Introduction
Numerous clinical studies have demonstrated that
diabetes is associated with the chronically elevated production of
reactive oxygen species (ROS), which exceeds the antioxidant
capacity of the tissue, resulting in oxidative stress, the
generation and accumulation of deleterious oxidatively modified
products, and tissue injury (1–3).
Advanced oxidation protein products (AOPPs) are the
dityrosine-containing and crosslinking protein products formed
during oxidative stress by the combined reactions of plasma
proteins with chlorinated oxidants and have been considered to be
markers of oxidant-mediated protein damage (4). Their accumulation has been
demonstrated in subjects with obesity and metabolic syndrome, and
in diabetic patients with or without vascular complications
(5–8).
A number of studies have shown that in addition to
being products formed by chronic oxidative stress, AOPPs can also
trigger oxidative stress and further stimulate ROS generation in a
variety of cells through NADPH oxidases (9–11).
An increase in the concentration of plasma AOPPs found in diabetic
patients, has been shown to deteriorate the urinary excretion of
albumin in both normal rats and rats with streptozotocin-induced
diabetes (12,13). As the advanced glycation
end-products (AGEs), AOPPs play a pathogenic role via the receptor
for AGEs (RAGE) in endothelial cells and induce vascular
endothelial dysfunction and accelerate atherosclerosis by elevating
the level of oxidative stress and inducing the overexpression of
inflammatory factors (14–16).
It is widely accepted that pancreatic microvascular endothelial
dysfunction and subsequent islet ischemia may be the main cause for
the initial dysfunction and apoptosis of β-cells in type 2 diabetes
(17). The apoptosis of islet
microvascular endothelial cells (IMECs) likely plays an important
role in the pathogenesis of diabetes (18). However, whether AOPPs affect the
survival of IMECs and the mechanisms involved have not been
reported to date, at least to the best of our knowledge.
Glucagon-like peptide-1 (GLP-1), a brain-gut
insulinotropic peptide secreted by intestinal L cells in response
to food ingestion, has been proposed as a prospective target for
the clinical treatment of type 2 diabetes (19). In addition to its important role
in regulating glucose homeostasis, GLP-1 has also been suggested to
exert beneficial effects on the cardiovascular system, such as
improving blood pressure, vascular tone and myocardial function
(20). Recent studies have
demonstrated that GLP-1 attenuates the AGE-induced ROS generation
in many cell cultures; the protective effect of GLP-1 on oxidative
stress is mainly related to its ability to downregulate the mRNA
expression of RAGE (21–23). The addition of GLP-1 to a culture
medium of AGEs has been shown to restore the redox balance,
attenuate AGE-induced RAGE expression and protect β-cells from the
detrimental effects of AGEs (21). However, it remains unknown whether
GLP-1 can ameliorate the detrimental effects of AOPPs on IMECs.
Therefore, the present study was conducted to
investigate the pathobiological effects of AOPPs on the cellular
functions of cultured IMECs and the potential mechanisms
responsible for these effects. Additionally, this study aimed to
identify the potential protective pathways that are triggered by
GLP-1 to counteract AOPP-mediated damage in IMECs.
Materials and methods
Chemicals and reagents
All reagents for cell culture, GLP-1-(7–36)
amide, Hoechst 33258 and apocynin (NADPH oxidase inhibitor) were
purchased from Sigma (St. Louis, MO, USA). The Annexin
V-FITC/propidium iodide (PI) apoptosis detection kit was purchased
from Invitrogen (Carlsbad, CA, USA). The cell counting kit-8
(CCK-8), ROS and superoxide anion assay kits were purchased from
the Beyotime Institute of Biotechnology (Jiangsu, China). Rabbit
anti-p47phox (SC-14015), rabbit anti-p22phox
(SC-20781), β-actin (SC-47778), and primary antibodies against RAGE
(SC-5563), p53 (SC-126), Bax (SC-23959) and exendin(9–39)
(SC-364387), the antagonist for receptor of GLP-1 (GLP-1R), were
all purchased from Santa Cruz Biotechnology, Inc. (Delaware, CA,
USA). The caspase-3 and caspase-9 activity assay kits were obtained
from BD Biosciences (Franklin Lakes, NJ, USA).
AOPP preparation
AOPP-rat serum albumin (AOPP-RSA) was prepared as
previously described (12,16,24).
Briefly, RSA was exposed to 200 mmol/l of HOCl for 30 min and
dialyzed against phosphate-buffered saline (PBS) to remove free
HOCl overnight. The AOPP preparation consisted of passing through a
Detoxi-Gel column to remove any contaminated endotoxins. Endotoxin
levels during the preparation were determined with an amebocyte
lysate assay kit and were found to be below 0.025 EU/ml. The
content of AOPPs in the preparation was determined as described
previously (12). The content of
AOPPs was 72.4±9.8 nmol/mg protein in the prepared AOPP-RSA and
0.2±0.02 nmol/mg protein in the native RSA.
Isolation and purification of IMECs and
cell treatment
All animal experiments were approved by the
Committee on Animal Experimentation of Southern Medical University,
Guangzhou, China and performed in compliance with the university's
Guidelines for the Care and Use of Laboratory Animals. Rat islets
were isolated from Wistar rats and purified using a previously
described standard method (25).
Briefly, we used a modified method of collagenase digestion and
Ficoll density gradient separation for the isolation and digestion
of islets from rats. The islets were stained with DTZ and typan
blue; the concentration of the cells was adjusted to 500 IU/ml. The
cells were then resuspended in DMEM medium containing 20% fetal
calf serum, 100 µg/ml penicillin/streptomycin and 2 mmol/l
L-glutamine, followed by culture in a 2% gelatin-coated T25 flask
at 37°C. After a 5-day culture, the IMECs and fibroblasts grew out
from adherent islets, and the purification for IMECs was carried
out using UEA-1-coated Dynabeads as previously described by Lou
et al (26). The final purified rat IMECs were cultured in
DMEM containing 20% FCS, 100 µg/ml penicillin/streptomycin,
2 mmol/l L-glutamine, 4 U/ml insulin, 40 U/ml heparin and 100
µg/ml endothelial growth supplement and then seeded in a
gelatin-coated T25 flask. The cells were cultured at 37°C in a 5%
CO2 incubator. The IMECs were firstly treated with RSA
(200 µg/ml), 0, 50, 100 and 200 µg/ml AOPPs and 200
µg/ml AOPPs together with apocynin (10 µmol/l) for
0–72 h to investigate the dose and time-effect association of AOPPs
on the apoptosis of the cells. Then, in order to investigate the
protective effect of GLP-1 against the apoptosis of IMECs, the
cells were divided into the negative control group (200
µg/ml RSA), AOPPs 200 µg/ml group, AOPPs 200
µg/ml + 100 nmol/l GLP-1 group and AOPPs 200 µg/ml +
100 nmol/l GLP-1 + 100 µmol/l exendin(9–39)
group [added AOPP-RSA and GLP-1 after preprocessing by
exendin(9–39) for 2 h].
Hoechst 33258 staining for apoptosis
The apoptosis of the IMECs was identified under a
fluorescence microscope (Olympus BX51; Olympus, Tokyo, Japan) after
staining with Hoechst 33258 at a dilution of 1:200 (1 mg/ml stock
solution) for 5 min in the dark. At least 1,000 cells were counted
for each experimental condition. The cells treated as indicated
were fixed with 4% paraformaldehyde in PBS, rinsed with PBS, and
permeabilized by 0.1% Triton X-100 for FITC end-labeling of the
fragmented DNA of the apoptotic IMECs.
Determination of apoptotic cells by
Annexin V-FITC/PI staining
The cells were trypsinized and resuspended at a
concentration of 1×106 cells/ml in diluted binding
buffer and were then labeled with Annexin V and PI and examined
using the Annexin V-FITC apoptosis detection kit according to the
manufacturer's instructions. Flow cytometric analysis was performed
with the excitation at 488 nm as soon as possible.
CCK-8 assay for cell viability
The treated IMECs were cultured in Corning 96-well
flat-bottomed microtiter plates. A total of 10 µl of CCK-8
was then added followed by incubation in a high humidity
environment at 37°C and 5% CO2 for 1 h, and the optical
difference (OD) was read at 460 nm with a microplate reader
(BIO-RAD689; Bio-Rad, Hercules, CA, USA). The OD value represents
the proliferative activity.
Assay for measuring intracellular ROS
levels
Intracellular ROS generation was measured using the
fluorescent probe, dihydroethidium (DHE). Intracellular DHE is
oxidized to ethidium, which binds DNA and stains nuclei bright
fluorescent red. The IMECs treated in the 24-well plates were
incubated with a fresh working solution containing 5 mM DHE in PBS
for 30 min at 37°C. After chilling on ice, the cultures were washed
twice with ice-cold PBS and then visualized using a fluorescence
microscope (Olympus BX51; Olympus). The total red fluorescence
intensities were quantified using image analysis software from
NIH.
Estimation of NADPH oxidase activity and
the expression of NADPH oxidase subunits
NADPH oxidase activity was assessed by measuring
superoxide production. NADPH-dependent O2−
production by homogenates from cultured IMECs was assessed by
lucigenin-enhanced chemiluminescence as previously described
(27). The chemiluminescence
value was recorded every minute for 30 min. The readings for each
of the last 5 min were averaged and expressed as counts per
second.
The expression of NADPH oxidase subunits in the
membrane was analyzed by western blot analysis as previously
described (28). Briefly,
membrane proteins were extracted using a ProteoExtract kit
according to the manufacturer's instructions. Proteins (40
µg) were loaded per lane and electrotransferred onto PVDF
membranes by semi-dry transfer. The PVDF membranes were then
blocked in 5% non-fat milk in TBS-Tween-20 for 1 h at room
temperature and incubated overnight at 4°C with the primary
antibodies, anti-p47phox and anti-p22phox
(dilution 1:2,000). Afterwards, the membranes were washed 3 times
and incubated for 1 h at room temperature with appropriate
HRP-linked secondary antibodies (dilution 1:2,000; A0208; Beyotime
Institute of Biotechnology). The relative protein levels were
determined by densitometry using Total Lab 2.0 software.
Measurement of caspase-3 and caspase-9
activity
Caspase-3 and caspase-9 activity was measured using
respective kits according to the manufacturer's instructions. The
cells were washed twice with PBS and pelleted via centrifugation.
Cell pellets were then resuspended with iced lysis buffer for 10
min. Following centrifugation, cell extracts were transferred to
fresh tubes. Specific substrates for caspase-3 or caspase-9 were
added, and the tubes were incubated at 37°C overnight. The activity
of caspase-3 and caspase-9 was assessed by calculating the ratio at
OD 405 nm of the treated cells to the untreated cells.
Western blot analysis for p53, Bax and
RAGE
The treated cells were collected, and proteins were
isolated as previously described (28). The nuclear and cytosolic proteins
were extracted using the cytosolic and nuclear extraction kit
according to the manufacturer's instructions (P0028; Beyotime
Institute of Biotechnology). First, 40 µg protein were
electro-phoresed on 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) gels and transferred onto PVDF
membranes. After blocking with 5% (w/v) non-fat milk and washing
with Tris-buffered saline-Tween-20 solution, the membranes were
incubated with β-actin (1:400), p53 (1:1,000), Bax (1:300), and
RAGE (1:1,000) antibodies. After washing, the blots were incubated
with an appropriate HRP-linked secondary antibody (dilution
1:2,000). The relative protein levels were determined by
densitometry using Total Lab 2.0 software.
Statistical analysis
All experiments were carried out in triplicate.
Continuous variables and data are expressed as the means ± SD. The
data were compared using one-way analysis of variance (ANOVA).
Pairwise comparisons were evaluated by the Student-Newman-Keuls
test. A two-tailed P-value <0.05 was considered to indicate a
statistically significant difference. Statistical analyses were
conducted using SPSS 13.0 software.
Results
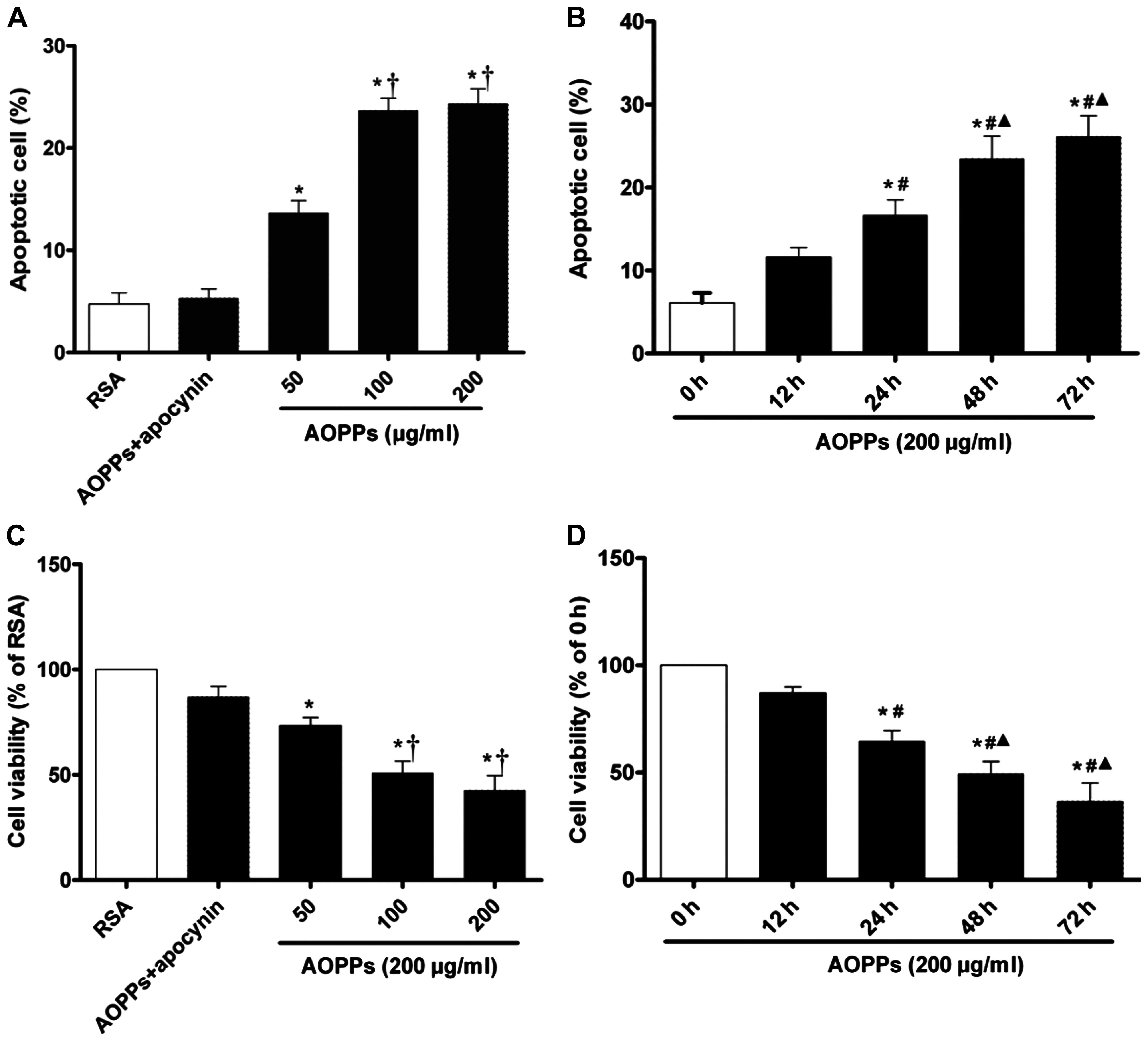
AOPPs increases the apoptosis of cultured
IMECs
To determine whether AOPP accumulation induces IMEC
apoptosis, the cells were exposed to the AOPPs at various
concentrations (0–200 µg/ml) for 0–72 h. We quantified the
rates of cell apoptosis using Annexin V-FITC/PI double staining.
The rate of apoptosis was significantly increased in the IMECs
exposed to the AOPPs than those exposed to the RSA control. The
apoptotic rate in the cells exposed to 100 or 200 µg/ml
AOPPs was higher than in those exposed to 50 µg/ml AOPPs,
with no significant difference observed between the cells treated
withy 100 and 200 µg/ml AOPPs. Treatment with apocynin
significantly protected the IMECs from AOPP-induced apoptosis,
indicating that the apoptotic processes are dependent on the
activation of NADPH oxidase (Fig.
1A). We found that AOPPs (200 µg/ml) induced the
apoptosis of IMECs in a time-dependent manner; the apoptotic rate
of the cells exposed to the AOPPs for 48 h was significantly higher
than that of the cells exposed for 0, 12 and 24 h; however, there
was no significant difference when compared to the cells exposed to
the AOPPS for 72 h (Fig. 1B).
Decrease in cell viability induced by
AOPPs
Cell viability was measured using the CCK-8 assay.
The results revealed that AOPPs had a significant effect on the
viability of the IMECs. A significant decrease in viability was
observed in the cells incubated with various concentrations of
AOPPs compared with those incubated with RSA only (p<0.05;
Fig. 1C). Treatment with apocynin
significantly protected the IMECs from the AOPPs-induced decrease
in cell viability. We also found that there was a time-dependent
effect of AOPPs on the viability of the IMECs; the viability in the
group of cells incubated for 48 h was notably decreased compared
with that of the cells incubated for 0, 12 and 24 h; however, there
was no significant difference when compared to the cells exposed to
the AOPPS for (Fig. 1D).
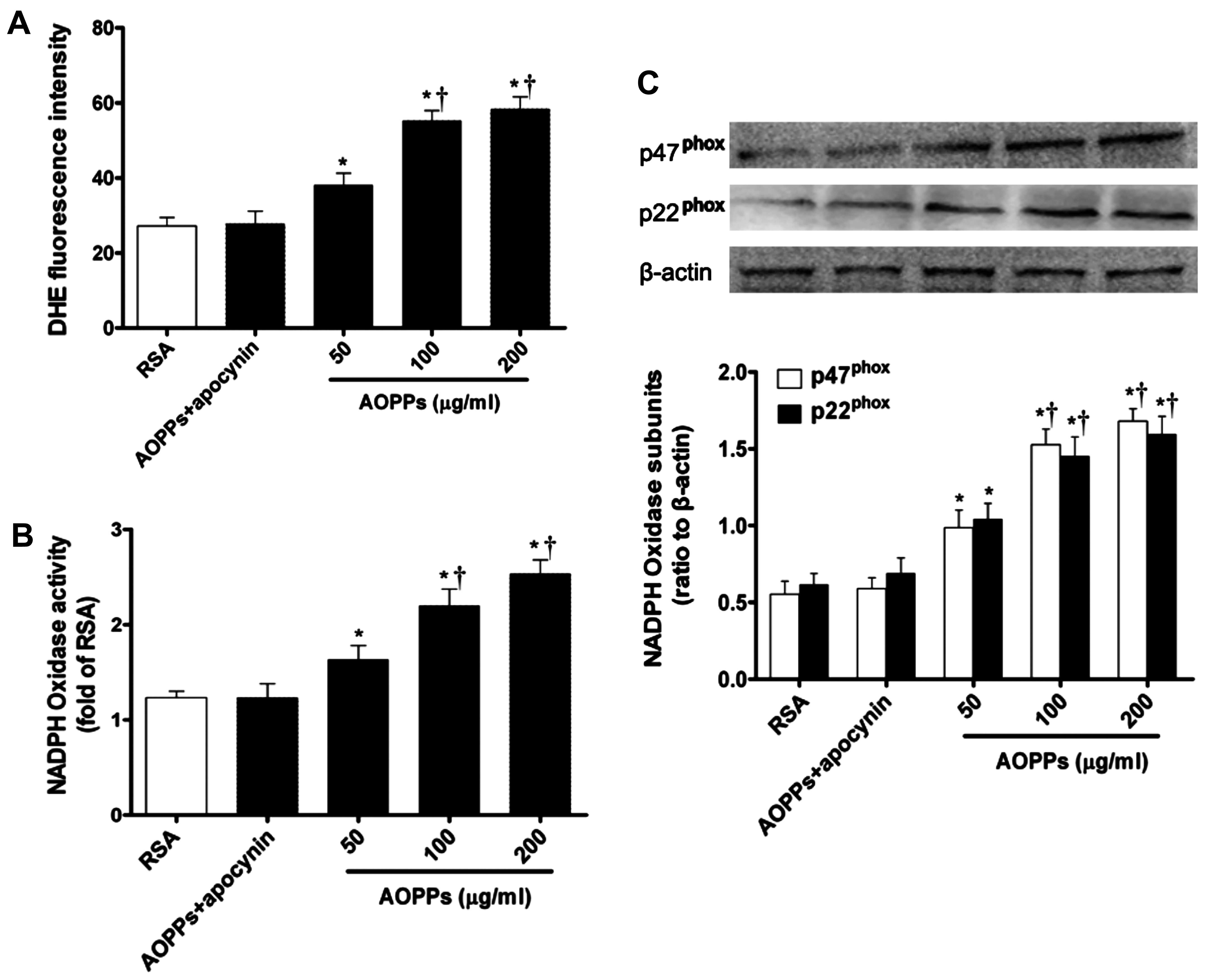
AOPPs induce NADPH oxidase-dependent ROS
production in IMECs
To examine the effect of AOPPs on intracellular ROS
production, the fluorescence intensity of the intracellular
fluorescent probe, DHE, was evaluated. ROS production was
significantly increased in the cells exposed to the AOPPs in a
dose-dependent manner compared with those exposed to RSA only
(Fig. 2A). However, ROS
production was completely suppressed by the NADPH oxidase
inhibitor, apocynin. These data indicate that NADPH oxidase plays a
central role in AOPP-induced ROS generation.
The effect of AOPPs on NADPH oxidase activity was
further estimated by measuring NADPH-dependent super-oxide
production. O2− production derived from NADPH
was significantly enhanced in the AOPP-exposed IMECs compared with
the cells incubated with RSA only (Fig. 2B). Furthermore, AOPP-induced
O2− generation was almost completely blocked
by treatment with apocynin (Fig.
2B).
The increased expression of NADPH oxidase subunits
may be necessary for NADPH oxidase sustained activity. We then
examined the effect of AOPPs on the expression of NADPH oxidase
subunits by western blot analysis. Compared with the RSA-exposed
control cells, the expression levels of the essential subunits of
NADPH oxidase, p47phox and p22phox, in the
IMECs were significantly upregulated following exposure to the
AOPPs (Fig. 2C). However,
treatment with apocynin reversed these effects (Fig. 2C).
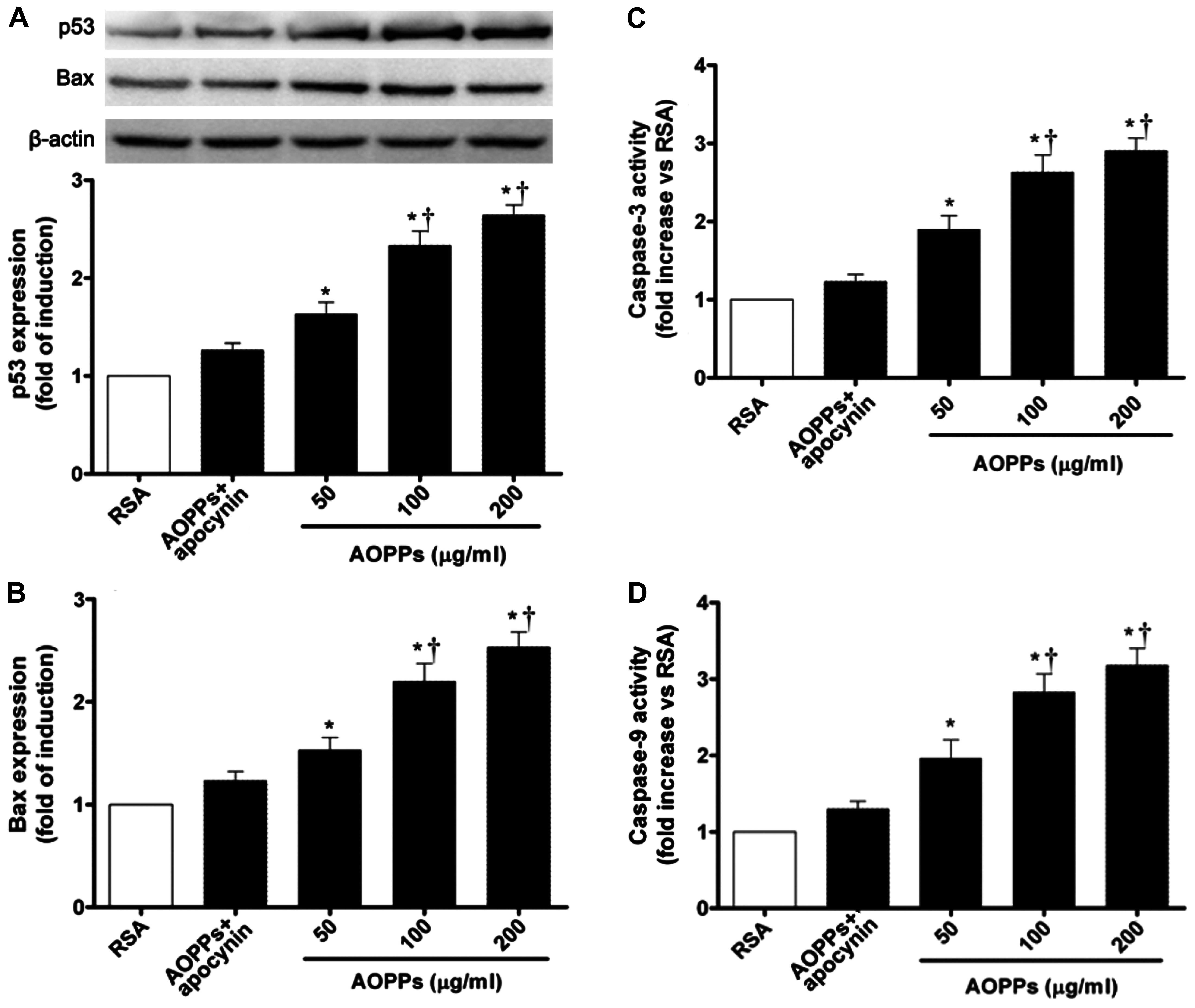
RAGE-mediated activation of the p53, Bax
and caspase-3 pathways
The Bcl-2 family regulates cell growth and cell
apoptosis in many types of models (9,33).
The increased expression of p53 has been shown to mediate apoptosis
through Bax expression in response to a number of stress signals.
Thus, to examine the potential pathways involved in AOPPs-induced
apoptosis, we examined the abundance of p53 and Bax proteins by
western blot analysis. The AOPP challenge increased p53 expression
in the cultured IMECs. The expression of the pro-apoptotic protein,
Bax, was also significantly increased compared with that of cells
exposed to RSA only (Fig. 3A and
B).
To further elucidate the influence of AOPPs on cell
apoptosis, the activity of caspase-3 and caspase-9 was measured as
described in the Materials and methods. As shown in Fig. 3C and D, the activity of caspase-3
and caspase-9 was increased significantly in the cells exposed to
the AOPPs when compared with those exposed to RSA only
(p<0.05).
As AOPPs have been shown to signal via RAGE and
vascular endothelial cells are known to express RAGE (9), we examined the effects of AOPPs on
the expression of RAGE. The AOPP challenge increased RAGE
expression in the cultured IMECs in a dose-dependent manner
compared to the cells exposed to RSA only (p<0.05; Fig. 5D). These results demonstrated that
AOPP-induced apoptosis is mainly associated with the increased
activity of caspase-3 and caspase-9, involved in the RAGE-mediated
p53/Bax pathway.
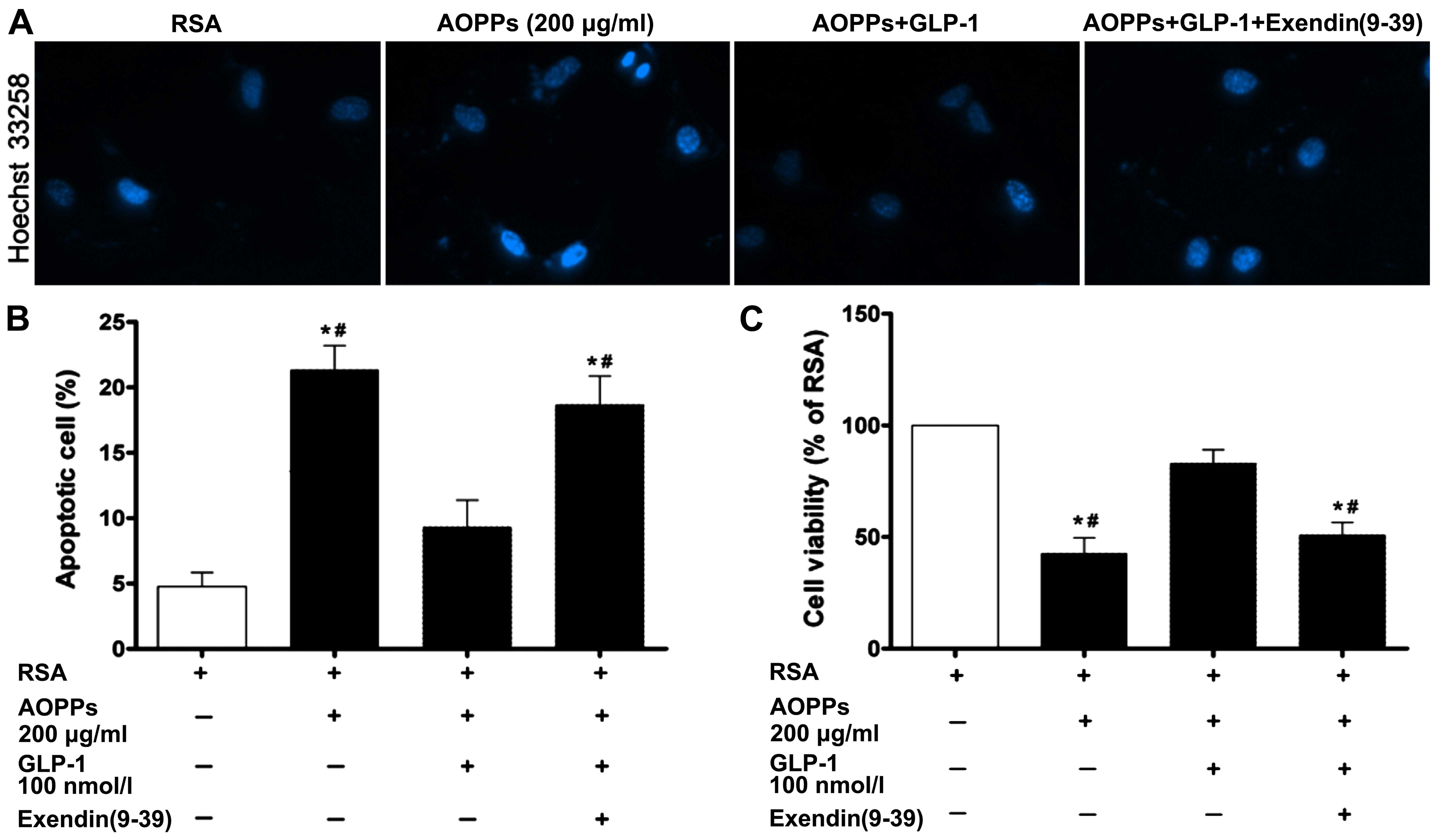
Effects of GLP-1 on AOPP-induced
apoptosis and cell viability in IMECs
To determine whether GLP-1 treatment alleviates the
apoptosis induced by AOPPs, the cells were treated with AOPPs (200
µg/ml) for 48 h in the presence or absence of GLP-1 (100
nmol/l). The number of Hoechst-positive cells in the cells exposed
to the AOPPs was significantly decreased in the presence of GLP-1
compared with the cells exposed to the AOPPS and not treated with
GLP-1 (Fig. 4A). The results from
Annexin V-FITC/PI double staining revealed that a significantly
lower apoptotic rate was observed after the addition of GLP-1 to
the culture medium (Fig. 4B). We
also evaluated the effects of GLP-1 (100 nmol/l) on the viability
of IMECs exposed to AOPPs. The IMECs exposed to the AOPPs exhibited
a significant decrease in viability compared with those exposed to
RSA only (p<0.05; Fig. 4C).
Following co-incubation with GLP-1, cell viability was
significantly increased (p<0.05). However, the protective
effects of GLP-1 on IMECs were blocked by treatment with
exendin(9–39), an antagonist for GLP-1R. These
data demonstrated that GLP-1 partially attenuated the cell
apoptosis and the decrease in cell viability induced by AOPPs.
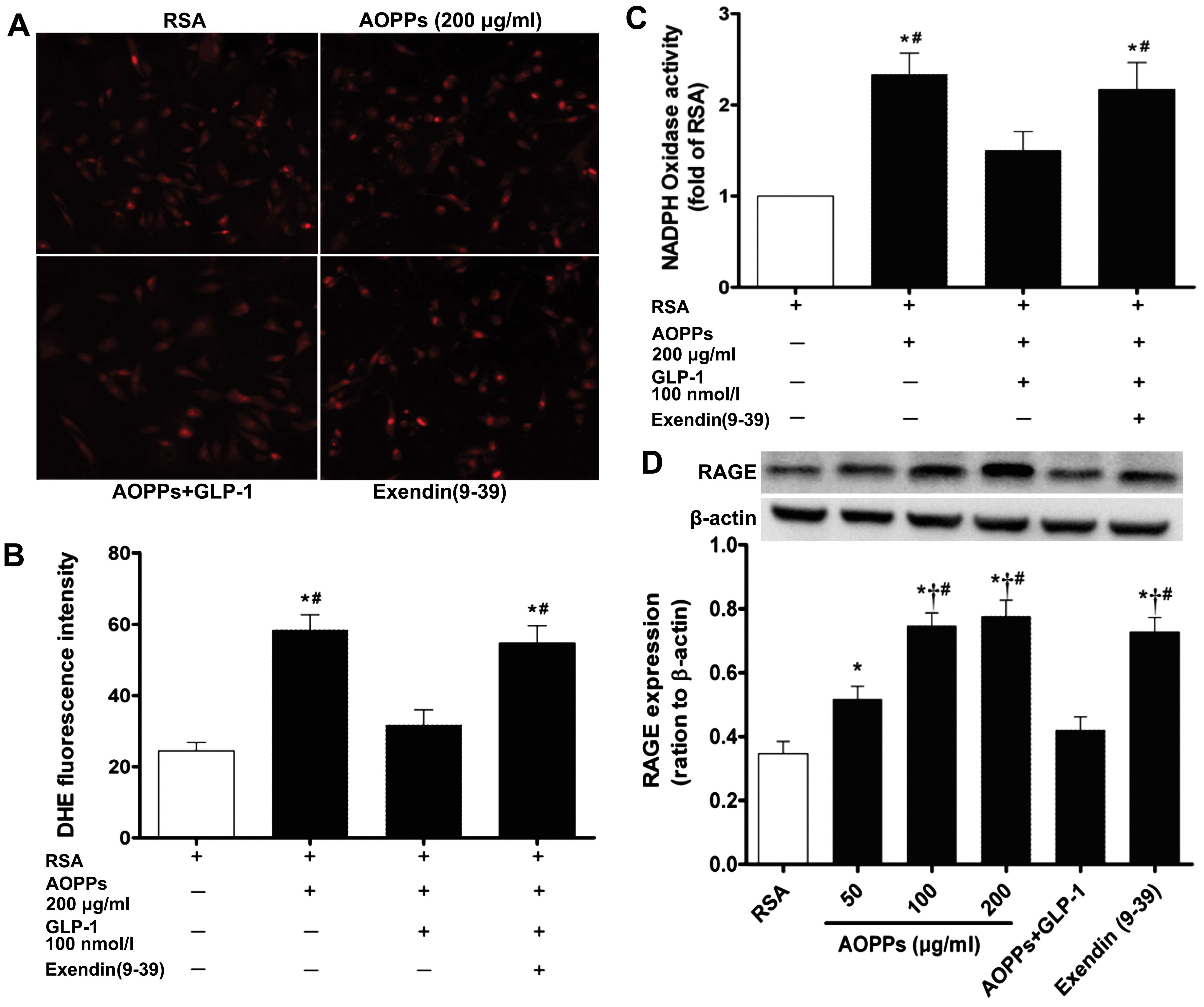
GLP-1 plays its protective role mainly by
regulating RAGE-mediated NADPH oxidase activity and ROS
generation
Intracellular ROS generation was measured using the
fluorescent probe, DHE. Intracellular DHE is oxidized to ethidium,
which binds DNA and stains nuclei bright fluorescent red. The level
of oxidative stress was evaluated by the fluorescent intensity of
DHE in the IMECs. GLP-1 markedly abrogated the AOPP-mediated ROS
generation in the IMECs (Fig. 5A and
B). We also examined the effect of GLP-1 on NADPH oxidase
activity by measuring NADPH-dependent superoxide production.
O2− production derived from NADPH was
significantly enhanced in the AOPP-exposed IMECs (Fig. 5C). However, following
co-incubation with GLP-1, NADPH oxidase activity was significantly
decreased (p<0.05). As it is well known that the intracellular
effects of AOPPs are mediated by RAGE, we further investigated the
effect of GLP-1 on the expression of RAGE in the AOPP-exposed
cells. The expression of RAGE increased significantly in the IMECs
cultured with the AOPPs, and the addition of GLP-1 to the AOPP
culture medium counter acted the AOPP-induced increase in RAGE
expression (Fig. 5D). These data
demonstrate that GLP-1 exerts a protective effect against
AOPP-induced cell damage by downregulating RAGE expression and
inhibiting the activity of NADPH oxidase.
Discussion
Increased recognition of vascular endothelial cell
dysfunction as a link between diabetes and its vascular
complications has highlighted the importance of determining the
mechanisms underlying the pathophysiological abnormalities in
microvascular endothelial cells and the development of diabetes
(17,29). Pancreatic microvascular
endothelial dysfunction and subsequent islet ischemia may be the
main cause of the initial dysfunction and the apoptosis of β-cells
in type 2 diabetes. AOPPs, a typical representation of oxidized
protein compounds, are not only considered to produce ROS, but are
also known as pro-inflammatory and pro-oxidative compounds that may
play a major role in increasing the prevalence of endothelial
dysfunction (30–32).
However, whether and how AOPPs affect the survival
of IMECs remains unknown. In this in vitro study, the
results revealed that a higher apoptotic rate of cultured IMECs, as
well as increased ROS production, were induced by exposure to AOPPs
in a dose-dependent manner. Increasing the concentration of AOPPs
also had a significant effect on IMEC cell viability; a significant
decrease in viability was observed in cells incubated with various
concentrations of AOPPs compared with those exposed to native
RSA.
We then sought to uncover the mechanism underlying
the induction of apoptosis by AOPPs in IMECs. AOPPs, as well as
AGEs, signal via RAGE and induce endothelial dysfunction. Early
studies have demonstrated that AOPPs stimulate ROS generation from
a variety of cells through a mechanism that involves NADPH oxidases
(10,12). AOPPs have been shown to induce
inflammatory responses and insulin resistance in cultured
adipocytes via the induction of endoplasmic reticulum stress
mediated by ROS, which were generated by the activation of NADPH
oxidase (11). Zhou et al
demonstrated that AOPPs co-localized and interacted with the
receptor of AGEs on podocytes; increasing the amount of AOPPs in
the medium rapidly triggered the generation of intracellular
superoxide by the activation of NADPH oxidase, and in turn resulted
in the upregulation of p53, Bax, caspase-3 activity and apoptosis.
Blocking or silencing RAGE significantly protected podocytes from
AOPP-induced apoptosis both in vitro and in vivo
(9,33).
In the present study, our data indicated that: i)
AOPPs induced NADPH oxidase-dependent ROS production in IMECs; ii)
NADPH oxidase activity was significantly enhanced in AOPP-exposed
IMECs; iii) the expression levels of p47phox and
p22phox, the essential subunits of NADPH oxidase in
IMECs, were significantly upregulated following exposure to AOPPs.
It was interesting that AOPP-triggered NADPH oxidase-dependent ROS
production was almost completely blocked by treatment with the
NADPH oxidase inhibitor, apocynin. We further found that AOPPs not
only increased RAGE expression in cultured IMECs in a
dose-dependent manner, but also increased the abundance of p53 and
Bax protein expression. The activity of caspase-3 and caspase-9 was
simultaneously significantly enhanced in the cells treated with
AOPPs. All these results demonstrated that the AOPP-induced
apoptosis of IMECs is mainly associated with the increased activity
of caspase-3 and caspase-9 involved in the RAGE-mediated p53/Bax
pathway, which is consistent with the findings of previous studies
(9,33).
GLP-1 and its long-acting peptide analog, exendin-4,
both well-known prospective therapeutic candidates, have
pleiotropic effects that include the enhancement of
glucose-dependent insulin release, as well as β-cell proliferation
and survival (34,35). In addition to its important role
in regulating glucose homeostasis, GLP-1 has also been suggested to
exert beneficial effects on the cardiovascular system, such as
improvements in blood pressure, vascular tone and myocardial
function (20). However, it is
not clear whether GLP-1 can ameliorate the detrimental effects of
AOPPs on IMECs.
In this study, we demonstrated in vitro that
treatment with GLP-1 significantly decreased AOPP-induced
apoptosis, as well as ROS generation in the IMECs, and markedly
improved cell viability. We then investigated the potential
mechanism through which GLP-1 exerts its protective effects on
IMECs, and we found that RAGE expression in the IMECs, which was
induced by AOPPs, was decreased in the presence of GLP-1. Of note,
NADPH oxidase activity measured by NADPH oxidase-dependent
superoxide production was also markedly inhibited by the
intervention of GLP-1. This protective effect of GLP-1 on IMECs was
inhibited by treatment with exendin(9–39),
an antagonist of GLP-1R.
During the past decade, a growing body of evidence
has shown that the addition of GLP-1 can protect β-cells from the
detrimental effects of AGEs by downregulating AGE-induced RAGE
expression (21). Co-incubation
with GLP-1 has been shown to reverse the glycated serum-mediated
detrimental effects by decreasing oxidative stress and triggering
protective intercellular pathways in human umbilical vein
endothelial cells (HUVECs) and HIT-T15 cells (36,37). GLP-1 intervention prevented the
AGE-induced impairement in viability in many cell types; this
important effect was related to the reduction of oxidative stress
and alterations in Bcl-2- and caspase-mediated pathways (38–40). Our results are in accordance with
those of previous studies (36,37,40) and demonstrate that GLP-1 mainly
plays a protective role via RAGE-mediated NADPH oxidase
activity.
In conclusion, in this study, we provide insight
into the pathological processes which may take place within
pancreatic microvascular endothelial cells as a result of
AOPP-induced cytotoxicity. By virtue of their participation in
pancreatic β-cell development and pathophysiology, IMECs have been
regarded as a target and an effector for the damage induced by
AOPPs, finally contributing to progressive islet dysfunction.
Treatment with GLP-1 not only targets the accumulation of AOPPs,
but may also attenuate the progression of diabetes and
diabetes-related complications.
Acknowledgments
This study was supported by the Guangdong Provincial
Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation,
the Sun Yat-Sen Memorial Hospital, the Sun Yat-Sen University. This
study was supported by a grant from the National Natural Science
Foundation of China (no. 81500623) and the special funds for public
welfare research and capacity building in Guangdong province (no.
2014A020212489).
References
|
1
|
Son SM: Role of vascular reactive oxygen
species in development of vascular abnormalities in diabetes.
Diabetes Res Clin Pract. 77(Suppl 1): S65–S70. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maejima Y, Kuroda J, Matsushima S, Ago T
and Sadoshima J: Regulation of myocardial growth and death by NADPH
oxidase. J Mol Cell Cardiol. 50:408–416. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Giacco F and Brownlee M: Oxidative stress
and diabetic complications. Circ Res. 107:1058–1070. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Witko-Sarsat V, Friedlander M,
Capeillère-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, Jungers
P and Descamps-Latscha B: Advanced oxidation protein products as a
novel marker of oxidative stress in uremia. Kidney Int.
49:1304–1313. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Krzystek-Korpacka M, Patryn E, Boehm D,
Berdowska I, Zielinski B and Noczynska A: Advanced oxidation
protein products (AOPPs) in juvenile overweight and obesity prior
to and following weight reduction. Clin Biochem. 41:943–949. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sakul A, Cumaoğlu A, Aydin E, Ari N,
Dilsiz N and Karasu C: Age- and diabetes-induced regulation of
oxidative protein modification in rat brain and peripheral tissues:
Consequences of treatment with antioxidant pyrindole. Exp Gerontol.
48:476–484. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martín-Gallán P, Carrascosa A, Gussinyé M
and Domínguez C: Biomarkers of diabetes-associated oxidative stress
and antioxidant status in young diabetic patients with or without
subclinical complications. Free Radic Biol Med. 34:1563–1574. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Atabek ME, Keskin M, Yazici C, Kendirci M,
Hatipoglu N, Koklu E and Kurtoglu S: Protein oxidation in obesity
and insulin resistance. Eur J Pediatr. 165:753–756. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou LL, Cao W, Xie C, Tian J, Zhou Z,
Zhou Q, Zhu P, Li A, Liu Y, Miyata T, et al: The receptor of
advanced glycation end products plays a central role in advanced
oxidation protein products-induced podocyte apoptosis. Kidney Int.
82:759–770. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wei XF, Zhou QG, Hou FF, Liu BY and Liang
M: Advanced oxidation protein products induce mesangial cell
perturbation through PKC-dependent activation of NADPH oxidase. Am
J Physiol Renal Physiol. 296:F427–F437. 2009. View Article : Google Scholar
|
|
11
|
Zhou QG, Zhou M, Lou AJ, Xie D and Hou FF:
Advanced oxidation protein products induce inflammatory response
and insulin resistance in cultured adipocytes via induction of
endoplasmic reticulum stress. Cell Physiol Biochem. 26:775–786.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li HY, Hou FF, Zhang X, Chen PY, Liu SX,
Feng JX, Liu ZQ, Shan YX, Wang GB, Zhou ZM, et al: Advanced
oxidation protein products accelerate renal fibrosis in a remnant
kidney model. J Am Soc Nephrol. 18:528–538. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi XY, Hou FF, Niu HX, Wang GB, Xie D,
Guo ZJ, Zhou ZM, Yang F, Tian JW and Zhang X: Advanced oxidation
protein products promote inflammation in diabetic kidney through
activation of renal nicotinamide adenine dinucleotide phosphate
oxidase. Endocrinology. 149:1829–1839. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu SX, Hou FF, Guo ZJ, Nagai R, Zhang WR,
Liu ZQ, Zhou ZM, Zhou M, Xie D, Wang GB and Zhang X: Advanced
oxidation protein products accelerate atherosclerosis through
promoting oxidative stress and inflammation. Arterioscler Thromb
Vasc Biol. 26:1156–1162. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen S, Liu L, Sun X, Liu Y and Song T:
Captopril restores endothelium-dependent relaxation induced by
advanced oxidation protein products in rat aorta. J Cardiovasc
Pharmacol. 46:803–809. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo ZJ, Niu HX, Hou FF, Zhang L, Fu N,
Nagai R, Lu X, Chen BH, Shan YX, Tian JW, et al: Advanced oxidation
protein products activate vascular endothelial cells via a
RAGE-mediated signaling pathway. Antioxid Redox Signal.
10:1699–1712. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tal MG: Type 2 diabetes: Microvascular
ischemia of pancreatic islets? Med Hypotheses. 73:357–358. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zanone MM, Favaro E and Camussi G: From
endothelial to beta cells: Insights into pancreatic islet
microendothelium. Curr Diabetes Rev. 4:1–9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baggio LL and Drucker DJ: Biology of
incretins: GLP-1 and GIP. Gastroenterology. 132:2131–2157. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abu-Hamdah R, Rabiee A, Meneilly GS,
Shannon RP, Andersen DK and Elahi D: Clinical review: The
extrapancreatic effects of glucagon-like peptide-1 and related
peptides. J Clin Endocrinol Metab. 94:1843–1852. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Puddu A, Storace D, Durante A, Odetti P
and Viviani GL: Glucagon-like peptide-1 counteracts the detrimental
effects of advanced glycation end-products in the pancreatic beta
cell line HIT-T 15. Biochem Biophys Res Commun. 398:462–466. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ishibashi Y, Nishino Y, Matsui T, Takeuchi
M and Yamagishi S: Glucagon-like peptide-1 suppresses advanced
glycation end product-induced monocyte chemoattractant protein-1
expression in mesangial cells by reducing advanced glycation end
product receptor level. Metabolism. 60:1271–1277. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ishibashi Y, Matsui T, Takeuchi M and
Yamagishi S: Sitagliptin augments protective effects of GLP-1
against advanced glycation end product receptor axis in endothelial
cells. Horm Metab Res. 43:731–734. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Capeillere-Blandin C, Gausson V,
Descamps-Latscha B and Witko-Sarsat V: Biochemical and
spectrophotometric significance of advanced oxidized protein
products. Biochim Biophys Acta. 1689:91–102. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arbet-Engels C, Darquy S, Capron F and
Reach G: Isolation of islets of Langerhans from the rat and pig
pancreas using a modified UW solution from organ storage to islet
purification. Diabete Metab. 19:590–596. 1993.PubMed/NCBI
|
|
26
|
Lou J, Triponez F, Oberholzer J, Wang H,
Yu D, Buhler L, Cretin N, Mentha G, Wollheim CB and Morel P:
Expression of alpha-1 proteinase inhibitor in human islet
microvascular endothelial cells. Diabetes. 48:1773–1778. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li JM, Mullen AM, Yun S, Wientjes F,
Brouns GY, Thrasher AJ and Shah AM: Essential role of the NADPH
oxidase subunit p47(phox) in endothelial cell superoxide production
in response to phorbol ester and tumor necrosis factor-alpha. Circ
Res. 90:143–150. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zheng S, Zhong ZM, Qin S, Chen GX, Wu Q,
Zeng JH, Ye WB, Li W, Yuan K, Yao L, et al: Advanced oxidation
protein products induce inflammatory response in fibroblast-like
synoviocytes through NADPH oxidase -dependent activation of NF-κB.
Cell Physiol Biochem. 32:972–985. 2013. View Article : Google Scholar
|
|
29
|
Garcia Soriano F, Virág L, Jagtap P, Szabó
E, Mabley JG, Liaudet L, Marton A, Hoyt DG, Murthy KG, Salzman AL,
et al: Diabetic endothelial dysfunction: The role of
poly(ADP-ribose) polymerase activation. Nat Med. 7:108–113. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barsotti A, Fabbi P, Fedele M, Garibaldi
S, Balbi M, Bezante GP, Risso D, Indiveri F, Ghigliotti G and
Brunelli C: Role of advanced oxidation protein products and Thiol
ratio in patients with acute coronary syndromes. Clin Biochem.
44:605–611. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Simm A, Wagner J, Gursinsky T, Nass N,
Friedrich I, Schinzel R, Czeslik E, Silber RE and Scheubel RJ:
Advanced glycation endproducts: A biomarker for age as an outcome
predictor after cardiac surgery? Exp Gerontol. 42:668–675. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gradinaru D, Borsa C, Ionescu C and
Margina D: Advanced oxidative and glycoxidative protein damage
markers in the elderly with type 2 diabetes. J Proteomics.
92:313–322. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou LL, Hou FF, Wang GB, Yang F, Xie D,
Wang YP and Tian JW: Accumulation of advanced oxidation protein
products induces podocyte apoptosis and deletion through
NADPH-dependent mechanisms. Kidney Int. 76:1148–1160. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tschen SI, Dhawan S, Gurlo T and Bhushan
A: Age-dependent decline in beta-cell proliferation restricts the
capacity of beta-cell regeneration in mice. Diabetes. 58:1312–1320.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim W and Egan JM: The role of incretins
in glucose homeostasis and diabetes treatment. Pharmacol Rev.
60:470–512. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ishibashi Y, Matsui T, Takeuchi M and
Yamagishi S: Glucagon-like peptide-1 (GLP-1) inhibits advanced
glycation end product (AGE)-induced up-regulation of VCAM-1 mRNA
levels in endothelial cells by suppressing AGE receptor (RAGE)
expression. Biochem Biophys Res Commun. 391:1405–1408. 2010.
View Article : Google Scholar
|
|
37
|
Puddu A, Sanguineti R, Durante A, Nencioni
A, Mach F, Montecucco F and Viviani GL: Glucagon-like peptide-1
triggers protective pathways in pancreatic beta-cells exposed to
glycated serum. Mediators Inflamm. 2013:3171202013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Luciano Viviani G, Puddu A, Sacchi G,
Garuti A, Storace D, Durante A, Monacelli F and Odetti P: Glycated
fetal calf serum affects the viability of an insulin-secreting cell
line in vitro. Metabolism. 57:163–169. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Matsui T, Nishino Y, Takeuchi M and
Yamagishi S: Vildagliptin blocks vascular injury in thoracic aorta
of diabetic rats by suppressing advanced glycation end
product-receptor axis. Pharmacol Res. 63:383–388. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhan Y, Sun HL, Chen H, Zhang H, Sun J,
Zhang Z and Cai DH: Glucagon-like peptide-1 (GLP-1) protects
vascular endothelial cells against advanced glycation end products
(AGEs)-induced apoptosis. Med Sci Monit. 18:BR286–BR291. 2012.
View Article : Google Scholar : PubMed/NCBI
|