1. Introduction
Although the differentiation potential of
mesenchymal stem cells (MSCs) is relatively restricted compared to
pluripotent stem cells such as embryonic stem cells (ESCs) and
induced pluripotent stem cells (iPSCs), MSCs are more feasible and
a much safer source for cell therapy in regards to the risk of
transplanted stem cells forming tumors and becoming cancerous. To
date, cultures of MSCs have been successfully established from bone
marrow, umbilical cord blood, trabecular bone, periosteum,
synovium, placenta, pancreas, adipose tissue, skin, lung and thymus
(1,2). MSCs have the property of
self-renewal and commit to multiple cell lineages, such as bone,
cartilage, adipose, muscle, tendon, stroma and neuronal cells
(3), which make them
overwhelmingly useful tools in tissue engineering and regenerative
medicine. MSCs show immunomodulatory effects, which attenuate the
functions of dendritic cells (DCs) through the suppression of the
MHC II molecule and co-stimulatory molecules (4,5).
MSCs were also found to be immune suppressive in mixed lymphocyte
assays by inhibiting T-cell proliferation (6). They also own the ability to support
parenchymal cells as a niche with which to maintain cell pools and
constitutively secrete a distinct set of cytokines and growth
factors for maintaining homeostasis of the microenvironment
throughout their lifetime (7).
Thus, MSCs have been broadly applied in the treatment of various
diseases, including graft-versus-host disease (GVHD), Crohn's
disease (CD), diabetes mellitus (DM), multiple sclerosis (MS),
myocardial infarction (MI), liver failure, and rejection after
liver transplant (8).
Unfortunately, the function of MSCs is known to decline with age, a
process that may be implicated in the loss of maintenance of tissue
homeostasis leading to organ failure and diseases of aging
(9). Therefore, the proper use of
MSCs for clinical applications requires a general understanding of
the MSC aging process. Understanding the molecular processes
controlling MSC proliferation, senescence and commitment to
specific differentiated cell lineages is not only crucial to
determining the drivers and effectors of age-associated MSC
dysfunction but essential for the development of therapeutic
interventions that can slow, even reverse, age-related degenerative
changes to enhance repair processes and maintain healthy function
in aging tissues.
2. Markers of senescent MSCs
Earlier passage MSCs have been demonstrated to have
better colony efficiency compared to later passage MSCs (10). The classic features characterizing
the senescence phenotype of MSCs include growth arrest in the G1
phase of the cell cycle, enlarged or flattened morphology,
increased expression of senescence-associated β-galactosidase
(SA-β-gal) and senescence-associated lysosomal α-L-fucosidase
(SA-α-Fuc), and surface marker alteration (11).
Senescent MSCs usually become flat and hypertrophic
with constrained nuclei and granular cytoplasm resulting from
accumulation of cell debris. They have an excess of actin fibers
and decreased adherence to plastic surfaces. The level of
autofluorescence is increased due to lipofuscin aggregation.
The colony-forming unit-fibroblast (CFU-F) assay can
be used to estimate the proliferative and clonogenic potential of
MSCs in culture. MSCs are plated on plastic culture dishes at a low
density and cultured under conditions that allow individual CFU-F
to adhere and proliferate. The number of colonies present indicates
the ability to proliferate, and the senescent MSCs show a decreased
level of CFU-F.
Senescence marker, SA-β-gal, is widely used in the
assay of senescent cells. Histochemical detection of SA-β-gal
expression by senescent cells was first published in 1995, and
continues to be one of the most common assays used to assess the
senescence of MSCs. The percentage of SA-β-gal-positive MSCs is
obviously increased during the process of aging, which is caused by
increased lysosomal activities and altered cytosolic pH (12,13).
Recently, SA-α-Fuc has been identified as a novel
marker of senescence that is upregulated in response to
replicative, DNA damage-, and oncogene-induced senescence.
Furthermore, as a marker of senescence, SA-α-Fuc is a more
sensitive and robust biomarker for cell senescence when compared
with SA-β-gal at the transcriptional and enzymatic levels (14).
The International Society for Cellular Therapy
(ISCT) has proposed the minimal criteria for the definition of MSCs
in 2006: i) adherence to plastic; ii) expression of surface markers
CD90, CD73 and CD105, in the absence of CD45, CD34, CD14, CD11b,
CD79α, CD19 and HLA-DR; and iii) multipotent differentiation
potential of chondrocytes, osteoblasts, and adipocytes under
different standard conditions in vitro (15). These markers are expressed at
similar levels in early-passage, young and late-passage senescent
MSCs indicating that their value may be limited only to basic MSC
characterization (16). Other
potential positive and negative antigenic markers have been
suggested to identify senescence since Stro-1 (17), CD106 (VCAM-1) (18) and CD146 (MCAM) (19) expression levels are downregulated
during prolonged culture. Moreover, CD106 expression is also
strongly downregulated in MSCs after differentiation in
adipogenesis, osteogenesis, and chondrogenesis, suggesting that
CD106 may be a marker of undifferentiated cells within expanded MSC
cultures. Meanwhile, CD295 (leptin receptor) was found to increase
as a function of intrinsic cellular aging, suggesting its ability
to mark apoptotic cells (20).
Based on the above-mentioned findings, these markers of MSCs can be
classified into two categories. One category includes molecules
that are stably expressed, such as CD73, CD90 and CD105 showing
little association with culture history and aging status. The
second group of markers, such as Stro-1 or CD106, contains
molecules which show dependency on donor, culture passage or other
variables including cell seeding density, cell homing or attachment
properties.
3. Changes in the differentiation potential
of senescent MSCs
MSCs are known as having high osteogenesis and
adipogenesis potential, and the switch between osteogenetic and
adipogenetic commitment and differentiation is mediated through
numerous transcription factors and signaling pathways. The mutual
exclusivity of cell fate is thought to be determined by factors
such as the CCAAT/enhancer binding protein (C/EBP), as well as
peroxisome proliferator-activated receptor γ (PPARγ), which promote
adipocyte maturation, or runt-related transcription factor/core
binding factor α1 (RUNX2/CBFA1), an osteoblastic transcriptional
mediator (21).
Some studies indicate that the osteogenic activity
of MSCs deteriorates as a function of increasing lifespan, which is
in line with the loss of bone-forming efficiency. This osteogenesis
is attributed to the expression of RUNX2/CBFA1 through the PI3K-AKT
pathway. It is an essential transcription factor for the
osteogenic/chondrogenic lineages as an activator and marker of
osteogenesis of MSCs (22,23)
and a slight decline is ob served in its expression with age
(24). Receptor activator of
nuclear factor-κB ligand (RANKL), which is essential for the
differentiation and maintenance of osteoclasts, was observed to be
highly expressed in late-passage MSCs. The transforming growth
factor-β (TGF-β)/SMAD3 signaling pathway is critical for osteogenic
differentiation and can induce ERK phosphorylation. In addition, an
inhibitor of ERK was found to suppress osteogenic differentiation
induced by TGF-β alone (25,26).
According to reports, the adipogenic differentiation
potential of MSCs remained unchanged, became worse or was enhanced.
However, there is a general agreement that the adipogenesis
potential of MSCs tends to decline with consecutive passages under
standard culture conditions. PPARγ, a member of the
ligand-activated nuclear receptor superfamily, is regarded as an
adipogenic-specific transcription factor, inducing the
transcriptional activation of many different target genes involved
in lipid metabolism and adipocyte differentiation. It has been
reported that expression of PPARγ decreases during aging, and
impaired PPARγ and C/EBP shift the fate of MSCs toward the
osteoblast lineage. The WNT/β-catenin signaling pathway can
suppress C/EBP and PPARγ, favoring MSC differentiation to
osteoblasts, and therefore are regarded as master moderators of
adipogenesis and osteogenesis (27). The phosphorylation of AKT by
insulin was found to suppress FOXO3 expression and activate PPAR,
which reverses differentiation balance and promotes adipogenesis
potential. Activation of the PPARγ transcription factors by
agonists such as thiazolidinedione compounds may be a strategy
worthy of trial for the functional expansion of MSCs. Leptin, an
important paracrine signaling molecule, may modulate the reciprocal
differentiation of stromal cells between the osteoblastic and
adipogenic pathway, and is associated with aging (28). It increases proliferation,
differentiation toward osteoblasts, and inhibits differentiation to
adipocytes (21). However, some
studies demonstrated that blockade of leptin in MSC cultures
abolished induced senescence through the PI3K/AKT signaling
pathway. Recent findings on glucagon-like peptide-1 (GLP-1) also
indicate that it has effects on the differentiation of MSCs into
osteoblasts and adipocytes. GLP-1 upregulates the activity and mRNA
expression of osteoblast-specific marker, alkaline phosphatase
(ALP) and the mineralization of calcium, while suppressing the
expression of PPARγ, LPL and adipocyte protein 2 (AP2) (29). Factors such as lipoprotein lipase
(LPL), which are involved in the metabolism of fat, also induce the
differentiation of MSCs away from an osteoblast phenotype to
adipocytes (30).
4. ROS accumulation: base of senescence
The free radical theory of aging was first proposed
in 1956 (31). Free radicals are
unstable molecules that have an unpaired electron, including nitric
oxide (NO), several of the reactive oxygen species (ROS), and their
reactive products. ROS include superoxide anions
(O2−), hydrogen peroxide
(H2O2), and hydroxyl radicals (OH•), ~90% of
which are generated in cells by the mitochondrial respiratory chain
(32,33). The NADPH Nox family of oxidases is
another major source of ROS (34). Other extrinsic factors (radiation,
ultraviolet light and growth factors) also cause ROS
production.
Oxidative stress is defined as an imbalance between
the production of free radicals/ROS and antioxidants (35). Superoxide dismutase (SOD),
catalase, peroxiredoxin, thioredoxin and glutathione systems are
known as antioxidative enzymes (36). Among these antioxidants, SOD is a
class of enzymes that catalyzes the dismutation of superoxide into
oxygen and hydrogen peroxide, which plays the most important role.
Studies have found reduced SOD ac tivity along with increasing NO,
ROS, oxidized and glycated protein levels in late-passage MSCs
(37,38).
The markers of stress resistance, heat shock
proteins (HSPs), play a central part in stem cell differentiation
and self-renewal. The responsiveness of MSCs to HSPs decreases with
age. Thus impaired HSPs and stress response lead to reduced
protective mechanisms, proliferation and differentiation capacity
(39). Ataxia telangiectasia
mutated (ATM) serine threonine protein kinase is a critical enzyme
in the regulation of the stress response to DNA damage,
specifically double-strand DNA breaks. Loss-of-function mutations
in ATM are associated with MSC senescence (40).
Nuclear factor erythroid 2-related factor 2 (NRF2)
is a master regulator and transcription factor in response to
oxidative stress, and activates various antioxidant responsive
element (ARE)-dependent genes encoding the above-mentioned
antioxidants. NRF2 has been shown to critically control MSC
survival under oxidative damage. The activity of NRF2 declines
during senescence (32,37) while its activation induces
lifespan extension of MSCs (41).
The PI3K/AKT/mTOR/FOXO3 signaling pathway is a
critical regulator of stress responses. It is reported that in the
presence of high levels of ROS, FOXO3 expression is activated, and
inhibition of FOXO3 results in fewer apoptotic cells and better
viability (42). Moreover, the
p38/MAPK signaling pathways are also responsible for establishing
an irreversible ROS-induced MSC senescence, and suppression of the
p38/MAPK pathway results in partial prevention.
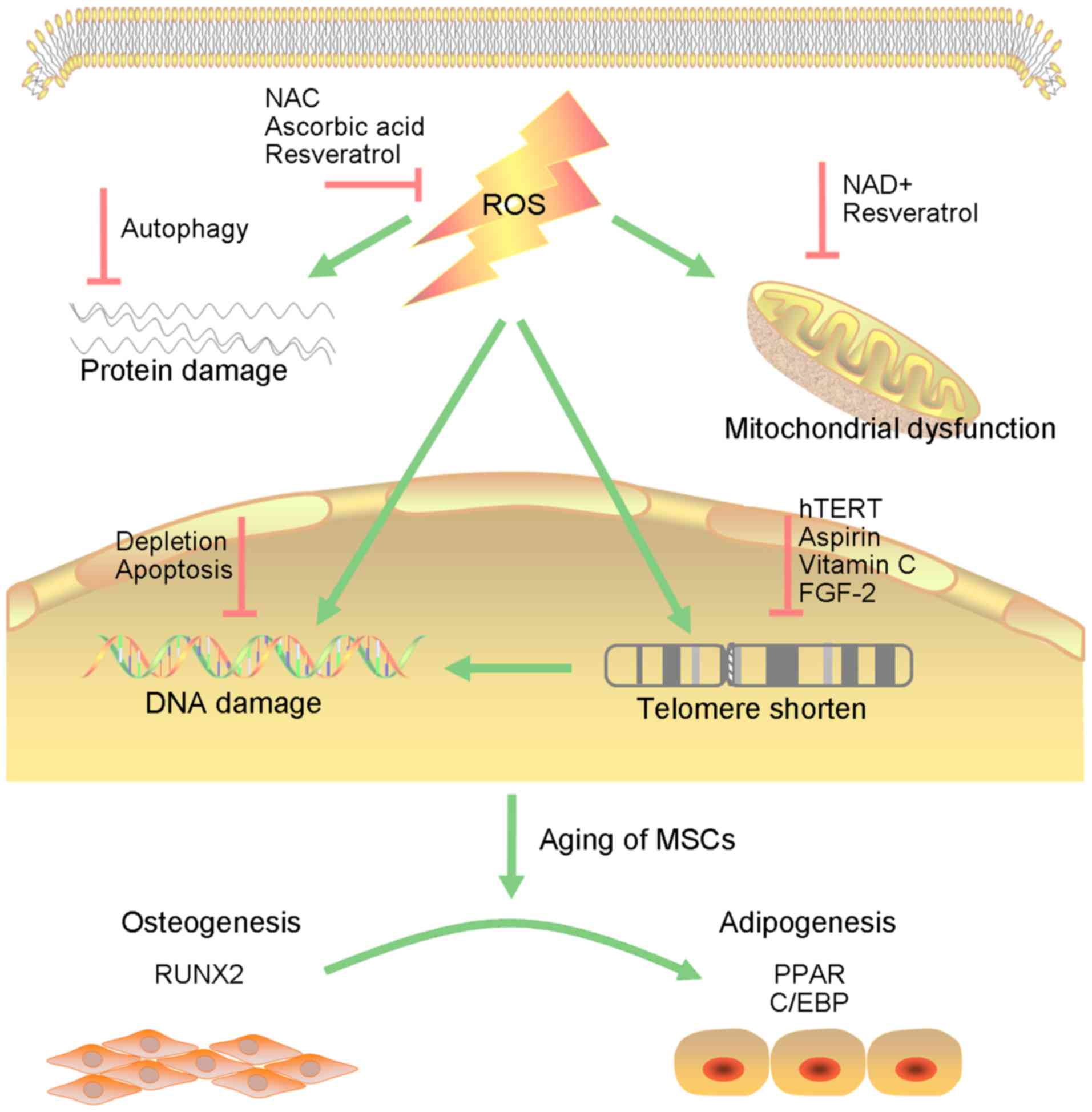
The ongoing oxidative process leads to DNA damage,
protein damage and mitochondrial dysfunction, which are
interconnected and cause reciprocal transformation (Fig. 1). Defects in proteostasis commonly
lead to aberrant folding, toxic aggregation and accumulation of
damaged proteins, which can in turn cause cellular damage and
tissue dysfunction (43). In
brief, ROS accumulation in MSCs contributes to the loss of
homeostasis, leading to senes cence (44).
5. Intrinsic changes in senescent MSCs
Telomere and telomerase
Hayflick and Moorhead uncovered the fact that
primary fibroblasts exhibit only a finite proliferative capacity in
culture, before they exit the cell cycle in a state known as
replicative senescence and the term has been coined the Hayflick
phenomenon. It has been demonstrated that progressive telomeric
erosion associated with the accumulation of DNA damage causes this
phenomenon (45). The in
vitro lifespan of MSCs reportedly ranges from ~20–40 doublings
(46). Telomere shortening is
observed over a lifetime, and it is estimated that every year
telomeres shorten by ~17 bp during aging (47). In MSCs, the average telomere
length in early-passage MSCs depends on the age of the tissue donor
and it has been postulated to range from 10 to 11 kb in cells from
fetal tissues to 7 kb in cells from postnatal sources (48). Other studies have reported longer
telomere lengths varying from 11–13 to 9–10 kb in early MSC
cultures (49,50). Telomere-dependent MSC senescence
has been confirmed. MSCs ultimately cease growth when telomeres
reach the length of 5.8–10.5 kb (51) and DNA damage occurs to the
telomeric DNA (52). Telomerase
prevents telomere erosion and induces telomere elongation by
continuous restitution of the lost TTAGGG repeats at the chromosome
termini (53), but the expression
of telomerase in MSCs is undetectable throughout the entire cell
lifespan (54,55). The function of telomerase may
relate to p53 (56) and TGF-β1
(57), which are both significant
repressors (58). Although
telomere shortening is a hallmark of cell senescence, the length of
telomeres varies from donor to donor, making telomere length an
unreliable measure of MSC senescence. In addition, it can extent
cell lifespan beyond the natural limit upon introduction of the
telomerase catalytic subunit hTERT (59).
Epigenetic changes
Histone deacetylases (HDACs) are a class of enzymes
that catalyze the removal of acetyl groups from the ε-amino group
of lysine residues in the histone tail. HDACs can act as
transcription regulators by sustaining the balance between the
chromatin status of acetylated and deacetylated histones and
regulate stem cell properties. Thus, the DNA status can be
modulated as relaxed euchromatin forms, or condensed
heterochromatin forms (60). MSCs
undergo aging and spontaneous differentiation with the epigenetic
dysregulation of histone H3 acetylation on K9 and K14, without
affecting the methylation of their promoter sites (61). During the progression of MSC
senescence, HDAC inhibitors were found to downregulate polycomb
group genes (PcGs) including BMI1, EZH2and SUZ12, and upregulate
jumonji domain-containing 3 (JMJD3). The expression of EZH2 and
SUZ12 was also regulated by the phosphorylation status of the
retinoblastoma (RB) protein following HDAC inhibitor treatment
(62). HDAC inhibitors also
activate a set of microRNAs (miRNAs) (let-7a1, let-7d, let-7f1,
miR-23a, miR-26a and miR-30a) by altering the histone modification
patterns within the vicinity of miRNA and RNA polymerase coding
regions. Activated miRNAs strongly suppress the translation of high
mobility group A2 (HMGA2), which in turn regulates cellular
senescence genes, including p16INK4a/CDKN2A (63). Sirtuin 1 (SIRT), an NADH-dependent
protein deacetylase, plays a preventive role in many
aging-associated disorders. It has been reported that SIRT1
expression is reduced in senescent MSCs, while its overexpression
delays the onset of senescence and the loss of differentiation
capacity (64).
The DNA methylation status of MSCs is proposed to be
a better method for monitoring aging of MSCs. Gene expression can
be regulated by DNA methylation through interference with
transcription factors or methyl-CpG binding proteins, leading to
the silencing of respective promoter regions (65). A correlation between histone
acetylation and DNA methylation suggests that histone acetylation
possibly determines DNA methylation (66). DNA methyltransferases (DNMTs)
modulate the patterns of polycomb-mediated histone acetylation and
methylation. Expression levels of DNMT1 and DNMT3B are
significantly decreased during the replicative senescence of MSCs,
leading to a decrease in the DNA methylation level, called
hypomethylation, which is a distinct feature of senescent cells. In
contrast, DNMT3a expression was found to be increased during
replicative senescence, participating in the new methylation
associated with senescence. DNMT inhibitors, such as 5-azacytidine,
can upregulate cyclin-dependent kinase (CDK) inhibitors,
p16INK4a/CDKN2A, p21CIP1/WAF1 and miRNAs
targeting EZH1, and the induction of cellular senescence in MSCs
(67).
Gene expression
The INK4a/ARF locus on chromosome 9p21 encodes two
tumor-suppressor proteins, p16INK4a/CDKN2A and
p14/p19ARF, which are involved in growth arrest,
cellular senescence and apoptosis in most mammalian tissues
(68). In MSCs,
p16INK4a/CDKN2A-positive cells show growth retardation
and increased activity of SA-β-gal. Furthermore, transfection of
small interfering RNA (siRNA) targeting p16INK4a/CDKN2A
in senescent MSCs results in the reduction of senescent cells and
the ability of cells to maintain proliferation, suggesting that
p16INK4a/CDKN2A is an important regulator of MSC aging
(69).
HMGA2 encodes a protein that belongs to the
nonhistone chromosomal HMGA protein family. It consists of three
DNA binding domains containing 8–9 amino acids (AA) and shows high
affinity for short AT-rich sequence (70). As an architectural transcription
factor, HMGA2 is involved in gene regulation. HMGA2 overexpression
activates genes related to cell proliferation, such as cyclin A,
cyclin F, cyclin E1 and CD25A (72). It also induces AKT phosphorylation
and its downstream effectors in the mammalian target of rapamycin
(mTOR)/p70S6K pathway, which is a serine/threonine protein kinase
that regulates cell growth, cell proliferation and cell survival
(71). The expression of HMGA2
decreases with age in MSCs, combined with the increased expression
of p16INK4a/CDKN2A, p19ARF,
p21CIP1/WAF1 and p27KIP1 (73).
RB encodes the RB protein, which controls cell cycle
progression from G1 into S phase by binding to E2F and inhibiting
its activity (72). Recently, the
mechanism of RB mediation was shown to play a positive role in
regulating MSC properties by affecting DNA methylation via
upregulation of DNA methyltransferase 1 (DNMT1) (73). In contrast, knockdown of the RB
gene induces senescence (74).
Thus, the RB gene is involved in the control of the MSC properties
of aging and stemness (75).
The LMNA gene encodes nuclear lamina, which is
composed of the A- and B-type lamins. Lamins A and C are members of
the A-type lamins, which are derived from alternative splicing and
are localized to the nuclear envelope and nucleoplasm. Lamins A and
C can regulate important cellular events including DNA replication,
cell division, transcriptional regulation and structural support
(76). Usually, prelamin A, the
precursor of lamin A, is catalyzed by ZMPSTE24/FACE-1, which is a
membrane zinc metalloproteinase (77). Mutation of the LMNA gene produces
progerin, which lacks the cleavage site for ZMPSTE24/FACE-1,
resulting in the accumulation of farnesylated prelamin A. The
accumulation of progerin and prelamin A leads to premature
senescence characterized by nuclear blebbing, heterochromatin
damage of HP1 and LAP2 and defects in DNA replication,
transcription and repair (78).
The introduction of progerin into MSCs interferes with cellular
function by activating the major downstream effectors of the Notch
signaling pathway and alters the differentiation potential
(79). It has been reported that
ZMPSTE24 expression is severely affected by replicative or HDAC
inhibitor-mediated senescence in MSCs (80).
Immunological properties
Many cytokines and growth factors are secreted by
MSCs and self-regulate their proliferation in culture, including
interleukin-1 (IL-1), IL-3, IL-4, IL-6, IL-8, IL-17, epidermal
growth factor (EGF), fibroblast growth factors-2 (FGF-2), FGF-4 and
FGF-8, hepatocyte growth factor (HGF), insulin growth factor-1
(IGF-1), platelet-derived growth factor (PDGF), TGF-β and vascular
endothelial growth factor (VEGF) (81). These factors are called
senescence-associated secretory phenotype (SASP) factors (82), and some are crucial to MSC
senescence as well. In addition, SASP mediated-inflammatory factors
are also interconnected with inflammatory process. For example, in
the presence of an inflammatory environment [high levels of tumor
necrosis factor-α (TNF-α) and interferon-γ (IFN-γ)], MSCs become
activated through Toll-like receptor (TLR)4 (83) and adopt an immune-suppressive
phenotype by secreting high levels of soluble factors, including
IDO, PGE2, NO, TGF-β, HGF and hemoxygenase (HO) (84). Moreover, TNF-α and IFN-γ activate
CD106 expression in senescent MSCs. HO-1 degrades production of
hemebiliverdin and carbon monoxide and exerts strong positive
effects on osteogenesis while suppressing adipogenesis in MSCs
(85). Thus, it plays a key
factor as a stress-responsive, cytoprotective and immunoregulatory
molecule.
The employment of exogenous TGF-β expression was
found to trigger premature cell senescence in MSCs confirming the
role of TGF-β, with concomitant activation of p16INK4a
and p21CIP1 checkpoints (86). Analysis of the gene expression
profile in senescent MSCs has shown that TGF-β is increased in a
dose-dependent manner (87). This
effect coincided with upregulated SMAD3, a major signaling molecule
for TGF-β. Inhibition of TGF-β receptor signaling was found to
promote the culture expansion of undifferentiatied MSCs (88).
6. Strategies to prevent senescence
For the use of MSCs in therapy, methods that allow
the generation of large populations of MSCs without affecting their
properties of differentiation or immunomodulation need to be
established. The information described in this review may suggest a
possible method to improve the therapeutic efficacy by regulating
specific factors or the microenvironment associated with the MSC
aging process. A number of senescent suppressors that function in
the damage response or defense against oxidative stress are
involved in modulation of the lifespan of human primary cells. Some
of these suppressors, when overexpressed in human MSCs, induce
substantial expansion of the proliferative potential and delay
replicative senescence to an extent worthy of practical
consideration.
The antioxidant N-acetyl-L-cysteine (NAC), a
precursor of glutathione and a direct ROS scavenger, has been used
as a therapeutic agent to ameliorate the damaging effects of ROS
(89). Other antioxidants such as
ascorbic acid and inhibitors of p38/MAPK or mTOR can markedly
improve ROS-mediated injury in MSCs and lead to full recovery
(90). NRF2 activation may also
be an alternatively excellent strategy to extend the lifespan of
BMSCs (91).
The introduction of hTERT into MSCs resulted in a
substantial multiplication of their replicative lifespan
accompanied by the preservation of a normal karyotype, elongation
of telomeres and loss of the senescent phenotype without impact on
differentiation ability (92,93). Several small molecular compounds,
such as aspirin and vitamin C, as well as FGF-2, have been
developed to activate the endogenous telomerase of MSCs, achieving
similar effects of improved proliferative and osteogenic potential
in recent research (94).
However, this is ill-advised for clinical applications given the
small but possible risk of malignant transformation.
Genetic engineering of cells is one possible
approach for preventing MSC aging (95). Knockdown of
p16INK4a/CDKN2A (96)
or silencing of RB (75) in MSCs
rescues the senescent phenotype and increases the proliferation
rate and clonogenicity. However, silencing of these
tumor-suppressor-genes disrupts differentiation potential and
increases tumorigenesis risks. Knockdown or silencing of miR-195
significantly increases hTERT, phosphorylation of AKT and FOXO3
expression and induces telomere relengthening in senescent MSCs
(97).
Selective growth factors have also been used to
maintain the self-renewal and differentiation potential of MSCs.
For instance, employing exogenous FGF-2, PDGF and EGF has been
reported to increase proliferation ability and delay MSC
senescence, without affecting osteogenesis and adipogenesis
(98) for therapeutic use.
7. Conclusion
Recent studies have demonstrated promising results
for the therapeutic utilization of MSCs in regenerative medicine.
They show strong advantages and ability in cell therapy. However,
the number of MSCs is usually 108–1010 for
cell transplantation, which means a single cell in 105
primary MSCs has to undergo 17 doublings and is evitable to consume
its potential. The proliferative and functional activity of MSCs is
destined to decline during the process of senescence, hindering the
preparation of sufficient cells for clinical application. This
calls for the investigation of the mechanisms of senescence and the
development of efficient means to reverse it. This review provides
a better understanding of the underlying mechanisms and
significance of cellular senescence, facilitating ways to
manipulate the replicative lifespan of MSCs. First, histological
markers such as SA-β-gal and CFU-F, CD106 and STRO-1, as well as
differentiation potential of osteogenesis and adipogen-esis are
used in detecting MSC aging and senescence. Second, the mechanisms
of free radicals and oxidative stress on MSC senescence are
illustrated. ROS and the consequent oxidative stress is at the base
of aging. It leads to DNA damage, protein damage and mitochondrial
dysfunction, which triggers the inner process of senescence,
including changes in HDAC and DNA methyltransferase, imbalance of
telomere and telomerase, genes and signaling pathways, as well as
the secretory phenotype. Considering the above aspects, these
strategies could be further investigated to prevent or reverse the
MSC aging process.
Acknowledgments
This study was supported by the National Natural
Scientific Foundations of China (no. 81200315).
References
|
1
|
Payushina O, Domaratskaya E and Starostin
V: Mesenchymal stem cells: Sources, phenotype, and differentiation
potential. Biol Bull. 33:2–18. 2006. View Article : Google Scholar
|
|
2
|
Musina RA, Bekchanova ES and Sukhikh GT:
Comparison of mesenchymal stem cells obtained from different human
tissues. Bull Exp Biol Med. 139:504–509. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mareschi K, Ferrero I, Rustichelli D,
Aschero S, Gammaitoni L, Aglietta M, Madon E and Fagioli F:
Expansion of mesenchymal stem cells isolated from pediatric and
adult donor bone marrow. J Cell Biochem. 97:744–754. 2006.
View Article : Google Scholar
|
|
4
|
Zhang B, Liu R, Shi D, Liu X, Chen Y, Dou
X, Zhu X, Lu C, Liang W, Liao L, et al: Mesenchymal stem cells
induce mature dendritic cells into a novel Jagged-2–dependent
regulatory dendritic cell population. Blood. 113:46–57. 2009.
View Article : Google Scholar
|
|
5
|
Trivedi P and Hematti P: Derivation and
immunological characterization of mesenchymal stromal cells from
human embryonic stem cells. Exp Hematol. 36:350–359.
2008.PubMed/NCBI
|
|
6
|
Anzalone R, Lo Iacono M, Corrao S, Magno
F, Loria T, Cappello F, Zummo G, Farina F and La Rocca G: New
emerging potentials for human Wharton's jelly mesenchymal stem
cells: immunological features and hepatocyte-like differentiative
capacity. Stem Cells Dev. 19:423–438. 2010. View Article : Google Scholar
|
|
7
|
Francese R and Fiorina P: Immunological
and regenerative properties of cord blood stem cells. Clin Immunol.
136:309–322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Patel DM, Shah J and Srivastava AS:
Therapeutic potential of mesenchymal stem cells in regenerative
medicine. Stem Cells Int. 2013:4962182013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rubin H: Promise and problems in relating
cellular senescence in vitro to aging in vivo. Arch Gerontol
Geriatr. 34:275–286. 2002. View Article : Google Scholar
|
|
10
|
Digirolamo CM, Stokes D, Colter D, Phinney
DG, Class R and Prockop DJ: Propagation and senescence of human
marrow stromal cells in culture: A simple colony-forming assay
identifies samples with the greatest potential to propagate and
differentiate. Br J Haematol. 107:275–281. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Campisi J and d'Adda di Fagagna F:
Cellular senescence: When bad things happen to good cells. Nat Rev
Mol Cell Biol. 8:729–740. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stenderup K, Justesen J, Clausen C and
Kassem M: Aging is associated with decreased maximal life span and
accelerated senescence of bone marrow stromal cells. Bone.
33:919–926. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou S, Greenberger JS, Epperly MW, Goff
JP, Adler C, Leboff MS and Glowacki J: Age-related intrinsic
changes in human bone-marrow-derived mesenchymal stem cells and
their differentiation to osteoblasts. Aging Cell. 7:335–343. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singh M and Piekorz RP:
Senescence-associated lysosomal α-L-fucosidase (SA-α-Fuc): A
sensitive and more robust biomarker for cellular senescence beyond
SA-β-Gal. Cell Cycle. 12:19962013. View
Article : Google Scholar
|
|
15
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop DJ and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu Y, Park YS, Kim HS, Kim HY, Jin YM,
Jung SC, Ryu KH and Jo I: Characterization of long-term in vitro
culture-related alterations of human tonsil-derived mesenchymal
stem cells: Role for CCN1 in replicative senescence-associated
increase in osteogenic differentiation. J Anat. 225:510–518. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Simmons PJ and Torok-Storb B:
Identification of stromal cell precursors in human bone marrow by a
novel monoclonal antibody, STRO-1. Blood. 78:55–62. 1991.PubMed/NCBI
|
|
18
|
Jung EM, Kwon O, Kwon KS, Cho YS, Rhee SK,
Min JK and Oh DB: Evidences for correlation between the reduced
VCAM-1 expression and hyaluronan synthesis during cellular
senescence of human mesenchymal stem cells. Biochem Biophys Res
Commun. 404:463–469. 2011. View Article : Google Scholar
|
|
19
|
Lv FJ, Tuan RS, Cheung KM and Leung VY:
Concise review: The surface markers and identity of human
mesenchymal stem cells. Stem Cells. 32:1408–1419. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Laschober GT, Brunauer R, Jamnig A, Fehrer
C, Greiderer B and Lepperdinger G: Leptin receptor/CD295 is
upregulated on primary human mesenchymal stem cells of advancing
biological age and distinctly marks the subpopulation of dying
cells. Exp Gerontol. 44:57–62. 2009. View Article : Google Scholar
|
|
21
|
Astudillo P, Ríos S, Pastenes L, Pino AM
and Rodríguez JP: Increased adipogenesis of osteoporotic
humanmesenchymal stem cells (MSCs) characterizes by impaired leptin
action. J Cell Biochem. 103:1054–1065. 2008. View Article : Google Scholar
|
|
22
|
Atashi F, Modarressi A and Pepper MS: The
role of reactive oxygen species in mesenchymal stem cell adipogenic
and osteogenic differentiation: A review. Stem Cells Dev.
24:1150–1163. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu J, Qian J, Xie X, Lin L, Zou Y, Fu M,
Huang Z, Zhang G, Su Y and Ge J: High density lipoprotein protects
mesenchymal stem cells from oxidative stress-induced apoptosis via
activation of the PI3K/Akt pathway and suppression of reactive
oxygen species. Int J Mol Sci. 13:17104–17120. 2012. View Article : Google Scholar
|
|
24
|
Jiang Y, Mishima H, Sakai S, Liu YK,
Ohyabu Y and Uemura T: Gene expression analysis of major
lineage-defining factors in human bone marrow cells: effect of
aging, gender, and age-related disorders. J Orthop Res. 26:910–917.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Komori T: Signaling networks in
RUNX2-dependent bone development. J Cell Biochem. 112:750–755.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tu B, Peng ZX, Fan QM, Du L, Yan W and
Tang TT: Osteosarcoma cells promote the production of pro-tumor
cytokines in mesenchymal stem cells by inhibiting their osteogenic
differentiation through the TGF-β/Smad2/3 pathway. Exp Cell Res.
320:164–173. 2014. View Article : Google Scholar
|
|
27
|
Xu C, Wang J, Zhu T, Shen Y, Tang X, Fang
L and Xu Y: Cross-talking between PPAR and WNT signaling and its
regulation in mesenchymal stem cell differentiation. Curr Stem Cell
Res Ther. 11:247–254. 2016. View Article : Google Scholar
|
|
28
|
Isidori AM, Strollo F, Morè M, Caprio M,
Aversa A, Moretti C, Frajese G, Riondino G and Fabbri A: Leptin and
aging: Correlation with endocrine changes in male and female
healthy adult populations of different body weights. J Clin
Endocrinol Metab. 85:1954–1962. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee HM, Joo BS, Lee CH, Kim HY, Ock JH and
Lee YS: Effect of glucagon-like peptide-1 on the differentiation of
adipose-derived stem cells into osteoblasts and adipocytes. J
Menopausal Med. 21:93–103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stringer B, Waddington R, Houghton A,
Stone M, Russell G and Foster G: Serum from postmenopausal women
directs differentiation of human clonal osteoprogenitor cells from
an osteoblastic toward an adipocytic phenotype. Calcif Tissue Int.
80:233–243. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Harman D: Aging: a theory based on free
radical and radiation chemistry. J Gerontol. 11:298–300. 1956.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Poyton RO, Ball KA and Castello PR:
Mitochondrial generation of free radicals and hypoxic signaling.
Trends Endocrinol Metab. 20:332–340. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Balaban RS, Nemoto S and Finkel T:
Mitochondria, oxidants, and aging. Cell. 120:483–495. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bedard K and Krause K-H: The NOX family of
ROS-generating NADPH oxidases: Physiology and pathophysiology.
Physiol Rev. 87:245–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Reuter S, Gupta SC, Chaturvedi MM and
Aggarwal BB: Oxidative stress, inflammation, and cancer: How are
they linked? Free Radic Biol Med. 49:1603–1616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Boutten A, Goven D, Boczkowski J and Bonay
M: Oxidative stress targets in pulmonary emphysema: Focus on the
Nrf2 pathway. Expert Opin Ther Targets. 14:329–346. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jeong SG and Cho GW: Endogenous ROS levels
are increased in replicative senescence in human bone marrow
mesenchymal stromal cells. Biochem Biophys Res Commun. 460:971–976.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Benameur L, Charif N, Li Y, Stoltz JF and
de Isla N: Toward an understanding of mechanism of aging-induced
oxidative stress in human mesenchymal stem cells. Biomed Mater Eng.
25(Suppl 1): 41–46. 2015.
|
|
39
|
Stolzing A, Jones E, McGonagle D and Scutt
A: Age-related changes in human bone marrow-derived mesenchymal
stem cells: consequences for cell therapies. Mech Ageing Dev.
129:163–173. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tsai WB, Chung YM, Takahashi Y, Xu Z and
Hu MC: Functional interaction between FOXO3a and ATM regulates DNA
damage response. Nat Cell Biol. 10:460–467. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Milani P, Ambrosi G, Gammoh O, Blandini F
and Cereda C: SOD1 and DJ-1 converge at Nrf2 pathway: a clue for
antioxidant therapeutic potential in neurodegeneration. Oxid Med
Cell Longev. 2013:8367602013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang F, Cui J, Liu X, Lv B, Liu X, Xie Z
and Yu B: Roles of microRNA-34a targeting SIRT1 in mesenchymal stem
cells. Stem Cell Res Ther. 6:1952015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bucciantini M, Giannoni E, Chiti F, Baroni
F, Formigli L, Zurdo J, Taddei N, Ramponi G, Dobson CM and Stefani
M: Inherent toxicity of aggregates implies a common mechanism for
protein misfolding diseases. Nature. 416:507–511. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Passos JF, Saretzki G, Ahmed S, Nelson G,
Richter T, Peters H, Wappler I, Birket MJ, Harold G, Schaeuble K,
et al: Mitochondrial dysfunction accounts for the stochastic
heterogeneity in telomere-dependent senescence. PLoS Biol.
5:e1102007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hayflick L and Moorhead PS: The serial
cultivation of human diploid cell strains. Exp Cell Res.
25:585–621. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hwang ES: Senescence suppressors: Their
practical importance in replicative lifespan extension in stem
cells. Cell Mol Life Sci. 71:4207–4219. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Harley CB, Futcher AB and Greider CW:
Telomeres shorten during ageing of human fibroblasts. Nature.
345:458–460. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Guillot PV, Gotherstrom C, Chan J, Kurata
H and Fisk NM: Human first-trimester fetal MSC express pluripotency
markers and grow faster and have longer telomeres than adult MSC.
Stem Cells. 25:646–654. 2007. View Article : Google Scholar
|
|
49
|
Bonab MM, Alimoghaddam K, Talebian F,
Ghaffari SH, Ghavamzadeh A and Nikbin B: Aging of mesenchymal stem
cell in vitro. BMC Cell Biol. 7:142006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Parsch D, Fellenberg J, Brümmendorf TH,
Eschlbeck AM and Richter W: Telomere length and telomerase activity
during expansion and differentiation of human mesenchymal stem
cells and chondrocytes. J Mol Med (Berl). 82:49–55. 2004.
View Article : Google Scholar
|
|
51
|
Baxter MA, Wynn RF, Jowitt SN, Wraith JE,
Fairbairn LJ and Bellantuono I: Study of telomere length reveals
rapid aging of human marrow stromal cells following in vitro
expansion. Stem Cells. 22:675–682. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Raz V, Vermolen BJ, Garini Y, Onderwater
JJ, Mommaas-Kienhuis MA, Koster AJ, Young IT, Tanke H and Dirks RW:
The nuclear lamina promotes telomere aggregation and centromere
peripheral localization during senescence of human mesenchymal stem
cells. J Cell Sci. 121:4018–4028. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Masutomi K, Yu EY, Khurts S, Ben-Porath I,
Currier JL, Metz GB, Brooks MW, Kaneko S, Murakami S, DeCaprio JA,
et al: Telomerase maintains telomere structure in normal human
cells. Cell. 114:241–253. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Graakjaer J, Christensen R, Kolvraa S and
Serakinci N: Mesenchymal stem cells with high telomerase expression
do not actively restore their chromosome arm specific telomere
length pattern after exposure to ionizing radiation. BMC Mol Biol.
8:492007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ryu E, Hong S, Kang J, Woo J, Park J, Lee
J and Seo JS: Identification of senescence-associated genes in
human bone marrow mesenchymal stem cells. Biochem Biophys Res
Commun. 371:431–436. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Serakinci N, Christensen R, Graakjaer J,
Cairney CJ, Keith WN, Alsner J, Saretzki G and Kolvraa S:
Ectopically hTERT expressing adult human mesenchymal stem cells are
less radio-sensitive than their telomerase negative counterpart.
Exp Cell Res. 313:1056–1067. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sawada R, Ito T and Tsuchiya T: Changes in
expression of genes related to cell proliferation in human
mesenchymal stem cells during in vitro culture in comparison with
cancer cells. J Artif Organs. 9:179–184. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li H, Xu D, Li J, Berndt MC and Liu JP:
Transforming growth factor β suppresses human telomerase reverse
transcriptase (hTERT) by Smad3 interactions with c-Myc and the
hTERT gene. J Biol Chem. 281:25588–25600. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bodnar AG, Ouellette M, Frolkis M, Holt
SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S and
Wright WE: Extension of life-span by introduction of telomerase
into normal human cells. Science. 279:349–352. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sengupta N and Seto E: Regulation of
histone deacetylase activities. J Cell Biochem. 93:57–67. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Li Z, Liu C, Xie Z, Song P, Zhao RC, Guo
L, Liu Z and Wu Y: Epigenetic dysregulation in mesenchymal stem
cell aging and spontaneous differentiation. PLoS One. 6:e205262011.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Jung JW, Lee S, Seo MS, Park SB, Kurtz A,
Kang SK and Kang KS: Histone deacetylase controls adult stem cell
aging by balancing the expression of polycomb genes and jumonji
domain containing 3. Cell Mol Life Sci. 67:1165–1176. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lee S, Jung JW, Park SB, Roh K, Lee SY,
Kim JH, Kang SK and Kang KS: Histone deacetylase regulates high
mobility group A2-targeting microRNAs in human cord blood-derived
multi-potent stem cell aging. Cell Mol Life Sci. 68:325–336. 2011.
View Article : Google Scholar
|
|
64
|
Yuan H-F, Zhai C, Yan X-L, Zhao DD, Wang
JX, Zeng Q, Chen L, Nan X, He LJ, Li ST, et al: SIRT1 is required
for long-term growth of human mesenchymal stem cells. J Mol Med
(Berl). 90:389–400. 2012. View Article : Google Scholar
|
|
65
|
Jaenisch R and Bird A: Epigenetic
regulation of gene expression: How the genome integrates intrinsic
and environmental signals. Nat Genet. 33(Suppl): 245–254. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cervoni N and Szyf M: Demethylase activity
is directed by histone acetylation. J Biol Chem. 276:40778–40787.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
So AY, Jung JW, Lee S, Kim HS and Kang KS:
DNA methyl-transferase controls stem cell aging by regulating BMI1
and EZH2 through microRNAs. PLoS One. 6:e195032011. View Article : Google Scholar
|
|
68
|
Rayess H, Wang MB and Srivatsan ES:
Cellular senescence and tumor suppressor gene p16. Int J Cancer.
130:1715–1725. 2012. View Article : Google Scholar :
|
|
69
|
Shibata KR, Aoyama T, Shima Y, Fukiage K,
Otsuka S, Furu M, Kohno Y, Ito K, Fujibayashi S, Neo M, et al:
Expression of the p16INK4A gene is associated closely with
senescence of human mesenchymal stem cells and is potentially
silenced by DNA methylation during in vitro expansion. Stem Cells.
25:2371–2382. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Grosschedl R, Giese K and Pagel J: HMG
domain proteins: Architectural elements in the assembly of
nucleoprotein structures. Trends Genet. 10:94–100. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yu KR, Park SB, Jung JW, Seo MS, Hong IS,
Kim HS, Seo Y, Kang TW, Lee JY, Kurtz A, et al: HMGA2 regulates the
in vitro aging and proliferation of human umbilical cord
blood-derived stromal cells through the mTOR/p70S6K signaling
pathway. Stem Cell Res (Amst). 10:156–165. 2013. View Article : Google Scholar
|
|
72
|
Li Y, Nichols MA, Shay JW and Xiong Y:
Transcriptional repression of the D-type cyclin-dependent kinase
inhibitor p16 by the retinoblastoma susceptibility gene product
pRb. Cancer Res. 54:6078–6082. 1994.PubMed/NCBI
|
|
73
|
Lin SP, Chiu FY, Wang Y, Yen ML, Kao SY
and Hung SC: RB maintains quiescence and prevents premature
senescence through upregulation of DNMT1 in mesenchymal stromal
cells. Stem Cell Rep. 3:975–986. 2014. View Article : Google Scholar
|
|
74
|
Alessio N, Bohn W, Rauchberger V, Rizzolio
F, Cipollaro M, Rosemann M, Irmler M, Beckers J, Giordano A and
Galderisi U: Silencing of RB1 but not of RB2/P130 induces cellular
senescence and impairs the differentiation potential of human
mesenchymal stem cells. Cell Mol Life Sci. 70:1637–1651. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Galderisi U, Cipollaro M and Giordano A:
The retinoblastoma gene is involved in multiple aspects of stem
cell biology. Oncogene. 25:5250–5256. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hutchison CJ and Worman HJ: A-type lamins:
Guardians of the soma? Nat Cell Biol. 6:1062–1067. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Corrigan DP, Kuszczak D, Rusinol AE,
Thewke DP, Hrycyna CA, Michaelis S and Sinensky MS: Prelamin A
endoproteolytic processing in vitro by recombinant Zmpste24.
Biochem J. 387:129–138. 2005. View Article : Google Scholar :
|
|
78
|
Liu B, Wang J, Chan KM, Tjia WM, Deng W,
Guan X, Huang JD, Li KM, Chau PY, Chen DJ, et al: Genomic
instability in laminopathy-based premature aging. Nat Med.
11:780–785. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
79
|
Scaffidi P and Misteli T: Lamin
A-dependent misregulation of adult stem cells associated with
accelerated ageing. Nat Cell Biol. 10:452–459. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Yu KR and Kang KS: Aging-related genes in
mesenchymal stem cells: A mini-review. Gerontology. 59:557–563.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ksiazek K: A comprehensive review on
mesenchymal stem cell growth and senescence. Rejuvenation Res.
12:105–116. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Rodier F and Campisi J: Four faces of
cellular senescence. J Cell Biol. 192:547–556. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Liotta F, Angeli R, Cosmi L, Filì L,
Manuelli C, Frosali F, Mazzinghi B, Maggi L, Pasini A, Lisi V, et
al: Toll-like receptors 3 and 4 are expressed by human bone
marrow-derived mesenchymal stem cells and can inhibit their T-cell
modulatory activity by impairing Notch signaling. Stem Cells.
26:279–289. 2008. View Article : Google Scholar
|
|
84
|
Trento C and Dazzi F: Mesenchymal stem
cells and innate tolerance: Biology and clinical applications.
Swiss Med Wkly. 140:w131212010.PubMed/NCBI
|
|
85
|
Barbagallo I, Vanella A, Peterson SJ, Kim
DH, Tibullo D, Giallongo C, Vanella L, Parrinello N, Palumbo GA, Di
Raimondo F, et al: Overexpression of heme oxygenase-1 increases
human osteoblast stem cell differentiation. J Bone Miner Metab.
28:276–288. 2010. View Article : Google Scholar
|
|
86
|
Ito T, Sawada R, Fujiwara Y, Seyama Y and
Tsuchiya T: FGF-2 suppresses cellular senescence of human
mesenchymal stem cells by down-regulation of TGF-beta2. Biochem
Biophys Res Commun. 359:108–114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wu J, Niu J, Li X, Wang X, Guo Z and Zhang
F: TGF-β1 induces senescence of bone marrow mesenchymal stem cells
via increase of mitochondrial ROS production. BMC Dev Biol.
14:212014. View Article : Google Scholar
|
|
88
|
Gurung S, Werkmeister JA and Gargett CE:
Inhibition of transforming growth factor-β receptor signaling
promotes culture expansion of undifferentiated human endometrial
mesenchymal stem/stromal cells. Sci Rep. 5:150422015. View Article : Google Scholar
|
|
89
|
Lin TM, Tsai JL, Lin SD, Lai CS and Chang
CC: Accelerated growth and prolonged lifespan of adipose
tissue-derived human mesenchymal stem cells in a medium using
reduced calcium and antioxidants. Stem Cells Dev. 14:92–102. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Choi KM, Seo YK, Yoon HH, Song KY, Kwon
SY, Lee HS and Park JK: Effect of ascorbic acid on bone
marrow-derived mesenchymal stem cell proliferation and
differentiation. J Biosci Bioeng. 105:586–594. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Su ZY, Shu L, Khor TO, Lee JH, Fuentes F
and Kong AN: A perspective on dietary phytochemicals and cancer
chemo-prevention: oxidative stress, nrf2, and epigenomics. Top Curr
Chem. 329:133–162. 2013. View Article : Google Scholar
|
|
92
|
Takeuchi M, Takeuchi K, Kohara A, Satoh M,
Shioda S, Ozawa Y, Ohtani A, Morita K, Hirano T, Terai M, et al:
Chromosomal instability in human mesenchymal stem cells
immortalized with human papilloma virus E6, E7, and hTERT genes. In
Vitro Cell Dev Biol Anim. 43:129–138. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Simonsen JL, Rosada C, Serakinci N,
Justesen J, Stenderup K, Rattan SI, Jensen TG and Kassem M:
Telomerase expression extends the proliferative life-span and
maintains the osteogenic potential of human bone marrow stromal
cells. Nat Biotechnol. 20:592–596. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Wei F, Qu C, Song T, Ding G, Fan Z, Liu D,
Liu Y, Zhang C, Shi S and Wang S: Vitamin C treatment promotes
mesenchymal stem cell sheet formation and tissue regeneration by
elevating telomerase activity. J Cell Physiol. 227:3216–3224. 2012.
View Article : Google Scholar :
|
|
95
|
Gharibi B, Farzadi S, Ghuman M and Hughes
FJ: Inhibition of Akt/mTOR attenuates age-related changes in
mesenchymal stem cells. Stem Cells. 32:2256–2266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Gu Z, Cao X, Jiang J, Li L, Da Z, Liu H
and Cheng C: Upregulation of p16INK4A promotes cellular senescence
of bone marrow-derived mesenchymal stem cells from systemic lupus
erythematosus patients. Cell Signal. 24:2307–2314. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Okada M, Kim HW, Matsu-Ura K, Wang YG, Xu
M and Ashraf M: Abrogation of age-induced MicroRNA-195 rejuvenates
the senescent mesenchymal stem cells by reactivating telomerase.
Stem Cells. 34:148–159. 2016. View Article : Google Scholar :
|
|
98
|
Gharibi B and Hughes FJ: Effects of medium
supplements on proliferation, differentiation potential, and in
vitro expansion of mesenchymal stem cells. Stem Cells Transl Med.
1:771–782. 2012. View Article : Google Scholar : PubMed/NCBI
|