Introduction
Many botanical molecules are known to possess
anti-inflammatory and analgesic effects. β-caryophyllene is a
sesquiterpene, which is the essential oil of many medical plants,
particularly clove (Syzygium aromaticum), hemp (Cannabis
sativa), rosemary (Rosmarinus officinalis), cinnamon and
hops (1–4). β-caryophyllene is natural dietary
ingredient found in many edible plants ingested daily, and has been
approved as a food additive by the Food and Drug Administration
(FDA).
β-caryophyllene has been known to be a selective
agonist of cannabinoid receptor type-2 and to exert cannabimimetic
anti-inflammatory and analgesic effects in animals (2,4)
for both acute and chronic pain with inflammation (4–6).
The anti-inflammatory effects of β-caryophyllene are not implicated
in its inhibitory effects on the production of tumor necrosis
factor (TNF)-α and interleukin (IL)-1β, that are related to opioid
receptors (7). RAW267.4 cells are
murine macrophages that produce various inflammatory cytokines,
including TNF-α and IL-1β (8).
However, the effects of β-caryophyllene on RAW267.4 macrophages
have not been investigated to date, at least to the best of our
knowledge.
This study was undertaken to determine whether
β-caryophyllene exerts suppressive effects on mouse RAW267.4
macrophages in vitro. Moreover, we compared the interactive
effects of β-caryophyllene with other botanical molecules, which
exert antioxidant and anti-inflammatory effects on mouse RAW267.4
macrophages in vitro. Of note, this study demonstrated that
the combination of β-caryophyllene, baicalin and (+)-catechin at
concentrations, which independently did not exert a significant
effect on RAW267.4 cells, exerted synergistic suppressive effects
on the proliferation and synergistic stimulatory effects on the
death of RAW267.4 cells in vitro. To the best of our
knowledge, this is the first time that such a finding has been
described. This composition of agents may be a potent and useful
tool as an anti-inflammatory therapeutic strategy with less
toxicity.
Materials and methods
Materials
Dulbecco's modification of Eagle's medium (DMEM)
with 4.5 g/l glucose, L-glutamine and sodium pyruvate and
antibiotics [penicillin and streptomycin (P/S)] were purchased from
Corning (Mediatech, Inc., Manassas, VA, USA). Fetal bovine serum
(FBS) was from HyClone (Logan, UT, USA). β-caryophyllene, baicalin,
(+)-catechin, curcumin, and (+)-aromatic turmerone were obtained
from Cayman Chemical (Ann Arbor, MI, USA). TNF-α was obtained from
R&D Systems (Minneapolis, MN, USA). Sodium butyrate,
roscovitine, sulforaphane, PD98059, wortmannin, dibucaine, and all
other reagents were purchased from Sigma-Aldrich (St. Louis, MO,
USA) unless otherwise specified. The caspase-3 inhibitor (Caspase
3/CPP32 inhibitor W-1, biotin conjugate; Wako Pure Chemical
Industries, Ltd., Osaka, Japan) was diluted in phosphate-buffered
saline (PBS) and the other reagents were dissolved in 100% ethanol
or dimethyl sulfoxide for use in the experiments.
RAW267.4 cells
Mouse RAW267.4 cells (murine macrophages) were
obtained from the American Type Culture Collection (ATCC,
Rockville, MD, USA) (9,10).
Cell proliferation assay
The RAW267.4 cells (1×105/ml/well) cells
were cultured using a 24-well plate in DMEM containing 10% FBS and
1% P/S for 1, 2, 3 or 7 days in a water-saturated atmosphere
containing 5% CO2 and 95% air at 37°C, as previously
described (11). The RAW267.4
cells were cultured in DMEM containing 10% FBS and 1% P/S in the
presence or absence of absence of either the vehicle (0.1% ethanol
as a final concentration), β-caryophyllene [1 (5 µM), 10,
50, 100 or 200 µg/ml of medium], baicalin [1 (2.24
µM), 10, 50, 100 or 200 µg/ml], (+)-catechin [1 (3.45
µM), 10, 50, 100 or 200 µg/ml], curcumin [1 (2.72
µM), 10, 50, 100 or 200 µg/ml], or (+)-aromatic
turmerone [1 (4.62 µM), 5, 10, 25 or 50 µg/ml] for 3
days. In separate experiments, the RAW267.4 cells were cultured in
DMEM containing 10% FBS and 1% P/S with either the vehicle (0.1%
ethanol as a final concentration) or mixture of β-caryophyllene (10
µg/ml of medium), baicalin (10 µg/ml) and
(+)-catechin (10 µg/ml) in the presence or absence of either
sodium butyrate (10 and 100 µM), roscovitine (10 and 100
nM), sulphoraphane (1 and 10 nM), dibucaine (0.1 or 1 µM),
PD98059 (1 or 10 µM), or wortmannin (0.1 or 1 µM) for
3 days. Following culture, the cells were detached from the culture
dishes and counted using the same method as described below in
'Cell counting'.
Cell death assay
The RAW267.4 cells (1×105/ml/well) were
cultured using a 24-well plate in DMEM containing 10% FBS and 1%
P/S for 3 days until they reached confluence, as previously
described (12), and the cells
were then cultured for an additional 2 days in the presence or
absence of absence of either the vehicle (0.1% ethanol as a final
concentration), β-caryophyllene (1 or 10 µg/ml of medium)
and/or baicalin or (+)-catechin (1 or 10 µg/ml of medium)
with or without caspase-3 inhibitor (10 µM). Following
culture, the cells were detached from the culture dishes.
Cell counting
Following trypsinization of each culture dish using
0.2% trpysin plus 0.02% EDTA in
Ca2+/Mg2+-free PBS for 2 min at 37°C, the
detached cells from the dish were collected following
centrifugation at 150 × g for 5 min at a room temperature, as
previously described (12,13).
The cells were resuspended in PBS solution and stained with eosin.
Cell numbers were counted under a microscope (Olympus MTV-3;
Olympus, Tokyo, Japan) using a hemocytometer plate (12,13). For each dish, we took the average
of two countings. The cell number was calculated as the number of
cells/well of each plate.
Western blot analysis
The RAW267.4 cells (1×105 cells/well in 2
ml of medium) were cultured in DMEM containing 10% FBS and 1% P/S
in the presence or absence of either the vehicle (0.1% ethanol as a
final concentration), β-caryophyllene (1 µg/ml of medium),
baicalin (1 µg/ml), and/or (+)-catechin (1 µg/ml) for
3 days. In a separate experiment, the cells were cultured in the
presence or absence of a combination of β-caryophyllene (1
µg/ml), baicalin (1 µg/ml) and (+)-catechin (1
µg/ml) for 3 days, and then were they cultured for an
additional 24 h with or without the addition of TNF-α (10 ng/ml).
Following culture, the cells were washed twice with ice-cold PBS
and removed from the dish by scraping in cell lysis buffer
containing protein inhibitors. The lysed cells were homogenized by
sonication in 0.4 ml of ice-cold cell lyses buffer containing
protein inhibitors. The homogenate was centrifuged for 5 min at
1,500 × g, and the protein concentration of the supernatant was
determined for western blotting using bovine serum albumin as a
standard. Samples of supernatant protein (30 µg/lane) were
separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred to nylon membranes for
western blotting using specific rabbit antibodies against Akt,
mitogen-activated protein kinase (MAPK), caspase-3, cyclooxygenase
(COX)-1, -2, p65, and β-actin (all form Cell Signaling Technology,
Danvers, MA, USA). β-actin was used as a loading control. Donkey
anti-rabbit IgG-HRP (Santa Cruz Biotechnology, Santa Cruz, CA, USA)
was used as the secondary antibody for all the above antibodies. A
minimum of 2 blots from independent experiments was scanned on an
Epson Perfection 1660 Photo scanner, and bands quantified using
ImageJ. Data from independent experiments were normalized to a
percentage of control before averaging.
Statistical analysis
Statistical significance was determined using
GraphPad InStat version 3 for Windows XP (GraphPad Software Inc.,
La Jolla, CA, USA). Multiple comparisons were performed by one-way
analysis of variance (ANOVA) with Tukey-Kramer multiple comparisons
post test for parametric data as indicated. A value of p<0.05
was considered to indicate a statistically significant
difference.
Results
Effects of various botanical molecules on
the proliferation of RAW267.4 cells
The effects of various botanical molecules
[β-caryophyllene, baicalin, (+)-catechin, curcumin and (+)-aromatic
turmerone], which exert anti-inflammatory effects on RAW267.4 cells
in vitro have not been investigated thus far. Thus, to
determine these effects, the cells were cultured for 3 days in the
presence or absence of each compound. The proliferation of the
RAW267.4 cells was suppressed in the presence of β-caryophyllene
(50, 100 and 200 µg/ml of medium) (Fig. 1A) or curcumin (100 and 200
µg/ml) (Fig. 1B). Culture
with baicalin (1, 10, 50, 100 and 200 µg/ml) (Fig. 1C) or (+)-catechin (1, 10, 50, 100
and 200 µg/ml) (Fig. 1D)
did not exert a significant effect on RAW267.4 cell proliferation.
Culture with (+)-aromatic turmerone (25 and 50 µg/ml)
significantly increased the proliferation of RAW267.4 cells (data
not shown).
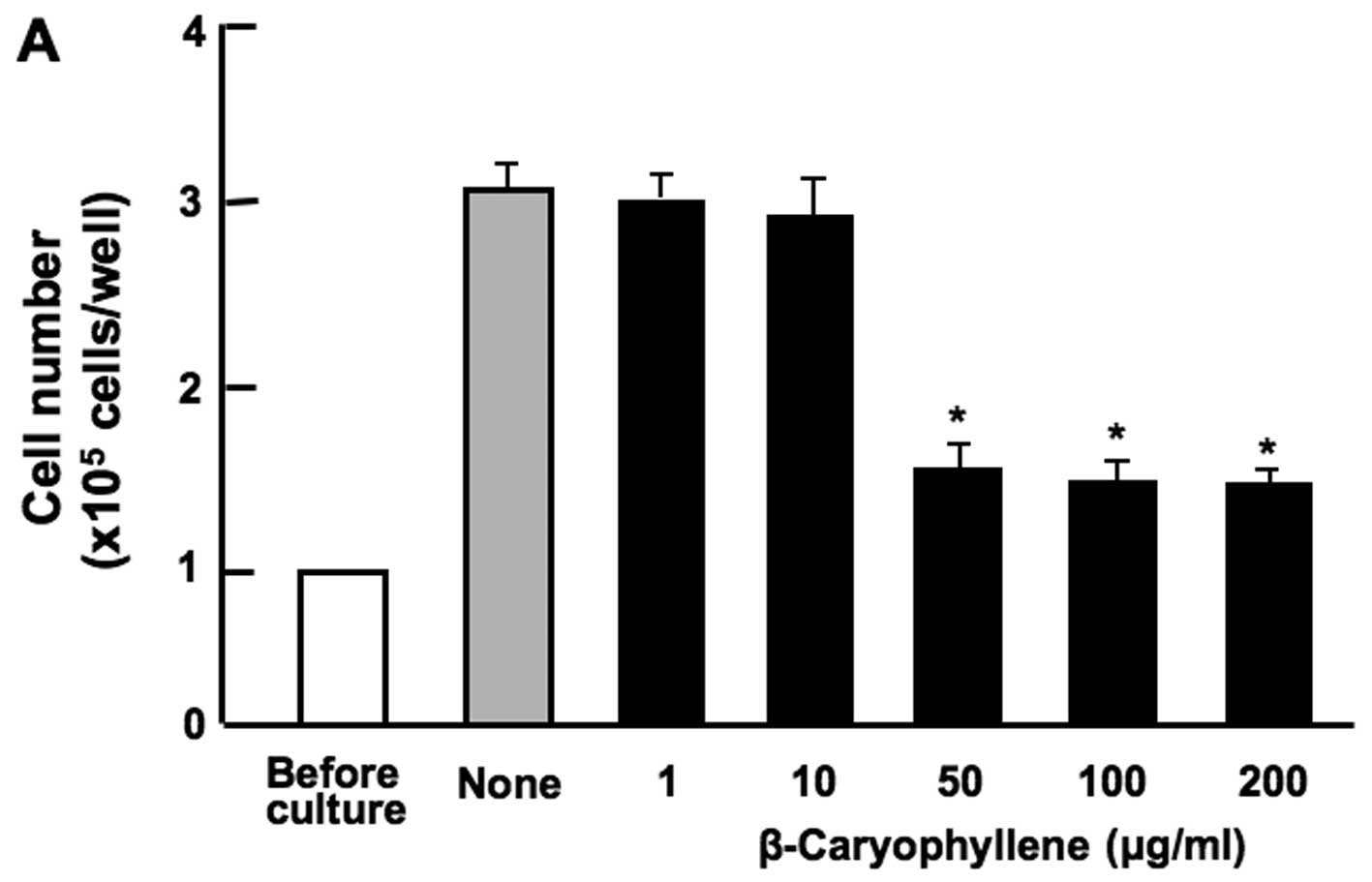 | Figure 1Effects of various botanical molecules
on the proliferation of RAW267.4 cells in vitro. RAW267.4
cells were cultured for 3 days in Dulbecco's modification of
Eagle's medium (DMEM) containing either the vehicle,
β-caryophyllene [(A) 1, 10, 50, 100 and 200 µg/ml of
medium], curcumin [(B) 1, 10, 50, 100 and 200 µg/ml],
(+)-catechin [(C) 1, 10, 50, 100 and 200 µg/ml] or baicalin
[(D) 1, 10, 50, 100 and 200 µg/ml of medium]. Following
culture, the number of attached cells on the dish was counted. Data
are presented as the means ± SD of 2 replicate wells/data set using
different dishes and cell preparation. *p<0.001 as
compared with the control group (grey bar), as shown by one way
ANOVA and Tukey-Kramer post-test. |
The combination of β-caryophyllene,
baicalin and (+)-catechin exerts a synergistic suppressive effect
on the proliferation of RAW267.4 cells
Subsequently, the effects of the combination with
various botanical molecules on the proliferation of RAW267.4 cells
in vitro were determined. Cell proliferation was not
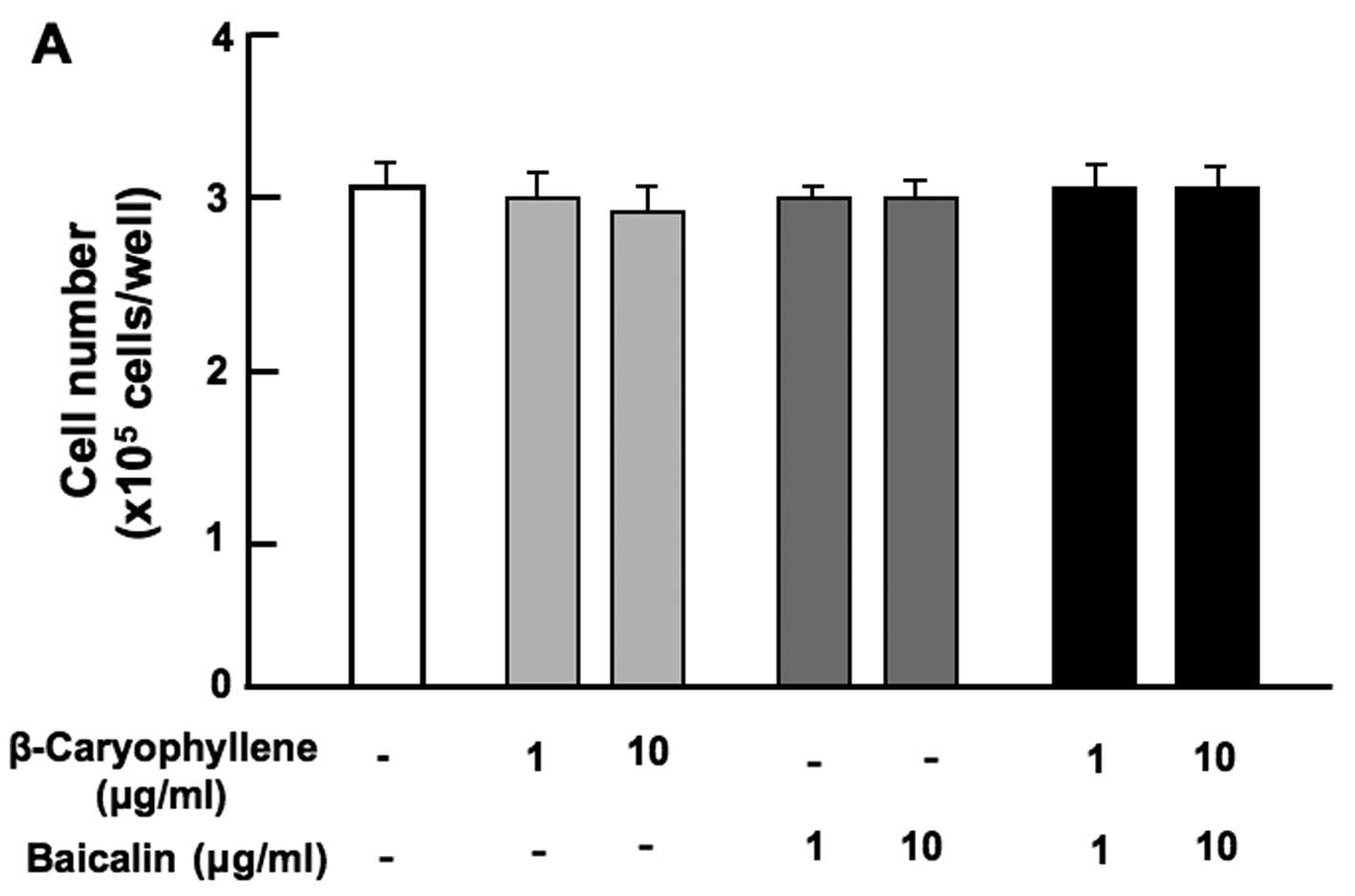
suppressed by the combination of β-caryophyllene (1 or 10
µg/ml) with either baicalin (1 or 10 µg/ml) (Fig. 2A), (+)-catechin (1 or 10
µg/ml) (Fig. 2B), curcumin
(1 or 10 µg/ml) (Fig. 2C)
or (+)-aromatic turmerone (1 and 10 µg/ml) (Fig. 2D).
Notably, the combination of baicalin (80 or 160
µg/ml) and (+)-catechin (20 or 40 µg/ml) at
comparatively higher concentrations exerted a significant
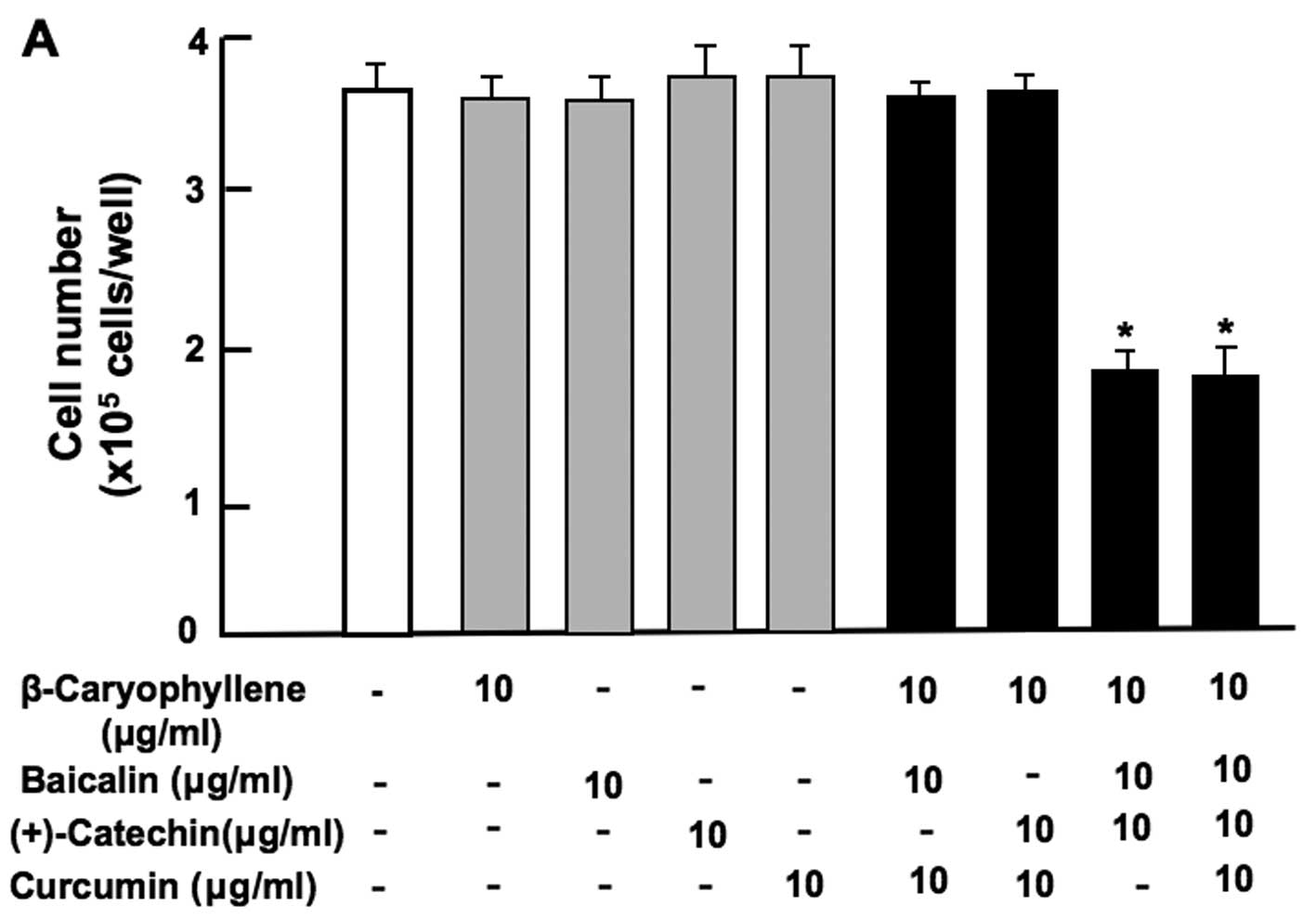
suppressive effect on cell proliferation (Fig. 3A). Moreover, the combination of
β-caryophyllene, baicalin and (+)-catechin at a lower concentration
of each (1 or 10 µg/ml), which did not independently exert a
significant effect on cell proliferation, was found to exert a
potent synergistic suppressive effect on cell proliferation
(Fig. 3B). Such an effect was
also observed with a higher concentration (100 µg/ml) of
β-caryophyllene, baicalin and (+)-catechin (Fig. 3B). In addition, the synergistic
effects of β-caryophyllene, baicalin and (+)-catechin on cell
proliferation were also observed with the combination of
β-caryophyllene (1 and 10 µg/ml), baicalin (0.8 or 8
µg/ml) and (+)-catechin (0.2 or 2 µg/ml) (Fig. 3C). The synergistic effects
observed with the combination of β-caryophyllene, baicalin and
(+)-catechin on cell proliferation were not enhanced in the
presence of curcumin (10 µg/ml) (Fig. 3D). Thus, the combination of
β-caryophyllene, baicalin and (+)-catechin was shown to exert a
potent synergistic suppressive effect on RAW267.4 cell
proliferation in vitro.
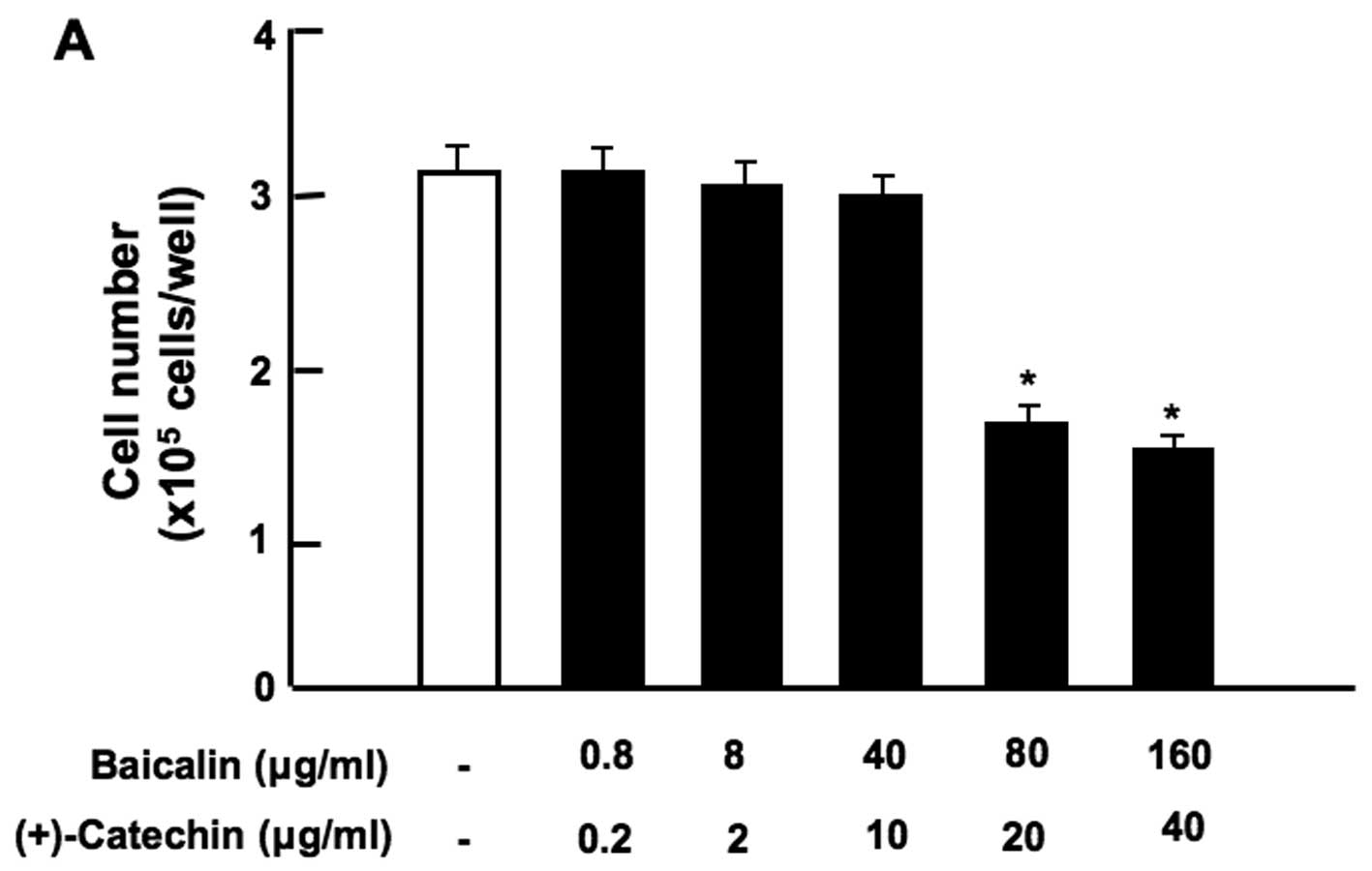 | Figure 3Combination of β-caryophyllene,
(+)-catechin and baicalin exerts a synergistic suppressive effect
on the proliferation of RAW267.4 cells in vitro. RAW267.4
cells were cultured for 3 days in Dulbecco's modification of
Eagle's medium (DMEM) containing various botanical molecules: (A)
both baicalin (0.8, 8, 40, 80 and 160 µg/ml of medium)
and/or (+)-catechin (0.2, 2, 10, 20 and 40 µg/ml), (B)
baicalin (1, 10 or 100 µg/ml) and/or (+)-catechin (1 or 10
µg/ml) in the presence or absence of β-caryophyllene (1, 10
or 100 µg/ml), (C) baicalin (0.8 or 8 µg/ml) and/or
(+)-catechin (0.2 or 2 µg/ml) in the presence or absence of
β-caryophyllene (1 or 10 µg/ml), (D) curcumin (10
µg/ml), baicalin (10 µg/ml) and/or (+)-catechin (10
µg/ml) in the presence or absence of β-caryophyllene (10
µg/ml). Following culture, the number of attached cells on
the dish was counted. Data are presented as the means ± SD of 2
replicate wells/data set using different dishes and cell
preparation. *p<0.001 as compared with the control
group (white bar) (A, B, C or D). #p<0.001 as
compared with the group (grey bar) treated with baicalin (1, 10 or
100 µg/ml) plus (+)-catechin (1, 10 or 100 µg/ml)
(B), as shown by one way ANOVA and Tukey-Kramer post-test. |
Mechanistic characterization of the
synergistic suppressive effects of β-caryophyllene, catechin and
baicalin on the proliferation of RAW267.4 cells
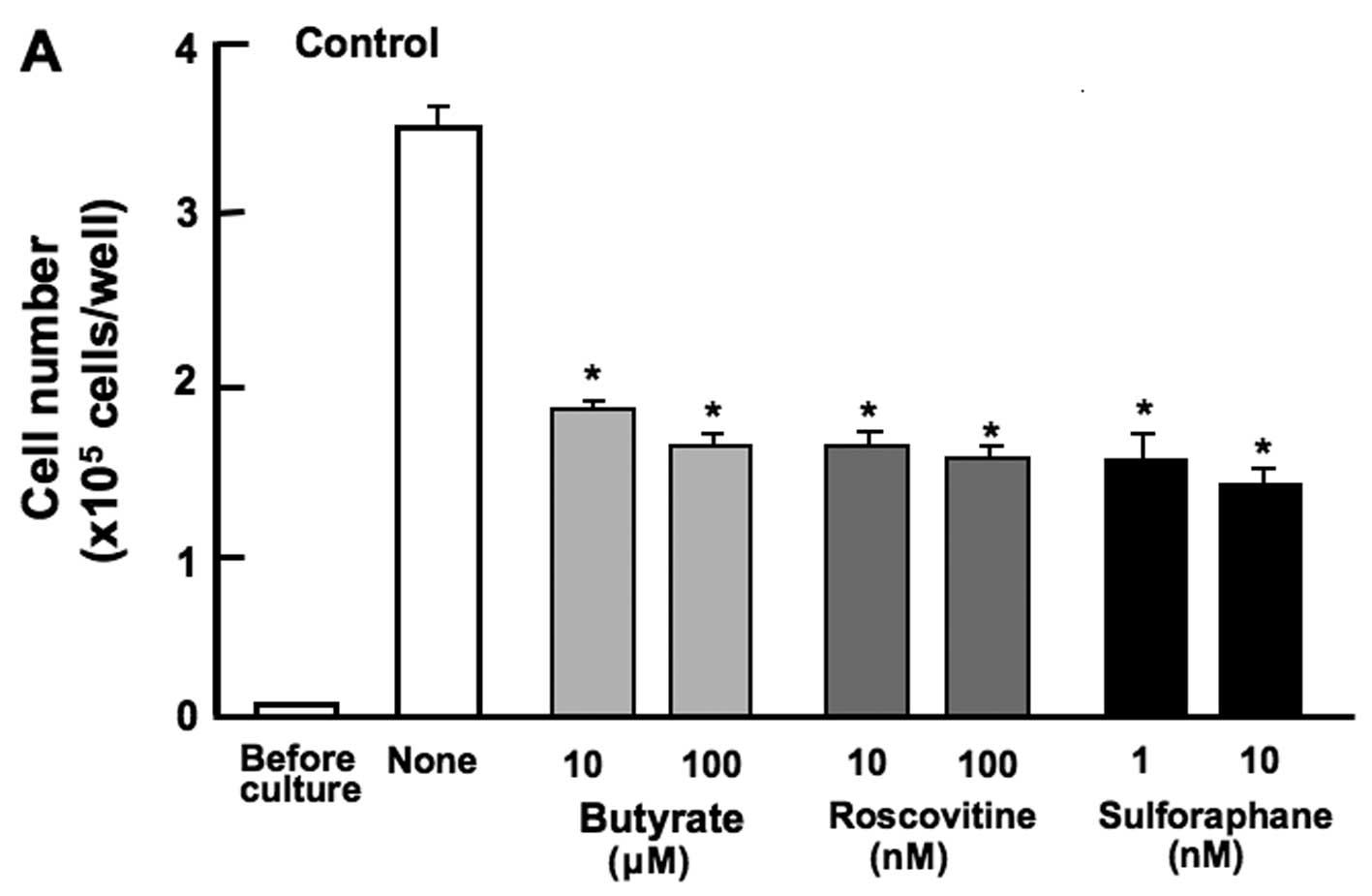
The proliferation of the RAW267.4 cells was
determined in the presence of various inhibitors that induce cell
cycle arrest in vitro (Fig.
4). The cells were cultured for 3 days in the presence of
butyrate (10 and 100 µM), roscovitine (10 and 100 nM), or
sulforaphane (1 and 10 nM). The proliferation of RAW267.4 cells was
suppressed in the presence of these inhibitors (Fig. 4A). Such effects were not altered
in the cells cultured in the presence of the combination of
β-caryophyllene, catechin and baicalin (Fig. 4B). Thus, the suppressive effects
of the combined chemicals on the proliferation of RAW267.4 cells
were not altered in the presence of butyrate, roscovitine or
sulphoraphan, that induce cell cycle arrest. Roscovitine is a
potent and selective inhibitor of the cyclin-dependent kinases,
cdc2, cdk2m and cdk5 (13).
Sulforaphane induces G2/M phase cell cycle arrest (14). Butyrate inhibits G1 progression
(11). In this study, the
combined use of the botanical molecules was found to induce G1 and
G2/M phase cell cycle arrest in RAW267.4 cells.
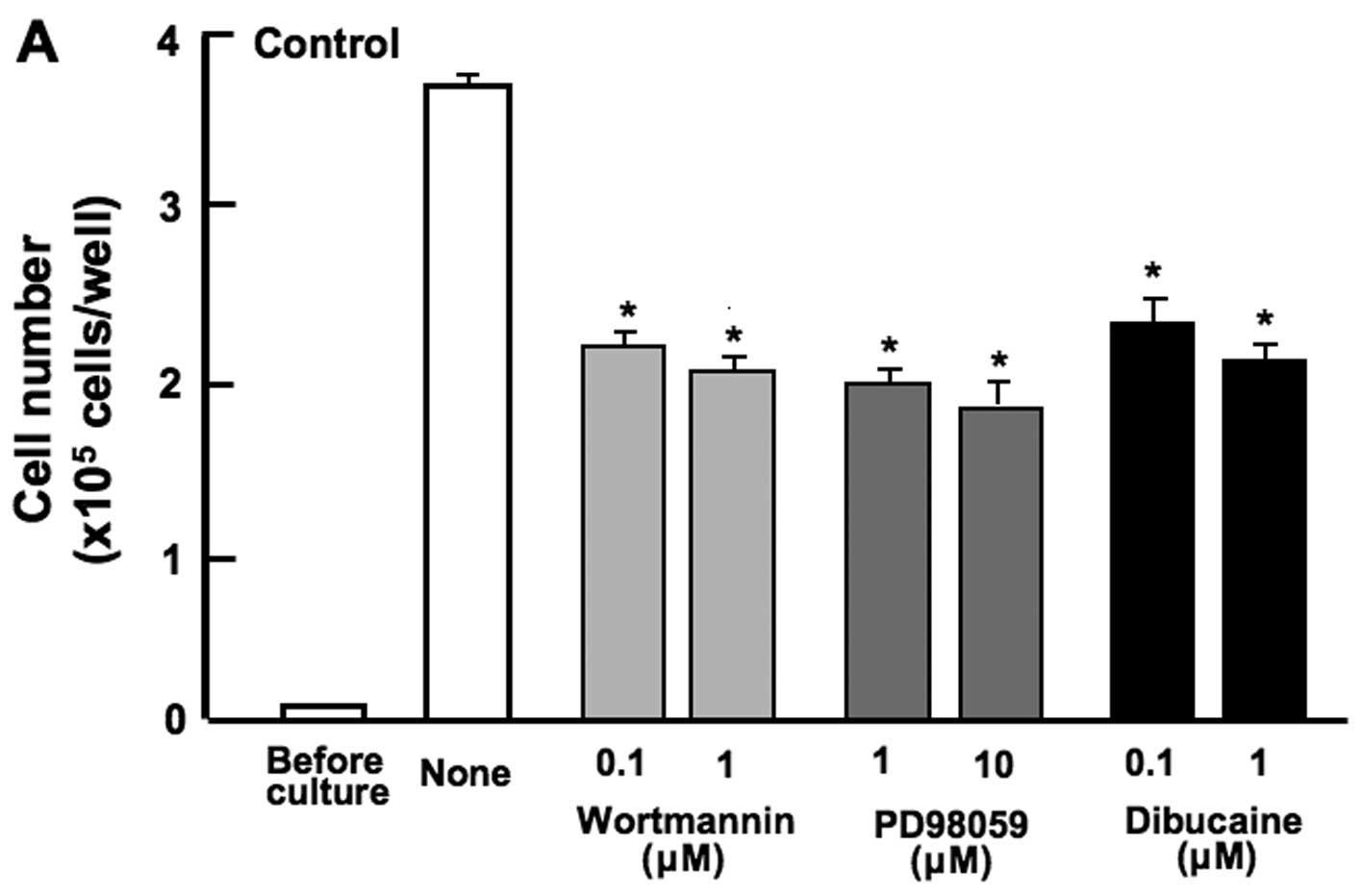
Subsequently, to further determine the mechanistic
characteristics responsible for the suppressive effects of the
combined use of botanical molecules on cell proliferation, we
examined whether the suppressive effects of the combined chemicals
on the proliferation of RAW267.4 cells are modulated through
various signaling factors that suppress cell proliferation. The
proliferation of RAW267.4 cells was suppressed in the presence of
wortmannin (0.1 or 1 µM), an inhibitor of
phosphatidylinositol 3-kinase (PI3K) (15), PD98059 (1 or 10 µM), an
inhibitor of extracellular signal-regulated kinase (ERK) and
mitogen-activated protein kinase (MAPK) (16), or dibucaine (0.1 or 1 µM),
an inhibitor of Ca2+/calmodulin-dependent protein kinase
(11) (Fig. 5A). The suppressive effects of
these inhibitors on cell proliferation were not observed when used
in combination with β-caryophyllene, catechin and baicalin
(Fig. 5B). The combined use of
the botanical molecules was found to exert suppressive effects on
cell proliferation by inhibiting various intracellular signaling
pathways, such as the PI3K/Akt, ERK/MAPK and Ca2+
pathways in RAW267.4 cells.
Combination of β-caryophyllene, baicalin
and (+)-catechin promotes the death of RAW267.4 cells in vitro
To determine whether the combination of
β-caryophyllene, baicalin and (+)-catechin induces apoptotic cell
death in vitro, RAW267.4 cells were cultured for 3 days
until they reached confluence, and the cells were then cultured for
an additional 2 days in the presence or absence of either
β-caryophyllene (10 µg/ml), baicalin (10 µg/ml), or
(+)-catechin (10 µg/ml) with or without caspase-3 inhibitor
(10 µM) (Fig. 6). The
addition of β-caryophyllene, baicalin or (+)-catechin alone did not
cause a significant alteration in the number of RAW267.4 cells
(Fig. 6A). However, the
combination of β-caryophyllene (10 µg/ml), baicalin (10
µg/ml) and (+)-catechin (10 µg/ml) caused a
significant decrease in cell number, indicating that this treatment
induces cell death. Culture with curcumin (10 µg/ml) did not
exert an effect on cell death (Fig.
6A). Of note, the suppressive effects of combination of the
botanical molecules on cell death were completely prevented in the
presence of caspase-3 inhibitor (Fig.
6B). These results suggest that the combined use of the
botanical molecules induce cell death related to caspase-3, that
activates nuclear DNA fragmentation, which induces apoptotic cell
death (17).
Effects of β-caryophyllene, baicalin and
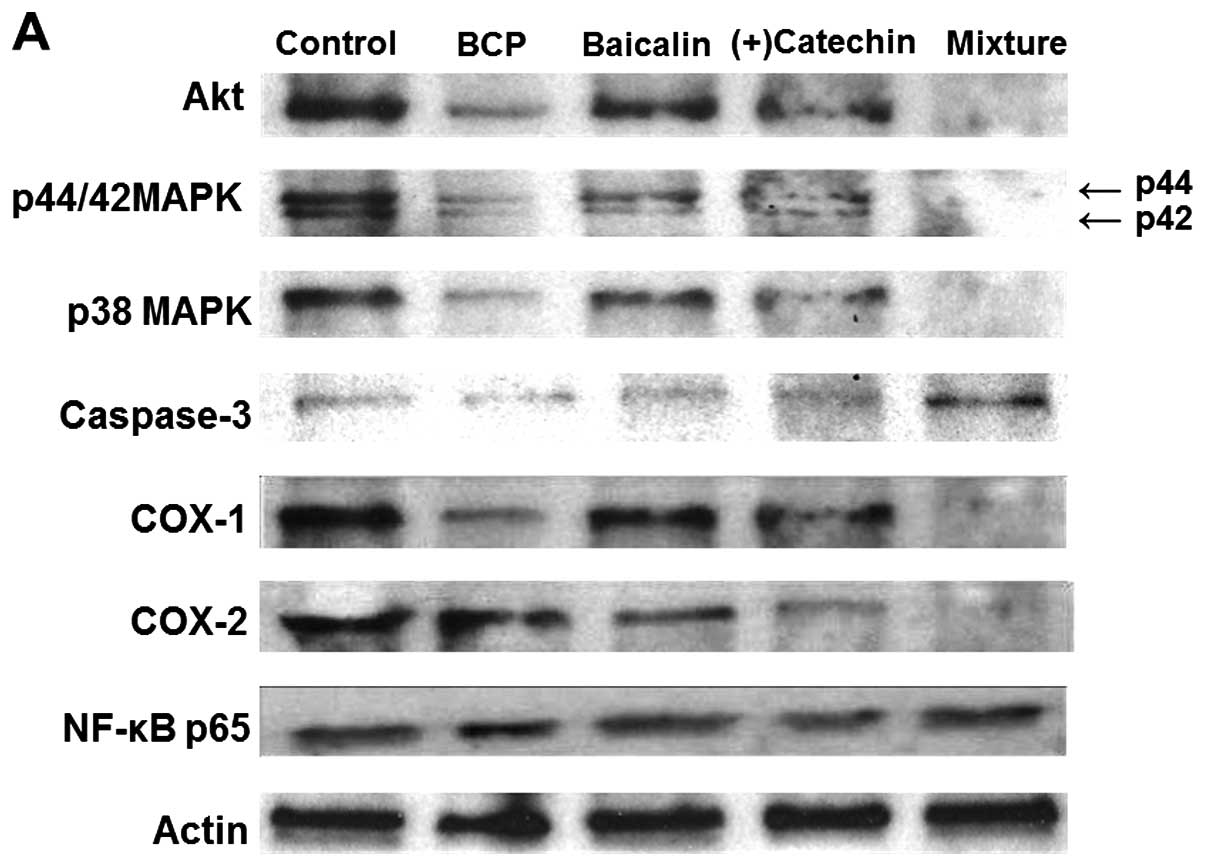
(+)-catechin on various protein levels in RAW267.4 cells
Alterations in the expression of key proteins
related to cell proliferation, apoptotic cell death and
inflammation were examined by western blot analysis (Fig. 7). The combination of
β-caryophyllene (1 or 10 µg/ml), baicalin (1 or 10
µg/ml) and (+)-catechin (1 or 10 µg/ml) exerted a
suppressive effect on Akt, p44/42 MAPK, p38 MAPK and COX-1 and -2
expression (Fig. 7A). Culture
with this combination increased caspase-3 expression, but did not
alter the levels of nuclear factor-κB (NF-κB) p65 (Fig. 7A). Moreover, culture with TNF-α
(10 ng/ml) caused an increase in COX-2 and NF-κB p65 expression
(Fig. 7B). This effect was
suppressed by the combination of β-caryophyllene, baicalin and
(+)-catechin at the concentration of 1 µg/ml (Fig. 7B).
Discussion
β-caryophyllene is a cannabinoid receptor 2 agonist,
which exerts anti-inflammatory effects (1–5).
This study was undertaken to determine the effects of
β-caryophyllene and other herbal molecules, which exert antioxidant
and anti-inflammatory effects on mouse RAW267.4 macrophages in
vitro. Of note, we found that the combined use of the three
botanical molecules [β-caryophyllene, baicalin and (+)-catechin] at
comparatively lower concentrations, which alone did not exert any
effects on cell proliferation, exerted a potent
synergistic-suppressive effect on the proliferation of RAW267.4
cells in vitro. These synergistic effects may provide a
useful pharmacologic tool for managing inflammatory pain.
Mechanistically, the combination of β-caryophyllene,
baicalin and (+)-catechin was found to induce G2/M phase cell cycle
arrest in RAW267.4 cells using various inhibitors that induce cell
cycle arrest in vitro. Moreover, the combined use of the
botanical molecules exerted suppressive effects on cell
proliferation by inhibiting various intracellular signaling
pathways, such as the PI3K/Akt, ERK/MAPK and Ca2+
pathways in RAW267.4 cells, using various inhibitors (11,13–16). In addition, the combined use of
the three agents was found to decrease the protein levels of Akt
and MAPKs (p44/42 and p38) in RAW267.4 cells.
β-caryophyllene, baicalin and (+)-catechin did not,
individually, exert a significant effect on the death of RAW267.4
cells in vitro. Importantly, the combination of
β-caryophyllene, baicalin and (+)-catechin at comparatively lower
concentrations of each agent was found to promote the death of
RAW267.4 cells in vitro. This finding suggests that the
suppressive effects on the proliferation of RAW267.4 cells exerted
by the combined use of β-caryophyllene, baicalin and (+)-catechin,
may be partly dependent on a promoting effect on cell death.
Mechanistically, suppressive effects of the combined use of the
botanical molecules on cell death were completely prevented in the
presence of caspase-3 inhibitor. In addition, the combined use of
the three agents increased caspase-3 expression in RAW267.4 cells.
These findings suggest that the cell death induced by the combined
use of the three botanical molecules is related to the activation
of caspase-3, that activates nuclear DNA fragmentation, which
induces apoptotic cell death (17).
Whether or not the combined effect of
β-caryophyllene, baicalin and (+)-catechin on the proliferation and
death of RAW267.4 cells influences inflammatory conditions, is
unknown. However, we found that the combination of β-caryophyllene,
baicalin and (+)-catechin decreased COX-1 and COX-2 expression in
RAW267.4 cells in vitro. Inflammation-inducing factors have
been reported to increase COX-2 and NF-κB p65 expression, which is
related to MAPK signaling pathways (18–21). Furthermore, we found that the
TNF-α-induced increased in the COX-2 and NF-κB p65 levels was
suppressed by culture with the combination of the botanical
molecules. These findings support the view that the combination of
β-caryophyllene, baicalin and (+)-catechin exerts potent inhibitory
effects on the inflammatory condition related to the function of
activated macrophages.
In conclusion, this study demonstrates that a
composition of botanical molecules, including β-caryophyllene,
baicalin and (+)-catechin at comparatively low levels exerts
potential synergistic suppressive effects on the function of
macrophages related to inflammation. This composition may prove to
be usefule as a novel anti-inflammatory therapy.
References
|
1
|
Ghelardini C, Galeotti N, Di Cesare
Mannelli L, Mazzanti G and Bartolini A: Local anaesthetic activity
of beta-caryophyllene. Farmaco. 56:387–389. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gertsch J, Leonti M, Raduner S, Racz I,
Chen JZ, Xie XQ, Altmann KH, Karsak M and Zimmer A:
Beta-caryophyllene is a dietary cannabinoid. Proc Natl Acad Sci
USA. 105:9099–9104. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ormeño E, Baldy V, Ballini C and Fernandez
C: Production and diversity of volatile terpenes from plants on
calcareous and siliceous soils: Effect of soil nutrients. J Chem
Ecol. 34:1219–1229. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Katsuyama S, Mizoguchi H, Kuwahata H,
Komatsu T, Nagaoka K, Nakamura H, Bagetta G, Sakurada T and
Sakurada S: Involvement of peripheral cannabinoid and opioid
receptors in β-caryophyllene-induced antinociception. Eur J Pain.
17:664–675. 2013. View Article : Google Scholar
|
|
5
|
Paula-Freire LI, Andersen ML, Gama VS,
Molska GR and Carlini EL: The oral administration of
trans-caryophyllene attenuates acute and chronic pain in mice.
Phytomedicine. 21:356–362. 2014. View Article : Google Scholar
|
|
6
|
Chavan MJ, Wakte PS and Shinde DB:
Analgesic and anti-inflammatory activity of Caryophyllene oxide
from Annona squamosa L. bark. Phytomedicine. 17:149–151. 2010.
View Article : Google Scholar
|
|
7
|
Martinez RM, Zarpelon AC, Cardoso RD,
Vicentini FT, Georgetti SR, Baracat MM, Andrei CC, Moreira IC,
Verri WA Jr and Casagrande R: Tephrosia sinapou ethyl acetate
extract inhibits inflammatory pain in mice: Opioid receptor
dependent inhibition of TNFα and IL-1β production. Pharm Biol.
51:1262–1271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pomari E, Stefanon B and Colitti M: Effect
of plant extracts on H2O2-induced
inflammatory gene expression in macrophages. J Inflamm Res.
7:103–112. 2014.PubMed/NCBI
|
|
9
|
Yamaguchi M, Vikulina T, Arbiser JL and
Weitzmann MN: Suppression of NF-κB activation by gentian violet
promotes osteoblastogenesis and suppresses osteoclastogenesis. Curr
Mol Med. 14:783–792. 2014. View Article : Google Scholar
|
|
10
|
Yamaguchi M and Weitzmann MN: The bone
anabolic carotenoid p-hydroxycinnamic acid promotes osteoblast
mineralization and suppresses osteoclast differentiation by
antagonizing NF-κB activation. Int J Mol Med. 30:708–712.
2012.PubMed/NCBI
|
|
11
|
Yamaguchi M and Daimon Y: Overexpression
of regucalcin suppresses cell proliferation in cloned rat hepatoma
H4-II-E cells: Involvement of intracellular signaling factors and
cell cycle-related genes. J Cell Biochem. 95:1169–1177. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Izumi T and Yamaguchi M: Overexpression of
regucalcin suppresses cell death in cloned rat hepatoma H4-II-E
cells induced by tumor necrosis factor-alpha or thapsigargin. J
Cell Biochem. 92:296–306. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meijer L, Borgne A, Mulner O, Chong JP,
Blow JJ, Inagaki N, Inagaki M, Deleros JG and Moulinoux JP:
Biochemical and cellular effects of roscovitine, a potent and
selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and
cdk5. Eur J Biochem. 243:527–536. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singh SV, Herman-Antosiewicz A, Singh AV,
Lew KL, Srivastava SK, Kamath R, Brown KD, Zhang L and Baskaran R:
Sulforaphane-induced G2/M phase cell cycle arrest involves
checkpoint kinase 2-mediated phosphorylation of cell division cycle
25C. J Biol Chem. 279:25813–25822. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Serrano-Nascimento C, da Silva Teixeira S,
Nicola JP, Nachbar RT, Masini-Repiso AM and Nunes MT: The acute
inhibitory effect of iodide excess on sodium/iodide symporter
expression and activity involves the PI3K/Akt signaling pathway.
Endocrinology. 155:1145–1156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen S, Wang Y, Ruan W, Wang X and Pan C:
Reversing multidrug resistance in hepatocellular carcinoma cells by
inhibiting extracellular signal-regulated kinase/mitogen-activated
protein kinase signaling pathway activity. Oncol Lett. 8:2333–2339.
2014.PubMed/NCBI
|
|
17
|
Zhao Y, Jing Z, Li Y and Mao W: Berberine
in combination with cisplatin suppresses breast cancer cell growth
through induction of DNA breaks and caspase-3-dependent apoptosis.
Oncol Rep. 36:567–572. 2016.PubMed/NCBI
|
|
18
|
Echizen K, Hirose O, Maeda Y and Oshima M:
Inflammation in gastric cancer: Interplay of the
COX-2/prostaglandin E2 and Toll-like receptor/MyD88
pathways. Cancer Sci. 107:391–397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin CC, Chan CM, Huang YP, Hsu SH, Huang
CL and Tsai SJ: Methylglyoxal activates NF-κB nuclear translocation
and induces COX-2 expression via a p38-dependent pathway in
synovial cells. Life Sci. 149:25–33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li N, Liu BW, Ren WZ, Liu JX, Li SN, Fu
SP, Zeng YL, Xu SY, Yan X, Gao YJ, Liu DF and Wang W: GLP-2
attenuates LPS-induced inflammation in BV-2 cells by inhibiting
ERK1/2, JNK1/2 and NF-κB signaling pathway. Int J Mol Sci.
17:E1902016. View Article : Google Scholar
|
|
21
|
Cha SM, Cha JD, Jang EJ, Kim GU and Lee
KY: Sophoraflavanone G prevents Streptococcus mutans surface
antigen I/II-induced production of NO and PGE2 by
inhibiting MAPK-mediated pathways in RAW 264.7 macrophages. Arch
Oral Biol. 68:97–104. 2016. View Article : Google Scholar : PubMed/NCBI
|