Introduction
Stroke remains a leading cause of death and
long-term disability worldwide, thus representing a major concern
to public health and the economy (1). According to statistics, 60–70% of
all stroke victims suffer an ischemic stroke (2,3),
which is characterized by the occlusion of blood vessels by the
formation of an obstructive thrombus or embolus in the brain,
consequently resulting in an inadequate supply of blood and oxygen
to the brain (4).
Re-establishment of blood circulation to the ischemic brain as soon
as possible is the most effective therapy for patients with
ischemic stroke. However, reperfusion itself has a latent risk, as
it can cause further damage to brain tissue, such as hemorrhagic
transformation, cerebral edema, blood-brain barrier (BBB) leakage
and neuronal death (3,5). This phenomenon is termed cerebral
ischemia/reperfusion (I/R) injury, which is accompanied by a
cascade of mechanisms, including glutamate excitotoxicity, calcium
overload, oxidative stress, inflammation and apoptosis, eventually
leading to cell death (6,7). However, due to the complex
mechanisms involved in the I/R process, to date, the only effective
American Food and Drug Administration (FDA)-approved
pharmacological treatment for ischemic stroke is the intravenous
recombinant tissue plasminogen activator (rtPA), the effectiveness
of which is extremely limited in clinical therapeutics, owing to
the short therapeutic window and an increased risk of subarachnoid
hemorrhage (8). Therefore, the
investigation of the pathological mechanisms and the search for and
development of safe and effective neuroprotective agents for the
treatment of ischemic stroke is of critical clinical
significance.
In recent years, natural products, due the abundant
resources, multi-targeted mechanisms of activity, few side-effects,
and no drug resistance have been increasingly used, such as
aloperine (9). Thus, natural
products used in traditional Chinese medicine have received
increasing attention and their use has been investigated in
cerebral I/R injury, revealing the neuroprotective effects of these
products (3,10,11). Matrine (Mat;
C15H24N2O) and oxymatrine
(C15H24N2O2), as the
main alkaloids extracted from the traditional Chinese herb,
Sophora flavescens Ait., have been shown to possess a
similar molecular structure (Fig.
1) and have been shown to have a variety of pharmacological
activities, such as antitumor (12), antioxidant, anti-inflammatory
(13) and antiviral properties
(14). It has been demonstrated
that Mat not only reduces brain edema induced by focal cerebral
ischemia (15), but directly
protects neurons and astrocytes against focal cerebral ischemia
through the inhibition of the nuclear factor (NF)-κB signaling
pathway (16). Studies have also
indicated that oxymatrine exerts protective effects against
myocardial ischemic injury (17),
as well as against liver and intestinal I/R injury in animal models
(18,19), and that exerts neuroprotective
effects against focal cerebral ischemia through the regulation of
Bcl-2/Bax expression and the inhibition of caspase-3 activation in
ischemic brain tissue (20,21). Furthermore, it has been reported
that Mat exerts protective effects against heart failure by
inhibiting the upregulation of Bax, caspase-3 and increasing the
expression of Bcl-2 in rats (22). However, whether Mat directly
protects ischemic neurons against damage by inhibiting the
overexpression of caspase-3 and modulating the Bcl-2/Bax ratio in
ischemic stroke has not yet been addressed. Thus, the present study
was undertaken to assess neuroprotective potential and possible
mechanisms of action of Mat by detecting the activities of oxygen
radical scavenging enzymes and the expression of the
apoptosis-associated proteins, caspase-3, Bax and Bcl-2, in a mouse
model of focal cerebral I/R injury induced by middle cerebral
artery occlusion (MCAO).
Materials and methods
Experiment animals
Male, Institute of Cancer Research (ICR) mice
(n=108) weighing between 20.0 to 25.0 g were obtained from the
Experimental Animal Center of Ningxia Medical University, Yinchuan,
China (certificate no. SYXK Ningxia 20050001). The animals were
housed in a temperature-controlled environment (22–24°C) under a 12
h light and dark cycle and had access to food and water ad
libitum. The experiments were performed as approved by the
Institutional Animal Ethics Committee of Ningxia Medical
University. This study complied with the internationally accredited
guidelines and ethical regulations on animal research. All
surgerical procedures were performed under chloral hydrate
anesthesia, and all efforts were made to minimize suffering.
Drug administration
Mat (purity ≥98.0%) and nimodipine were purchased
from the Ningxia Institute of Materia Medica, Yinchuan, China and
Bayer Pharma AG, Berlin, Germany, respectively. Both compounds were
dissolved in saline solution (0.9% NaCl) and injected by an
intraperitoneal (i.p.) injection in an application volume of 0.1
ml/10 g body weight and administered 15 min prior to testing for 7
consecutive days.
The mice were randomly assigned into the following 6
experimental groups (n=42 in each group): i) the sham-operated
group (sham); ii) the vehicle-treated group (vehicle); iii) the
MCAO + Mat (L) group (low dose, L = 7.5 mg/kg); iv) the MCAO + Mat
(M) group (medium dose, M = 15 mg/kg); v) the MCAO + Mat (H) group
(high dose, H = 30 mg/kg); and vi) the MCAO + nimodipine group
(nimodipine = 1 mg/kg).
Mat and nimodipine were administered by an i.p.
injection for 7 consecutive days prior to MCAO. The sham and
vehicle groups were treated with physiological saline under the
same conditions.
Mouse model of MCAO
Focal cerebral ischemia was induced using the
intraluminal filament technique as previously described by Longa
et al (23). The mice in
the sham-operated group were not subjectd to MCAO. Briefly, male
mice were anesthetized with an intraperitoneal injection of 3.5%
chloral hydrate. Via a midline neck incision, the left common
carotid artery (CCA), external carotid artery (ECA) and the
internal carotid artery (ICA) were surgically exposed. The ECA was
then isolated and ligated. A 4-0 monofilament nylon suture was
inserted into the ICA through the ECA to occlude the origin of the
left middle cerebral artery (MCA), almost 15–17 mm from the carotid
bifurcation. At 2 h following ischemia, the filament was removed
for reperfusion. The sham-operated group mice were subjected to the
same surgical procedure, but the MCA was not occluded.
Evaluation of neurological deficits
Neurological deficit scores were evaluated by an
examiner who was blinded to the experimental groups at 24 h after
MCAO, following a grading system carried out according to a
five-point scale (24) as
follows: no neurological deficits, 0; unable to extend the
contralateral forelimb fully, 1; circling to the contralateral
side, 2; falling to the contralateral side, 3; unable to walk
spontaneously and depression of consciousness, 4. The higher the
neurological deficit score, the more severe the impairment of motor
motion injury.
Measurement of infarct volume
After neurological scoring, 6 rats (randomly
selected) from each group were decapitated to remove the brain. The
brains were cut into 5 coronal sections (1-mm-thick each) and
stained with a 2% solution of 2,3,5-triphenyltetrazolium chloride
(TTC) (Sigma, St. Louis, MO, USA) at 37°C for 20 min, followed by
fixation with 4% formaldehyde solution overnight. The infarct
volumes were calculated using microscope image-analysis software
(Image-Pro Plus; Media Cybernetics, Rockville, MD, USA). To
compensate for the effects of brain edema, the exact infarct
volumes were calculated according to the following equation:
percentage of corrected infarct volume = (normal hemisphere volume
− non-infarct volume of infarct side)/normal hemispheric volume
×100.
Histopathological analysis
After 2 h of ischemia followed by 24 h of
reperfusion, the mice (n=6 from each group) were anesthetized with
3.5% chloral hydrate, and perfused with physiological saline via an
aortic root catheter until the liver appeared to be white, followed
by 4% paraformaldehyde solution that had been cooled to 4°C. The
brains were removed and post-fixed in 4% formaldehyde solution
overnight at 4°C. After being dehydrated and embedded with
paraffin, the brain tissues were cut into 5-µm-thick coronal
sections. The paraffin sections were deparaffined in xylene and
rehydrated in gradient ethanol from 100 to 70%. Finally, they were
stained with hematoxylin and eosin (H&E). The histopathological
changes of the cortex were observed under a light microscope
(Olympus, Tokyo, Japan) at a magnification of ×400 and images were
acquired.
Morphological evaluation by electron
microscopy
The parietal cortex of the ischemic hemisphere of
the mice (n=6 from each group) was collected and fixed for 2 h at
4°C with 2.5% glutaraldehyde, immediately following rinsing with
phosphate-buffered saline (PBS) and soaking in 2% osmium tetroxide.
This was followed by dehydration and embedding in epon. Ultrathin
(60-nm-thick) sections were cut using a diamond knife and placed
onto colloid-coated copper grids, and finally, were stained with
0.4% uranyl acetate and 2% lead citrate. The morphological changes
of the neurons were then observed and photographed using a
transmission electron microscope (H-7650; Hitachi, Tokyo,
Japan).
Determination of indicators of oxidative
stress
Following MCAO, the mice (n=6 from each group) were
decapitated, and the parietal cortexes of the ischemic hemisphere
were quickly removed and washed in chilled saline, and were then
homogenized in ice-cold saline (9 volumes) for 20 min to prepare a
10% (w/v) homogenate. The homogenate was centrifuged at 3,500 rpm
and 4°C for 15 min. The level of malondialdehyde (MDA), as well as
the activities of superoxide dismutase (SOD), glutathione
peroxidase (GSH-Px) and catalase (CAT), and the total antioxidant
capacity (T-AOC) in the supernatant were investigated using a
microplate reader (1510; Thermo Fisher Scientific, Waltham, MA,
USA) according to the instructions provided with the assay kits
(Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The
assay results were normalized to the protein concentration in each
sample, and expressed as U/mg protein or nmol/mg protein.
Apoptosis assay by flow cytometry
Following 24 h of reperfusion, the ischemic penumbra
areas of the parietal cortex of the mice (n=6 from each group) was
taken removed and place on ice, shredded and treated with trypsin
at 37°C for 15 min. This was followed by washing with ice-cold PBS,
and filtering through a 400 mesh nylon net 2 times to yield a
single cell suspension. The cell suspension was stained with
Annexin V-FITC staining solution at 4°C for 15 min, followed by
propidium iodide staining solution at 4°C for 5 min in the dark.
The samples were then immediately analyzed using a flow cytometer
(BD Biosciences, San Jose, CA, USA) and the data were analyzed
using CellQuest Pro software.
Immunofluorescence staining
Paraffin-embedded coronal brain sections
(5-µm-thick) were subjected to deparaffinization,
rehydration and then to microwave irradiation antigen retrieval
(microwave method). The sections (n=6 from each group) were then
incubated with the appropriate primary antibodies: caspase-3
(19677-1-AP), Bax (50599-2-lg) and Bcl-2 (12789-1-AP) (caspase-3,
1:50; Bax, 1:50; Bcl-2, 1:50; Proteintech Group, Chicago, IL, USA)
overnight at 4°C. The following day, the brain sections were rinsed
with cold PBS in order to remove the unbound antibodies and
incubated with FITC-labeled goat anti-rabbit IgG (SA00003-2; 1:200;
Proteintech Group) for 1 h at room temperature followed by
4′,6-diamidino-2-phenylindole (DAPI) for 5 min at room temperature.
Finally, the mean density of Bax, Bcl-2 and caspase-3 in the mouse
brains (per section; ×400 magnification) was measured using
microscope image-analysis software (Image-Pro Plus; Media
Cybernetics) by a single investigator who was blind to the sample
identity.
Western blot analysis
After 24 h of reperfusion, the mice (n=6 from each
group) were selected randomly and decapitated, adn the ischemic
penumbra areas of the parietal cortex were rapidly collected onto
ice and kept at −20°C. The prepared brain tissues were weighed and
homogenized in 1:10 (w/v) ice-cold whole cell lysis buffer (Nanjing
KeyGen Biotech Co., Ltd., Nanjing, China) using a glass
homogenizer. Soluble proteins were collected and centrifuged at
12,000 × g for 10 min at 4°C. Tissue total protein concentrations
were determined by a BCA Protein assay reagent kit (Beijing
TransGen Biotech Co., Ltd.). Tissue total protein (50 µl;
caspase-3, Bax and Bcl-2) was separated by 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then
transferred onto a nitrocellulose membrane. The membrane was
blocked with PBST containing 5% non-fat dry milk for 1 h, and then
incubated overnight at 4°C with the corresponding primary
antibodies (Bax, 1:500; Bcl-2, 1:500; caspase-3, 1:1,000;
Proteintech Group). After washing 3 times with PBST, the membrane
was incubated with secondary antibody (anti-rabbit IgG, SA00001-2;
1:3,000; Proteintech Group). An anti-actin antibody (20536-1-AP;
1:1,000; Proteintech Group) served as the control. The protein band
was visualized using an enhanced chemiluminescence (ECL) kit and
the density of each band was quantified using a western blotting
detection system (Quantity One software; Bio-Rad Laboratories,
Hercules, CA, USA).
Statistical analysis
All statistical analyses were performed using SPSS
17.0 statistical software (SPSS Inc., Chicago, IL, USA). The
results are expressed as the means ± standard deviation. The
statistical significance of the differences between the various
groups was assessed using one-way analysis of variance (ANOVA)
followed by the LSD post hoc test. Data of 2 groups were analyzed
by an unpaired t-test. Differences were considered statistically
significant at values of p<0.05.
Results
Mat exerts neuroprotective effects
against cerebral ischemia Infarct volume
As shown form the images of the TTC-stained brain
sections (Fig. 2A), the infarcted
brain tissue appeared white, whereas the normal region appeared
red. No infarction was observed in the sham-operated group and an
extensive infract area (36.01±5.33%) was observed in the
vehicle-treated group. The administration of Mat (7.5, 15 and 30
mg/kg) and nimodipine significantly decreased the percentage of the
infarct area to 28.39±6.65% (p<0.05), 19.62±2.85% (p<0.01),
15.76±3.60 % (p<0.01) and 13.31±2.58% (p<0.01), respectively
(Fig. 2B).
Neurological deficit scores
The examination of neurological function was carried
out on the mice subjected to 2 h of ischemia followed by 24 h of
reperfusion. Compared with the sham-operated group, the
neurological deficits were significantly increased in the
vehicle-treated group (p<0.01). However, the neurological
deficit scores were markedly reduced in the groups treated with Mat
(7.5, 15 and 30 mg/kg) and nimodipine (p<0.01). The range in the
neurological deficit scores for the different groups is shown in
Fig. 2C.
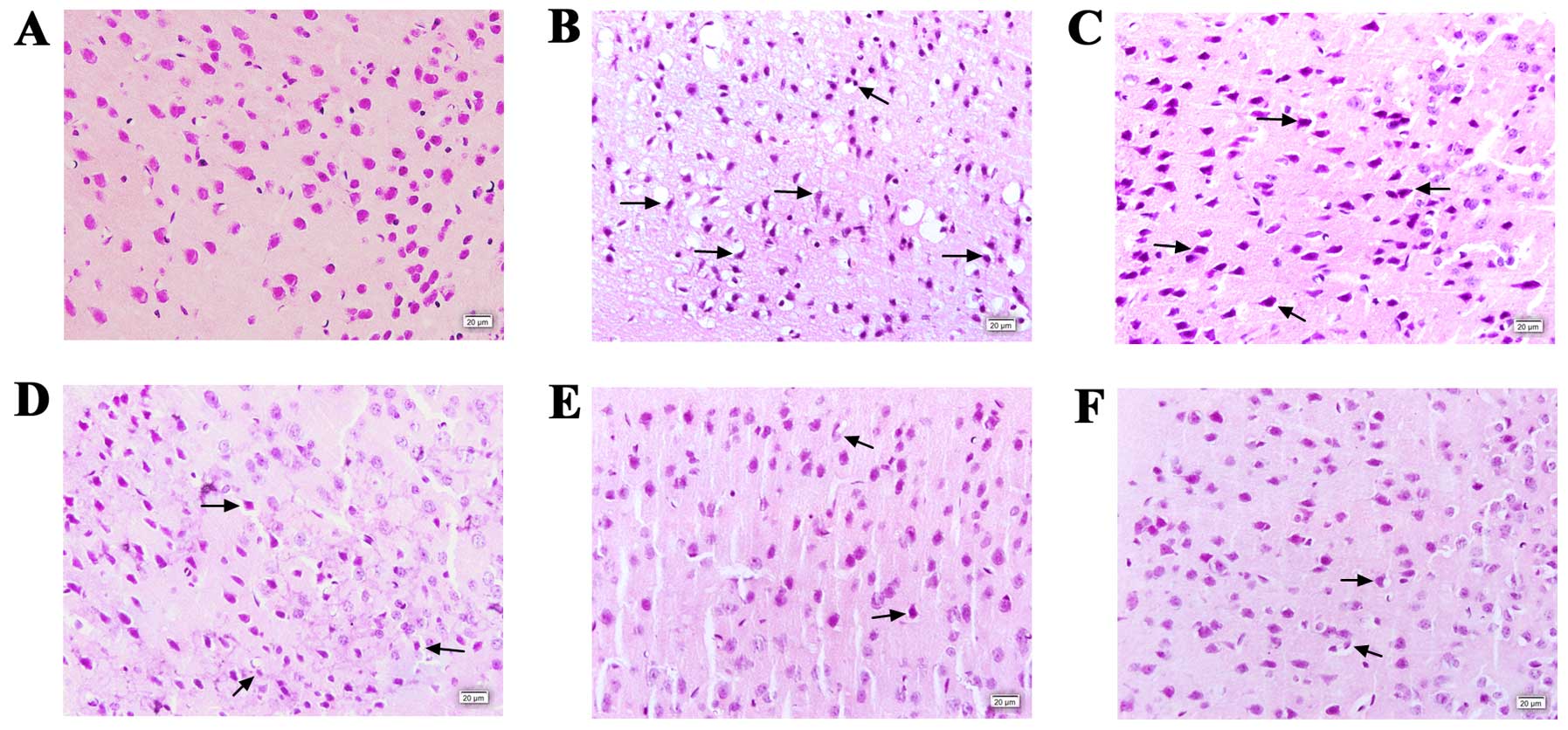
Histopathological examination
H&E staining was performed to observe the
histopathological changes in the neurons of the mouse brains in the
different groups (Fig. 3). In the
sham-operated group, the cortex tissue remained intact and the
neurons remained well-arranged, and the nuclei were centered with
clear staining. However, in the vehicle-treated group (Fig. 3B), a large number of neurons
appeared shrunken, swollen, and karyopyknosis and interstitial
edema were observed. In addition, neuron arrangement was disordered
with loosened and vacuolar neural fibers. However, in the groups
pre-treated with Mat and nimodipine, the extent of damage was
significantly alleviated, and the number of normal neurons was also
markedly increased.
Morphological evaluation
We evaluated the ultrastructural changes of the
cortex neurons by transmission electron microscopy, and images were
acquired (Fig. 4). The normal
cortex neuron contained a large round or oval nucleus with a clear
and integrated double nuclear membrane with homogeneous euchromatin
and abundant cellular organelles. After 24 h of reperfusion, the
cortex neurons showed severe damage. The majority of the nuclei
were irregular rather than round or oval in shape with uneven
chromatin and a damaged double nuclear membrane, and swollen or
vacuolated cellular organelles were observed in the vehicle-treated
group (Fig. 4B). By comparison,
the morphology of the cortex neurons in the groups pre-treated with
Mat and nimodipine showed varying degrees of recovery, displaying
relatively normal nuclear membranes, a regular-shaped nucleus and
slightly broken cellular organelles.
 | Figure 4Ultrastructural changes induced by
cerebral ischemia and inhibition by matrine (Mat) (×3,000
magnification). (A) Sham-operated group. In the hippocampus, the
nerve cell (NC) shows a normal ultrastructure. The nucleus (N),
granular endoplasmic reticulum (ER), mitochondrion (M), and Golgi
apparatus (G) are indicated. (B) Vehicle-treated group. In the
hippocampus, the nerve cell exhibits nuclear chromatine clumping,
enlargement of granular ER cisternae, increase in lysosomes (L) and
cytoplasmic blebbing. The Golgi apparatus (G) and mitochondrion (M)
are indicated. (C–F) Mat (7.5, 15 and 30 mg/kg)- and
nimodipine-treated group, respectively. In the hippocampus, the
nerve cell (NC) shows nuclear (N) chromatine clumping and slight
dilatation of the granular ER cisternae and mitochondrion (M).
Lysosomes (L) are indicated. In the Mat 30 mg/kg- and
nimodipine-treated groups, the nerve cells (NC) had a relatively
normal ultrastructure. |
All of these results demonstrated that Mat
alleviated cerebral I/R injury. Moreover, these observations
indicated that the most prominent protective effects were observed
in the MCAO + Mat (H) (H = 30 mg/kg) group.
Antioxidant activity of Mat in mice with
cerebral I/R injury
To evaluate the effects of Mat on oxidative stress
induced by MCAO, the levels of MDA, as well as the activity of SOD,
GSH-Px and CAT, and T-AOC were measured after 24 h of reperfusion.
When compared with the sham-operated group, SOD, GSH-Px and CAT
activity, and T-AOC were markedly reduced (p<0.01) and the MDA
concentration increased significantly (p<0.01) in the
vehicle-treated group. Pre-treatment of the mice with Mat exerted
antioxidant effects as evidenced by the resoration of SOD, GSH-Px
and CAT activity, and T-AOC (Fig.
5B–E) and a decrease in the MDA levels (p<0.05 and
p<0.01; Fig. 5A) in a
dose-dependent manner after 24 h of reperfusion. Nimodipine
produced similar effects.
Effect of Mat on neuronal apoptosis in
mice with cerebral I/R injury
In order to assess cortex neuronal apoptosis, flow
cytometry was carried out. After 24 h of reperfusion, the results
revealed that, in the sham-operated group, there was a small amount
of apoptotic cells in the left cortex. Compared to the
sham-operated group, the cortex neuronal apoptotic rate was
significantly increased following MCAO (p<0.01). However, the
increase in neuronal apoptosis following MCAO was markedly reduced
in the groups pre-treated with Mat (H) and nimodipine (p<0.05;
Fig. 6).
Mat affects the expression of
apoptosis-associated proteins
To investigate the mechanisms through which Mat
inhibits MCAO-induced neuronal apoptosis, the expression of the
apoptosis-related proteins, Bax, Bcl-2 and caspase-3, in the
hippocampus CA1 and cortex regions of the mice were examined by
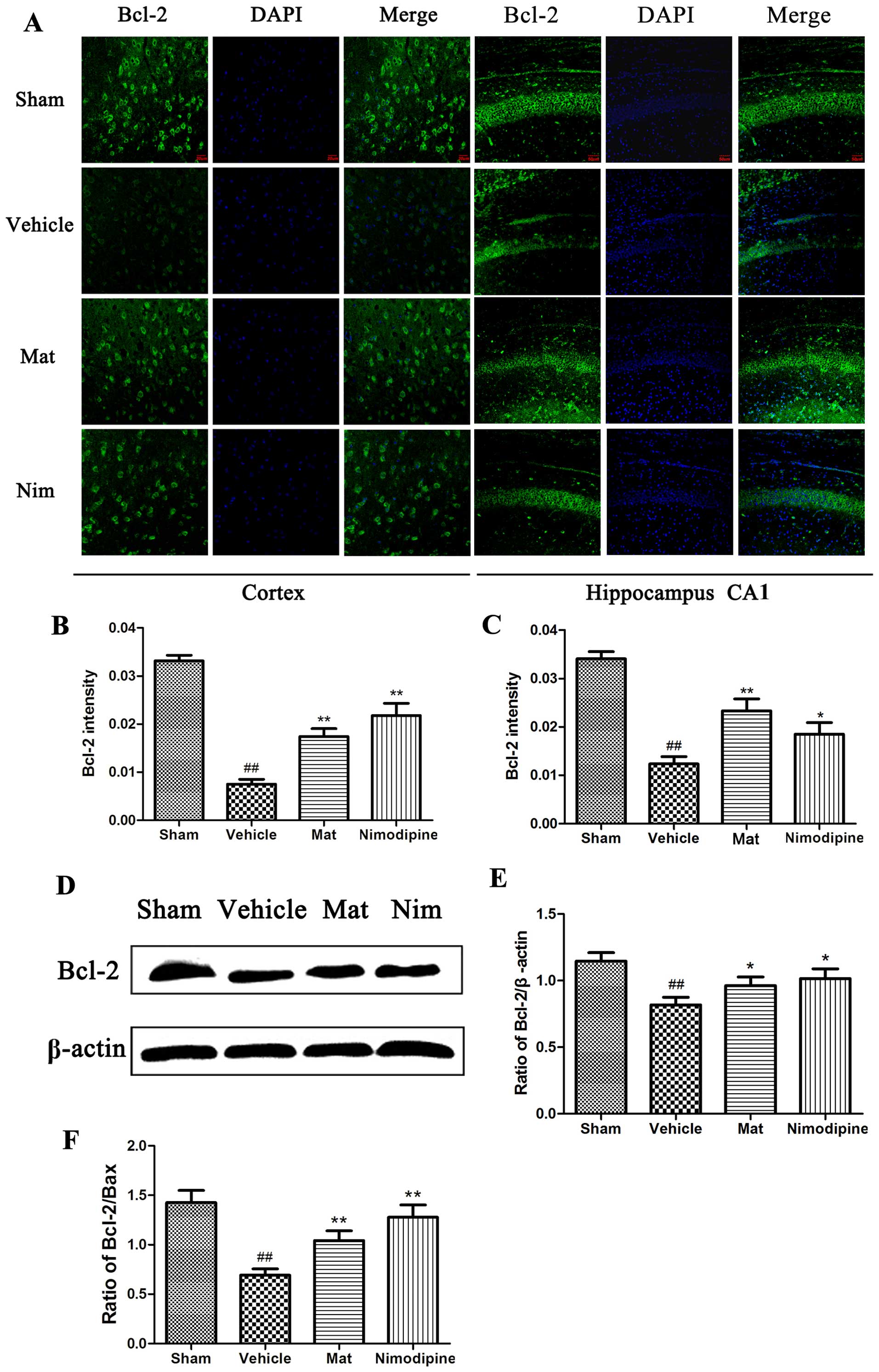
immunofluorescence staining and western blot analysis. As shown in
the images of immunofluorescence staining (Fig. 7A), compared with the sham-operated
group, the fluorescence intensity measurements of the protein
expression levels of caspase 3 were significantly greater following
MCAO (p<0.01). Pre-treatment with Mat (H) and nimodipine
significantly reduced the intensity of caspase-3 protein expression
(p<0.05 and p<0.01; Fig. 7B and
C) in comparison to the vehicle-treated group. On the other
hand, compared with the sham-operated group, the vehicle-treated
group displayed a higher fluorescence intensity of Bax (Fig. 8A) and a lower fluorescence
intensity of Bcl-2 protein (Fig.
9A). However, pre-treatment with Mat (H) or nimodipine resulted
in a significant increase in Bcl-2 expression (p<0.05 and
p<0.01; Fig. 9B and C) and a
marked decrease in Bax expression (p<0.05 and p<0.01;
Fig. 8B and C). In line with the
results from immunofluorescence staining, the results from western
blot analysis demonstrated that the caspase-3 protein level in the
vehicle-treated group was markedly increased compared to that of
the sham-operated group (p<0.01; Fig. 7D and E). The groups treated with
Mat (H) and nimodipine showed a significantly reduced protein
expression of caspase-3 in comparison to the vehicle-treated group
(p<0.05 and p<0.01; Fig.
7E). Similarly, the protein expression of Bcl-2 was markedly
decreased (p<0.01; Fig. 9E),
Bax protein expression was significantly increased (p<0.01;
Fig. 8E), and the Bcl-2/Bax ratio
was significantly decreased (p<0.01; Fig. 9F) in vehicle-treated group
compared to the sham-operated group. However, the Bcl-2/Bax ratio
returned to approximately normal levels (those of the control) in
the groups pre-treated with Mat (H) and nimodipine (p<0.01;
Fig. 9F).
Discussion
In the present study, we provide evidence that Mat
is an effective neuroprotectant against focal cerebral ischemia. We
demonstrated that pre-treatment with Mat reduced the infarct
volume, improved neurological deficits and alleviated
histopathology and morphological injury in mice subjected to MCAO,
which indicated that Mat has the ability to protect the mouse brain
against I/R injury. Moreover, we explored the mechanisms
responsible for the neuroprotective effects of Mat against focal
cerebral ischemia by determining the levels of oxidative stress and
apoptotic biomarkers in the ischemic brain. Our data demonstrated
that the neuroprotective effects of Mat may be associated with the
suppression of the cell apoptosis through the regulation of the
expression of Bax, Bcl-2 and caspase-3 proteins and an increase in
the Bcl-2/Bax ratio, as well as the suppression of oxidative
stress, as evidenced by a decrease in the MDA content, and an
increase in SOD, GSH-Px and CAT activity and T-AOC in mice
subjected to MCAO.
MCAO is a classical stroke model of temporary
regional ischemia and is considered reliable and less invasive
(21,23,24); it is extensively used to study
neurological, histopathological and biochemical changes and the
mechanisms of cerebral ischemic injury in mice. The infarct volume
and neurological deficit score play important roles in evaluating
the validity of cerebrovascular drugs in the treatment of ischemic
brain disease. In the present study, pre-treatment with Mat
significantly decreased the percentage of the brain infarct volume
and improved neurological deficit scores at 24 h following ischemia
in a mouse model of cerebral infarction. In addition, the degree of
ischemic damage was observed by H&E staining, a method commonly
used to identify the histopathological changes associated with the
development of I/R injury (25).
Our results demonstrated that the size of the ischemia-affected
regions and neuronal necrosis were significantly decreased by
pre-treatment with Mat. Another important observation was shown by
electron microscopy; the cortex neurons showed prominent
morphological injuries in the mice following MCAO. However, these
morphological changes and damage were mitigated in the group
pre-treated with Mat. Therefore, the results from behavioral and
morphological analysis suggested that pre-treatment with Mat
exerted a protective effect against cerebral I/R injury.
Ischemic stroke remains an urgent public health
concern and is the major cause of mortality and permanent
neurological disability worldwide (26). During the past two decades,
accumulating research into the complex mechanisms of ischemic stoke
has indicated that excessive reactive oxygen species (ROS)
production and subsequent oxidative stress play harmful roles
during cerebral I/R injury (27,28). Along with the occurrence and
development of reperfusion, multifarious pernicious processes,
including the overproduction of oxygen radicals, the inactivation
of detoxification systems, the consumption of antioxidants and the
failure to adequately replenish antioxidants occur in ischemic
brain tissue (29), which
contributes to oxidative damage to cellular macromolecules such as
lipids, proteins and nucleic acids in the ischemic tissue, leading
to membrane damage, cell death and brain dysfunction (30,31). As the toxic final product of lipid
peroxidation, MDA is a sensitive marker of oxidative stress and is
responsible for cytotoxic effects and neuronal death (32). An efficient antioxidant defense
system involving endogenous antioxidant enzymes, such as SOD,
GSH-Px and CAT (33) plays an
important role in the maintenance of low concentrations of oxidants
and redox homeostasis in tissue through the scavenging of oxidants,
preventing deleterious ROS generation (34). In the present study, we
demonstrated that pre-treatment with Mat for 7 consecutive days
significantly increased SOD, GSH-Px, CAT and activity, and T-AOC,
and decreased MDA levels in a dose-dependent manner. These results
suggest that Mat protects the brain from cerebral I/R injury by
exerting antioxidant effects.
On the other hand, apoptosis, a form of programmed
cell death characterized by DNA fragmentation (35), is now being regarded as a key
event in the acceleration of tissue injury and cell death following
cerebral ischemia (36). As an
actively regulated form of cell death, apoptosis is mediated by two
pathways following cerebral ischemia: the intrinsic and extrinsic
pathways (37). The intrinsic
pathway originates from the mitochondrial release of cytochrome
c. The release of cytochrome c into the cytosol
promotes the formation of the apoptosome, a complex composed of the
apoptotic protease activating factor-1 (Apaf-1), procaspase-9 and
ATP (38). The apoptosome permits
the autoactivation of procaspase-9, which is followed by the
activation of procaspase-3. Ultimately, the activation of caspase-3
leads to DNA fragmentation (39).
In present study, the results of H&E staining and transmission
electron microscopy indicated that apoptotic morphological
characteristics, such as the breakup of the nuclear membrane,
pyknosis of the nucleolus and the disruption of the mitochondrial
ridge were evident in the neurons following cerebral ischemia.
Pre-treatment with Mat alleviated these effects.
It is well known that caspases are a family of
intracellular cysteine proteases involved in the initiation and
execution of cell apoptosis (40). Sudies have identified that
caspase-3 is a potent, terminal caspase that plays a crucial role
in executing apoptosis through the mitochondrial-dependent pathway
(41) and increased neuronal
caspase-3 expression has been observed in transient ischemic injury
(42). Moreover, the Bcl-2 family
proteins, composed of pro-apoptotic and anti-apoptotic members, are
vital to the intrinsic apoptotic pathway and control the activation
of downstream caspases (43).
Further evidence of the crucial role of Bcl-2 family proteins in
neuronal cell death has been provided by recent studies on cerebral
ischemia in rats, showing that the dysregulation of the Bcl-2
family proteins exacerbates ischemic neuronal injury (5,44,45). As a member of the Bcl-2 family,
the anti-apoptotic protein, Bcl-2, is localized to the
mitochondrial membrane, helping maintain membrane integrity and
preventing cytochrome c from being released into the
cytoplasm, which is a central step in the apoptotic process
(36). By contrast, the
pro-apoptotic protein, Bax, is a cytoplasmic protein, and when
cells are exposed to various apoptotic stimuli, the protein
translocates specifically to the mitochondria, which causes the
disequilibrium between Bax and Bcl-2 (46). This disequilibrium leads to
mitochondrial permeability changes and promotes the release of
cytochrome c from the mitochondria to cytoplasm. The
subsequent activation of caspase-3 eventually leads to cerebral
ischemia-induced apoptosis (47).
In the present study, we demonstrated that the decrease in the
expression of Bax and caspase-3 and the concurrent increase in the
expression of Bcl-2 and the Bcl-2/Bax ratio by 7 days of
pre-treatment with Mat (30 mg/kg), which strongly favors the notion
that Mat has anti-apoptotic activity as recently reported (22), occurred least in part, through the
modulation of the Bcl-2/Bax ratio and the inhibition of caspase-3
expression. Increasing evidence indicates that Mat induces
apoptosis in a number of cancer cell lines (12,48–50). The dual regulation and control of
Mat as regards apoptosis may be due to its differential effects on
dividing cells and non-dividing cells.
In this study, focal cerebral ischemia reperfusion
was evident by 2 h of MCAO and reperfusion for 24 h in mice.
However, the therapeutic window of Mat is and its maximal
protective effects against cerebral I/R injury were not
sufficiently elucidated. A major limitation of the present study
was that multiple pathways are involved in the apoptotic process
following cerebral I/R injury and additional investigations are
required to determine the detailed molecular targets mediating the
neuroprotective effects of Mat.
In conclusion, this study demonstrated that Mat
exerted neuroprotective effects against cerebral I/R injury mice.
Mat significantly improved neurological deficits, reduced the
infarct volume and the percentage of apoptotic neurons, and
inhibited histopathologicla and morphological changes. Taken
together, the findings of this study suggest that the mechanisms
underlying the neuroprotective effects of Mat are associated with
its antioxidant and anti-apoptotic properties by targeting the
apoptosis-related proteins, caspase-3, Bax and Bcl-2. Therefore,
Mat may be used as an effective neuroprotective agent for the
treatment of stroke in clinical trials.
References
|
1
|
Liu Y, Zhang XJ, Yang CH and Fan HG:
Oxymatrine protects rat brains against permanent focal ischemia and
downregulates NF-kappaB expression. Brain Res. 1268:174–180. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu Q, Yang JW, Cao Y, Zhang LW, Zeng XH,
Li F, Du SQ, Wang LP and Liu CZ: Acupuncture improves locomotor
function by enhancing GABA receptor expression in transient focal
cerebral ischemia rats. Neurosci Lett. 588:88–94. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yao Y, Chen L, Xiao J, Wang C, Jiang W,
Zhang R and Hao J: Chrysin protects against focal cerebral
ischemia/reperfusion injury in mice through attenuation of
oxidative stress and inflammation. Int J Mol Sci. 15:20913–20926.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang M, Li J, Peng Q, Liu Y, Liu W, Luo
C, Peng J, Li J, Yung KK and Mo Z: Neuroprotective effects of
bilobalide on cerebral ischemia and reperfusion injury are
associated with inhibition of pro-inflammatory mediator production
and down-regulation of JNK1/2 and p38 MAPK activation. J
Neuroinflammation. 11:1672014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma Y, Li Y, Zhang C, Zhou X and Wu Y:
Neuroprotective effect of 4-methylcyclopentadecanone on focal
cerebral ischemia/reperfusion injury in rats. J Pharmacol Sci.
125:320–328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang L, Zhao H, Zhang X, Chen L, Zhao X,
Bai X and Zhang J: Nobiletin protects against cerebral ischemia via
activating the p-Akt, p-CREB, BDNF and Bcl-2 pathway and
ameliorating BBB permeability in rat. Brain Res Bull. 96:45–53.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tabassum R, Vaibhav K, Shrivastava P, Khan
A, Ahmed ME, Ashafaq M, Khan MB and Islam F, Safhi MM and Islam F:
Perillyl alcohol improves functional and histological outcomes
against ischemia-reperfusion injury by attenuation of oxidative
stress and repression of COX-2, NOS-2 and NF-κB in middle cerebral
artery occlusion rats. Eur J Pharmacol. 747:190–199. 2015.
View Article : Google Scholar
|
|
8
|
Micieli G, Marcheselli S and Tosi PA:
Safety and efficacy of alteplase in the treatment of acute ischemic
stroke. Vasc Health Risk Manag. 5:397–409. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu YQ, Jin SJ, Liu N, Li YX, Zheng J, Ma
L, Du J, Zhou R, Zhao CJ, Niu Y, et al: Aloperine attenuated
neuropathic pain induced by chronic constriction injury via
anti-oxidation activity and suppression of the nuclear factor kappa
B pathway. Biochem Biophys Res Commun. 451:568–573. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang T, Li Y, Wang Y, Zhou R, Ma L, Hao Y,
Jin S, Du J, Zhao C, Sun T, et al: Lycium barbarumpolysaccharide
prevents focal cerebral ischemic injury by inhibiting neuronal
apoptosis in mice. PLoS One. 9:e907802014. View Article : Google Scholar
|
|
11
|
Dong XQ, Du Q, Yu WH, Zhang ZY, Zhu Q, Che
ZH, Chen F, Wang H and Chen J: Anti-inflammatory effects of
oxymatrine through inhibition of nuclear factor-kappa B and
mitogen-activated protein kinase activation in
lipopolysaccharide-induced BV2 microglia cells. Iran J Pharm Res.
12:165–174. 2013.PubMed/NCBI
|
|
12
|
Cao HW, Zhang H, Chen ZB, Wu ZJ and Cui
YD: Chinese traditional medicine matrine: A review of its antitumor
activities. J Med Plants Res. 5:1806–1810. 2011.
|
|
13
|
Zhang HF, Shi LJ, Song GY, Cai ZG, Wang C
and An RJ: Protective effects of matrine against progression of
high-fructose diet-induced steatohepatitis by enhancing antioxidant
and anti-inflammatory defences involving Nrf2 translocation. Food
Chem Toxicol. 55:70–77. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Long Y, Lin XT, Zeng KL and Zhang L:
Efficacy of intramuscular matrine in the treatment of chronic
hepatitis B. Hepatobiliary Pancreat Dis Int. 3:69–72.
2004.PubMed/NCBI
|
|
15
|
Hu ZL, Tan YX, Zhang JP and Qian DH:
Effects of inhibitor of protein kinase C on brain edema formation
evoked by experimental cerebral ischemia in gerbils and rats. Yao
Xue Xue Bao. 31:886–890. 1996.In Chinese.
|
|
16
|
Xu M, Yang L, Hong LZ, Zhao XY and Zhang
HL: Direct protection of neurons and astrocytes by matrine via
inhibition of the NF-κB signaling pathway contributes to
neuroprotection against focal cerebral ischemia. Brain Res.
1454:48–64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hong-Li S, Lei L, Lei S, Dan Z, De-Li D,
Guo-Fen Q, Yan L, Wen-Feng C and Bao-Feng Y: Cardioprotective
effects and underlying mechanisms of oxymatrine against Ischemic
myocardial injuries of rats. Phytother Res. 22:985–989. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang H, Meng F, Li J and Sun X:
Anti-apoptosis effects of oxymatrine protect the liver from warm
ischemia reperfusion injury in rats. World J Surg. 29:1397–1401.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao J, Yu S, Tong L, Zhang F, Jiang X,
Pan S, Jiang H and Sun X: Oxymatrine attenuates intestinal
ischemia/reperfusion injury in rats. Surg Today. 38:931–937. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park SJ, Nam KW, Lee HJ, Cho EY, Koo U and
Mar W: Neuroprotective effects of an alkaloid-free ethyl acetate
extract from the root of Sophora flavescens Ait. against focal
cerebral ischemia in rats. Phytomedicine. 16:1042–1051. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang K, Li YJ, Yang Q, Gerile O, Yang L,
Li XB, Guo YY, Zhang N, Feng B, Liu SB, et al: Neuroprotective
effects of oxymatrine against excitotoxicity partially through
down-regulation of NR2B-containing rceptors. Phytomedicine.
20:343–350. 2013. View Article : Google Scholar
|
|
22
|
Yu J, Yang S, Wang X and Gan R: Matrine
improved the function of heart failure in rats via inhibiting
apoptosis and blocking β3 adrenoreceptor/endothelial nitric oxide
synthase pathway. Mol Med Rep. 10:3199–3204. 2014.PubMed/NCBI
|
|
23
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bederson JB, Pitts LH, Tsuji M, Nishimura
MC, Davis RL and Bartkowski H: Rat middle cerebral artery
occlusion: evaluation of the model and development of a neurologic
examination. Stroke. 17:472–476. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Okuno S NH and Sakaki T: Comparative study
of 2,3,5-triphenyltetrazolium chloride (TTC) and hematoxylin-eosin
staining for quantification of early brain ischemic injury in cats.
Neurol Res. 23:657–661. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang HB, Li YX, Hao YJ, Wang TF, Lei Z, Wu
Y, Zhao QP, Ang H, Ma L, Liu J, et al: Neuroprotective effects of
LBP on brain ischemic reperfusion neurodegeneration. Eur Rev Med
Pharmacol Sci. 17:2760–2765. 2013.PubMed/NCBI
|
|
27
|
Gilgun-Sherki Y, Rosenbaum Z, Melamed E
and Offen D: Antioxidant therapy in acute central nervous system
injury: Current state. Pharmacol Rev. 54:271–284. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu R, Gao M, Yang ZH and Du GH:
Pinocembrin protects rat brain against oxidation and apoptosis
induced by ischemia-reperfusion both in vivo and in vitro. Brain
Res. 1216:104–115. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chan PH: Reactive oxygen radicals in
signaling and damage in the ischemic brain. J Cereb Blood Flow
Metab. 21:2–14. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kontos HA: Oxygen radicals in cerebral
ischemia: The 2001 Willis lecture. Stroke. 32:2712–2716. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dröge W: Free radicals in the
physiological control of cell function. Physiol Rev. 82:47–95.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zimmermann C, Winnefeld K, Streck S,
Roskos M and Haberl RL: Antioxidant status in acute stroke patients
and patients at stroke risk. Eur Neurol. 51:157–161. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ozkan A, Sen HM, Sehitoglu I, Alacam H,
Guven M, Aras AB, Akman T, Silan C, Cosar M and Karaman HI:
Neuroprotective effect of humic Acid on focal cerebral ischemia
injury: An experimental study in rats. Inflammation. 38:32–39.
2015. View Article : Google Scholar
|
|
34
|
Chen H, Yoshioka H, Kim GS, Jung JE, Okami
N, Sakata H, Maier CM, Narasimhan P, Goeders CE and Chan PH:
Oxidative stress in ischemic brain damage: Mechanisms of cell death
and potential molecular targets for neuroprotection. Antioxid Redox
Signal. 14:1505–1517. 2011. View Article : Google Scholar :
|
|
35
|
Yuan J and Yankner BA: Apoptosis in the
nervous system. Nature. 407:802–809. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kong LL, Wang ZY, Hu JF, Yuan YH, Han N,
Li H and Chen NH: Inhibition of chemokine-like factor 1 protects
against focal cerebral ischemia through the promotion of energy
metabolism and anti-apoptotic effect. Neurochem Int. 76:91–98.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang F, Yin W and Chen J: Apoptosis in
cerebral ischemia: Executional and regulatory signaling mechanisms.
Neurol Res. 26:835–845. 2004. View Article : Google Scholar
|
|
38
|
Li P, Nijhawan D, Budihardjo I,
Srinivasula SM, Ahmad M, Alnemri ES and Wang X: Cytochrome c and
dATP-dependent formation of Apaf-1/caspase-9 complex initiates an
apoptotic protease cascade. Cell. 91:479–489. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li Y, Chopp M, Jiang N, Yao F and Zaloga
C: Temporal profile of in situ DNA fragmentation after transient
middle cerebral artery occlusion in the rat. J Cereb Blood Flow
Metab. 15:389–397. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Han BH, D'Costa A, Back SA, Parsadanian M,
Patel S, Shah AR, Gidday JM, Srinivasan A, Deshmukh M and Holtzman
DM: BDNF blocks caspase-3 activation in neonatal hypoxia-ischemia.
Neurobiol Dis. 7:38–53. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gill R, Soriano M, Blomgren K, Hagberg H,
Wybrecht R, Miss MT, Hoefer S, Adam G, Niederhauser O, Kemp JA, et
al: Role of caspase-3 activation in cerebral ischemia-induced
neurodegeneration in adult and neonatal brain. J Cereb Blood Flow
Metab. 22:420–430. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Namura S, Zhu J, Fink K, Endres M,
Srinivasan A, Tomaselli KJ, Yuan J and Moskowitz MA: Activation and
cleavage of caspase-3 in apoptosis induced by experimental cerebral
ischemia. J Neurosci. 18:3659–3668. 1998.PubMed/NCBI
|
|
43
|
Peng Z, Wang S, Chen G, Cai M, Liu R, Deng
J, Liu J, Zhang T, Tan Q and Hai C: Gastrodin alleviates cerebral
ischemic damage in mice by improving anti-oxidant and
anti-inflammation activities and inhibiting apoptosis pathway.
Neurochem Res. 40:661–673. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jia D, Han B, Yang S and Zhao J: Anemonin
alleviates nerve injury after cerebral ischemia and reperfusion
(i/r) in rats by improving antioxidant activities and inhibiting
apoptosis pathway. J Mol Neurosci. 53:271–279. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Abas F, Alkan T, Goren B, Taskapilioglu O,
Sarandol E and Tolunay S: Neuroprotective effects of
postconditioning on lipid peroxidation and apoptosis after focal
cerebral ischemia/reperfusion injury in rats. Turk Neurosurg.
20:1–8. 2010.PubMed/NCBI
|
|
46
|
Hetz C, Vitte PA, Bombrun A, Rostovtseva
TK, Montessuit S, Hiver A, Schwarz MK, Church DJ, Korsmeyer SJ,
Martinou JC, et al: Bax channel inhibitors prevent
mitochondrion-mediated apoptosis and protect neurons in a model of
global brain ischemia. J Biol Chem. 280:42960–42970. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sugawara T, Fujimura M, Morita-Fujimura Y,
Kawase M and Chan PH: Mitochondrial release of cytochrome c
corresponds to the selective vulnerability of hippocampal CA1
neurons in rats after transient global cerebral ischemia. J
Neurosci. 19:RC391999.PubMed/NCBI
|
|
48
|
Ma L, Wen S, Zhan Y, He Y, Liu X and Jiang
J: Anticancer effects of the Chinese medicine matrine on murine
hepatocellular carcinoma cells. Planta Med. 74:245–251. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tan C, Qian X, Jia R, Wu M and Liang Z:
Matrine induction of reactive oxygen species activates p38 leading
to caspase-dependent cell apoptosis in non-small cell lung cancer
cells. Oncol Rep. 30:2529–2535. 2013.PubMed/NCBI
|
|
50
|
Niu H, Zhang Y, Wu B, Zhang Y, Jiang H and
He P: Matrine induces the apoptosis of lung cancer cells through
downregulation of inhibitor of apoptosis proteins and the Akt
signaling pathway. Oncol Rep. 32:1087–1093. 2014.PubMed/NCBI
|