Introduction
Epidermal growth factor (EGF)-like domain 8 (EGFL8)
is a member of the EGFL domain family and was identified in mice as
a paralog of EGFL7 using the Basic Local Alignment Search Tool
(1). EGFL8 displays the same
overall domain structure as that of EGFL7 (1). EGFL domain, which consists of 30–40
amino acids and has a significant identity with EGF, is a common
structural module in numerous secreted or transmembrane proteins
and is generally involved in protein-protein interactions (2). The EGFL domain of each protein has
diverse physiological functions. For instance, the EGFL domain of
CD93 has a central role in eliciting angiogenesis by stimulating
proliferation, migration and in vitro tube formation of
human umbilical vein endothelial cells (3). The EGFLs in thrombospondins induce
synaptogenesis by interacting with neuronal cell-surface receptors
such as α2δ-1 (4). The EGFL
structures in a large number of the coagulation factors as well as
in thrombomodulin mediate complex formation, which is a critical
process in the blood coagulation cascade and in the
thrombomodulin-protein C anti-coagulant pathway (5). In addition, the EGFLs of the Notch
family proteins have essential roles in controlling the Notch
signaling pathway by binding their ligands on adjacent cells, and
their mutations and polymorphisms are implicated in the development
of severe diseases (6). For
example, polymorphisms in the EGFL domain of Notch-3 result in
symptomatic ischemic cerebrovascular disease (7).
To date, several EGFL proteins have been identified
using a complementary DNA (cDNA) library from human brain tissue,
but less is known about their physiological features. EGFL7 is
secreted by endothelial cells and is implicated in the regulation
of blood vessel formation and cell migration through interaction
with receptors of the Notch family (1,8–11).
EGFL7 binds to the extracellular domains of all four Notch receptor
isoforms and inhibits Jagged-induced Notch signaling (12). EGFL7 has recently been suggested
as a novel target for the design of therapies aimed at preventing
cancer progression by interfering with the processes of tumor
immune evasion, as the regulatory role of EGFL7 in tumor
endothelial cell activities has been implicated in tumor escape
from immunity through downregulation of immune cell extravasation
(13). EGFL7 expression is
significantly higher in low-grade invasive lesions and is
correlated with favorable prognosis in human breast cancer.
Furthermore, EGFL7 has been identified as an inhibitor of neural
stem cell maintenance (14).
EGFL6, an extracellular matrix protein, has been found in human
subcutaneous adipose tissue and is a paracrine/autocrine growth
factor of adipose tissue in obesity (15). EGFL6 is overexpressed in benign
meningioma tissues and serum (16).
Downregulation of EGFL8 expression has been observed
in gastric cancer, and is significantly correlated with high
tumor-node-metastasis stage and poor prognosis in gastric and
colorectal cancer (17,18). Recent studies by our group have
demonstrated that EGFL8 has inhibitory effects on mouse thymic
epithelial cells and thymocytes, indicating that it is a negative
regulatory molecule in T-cell development in the mouse thymus
(19,20). However, the physiological
characteristics and biological significance of EGFL8 have remained
to be elucidated. In addition, little information is available on
the molecular characterization and expression profile of EGFL8
(1). Thus, the present study was
performed to investigate the molecular and expressional
characterization of EGFL8 in various mouse tissue types.
Materials and methods
Cell lines and cell culture
The generation, maintenance and functional
characterization of mouse thymic sub-capsular cortex or thymic
nurse epithelial cells (SNECs) have been described previously
(21). SNECs constitutively
express the SV40 T antigen transgene and class I antigens of the
major histocompatibility complex, and they can be induced to
express major histocompatibility complex class II antigens via
stimulation with recombinant interferon-γ (IFN-γ) as well as
produce granulocyte-macrophage colony-stimulating factor (GM-CSF)
(21). SNECs were provided by Dr
Barbara B. Knowles (Jackson Laboratory, Bar Harbor, ME, USA) and
were cultured in Dulbecco’s modified Eagle’s medium containing 10%
(v/v) fetal bovine serum (both from Gibco-BRL, Invitrogen Life
Technologies, Carlsbad, CA, USA), 2 mM glutamine (Sigma-Aldrich,
St. Louis, MO, USA), 100 U/ml penicillin, and 100 μg/ml
streptomycin (both from Invitrogen Life Technologies) at 37°C in a
5% CO2-enriched atmosphere.
RNA extraction
Total RNA was extracted from each tissue or cell
sample. In case of tissues, they were transferred to a mortar
containing liquid nitrogen and ground to fine powder using a
pestle. Total RNA from tissue or cell samples was isolated using
TRIzol reagent (Invitrogen Life Technologies) following the
manufacturer’s instructions. After the addition of chloroform, the
mixture was vigorously shaken by hand for 15–30 sec and incubated
at room temperature for 5 min. The mixture was then centrifuged at
15,300 × g for 15 min at 4°C. The upper aqueous phase containing
the total RNA was carefully removed and placed in a fresh 50 ml
tube, where it was precipitated with isopropanol, washed with
ethanol and re-suspended in diethylpyrocarbonate-treated water. RNA
concentration and purity were determined using a spectrophotometer
(NanoDrop 2000c; Thermo Scientific, Wilmington, DE, USA) at
absorbances of 260 and 280 nm. Samples exhibiting a (260/280)
absorbance ratio of ≥1.7 and strong 28S- and 18S-ribosomal RNA
bands on 1% agarose gels were used for further analysis. RNA
samples were stored at −80°C until required. Polyadenylated
[poly(A)] RNA was purified from total RNA using a messenger RNA
(mRNA) isolation kit (200347; Stratagene, La Jolla, CA, USA)
according to the manufacturer’s instructions. Briefly, 5 mg total
RNA was mixed with 5 ml elution buffer and the mixture was
hybridized to 0.2 g oligo(dT) cellulose at room temperature with
gentle agitation followed by high-salt (low-stringency) and
low-salt (high-stringency) washes. These washes removed unwanted
components of the crude lysate, including proteins, carbohydrates,
lipids, DNA, transfer RNA and a significant amount of ribosomal RNA
from poly(A) mRNA. The oligo(dT) cellulose was loaded into a push
column and mRNA was eluted with 65°C elution buffer. The mRNA was
concentrated via ethanol precipitation, and the mRNA concentration
was determined by measuring the absorbance at 260 nm.
Cloning cDNA of the open reading frame
(ORF) region of mouse EGFL8
The first strand of cDNA was synthesized by the
reverse transcription using 1–2 μg of poly(A) mRNA. The ORF
region of mouse EGFL8 was amplified with the following primers:
EGFL8-sense (5′-AGC CCG CTC CCG CAC CAT-3′) and EGFL8-anti-sense
(5′-gcc ctt ggc tgc acg ctc a-3′), derived from mouse EGFL8
(GenBank accession no. NM_152922). PCR amplification of the cDNA
was performed for 25 cycles at 94°C for 30 sec, 60°C for 30 sec and
72°C for 60 sec in an automated thermal cycler (TC-312; Techne,
Teddington, UK) in a final volume of 25 μl containing 4
μl cDNA solution, 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 1.5 mM
MgCl2, 0.1% Triton X-100, 0.2 mM deoxynucleotide
triphosphate mixture (Invitrogen Life Technologies), 0.5 pmol of
each primer and 5 units TaqDNA polymerase (Promega). After PCR, the
amplified products were separated on a 1.5% agarose gel, purified
with a QIAquick gel extraction kit (Qiagen, Hilden, Germany) and
cloned into a pGEM-T-easy vector (Promega, Madison, WI, USA). The
cloned DNA was isolated using a High Pure plasmid isolation kit
(Roche, Basel, Switzerland).
Sequence analysis
Sequence alignments of EGFL8 and other
homologous EGFL family genes were performed using the
BioEdit sequence alignment editor program. To analyze functional
domains, hydrophobicity, nucleotide and amino acid sequence
composition, and synonymous/non-synonymous substitution of EGFL8,
online tools were used, including Pfam (22), ExPASy (23), MEGA5 (24) and the National Center for
Biotechnology Information (NCBI) Conserved Domain Database (CDD;
https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi).
Neighbor-joining phylogenetic analysis (25) was performed using CLUSTAL W
(26) and MEGA (24). EGFL sequences were retrieved from
the GenBank database (http://www.ncbi.nlm.nih.gov/genbank/)with the aid of
the Basic Local Alignment Search Tool network server (27).
RT-PCR amplification
Mouse EGFL8 transcripts were analyzed through RT-PCR
amplification. First-strand cDNA was obtained via reverse
transcription using 2 μg total RNA. The reaction was
performed in 20 μl buffer containing 0.5 μg
oligo(dT)12–18 primer, 50 mM Tris-HCl (pH 8.3), 75 mM
KCl, 3 mM MgCl2, 40 mM dithiothreitol, 0.5 mM
deoxynucleotide triphosphate mixture, 10 units RNase inhibitor and
200 units Moloney murine leukemia virus reverse transcriptase (all
from Invitrogen Life Technologies). After incubation at 37°C for 60
min, the reaction was stopped via heating at 70°C for 15 min. To
remove the remaining RNA, 1 μl Escherichia coli RNase
H (4 mg/ml; Promega) was added to the reaction mixture followed by
incubation at 37°C for 30 min. The cDNA was used as a template for
PCR amplification with gene-specific primers. As a standard
control, GAPDH was amplified using the primer pair GAPDH-sense
(5′-GAA ATC CCA TCA CCA TCT TCC AGG-3′) and GAPDH-anti-sense
(5′-GAG CCC CAG CCT TCT CCA TG-3′), derived from mouse GAPDH
(GenBank accession no. NM_002046). The mouse full-length EGFL8
transcript was amplified using the primer pair EGFL8-sense (5′-TTT
CAA AGA GAG TTT GGG AGT G-3′) and EGFL8-anti-sense (5′-CAC CAC GTG
TGT CTG TGG TA-3′), derived from mouse EGFL8 (GenBank accession no.
NM_152922). PCR amplification of the cDNA was performed in an
automated thermal cycler (TC-312; Techne) in a final volume of 25
μl containing 4 μl cDNA solution, 20 mM Tris-HCl (pH
8.4), 50 mM KCl, 1.5 mM MgCl2, 0.1% Triton X-100, 0.2 mM
deoxynucleotide triphosphate mixture (Invitrogen Life
Technologies), 0.5 pmol of each primer and 5 units TaqDNA
polymerase (Promega). All PCR reactions were performed in 30–35
cycles at 94°C for 30 sec, 57°C for 30 sec and 72°C for 30 sec.
After PCR, the amplified products were separated by 1.5% agarose
gel and visualized using ethidium bromide staining under
ultraviolet light using a Gel Doc XR system (Bio-Rad, Hercules, CA,
USA).
Molecular cloning, sequencing and
nucleotide sequence accession numbers
Since an additional band to the specific EGFL8 band
was identified in certain mouse organs when RT-PCR analysis of the
mouse EGFL8 was performed in various mouse tissue types, it was
hypothesized that this band may represent an isoform of mouse
EGFL8. To test the hypothesis, the RT-PCR products were separated
using a 1.5% agarose gel and purified using the
QIAquick® Gel Extraction kit (Qiagen) and cloned into a
pGEM-T-easy vector (Promega). The cloned DNA was isolated using a
High Pure plasmid isolation kit (Roche), amplified using the primer
pair, EGFL8-2-sense (5′-TTG GGG TCC CTT TGA GAC CT-3′) and
EGFL8-2-anti-sense (5′-TGC TGG TTC CTC TCC GTT AC-3′), and
sequenced by Macrogen (Seoul, Korea) to confirm their identities.
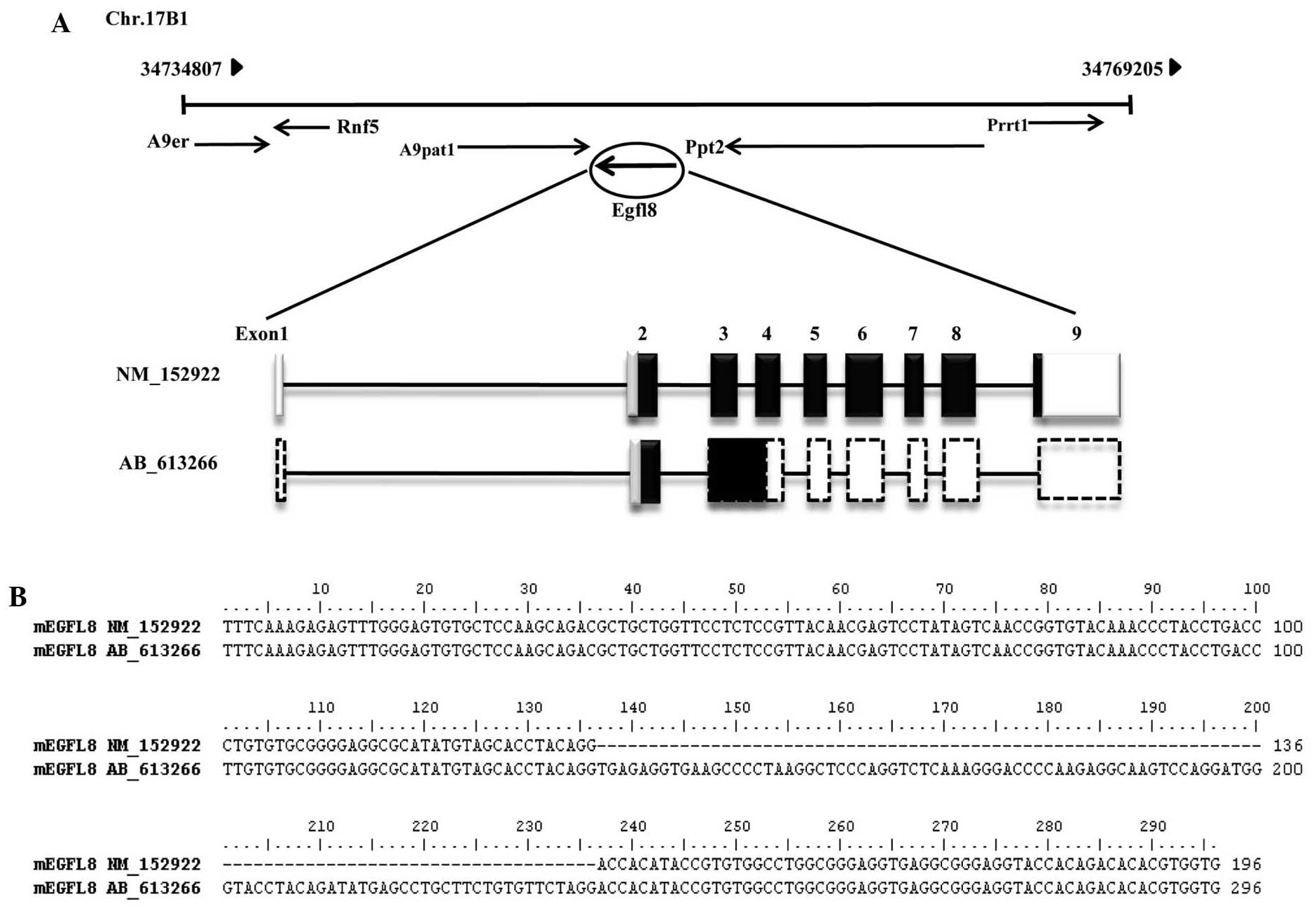
The nucleotide sequences of the EGFL8 isoform (EGFL8-2) from mouse
thymic epithelial cells reported herein have been deposited in the
DNA Data Bank of Japan/European Molecular Biology
Laboratory/GenBank nucleotide sequence databases with the accession
number AB613266.
Experimental animals
Adult male specific pathogen-free C57BL/6 mice
(weighing 22–25 g) were purchased from Dae Han Bio Link (Seoul,
Korea). Six animals were sacrificed by cervical dislocation. Mice
were used at 8–10 weeks of age. Animal care and all experimental
procedures were performed in accordance with the Guide for Animal
Experiments edited by the Korean Academy of Medical Sciences.
Thymocyte and thymic stromal cell
isolation
For the isolation of mouse thymocytes and thymic
stromal cells, 2–3 thymi were dissected from mice immediately after
sacrification and trimmed of fat and connective tissues. Small
incisions (2–3 mm) were made into the capsules with a pair of
razors, and the thyme were gently agitated in 30 ml RPMI-1640
(Gibco-BRL) using a magnetic stirrer at 4°C for 40 min. The
resulting thymic fragments and supernatant were transferred into
separate tubes. For the isolation of thymocytes, the supernatant
was passed three times through 70 μm mesh and centrifuged at
3,800 × g for 2 min at 4°C. The cell pellet was re-suspended in 20
ml ammonium chloride-potassium lysis solution (0.15 M
NH4Cl, 1 mM KHCO3 and 0.1 mM disodium EDTA)
to remove red blood cells. The thymocyte suspension was washed
three times with Hank’s buffered salt solution (HBSS) buffer. The
thymocytes were then re-suspended in HBSS buffer, and viable cells
were counted using a hemocytometer after trypan blue staining. For
the isolation of thymic stromal cells, the thymic fragments were
transferred into 5 ml RPMI-1640 containing 0.125% (w/v) collagenase
D and 0.1% (w/v) DNase I (both from Roche) and incubated for 15 min
with gentle shaking in a water bath at 37°C. The thymic fragments
in the enzyme mixtures were carefully dispersed several times with
a Pasteur pipette, and the supernatant was removed after fragments
had settled and replaced with fresh enzyme mixture. Gentle
mechanical agitation was provided using a 5-ml syringe and 18G, 21G
and 23G needles. Tissue fragments were allowed to settle, and the
supernatant was discarded. This digestion process was repeated four
times until the tissue was fully digested. Cells liberated by the
fourth, fifth and sixth digest were saved, filtered through
100-μm mesh to remove undigested particles and washed three
times with HBSS buffer. They were then re-suspended in HBSS buffer
and viable cells were counted using a hemocytometer after trypan
blue staining.
Western blot analysis
Tissue proteins were isolated using a protein
extraction solution (PRO-PREP Protein Extraction Solution; Intron
Biotechnology, Seoul, Korea) from 27 adult mouse tissues, including
the thymus, spleen, lymph nodes, stomach, ileum, colon, esophagus,
tongue, liver, kidney, testis, epididymis, penis, ductus deferens,
ovary, uterus, urinary bladder, adrenal glands, trachea, heart,
submandibular gland, skin, fat, lung, pancreas, aorta and skeletal
muscle. Total proteins from the cultured cells were then extracted
using a protein extraction solution (Intron Biotechnology)
supplemented with a protease inhibitor mixture (Sigma-Aldrich). The
lysates were centrifuged at 17,900 × g for 15 min at 4°C. Protein
concentrations were determined using the bicinchoninic acid protein
assay method (B9643; Sigma-Aldrich). Equal amounts of protein
samples were heated for 10 min at 95°C in sample buffer and
separated by 10% SDS-PAGE, using a Mini-Protean III system
(Bio-Rad). The proteins were transferred onto a polyvinylidene
fluoride membrane via semi-dry transfer (both from Bio-Rad), and
the membrane was incubated overnight at 4°C with rabbit polyclonal
anti-EGFL8 (AV42656; Sigma-Aldrich) at a dilution of 1:500 and
mouse monoclonal anti-β-actin-horseradish peroxidase (HRP) (Ab8226;
Abcam, Cambridge, UK) antibody at a dilution of 1:1,000 in
Tris-buffered saline (TBS; 20 mM Tris-HCl, 150 mM NaCl, pH 7.4)
containing 2% skimmed milk. After three washes with TBS containing
0.1% Tween-20 (TBS-T) and 1% skimmed milk, the membrane was
incubated for 2 h at room temperature with secondary antibodies,
donkey anti-rabbit immunoglobulin G-HRP (sc-2313; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), diluted 1:10,000 and washed
three times with TBS-T. Immunoreactivity was detected with enhanced
chemiluminescence (SuperSignal West Pico Chemiluminescent Substrate
kit; Pierce, Rockford, IL, USA) according to manufacturer’s
instructions. Images were captured and quantified using the
LAS-3000 imaging system (Fujifilm, Tokyo, Japan).
Results and Discussion
Molecular cloning and characterization of
mouse EGFL8
To isolate the full-length EGFL8 cDNA, total RNA
from thymic epithelial cells from C57BL/6 mice was amplified using
RT-PCR, and the amplicon was cloned and sequenced. Searching the
full-length cDNA of EGFL8 in the mouse genome using NCBI server
(http://blast.ncbi.nlm.nih.gov/), mouse
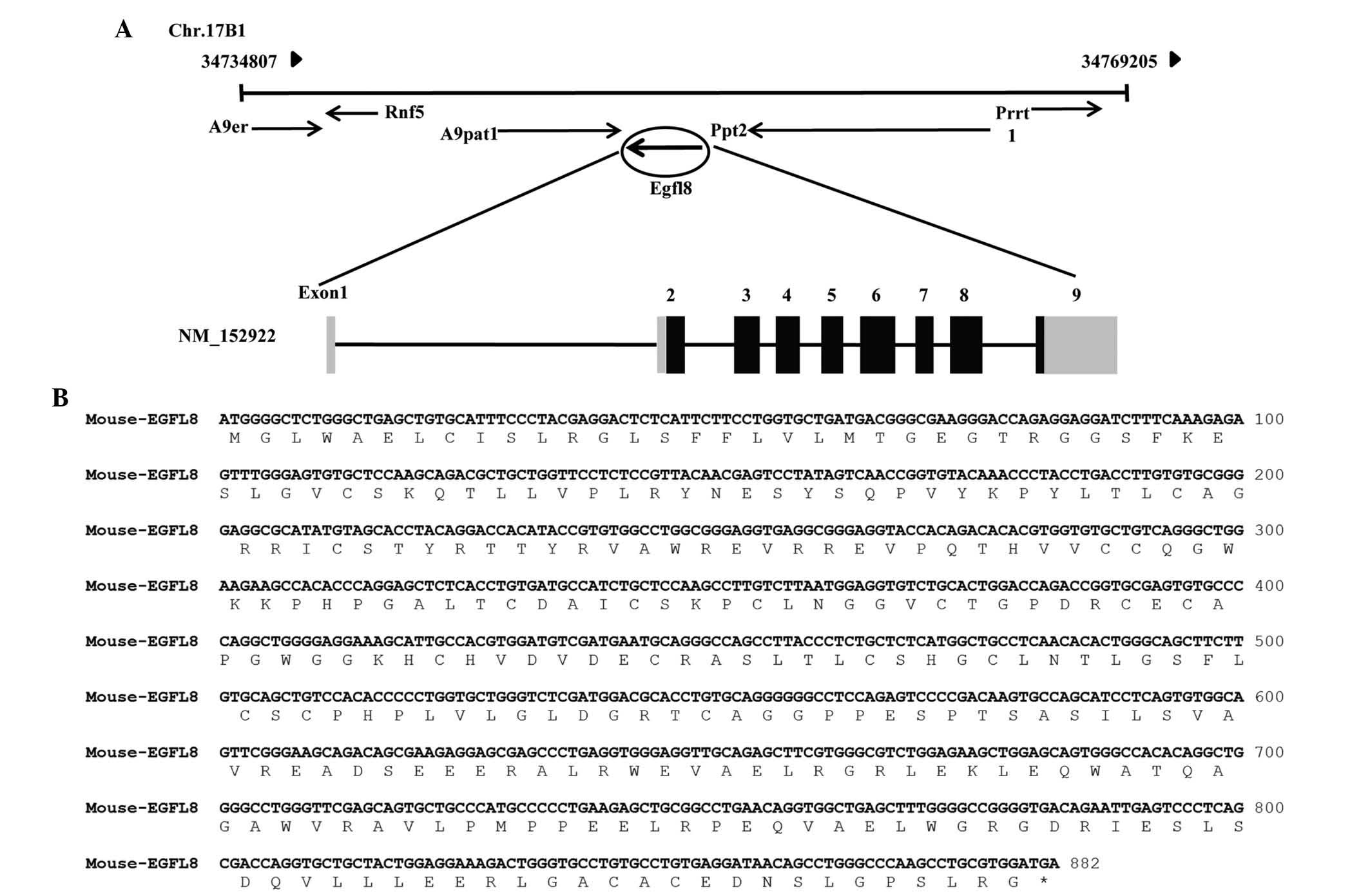
EGFL8 was located on chromosome 17B1 from 34,734,807 to 34,769,205
bp and was identified to consist of nine coding exons (Fig. 1A). The cDNA contains an ORF of 879
bp, which encodes a putative 32-kDa protein of 293 amino acids
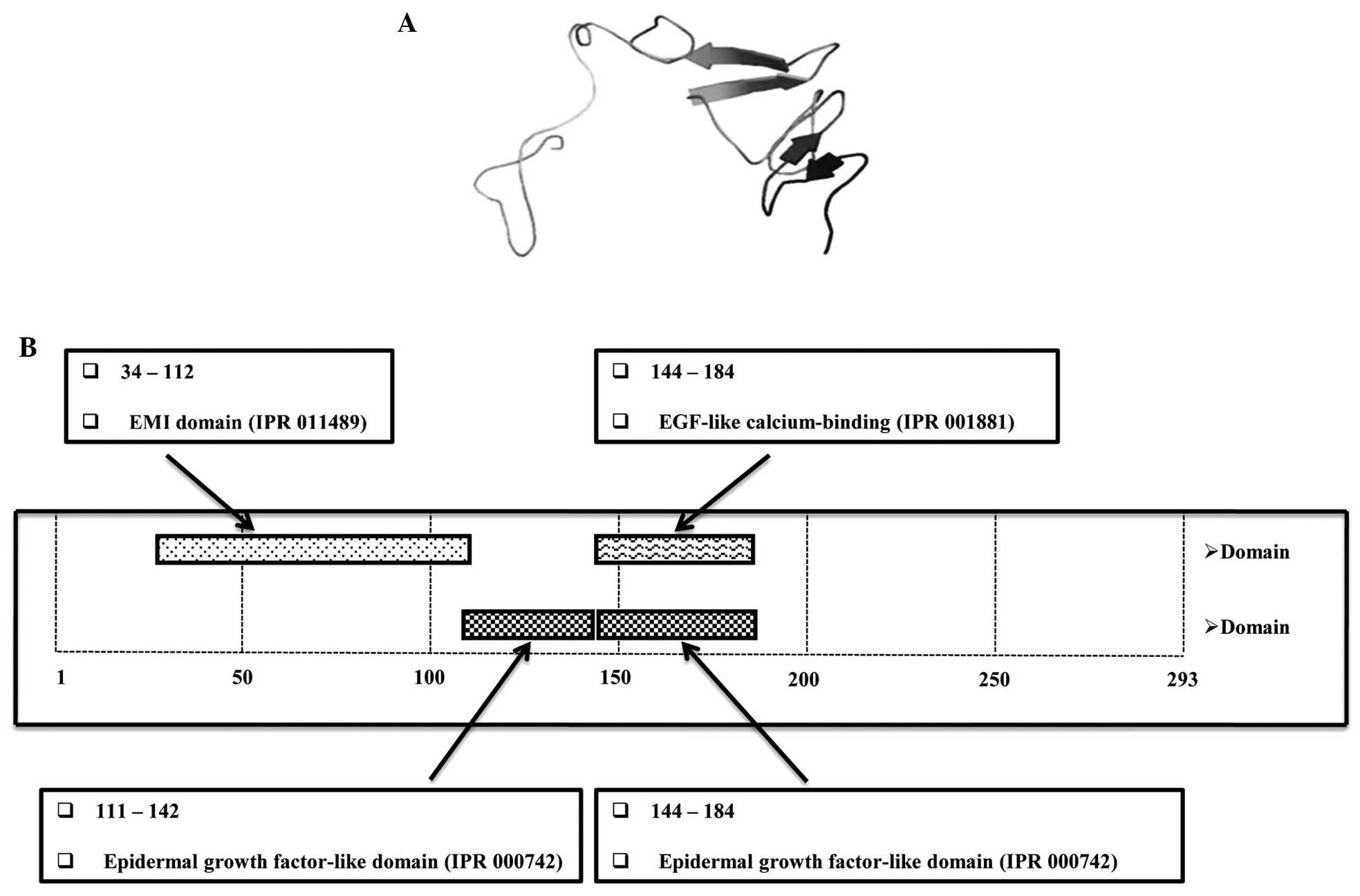
(Fig. 1B). Based on the deduced
amino acid sequence from the mature peptide region of this EGFL8
cDNA, a 3-dimensional molecular model (Fig. 2) was generated using the automated
protein-modeling server Swiss-Model (http://swissmodel.expasy.org). The gene product was a
slightly acidic protein with an isoelectric point of pH 6.13 and
had a molecular mass of 32 kDa (Fig.
3).
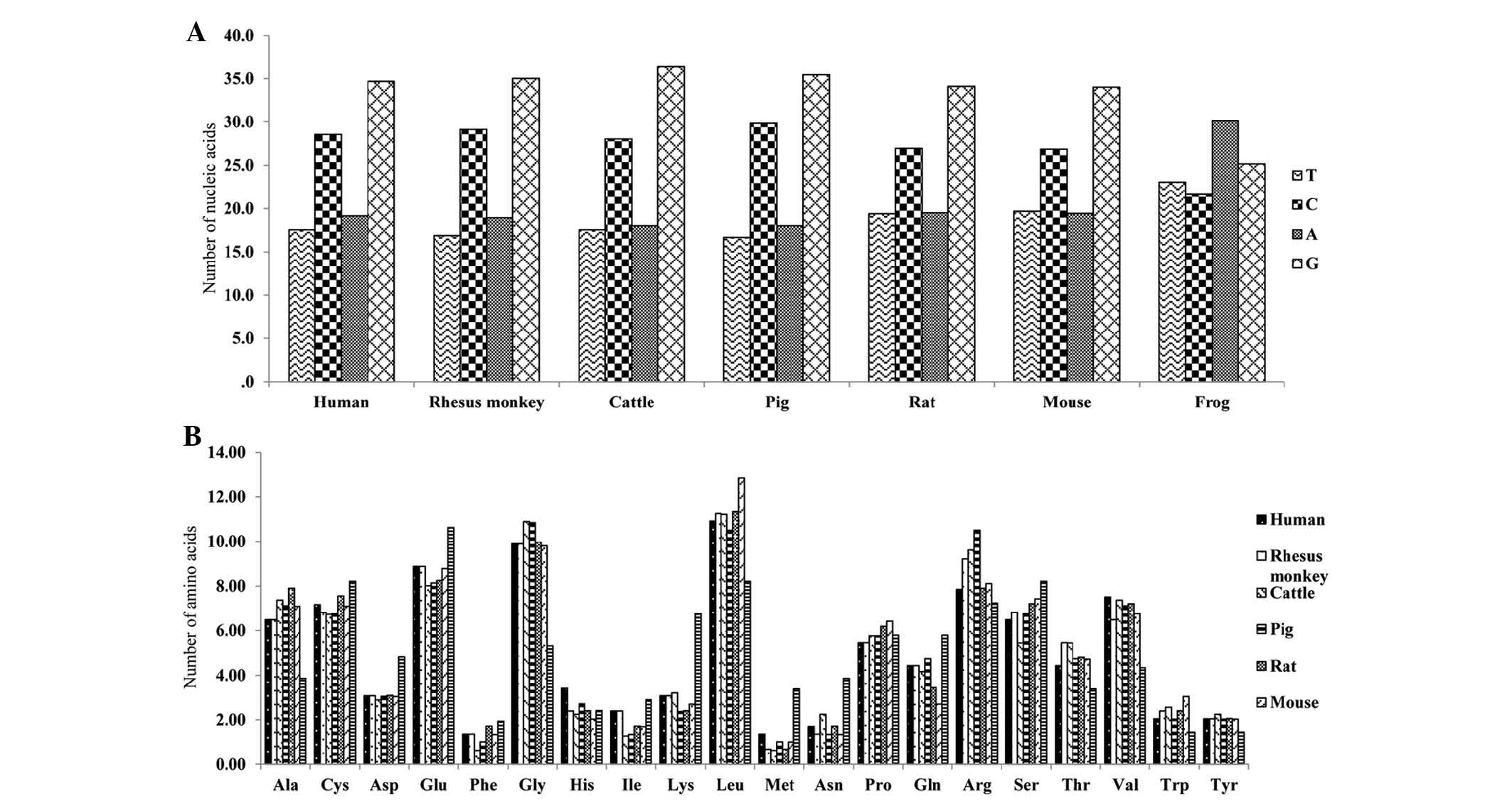
Base composition and putative amino acids were
calculated from the cDNA sequence data. The average G+C content of
EGFL8 was 61.4% in the species examined (Fig. 4A). Mouse EGFL8 sequences had a G+C
content of 60.8%, whereas human EGFL8 sequences had a G+C content
of 63.3%. Amino acids with high prevalence in mouse EGFL8 are
leucine (Leu) (12.84%), glycine (Gly) (9.8%) and glutamic acid
(Glu) (8.78%) (Fig. 4B).
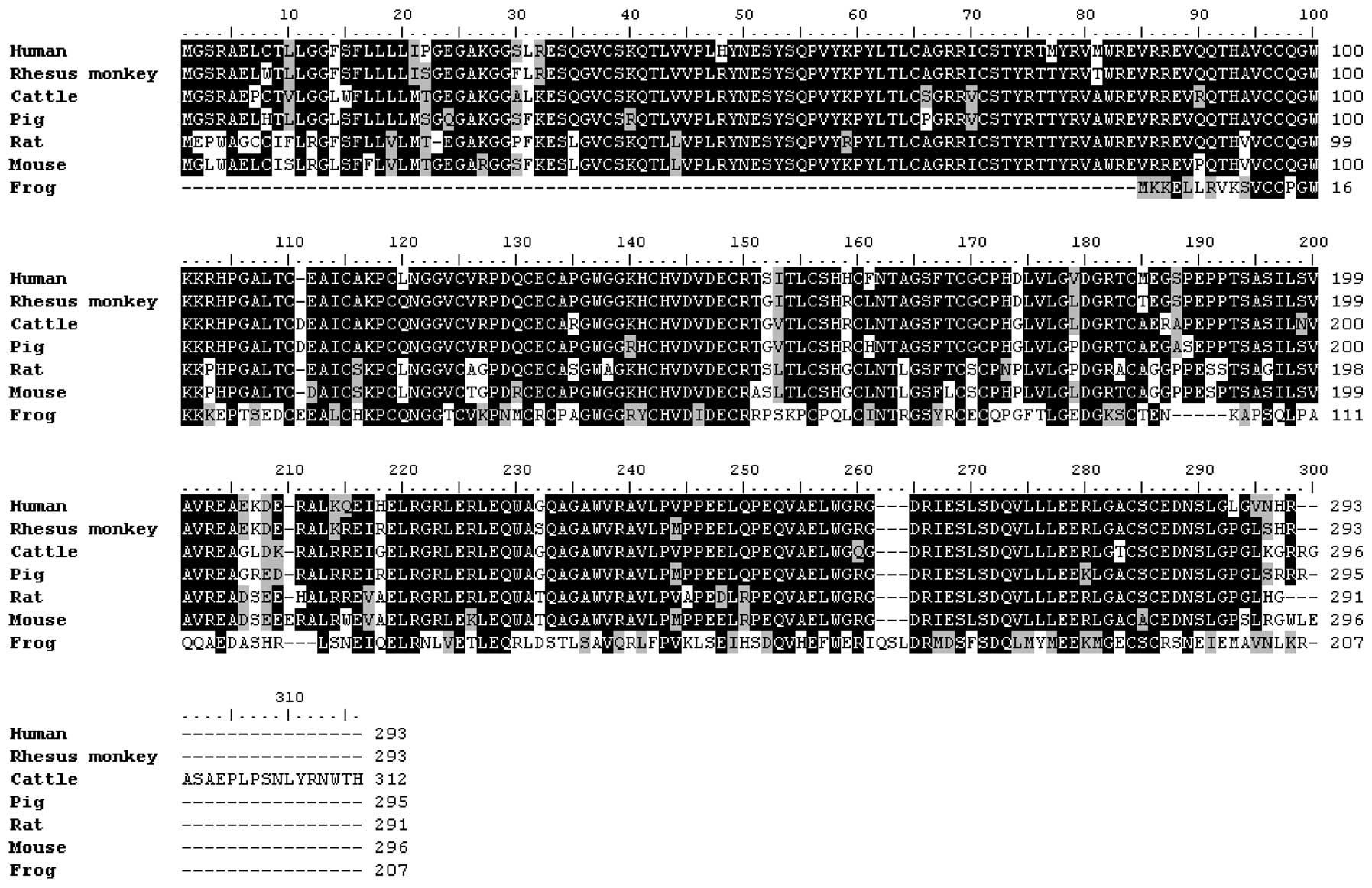
Multiple alignment of the amino acid sequences of
EGFL8 proteins from various vertebrates revealed that the EGF-like
domain is highly conserved in various species (human, monkey,
cattle, pig, rat and frog) (Fig.
5). This coding sequence shared 78.4% nucleotide sequence
similarity and 79.7% amino acid sequence identity with the
homologous sequences of human EGFL8 cDNA. Sequence identity of
amino acids was 87.5% between mouse EGFL8 and rat EGFL8 (Table I). The domain structure of EGFL8
was determined using the SignalP server (http://www.cbs.dtu.dk/services/SignalP/) (28) to predict signal sequences and the
Pfam database search (http://www.sanger.ac.uk/resources/databases/pfam.html)
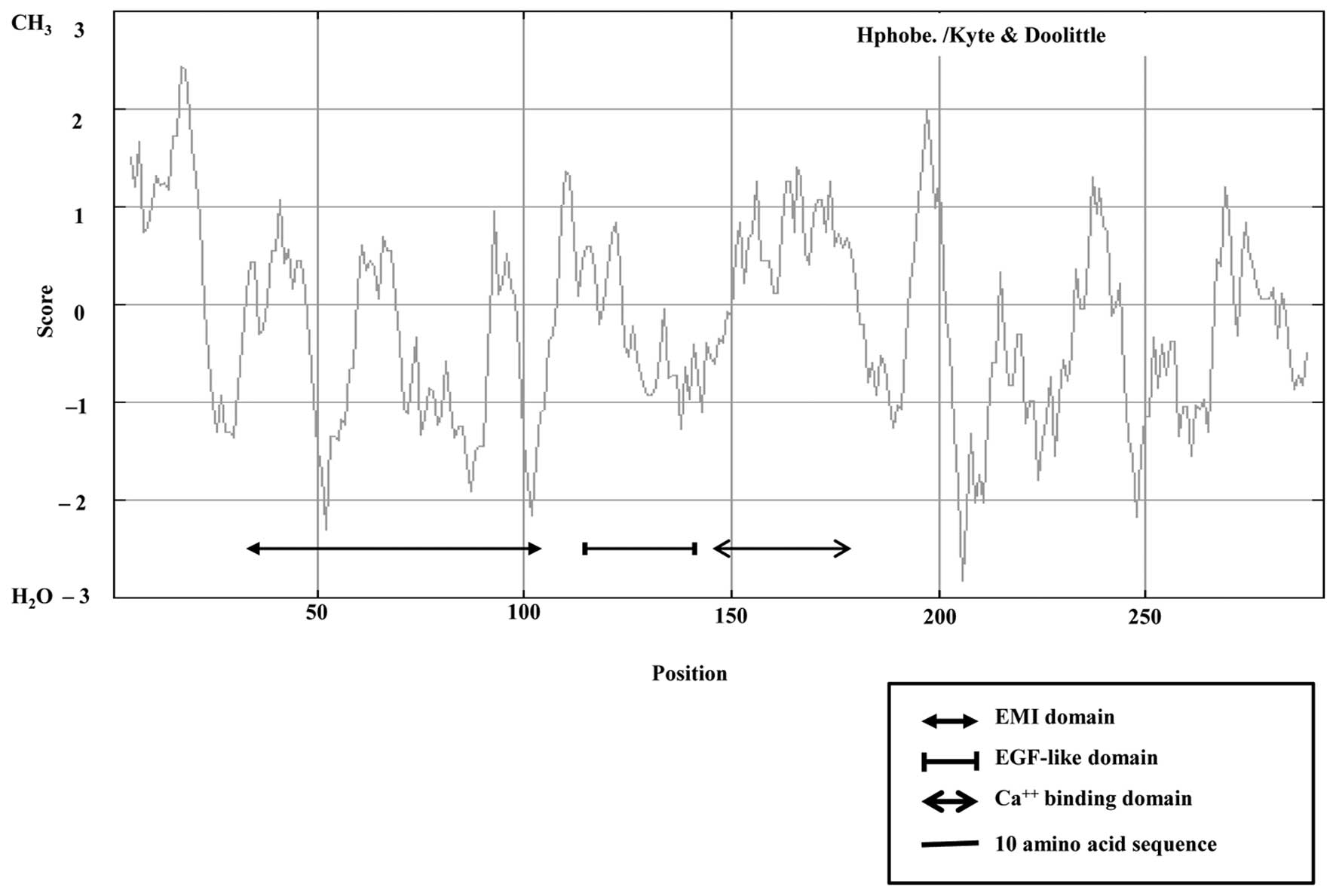
was used to identify protein domains. EGFL8 contained a predicted
N-terminal signal sequence, suggesting that EGFL8 is a secreted
protein composed mainly of an Emilin-like (EMI) domain, followed by
two EGFL domains and a sub-class of EGFL domains that bind
Ca2+ (Figs. 5 and
2B). Of note, EGFL8 contained an
amino acid sequence similar to that of the Delta:Serrate:LAG2
domain conserved in ligands of Notch receptors (29).
 | Table ISimilarity and identity of the open
reading frame region for epidermal growth factor-like domain 8
genes (%). |
Table I
Similarity and identity of the open
reading frame region for epidermal growth factor-like domain 8
genes (%).
| Species | Human | Rhesus monkey | Cattle | Pig | Rat | Mouse | Frog |
|---|
| Human | – | 93.5 | 81.7 | 86.7 | 79.5 | 79.7 | 29.1 |
| Rhesus monkey | 96.3 | – | 83.9 | 90.1 | 80.2 | 80.7 | 28.8 |
| Cattle | 85.5 | 86.5 | – | 85.5 | 75.0 | 75.7 | 27.6 |
| Pig | 85.8 | 86.8 | 90.4 | – | 78.9 | 80.1 | 29.8 |
| Rat | 79.3 | 80.0 | 80.1 | 78.6 | – | 87.5 | 26.9 |
| Mouse | 78.4 | 79.5 | 79.0 | 78.2 | 90.2 | – | 26.6 |
| Frog | 36.1 | 35.8 | 37.1 | 36.3 | 34.4 | 34.8 | – |
The level of synonymous substitutions per site (Ks)
and non-synonymous substitutions (Ka) in the EGFL8 genes was also
calculated (Table II). As Ks
values are influenced by selection to a greater extent than Ka
values, their ratio is a good indicator of selection. For the
comparison of mouse to human and mouse to rat, Ka/Ks values of 0.32
and 0.46 were determined, respectively. The number of synonymous
substitutions was higher than that of non-synonymous substitutions
in EGFL8 (Table II). Positive
selection has not occurred in EGFL8. Therefore, EGFL8 genes were
stable and amino acid changes were deleterious.
 | Table IISynonymous substitutions per site
(Ks) and non-synonymous substitutions per site (Ka) in epidermal
growth factor-like domain 8 genes (expressed as Ka/Ks). |
Table II
Synonymous substitutions per site
(Ks) and non-synonymous substitutions per site (Ka) in epidermal
growth factor-like domain 8 genes (expressed as Ka/Ks).
| Species | Human | Rhesus monkey | Cattle | Pig | Rat | Mouse | Frog |
|---|
| Human | – | 0.66 | 0.30 | 0.28 | 0.32 | 0.32 | 0.56 |
| Rhesus monkey | 0.03/0.05 | – | 0.26 | 0.18 | 0.29 | 0.29 | 0.50 |
| Cattle | 0.09/0.31 | 0.08/0.29 | – | 0.28 | 0.33 | 0.26 | 0.59 |
| Pig | 0.09/0.32 | 0.06/0.34 | 0.06/0.20 | – | 0.28 | 0.27 | 0.55 |
| Rat | 0.16/0.49 | 0.14/0.48 | 0.14/0.43 | 0.14/0.51 | – | 0.46 | 0.60 |
| Mouse | 0.16/0.50 | 0.14/0.49 | 0.13/0.50 | 0.14/0.51 | 0.07/0.15 | – | 0.65 |
| Frog | 0.61/1.08 | 0.60/1.20 | 0.60/1.01 | 0.61/1.10 | 0.68/1.12 | 0.68/1.04 | – |
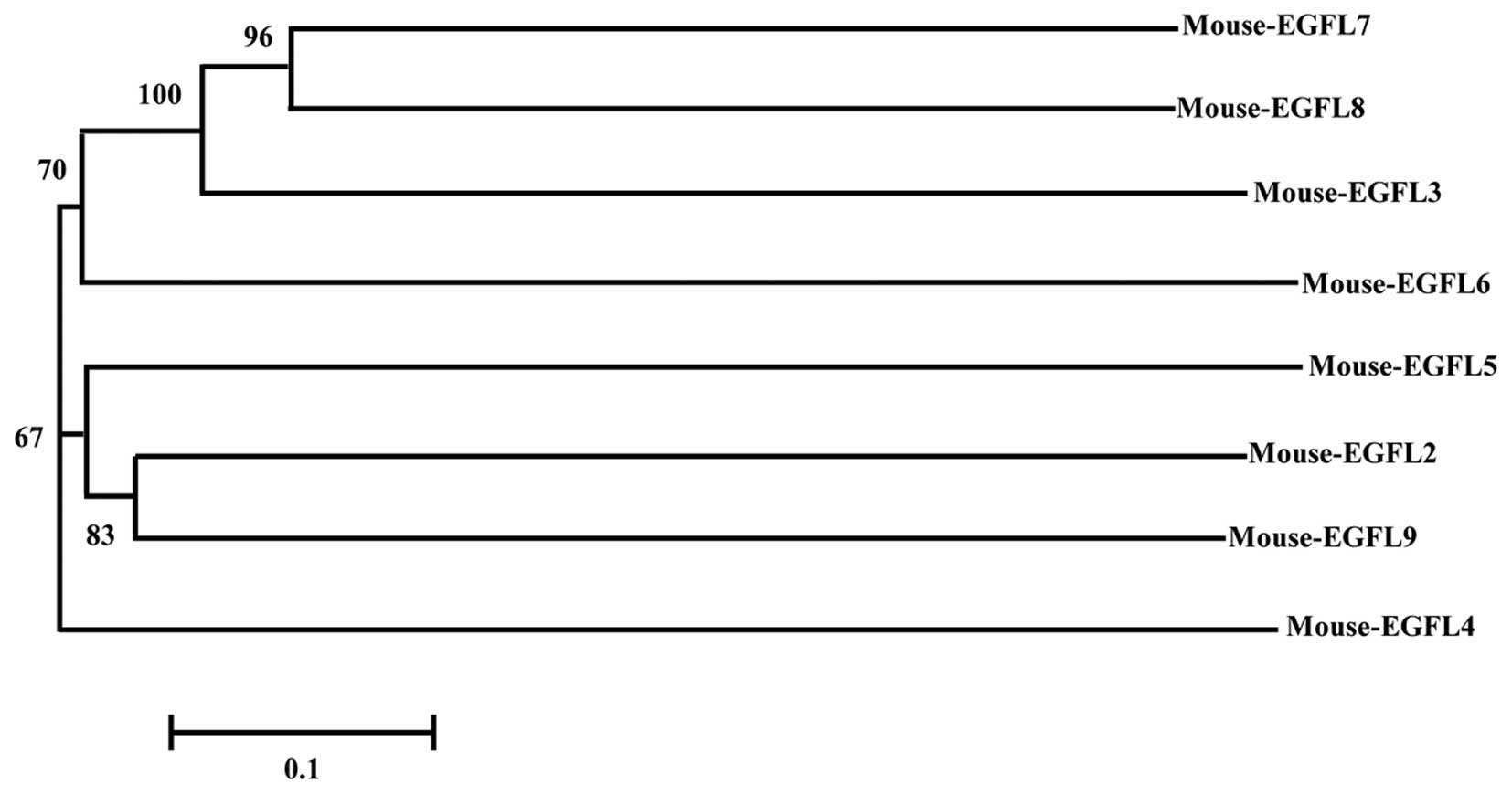
In order to illustrate the evolutionary associations
among mouse EGFL family members, a phylogenetic tree was
constructed by the neighbor-joining method using cDNA sequences.
Mouse EGFL8 and EGFL7 are more closely related to each other than
either is to EGFL3, and they cluster with EGFL6
(Fig. 6).
The EGFL domain, an evolutionarily conserved protein
domain found in large numbers in most animal proteins, constitutes
an expanding family of proteins involved in cellular activities,
including blood coagulation, fibrinolysis, cell adhesion, and
neural and vertebrate development (30,31). EGFL8, also known as C6 or f8, NG3
and VE-statin-2, is a secreted protein of 293 amino acids that
contains two EGFL domains, an EMI domain and a
Ca2+-binding EGFL domain. Via these domains, EGFL8 may
participate in protein-protein interactions that correlate with
cellular proliferation and developmental signaling events. Although
the characterization of EGFL8 and the presence of EGFL8 mRNA
in certain mouse tissues, including the thymus, lungs and kidney,
have been mentioned briefly in the literature (1), the complete characterization of
EGFL8 and the expression pattern of EGFL8 have remained to be
elucidated.
Tissue distribution of mouse EGFL8
EGFL8 was first identified in 2004 and shares a high
degree of structural similarity with EGFL7 (1). A previous study has described the
tissue distribution of EGFL8 in mice (19) but tested a limited panel of mouse
tissues. As little is known regarding the expression of EGFL8, the
present study investigated the expression pattern of EGFL8 protein
in normal C57BL/6 mouse tissues. To determine the distribution more
broadly, EGFL8 protein was examined in various tissues by western
blot analysis. The results showed that EGFL8 was expressed in a
wide variety of tissue types in C57BL/6 mice. Western blot analysis
of 27 mouse tissues showed a specific band of ~32 kDa, which
represented EGFL8. EGFL8 proteins were highly expressed in diverse
mouse tissues, including the thymus, lymph nodes, testis, ovary,
epididymis, ductus deferens, ileum, colon, stomach, esophagus,
lung, uterus, urinary bladder, skin, spleen, adrenal glands and
penis (Fig. 7). However, almost
no EGFL8 protein was expressed in the heart, kidney, submandibular
gland, aorta, fat and skeletal muscle, while EGFL8 protein was
expressed at low levels in the trachea, tongue and pancreas
(Fig. 7). At the same time, a
band that was smaller in size than the typical EGFL8-specific band
was detected only in the lung and trachea among all organs examined
in the present study. Although it is difficult to determine the
precise nature of the smaller-sized band, the anti-EFGL8 antibody
used in the present study appeared to be able to bind either
non-specifically or specifically to a certain protein present
particularly in the organs of the respiratory system including the
lung and trachea. Further studies are required to clarify whether
this band represents an EGFL8 isoform or not. Of note, high levels
of EGFL8 mRNA were detected in diverse organs. The expression
pattern of EGFL8 mRNA as determined by RT-PCR analysis was also
virtually in accord with that of EGFL8 protein as determined by
western blot analysis (data not shown). These findings supported
the validity of the results regarding EGFL8 expression in the
present study.
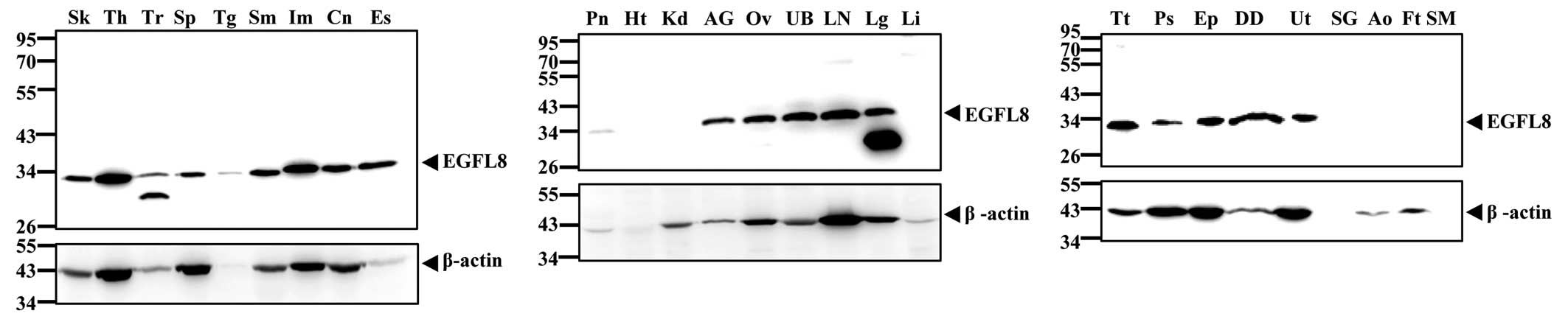 | Figure 7Representative western blot analysis
of mouse EGFL8 expression in various mouse tissues: Sk, skin; Th,
thymus; Tr, trachea; Sp, spleen; Tg, tongue; Sm, stomach; Im,
ileum; Cn, colon; Es, esophagus; Pn, pancreas; Hr, heart; Kd,
kidney; AG, adrenal gland; Ov, ovary; UB, urinary bladder; LN,
lymph node; Lg, lung; Li, liver; Tt, testis; Ps, penis; Ep,
epididymis; DD, ductus deferens; Ut, uterus; SG, submandibular
gland; Ao, aorta; Ft, fat; and SM, skeletal muscle. β-actin was
used as a loading control. EGFL, epidermal growth factor-like. |
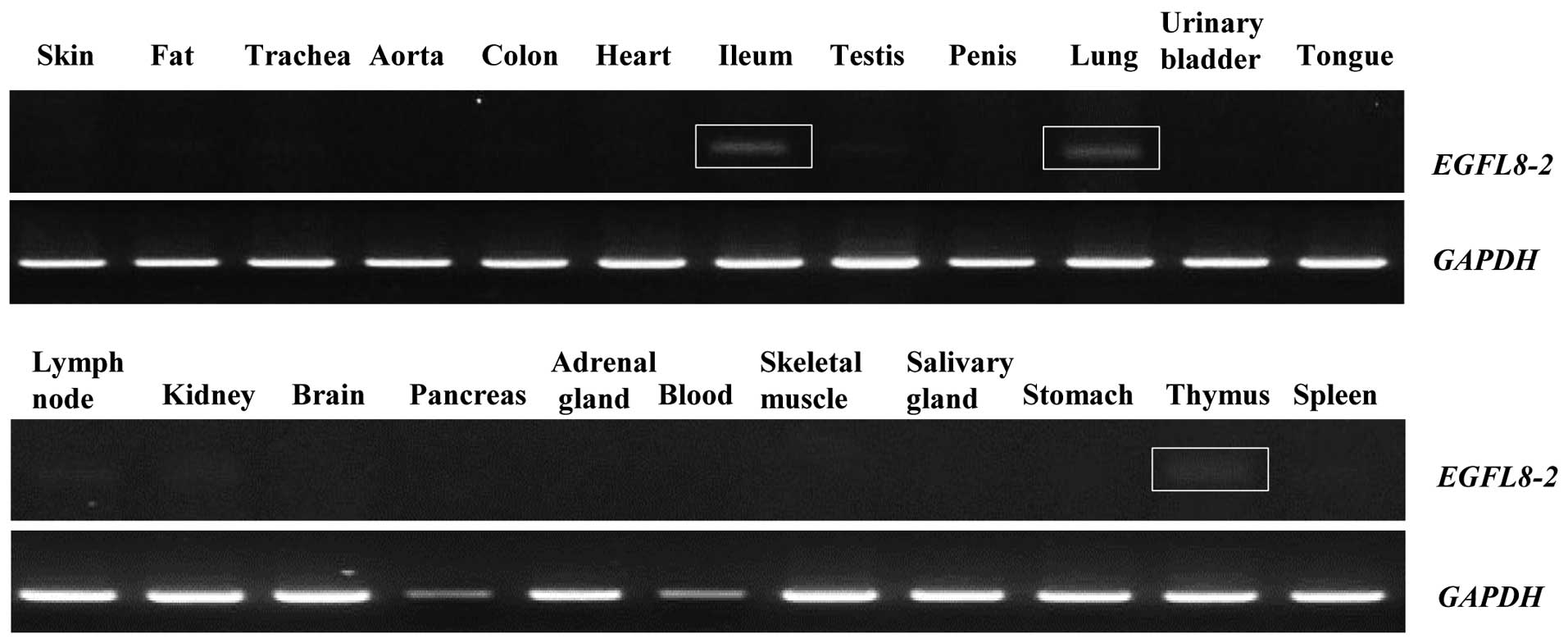
Alternative splicing variant of EGFL8 in
specific mouse organs
Of note, the 296-bp EGFL8 amplicon found in the
present study was present in the ileum, lung and thymus (Fig. 8). To determine whether this
abnormal phenomenon resulted from an alternative splicing process
of the EGFL8 gene, the amplicon was cloned and sequenced. The
296-bp EGFL8 isoform, EGFL8-2 (GenBank accession no. AB613266) was
shown to contain an ORF resulting from alternative splicing of the
mouse full-length EGFL8 gene transcript. Analysis with the NCBI CDD
indicated that mouse EGFL8-2 contains an EMI domain, which is also
present in the full-length EGFL8 (Fig. 9).
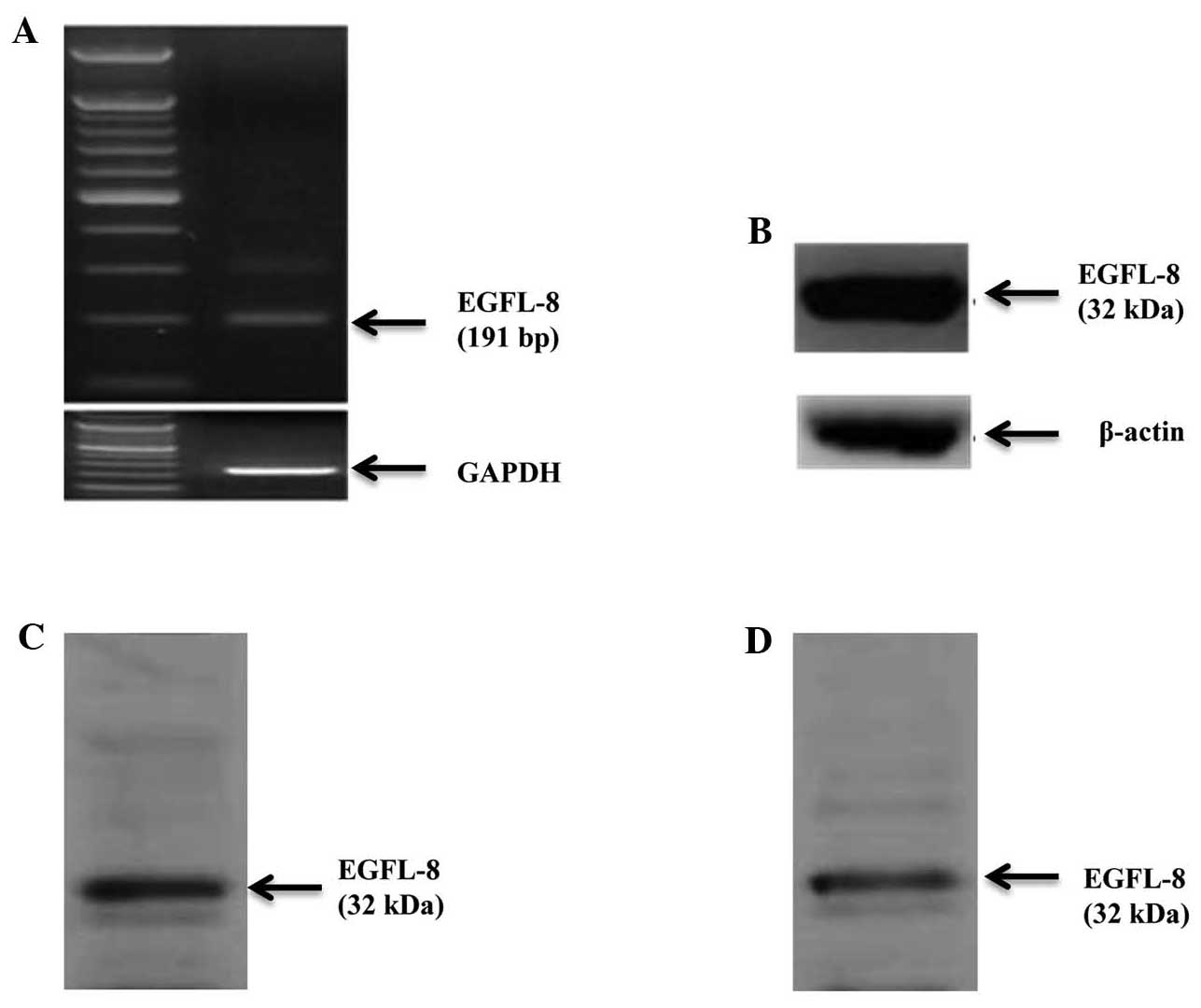
EGFL8 expression in the mouse thymus,
thymocytes and thymic stromal cells
The expression of EGFL8 was assessed in the mouse
thymus using RT-PCR and western blotting, which demonstrated the
presence of high levels of EGFL8 mRNA (Fig. 10A) and protein expression
(Fig. 10B). In addition, western
blot analysis of EGFL8 expression in the mouse thymus showed that
EGFL8 equally expressed in freshly isolated thymocytes (Fig. 10C) and thymic stromal cells in
the mouse (Fig. 10D).
The presence of EGFL8 expression in the thymus
implied that the protein may have a physiological role in T-cell
development, as evidenced by the findings of a recent study by our
group, which indicated that EGFL8 inhibits the expression of the
Notch downstream effectors Hes-1 and Hey-1 in the thymocytes and
thymic epithelial cells of mice, indicating that EGFL8 has a role
in the inhibition of T-cell development in the mouse thymus
(19,20). EGFL8 may have important
implications in the physiology of the thymus, since the Notch
signaling pathway is crucial in T-cell development (32); this is further supported by the
finding of the present study that the EGFL domain of EGFL8 contains
a domain structure similar to the Delta:Serrate:LAG2 domain
conserved in ligands of Notch receptors. These results also
suggested that EGFL8 may be a physiologically important inhibitory
ligand of Notch family proteins. Furthermore, it is of critical
importance to develop therapeutic strategies to modulate Notch
signaling, since it has been well recognized that alterations in
Notch signaling lead to the development of various diseases,
including T-cell leukemia, tumors, Alagille syndrome, Hajdu-Cheney
syndrome and cerebral autosomal dominant arteriopathy with
subcortical infarcts and leukoencephalopathy (CADASIL), a syndrome
associated with progressive dementia, mood disorders, migraine and
recurrent sub-cortical cerebral infarctions (33–37). In conclusion, the results of the
present study suggested that EGFL8 may have great potential as a
therapeutic target for various diseases associated with
de-regulated Notch signaling.
Acknowledgments
This study was supported by the Pioneer Research
Center Program through the National Research Foundation of Korea
funded by the Ministry of Science, ICT and Future Planning
(NRF-2012-000-9667).
References
|
1
|
Fitch MJ, Campagnolo L, Kuhnert F and
Stuhlmann H: Egfl7, a novel epidermal growth factor-domain gene
expressed in endothelial cells. Dev Dyn. 230:316–324. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Koeppe JR, Beach MA, Baerga-Ortiz A, Kerns
SJ and Komives EA: Mutations in the fourth EGF-like domain affect
thrombomodulin-induced changes in the active site of thrombin.
Biochemistry. 47:10933–10939. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kao YC, Jiang SJ, Pan WA, Wang KC, Chen
PK, Wei HJ, Chen WS, Chang BI, Shi GY and Wu HL: The epidermal
growth factor-like domain of CD93 is a potent angiogenic factor.
PLoS One. 7:e516472012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eroglu C, Allen NJ, Susman MW, et al:
Gabapentin receptor α2δ-1 is a neuronal thrombospondin receptor
responsible for excitatory CNS synaptogenesis. Cell. 139:380–392.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koutsi A, Papapanagiotou A and
Papavassiliou AG: Thrombomodulin: From haemostasis to inflammation
and tumourigenesis. Int J Biochem Cell Biol. 40:1669–1673. 2008.
View Article : Google Scholar
|
|
6
|
Kojika S and Griffin JD: Notch receptors
and hematopoiesis. Exp Hematol. 29:1041–1052. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ito D, Tanahashi N, Murata M, Sato H,
Saito I, Watanabe K and Fukuuchi Y: Notch3 gene polymorphism and
ischaemic cerebro-vascular disease. J Neurol Neurosurg Psychiatry.
72:382–384. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Campagnolo L, Leahy A, Chitnis S,
Koschnick S, Fitch MJ, Fallon JT, Loskutoff D, Taubman MB and
Stuhlmann H: EGFL7 is a chemoattractant for endothelial cells and
is up-regulated in angiogenesis and arterial injury. Am J Pathol.
167:275–284. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nichol D, Shawber C, Fitch MJ, Bambino K,
Sharma A, Kitajewski J and Stuhlmann H: Impaired angiogenesis and
altered Notch signaling in mice overexpressing endothelial Egfl7.
Blood. 116:6133–6143. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nichol D and Stuhlmann H: EGFL7: A unique
angiogenic signaling factor in vascular development and disease.
Blood. 119:1345–1352. 2012. View Article : Google Scholar :
|
|
11
|
Parker LH, Schmidt M, Jin SW, et al: The
endothelial-cell-derived secreted factor Egfl7 regulates vascular
tube formation. Nature. 428:754–758. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dikic I and Schmidt MH: Notch:
Implications of endogenous inhibitors for therapy. BioEssays.
32:481–487. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pinte S and Soncin F: Egfl7 promotes tumor
escape from immunity. Oncoimmunology. 1:375–376. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schmidt MH, Bicker F, Nikolic I, Meister
J, Babuke T, Picuric S, Müller-Esterl W, Plate KH and Dikic I:
Epidermal growth factor-like domain 7 (EGFL7) modulates Notch
signalling and affects neural stem cell renewal. Nat Cell Biol.
11:873–880. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oberauer R, Rist W, Lenter MC, Hamilton BS
and Neubauer H: EGFL6 is increasingly expressed in human obesity
and promotes proliferation of adipose tissue-derived stromal
vascular cells. Mol Cell Biochem. 343:257–269. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang X, Gong Y, Wang D, et al: Analysis of
gene expression profiling in meningioma: Deregulated signaling
pathways associated with meningioma and EGFL6 overexpression in
benign meningioma tissue and serum. PLoS One. 7:e527072012.
View Article : Google Scholar
|
|
17
|
Wu F, Shirahata A, Sakuraba K, et al:
Down-regulation of EGFL8: A novel biomarker for advanced gastric
cancer. Anticancer Res. 31:3377–3380. 2011.PubMed/NCBI
|
|
18
|
Wu F, Shirahata A, Sakuraba K, et al:
Down-regulation of EGFL8: A novel prognostic biomarker for patients
with colorectal cancer. Anticancer Res. 31:2249–2254.
2011.PubMed/NCBI
|
|
19
|
Choi H-J, Yoon TD, Muhammad I, Jeong MH,
Lee J, Baek SY, Kim BS and Yoon S: Regulatory role of mouse
epidermal growth factor-like protein 8 in thymic epithelial cells.
Biochem Biophys Res Commun. 425:250–255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Subhan F, Yoon TD, Choi HJ, Muhammad I,
Lee J, Hong C, Oh SO, Baek SY, Kim BS and Yoon S: Epidermal growth
factor-like domain 8 inhibits the survival and proliferation of
mouse thymocytes. Int J Mol Med. 32:952–958. 2013.PubMed/NCBI
|
|
21
|
Faas SJ, Rothstein JL, Kreider BL, Rovera
G and Knowles BB: Phenotypically diverse mouse thymic stromal cell
lines which induce proliferation and differentiation of
hematopoietic cells. Eur J Immunol. 23:1201–1214. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sonnhammer EL, Eddy SR and Durbin R: Pfam:
A comprehensive database of protein domain families based on seed
alignments. Proteins. 28:405–420. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Appel RD, Bairoch A and Hochstrasser DF: A
new generation of information retrieval tools for biologists: The
example of the ExPASy WWW server. Trends Biochem Sci. 19:258–260.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tamura K, Peterson D, Peterson N, Stecher
G, Nei M and Kumar S: MEGA5: molecular evolutionary genetics
analysis using maximum likelihood, evolutionary distance, and
maximum parsimony methods. Mol Biol Evol. 28:2731–2739. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saitou N and Nei M: The neighbor-joining
method: A new method for reconstructing phylogenetic trees. Mol
Biol Evol. 4:406–425. 1987.PubMed/NCBI
|
|
26
|
Thompson JD, Higgins DG and Gibson TJ:
CLUSTAL W: Improving the sensitivity of progressive multiple
sequence alignment through sequence weighting, position-specific
gap penalties and weight matrix choice. Nucleic Acids Res.
22:4673–4680. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Altschul SF, Madden TL, Schäffer AA, Zhang
J, Zhang Z, Miller W and Lipman DJ: Gapped BLAST and PSI-BLAST: A
new generation of protein database search programs. Nucleic Acids
Res. 25:3389–3402. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Emanuelsson O, Brunak S, von Heijne G and
Nielsen H: Locating proteins in the cell using TargetP, SignalP and
related tools. Nat Protoc. 2:953–971. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lindsell CE, Shawber CJ, Boulter J and
Weinmaster G: Jagged: A mammalian ligand that activates Notch1.
Cell. 80:909–917. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Downing AK, Knott V, Werner JM, Cardy CM,
Campbell ID and Handford PA: Solution structure of a pair of
calcium-binding epidermal growth factor-like domains: Implications
for the Marfan syndrome and other genetic disorders. Cell.
85:597–605. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nagata K, Kohda D, Hatanaka H, Ichikawa S,
Matsuda S, Yamamoto T, Suzuki A and Inagaki F: Solution structure
of the epidermal growth factor-like domain of heregulin-alpha, a
ligand for p180erbB-4. EMBO J. 13:3517–3523. 1994.PubMed/NCBI
|
|
32
|
Shah DK and Zúñiga-Pflücker JC: An
overview of the intrathymic intricacies of T cell development. J
Immunol. 192:4017–4023. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Andersson ER and Lendahl U: Therapeutic
modulation of Notch signalling - are we there yet? Nat Rev Drug
Discov. 13:357–378. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lobry C, Oh P, Mansour MR, Look AT and
Aifantis I: Notch signaling: Switching an oncogene to a tumor
suppressor. Blood. 123:2451–2459. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hernandez Tejada FN, Galvez Silva JR and
Zweidler-McKay PA: The challenge of targeting notch in hematologic
malignancies. Front Pediatr. 2:542014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li Y, Ma J, Qian X, Wu Q, Xia J, Miele L,
Sarkar FH and Wang Z: Regulation of EMT by Notch signaling pathway
in tumor progression. Curr Cancer Drug Targets. 13:957–962. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Aster JC: In brief: Notch signalling in
health and disease. J Pathol. 232:1–3. 2014. View Article : Google Scholar
|