days. Following incubation of the cells under the
cell culture conditions for 5 days, the 6-well cell culture plates
were observed under an optical microscope. Three parallel wells
were established for each treatment condition. After rinsing in PBS
(pH 7.4; Gibco-BRL), a cytolysate (RIPA; Wuhan Boster Biological
Technology, Ltd.) was added to each well at 0.4 ml/well and
vortexed for 10 sec. An aliquot (40 µl) of the digest was
assayed for the total GAG content by the addition of 200 µl
of 1,9-dimethylmethylene blue dye solution (Beyotime Institute of
Biotechnology). The absorbance was determined at 595 nm using a
microplate reader (Bio-Rad 680; Bio-Rad). The amount of GAG was
extrapolated from a standard curve based on shark chondroitin
sulfate.
Col II content assay and enzyme-linked
immunosorbent assay (ELISA)
The chondrocytes were treated as described above.
The cells were seeded in 12-well plates at a density of
5×104 cells/well. The cells were then incubated for 24 h
to allow attachment. The medium was then changed with 2 ml of
extraction medium every 2 days. Following incubation of the cells
in a humidified atmosphere (95%) with 5% CO2 at 37°C for
5 days, the 12-well cell culture plates were observed under an
optical microscope. Three parallel wells were established for each
treatment condition. After the cell culture plates were placed on
ice and rinsed in PBS (pH 7.4; Gibco-BRL), a cytolysate (RIPA;
Wuhan Boster Biological Technology, Ltd.) was added to each well at
0.2 ml/well, vortexed for 10 sec, and centrifuged for 10 min at
10,000 x g according to the manufacturer’s instructions. An ELISA
kit (R&D Systems, Minneapolis, MN, USA) was used for the
quantitative determination of rabbit Col II levels in the cell
cultures from each well. ELISA was conducted according to the
manufacturer’s instructions. The spectrophotometric absorbance of
the samples at 450 nm was measured using a microplate reader
(Bio-Rad 680; Bio-Rad). The standard curve was calculated according
to the concentration and absorbance of the standard, asw ell as
based on the curve to extrapolate the corresponding concentration
of the sample.
RNA extraction and quantitative-reverse
transcription PCR (RT-qPCR)
The chondrocytes were seeded into 25 cm2
culture flasks at a density of 4×105 cells per flask and
incubated for 24 h to allow attachment. The medium was then changed
with 5 ml of the respective extracts every 2 days. DMEM/F12 medium
was used as a negative control. Following incubation of the cells
in a humidified atmosphere (95%) with 5% CO2 at 37°C for
5 days, the culture flasks were observed under an optical
microscope. Three parallel wells were established for each
treatment condition. Total RNA from the chondrocytes in the
different groups was isolated using TRIzol reagent (Invitrogen Life
Technologies). First-strand cDNA was synthesized using the
RevertAid First Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc., Waltham, MA, USA), as previously described
(12). Quantitative PCR (qPCR)
was performed to amplify rabbit GAPDH, aggrecan and Col II using
the LightCycler DNA Master SYBR-Green I kit (Roche, Basel,
Switzerland) according to the manufacturer’s instructions. The
copies of target cDNA were normalized to GAPDH expression
(housekeeping gene). Each PCR reaction was repeated thrice for each
independent sample. The primers used were the same as those in a
previous study of ours (12).
Statistical analysis
Three replicates were conducted for each test
(cytotoxicity test, N=6) and are presented as the mean values ±
standard deviation (SD). ANOVA for repeated measurements was used
for the MTT assay data. Multivariate ANOVA and the
multiple-comparison post hoc test [least significant difference
(LSD)] were subsequently conducted for pairwise comparisons between
groups at each time point. One-way ANOVA, followed by the
multiple-comparison post hoc test [Student-Newman-Keul (SNK) test;
Tukey’s test], was performed using SPSS (version 16.0) software for
other group comparisons. Mauchly’s test of sphericity was used to
determine whether there were any associations among the repeatedly
measured data. If any (p≤0.05), multivariate ANOVA was then
performed, or the Greenhouse-Geisser corrected results were taken
into consideration. The effects of treatment were evaluated by
estimating between subject variance. The repeated measurement
effect or its interactive effect with the treatment group was
evaluated by estimating within subject variance. The method of
Bonferroni was used to perform pairwise comparisons of the
repeatedly measured data at different measurement times in each
treatment group. A value of p<0.05 was considered to indicate a
statistically significant difference.
Results
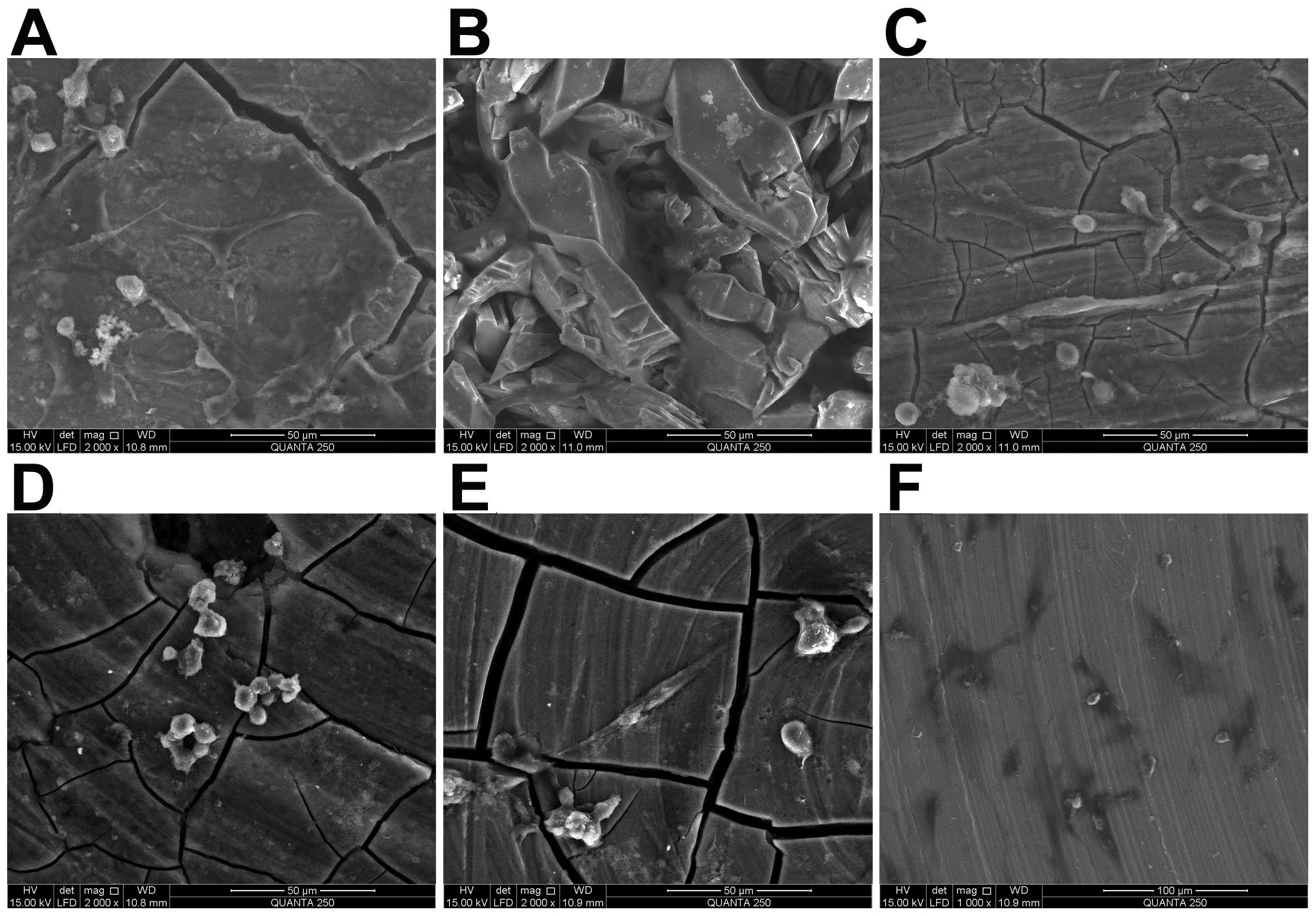
Cell adhesion and morphology
The morphologies of the chondrocytes cultured on the
discs of JDBM, C-JDBM, AZ31, WE43, pure Mg and TC4 for 1 or 3 days
are illustrated in Figs. 1 and
2.
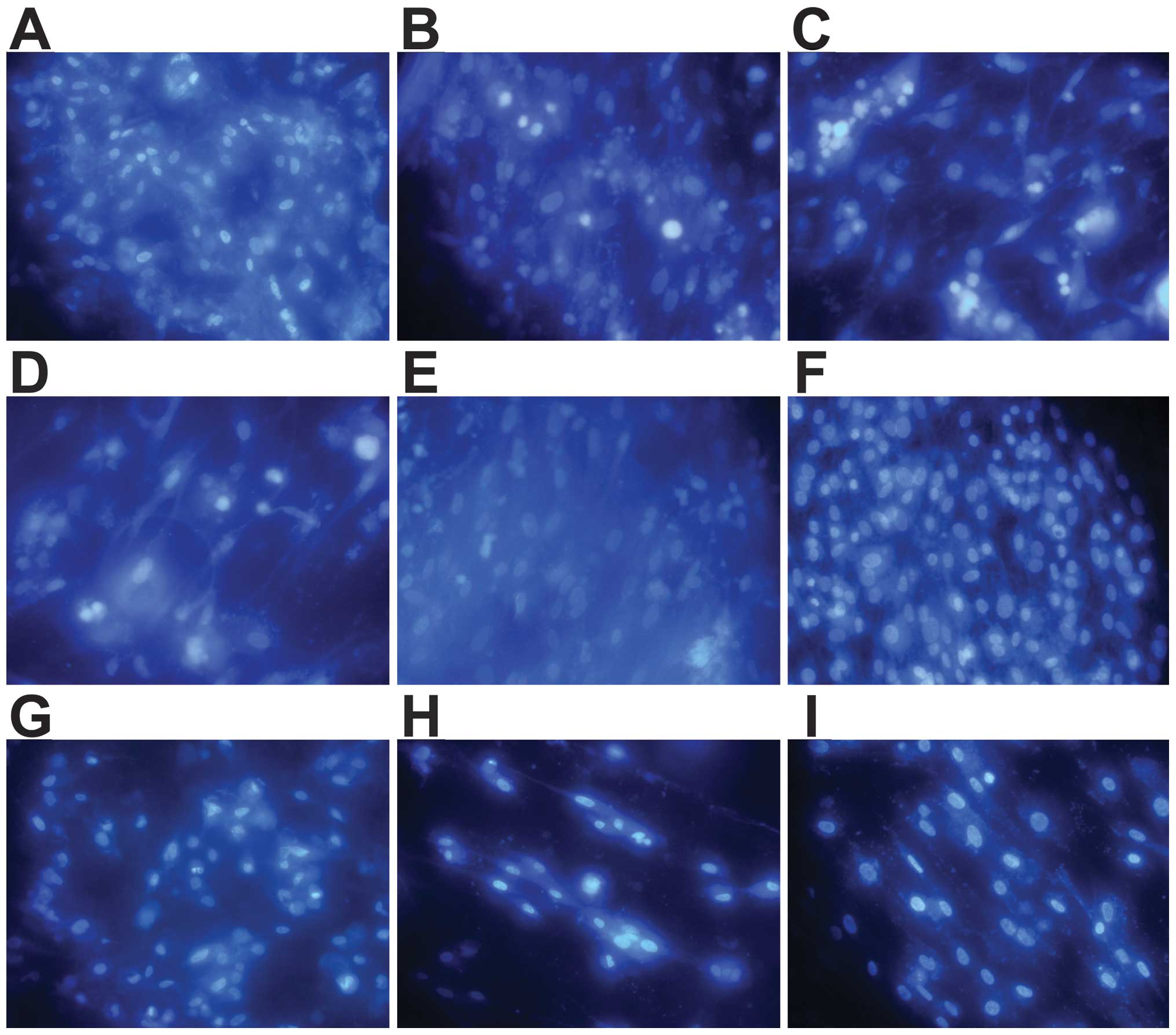 | Figure 24′,6-Diamidino-2-phenylindole (DAPI)
staining of chondrocytes (x400 magnification). Cells cultured for 3
days on (A) brushite
(CaHPO4·2H2O)-co ated JDBM
(C-JDBM), (B) Mg-Nd-Zn-Zr (JDBM), (C) AZ31, (D) WE43, (E) pure
magnesium (Mg), and (F) TC4. Cells cultured for 1 day on (G)
C-JDBM, (H) pure Mg, and (I) TC4. A few cells were already observed
on the samples after 1 day of culture (G–I). After 3 days of
culture, the number of cells on the discs increased (A–F). Among
the cultures, a significantly greater number of adhered cells was
observed on the surfaces of the C-JDBM and TC4 discs (A and F,
respectively), whereas the least number of cells was observed on
the WE43 disc (D). |
The chondrocytes cultured on the discs presented
with a highly elongated, irregular and round shape. A few cells
were already observed on the samples after 1 day of culture
(Fig. 2G–I). After 3 days of
culture, the number of cells on the discs increased (Fig. 2A–F). Among the cultures, a
significantly greater number of adhered cells was observed on the
surfaces of the C-JDBM and TC4 discs (Fig. 2A and F, respectively), whereas the
least number of cells was observed on the WE43 disc (Fig. 2D).
Indirect cytotoxicity and proliferation
assay
The absorbance of the chondrocytes cultured on the
different extracts, DMEM/F12 and 10% DMSO media for 1, 3, and 5
days is shown in Fig. 3. The
morphologies of the chondrocytes after 1, 3, and 5 days of
incubation are presented in Fig.
4. As shown in Fig. 3, the
absorbance of all groups of cells increased with time. As shown in
Table I, the time effect (day)
and the effects of day x group interaction were statistically
significant (p<0.05), indicating that the research target
changed with time. Furthermore, the role of the time factor varied
within each group. Tests on the between-subject effects indicated
that the grouping factor influenced the results, and each group of
targets generally differed (p<0.05). Likewise, the absorbance of
C-JDBM (Fig. 3) was greater than
that of the negative control and TC4, although the difference was
not significant (p>0.05). The absorbance of the remaining groups
showed significantly lower results (p<0.05). By contrast, no
significant differences were observed among the JDBM, AZ31, WE43
and pure Mg groups (p>0.05), thereby indicating that these
alloys have high cytocompatibility with chondrocytes.
 | Figure 3Results of cytotoxicity assay of the
Mg-Nd-Zn-Zr (JDBM), brushite
(CaHPO4·2H2O)-coated JDBM
(C-JDBM), AZ31, WE43, pure magnesium (Mg), and TC4 extraction
media, as wel as the Dulbecco’s modified Eagle’s medium with F12
(DMEM/F12) culture media, with and without 10% DMSO [mean ±
standard deviation (SD)] on chondrocytes after 1, 3 and 5 days.
*P<0.05 (positive control vs. all treatments;
negative control, C-JDBM, and TC4 vs. the other Mg alloys and Mg).
#P>0.05 (negative control vs. C-JDBM vs. TC4; JDBM
vs. AZ31 vs. WE43 vs. Mg). Negative control, cells cultured in
DMEM/F12 alone; positive control, cells cultured in DMEM/F12 medium
with 10% DMSO. |
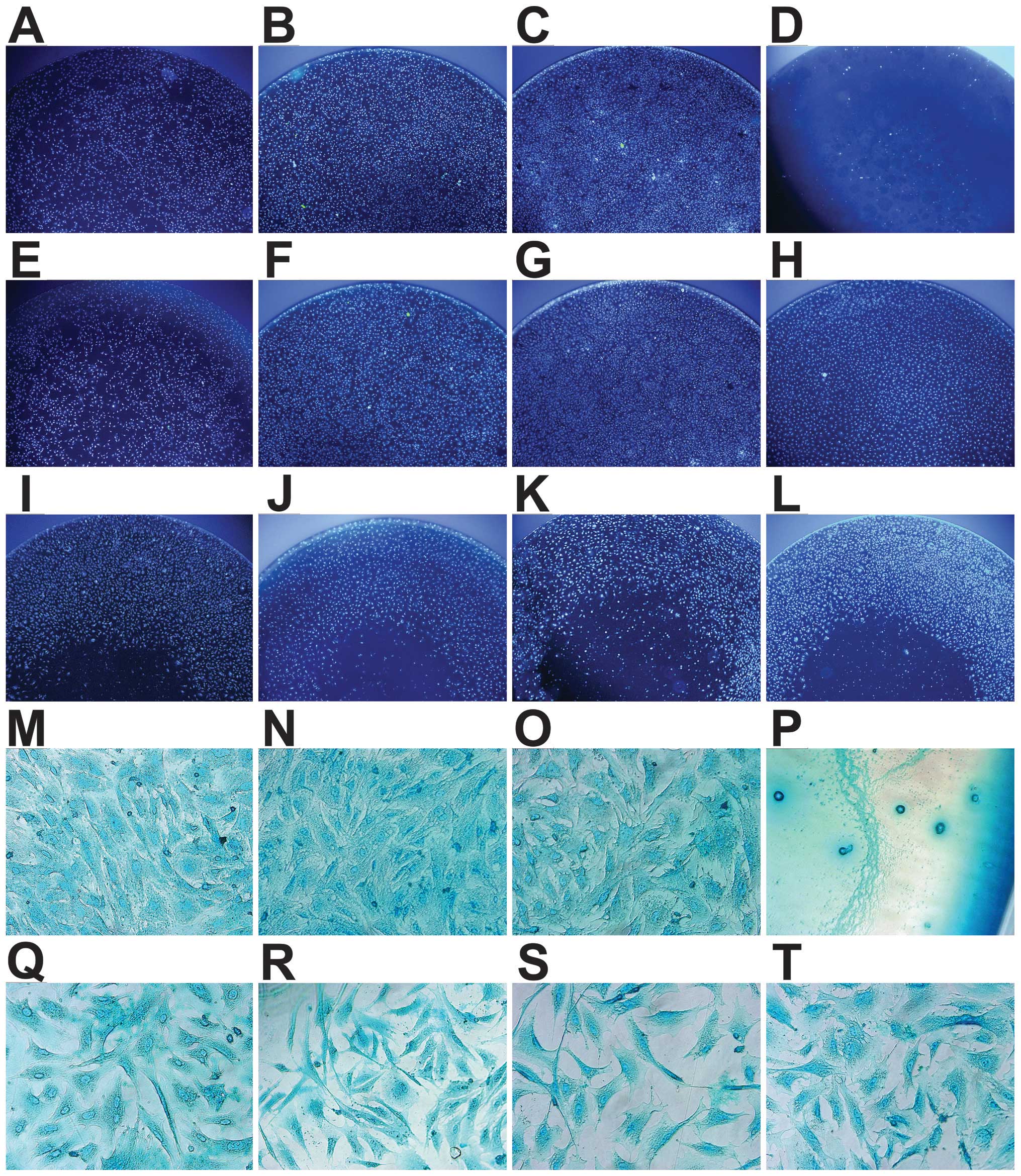 | Figure 4Morphology of the chondrocytes
follwoing 1, 3 and 5 days of incubation with: (A–C and M)
Dulbecco’s modified Eagle’s medium with F12 (DMEM/F12), (D and P)
10% DMSO medium, (E–G and N) brushite
(CaHPO4·2H2O)-coated JDBM
(C-JDBM), (H and O) Ti alloy (TC4), (I and Q) Mg-Nd-Zn-Zr (JDBM),
(J and R) AZ31, (K and S) WE43, and (L and T) pure magnesium (Mg).
(A–C and E–G) Cells were incubated for 1, 3 and 5 days,
respectively. (D and H–T) Cells were incubated for 3 days. (A–L)
Cells were stained with 4′,6-diamidino-2-phenylindole (DAPI) (x40
magnification). (M–T) Cells were stained with Alcian blue (x400
magnification). A greater number of cells was observed in the
negative control group (B), C-JDBM (F) and TC4 (H) groups after 3
days, as compared with the other groups (I–L), due to the loss of
cells in the center of the other culture plates, while no other
differences were observed between them. (A–C and E–G) The number of
cells was directly associated with the incubation time. The
chondrocytes in the positive control group were extremely scarce (D
and P), and unlike the elongated, polygonal, deltoid or irregular
shape of the other chondrocytes (M–O and Q–T), their morphology had
changed to a small and round shape (P), whereas the cells from all
the other treatment groups appeared normal. |
 | Table IStatistical analysis of time, group
factor effect and day x group interaction effect. |
Table I
Statistical analysis of time, group
factor effect and day x group interaction effect.
| Mauchly’s test of
sphericitya |
|---|
|
|---|
| Within subjects
effect | Mauchly’s W | Approx.
Chi-square | Df | Sig. | εb
|
|---|
| | | | |
Greenhouse-Geisser | Huynh-Feldt | Lower-bound |
|---|
|
|---|
| Day | 0.917 | 3.380 | 2 | 0.185 | 0.923 | 1.000 | 0.500 |
|---|
| Tests of within
subjects effects |
|
| Source | Type III sum of
squares | | Df | Mean square | F-value | Sig. |
|
| Day | | | | | | |
| Sphericity
assumption | 1.597 | | 2 | 0.798 | 475.791 | 0.000 |
|
Greenhouse-Geisser | 1.597 | | 1.847 | 0.865 | 475.791 | 0.000 |
| Huynh-Feldt | 1.597 | | 2.000 | 0.798 | 475.791 | 0.000 |
| Lower-bound | 1.597 | | 1.000 | 1.597 | 475.791 | 0.000 |
| Day x group | | | | | | |
| Sphericity
assumption | 0.802 | | 14 | 0.057 | 34.132 | 0.000 |
|
Greenhouse-Geisser | 0.802 | | 12.927 | 0.062 | 34.132 | 0.000 |
| Huynh-Feldt | 0.802 | | 14.000 | 0.057 | 34.132 | 0.000 |
| Lower-bound | 0.802 | | 7.000 | 0.115 | 34.132 | 0.000 |
| Error (day) | | | | | | |
| Sphericity
assumption | 0.134 | | 80 | 0.002 | | |
|
Greenhouse-Geisser | 0.134 | | 73.868 | 0.002 | | |
| Huynh-Feldt | 0.134 | | 80.000 | 0.002 | | |
| Lower-bound | 0.134 | | 40.000 | 0.003 | | |
|
| Tests of between
subjects effects |
|
| Source | Type III sum of
squares | | Df | | Mean square | F-value | Sig. |
|
| Intercept | 15.060 | | 1 | | 15.060 | 3.608E3 | 0.000 |
| Group | 4.437 | | 7 | | 0.634 | 151.828 | 0.000 |
| Error | 0.167 | | 40 | | 0.004 | | |
The analysis of cell morphology (Fig. 4) presented similar results as
those of the MTT assay. A greater number of cells was observed in
the negative control group (Fig.
4B), C-JDBM (Fig. 4F) and TC4
(Fig. 4H) groups after 3 days, as
compared with the other groups (Fig.
4I–L), due to the loss of cells in the center of the other
culture plates, while no other differences were observed between
them. As shown in Fig. 4A–C and
E–G, the number of cells was directly associated with the
incubation time. The chondrocytes in the positive control group
were extremely scarce (Fig. 4D and
P), and unlike the elongated, polygonal, deltoid or irregular
shape of the other chondrocytes (Fig.
4M–O and Q–T), their morphology had changed to a small and
round shape (Fig. 4P), whereas
the cells from all the other treatment groups appeared normal.
Apoptosis assay
The results from cell apoptosis assay (Fig. 5) were similar to those obtained by
MTT assay and the analysis of cell morphology (Figs. 3 and 4). The cells cultured on C-JDBM and TC4
were comparable with those of the negative control group
(p>0.05), in terms of the apoptotic rate, whereas the apoptotic
rate of the cells in the other groups was higher (p<0.05). There
were no statistically significant differences observed among the
JDBM, AZ31, WE43 and pure Mg groups (p> 0. 05).
 | Figure 5Apoptotic rate of chondrocytes
cultured in 25 cm2 plates after 5 days of incubation
with Mg-Nd-Zn-Zr (JDBM), brushite
(CaHPO4·2H2O)-coated JDBM
(C-JDBM), AZ31, WE43, pure magnesium (Mg), and TC4 extraction
media, as well as in Dulbecco’s modified Eagle’s medium with F12
(DMEM/F12). *P<0.05 (DMEM/F12, C-JDBM and TC4 vs.
JDBM, AZ31, WE43, and Mg). #P>0.05 (DMEM/F12 vs.
C-JDBM vs. TC4, JDBM vs. AZ31 vs. WE43 vs. Mg). Negative control,
cells cultured in DMEM/F12 alone; positive control, cells cultured
in DMEM/F12 medium with 10% DMSO. |
Total GAG quantification assay
The results obtained from the analysis of the GAG
content (Fig. 6) were comparable
to those obtained by MTT assay and the analysis of cell morphology
(Figs. 3 and 4) and apoptosis assay (Fig. 5). The GAG content in the cells
cultured on C-JDBM and TC4 was comparable to that of the negative
control, while the GAG content did not differ between the cells in
the other groups. There was no statistically significant difference
observed in the GAG content between the JDBM, AZ31, WE43 and pure
Mg groups (p>0.05).
 | Figure 6Total glycosaminoglycan (GAG) content
of chondrocytes cultured in 25 cm2 plates after 5 days
of incubation with Mg-Nd-Zn-Zr (JDBM), brushite
(CaHPO4·2H2O)-coated JDBM
(C-JDBM), AZ31, WE43, pure magnesium (Mg), and TC4 extraction
mediums, as well as Dulbecco’s modified Eagle’s medium with F12
(DMEM/F12). *P<0.05 (DMEM/F12, C-JDBM and TC4 vs.
JDBM, AZ31, WE43, and Mg). #P>0.05 (DMEM/F12 vs.
C-JDBM vs. TC4, JDBM vs. AZ31 vs. WE43 vs. Mg). Negative control,
cells cultured in DMEM/F12 alone; positive control, cells cultured
in DMEM/F12 medium with 10% DMSO. |
Col II content and ELISA
The results from ELISA were not satisfactory as the
values obtained for the JDBM, AZ31, WE43 and Mg samples were
extremely low, much lower than those of the kit (0.1–30 ng/ml).
Moreover, the negative control, as well as the C-JDBM and TC4
groups, only had values of 0.112±0.0083, 0.122±0.0068 and
0.101±0.0089 ng/ml, respectively (data not shown, as these results
were lower than the lower limit of the measuring range of the
kit).
RT-qPCR
As shown in Fig.
7, the results of the analysis of the relative mRNA expression
levels of aggrecan and Col II in the chondrocytes following 5 days
of incubation under the different treatment conditions were
consistent with those obtained form the other assays (Figs. 3Figure 4Figure 5–6) and with the results of our previous
study (12). The results revealed
that the cytocompatibility of JDBM was comparable to that of AZ31,
WE43 and pure Mg. Furthermore, the
CaHPO4·2H2O coating significantly
improved its compatibility.
 | Figure 7Aggrecan and collagen II (Col II)
gene expression relative to GAPDH after 5 days of incubation with
the Mg-Nd-Zn-Zr (JDBM), brushite
(CaHPO4·2H2O)-coated JDBM
(C-JDBM), AZ31, WE43, pure magnesium (Mg), and TC4 extraction
media, with Dulbecco’s modified Eagle’s medium with F12 (DMEM/F12)
as the control. *P<0.05 (DMEM/F12, C-JDBM and TC4 vs. JDBM,
AZ31, WE43, and Mg). #P>0.05 (DMEM/F12 vs. C-JDBM vs.
TC4, JDBM vs. AZ31 vs. WE43 vs. Mg). Negative control, cells
cultured in DMEM/F12 alone; positive control, cells cultured in
DMEM/F12 medium with 10% DMSO. |
Discussion
Despite numerous studies on Mg alloys for biomedical
applications, the majority of these studies focused on the
commercial Mg alloys that may be harmful to the human body
(8–10). The JDBM alloy was originally
developed as a Mg alloy for medical implants (11,12,15). The main aim of this study was to
evaluate the cytocompatibility of JDBM to chondrocytes, which may
be used for cartilage tissue engineering. The biocompatibility of
different cell lines with the same Mg alloy may be significantly
different (16). However,
chondrocytes, widely used as ‘seed’ cells in cartilage tissue
engineering, have been rarely used to study the biocompatibility of
Mg alloys. Our preliminary study presented positive results
(12); thus, further
investigation was performed. In the present study, JDBM and C-JDBM
served as the test groups, whereas the AZ31, WE43, pure Mg and TC4
alloy group were set up as the control group, aside from the
negative and positive controls. Comparisons among groups revealed
that JDBM and C-JDBM had better reference values as regards their
cytocompatibility, based on previous positive results obtained for
AZ31, WE43, and TC4 alloys, as well as pure Mg (1,2,17–19).
Cell direct adhesion experiments revealed efficient
chondrocyte growth, distribution and adhesion to all material
surfaces (Figs. 1 and 2). The adhesion behavior of the
chondrocytes is similar to that of MG-63 cells, L929 cells, human
bone marrow stromal cells (hBMSCs) and MC3T3-E1 cells on the
surface of Mg alloys (7,17,20,21). Furthermore, as shown in Fig. 2, the number of cells that had
adhered to the surface of the C-JDBM and TC4 discs (Figs. 2A and F) was greater than that of
the other groups (Fig. 2B–E),
thereby indicating the better biocompatibility of chondrocytes with
C-JDBM and TC4 than the others. Although the cells cultured on the
WE43 alloy appeared to be fewer (Fig.
2D), the biocompatibility of WE43 is not necessarily worse than
that of other non-coating materials. According to the study by
Witte et al (22), the
direct cell assay reduces cell viability more rapidly than the
indirect cytotoxicity tests. Cells are known to be very sensitive
to environmental fluctuations, including ion release, changes in pH
and hydrogen evolution. The disintegrated particles and corrosion
product Mg(OH)2 for Mg-based biomaterials, as well as
the influencing factors increased when the cells were directly
exposed to the material. To be specific, the significant increase
in the pH of cell culture media caused by Mg alloy degradation may
have an adverse effect on the cells (8,18,22-24). Besides, a corrosion product layer
is formed during the corrosion process of Mg and Mg alloys. During
immersion, the corrosion product gradually falls from the surface
due to the severe mismatch between the substrate and the corrosion
product layer (1). The formed
corrosion product and departure process make it difficult for the
cells to attach to the surface. Furthermore, the high hydrogen
evolution rate affects cell attachment and proliferation. Once a
Mg-based material is immersed in the cell culture medium, the
hydrogen gas evolves from the sample surface, which may deteriorate
cell adhesion and the ensuing proliferation process. With a high pH
value, Mg(OH)2 and zinc hydroxide [Zn(OH)2]
precipitate easily during the incubation period from the surface of
the sample, but also in the culture medium. However, the toxicity
of micro-sized Zn(OH)2 may be related to the particle
concentration. Nair et al (25) indicated that micro-ZnO is
extremely toxic to MG63 cells at a concentration above 100
µm (with an ~50% loss in cell viability). There were
different environment fluctuations for each sample. Due to the
aforementioned reasons, we changed the medium once a day to reduce
the influence of these fluctuations on the cells.
The SEM micrograph revealed few chondrocytes on the
TC4 disc. However, a few cells were fluorescently stained on the
parallel sample (Fig. 2F). The
lower adhesive force of chondrocytes, as compared with other cells
may account for this result. Chondrocyte digestion possibly occurs
more rapidly than other cells, including MC3T3-E1 cells in flask
cultures; it only needs approximately 30–60 sec. Thus, the
chondrocytes may have easily dropped off from the surface of the
TC4 samples due to their complex treatment process prior to SEM.
The shadows in Fig. 1F show the
chondrocytes spreading and falling off. Moreover, Mg alloys have
rougher surfaces following degradation than the Ti alloy, which
then favors chondrocyte adhesion onto Mg alloys.
Based on indirect cell cytotoxicity and
proliferation assay, the cytocompatibility of JDBM was comparable
with that of AZ31, WE43 and pure Mg; however, it may be
significantly improved by
CaHPO4·2H2O coating. Cell
viability increased with time, which was in accordance with the
results obtained by direct and indirect adhesion assays (Figs. 2 and 4), as well as those of previous studies
(7,16,17,20). The cell counts in parallel 96-well
plates were consistent with those obtained by MTT assay and the
anlaysis of cell morphology (Figs.
3 and 4). A greater number of
cells was observed in the negative control, C-JDBM and TC4 groups.
Some cells had disappeared from the center of the plates in the
JDBM, AZ31, WE43 and pure Mg groups. The following reasons may
account for these results: firstly, the obvious increase in the pH
in the media caused by Mg alloy corrosion had an adverse effect on
cell viability, as previously mentioned (7,8,17,19,23,24). Our preliminary results presented
high pH values for uncoated samples (12); however, further and more detailed
investigations are warranted to clarify this issue. Secondly,
chondrocytes can be expanded in vitro in monolayer culture,
although this multiplication may lead to dedifferentiation
beginning from 3 or 4 passages. This process makes the cells become
fibroblast-like as they lose their round phenotype and become
spindle-shaped, while switching their collagen production from
types II, IX and XI to types I, III and V (26–29). Given the limitations of a low
initial number of cells and their dedifferentiation, chondrocytes
are multiplied in monolayer culture to increase the number of cells
and are then transferred to a three-dimensional culture system to
regain their phenotype (26,27,29). Thus, in the present study, we used
cells that were passaged twice to eliminate such limitations.
Apparently, the viability of the chondrocytes is inferior to that
of other cells (26,27,29). Thirdly, the wells of 96-well
plates are small, and surface tension brings more cell suspensions
to the periphery, and more cells gathered together are more
susceptible to survival than sparse ones.
The results obtained by MTT and apoptosis assays, as
well as the from the analysis of GAG and Col II content alongside
the relative mRNA expression of aggrecan and Col II were likewise
similar. GAG, Col II, aggrecan and Col II mRNA expression are
unique to chondrocytes (14,26–29). The cells were visibly well
attached to the coating and proliferated normally. These results
can be explained by the corrosion protecting effect of the brushite
coating, as discussed above, thereby improving the
cytocompatibility. The purity of Mg alloys and surface modification
may reduce the degradation rate of Mg and its alloys (7,17,19,20,22,30). Mg purity has improved as
metallurgical techniques have improved. Surface modification
includes alkali-heat treatment, carbonate treatment, ion plating
deposition of Ti, surface plasma immersion ion implantation,
micro-arc oxidation, fluoride coating and phosphate coating.
Calcium phosphate (Ca-P) coatings are widely used on bone implant
materials due to their favorable biocompatibility and
osteoconductive properties (7,31).
The brushite (CaHPO4·2H2O) coating
has been reported to significantly improve the biocorrosion
resistance and osseous integration of Mg alloys (7). In this study,
CaHPO4·2H2O was coated onto JDBM
alloys through chemical deposition. Compared with other surface
treatment methods, the CaHPO4·2H2O
coating is simpler and easier to control. Furthermore,
CaHPO4·2H2O is applicable to
implants of complex shapes and low-temperature processes. This last
feature is of particular importance during the surface modification
of Mg implants due to the low melting point of Mg (17,30). The Ca-P coating can effectively
reduce the degradation rate of Mg and its alloys. Our findings
confirm the excellent biocompatibility and desirable protective
effects of the coated sample, which is in agreement with the
results previously reported (7,17,20,30,31).
The property difference of our proposed coated and
uncoated alloys may be attributed to the following reasons:
firstly, the alkalization effect caused by the rapid corrosion of
Mg alloys is undesirable for cell adhesion, growth and
proliferation; this alkalization effect rapidly increases the pH of
the SBF until pH >9.0 within approximately 2 h, which is beyond
the pH range suitable for cell survival, before it finally reaches
a relatively stable value of 10.5 (32). According to previously reported
results, the Ca-P coating can serve as an effective corrosion
inhabiting layer and reduce the increase in pH of simulated body
fluid (33). Although cells are
very sensitive to environmental fluctuations, particularly
fluctuations in pH (22–24), a Ca-P coating can improve the
corrosion resistance of JDBM alloys and provide an environment for
cells with a suitable pH (12,30). Thus, the Ca-P coating would
likewise benefit cell adhesion and growth. The in vitro cell
assays, including cell adhesion, MTT, apoptosis, GAG content assay
and RT-PCR, demonstrated that C-JDBM had an excellent cellular
response due to the CaHPO4·2H2O
coating.
Mg and Ca ions have been previouly demonstrated to
promote cell viability and proliferation, which are known to
promote cell differentiation (7,34).
Mg ions at an appropriate concentration can activate bone cells by
influencing the protein synthesis and ancillary processes (2,34).
Furthermore, Mg ion concentrations of up to 10.286 mM have been
reported to be safe to human bone marrow-derived stromal cells
(24). Another study demonstrated
that physiologically high extracellular Mg concentrations (10 mM)
enhance chondrocyte proliferation and redifferentiation in distinct
concentration ranges (16). Mg is
likewise known to be active in cell adhesion mechanisms (35). Furthermore, Mg hydroxide may
enhance osteoblast activity and decrease the osteoclast number
temporarily in peri-implant bone remodeling (36). Overall, the Mg ion and
Mg(OH)2 may enhance the viability, proliferation, and
adhesion ability of chondrocytes.
Finally, calcium (Ca) ions are essential in chemical
signaling for cells (37). Ca
ions on the material surfaces favor protein absorption, e.g.,
fibronectin and vitronectin, which are important cell
attachment-promoting proteins. Therefore, Ca ions improve cell
attachment and spreading onto the surface (38). The enhanced Mg ion concentration
elevates the calcium concentration (39), which is suitable for the
nucleation of the Ca-P containing compounds. This mechanism
verifies that Mg is capable of osteoconductivity (39,40). Bone growth on an implant surface
requires the presence of sufficient amounts of Ca and phosphate
ions (17). Therefore,
CaHPO4·2H2O enhances the cellular
response.
The combined results show that the presence of Ca
and Mg ions, together with a more stable pH, may increase cell
proliferation with C-JDBM, as compared with the uncoated JDBM,
AZ31, WE43 and Ti alloys or pure Mg (12).
In conclusion, in this study, the Mg alloy, JDBM,
was investigated as a medical biodegradable material in terms of
its cytocompatibility to chondrocytes in vitro. JDBM
demonstrated high biocompatibility to chondrocytes, which was
similar to the performance of AZ31, WE43 and pure Mg. The
CaHPO4·2H2O coating may
significantly improve its biocompatibility, which was attributed to
the presence of Mg and Ca ions, as well as its more stable pH.
Acknowledgments
The present study was sponsored by grants from the
Science and Technology Commission of Shanghai Municipality
(11DJ1400300), the National Natural Science Foundation of China
(nos. 81271961 and 51174136), and the National Key Technology
Research and Development Program of China (2012BAI18B01).
References
|
1
|
Song G, Atrens A, St John D, Wu X and
Nairn J: The anodic dissolution of magnesium in chloride and
sulphate solutions. Corros Sci. 39:1981–2004. 1997. View Article : Google Scholar
|
|
2
|
Witte F, Kaese V, Haferkamp H, Switzer E,
Meyer-Lindenberg A, Wirth CJ and Windhagen H: In vivo corrosion of
four magnesium alloys and the associated bone response.
Biomaterials. 26:3557–3563. 2005. View Article : Google Scholar
|
|
3
|
Witte F, Fischer J, Nellesen J, Crostack
HA, Kaese V, Pisch A, Beckmann F and Windhagen H: In vitro and in
vivo corrosion measurements of magnesium alloys. Biomaterials.
27:1013–1018. 2006. View Article : Google Scholar
|
|
4
|
Xu L, Yu G, Zhang E, Pan F and Yang K: In
vivo corrosion behavior of Mg-Mn-Zn alloy for bone implant
application. J Biomed Mater Res A. 83:703–711. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kannan MB and Raman RKS: In vitro
degradation and mechanical integrity of calcium-containing
magnesium alloys in modified-simulated body fluid. Biomaterials.
29:2306–2314. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Staiger MP, Pietak AM, Huadmai J and Dias
G: Magnesium and its alloys as orthopedic biomaterials: A review.
Biomaterials. 27:1728–1734. 2006. View Article : Google Scholar
|
|
7
|
Xu L, Pan F, Yu G, Yang L, Zhang E and
Yang K: In vitro and in vivo evaluation of the surface bioactivity
of a calcium phosphate coated magnesium alloy. Biomaterials.
30:1512–1523. 2009. View Article : Google Scholar
|
|
8
|
Witte F, Hort N, Vogt C, Cohen S, Kainer
KU, Willumeit R and Feyerabend F: Degradable biomaterials based on
magnesium corrosion. Curr Opin Solid State Mater Sci. 12:63–72.
2008. View Article : Google Scholar
|
|
9
|
El-Rahman SS: Neuropathology of aluminum
toxicity in rats (glutamate and GABA impairment). Pharmacol Res.
47:189–194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hirano S and Suzuki KT: Exposure,
metabolism, and toxicity of rare earths and related compounds.
Environ Health Perspect. 104(Suppl 1): S85–S95. 1996. View Article : Google Scholar
|
|
11
|
Yuan G, Zhang X, Niu J, Tao H, Chen D, He
Y, Jiang Y and Ding W: Research progress of new type of degradable
biomedical magnesium alloys JDBM. Chin J Nonferrous Met.
21:2476–2488. 2011.
|
|
12
|
Liao Y, Ouyang Y, Niu J, Zhang J, Wang Y,
Zhu Z, Yuan G, He Y and Jiang Y: In vitro response of chondrocytes
to a biodegradable Mg-Nd-Zn-Zr alloy. Mater Lett. 83:206–208. 2012.
View Article : Google Scholar
|
|
13
|
Niu J, Yuan G, Liao Y, Mao L, Zhang J,
Wang Y, Huang F, Jiang Y, He Y and Ding W: Enhanced biocorrosion
resistance and biocompatibility of degradable Mg-Nd-Zn-Zr alloy by
brushite coating. Mater Sci Eng C Mater Biol Appl. 33:4833–4841.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin YJ, Yen CN, Hu YC, Wu YC, Liao CJ and
Chu IM: Chondrocytes culture in three-dimensional porous alginate
scaffolds enhanced cell proliferation, matrix synthesis and gene
expression. J Biomed Mater Res A. 88:23–33. 2009. View Article : Google Scholar
|
|
15
|
Zhang XB, Yuan GY, Mao L, Niu JL and Ding
WJ: Biocorrosion properties of as-extruded Mg-Nd-Zn-Zr alloy
compared with commercial AZ31 and WE43 alloys. Mater Lett.
66:209–211. 2012. View Article : Google Scholar
|
|
16
|
Feyerabend F, Fischer J, Holtz J, Witte F,
Willumeit R, Drücker H, Vogt C and Hort N: Evaluation of short-term
effects of rare earth and other elements used in magnesium alloys
on primary cells and cell lines. Acta Biomater. 6:1834–1842. 2010.
View Article : Google Scholar
|
|
17
|
Geng F, Tan LL, Jin XX, Yang JY and Yang
K: The preparation, cytocompatibility, and in vitro biodegradation
study of pure β-TCP on magnesium. J Mater Sci Mater Med.
20:1149–1157. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Popat KC, Leoni L, Grimes CA and Desai TA:
Influence of engineered titania nanotubular surfaces on bone cells.
Biomaterials. 28:3188–3197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Keim S, Brunner JG, Fabry B and Virtanen
S: Control of magnesium corrosion and biocompatibility with
biomimetic coatings. J Biomed Mater Res B Appl Biomater. 96:84–90.
2011. View Article : Google Scholar
|
|
20
|
Li J, Song Y, Zhang S, Zhao C, Zhang F,
Zhang X, Cao L, Fan Q and Tang T: In vitro responses of human bone
marrow stromal cells to a fluoridated hydroxyapatite coated
biodegradable Mg-Zn alloy. Biomaterials. 31:5782–5788. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang SX, Li JA, Song Y, Zhao C, Zhang X,
Xie C, Zhang Y, Tao H, He Y, Jiang Y and Bian YJ: In vitro
degradation, hemolysis and MC3T3-E1 cell adhesion of biodegradable
Mg-Zn alloy. Mater Sci Eng C Mater Biol Appl. 29:1907–1912. 2009.
View Article : Google Scholar
|
|
22
|
Witte F, Feyerabend F, Maier P, Fischer J,
Störmer M, Blawert C, Dietzel W and Hort N: Biodegradable
magnesium-hydroxy-apatite metal matrix composites. Biomaterials.
28:2163–2174. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Serre CM, Papillard M, Chavassieux P,
Voegel JC and Boivin G: Influence of magnesium substitution on a
collagen-apatite biomaterial on the production of a calcifying
matrix by human osteoblasts. J Biomed Mater Res. 42:626–633. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang C, Yuan G, Zhang J, Tang Z, Zhang X
and Dai K: Effects of magnesium alloys extracts on adult human bone
marrow-derived stromal cell viability and osteogenic
differentiation. Biomed Mater. 5:0450052010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nair S, Sasidharan A, Divya Rani VV, Menon
D, Nair S, Manzoor K and Raina S: Role of size scale of ZnO
nanoparticles and microparticles on toxicity toward bacteria and
osteoblast cancer cells. J Mater Sci Mater Med. 20(Suppl 1):
S235–S241. 2009. View Article : Google Scholar
|
|
26
|
Chaipinyo K, Oakes BW and van Damme MPI:
Effects of growth factors on cell proliferation and matrix
synthesis of low-density, primary bovine chondrocytes cultured in
collagen I gels. J Orthop Res. 20:1070–1078. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brodkin KR, García AJ and Levenston ME:
Chondrocyte phenotypes on different extracellular matrix
monolayers. Biomaterials. 25:5929–5938. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Martin I, Suetterlin R, Baschong W,
Heberer M, Vunjak-Novakovic G and Freed LE: Enhanced cartilage
tissue engineering by sequential exposure of chondrocytes to FGF-2
during 2D expansion and BMP-2 during 3D cultivation. J Cell
Biochem. 83:121–128. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gagne TA, Chappell-Afonso K, Johnson JL,
McPherson JM, Oldham CA, Tubo RA, Vaccaro C and Vasios GW: Enhanced
proliferation and differentiation of human articular chondrocytes
when seeded at low cell densities in alginate in vitro. J Orthop
Res. 18:882–890. 2000. View Article : Google Scholar
|
|
30
|
Du H, Wei Z, Wang H, Zhang E, Zuo L and Du
L: Surface microstructure and cell compatibility of calcium
silicate and calcium phosphate composite coatings on Mg-Zn-Mn-Ca
alloys for biomedical application. Colloids Surf B Biointerfaces.
83:96–102. 2011. View Article : Google Scholar
|
|
31
|
Hench LL: Bioceramics. J Am Ceram Soc.
81:1705–1728. 1998. View Article : Google Scholar
|
|
32
|
Song GL and Song SZ: A possible
biodegradable magnesium implant material. Adv Eng Mater. 9:298–302.
2007. View Article : Google Scholar
|
|
33
|
Xu L, Zhang E and Yang K: Phosphating
treatment and corrosion properties of Mg-Mn-Zn alloy for biomedical
application. J Mater Sci Mater Med. 20:859–867. 2009. View Article : Google Scholar
|
|
34
|
Rude RK, Gruber HE, Wei LY, Frausto A and
Mills BG: Magnesium deficiency: Effect on bone and mineral
metabolism in the mouse. Calcif Tissue Int. 72:32–41. 2003.
View Article : Google Scholar
|
|
35
|
Paul W and Sharma CP: Nanoceramic
matrices: Biomedical applications. Am J Biochem Biotechnol.
2:41–48. 2006. View Article : Google Scholar
|
|
36
|
Janning C, Willbold E, Vogt C, Nellesen J,
Meyer-Lindenberg A, Windhagen H, Thorey F and Witte F: Magnesium
hydroxide temporarily enhancing osteoblast activity and decreasing
the osteoclast number in peri-implant bone remodelling. Acta
Biomater. 6:1861–1868. 2010. View Article : Google Scholar
|
|
37
|
Ilich JZ and Kerstetter JE: Nutrition in
bone health revisited: A story beyond calcium. J Am Coll Nutr.
19:715–737. 2000. View Article : Google Scholar
|
|
38
|
Feng B, Weng J, Yang BC, Qu SX and Zhang
XD: Characterization of titanium surfaces with calcium and
phosphate and osteoblast adhesion. Biomaterials. 25:3421–3428.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
TenHuisen KS and Brown PW: Effects of
magnesium on the formation of calcium-deficient hydroxyapatite from
CaHPO 4·2H2O and
Ca4(PO4)2O. J Biomed Mater Res.
36:306–314. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Witte F, Reifenrath J, Müller PP, Crostack
H-A, Nellesen J, Bach FW, Bormann D and Rudert M: Cartilage repair
on magnesium scaffolds used as a subchondral bone replacement.
Materialwiss Werkstofftech. 37:504–508. 2006. View Article : Google Scholar
|