Introduction
Hyperplasia of vascular smooth muscle cells (VSMCs)
plays an essential role in the pathogenesis of a variety of
cardiovascular diseases, such as hypertension and atherosclerosis
(1). The deregulation of core
genes palys a major role in the development of a wide spectrum of
cardiovascular diseases, such as hypertension, heart failure and
arteriosclerosis that are characterized by abnormally proliferating
VSMCs (2–4). In the case of hypertension, it is
generally accepted that the abnormally accelerated proliferation
and enhanced contractile ability of VSMCs, significantly contribute
to the pathophysiology of the disease (2,5).
Insulin-like growth factor 1 (IGF1) is known to be a potent
stimulator of VSMC proliferation (6). The expression levels of IGF1 have
been found to be substantially upregulated in blood vessels
following injury and in the aorta of hypertensive animals (7), and the ability of IGF1 to enhance
VSMC proliferation requires the ligand occupancy of
αVβ3 integrin (8). However, the molecular mechanisms
underlying the upregulation of IGF1 remain largely unknown.
MicroRNAs (miRNAs or miRs) are highly conserved
endogenous small non-coding RNAs (approximately 22–25 nucleotides
in length), and they are believed to be able to regulate the
expression of up to one-third of all protein-coding genes in humans
at the post-transcriptional level by binding to the 3′ untranslated
region (3′UTR) of the target genes, inhibiting the translation of
mRNAs or promoting the degradation of mRNAs (9). miRNAs have been reported to play a
role in various physiological and pathological processes, such as
metabolism, cell differentiation, development, apoptosis and
proliferation, as well as in oncogenesis (10). Accumulating evidence indicates
that several specific miRNAs play a role in the regulation of VSMC
proliferation, apoptosis and differentiation by targeting the
post-transcriptional expression of multiple genes (11–14). For example, miR-145 has been found
to be a regulator of VSMC phenotypic modulation and VSMC
proliferation by targeting Krüppel-like factor 5 (KLF5) (12). Additionally, it has been reported
that p27 (Kip1) and p57 (Kip2) are target genes of miR-221 and
miR-222 and that they are responsible for their regulatory effects
on VSMC proliferation and neointimal hyperplasia (14). These findings may lead to the
identification of promising novel therapeutic targets for the
treatment of a variety of proliferative vascular diseases,
including hypertension and atherosclerosis.
It has been observed that spontaneously hypertensive
rats (SHRs) and Wistar Kyoto (WKY) rats genetically share a common
lineage, but markedly differ in their vascular phenotype (15), and it has also been reported that
the proliferative activity of VSMCs isolated from SHRs (SHR-VSMCs)
is markedly greater than that of VSMCs isolated from WKY rats
(WKY-VSMCs) (16). In addition,
in a previous study, Yu et al (17) identified differentially expressed
miRNAs in SHR-VSMCs and WKY-VSMCs using microarray analysis. Thus,
in this study, we performed reverse transcription-quantitative PCR
(RT-qPCR) to verify the changes in the expression of the miRNAs
identified by Yu et al (17) to be downregulated in SHR-VSMCs;
these miRNAs include miR-1, miR-98, miR-34b and miR-500. We
confirmed that miR-1 was significantly downregulated in the
SHR-VSMCs compared with the controls. Using in silico
analysis based on online predicting tools, such as TargetScan and
miRDB, we identified IGF1 as a potential target gene of miR-1. Our
hypothesis was that miR-1 may play a role in regulating the
proliferation of VSMCs by targeting IGF1. To confirm this
hypothesis, we validated that IGF1 is a target gene of miR-1 by
luciferase assay and demonstrated that the exogenous overexpression
of miR-1 significantly attenuated the proliferation of VSMCs.
Materials and methods
Cell culture
Rat aortic smooth muscle cells SMCs were isolated
from the medial layer of the thoracic aorta of female SHRs and WKY
rats (10 weeks old) and cultured in Dulbecco's modified Eagle's
medium (DMEM; Lonza, Walkersville, MD, USA) supplemented with 10%
fetal bovine serum (FBS), penicillin and streptomycin (HyClone,
Logan, UT, USA). The cells of passage 4–6 were employed in the
experiment. The experimental protocol was approved by the Ethics
Committee of Shougang Hospital, Peking University, Beijing, China.
Human aortic SMCs (HASMCs) were cultured in SmGM-2 growth medium
(both from Lonza) supplemented with 5% FBS (HyClone) following the
manufacturer's instructions.
Patient demographics and
characteristics
Six patients who underwent open aortic arch
reconstruction that required aortic dissection at Shougang Hospital
were recruited in this study. Their demographic and clinical data
are presented in Table I. The
study protocol was approved by the Medical Ethics Committee of
Shougang Hospital. Informed consent was obtained from all patients
prior to enrollment. The SMCs were isolated as previously described
(18). Briefly, resected vascular
tissue was placed in DMEM with penicillin/streptomycin (5/500 ml)
and transferred to a super-clean bench. The vascular tissue was
rinsed 3 times with phosphate-buffered saline (PBS) and the intima
was removed. The tunica media was finely cut into 2–3-mm-thick
sections in another 100-mm culture dish, and 5 ml of 0.1% type I
collagenase (Invitrogen, Carlsbad, CA, USA) was added to the
culture dish. Subsequently, the dish was placed in an incubator for
2 h at 37°C. Digestion media were collected and filtrated using a
cell strainer to remove the undigested explants and then
centrifuged (1,000 rpm, 5 min, 4°C). The aforementioned procedures
were repeated 3 times in order to harvest more cells.
 | Table IDemographic data and clinical
characteristics of the patients recruited in this study. |
Table I
Demographic data and clinical
characteristics of the patients recruited in this study.
| Patient ID | Age (years) | Gender | Hypertension
(mmHg) | Diabetes |
|---|
| 1 | 39 | M | 168/98 | + |
| 2 | 56 | F | 173/105 | + |
| 3 | 63 | M | 182/112 | + |
| 4 | 58 | M | 118/75 | − |
| 5 | 42 | F | 119/73 | + |
| 6 | 65 | M | 116/78 | − |
Isolation of RNA and RT-qPCR
Total RNA was isolated using the miRNeasy Mini kit
(Qiagen, Valencia, CA, USA) following the manufacturer's
instructions, and the quality of the isolated RNA was evaluated
using a NanoDrop spectrophotometer (Thermo Fisher Scientific,
Waltham, MA, USA) and agarose electrophoresis. The PrimerScript RT
reagent kit (Takara, Dalian, China) was used to synthesize the
cDNA. The SYBR-Green real-time detection system was purchased from
Bio-Rad Co., Ltd. (Hercules, CA, USA). U6 was used as an internal
control to normalize the miR-1 and IGF1 mRNA expression levels. All
fold changes were calculated using the ΔΔCt method. The primer sets
used for amplification are listed in Table II.
 | Table IIPrimer sets used for qPCR, subcloning
and mutagenesis of IGF1. |
Table II
Primer sets used for qPCR, subcloning
and mutagenesis of IGF1.
| Name | Sequences |
|---|
| qPCR |
| miRNA-1 |
5′-CTCAACTGGTGTCGTGGAGTCGGC
AATTCAGTTGAGATACACAC-3′ |
|
5′-ACACTCCAGCTGGGTGGAATGTAAAGAAGT-3′ |
| miRNA-34b |
5′-GTGCTCGGTTTGTAGGCAGT-3′ |
|
5′-GTGCCTTGTTTTGATGGCAG-3′ |
| miRNA-500 |
5′-CTGGCCGCCGATATTCACC-3′ |
|
5′-GGCAATCAAGTCAGCAAAATACC-3′ |
| miRNA-98 |
5′-TGAGGTAGTAAGTTGTATTGTT-3′ |
|
5′-AGCGCAGATCAAAAGGAGACA-3′ |
| IGF1 |
5′-AAATCAGCAGTCTTCCAAC-3′ |
|
5′-CTTCTGGGTCTTGGGCATGT-3′ |
| U6 |
5′-CTCGCTTCGGCAGCACA-3′ |
|
5′-AACGCTTCACGAATTTGCGT-3′ |
| Subcloning IGF1
3′UTR |
| Forward |
5′-GAAGACCCTCCTGAGGAGTG-3′ |
| Reverse |
5′-GAACTAATTAATCAAACATG-3′ |
| Mutagenesis of
IGF1 |
| Forward |
5′-GAAATACACAAGTAATGTAAGGTACATTGTCTTTAGGAGT-3′ |
| Reverse |
5′-ACTCCTAAAGACAATGTACCTTACATTACTTGTGTATTTC-3′ |
Overexpression of target miRNA
miR-1 mimics or anti-IGF1 siRNA (Ambion, Austin, TX,
USA) were used in the experiments, and Lipofectamin 2000
(Invitrogen) was employed for the transfection of the HASMCs.
Fluorescently labeled control oligo was first transfected and the
transfection efficiency was evaluated by visual fluorescence
microscopic analysis and flow cytometry. When the transfection
efficiency was >85%, the transfection was considered successful.
The in vitro experiments described below included cohorts
transfected with 30 and 100 pmol of miR-1 mimics and 100 pmol of
anti-IGF1 siRNA or the fluorescently labeled scrambled control
oligo (negative control) known to have no effect on any human miRNA
in order to minimize the non-specific effect.
MicroRNA target prediction
We searched two major online microRNA databases:
TargetScan (http://www.targetscan.org) and miRDB
(http://www.mirdb.org) to identify the candidate
target genes of miR-1. Among all the candidate genes, we further
narrowed down a short list of candidates based on the
phyiopathological function of the genes.
Transwell invasion and migration
assay
Transwell invasion assay was carried out in 24-well
fitted inserts with membranes (8 mm pore size; Costar, Cambridge,
MA, USA). As previously described (20), cell invasion was examined using a
polycarbonate membrane cell culture insert (Costar, Corning Inc.,
Corning, NY, USA) coated with growth factor-reduced Matrigel (BD
Biosciences, Bedford, MA, USA). The cells transfected with the
control, miR-1 mimics and anti-IGF1 siRNA were treated with 10
mg/ml mitomycin C (Sigma-Aldrich, St. Louis, MO, USA) for 2 h and
placed into the top of the wells at a density of 2.5×105
cells/well.
Cell survival assays
A modified
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT)
assay was performed to examine HASMC viability. The HASMCs
transfected with miR-1 mimics or anti-IGF1 siRNA were cultured in
96-well plates for 24 h and 10 μl of MTT AB solution
(Millipore, Billerica, MA, USA) were then added, followed by
incubation for 4 h. The formazan product was dissolved by the
addition of 100 μl of acidic isopropanol, and the absorbance
was determined at 570 nm (reference wavelength 630 nm) using an
xMark Microplate Absorbance Spectrophotometer (Bio-Rad Co.,
Ltd.).
Apoptosis assays
Programmed cell death rates were assessed using a
commercially available apoptosis assay (Sigma-Aldrich). The HASMCs
were transfected with miR-1 mimics or anti-IGF1 siRNA, and
1×105 cells were harvested and stained with 10 μl
FITC Annexin V and 10 μl propidium iodide and sorted by FACS
within 1 h (BD Biosciences, San Jose, CA, USA).
Western blot analysis
The transfected HASMCs were harvested and lysed, and
the cell lysates were subjected to 10% PAGE. The separated proteins
were transferred onto PVDF membranes blocked with TBST (10 mM
Tris-Cl pH 8.0, 150 mM NaCl, and 0.05% Tween-20) containing 5%
non-fat dry milk powder at room temperature for 1 h. Subsequently,
the membranes were incubated with primary antibodies directed
against human IGF1 (1:2,000) and β-actin (1:1,500), and an
HRP-conjugated goat-anti-rabbit secondary antibody (1:1,000) (both
from Cell Signaling Technology, Danvers, MA, USA). Exposed films
were scanned and fluorescence signals were detected using an ECL
kit (Pierce, Rockford, IL, USA).
Luciferase assay
The PCR product was then cloned into the pRL-SV40
vector (Promega, Fitchburg, WI, USA) to replace the 3′UTR of
Renilla luciferase, and the QuickChange XL site-directed
mutagenesis kit (Stratagene, La Jolla, CA, USA) was used to
introduce the variant. Luciferase assay was conducted on the
HASMCs, and the cells were seeded at 1×105 cells/well in
24-well plates. Twelve hours later, the cells were transfected
using Lipofectamin 2000 (Invitrogen) according to the
manufacturer's instructions. In each well, 500 ng of the wild-type
or mutant construct and 50 ng of the control vector (control oligo)
were co-transfected with 100 pmol miR-1 mimics or the negative
control (Ambion). Twenty-four hours after transfection, the cells
were harvested by the addition of passive lysis buffer (Promega).
Luciferase activity in the cell lysates was determined using the
Dual Luciferase assay system (Promega) with a TD-20/20 luminometer
(Turner Biosystems, Sunnyvale, CA, USA).
Statistical analysis
All data are presented as the means ± SD. One-way
ANOVA was used for comparisons between samples (>2 groups), and
an independent t-test was used for comparisons between 2 groups. A
value of P<0.05 was considered to indicate a statistically
significant difference. All experiments were performed at least 3
times. Statistical analysis was performed using the SPSS software
package (version 19; SPSS, Inc., Chicago, IL, USA).
Results
Comparison of miRNA expression profiles
between SHR-VSMCs and WKY-VSMCs
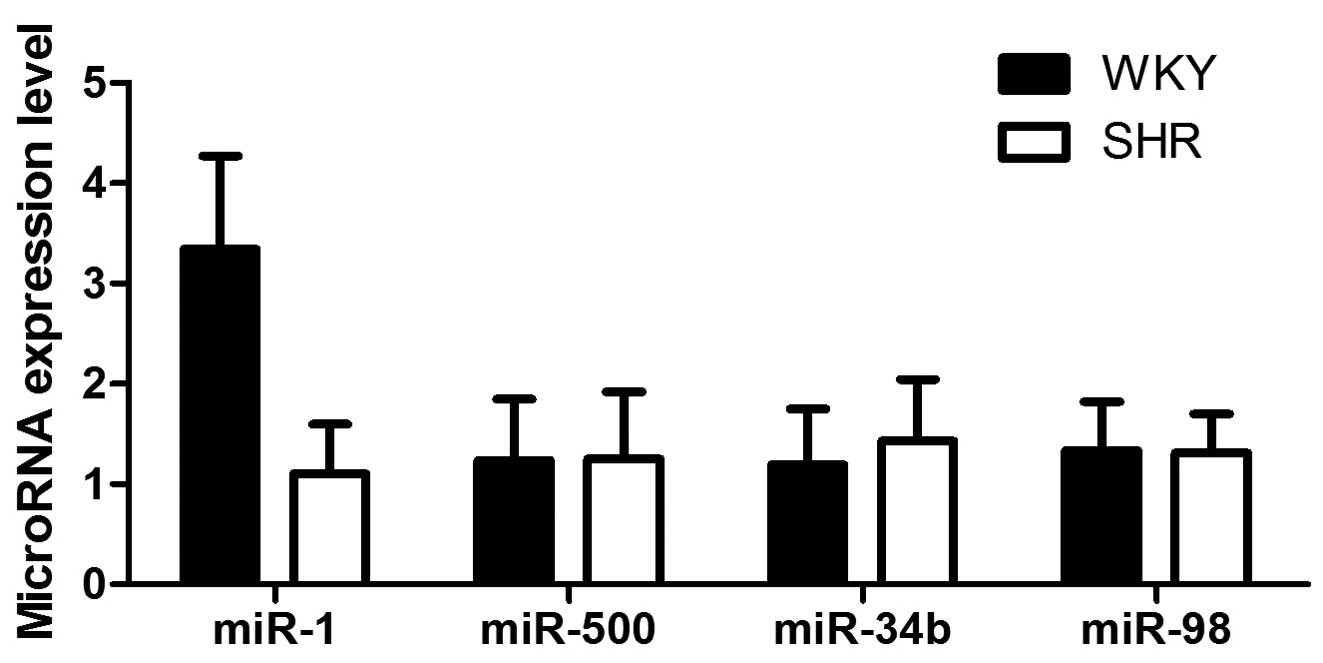
In the present study, we focused on miR-1, miR-500,
miR-34b and miR-98, as these miRNAs have been previously reported
to be significantly downregulated in SHR-VSMCs compared with
WKY-VSMCs, based on the results of microarray-based miRNA
expression profiling analysis (17). RT-qPCR was performed using the
primary VSMCs isolated from the medial layer of the thoracic aorta
obtained from SHRs and WKY rats, and we observed a 3.29-fold
decrease in the expression levels of miR-1 in the SHR-VSMCs
compared with the WKY-VSMCs (P<0.01; Fig. 1), whereas the expression levels of
the miRNAs were similar between the 2 groups. Thus, miR-1 was
further investigated to determine its potential biological
function.
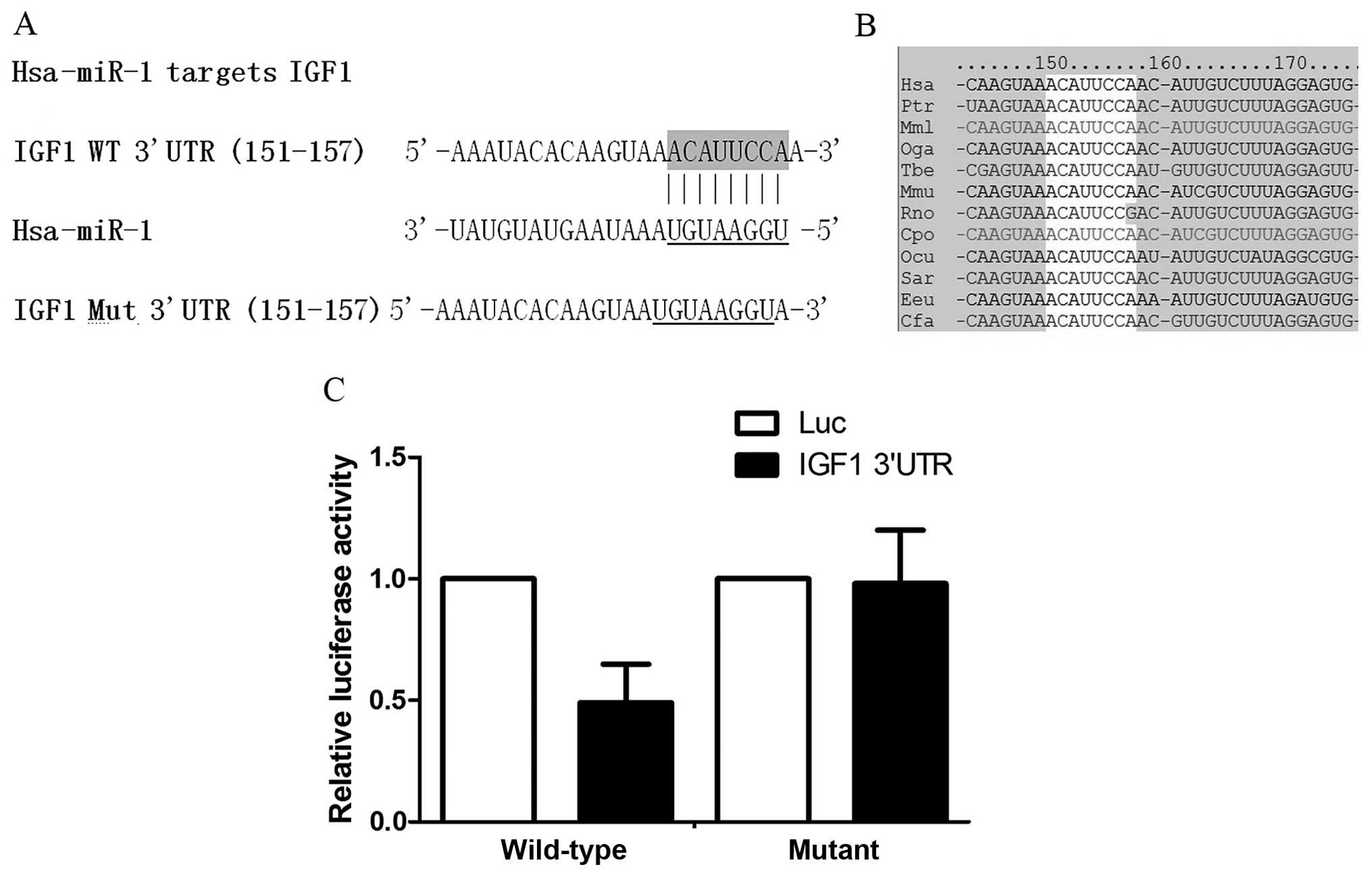
Validation of IGF1 as a target gene of
miR-1 using the luciferase reporter system
Using in silico analysis based on online
predicting tools, such as TargetScan and miRDB, we identified IGF1
as a potential target gene of miR-1 (Fig. 2A), and the 'seed sequence' in the
3′UTR of IGF1 was found to be highly conserved among species
(Fig. 2B), suggesting that such a
'seed sequence' may have an important function. To validate the
target gene, we subcloned the 3′UTR of IGF1 into the pRL-SV40
vector to replace the 3′UTR of Renilla luciferase, and
performed luciferase assay to compare the inhibitory effects
between the wild-type 3′UTR and the mutant as described in the
Materials and methods. The results revealed that co-transfection
with the miR-1 mimic significantly suppressed luciferase activity
when transfected together with the wild-type 3′UTR of IGF1, but not
the mutant one (Fig. 2C).
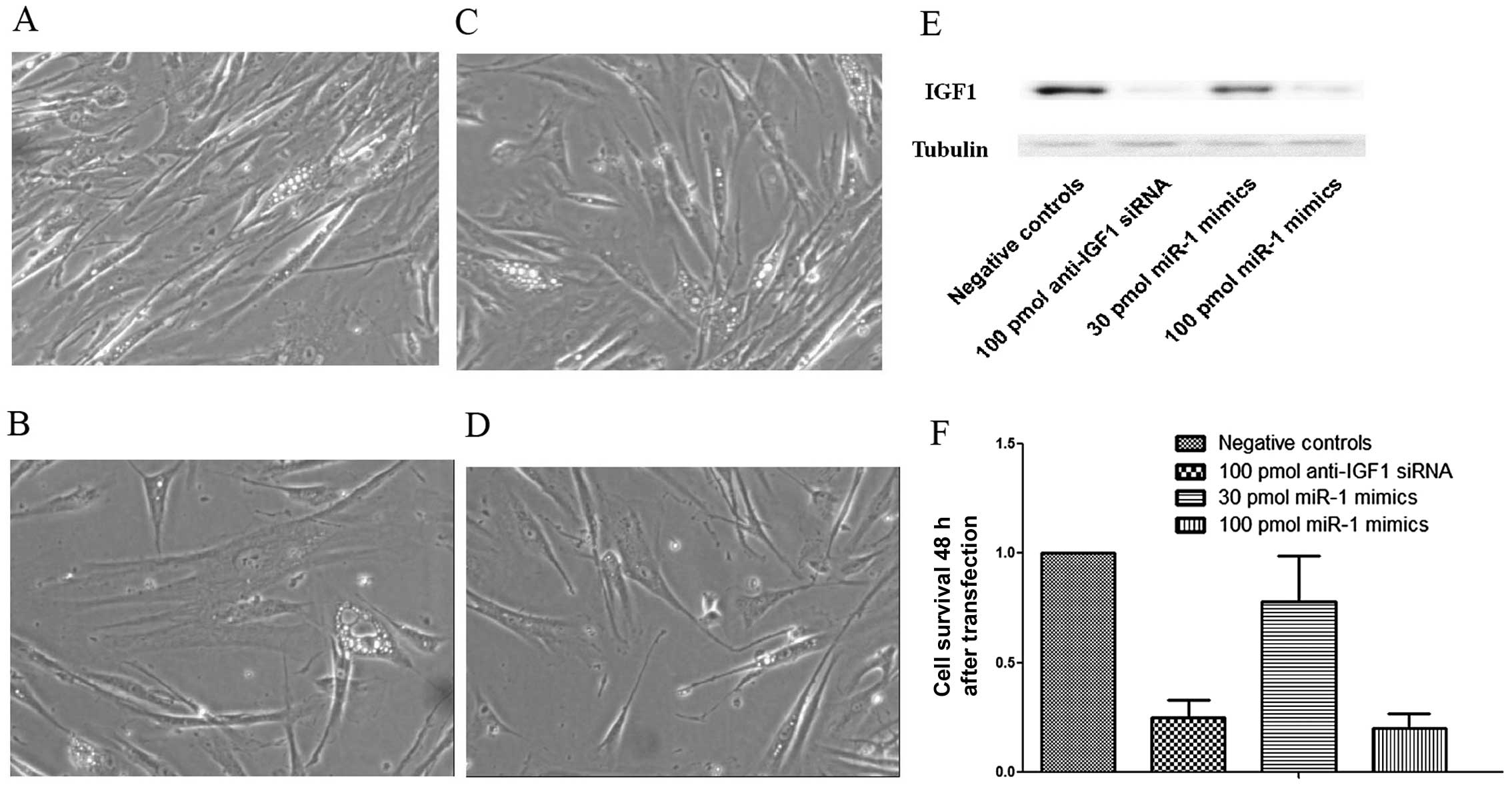
Inhibition of IGF1 expression in VSMCs by
transfection with miR-1 mimics
Additionally, the HASMCs were transfected with miR-1
mimics (30 and 100 pmol) and IGF1-specific siRNA, and the IGF1
protein levels were quantified by western blot analysis. Fig. 3E shows the step-wise
downregulation of IGF1 expression in the HASMCs transfected with an
increasing amount of miR-1 mimics (30 and 100 pmol). The silencing
effect of transfection with 100 pmol of the miR-1 mimics was
comparable with that of transfection with 100 pmol of the anti-IGF1
siRNA.
Effects of the overexpression of miR-1 on
VSMC proliferation
The HAMSCs were transfected with the miR-1 mimics
(30 and 100 pmol) and anti-IGF1 siRNA prior to the proliferation
assay. MTT assay was used to measure cell proliferation. The
results revealed that the exogenous overexpression of miR-1
significantly inhibited the proliferation of the HAMSCs (Fig. 3). After incubation for 48 h,
significant inhibitory effects on cell proliferation were observed
in the cells transfected with the miR-1 mimis or with anti-IGF1
siRNA compared with those transfected with the negative control. In
the HASMCs, the inhibitory rates were 75% (anti-IGF1 siRNA), 22%
(30 pmol miR-1 mimics) and 80% (100 pmol miR-1 mimics) following
incubation for 48 h (Fig. 3F).
This suggested that the overexpression of miR-1 inhibited the
proliferation of the VSMCs in vitro. Furthermore, we
performed Transwell invasion assay with the cells being treated
with mitomycin C that was used to eliminate the possible effect of
cell growth on cell invasion. The results revealed that the
inhibition of miR-1 in the HASMCs did not affect cell invasiveness
(data not shown).
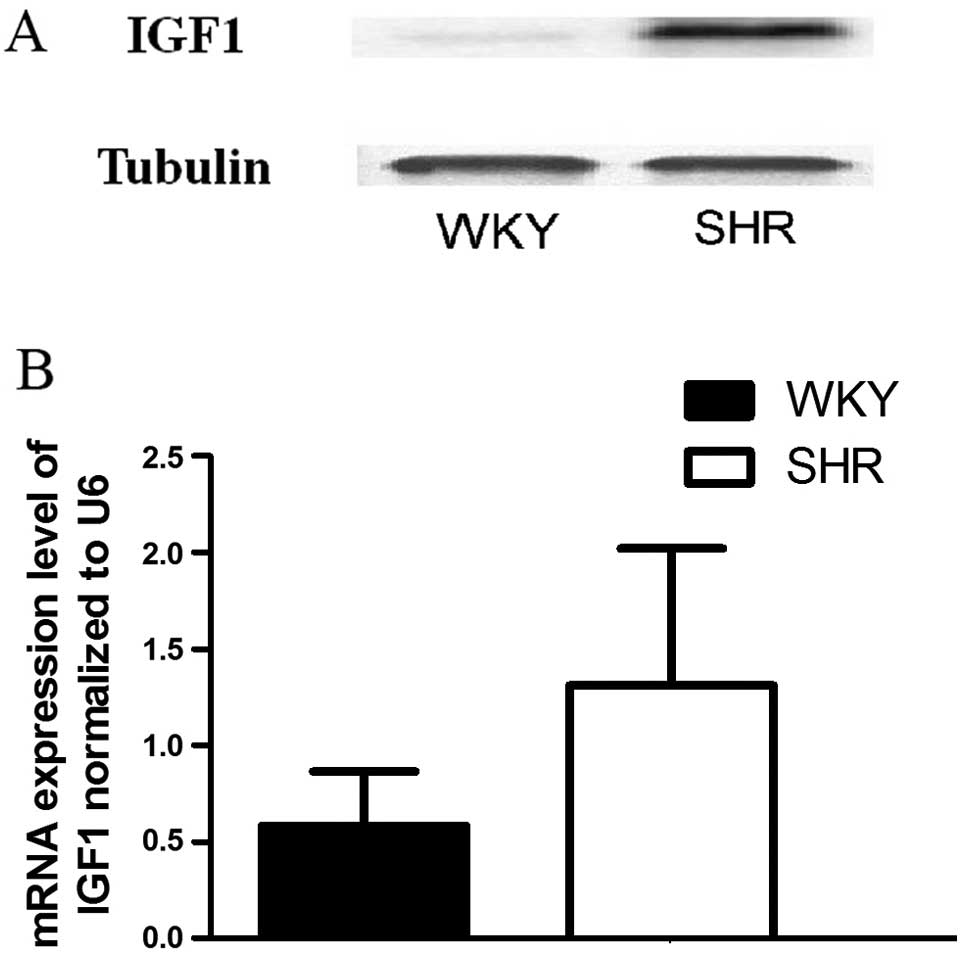
Comparison of IGF1 expression between the
SHR-VSMCs and WKY-VSMCs
We examined the SHR-VSMCs and WKY-VSMCs to determine
the expression patterns of IGF1 in these cells. The IGF1 mRNA
expression levels were measured by RT-qPCR, and we found that the
mRNA levels of IGF1 in the WKY-VSMCs were significantly lower than
those in the SHR-VSMCs (Fig. 4B).
The IGF1 protein levels were also measured using western blot
anlaysis. The results revealed that the protein expression levels
of IGF1 in the SHR-VSMCs were substantitally higher than those in
the WKY-VSMCs (Fig. 4A).
Comparison of IGF1 expression levels
between VSMCs collected from patients with severe hypertension and
the normal blood pressure control group
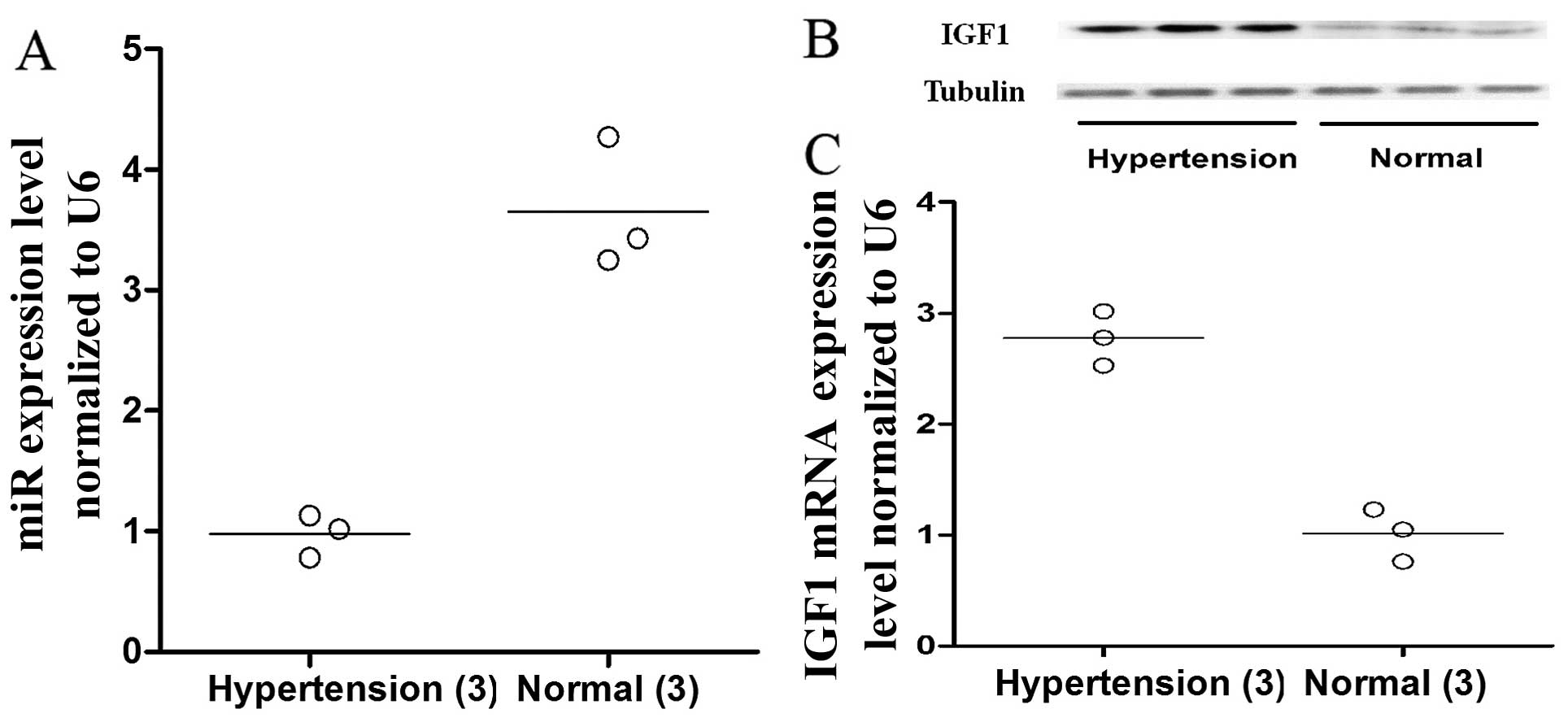
VSMCs were isolated from the vascular tissue
collected from 6 patients (hypertension, 3; normal blood pressure,
3) who had undergone open aortic arch reconstruction that required
aortic dissection (HTN-VSMCs vs. normal-VSMCs). The mRNA and
protein expression levels of IGF1 were compared between the 2
groups of cells by RT-qPCR and western blot analysis. We found that
both the protein and mRNA expression levels of IGF1 were markedly
higher in the HTN-VSMCs than in the normal-VSMCs (Fig. 5B and C, respectively). Similarly,
the expression levels of miR-1 were significantly lower in the
HTN-VSMCs than in the normal-VSMCs (Fig. 5A).
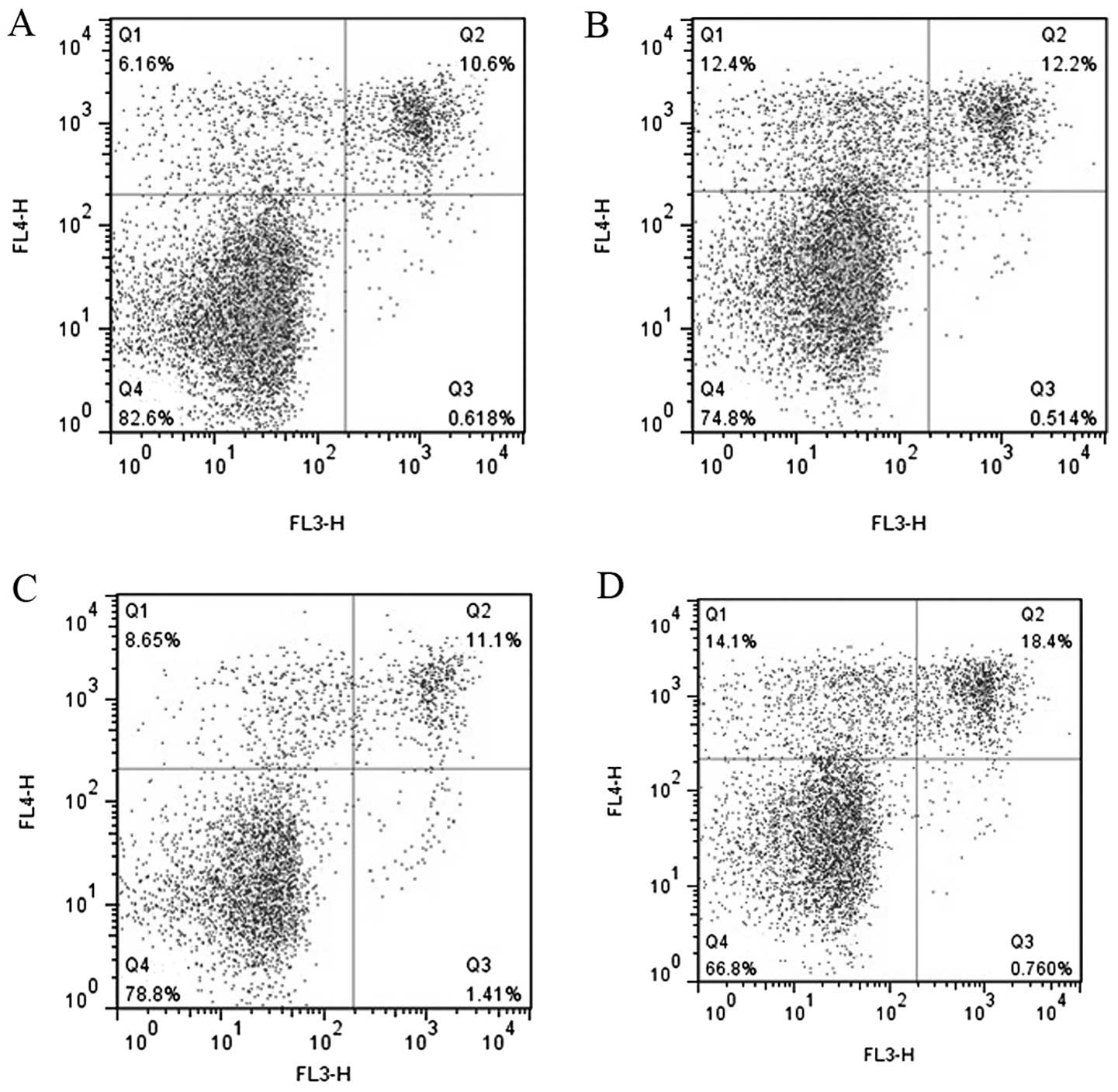
Increased expression of miR-1 induces the
apoptosis of HASMCs
As IGF1 functions as an anti-apoptotic protein and
the exogenous expression of miR-1 causes a reduction in the enzyme
(21), we examined the effects of
miR-1 on the apoptosis of HASMCs by flow cytometry. We found that
transfection of the cells with miR-1 mimics and anti-IGF1 siRNA
promoted the apoptosis of HASMCs (Fig. 6).
Discussion
The regulatory role of miRNAs in the control of VSMC
proliferation and the phenotypic switch has been well described in
the literature (12).
Furthermore, it has been demonstrated that proliferating VSMCs
significantly contribute to the pathogenesis of a wide spectrum of
cardiovascular diseases, such as atherosclerosis and hypertension
(3,4). In the present study, we demonstrated
that the expression of miR-1 was substantially downregulated in the
SHR-VSMCs compared with the WKY-VSMCs, and that the exogenous
overexpression of miR-1 markedly inhibited the proliferation of the
VSMCs; these effects were mediated by the downregulation of its
target gene, IGF1. Our results are consistent with the results of
previous studies on the role of miR-1 in the growth and
proliferation of cardiomyocytes and skeletal myoblasts (19,20,22,23).
The results of the present study demonstrated that
transfection of the cells with miR-1 inhibited VSMC proliferation;
these results are consistent with the inhibitory role of miR-1 in
the proliferation of other types of muscle cells, such as
cardiomyocytes and skeletal myoblasts (22,23). miR-1 has also been reported to
play a similar role in regulating the growth of cancer cells
(24). Our data suggest that the
downregulation of miR-1 contributes to a higher proliferation rate
of VSMCs isolated from SHRs, providing a potential explanation for
the lower proliferation rate of VSMCs obtained from WKY rats
compared to those from SHRs. Furthermore, considering the
inhibitory role of miR-1 in the control of the proliferation of
VSMCs, the deregulation of miR-1 signaling may also facilitate VSMC
proliferation in some pathophysiological context, such as
atherosclerosis and restenosis following angioplasty. A number of
genes have been suggested to be potential targets of miR-1 in
various types of cells and tissues, including BRG1, which is a
subunit of the SWI/SNF complex (25), morphogenetic protein 2 (BMP2)
(26), bone morphogenetic protein
receptor type-1B (BMPR1B), transforming growth factor (TGF)-β
(27), transforming growth
factor, β receptor III (TGFBR3) and the downstream signaling
mediators, SMAD2 and SMAD4 (TargetScan). Using in silico
analysis based on online predicting tools, such as TargetScan and
miRDB, we identified IGF1 as potential target gene of miR-1, and we
subsequently validated IGF1 as a target gene of miR-1 by luciferase
assay, and demonstrated that the exogenous overexpression of miR-1
significantly suppressed the expression of IGF1.
IGF1 is an ubiquitous factor which exerts
pleiotropic effects on a variety of cell types and can activate the
signaling pathway downstream of the IGF1 receptor (IGF1R) by
binding to the receptor, which has been shown to play a crucial
role in the control of cell proliferation, differentiation,
transformation and survival (28). IGF1R-dependent signaling is
crucial for the survival of many cell types, including VSMCs
(28). In this study, we
demonstrated that the downregulation of IGF1 by the introduction of
miR-1 attenuated the proliferation of the VSMCs, and the inhibitory
effect of miR-1 on cell proliferation was comparable to that
observed with transfection with anti-IGF1 siRNA, suggesting that
IGF1 is a target gene of miR-1 and that the anti-proliferative
effects of miR-1 are mediated through IGF1. It has been previously
demonstrated that VSMCs derived from atherosclerotic plaques
(pVSMCs) have a defect in IGF1-dependent survival signaling
(29), and are more sensitive to
apoptosis than cells derived from normal control vessels (30). Furthermore, it has been
demonstrated that oxidative stress reduces IGF1R expression and
induces VSMC apoptosis in culture (31–34). The downregulation of IGF1/IGF1R
signaling has also been observed in atherosclerotic lesions,
indicating that IGF1/IGF1R-dependent survival regulates apoptosis
in vivo (6,35). VSMCs lacking IGF1/IGF1R also
represent an increased sensitivity to multiple pro-apoptotic
stimuli, such as tumor suppressor genes and death receptor ligation
(36,37), suggesting that the defective
IGF1/IGF1R signaling pathway alone is an important cause of the
reduced survival of VSMCs. In this study, we found that the
inhibitory effect of miR-1 on the proliferation of VSMCs was
mediated by its ability to induce apoptosis by suppressing the
expression of IGF1. A major downstream effector of IGF1R signaling
is the serine/threonine kinase, Akt [also known as protein kinase B
(PKB)]. Akt phosphorylates a variety of targets involved in glucose
metabolism and cell differentiation, proliferation and survival
(38). It has been shown that
VSMCs in plaque also exhibit a reduced activation of Akt in
response to IGF1 treatment (29),
suggesting that Akt mediates IGF1R-dependent signaling in these
cells. It has also been demonstrated that the activation of Akt is
necessary and/or sufficient for VSMC survival in response to
apoptotic stimuli, and that Akt-dependent phosphorylation and the
subsequent inactivation of forkhead box O3a (FOXO3a) and glycogen
synthase kinase-3 (GSK3) is important for VSMC survival (28).
To further establish the role of IGF1 in mediating
the effects of miR-1 in VSMCs, we evaluated and compared the
effects of miR-1 mimics and anti-IGF1 siRNA on the expression of
IGF1 and on the proliferation status of VSMCs. As shown in Fig. 3, transfection with 100 pmol of
miR-1 mimics and 100 pmol of anti-IGF1 siRNA had a similar effect
on the knockdown the protein expression levels of IGF1, whereas the
inhibitory effects of miR-1 mimics on cell proliferation were more
prominent than those of anti-IGF1 siRNA. We reasoned that the
inhibitory effects of miR-1 on VSMC proliferation were mediated, at
least in part, through some other mediators, such as Pim-1, a gene
encoding an oncogenic serine/threonine kinase, which is required
for injury-induced neointima formation and VSMC proliferation
(39).
Additionally, miR-1 has been reported to be involved
in regulating the contractile phenotype of VSMCs in a
myocardin-dependent manner which has important physiological
relevance to the control of blood pressure in vivo (40). Phenotypic plasticity is an
important characteristic of mature VSMCs. Different phenotypes of
VSMCs exist in the normal blood vessel wall and the dysregulation
of the phenotype differentiation contributes to the development of
hypertension (41–43).
In conclusion, in the present study, we demonstrate
that the downregulation of miR-1 inhibits VSMC proliferation in
SHR-VSMCs, at least in part through the downregulation of IGF1. It
is crucial to determine whether the miR-1-IGF1 pathway serves as a
therapeutic target in proliferative vascular diseases.
Acknowledgments
The study was fully sponsored by the National
Natural Science Foundation of China (grant no. 81100288).
References
|
1
|
Yu X and Li Z: MicroRNAs regulate vascular
smooth muscle cell functions in atherosclerosis (Review). Int J Mol
Med. 34:923–933. 2014.PubMed/NCBI
|
|
2
|
Bornfeldt KE and Krebs EG: Crosstalk
between protein kinase A and growth factor receptor signaling
pathways in arterial smooth muscle. Cell Signal. 11:465–477. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Somjen D, Knoll E, Sharon O, Many A and
Stern N: Calciotrophic hormones and hyperglycemia modulate vitamin
D receptor and 25 hydroxyy vitamin D 1-α hydroxylase mRNA
expression in human vascular smooth muscle cells. J Steroid Biochem
Mol Biol. 148:210–213. 2015. View Article : Google Scholar
|
|
4
|
Liu N, Bezprozvannaya S, Williams AH, Qi
X, Richardson JA, Bassel-Duby R and Olson EN: microRNA-133a
regulates cardiomyocyte proliferation and suppresses smooth muscle
gene expression in the heart. Genes Dev. 22:3242–3254. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Doe Z, Fukumoto Y, Takaki A, et al:
Evidence for Rho-kinase activation in patients with pulmonary
arterial hypertension. Circ J. 73:1731–1739. 2009. View Article : Google Scholar
|
|
6
|
Delafontaine P, Song YH and Li Y:
Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1
binding proteins in blood vessels. Arterioscler Thromb Vasc Biol.
24:435–444. 2004. View Article : Google Scholar
|
|
7
|
Fath KA, Alexander RW and Delafontaine P:
Abdominal coarctation increases insulin-like growth factor I mRNA
levels in rat aorta. Circ Res. 72:271–277. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maile LA, Busby WH, Sitko K, et al:
Insulin-like growth factor-I signaling in smooth muscle cells is
regulated by ligand binding to the 177CYDMKTTC184 sequence of the
beta3-subunit of alphaVbeta3. Mol Endocrinol. 20:405–413. 2006.
View Article : Google Scholar
|
|
9
|
Valinezhad Orang A, Safaralizadeh R1 and
Kazemzadeh-Bavili M: Mechanisms of miRNA-mediated gene regulation
from common downregulation to mRNA-specific upregulation. Int J
Genomics. 2014:9706072014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cho WC: OncomiRs: the discovery and
progress of microRNAs in cancers. Mol Cancer. 6:602007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chan MC, Hilyard AC, Wu C, et al:
Molecular basis for antagonism between PDGF and the TGFbeta family
of signalling pathways by control of miR-24 expression. EMBO J.
29:559–573. 2010. View Article : Google Scholar :
|
|
12
|
Elia L, Quintavalle M, Zhang J, et al: The
knockout of miR-143 and -145 alters smooth muscle cell maintenance
and vascular homeostasis in mice: correlates with human disease.
Cell Death Differ. 16:1590–1598. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang C: MicroRNA-145 in vascular smooth
muscle cell biology: a new therapeutic target for vascular disease.
Cell Cycle. 8:3469–3473. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu X, Cheng Y, Zhang S, et al: A
necessary role of miR-221 and miR-222 in vascular smooth muscle
cell proliferation and neointimal hyperplasia. Circ Res.
104:476–487. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Louis WJ and Howes LG: Genealogy of the
spontaneously hypertensive rat and Wistar-Kyoto rat strains:
Implications for studies of inherited hypertension. J Cardiovasc
Pharmacol. 16(Suppl 7): S1–S5. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Uehara Y, Numabe A, Kawabata Y, et al:
Rapid smooth muscle cell growth and endogenous prostaglandin system
in spontaneously hypertensive rats. Am J Hypertens. 4:806–814.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu ML, Wang JF, Wang GK, et al: Vascular
smooth muscle cell proliferation is influenced by let-7d microRNA
and its interaction with KRAS. Circ J. 75:703–709. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu S, Sun X, Hong T, et al: Isolation and
culture of smooth muscle cells from human acute type A aortic
dissection. J Cardiothorac Surg. 8:832013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao Y, Ransom JF, Li A, et al:
Dysregulation of cardiogenesis, cardiac conduction, and cell cycle
in mice lacking miRNA-1–2. Cell. 129:303–317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van Rooij E, Sutherland LB, Qi X, et al:
Control of stress-dependent cardiac growth and gene expression by a
microRNA. Science. 316:575–579. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Walenkamp MJ, Losekoot M and Wit JM:
Molecular IGF-1 and IGF-1 receptor defects: from genetics to
clinical management. Endocr Dev. 24:128–137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen JF, Mandel EM, Thomson JM, et al: The
role of microRNA-1 and microRNA-133 in skeletal muscle
proliferation and differentiation. Nat Genet. 38:228–233. 2006.
View Article : Google Scholar
|
|
23
|
Zhao Y, Samal E and Srivastava D: Serum
response factor regulates a muscle-specific microRNA that targets
Hand2 during cardiogenesis. Nature. 436:214–220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nasser MW, Datta J, Nuovo G, et al:
Down-regulation of micro-RNA-1 (miR-1) in lung cancer. Suppression
of tumorigenic property of lung cancer cells and their
sensitization to doxorubicin-induced apoptosis by miR-1. J Biol
Chem. 283:33394–33405. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rodriguez-Nieto S and Sanchez-Cespedes M:
BRG1 and LKB1: tales of two tumor suppressor genes on chromosome
19p and lung cancer. Carcinogenesis. 30:547–554. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hou X, Tang Z, Liu H, et al: Discovery of
microRNAs associated with myogenesis by deep sequencing of serial
developmental skeletal muscles in pigs. PLoS One. 7:e521232012.
View Article : Google Scholar
|
|
27
|
Luong HT, Chaplin J, McRae AF, Medland SE,
Willemsen G, Nyholt DR, Henders AK, Hoekstra C, Duffy DL, et al:
Variation in BMPR1B, TGFRB1 and BMPR2 and control of dizygotic
twinning. Twin Res Hum Genet. 14:408–416. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Allard D, Figg N, Bennett MR and
Littlewood TD: Akt regulates the survival of vascular smooth muscle
cells via inhibition of FoxO3a and GSK3. J Biol Chem.
283:19739–19747. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Patel VA, Zhang QJ, Siddle K, et al:
Defect in insulin-like growth factor-1 survival mechanism in
atherosclerotic plaque-derived vascular smooth muscle cells is
mediated by reduced surface binding and signaling. Circ Res.
88:895–902. 2011. View Article : Google Scholar
|
|
30
|
Bennett MR, Evan GI and Schwartz SM:
Apoptosis of human vascular smooth muscle cells derived from normal
vessels and coronary atherosclerotic plaques. J Clin Investig.
95:2266–2274. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kavurma MM, Figg N, Bennett MR, et al:
Oxidative stress regulates IGF1R expression in vascular
smooth-muscle cells via p53 and HDAC recruitment. Biochem J.
407:79–87. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pedruzzi E, Guichard C, Ollivier V, et al:
NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced
endoplasmic reticulum stress and apoptosis in human aortic smooth
muscle cells. Mol Cell Biol. 24:10703–10717. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sandberg EM and Sayeski PP: Jak2 tyrosine
kinase mediates oxidative stress-induced apoptosis in vascular
smooth muscle cells. J Biol Chem. 279:34547–34552. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Song HJ, Lee TS, Jeong JH, Min YS, Shin CY
and Sohn UD: Hydrogen peroxide-induced extracellular
signal-regulated kinase activation in cultured feline ileal smooth
muscle cells. J Pharmacol Exp Ther. 312:391–398. 2005. View Article : Google Scholar
|
|
35
|
Okura Y, Brink M, Zahid AA, Anwar A and
Delafontaine P: Decreased expression of insulin-like growth
factor-1 and apoptosis of vascular smooth muscle cells in human
atherosclerotic plaque. J Mol Cell Cardiol. 33:1777–1789. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bennett MR, Littlewood TD, Schwartz SM and
Weissberg PL: Increased sensitivity of human vascular smooth muscle
cells from atherosclerotic plaques to p53-mediated apoptosis. Circ
Res. 81:591–599. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chan SW, Hegyi L, Scott S, et al:
Sensitivity to Fas-mediated apoptosis is determined below receptor
level in human vascular smooth muscle cells. Circ Res.
86:1038–1046. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liang Y, Jing Z, Deng H, Li Z, Zhuang Z,
Wang S and Wang Y: Soluble epoxide hydrolase inhibition ameliorates
proteinuria-induced epithelial-mesenchymal transition by regulating
the PI3K-Akt-GSK-3β signaling pathway. Biochem Biophys Res Commun.
463:70–75. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu Y, Zhang J, Yi B, Chen M, Qi J, Yin Y,
Lu X, Jasmin JF and Sun J: Nur77 suppresses pulmonary artery smooth
muscle cell proliferation through inhibition of the
STAT3/Pim-1/NFAT pathway. Am J Respir Cell Mol Biol. 50:379–388.
2014.
|
|
40
|
Hamfjord J, Stangeland AM, Hughes T, et
al: Differential expression of miRNAs in colorectal cancer:
comparison of paired tumor tissue and adjacentnormal mucosa using
high-throughput sequencing. PLoS One. 7:e341502012. View Article : Google Scholar
|
|
41
|
Mitra S, Goyal T and Mehta JL: Oxidized
LDL, LOX-1 and atherosclerosis. Cardiovasc Drugs Ther. 25:419–429.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stoneman VE and Bennett MR: Role of
apoptosis in atherosclerosis and its therapeutic implications. Clin
Sci (Lond). 107:343–354. 2004. View Article : Google Scholar
|
|
43
|
Conover CA, Bale LK, Harrington SC, et al:
Cytokine stimulation of pregnancy-associated plasma protein A
expression in human coronary artery smooth muscle cells: inhibition
by resveratrol. Am J Physiol Cell Physiol. 290:C183–C188. 2006.
View Article : Google Scholar
|