Introduction
Glucocorticoids (GCs) are widely used in the
treatment of a variety of diseases, including autoimmune diseases,
bronchial asthma and skin diseases. Although GCs are potent
antiinflammatory agents, long-term use can lead to various adverse
effects, including osteoporosis (3). GC-induced osteoporosis (GIOP), the
major cause of which is considered to be impairment of bone
formation (1,2) is the most common form of secondary
osteoporosis (3). Excess GCs
inhibits osteoblast maturation and differentiation, and promotes
the apoptosis of osteoblasts (4),
which is considered to be the third major cause of GIOP (4). Therefore, anti-apoptosis of
osteoblasts is generally served as the pivotal target for the
prevention of GCs-induced osteoporosis.
Puerarin, a C-glycoside compound, is a major
component derived from Pueraria lobata (Willd.), which is a
commonly used Chinese herbal medicine (5). A number of investigations have been
performed internationally to identify the pharmacology functions of
puerarin. Previous studies have reported that puerarin possesses
antioxidant (6,7), anti-inflammatory (8) and anti-apoptotic properties
(7). In addition, investigations
have revealed that puerarin has the ability to resist apoptosis in
a variety of cells, including liver cells (9), osteoblasts (10), kidney cells (11) and nerve cells (12). Although it acts as an important
regulator of cell death, the role of puerarin in the GC-induced
apoptosis of osteoblasts and the underlying mechanisms remain to be
fully elucidated.
Apoptosis is an essential process in maintaining
homeostasis under normal conditions (13). To date, studies have indicated
that there are two predominant apoptotic pathways: the extrinsic,
or death receptor, pathway and the intrinsic, or mitochondrial,
pathway (14), B-cell-associated
X protein (Bax) and B-cell lymphoma (Bcl)-2 are members of the
Bcl-2 family, which is closely associated with the mitochondrial
apoptotic pathway (15). Bcl-2
protein forms heterodimer complexes with Bax proteins, leading to
the release of cytochrome c from the mitochondria and the
induction of apoptosis (16). Liu
et al (17) found that
puerarin suppresses the apoptosis of human osteoblasts by
increasing the protein levels of Bcl-2, while decreasing the levels
of Bax in a dose-dependent manner. Wang et al (18) reported that puerarin prevents
MPP-induced apoptosis of PC12 cells by suppressing the release of
mitochondrial cytochrome c and activation of caspase-3.
Several signaling pathways, including the phosphoinositide 3-kinase
(PI3K)/Akt pathway and c-Jun N-terminal kinase (JNK) pathway, have
also been demonstrated to regulate the apoptosis of osteoblastic
cells (18–22). It has been observed that puerarin
inhibits serum-free-induced apoptosis of human osteoblast cells
(23), however, whether puerarin
has an effect on the GC-induced apoptosis of osteoblast remains to
be elucidated.
The present study aimed to investigate the effects
of puerarin on dexamethasone (DEX)-induced apoptosis in hFBO1.19
cells and examine the expression of several apoptosis-associated
proteins, including Bcl-2, Bax, caspase-3 and cytochrome c
were examined, to determine whether the effects were mediated by
the JNK and PI3K/Akt signaling pathways.
Materials and methods
Reagents
DEX and 17β-estradiol (E2) were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Puerarin (dissolved in dimethyl
sulfoxide (DMSO; molecular weight 416.38; purity >98%) was
obtained from the National Institute for the Control of
Pharmaceutical and Biological Products (Beijing, China). DEX and
pueranrin were used in a concentration gradient between
10−5 and 10−10 M. The final concentration of
DEX (10−5 M) and of E2 (10−5 M) was dissolved
in ethanol, and puerarin (10−5 M) was dissolved in DMSO
(Sigma-Aldrich). The DEX, puerarin and E2 were stored at −20°C.
Cell culture
The conditionally immortalized human fetal
osteoblastic cell line, hFOB1.19, was provided by Dr Meng (China
Medical University, Shenyang, China). The cells were cultured,
according to the procedures of American Type Culture Collection
(Manassas, VA, USA) and those previously described (24). For in vitro proliferation,
the hFOB1.19 cells were maintained in non-differentiation medium
consisting of 1:1 Dulbecco’s modified Eagle’s medium (DMEM)/F-12
(GE Healthcare Life Sciences, Logan, UT, USA) medium with 10% fetal
bovine serum (FBS; Biological Industries, Beit-Haemek, Israel), and
0.3 g/l G418 (Sigma-Aldrich), and were cultured in a humidified
incubator with 5% CO2 at 33.4°C. The medium was replaced
twice a week and the cells were cultured using 0.25% trypsin with
0.02% ethylenediaminetetraacetic acid (Sigma-Aldrich).
Cell proliferation assay
The hFOB1.19 cells, at 80–90% confluency, were
inoculated at 3×103 cells/well in 96-well plates and the
number of cells were quantified using an MTS assay, according to
the manufacturer’s instructions (Promega Corporation, Madison, WI,
USA). Briefly, 20 µl MTS solution was added to 100 µl
culture medium in each well. Following incubation for 2 h at 37°C,
the absorbance was read at a wavelength of 490 nm on a microplate
reader (Varioskan LUX; Thermo Fisher Scientific, Vantaa, Finland),
with measurements presented as the mean of at least three
independent experiments, with each data point based upon three
replicates.
To assess the effects of puerarin on cell
proliferation, the cells (5×103 per well) were incubated
in conditioned medium at 37°C for 3, 12 and 24 h at a concentration
gradient between 0, 10−5 and 10−9 M. Cells
were incubated with DEX in conditioned medium for 24, 48 and 72 h
at a concentration gradient between 0, 10−5 and
10−10 M.
Determination of cell death
Enzyme-linked immunosorbent assay (ELISA) detection
was performed to detect the levels of apoptosis, as previously
described (25). According to the
kit protocol (Roche Molecular Biochemicals, Mannheim, Germany), the
cells were plated at a density of 1×104 cells/well in
24-well plates at 37°C for 1 day and then cultured in the absence
or presence of puerarin (10−6–10−9 M) and E2
(10−5 M) at 37°C for 3 h, followed by incubation with
10−5 M DEX medium at 37°C for 48 h. The cells were then
rinsed with phosphate-buffered saline (PBS) and incubated with 0.5
ml lysis buffer (Beyotime Institute of Biotechnology, Shanghai,
China) at 4°C for 30 min, and centrifuged at 4°C for 10 min at 200
× g. Aliquots (20 µl) of the supernatant were then assessed
for the rate of apoptosis using the Cell Death Detection
ELISAPLUS kit (Roche Molecular Biochemicals).
Quantitification of histone-associated DNA fragments were
determined at 405 nm using a microplate ELISA reader (Varioskan
LUX; Thermo Fisher Scientific).
Assessment of apoptosis
Annexin V-fluorescein isothiocyanate (FITC) and
propidium iodide (PI) (Beyotime Institute of Biotechnology,
Shanghai, China) staining were performed according to the
manufacturer’s instructions, and the cells were analyzed using flow
cytometry (BD FACSCalibur; BD Biosciences, San Jose, CA, USA).
Briefly, the cells were harvested by trypsinization and washed in
PBS, and 1×105 cells were resuspended in 195 µl
binding buffer (Roche Molecular Biochemicals). The cell solution
(195 µl; 1×105 cells) were transferred to a 5 ml
culture tube and incubated with 5 µl Annexin V-FITC and 10
µl PI for 15 min at 25°C in the dark. Following incubation,
300 µl binding buffer was added to each tube and the cells
were analyzed using flow cytometry within 1 h. The cells, which
stained positive for Annexin V-FITC and negative for PI were
considered to be undergoing early stage apoptosis. Cells that
stained positive for Annexin V-FITC and PI were considered to be
undergoing late stage apoptosis and those that stain negative for
Annexin V-FITC and PI remained alive.
A terminal deoxynucleotidyltransferase-mediated dUTP
nick-end labeling (TUNEL) assay was performed to identify apoptotic
cells with fragmented DNA, which was performed using a in
situ cell death detection kit, according to the manufacturer’s
instructions (Roche Molecular Biochemicals). Briefly, the hFOB1.19
cells (5×103) were plated onto coverslips and cultured
overnight at 37°C and reached 80–90% confluency. The cells were
then cultured in medium with different concentrations of puerarin
(10−8–10−9 M) at 37°C for 3 h, and then
treated with 10−5 M DEX at 37°C for 48 h. Subsequently,
the cells were fixed in immune stationary liquid (Beyotime
Institute of Biotechnology, Suzhou, China) at 37°C for 60 min at
room temperature and washed with immune washing liquid, containing
0.1% Triton X-100 (contained in immune washing liquid; Beyotime
Institute of Biotechnology, Suzhou, China), for 2 min on ice. For
counterstaining, 4-Diamino-2-phenylindole (DAPI) was used, and the
TUNEL+ and DAPI+ nuclei in the cells were
counted manually. The cells were observed using an Eclipse E600
fluorescence microscope (magnification, ×100; Nikon, Tokyo, Japan),
in which six fields were randomly selected. The percentage of
positive cells was calculated as the apoptotic index (AI) using the
following equation: AI = (number of positive cells/total number of
cells) × 100%, as previously described (26,27).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA was extracted from the cells using a
MiniBEST Universal RNA Extraction kit (Takara Bio, Inc., Dalian,
China). The concentration and purity of the total RNA were
calculated with absorbance of 260 and 280 nm. Single strand cDNA
synthesis was performed using a PrimeScript RT Master Mix kit
(Takara, Bio, Inc.). The qPCR was performed using SYBR Premix Ex
Taq (Takara, Bio, Inc.) on an Mx3000P Real-Time PCR system (Applied
Biosystems, Foster City, CA, USA) as follows: 50 cycles of 95°C for
10 sec and 60°C for 30 sec. Each reaction contains 10 µl
SYBR Green 1, 2 µl primer, 1 µl dNTP, 2 µl Taq
Polymerase, 5 µl cDNA and 30 µl ddH2O. All
the reactions were repeated at least three times. The expression
levels of the target genes, caspase-3, Bcl-2 and Bax, were
calculated relative to housekeeping β-actin using Stratagene
Mx3000P software (Applied Biosystems). The qPCR primers were
designed using Primer 5.0 software, the sequences of which are
listed in Table I.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Name | Forward primers
(5′→3′) | Reverse primers
(5′→3′) |
|---|
| Caspase-3 |
TGTGAGGCGGTTGTAGAAGTT |
GCTGCATCGACATCTGTACC |
| Bax |
GTCCAATGTCCAGCCCATGA |
ATCATGTTTGAGACCTTCAACA |
| Bcl-2 |
GGTGAACTGGGGGAGGATTG |
GGCAGGCATGTTGACTTCAC |
| β-actin |
ATCATGTTTGAGACCTTCAACA |
CATCTCTTGCTCGAAGTCCA |
Western blot analysis
Total protein was extracted from the cells using
radioimmunoprecipitation assay lysis buffer and quantified using a
bicinchoninic acid protein assay (Beyotime Institute of
Biotechnology, Suzhou, China). Equal quantities of protein were
separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE; 10% gel) and transferred onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were then blocked with 5% nonfat milk in PBS
for 1 h at room temperature and incubated with Bcl-2 (rabbit mAb;
2870), Bax (rabbit mAb; 5023), cytochrome c (rabbit mAb;
11940), Akt (rabbit mAb; 4691), phosphotylated (p)-Akt (Ser473;
rabbit mAb; 4060), ERK1/2 (Rabbit Ab; 9102), p-ERK (rabbit mAb;
4370), p38 (rabbit mAb; 8690), p-p38 (rabbit mAb; 4511), JNK
(rabbit Ab; 9252) and p-JNK (rabbit mAb; 4668) antibodies (1:1,000;
Cell Signaling Technology, Inc., Beverly, MA, USA) overnight at
4°C. Following extensive washing in washing liquid 3 times, the
membranes were re-probed with horseradish peroxidase
(HRP)-conjugated secondary antibodies (Cell Signaling Technology,
Inc.). The blots were then washed, and the signal was visualized
using an HRP chemiluminescent substrate reagent kit (Invitrogen
Life Technologies, Carlsbad, CA, USA), according to the
manufacturer’s instructions. The band intensity was quantified
using densitometric analysis with ImageJ 1.36 software (National
Institutes of Health, Bethesda, MD, USA), with the absorbance ratio
of each protein to the internal reference presented as the relative
quantity of target protein.
Statistical analysis
SPSS version 11.5 for Windows was used for all
statistical analyses. All data are expressed as the mean ± standard
deviation. Statistical analyses of the data were performed using
one-way analysis of variance. P<0.05 was considered to indicate
a statistically significant difference. All experiments were
repeated at least three times.
Results
Effects of puerarin on the viability of
hFOB1.19 cells
To examine the effect of puerarin on the survival of
hFOB1.19 cells, the present study performed an MTS assay using the
puerarin- and vehicle (E2)-treated cells. The cells were treated
with different concentrations of puerarin (0,
10−6–10−10 M) or E2 (10−5 M) for
different durations (3, 12 and 24 h; Fig. 1). Treatment with lower
concentrations of puerarin (10−8–10−9 M)
increased cell growth and viability at all time-points (P<0.05),
compared with the untreated control cells. Notably, the hFOB1.19
cells treated with puerarin at 10−8 M for 3 h exhibited
the highest proliferation rate. By contrast, treatment with a
higher dose of puerarin (10−6 M) had no significant
effects on cell viability, compared with E2-treated and untreated
cells. Therefore, the cells were treated with puerarin at a
concentration of 10−8 M for a duration of 3 h in the
subsequent experiments.
DEX inhibits the proliferation of
hFOB1.19 cells
The hFOB1.19 cells were exposed to various
concentrations (0 and 10−5–10−9 M) of DEX for
24, 48 or 72 h, and the cell viability was analyzed using an MTS
assay. DEX had significant inhibitory effects on cell proliferation
at concentration of 10−5 and 10−6 M (Fig. 2). Treatment with DEX at
10−5 M resulted in a 49, 37 and 29% reduction of
survival at 24, 48 and 72 h, respectively. Treatment with DEX at
10−6 M resulted in a 35, 30 and 25% reduction of
survival at 24, 48 and 72 h, respectively. Thus, the hFOB1.19 cells
were treated with DEX at a concentration of 10−5 M for a
duration of 48 h in the subsequent experiments.
Puerarin suppresses DEX-induced apoptosis
in hFOB1.19 cells, detected using ELISA and TUNEL
The present study used an ELISA to quantify DNA
fragmentation, a hallmark of apoptosis (28). The hFOB1.19 cells were cultured in
DEX (10−5 M)-containing medium for 48 h in the presence
of either 0–10−6 M puerarin or 10−5 M E2. The
hFOB1.19 cells in 10% FBS medium exhibited basal levels of
apoptosis (0.13±0.01 ELISA absorbance units), while DEX treatment
markedly increased the levels of apoptosis, compared with the
control (1.71±0.01 ELISA absorbance units; Fig. 3). However, following exposure to
puerarin the numbers of apoptotic cells in the cells treated with
puerarin at concentrations of 10−10 M (1.64±0.03),
10−9 M (0.83±0.05), 10−8 M (0.64±0.05),
10−7 M (1.16±0.05) and 10−6 M (1.33±0.04
ELISA absorbance units) were lower, compared with those in the
DEX-treated group and positive control group exposed to
10−5 M E2 (P<0.05). The maximal anti-apoptotic effect
of puerarin was observed at a concentration of 10−8 M,
and no anti-apoptotic effect was observed at a concentration of
10−10 M.
A TUNEL assay was also performed in the present
study, which also indicated that 10−8 M puerarin
significantly decrease DEX-induced apoptosis in the hOB1.19 cells.
The hFOB1.19 cells were treated with DEX (10−5 M) in the
absence or presence of 10−8 and 10−9 M
puerarin. The percentage of apoptotic cells exhibiting green
condensed or fragmented nuclei, identified by TUNEL staining, was
significantly lower in the cells treated with DEX in the presence
of puerarin (Fig. 4), compared
with the cells in the control group. The data from the above two
assays indicated that puerarin protected the hFOB1.19 cells against
DEX-induced apoptosis.
Effect of puerarin on the expression of
apoptosis-associated protein in hFOB1.19 cells
To clarify the possible mechanisms by which puerarin
attenuated DEX-induced apoptosis, western blot analysis and RT-qPCR
were performed to examine a panel of apoptosis-associated proteins,
including Bcl-2, Bax, caspase-3 and cytochrome c. The
hFOB1.19 cells were incubated with DEX 10−5 M in
combination with different concentrations of puerarin (0,
10−9, 10−8 and 10−7 M) for 48 h,
following which the protein and mRNA levels were examined.
The results demonstrated that puerarin increased the
protein expression of Bcl-2, and decreased the protein expression
of Bax in the hFOB1.19 cells in a dose-dependent manner (all
P<0.05; Fig. 5). As the
concentration of puerarin increased between 10−7 and
10−9 M, the protein expression of Bax decreased
gradually, and 10−9 M puerarin almost eradicated the
DEX-induced expression of Bax (P<0.05), compared with the cells
treated with DEX only (Fig. 5).
In addition, puerarin at concentrations of 10−8 and
10−9 M increased the protein expression of Bcl-2, and
the maximal effect was observed at 10−8 M (P<0.05,
compared with DEX alone; Fig. 5).
In accordance, the results of the qPCR revealed similar results to
the western blot analyses.
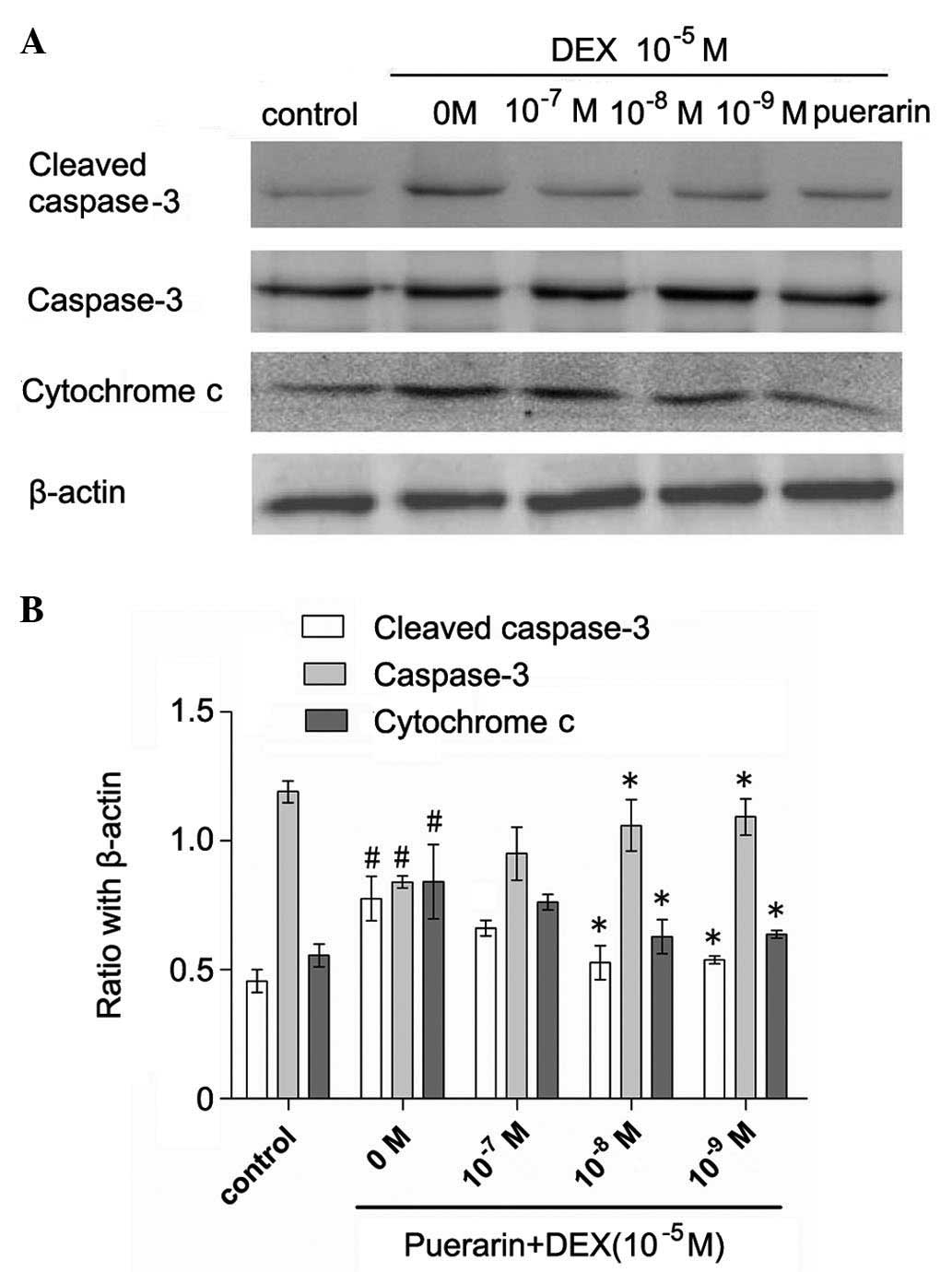
Cytochrome c was released into the cytoplasm
in the DEX-treated cells, whereas pretreatment with puerarin at
concentration between 10−7 and 10−9 M
attenuated the DEX-induced release of cytochrome c (Fig. 6). Additionally, puerarin
pretreatment significantly decreases the protein expression of
cleaved caspase-3. Consistent with the results of cellular caspase
activity, the results of the qPCR revealed that puerarin
pretreatment significantly decreased the mRNA levels of caspase-3
(Fig. 7).
 | Figure 7RT-qPCR analysis of caspase-3, Bax
and Bcl-2. The cells were treated with puerarin (0, 10–7,
10−8 and 10−9 M) for 3 h prior to incubation
with DEX (10−5 M) for 48 h. RT-qPCR was performed to
analyzed expression levels of caspase-3, Bax and Bcl-2. Data are
presented as the mean ± standard deviation. *P<0.05,
vs. DEX; #P<0.05, vs. control. RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; DEX,
dexamethasone; Bcl-2, B-cell lymphoma-2; Bax, B-cell-associated X
protein. |
Puerarin inhibits the JNK pathway and
activates the PI3K/Akt signaling pathway in hFOB1.19 cells
Puerarin exhibited no effects on p38 or
extracellular signal-regulated kinase (ERK) phosphorylation,
however, it increased the levels of p-Akt, and inhibited the levels
of p-JNK (Fig. 8). The hFOB1.19
cells were treated with DEX (10−5 M) alone or in
combination with puerarin (10−8 M) for 5, 30 or 60 min,
respectively. The results revealed that DEX significantly increased
the expression of p-JNK, with a maximal effect after 5 min,
however, no effect on the expression of p-Akt was observed.
Notably, the combined treatment with puerarin ameliorated the
DEX-induced activation of the JNK pathway, which became apparent at
30 min and peaked 60 min after puerarin treatment. In addition,
puerarin induced the phosphorylation of Akt, which peaked after 30
min.
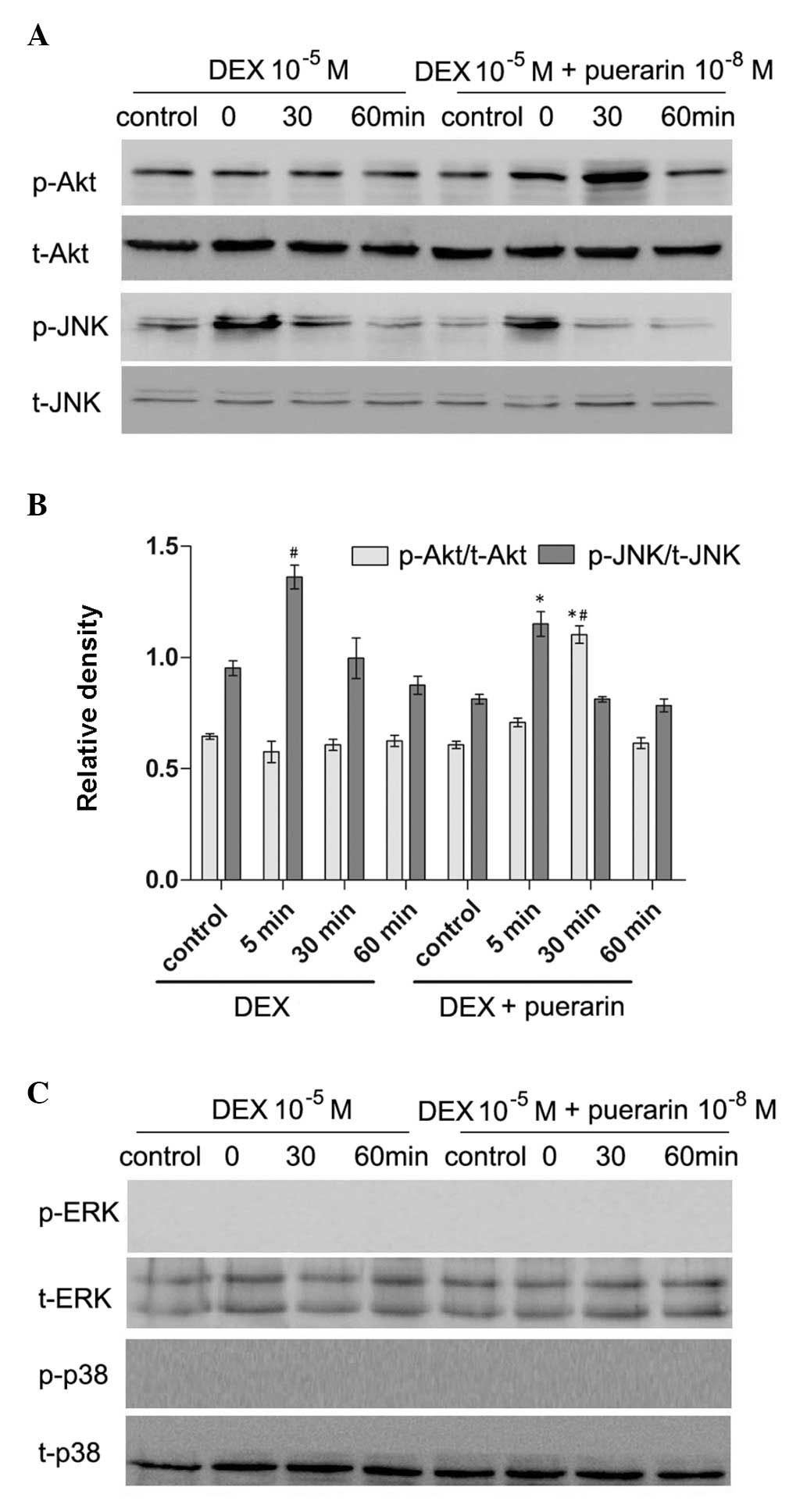 | Figure 8Effects of puerarin on the
phosphorylation of JNK and Akt in hFOB1.19 cells. (A and C) Cells
were treated with DEX (10−5 M) alone or in combination
with puerarin (10−8 M) for 5, 30 and 60 min. Western
blot analysis was performed to detect the expression levels of
p-JNK, JNK, p-Akt, Akt, p-ERK and ERK. (B) Relative expression
levels of p-Akt and p-JNK compared with t-Akt and t-JNK were
analyzed, respectively. Data are presented as the mean ± standard
deviation (n=3). *P<0.05, vs. DEX;
#P<0.05, vs. control. JNK, c-Jun N-terminal kinase;
ERK, extracellular signal-regulated kinase; p-, phosphorylated; t-,
total. |
Effect of LY294002and SP600125 on
puerarin-induced changes in the expression levels of p-Akt, p-JNK
in hFOB1.19 cells
In order to clarify whether the PI3K/Akt inhibitor,
LY294002, or JNK inhibitor, SP600125, had any effect on
puerarin-induced phosphoralation changes of Akt and JNK, the
present study incubated the cells with DEX, puerarin, LY294002 and
SP600125, alone or in combination, for 30 min (p-Akt) or 5 min
(p-JNK) (Fig. 9). The results
demonstrated that puerarin combined with DEX increased the
phosphorylation of Akt, compared with DEX alone, however, LY294002
partially abrogated this effect. The JNK inhibitor, SP600125,
ameliorated DEX-induced phosphorylation of JNK (Fig. 10). In addition, puerarin combined
with SP600125 almost eliminated the DEX-induced expression of
p-JNK, indicating puerarin as a potent JNK suppressor, which had
synergistic effect with SP600125.
JNK and PI3K/Akt signaling pathways
mediate the inhibitory effects of puerarin on apoptosis in hFOB1.19
cells
As puerarin treatment was observed to inhibit the
JNK signaling pathway and activate the PI3K/Akt signaling pathway
in the hFOB1.19 cells, and it is well known that these two pathways
are closely associated with the regulation of apoptosis, the
present study further examined whether these two pathways are
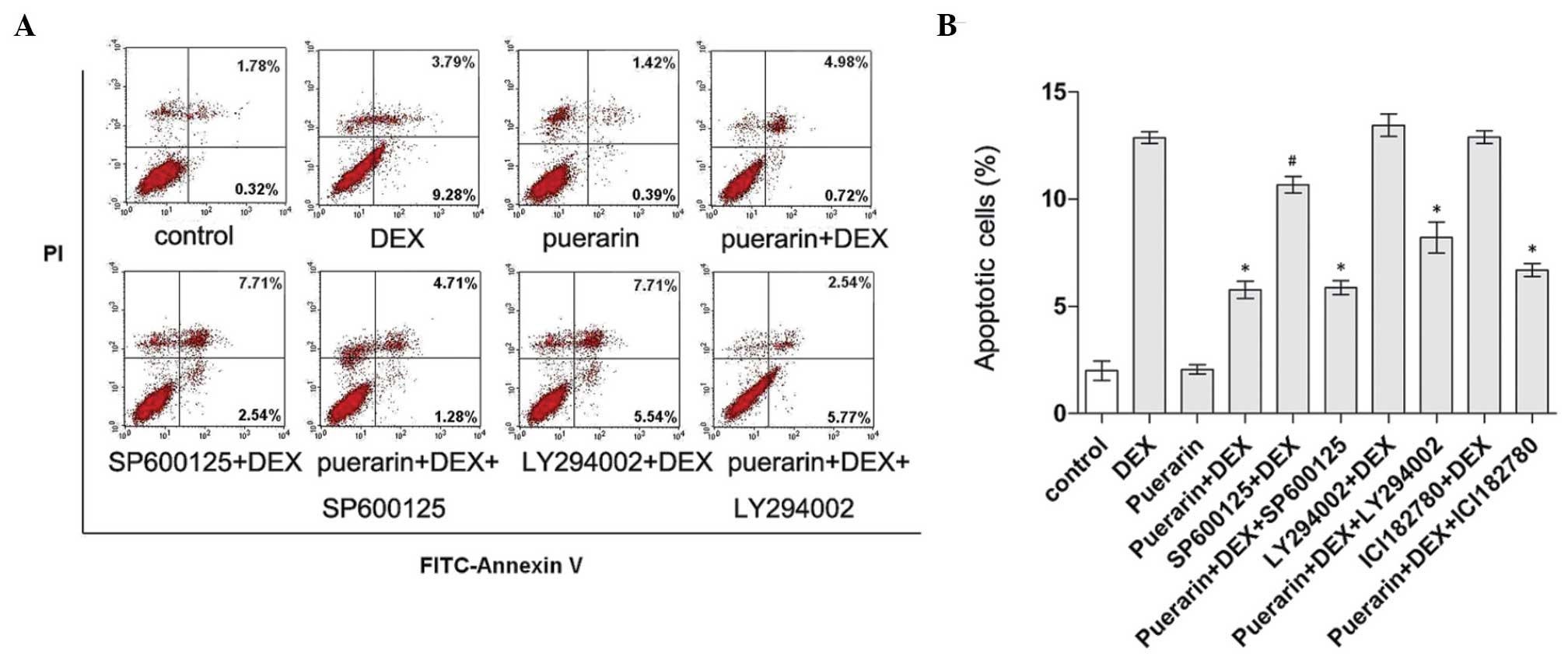
involved in the anti-apoptotic activity of puerarin. Cell apoptosis
was determined using Annexin V-FITC/PI flow cytometric analysis
(Fig. 10). According to the
results, DEX treatment significantly induced apoptosis, while
combined treatment with puerarin significantly attenuated this
effect. In addition, inhibition of the Akt pathway by LY294002
caused a marginal increase in DEX-induced apoptosis, and LY294002
partly eliminated the protective effect of puerarin on DEX-induced
apoptosis, indicating that puerarin exhibited anti-apoptotic
properties, partly dependent on the Akt signaling pathway. In line
with the above results, puerarin combined with SP600125 suppressed
DEX-apoptosis to a lesser extent, compared with SP600125 alone,
suggesting that puerarin enhanced the anti-apoptotic effect of the
JNK inhibitor, and that combination treatment may be a potentially
therapeutic option for GC-induced osteoporosis.
Discussion
GCs are widely used for their unsurpassed
anti-inflammatory and immunomodulatory effects, however, the
clinical applications are limited by substantial adverse outcomes,
including osteoporosis (29).
GIOP is the most common cause of secondary osteoporosis (30) and the pro-apoptotic effect of
high-dose GCs on osteoblasts has been identified as a major cause
for GIOP (4,31,32). However, the underlying molecular
mechanisms underlying this action remain to be elucidated, which
has impeded the prevention and cure of this side effect.
The identification of naturally occurring
phytochemicals, which can antagonize osteopo rosis has received
increased attention. Studies have demonstrated that dehydrocostus
lactone, puerarin and chemical constituents of the fruits of
Prunus mume can reverse GC-induced apoptosis in osteoblasts
by regulating of various signaling pathways (10,33,34). Puerarin, the major isoflavonoid in
the traditional Chinese herb pueraria lobata, is unique in
that it contains a C-C conjugated glucose at position 8 of the
isoflavonoid structure (35), and
has been widely used in traditional Chinese medicine for the
treatment of various diseases (36,37). Puerarin is reported to
significantly facilitate the survival rate of osteoblasts, and
regulate osteoblast proliferation and differentiation. In
vivo studies have demonstrated that osteoblast implants in the
puerarin-treated rats have a higher rate of bone formation
(38,39). In addition, puerarin in the diet
has been observed to effectively prevent osteoporosis in
ovariectomized rats by increasing bone mineral density and bone
mineral content (35,40). Furthermore, bone defects, created
in rabbit parietal bone grafted using a mixture of puerarin and
collagen, formed >5-fold more new bone, compared with defects
grafted with collagen alone (41). In addition, various studies have
reported that puerarin stimulates the proliferation and
differentiation of osteoblasts and protects osteoblasts against
cell death in vitro (10,17,35,42). In the present study, puerarin
attenuated DEX-induced apoptosis in hFOB1.19 cells through
activation of the PI3K/Akt signaling pathway and inhibition of the
JNK signaling pathway. In addition, puerarin upregulated Bcl-2,
downregulated Bax, and inhibited the cleavage of caspase-3 and
release of cytochrome c. These findings indicated that
puerarin exhibited anti-apoptotic properties through the
mitochondrial apoptotic pathway.
Mitochondria have been reported to be key in the
regulation of apoptosis. The activation of Bax triggers the release
of cytochrome c from the intermembrane space into the
cytoplasm, which finally activates caspase enzymes that mediate the
entrance in the execution phase of the apoptotic program (43). Anti-apoptotic Bcl-2-associated
proteins suppress the activities of Bax and prevent the release of
cytochrome c (44).
Previous studies have demonstrated that puerarin suppresses
apoptosis through regulation of the Bcl-2 family proteins in a
variety of cells, including vascular endothelial cells, osteobastic
cells, vascular smooth muscle cells and human neurons (10,45–47). In the present study, western blot
analysis and RT-qPCR were used to examine the effect of puerarin on
major members of the Bcl-2 family, including anti-apoptotic Bcl-2
and pro-apoptotic Bax (48). The
results demonstrated that DEX repressed the expression of Bcl-2 and
promoted the release of Bax. Puerarin attenuated these changes,
indicating that puerarin exerted its activities by modulating Bcl-2
and Bax in hFOB1.19 cells.
Caspase-3 is one of the major activated cysteine
proteases in the caspase family and is pivotal in apoptosis.
Caspase-3 was considered to be a central mediator of apoptosis, it
cleaves a number of substrates and activates endonucleases,
including the activation of caspase-activated DNase, which is
responsible for internucleosomal DNA fragmentation, a hallmark of
apoptosis (49–51). In addition to activation of the
caspase family, the release of cytochrome c is regarded as a
key regulatory event in apoptosis under certain conditions and is
released from mitochondria into the cytosol, which results in
caspase activation and cell apoptosis (52). It is reported that osteoblasts
undergoing apoptosis in response to GCs exhibit typical features,
including the activation of caspase-3 and release of cytochrome
c (53,54). In the present study, DEX induced
the cleavage of caspase-3 and the release of cytochrome c,
whereas treatment with puerarin suppressed the cleavage of
caspase-3 and release of cytochrome c, suggesting that
puerarin inhibited DEX-induced apoptosis in a
mitochondria-dependent manner.
To further investigate the detailed mechanisms and
signaling pathways involved in the anti-apoptotic activity of
puerarin, the present study investigated the responses of the
PI3K/Akt and MAPK serine/threonine kinase signaling pathways to
puerarin treatment in the hFOB1.19 cells.
MAPK pathways, including the ERK, JNK and p38
pathways, are activated by several stimuli, and one of their major
functions is to connect cell surface receptors to transcription
factors in the nucleus, which consequently triggers long-term
cellular responses (54). ERK1/2
is generally associated with proliferation and growth. By contrast,
the JNK and p38 pathways are pivotal in mediating apoptosis by
modulating the transcription of apoptosis-associated genes, and is
involved in the progression of apoptosis in osteoblasts (17–20,27,55–57). The present study provided the
first evidence, to the best of our knowledge, that the JNK-MAPK
pathways mediated the inhibitory effects of puerarin on DEX-induced
apoptosis in the hFOB1.19 cells.
Among the pathways linked to apoptosis resistance,
the PI3K/Akt pathway stands out as the convergent point for a
variety of stimuli generated at the cell surface (58,59). The PI3K/Akt pathway, which can be
activated by growth factors and certain extracellular signals,
regulates cell proliferation, differentiation and survival
(60). It has been reported that
the PI3K/Akt pathway is involved in the protection of osteoblasts
from apoptosis (21,22,61). The present study investigated
whether puerarin affected the activation of PI3K/Akt, and found
that puerarin increased the phosphorylation of Akt. In addition,
the involvement of the PI3K/Akt signaling pathway in the
anti-apoptotic activity of puerarin was examined. The results
revealed that PI3K inhibitor, LY294002, partially abrogated the
anti-apoptotic effects of puerarin, confirming that the PI3K/Akt
pathway is critical in the anti-apoptotic effect of puerarin
against DEX-induced apoptosis in hFOB1.19 cells.
In conclusion, the present study demonstrated that
puerarin prevented the DEX-induced apoptosis of hFOB1.19 cells via
inhibition of the JNK pathway and activation of the PI3K/Akt
signaling pathway in hFOB1.19 cells, which was dependent on the
mitochondrial apoptotic pathway. These results indicated puerarin
as a promising target in the treatment of GC-induced
osteoporosis.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (grant. nos. 81370981 and
81200714).
References
|
1
|
Canalis E: Clinical review 83: Mechanisms
of glucocorticoid action in bone: implications to
glucocorticoid-induced osteoporosis. J Clin Endocrinol Metab.
81:3441–3447. 1996.PubMed/NCBI
|
|
2
|
Manelli F and Giustina A:
Glucocorticoid-induced osteoporosis. Trends Endocrinol Metab.
11:79–85. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tamura Y, Okinaga H and Takami H:
Glucocorticoid-induced osteoporosis. Biomed Pharmacother.
58:500–504. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weinstein RS, Jilka RL, Parfitt AM and
Manolagas SC: Inhibition of osteoblastogenesis and promotion of
apoptosis of osteoblasts and osteocytes by glucocorticoids.
Potential mechanisms of their deleterious effects on bone. J Clin
Invest. 102:274–282. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu L, Qiao H, Li Y and Li L: Protective
roles of puerarin and Danshensu on acute ischemic myocardial injury
in rats. Phytomedicine. 14:652–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guerra MC, Speroni E, Broccoli M, Cangini
M, Pasini P, Minghett A, Crespi-Perellino N, Mirasoli M,
Cantelli-Forti G and Paolini M: Comparison between chinese medical
herb Pueraria lobata crude extract and its main isoflavone puerarin
antioxidant properties and effects on rat liver CYP-catalysed drug
metabolism. Life Sci. 67:2997–3006. 2000. View Article : Google Scholar
|
|
7
|
Dong LP and Wang TY: Effects of puerarin
against glutamate excitotoxicity on cultured mouse cerebral
cortical neurons. Zhongguo Yao Li Xue Bao. 19:339–342. 1998.
|
|
8
|
Huang F, Liu K, Du H, Kou J and Liu B:
Puerarin attenuates endothelial insulin resistance through
inhibition of inflammatory response in an IKKβ/IRS-1-dependent
manner. Biochimie. 94:1143–1150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu CM, Ma JQ and Sun YZ: Puerarin
protects the rat liver against oxidative stress-mediated DNA damage
and apoptosis induced by lead. Exp Toxicol Pathol. 64:575–582.
2012. View Article : Google Scholar
|
|
10
|
Wang Y, Wang WL, Xie WL, Li LZ, Sun J, Sun
WJ and Gong HY: Puerarin stimulates proliferation and
differentiation and protects against cell death in human
osteoblastic MG-63 cells via ER-dependent MEK/ERK and PI3K/Akt
activation. Phytomedicine. 20:787–796. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu CM, Ma JQ and Sun YZ: Puerarin
protects rat kidney from lead-induced apoptosis by modulating the
PI3K/Akt/eNOS pathway. Toxicol Appl Pharmacol. 258:330–342. 2012.
View Article : Google Scholar
|
|
12
|
Li J, Wang G, Liu J, Zhou L, Dong M, Wang
R, Li X, Li X, Lin C and Niu Y: Puerarin attenuates
amyloid-beta-induced cognitive impairment through suppression of
apoptosis in rat hippocampus in vivo. Eur J Pharmacol. 649:195–201.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abud HE: Shaping developing tissues by
apoptosis. Cell Death Differ. 11:797–799. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Adams JM and Cory S: The Bcl-2 protein
family: Arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang H, Liu Y, Lao M, Ma Z and Yi X:
Puerarin protects Alzheimer’s disease neuronal cybrids from
oxidant-stress induced apoptosis by inhibiting pro-death signaling
pathways. Exp Gerontol. 46:30–37. 2011. View Article : Google Scholar
|
|
17
|
Liu LJ, Liu LQ, Bo T, Li SJ, Zhu Z, Cui RR
and Mao DA: Puerarin suppress apoptosis of human osteoblasts via
ERK signaling pathway. Int J Endocrinol. 2013:7865742013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang G, Zhou L, Zhang Y, Dong M, Li X, Liu
J and Niu Y: Implication of the c-Jun-NH2-terminal kinase pathway
in the neuroprotective effect of puerarin against
1-methyl-4-phenylpyr-idinium (MPP+)-induced apoptosis in PC-12
cells. Neurosci Lett. 487:88–93. 2011. View Article : Google Scholar
|
|
19
|
Guo C, Yuan L, Wang JG, Wang F, Yang XK,
Zhang FH, Song JL, Ma XY, Cheng Q and Song GH: Lipopolysaccharide
(LPS) induces the apoptosis and inhibits osteoblast differentiation
through JNK pathway in MC3T3-E1 cells. Inflammation. 37:621–631.
2014. View Article : Google Scholar
|
|
20
|
Tang SY, Xie H, Yuan LQ, Luo XH, Huang J,
Cui RR, Zhou HD, Wu XP and Liao EY: Apelin stimulates proliferation
and suppresses apoptosis of mouse osteoblastic cell line MC3T3-E1
via JNK and PI3-K/Akt signaling pathways. Peptides. 28:708–718.
2007. View Article : Google Scholar
|
|
21
|
Liang QH, Liu Y, Wu SS, Cui RR, Yuan LQ
and Liao EY: Ghrelin inhibits the apoptosis of MC3T3-E1 cells
through ERK and AKT signaling pathway. Toxicol Appl Pharmacol.
272:591–597. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liang D, Xiang L, Yang M, Zhang X, Guo B,
Chen Y, Yang L and Cao J: ZnT7 can protect MC3T3-E1 cells from
oxidative stress-induced apoptosis via PI3K/Akt and MAPK/ERK
signaling pathways. Cell Signal. 25:1126–1135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feng R, Feng L, Yuan Z, Wang D, Wang F,
Tan B, Han S, Li T, Li D and Han Y: Icariin protects against
glucocorticoid-induced osteoporosis in vitro and prevents
glucocorticoid-induced osteocyte apoptosis in vivo. Cell Biochem
Biophys. 67:189–197. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo D, Li Q, Lv Q, Wei Q, Cao S and Gu J:
MiR-27a targets sFRP1 in hFOB cells to regulate proliferation,
apoptosis and differentiation. PLoS One. 9:e913542014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cui RR, Mao DA, Yi L, et al: Apelin
suppresses apoptosis of human vascular smooth muscle cells via
APJ/PI3-K/Akt signaling pathways. Amino Acids. 39:1193–1200. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kitamura T, Itoh M, Noda T, Matsuura M and
Wakabayashi K: Combined effects of cyclooxygenase-1 and
cyclooxygenase-2 selective inhibitors on intestinal tumorigenesis
in adenomatous polyposis coli gene knockout mice. Int J Cancer.
109:576–580. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu X, Jiang Y, Shan PF, et al: Vaspin
attenuates the apoptosis of human osteoblasts through ERK signaling
pathway. Amino Acids. 44:961–968. 2013. View Article : Google Scholar
|
|
28
|
Habata Y, Fujii R, Hosoya M, et al:
Apelin, the natural ligand of the orphan receptor APJ, is
abundantly secreted in the colostrum. Biochim Biophys Acta.
1452:25–35. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Seibel MJ, Cooper MS and Zhou H:
Glucocorticoid-induced osteoporosis: Mechanisms, management, and
future perspectives. Lancet Diabetes Endocrinol. 1:59–70. 2013.
View Article : Google Scholar
|
|
30
|
van Brussel MS, Bultink IE and Lems WF:
Prevention of glucocorticoid-induced osteoporosis. Expert Opin
Pharmacother. 10:997–1005. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Beavan S, Horner A, Bord S, Ireland D and
Compston J: Colocalization of glucocorticoid and mineralocorticoid
receptors in human bone. J Bone Miner Res. 16:1496–1504. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gohel A, McCarthy MB and Gronowicz G:
Estrogen prevents glucocorticoid-induced apoptosis in osteoblasts
in vivo and in vitro. Endocrinology. 140:5339–5347. 1999.PubMed/NCBI
|
|
33
|
Choi EM, Kim GH and Lee YS: Protective
effects of dehydrocostus lactone against hydrogen peroxide-induced
dysfunction and oxidative stress in osteoblastic MC3T3-E1 cells.
Toxicol In Vitro. 23:862–867. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yan XT, Lee SH, Li W, Sun YN, Yang SY,
Jang HD and Kim YH: Evaluation of the antioxidant and
anti-osteoporosis activities of chemical constituents of the fruits
of Prunus mume. Food Chem. 156:408–415. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Michihara S, Tanaka T, Uzawa Y, Moriyama T
and Kawamura Y: Puerarin exerted anti-osteoporotic action
independent of estrogen receptor-mediated pathway. J Nutr Sci
Vitaminol (Tokyo). 58:202–209. 2012. View Article : Google Scholar
|
|
36
|
Kim H: Neuroprotective herbs for stroke
therapy in traditional eastern medicine. Neurol Res. 27:287–301.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Q, Wu T, Chen X, Ni J, Duan X, Zheng
J, Qiao J, Zhou L and Wei J: Puerarin injection for unstable angina
pectoris. Cochrane Database Syst Rev. 3:CD0041962006.PubMed/NCBI
|
|
38
|
Hwang YP and Jeong HG: Mechanism of
phytoestrogen puerarin-mediated cytoprotection following oxidative
injury: Estrogen receptor-dependent up-regulation of PI3K/Akt and
HO-1. Toxicol Appl Pharmacol. 233:371–381. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang MY, Qiang H, Yang HQ, Dang XQ and
Wang KZ: In vitro and in vivo effects of puerarin on promotion of
osteoblast bone formation. Chin J Integr Med. 18:276–282. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Urasopon N, Hamada Y, Cherdshewasart W and
Malaivijitnond S: Preventive effects of Pueraria mirifica on bone
loss in ovariectomized rats. Maturitas. 59:137–148. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wong R and Rabie B: Effect of puerarin on
bone formation. Osteoarthritis Cartilage. 15:894–899. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tiyasatkulkovit W, Charoenphandhu N,
Wongdee K, Thongbunchoo J, Krishnamra N and Malaivijitnond S:
Upregulation of osteoblastic differentiation marker mRNA expression
in osteoblast-like UMR106 cells by puerarin and phytoestrogens from
Pueraria mirifica. Phytomedicine. 19:1147–1155. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cao XH, Wang AH, Wang CL, Mao DZ, Lu MF,
Cui YQ and Jiao RZ: Surfactin induces apoptosis in human breast
cancer MCF-7 cells through a ROS/JNK-mediated mitochondrial/caspase
pathway. Chem Biol Interact. 183:357–362. 2010. View Article : Google Scholar
|
|
44
|
Chipuk JE and Green DR: How do BCL-2
proteins induce mitochondrial outer membrane permeabilization?
Trends Cell Biol. 18:157–164. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shi RL and Zhang JJ: Protective effect of
puerarin on vascular endothelial cell apoptosis induced by chemical
hypoxia in vitro. Yao Xue Xue Bao. 38:103–107. 2003.In Chinese.
PubMed/NCBI
|
|
46
|
Zhu LH, Wang L, Wang D, Jiang H, Tang QZ,
Yan L, Bian ZY, Wang XA and Li H: Puerarin attenuates
high-glucose-and diabetes-induced vascular smooth muscle cell
proliferation by blocking PKCbeta2/Rac1-dependent signaling. Free
Radic Biol Med. 48:471–482. 2010. View Article : Google Scholar
|
|
47
|
Han JQ, Yu KY and He M: Effects of
puerarin on the neurocyte apoptosis and p-Akt (Ser473) expressions
in rats with cerebral ischemia/reperfusion injury. Zhongguo Zhong
Xi Yi Jie He Za Zhi. 32:1069–1072. 2012.In Chinese. PubMed/NCBI
|
|
48
|
Gu YX, Du J, Si MS, Mo JJ, Qiao SC and Lai
HC: The roles of PI3K/Akt signaling pathway in regulating MC3T3-E1
preosteoblast proliferation and differentiation on SLA and SLActive
titanium surfaces. J Biomed Mater Res A. 101:748–754. 2013.
View Article : Google Scholar
|
|
49
|
Widłak P: The DFF40/CAD endonuclease and
its role in apoptosis. Acta Biochim Pol. 47:1037–1044. 2000.
|
|
50
|
Chua CC, Chua BH, Chen Z, Landy C and
Hamdy RC: Dexamethasone induces caspase activation in murine
osteoblastic MC3T3-E1 cells. Biochim Biophys Acta. 1642:79–85.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xie H, Tang LL, Luo XH, Wu XY, Wu XP, Zhou
HD, Yuan LQ and Liao EY: Suppressive effect of dexamethasone on
TIMP-1 production involves murine osteoblastic MC3T3-E1 cell
apoptosis. Amino Acids. 38:1145–1153. 2010. View Article : Google Scholar
|
|
52
|
Quintavalle C, Brenca M, De Micco F, et
al: In vivo and in vitro assessment of pathways involved in
contrast media-induced renal cells apoptosis. Cell Death Dis.
2:e1552011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu Y, Porta A, Peng X, Gengaro K,
Cunningham EB, Li H, Dominguez LA, Bellido T and Christakos S:
Prevention of glucocorticoid-induced apoptosis in osteocytes and
osteoblasts by calbindin-D28k. J Bone Miner Res. 19:479–490. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bost F, Aouadi M, Caron L and Binétruy B:
The role of MAPKs in adipocyte differentiation and obesity.
Biochimie. 87:51–56. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Matsuguchi T, Chiba N, Bandow K, Kakimoto
K, Masuda A and Ohnishi T: JNK activity is essential for Atf4
expression and late-stage osteoblast differentiation. J Bone Miner
Res. 24:398–410. 2009. View Article : Google Scholar
|
|
56
|
Kim SW, Her SJ, Park SJ, Kim D, Park KS,
Lee HK, Han BH, Kim MS, Shin CS and Kim SY: Ghrelin stimulates
proliferation and differentiation and inhibits apoptosis in
osteoblastic MC3T3-E1 cells. Bone. 37:359–369. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Xie H, Tang SY, Li H, Luo XH, Yuan LQ,
Wang D and Liao EY: L-carnitine protects against apoptosis of
murine MC3T3-E1 osteoblastic cells. Amino Acids. 35:419–423. 2008.
View Article : Google Scholar
|
|
58
|
Díaz-Montero CM, Wygant JN and McIntyre
BW: PI3-K/Akt-mediated anoikis resistance of human osteosarcoma
cells requires Src activation. Eur J Cancer. 42:1491–1500. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kim BJ, Lee YS, Lee SY, et al: Afamin
secreted from nonresorbing osteoclasts acts as a chemokine for
preosteoblasts via the Akt-signaling pathway. Bone. 51:431–440.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cantrell DA: Phosphoinositide 3-kinase
signalling pathways. J Cell Sci. 114:1439–1445. 2001.PubMed/NCBI
|
|
61
|
Kitase Y, Barragan L, Qing H, Kondoh S,
Jiang JX, Johnson ML and Bonewald LF: Mechanical induction of PGE2
in osteocytes blocks glucocorticoid-induced apoptosis through both
the β-catenin and PKA pathways. J Bone Miner Res. 25:2657–2668.
2010. View Article : Google Scholar : PubMed/NCBI
|