Introduction
Osteoarthritis (OA) is an aging-associated disease
(1) that affects the hyaline
articular cartilage and all weight-bearing joint structures. The
main pathogenic events in OA include loss and abnormal remodeling
of cartilage extracellular matrix (2). Chondrocytes, the only cell type of
the articular cartilage, maintain tissue homeostasis, respond to
injury and perform the cartilage remodeling process that
characterizes OA. Apoptosis has been proposed as a possible pathway
for osteoarthritis pathology and a strong association has been
demonstrated between the programmed cell death in chondrocytes and
cartilage degradation in human osteoarthritis (3,4).
In addition, recent findings suggest that chondrocyte apoptosis is
closely associated with cartilage matrix integrity and development
of OA (5,6).
Apoptosis in chondrocytes results mainly from
mitochondrial and extrinsic pathways (7,8).
However, Archer and Francis-West (9) suggested that blocking these two
pathways cannot completely prevent chondrocyte apoptosis and
degradation of cartilage matrix, indicating that another pathway is
responsible for apoptosis in chondrocytes. Recently, the
endoplasmic reticulum (ER) pathway has been reported to be
associated with apoptosis of chondrocytes in animal models and
cultured cells. Yang et al (10) have reported that ER stress induces
rat chondrocyte apoptosis and decreases the mRNA expression of
extracellular matrix proteins, including aggrecan and type II
collagen in articular cartilage. In addition, ER stress has been
reported in chondrocytes in human osteoarthritis cartilage
(11,12). A recent study suggested the
existence of an endogenous autocrine/paracrine chondroprotective
mechanism against stimuli inducing chondrocyte apoptosis via the
intrinsic/mitochondrial pathway (13). However, it remains unclear whether
ER stress is involved in cartilage apoptosis and OA pathogenesis.
Therefore, the aim of the present study was to determine whether ER
stress is involved in chondrocyte apoptosis and whether it is
associated with the degree of cartilage degeneration in OA
patients.
Tauroursodeoxycholic acid (TDA) is a hydrophilic
bile acid that is normally produced endogenously in humans at
extremely low levels. TDA is formed in the conjugation pathway of
ursodeoxycholic acid, which is commonly used as a bile acid
replacement therapy for the treatment of certain cholestatic
syndromes (14). Notably, recent
studies have shown that TDA can inhibit ER stress in vitro
and in vivo (15). In
addition, Chen et al (16)
demonstrated that TDA regulates ER function and reduces cell
apoptosis by decreasing ER stress.
Therefore, we hypothesized that TDA may alleviate OA
by protecting chondrocytes and preventing apoptosis through
regulation of ER stress. Therefore, chondrocyte function was
assessed and the cartilage was observed under ER stress conditions
following treatment with tunicamycin. Of note, the effects of TDA
on tunicamycin-induced ER stress were evaluated.
In the present study treatment with tunicamycin
caused ER stress. Significant changes were observed in cells: High
expression of ER stress markers, reduced cell proliferation,
increased apoptosis and decreased synthesis of type II collagen.
These effects were alleviated by treatment with TDA. These findings
provide new insights on the mechanisms underlying ER stress in
cartilage degeneration and osteoarthritis development.
Additionally, the data provide a basis for new drug development for
the prevention and treatment of osteoarthritis.
Materials and methods
Study subjects
Patients were diagnosed according to the 1995
American Academy of Rheumatology established diagnostic criteria
for OA (17). Articular cartilage
samples were collected from 18 patients (9 males and 9 females)
aged between 18 and 65 years, who were diagnosed for OA and
underwent total knee arthroplasty in the Department of Orthopaedic
Surgery, Peking University First Hospital (Beijing, China) between
January and September 2009. All the patients were informed of the
purpose of the experiment and provided signed consent. All the
procedures were approved by the Institute Review Board and Human
Subjects Committee of Peking University First Hospital. The degree
of cartilage degeneration was graded using a modified Outerbridge
system (18).
Specimen processing
The cartilages obtained from surgery were rinsed
with phosphate-buffered saline (PBS) and several blocks of level
−I, −II and −III tissue (Outerbridge system) were cut aseptically
in a frozen state. Three blocks were obtained for each level with
cross-sectional areas of ~60 mm2/piece, and used for
histological examination, tissue protein extraction and RNA
extraction. The remaining specimens were stored at −70°C.
Histology
Cartilage blocks with subchondral bone were obtained
and immersed in decalcification solution composed of neutral
formalin and 10% ethylenediaminetetraacetic acid (EDTA). The medium
was changed weekly to soften the subchondral bone. Subsequently,
cartilages were embedded, dewaxed and cut into 5-µm
sections. Terminal deoxynucleotidyltransferase-mediated dUTP nick
end labelling (TUNEL) staining of the samples was carried out using
the TUNEL staining kit (Roche Diagnostics, Basel, Switzerland),
according to the manufacturer's instructions, to measure cartilage
cell apoptosis; apoptotic nuclei appeared brown. Analysis was
performed in a double-blinded manner through continuous observation
of 5–10 high power fields, where the cells below the tide line were
counted. The results are expressed as average number of apoptotic
cells/unit.
Cell culture and treatments
The articular cartilages were minced and digested at
37°C using 0.2% w/v collagenase II (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS). Chondrocytes were
collected after 12–16 h and cultured in DMEM supplemented with 10%
FBS, 50 mg/ml ascorbic acid, 100 U/ml penicillin and 100 mg/ml
streptomycin at 37°C in a 5% CO2 incubator. When cells
were close to 100% confluency, they were passaged following
trypsinization (0.05% trypsin and 0.02% EDTA). Subsequently, the
cells were harvested following the second passage and cultured in
6-well plates at a density of 0.6×106 cells/well. The
chondrocytes were divided into three groups: CON group, no
treatment; ERS group treatment with 2 µmol/l tunicamycin on
day 4 after seeding; and TDA group, treatment with 200
µmol/l TDA 4 h after tunicamycin treatment.
Cell proliferation assay
A CCK-8 Cell Proliferation assay kit (Promega,
Beijing, China) was used to determine viability of the cells,
according to the manufacturer's instructions. Briefly,
1×104 cells/well were seeded in 6-well plates and 20
µl of CCK-8 solution was added into each well at each
time-point (days 1–9) followed by incubation at 37°C for 4 h.
Finally, cell viability was measured using a microplate reader
(#M581789; Westingarea, Shanghai, China).
Apoptosis assay
Apoptotic cells were quantified by Annexin V-FITC
and propidium iodide (PI) staining. Detection of early apoptosis in
chondrocytes was performed using a kit from Nanjing KeyGen Biotech.
Co., Ltd. (Nanjing, China) according to the manufacturer's
instructions. In brief, chondrocytes (105 cells/ml) were
harvested and centrifuged at 450 × g for 5 min at 4°C. The cells
were washed twice with PBS and the pellets were resuspended in 100
µl ice-cold binding buffer. Subsequently, cells were
incubated with 5 µl Annexin V and 5 µl PI for 10 min
at room temperature in the dark. Following washing, cells were
resuspended in 400 µl binding buffer and analyzed on a
FACScan flow cytometer (BD Biosciences, San Jose, CA, USA). The
negative control was set for each sample, with the cells incubated
with binding buffer alone.
TUNEL staining
The apoptosis index of the chondrocytes was assessed
using a TUNEL staining kit (Maxim, Fujian, China) according to the
manufacturer's instructions. The samples were examined under
microscopy (Olympus CX31-12C04; Olympus, Tokyo, Japan) and stained
cells were counted in 6 random visual fields.
Western blot analysis
Cells collected by centrifugation and the cartilage,
which was ground to powder in liquid nitrogen, were resuspended in
radioimmunoprecipitation assay buffer in the presence of the
protease inhibitors. Following lysate clarification, protein
concentration was determined using the bicinchoninic acid assay
reagent kit (Pierce, Rockford, IL, USA) with bovine serum albumin
as the standard. Subsequently, 30 µg of total protein was
denatured at 95°C for 5 min in 2% SDS sample buffer in the presence
of NuPage reducing agent (Invitrogen, Carlsbad, CA, USA).
Equivalent amounts of protein were resolved on 12%
SDS-polyacrylamide gels and electroblotted onto polyvinylidene
difluoride (Pierce) using the Invitrogen X cell electrophoresis and
blotting system (Invitrogen). Following blocking with 5% (w/v)
non-fat milk in wash buffer (Tris-buffered saline containing 0.1%
Tween-20) for 2 h, membranes were incubated with rabbit monoclonal
anti-glucose regulated protein 78 (GRP78) (sc-13968; 1:500), rabbit
monoclonal anti-caspase-12 (1:1,000), goat monoclonal anti-growth
arrest and DNA-damage-inducible gene 153 (GADD153) (sc-575; 1:500),
rabbit anti-collagen II (sc-7763; 1:1,000), and rabbit monoclonal
anti-actin (sc-7210; 1:2,000) (all from Santa Cruz Biotechnology,
Inc.). Following four washes, the membranes were incubated with
horseradish peroxidase-conjugated anti-rabbit or anti-goat
secondary antibodies at 1:5,000 dilution and immunoreactivity was
visualized using an ECL system (Pierce).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was prepared from chondrocytes cultured
in vitro or harvested from the cartilage using the TRIzol
Reagent (Invitrogen) according to the manufacturer's instructions.
To amplify 133-base pair (bp) GRP787, 182-bp GADD153,
244-bp caspase-12, 286-bp collagen II and 200-bp glyceraldehyde
3-phosphate dehydrogenase (GAPDH) cDNA fragments, PCR
primers were synthesized by Genosys (The Woodlands, TX, USA):
GRP78 sense, 5′-TCC TAT GTC GCC TTC ACT-3′ and antisense,
5′-ACA GAC GGG TCA TTC CAC-3′; GADD153 sense, 5′-CTG ACC AGG
GAA GTA GAG G-3′ and antisense, 5′-TGC GTA TGT GGG ATT GAG-3′;
caspase-12 sense, 5′-AAT CTG TGG GAC CAA GCA-3′ and antisense,
5′-GAG CCT TTG TAA CAG CAT CA-3′); GAPDH sense, 5′-ACC CAG
AAG ACT GTG GAC TT-3′ and antisense, 5′-TTC TAG ACG GCA GGT CAG
GT-3′; and collagen II sense, 5′-CCA CAC TCA ATC CCT CAA C-3′ and
antisense, 5′-GCT GCT CCA CCA GTT CTT C-3′. The samples were first
denatured at 95°C for 30 sec, followed by 32 cycles of 95°C for 30
sec, 60°C for 30 sec and 72°C for 30 sec. The last cycle was
followed by an additional incubation for 7 min at 72°C. Analysis of
amplicons was accomplished on 1% agarose gel containing 0.2
µg/µl ethidium bromide and amplicons were visualized
under a UV transluminator. The densitometric analysis of the PCR
products was performed by Bio-Rad Quantity One on GS-800 Imaging
Densitometer (both from Bio-Rad, Hercules, CA, USA) and all the
values were standardized against the GAPDH product. The
cycle threshold (CT) was defined as the cycle number at which the
fluorescence generated by cleavage of the probe exceeded a fixed
threshold above the baseline. Final values, which were expressed as
fold difference relative to the GAPDH gene (N), were
calculated with the following formula: N =
2(C-T)gene/2(C-T)GAPDH, where C is the CT for
GRP78, GAPDH in control samples; and T is the CT for
GRP78, GAPDH in treatment samples. All the PCR
reactions were performed using the LightCycler FastStart DNA Master
SYBR-Green I kit and the Cepheid SmartCycler real-time PCR cycler
(Cepheid Inc., Sunnyvale, CA, USA). The cycling conditions were as
follows: Initial denaturation at 95°C for 10 min; 40 cycles at 95°C
for 15 sec, 60°C for 5 sec and 72°C for 10 sec. Experiments were
performed in triplicate for each data point, and for all the
experiments the controls were included without templates.
Statistical analysis
The data are presented as mean ± standard error for
the number of experiments indicated. One-way analysis of variance
was used to determine significant differences among groups. All the
statistical analyses were generated using the SPSS software 11.0
(SPSS Inc., Chicago, IL, USA) and figures were plotted using the
Origin 7.5 software (OriginLab Corp., Northampton, MA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Apoptosis of chondrocytes in the
cartilage of patients with OA associated with the grade of OA
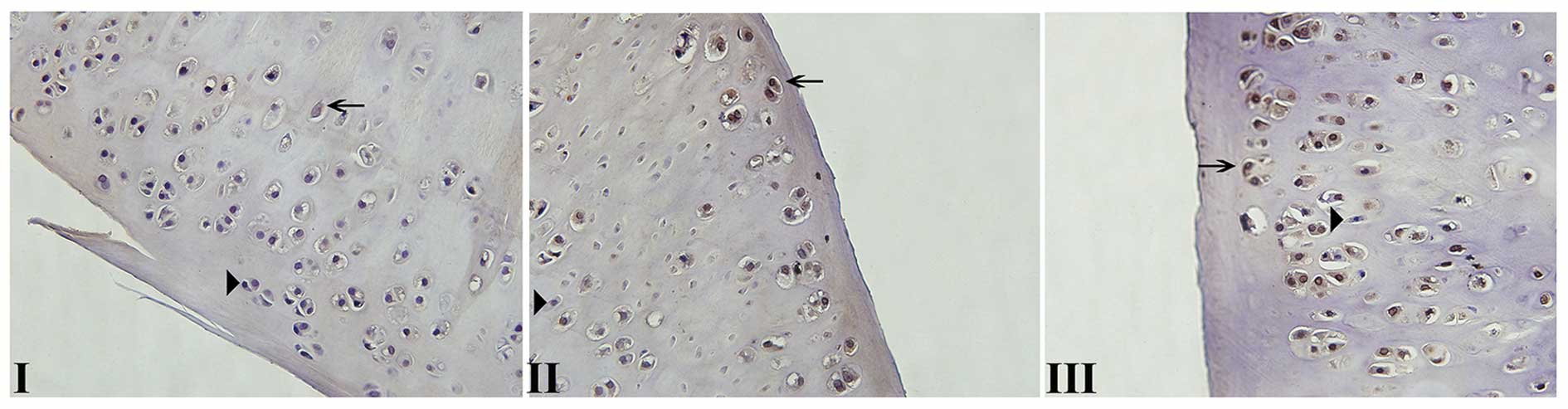
Normal cell nuclei stained in blue by hematoxylin
maintained a good shape (Fig. 1).
Apoptotic chondrocytes showed an uneven distribution in articular
cartilage and were mainly concentrated on the surface and shallow
regions. Apoptotic cell nuclei exhibited condensation, loss of the
oval shape and uneven chromatin distribution. Notably, the number
of apoptotic chondrocytes was increased with the aggravation of
cartilage degeneration: The apoptosis rate of chondrocytes in
cartilage specimens was 8.2, 14.1 and 23.7% in grades I, II and
III, respectively.
Type II collagen (Col II) expression and
ER stress in cartilage chondrocytes of patients with OA is
associated with the OA grade
The mRNA levels of Col II were decreased by 47 and
51% in grades II (P<0.01) and III (P<0.01), respectively, as
compared to grade I. However, no significant difference was
observed between grades II and III, indicating the reduced ability
of chondrocytes to synthesize the extracellular matrix. The
GRP78 gene expression was increased by 8 and 12% in grades
II (P<0.01) and III (P<0.01), respectively, as compared to
grade I. In the case of GADD153, gene expression was
increased by 104 and 210% in grades II (P<0.01) and III
(P<0.01), respectively, as compared to grade I. Finally,
caspase-12 gene expression was increased by 121 and 230% in grades
II (P<0.01) and III (P<0.01), respectively, as compared to
grade I (Fig. 2A).
As for gene expression, Col II protein levels were
decreased by 23 and 31% in grades II (P<0.01) and III
(P<0.01), respectively, as compared to grade I, and no
significant difference was obtained between grades II and III. The
protein expression of GRP78 was increased by 23 and 27% in grades
II (P<0.01) and III cartilage tissue (P<0.01), respectively,
as compared with grade I. As for caspase-12, protein levels were
increased by 143 and 210% in grades II (P<0.01) and III
(P<0.01), respectively, as compared to grade I. In the case of
GADD153, protein expression was increased by 98 and 180% in grades
II (P<0.01) and III (P<0.01), respectively, as compared to
grade I (Fig. 2B).
TDA treatment results in cell
proliferation recovery and a decrease in apoptosis
Cells that were adherent after 24 h of culture began
to form spindles and gradually became polygonal, with large round
nuclei and abundant cytoplasm containing secretory granules. Cell
processes began to resume over one week and cells were surrounded
by matrix-like material deposition. Medium floating apoptotic cells
were infrequently observed. Considering that tunicamycin stimulated
the growth of chondrocytes with a poor morphology, more cells were
collapsed with medium large apoptotic cells floating. TDA-treated
chondrocytes grew well and became more angular and adherent with
rich cytoplasm (Fig. 3A). The
number of medium floating apoptotic cells was significantly reduced
compared with the tunicamycin-treated group. Tunicamycin induced ER
stress in chondrocytes (Fig. 3B),
which resulted in a marked reduction in cell proliferation
(P<0.01). However, cell proliferation was significantly higher
in tunicamycin-treated cells following TDA treatment.
Following chondrocyte treatment, cells were stained
with Annexin V/PI and analyzed by flow cytometry. Apoptosis rates
were 9.2 and 46.7% in the CON and ERS groups, respectively.
However, the apoptosis rate decreased to 28.3% in the TDA group
(Fig. 3C).
Effect of tunicamycin and TDA treatment
on type II collagen expression in chondrocytes
Type II collagen mRNA levels were decreased by 82%
(P<0.01) in the ERS group compared to the CON group, and
increased by 170% (P<0.01) in the TDA group as compared to the
ERS group (Fig. 4A).
Similarly, type II collagen protein expression was
decreased by 84% (P<0.01) in the ERS group as compared to the
CON group. However, type II collagen protein levels were increased
by 245% (P<0.01) in the TDA group as compared to the ERS group
(Fig. 4B).
TDA inhibits ER stress induced by
tunicamycin in chondrocytes
The gene expression levels of GRP78,
GADD153 and caspase-12 were increased by 108, 112 and 113%
(P<0.01), respectively, in chondrocytes treated with tunicamycin
compared with the CON group (Fig.
5A). Of note, GRP78, GADD153 and caspase-12 mRNA
levels were decreased by 40, 44 and 34% (P<0.01), respectively,
in the TDA group as compared to chondrocytes treated with
tunicamycin only.
Similarly, GRP78, GADD153 and caspase-12 protein
expression was increased by 52, 71 and 54% (P<0.01),
respectively, in chondrocytes treated with tunicamycin associated
with the CON group. Notably, GRP78, GADD153 and caspase-12 protein
levels were decreased by 23.7, 36.3 and 30.1% (P<0.01),
respectively, in the TDA group as compared to the ERS group
(Fig. 5B).
Discussion
In the present study, the presence of ER stress was
demonstrated in the cartilage of patients with osteoarthritis and a
positive correlation was confirmed between ER stress and
osteoarthritis severity. Osteoarthritis was mainly graded using the
Outerbridge system and was manifested by mRNA and protein
expression of ER stress markers, such as GRP78, GADD153 and
caspase-12, indicating the involvement of ER stress in the
osteoarthritis pathological process.
The ER regulates protein synthesis, folding and
transport, and is involved in intracellular calcium homeostasis and
a variety of cell signaling pathways (19–21). Thus, changes in intracellular
calcium homeostasis results in accumulation of unfolded or
misfolded proteins in the ER, leading to ER stress (7,8).
GRP78 binds to unfolded proteins that aggregate in the ER and
activates downstream effectors, which suppress protein synthesis
and express stress response proteins to restore the ER protein
processing capacity, the redox balance and calcium homeostasis
(19–22,23). Of note, as cartilage degeneration
worsened, GRP78 expression increased gradually, as shown,
indicating that larger quantities of unfolded proteins could be
bound in order to protect chondrocytes during the stress. However,
due to the persistence of external stimulation, ER stress caused
apoptosis. The present study found that ER apoptosis markers were
highly expressed in level III cartilage, including GADD153 and
activated caspase-12. These observations indicated that during ER
stress, the chondrocytes decreased the synthesis of extracellular
matrix, decreasing the cartilage thickness and increasing the
fibrous tissue. With the ER stress pathway activation, chondrocyte
apoptosis gradually increased, resulting in decreased cell number
and the observed irregularly arranged cartilage.
With the Outerbridge grade increase, it was also
demonstrated that osteoarthritis became more serious, with
TUNEL-positive cells appearing more frequently, an indication of
increased apoptosis. In addition, reduced mRNA and protein
expression of type II collagen and the main matrix proteins was
shown in osteoarthritis-affected cartilages. These findings
indicated a significantly reduced condrocyte activity and increased
apoptosis during osteoarthritis development. The association
between condrocyte apoptosis and ER stress is well documented
(10–24,25). Notably, protein folding inhibition
in the ER lumen by tunicamycin resulted in an ER stress response as
evidenced by increased GADD153 protein and gene expression, and
PERK and eIF2-α phosphorylation, which resulted in apoptosis
(26). Therefore, the present
results suggest that ER stress could lead to cartilage cell
apoptosis by affecting chondrocyte activity along with a gradual
degeneration of cartilage and osteoarthritis development.
As shown, treatment of chondrocytes with tunicamycin
resulted in ER stress, evidenced by the increased expression of
GRP78 at the gene and protein levels. As a result, cell function
was damaged, leading to reduced type II collagen synthesis and
increased expression of caspase-12 and GADD153. Flow cytometry data
confirmed the increased apoptosis following ER stress induction by
tunicamycin. Of note, administration of TDA to chondrocytes
pretreated with tunicamycin caused a relative decline in GRP78
expression, i.e. decreased ER stress concomitantly with increased
type II collagen levels and decreased expression of apoptosis
markers such as caspase-12 and GADD153. Furthermore, flow cytometry
data showed that the tunicamycin-induced apoptosis was
significantly decreased following treatment with TDA. Therefore, we
believe that TDA reduces ER stress in chondrocytes, protects ER
function in protein synthesis and folding, and reduces
apoptosis.
Multiple studies have demonstrated the modulatory
role of TDA on the ER function, in vitro and in vivo.
TDA was shown to attenuate tunicamycin-induced ER stress, autophagy
and cell death in cultured hepatocytes (27,28). In addition, TDA protects rat
pancreatic β-cells (INS-1 cells) from palmitate-induced injury,
which may be due to the amelioration of ER stress among others
(29), demonstrating a potential
application in the treatment of type 2 diabetes (15). Chen et al (16) proposed that ER stress has an
important role in AGEs-induced apoptosis, which was prevented by
TDA following blocking of an ER stress-mediated apoptotic pathway
in chondrocytes. Taken together, these findings demonstrate the
important role of ER stress in chondrocyte apoptosis.
In conclusion, ER stress and chondrocyte apoptosis
are involved in the development of osteoarthritis. However, there
is a possibility of chondrocyte apoptosis induction by mechanisms
other than ER stress. In addition, whether the two other apoptotic
pathways were induced was not investigated. The ER markers GADD153,
caspase-12 and GRP78, which are independent and specific for ER
stress, were detected. Therefore, the study partly provides a
mechanism underlying ER stress in cartilage degeneration and
osteoarthritis development.
Acknowledgments
The present study was supported by the National
Natural Science Fundation of China (grant no. 30772198).
References
|
1
|
Loeser RF: Aging and osteoarthritis: The
role of chondrocyte senescence and aging changes in the cartilage
matrix. Osteoarthritis Cartilage. 17:971–979. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Creamer P and Hochberg MC: Osteoarthritis.
Lancet. 350:503–508. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blanco FJ, Guitian R, Vázquez-Martul E, de
Toro FJ and Galdo: Osteoarthritis chondrocytes die by apoptosis. A
possible pathway for osteoarthritis pathology. Arthritis Rheum.
41:284–289. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hashimoto S, Ochs RL, Komiya S and Lotz M:
Linkage of chondrocyte apoptosis and cartilage degradation in human
osteoarthritis. Arthritis Rheum. 41:1632–1638. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kühn K, D'Lima DD, Hashimoto S and Lotz M:
Cell death in cartilage. Osteoarthritis Cartilage. 12:1–16. 2004.
View Article : Google Scholar
|
|
6
|
Aigner T, Kurz B, Fukui N and Sandell L:
Roles of chondrocytes in the pathogenesis of osteoarthritis. Curr
Opin Rheumatol. 14:578–584. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boyce M and Yuan J: Cellular response to
endoplasmic reticulum stress: A matter of life or death. Cell Death
Differ. 13:363–373. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brostrom MA and Brostrom CO: Calcium
dynamics and endoplasmic reticular function in the regulation of
protein synthesis: Implications for cell growth and adaptability.
Cell Calcium. 34:345–363. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Archer CW and Francis-West P: The
chondrocyte. Int J Biochem Cell Biol. 35:401–404. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang L, Carlson SG, McBurney D and Horton
WE Jr: Multiple signals induce endoplasmic reticulum stress in both
primary and immortalized chondrocytes resulting in loss of
differentiation, impaired cell growth, and apoptosis. J Biol Chem.
280:31156–31165. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ruiz-Romero C, Carreira V, Rego I,
Remeseiro S, López-Armada MJ and Blanco FJ: Proteomic analysis of
human osteoarthritic chondrocytes reveals protein changes in stress
and glycolysis. Proteomics. 8:495–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nugent AE, Speicher DM, Gradisar I,
McBurney DL, Baraga A, Doane KJ and Horton WE Jr: Advanced
osteoarthritis in humans is associated with altered collagen VI
expression and upregulation of ER-stress markers Grp78 and bag-1. J
Histochem Cytochem. 57:923–931. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Intekhab-Alam NY, White OB, Getting SJ,
Petsa A, Knight RA, Chowdrey HS, Townsend PA, Lawrence KM and Locke
IC: Urocortin protects chondrocytes from NO-induced apoptosis: A
future therapy for osteoarthritis? Cell Death Dis. 4:e7172013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hardison WG and Grundy SM: Effect of
ursodeoxycholate and its taurine conjugate on bile acid synthesis
and cholesterol absorption. Gastroenterology. 87:130–135.
1984.PubMed/NCBI
|
|
15
|
Ozcan U, Yilmaz E, Ozcan L, Furuhashi M,
Vaillancourt E, Smith RO, Görgün CZ and Hotamisligil GS: Chemical
chap-erones reduce ER stress and restore glucose homeostasis in a
mouse model of type 2 diabetes. Science. 313:1137–1140. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Y, Liu CP, Xu KF, Mao XD, Lu YB, Fang
L, Yang JW and Liu C: Effect of taurine-conjugated ursodeoxycholic
acid on endoplasmic reticulum stress and apoptosis induced by
advanced glycation end products in cultured mouse podocytes. Am J
Nephrol. 28:1014–1022. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hochberg MC, Altman RD, Brandt KD, Clark
BM, Dieppe PA, Griffin MR, Moskowitz RW and Schnitzer TJ; American
College of Rheumatology: Guidelines for the medical management of
osteoarthritis. Part II Osteoarthritis of the knee. Arthritis
Rheum. 38:1541–1546. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Outerbridge RE: The etiology of
chondromalacia patellae. J Bone Joint Surg Br. 43-B:752–757.
1961.PubMed/NCBI
|
|
19
|
Xu C, Bailly-Maitre B and Reed JC:
Endoplasmic reticulum stress: Cell life and death decisions. J Clin
Invest. 115:2656–2664. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rao RV, Ellerby HM and Bredesen DE:
Coupling endoplasmic reticulum stress to the cell death program.
Cell Death Differ. 11:372–380. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Iwawaki T, Akai R, Kohno K and Miura M: A
transgenic mouse model for monitoring endoplasmic reticulum stress.
Nat Med. 10:98–102. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oyadomari S and Mori M: Roles of
CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ.
11:381–389. 2004. View Article : Google Scholar
|
|
23
|
Wang XZ, Lawson B, Brewer JW, Zinszner H,
Sanjay A, Mi LJ, Boorstein R, Kreibich G, Hendershot LM and Ron D:
Signals from the stressed endoplasmic reticulum induce
C/EBP-homologous protein (CHOP/GADD153). Mol Cell Biol.
16:4273–4280. 1996.PubMed/NCBI
|
|
24
|
Oliver BL, Cronin CG, Zhang-Benoit Y,
Goldring MB and Tanzer ML: Divergent stress responses to IL-1beta,
nitric oxide, and tunicamycin by chondrocytes. J Cell Physiol.
204:45–50. 2005. View Article : Google Scholar
|
|
25
|
Matsuo M, Nishida K, Yoshida A, Murakami T
and Inoue H: Expression of caspase-3 and -9 relevant to cartilage
destruction and chondrocyte apoptosis in human osteoarthritic
cartilage. Acta Med Okayama. 55:333–340. 2001.
|
|
26
|
Pelletier JP, Jovanovic DV, Lascau-Coman
V, Fernandes JC, Manning PT, Connor JR, Currie MG and
Martel-Pelletier J: Selective inhibition of inducible nitric oxide
synthase reduces progression of experimental osteoarthritis in
vivo: Possible link with the reduction in chondrocyte apoptosis and
caspase 3 level. Arthritis Rheum. 43:1290–1299. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xie Q, Khaoustov VI, Chung CC, Sohn J,
Krishnan B, Lewis DE and Yoffe B: Effect of tauroursodeoxycholic
acid on endoplasmic reticulum stress-induced caspase-12 activation.
Hepatology. 36:592–601. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang J, Morris MW Jr, Dorsett-Martin WA,
Drake LC and Anderson CD: Autophagy is involved in endoplasmic
reticulum stress-induced cell death of rat hepatocytes. J Surg Res.
183:929–935. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu Q, Zhong JJ, Jin JF, Yin XM and Miao
H: Tauroursodeoxycholate, a chemical chaperone, prevents
palmitate-induced apoptosis in pancreatic β-cells by reducing ER
stress. Exp Clin Endocrinol Diabetes. 121:43–47. 2013.
|