Introduction
Constipation is one of the most frequent health
afflications which causes discomfort and affects quality of life of
patients (1). A relatively large
number of subjects in the general population (ranging from
approximately 9% to more than 20%, depending on the geographical
area) is affected by this highly prevalent functional
gastrointestinal disorder (2). It
is a complex symptom and increases during aging. Constipation can
cause not only discomfort, but also restlessness, abdominal
distension, vomiting, gut obstruction and perforation, and even
aspiration or fatal pulmonary embolism (3). Slow-transit constipation (STC) is
the most common type of chronic constipation (4), which is a motility disorder
characterized by markedly increased total bowel transit time
(5). The pathogenesis of
constipation remains largely unknown. Earlier investigations have
focused on the quantitative alternations of the interstitial cells
of Cajal (ICCs), the pacemaker of the gastrointestinal tract. The
complete absence or a significant reduction in the number of ICCs
in colon specimens resected from patients with STC has been
reporeted in comparison with normal controls (6). In addition, a number of other
pathophysiological abnormalities, including the degeneration of the
myenteric plexus ganglia, the aberrant expression of smoothelin
protein, and abnormalities of the enteric neurotransmitters may be
associated with constipation (7,8).
However, the mechanisms through which these numerous abnormalities
contribute to the pathogenesis of constipation remain to be further
elucidated.
Naringenin (NAR), a natural flavonoid widely found
in citrus fruits and tomatoes, has been reported to exhibit various
pharmacological effects, such as anti-inflammatory,
anti-atherogenic, anti-mutagenic, hepatoprotective and anticancer
effects (9,10). There is increasing evidence to
indicate that NAR has potential for use in the treatment of
constipation. Yang et al reported that NAR stimulated
Cl− secretion in the colonic epithelium via a signaling
pathway involving cyclic adenosine monophosphate (cAMP) and protein
kinase A (PKA) (11). However,
the precise effects of NAR on constipation have not been
investigated thus far, at least to the best of our knowledge.
In this study, we examined the effect of NAR on
loperamide (Lop)-induced constipation in rats, indicating its
therapeutic potential for the treatment of constipation and other
related diseases.
Materials and methods
Ethics statement
This study was performed in strict accordance with
the recommendations of the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health. The animal protocol
used in this study was reviewed and approved by the Institutional
Animal Care and Use Committee (IACUC) according to the National
Institutes of Health Guidelines (permit no. A27-011-0). Mice were
anaesthetized with 50 mg/kg pentobarbital sodium during the
necessary operations, and every effort was made to minimize
suffering.
Experimental animals
Adult ICR mice were purchased from Charles River
Ltd. (Beijing, China). All mice were provided with standard
irradiated chow diet ad libitum and maintained in a specific
pathogen-free state under a strict light cycle (12/12-h) at a
temperature of 22±2°C and a relative humidity of 50±10%.
Induction of constipation and
experimental design
Constipation was induced in ICR male mice (8 weeks
old, 18–22 g) by the intragastric administration of Lop (Xi'an
Janssen Pharmaceutical Co., Ltd., Shanxi, China) (3 mg/kg weight)
in sterilized physiological saline once a day for 5 days, whereas
the non-constipation group was administered sterilized
physiological saline alone. For the animal experiment, the ICR mice
were assigned to either a non-constipation group (negative control
group) or a constipation group.
For the experiment, the ICR mice were divided into
the following groups: i) the negative control group (No group, n=6)
in which mice were treated with a consistent volume of sterilized
physiological saline; ii) the Lop + Vehicle group (n=6) in which
mice were treated with Lop and the vehicle at the same volume as
the NAR-treated groups; iii) different NAR-treated groups including
the Lop + NAR75 (n=6), Lop + NAR150 (n=6) and Lop + NAR300 (n=6)
groups, in which mice first were treated with Lop and 1 h later
treatment with 75, 150 and 300 mg/kg body weight of NAR (Dalian
Meilun Biotech Co., Ltd., Dalian, China) once a day for 5 days. The
Lop + Vehicle group also received a consistent volume of sterilized
physiological saline via gavage following treatment with Lop. At 5
days after NAR treatment, all animals were sacrificed and tissue
samples were acquired and stored in Eppendorf tubes at −70°C until
assay.
Measurement of fecal pellet parameters
and body weight
The excreted fecal pellets of individual mice were
collected at day 6 after the induction of constipation for 5 days.
The total number, weight and water content of the pellets were
determined. The water content was calculated as the difference
between the wet and dry weights of the pellet. Stool and body
weight was weighed 3 times per sample using an electric
balance.
Measurement of intestinal charcoal
transit ratio
The animals were fasted prior to the experiment, but
consumed water ad libitum. At day 6 after the induction of
constipation for 5 days, the animals were fed charcoal meal (1
mg/100 kg weight) (5% suspension of activated charcoal in 10% gum
arabic; Sigma-Aldrich, St. Louis, MO, USA). At 30 min after the
charcoal meal administration, the animals were first sacrified by
cervical dislocation and the abdominal cavity was then opened. The
intestines were removed and the total intestine length (from
pyloric sphincter to cecum) and charcoal meal transit distance were
measured. The intestinal charcoal transit ratio was calculated as
follows: charcoal transit ratio (%) = (distance travelled by the
charocal)/(total length of small intestine) ×100%.
Histological analysis
The proximal colons collected from the ICR mice were
fixed with 4% paraformaldehyde (Sinopharm Group Co., Ltd.,
Shanghai, China) for 30 min, embedded in paraffin wax, and then
sectioned into 5-µm-thick slices that were stained with
H&E (Hematoxylin, Solarbio Co., Ltd., Beijing, China; Eosin,
Sinopharm Group Co., Ltd.). Morphological features of these
sections were observed under a light microscope (DP73; Olympus,
Tokyo, Japan).
Real-time PCR
The proximal colons were chopped with scissors and
total RNA was extracted using a high-purity total RNA extraction
kit (RP1201; BioTeke Co., Ltd., Beijing, China) according to the
manufacturer's instructions. Gene expression was then examined by
RT-PCR using total RNA from each tissue. The complementary DNA
(cDNA) was synthesized from total RNA using a Super M-MLV reverse
transcriptase kit (PR6502; BioTeke Co., Ltd.). The reverse
transcription products were then amplified for PCR. Primers used in
RT-PCR are listed in Table I.
Forward primer, reverse primer and template cDNA were prepared; the
reaction conditions for reverse transcriptoin were as follows: 25°C
for 10 min, 42°C for 50 min and 95°C for 5 min. The PCR
amplification conditions were: 95°C for 10 min, 40 cycles of 95°C
for 10 sec, 60°C for 20 sec and 72°C for 30 sec, then 4°C for 5
min. The PCR results were verified by varying the number of PCR
cycles for each cDNA and set of primers. PCR reaction was performed
using Exicycler™ 96 (Bioneer Co., Daejeon, Korea) with β-actin as a
control. RT-PCR was performed at least in quadruplicate.
 | Table IThe primer sequences used in this
study. |
Table I
The primer sequences used in this
study.
| Genes | Primer
sequences |
|---|
| NOS | F:
5′-TCAGCGGTGATAGGATAAAGCA-3′ |
| R:
5′-CGCTGTGCTAAGTAGCCCTCG-3′-3′' |
| TRPV1 | F:
5′-TCTCGTGGAGCCCTTGAACCG-3′ |
| R:
5′-CCGATAGTAAGCAGCCGTGGT-3′ |
| GDNF | F:
5′-GACGCTTGGTGGTTGATTCTG-3′ |
| R:
5′-GTTTCTGAGGGCACGAAGGAG-3′ |
| BDNF | F:
5′-CAATCGCTTCATCTTAGGAGT-3′ |
| R:
5′-TAAACGGCACAAAACAATC-3′ |
| c-Kit | F:
5′-CCGACGCAACTTCCTTATGAT-3′ |
| R:
5′-TCAGGACCTTCAGTTCCGACA-3′ |
| SCF | F:
5′-ATAGTGGATGACCTCGTGTTA-3′ |
| R:
5′-GAATCTTTCTCGGGACCTAAT-3′ |
| AQP3 | F:
5′-GCCAAGGTAGGATAGCAAATAA-3′ |
| R:
5′-TTGAAAACTTGGTCCCTTGC-3′ |
| β-actin | F:
5′-CTGTGCCCATCTACGAGGGCTAT-3′ |
| R:
5′-TTTGATGTCACGCACGATTTCC-3′ |
ELISA
At day 6 after the induction of constipation for 5
days, the serum was obtained from the eyeball before sacrifice
under anesthesia by intraperitoneal injection with 50 mg/kg
pentobarbital sodium. The serum from the eyeball of constipated
mice treated with or without NAR was harvested and washed in cold
phosphate-buffered saline (PBS) twice. The concentrations of
gastrin (Gas), endothelin (ET), acetylcholinesterase (AChE),
substance P (SP) and vasoactive intestinal peptide (VIP) in the
serum from ICR mice were determined using corresponding detection
kits (USNC Life Science Inc., Wuhan, China), according to the
manufacturer's instructions. The concentration level of motilin
(MTL) in serum was determined using a Mice Motilin ELISA kit
(Shanghai Yanjing Biological Technology Co., Ltd., Shanghai,
China). The sensitivity of this assay, or the lower limit of
detection was defined as the lowest protein concentration that
could be differentiated from zero. The minimum detectable doses of
Gas, ET, AChE, SP, VIP and MTL are typically less than 4.77, 2.54,
0.28, 4.88, 2.61 and 9.27 ng/ml in this study, respectively.
Western blot analysis
Proteins collected from the proximal colons of
constipated mice treated with or without NAR were lysed in ice-cold
radioimmunoprecipitation (RIPA) buffer plus PMSF and protein
concentrations in the supernatant were determined using the BCA
Protein Assay kit (all agents from Beyotime Institute of
Biotechnology Co., Shanghai, China) following the manufacturer's
instructions. Total proteins were separated by 8, 12 and 14% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) for
2.5 h, after which the resolved proteins were transferred onto
polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA) for
1.5 h at 80 V. Each membrane was then incubated separately with the
primary antibodies, anti-transient receptor potential cation
channel subfamily V member 1 (TRPV1; BA2589), anti-glial cell
line-derived neurotrophic factor (GDNF; BA0890), anti-brain-derived
neurotrophic factor (BDNF; BA0565-2), anti-nitric oxide synthase
(NOS; BA0360) (1:400; Wuhan Boster Bio-Engineering Co., Ltd.,
Wuhan, China); anti-c-Kit (bs-0672R), anti-stem cell factor (SCF;
bs-0545R) (1:500; Bioss, Beijing, China); anti-aquaporin 3 (AQP3;
D260100) (1:500; Sangon Biotech Co., Ltd., Shanghai, China) or
anti-β-actin (1:1,000; sc-47778; Santa Cruz Biotechnology, Inc.,
Dallas, Texas, USA) overnight at 4°C. The membranes were then
washed with TBS-T buffer (10 mM Tris-HCl, 150 mM NaCl and 1%
Tween-20) for 30 min and incubated with horse-radish
peroxidase-conjugated goat anti-rabbit IgG (Beyotime Institute of
Biotechnology Co.) at a dilution of 1:5,000 at room temperature for
1 h. Finally, the membrane blots were developed with an enhanced
chemiluminescence detection kit ECL detection reagent (7 Sea
Biotech, Shanghai, China) according to the manufacturer's
instructions. Quantitative analysis for western blot analysis was
made by Gel-Pro Analyzer software (Media Cybernetics, Inc.,
Rockville, MD, USA).
Immunohistochemistry
Paraffin-embedded tissue sections of 5 µm
thickness were rehydrated first in xylene and then in graded
ethanol solutions. The slides were then blocked with 5% goat serum
for 15 min. The sections were then immunostained with primary
antibody anti-AQP3 (1:50; D260100; Sangon Biotech Co., Ltd.) and
incubated overnight at 4°C. After washing the slides with PBS 3
times, the sections were incubated with secondary antibody
(biotin-conjugated goat anti-rabbit IgG; A0277; Beyotime Institute
of Biotechnology Co.) at room temperature for 30 min. Sections were
then washed with PBS and incubated for 5–10 min in DAB Horseradish
Peroxidase Color Development kit (0.02% diaminobenzidine (DAB)
containing 0.01% hydrogen peroxide; Beyotime Institute of
Biotechnology Co.). Counter staining was performed using
hematoxylin (Solarbio Co., Ltd.) and the slides were visualized
under a light microscope (Olympus); all images were taken at ×400
magnification.
Statistical analysis
Data are presented as the means ± standard deviation
(SD) from at least 3 experiments. Statistical comparisons were
analyzed by Bonferroni's multiple comparison tests or analysis of
variance (ANOVA). A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
Body weight and fecal pellet
parameters
Constipation was assessed principally by fecal
pellet number, weight and water contents to determine the effects
of NAR on Lop-induced constipation. Although the body weight in the
No group was slightly higher than that of the other groups, there
was no significant differences in body weight among all the
experimental groups (No group, 20.33±1.21 g; Lop + Vehicle group,
19.50±1.38 g; Lop + NAR75 group, 19.67±1.75 g; Lop + NAR150 group,
19.83±1.47 g; Lop + NAR300 group, 19.33±1.37 g; Fig. 1A). Moreover the effects of any
treatment on constipation are generally determined based on altered
excretion from ICR mice.
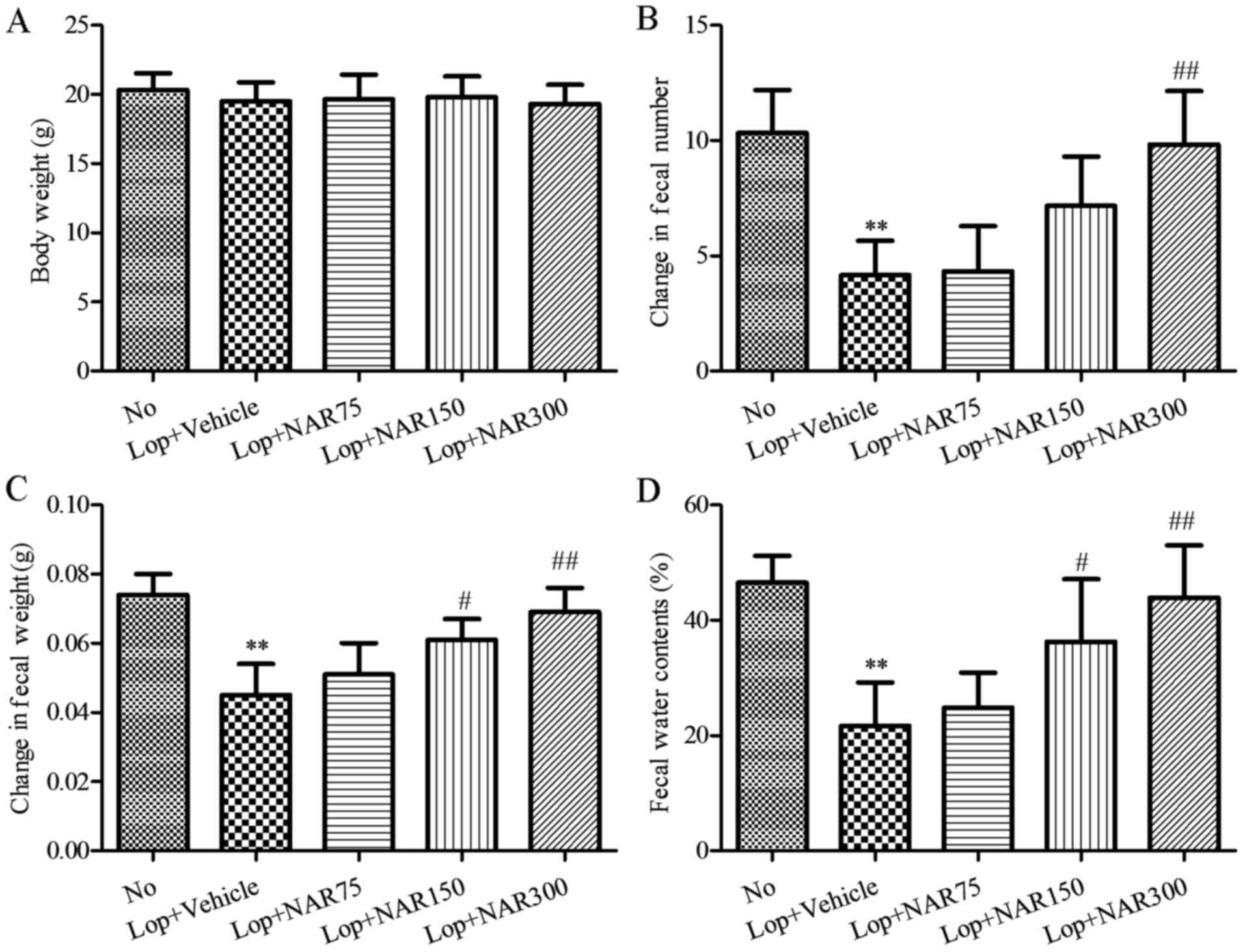 | Figure 1Body weight and fecal parameters
following intragastric administration with or without NAR in mice
with Lop-induced constipation. (A) Body weight, (B) fecal number,
(C) fecal weight and (D) water contents were measured at the same
time during the experiment. Six mice per group were assayed in
triplicate for body weight and fecal parameters analysis. Data
represent the means ± SD from 3 replicates. **P<0.01
compared to the No group; #P<0.05 and
##P<0.01 compared to Lop + Vehicle group. Lop,
loperamide; NAR, naringenin. The mouse groups were as follows: No,
negative control, mice treated with saline; Lop + Vehicle, mice
treated with Lop and the vehicle; Lop + NAR75, mice treated with
Lop and NAR at 75 mg/kg body weight; Lop + NAR150, mice treated
with Lop and NAR at 150 mg/kg body weight; Lop + NAR300, mice
treated with Lop and NAR at 300 mg/kg body weight. |
To investigate the laxative effects of NAR on stool
excretion, alterations in fecal pellet parameters were measured in
all groups of mice. The data indciated that the numbers of fecal
pellets were significantly reduced by more than half following the
administration of Lop when compared with the No group, whereas they
were increased by 3.99, 71.99 and 135.98% in the Lop + NAR75, Lop +
NAR150 and Lop + NAR300 groups, respectively, as compared with the
Lop + vehicle group (P<0.05; Fig.
1B). Indeed, the weight of stool was reduced approximately half
of the No group in the Lop + Vehicle group. However, following
treatment with NAR, this level was elevated significantly to that
of the No group (P<0.05; Fig.
1C). The total water content of the fecal pellets collected
over 24 h after 6 days decreased by almost 50% in the Lop + Vehicle
group when compared with the No group, and increased by 14.57,
67.43 and 102.69% in Lop + NAR75, Lop + NAR150 and Lop + NAR300
groups, respectively, as compared with the Lop + Vehicle group
(P<0.05; Fig. 1D). These
results suggest that NAR treatment relieves Lop-induced
constipation in ICR mice through the enhancement of stool
excretion.
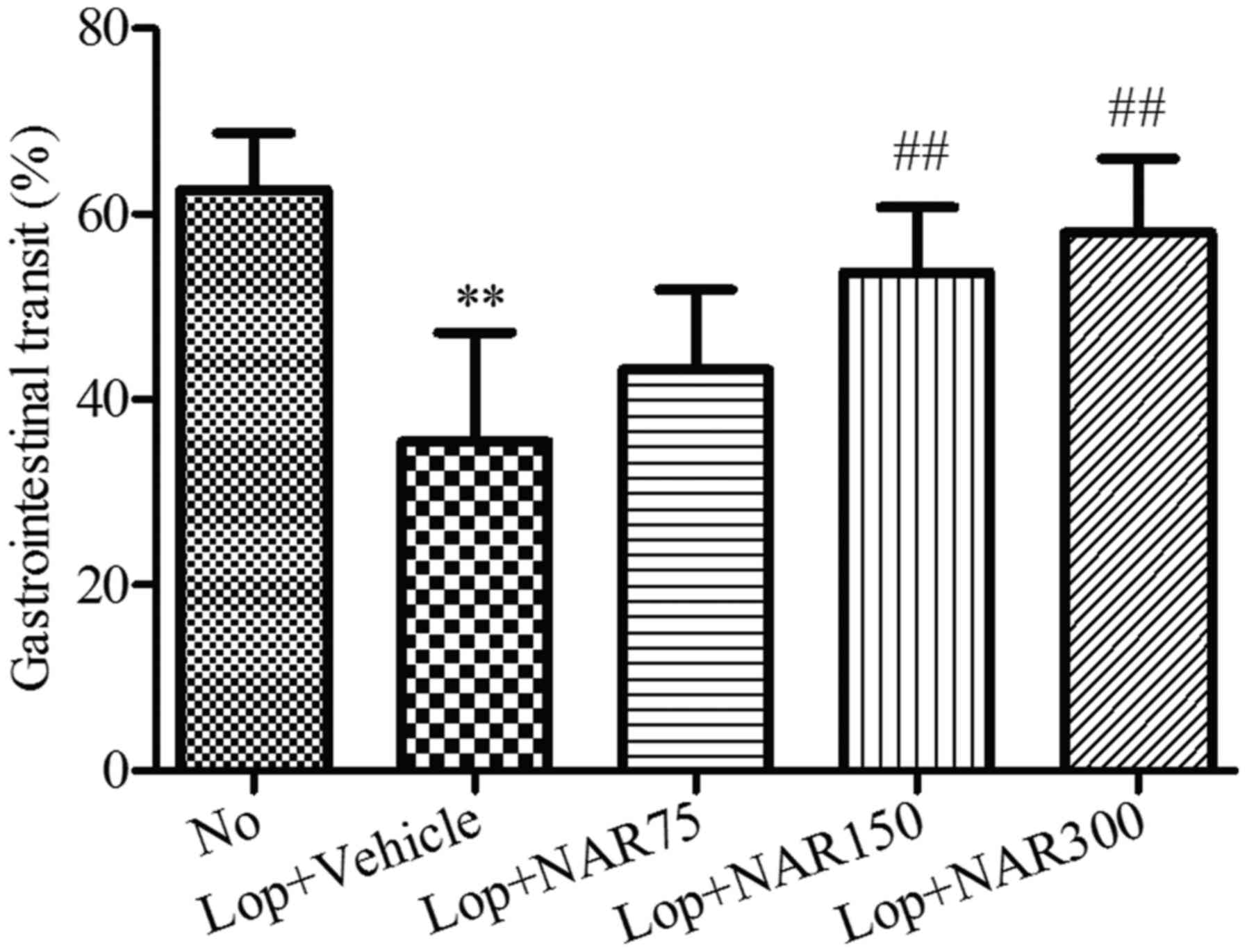
Effects on intestinal charcoal
transit
A statistically significant decrease in the
intestinal charcoal transit ratio was detected in the Lop + Vehicle
group when compared with the No group (from 62.67±6.13% to
35.44±11.78%). By contrast, statistically significant increases in
the intestinal charcoal transit ratio were detected after 6 days of
continuous treatment with various concentrations of NAR when
compared with the Lop + Vehicle group (Fig. 2). The intestinal charcoal transit
ratio increased from 35.44±11.78% in the Lop + Vehicle group to
43.25±8.61, 53.69±7.11 and 57.95±8.00% in the Lop + NAR75, Lop +
NAR150 and Lop + NAR300 groups, respectively (P<0.01; Fig. 2).
Parameters of serum
To evaluate the effects of NAR on serum biochemical
components in the constipated mice, alterations of several
components related to gastrointestinal metabolics in serum of the
Lop and/or NAR-treated mice were assessed by ELISA. As shown in
Fig. 3, serum parameters such as
MTL, Gas, ET, AChE, SP and VIP were significantly decreased in the
Lop + Vehicle group compared with the No group. However, these
parameters, in particular Gas, SP and AChE were increased following
treatment with NAR in a dose-dependent manner as compared with the
Lop + Vehicle group (P<0.05; Fig.
3). Therefore, these results suggest that NAR treatment may
increase the factors related to gastrointestinal movement to
relieve Lop-induced constipation in ICR mice.
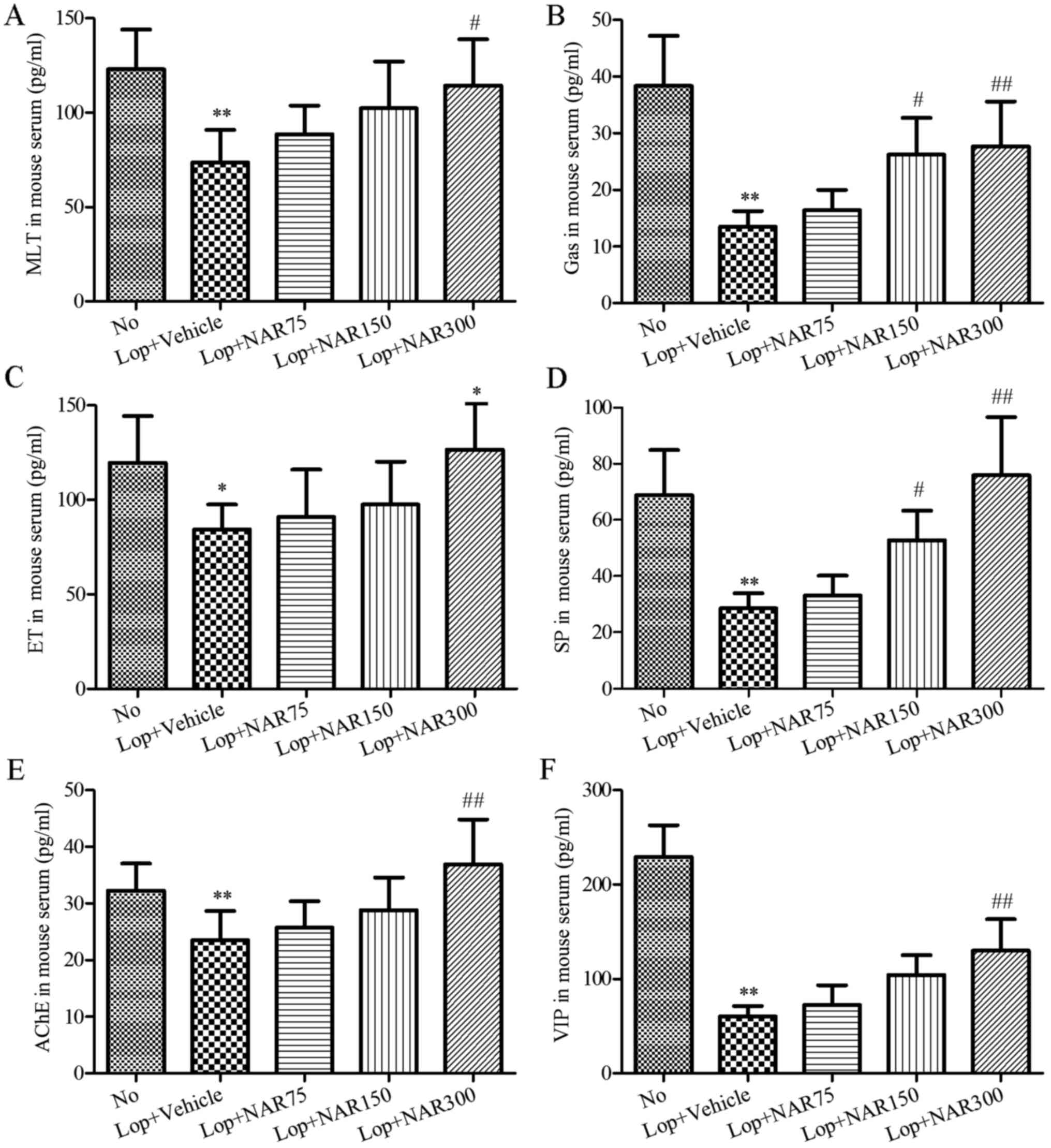 | Figure 3Effects of NAR on serum parameters in
mice with Lop-induced constipation. Consitipation was induced by
the intragastric administration of Lop followed by treatment with
or without NAR. The serum was collected separately for the analysis
of hte concentrations of (A) MTL, (B) Gas, (C) ET, (E) AChE, (D) SP
and (F) VIP in mice with Lop-induced constipation by ELISA.
**P<0.01 compared to the No group;
#P<0.05 and ##P<0.01 compared to Lop +
Vehicle group. NAR, naringenin; Lop, loperamide; MTL, motilin; Gas,
gastrin; AChE, acetylcholinesterase; SP, substance P; VIP,
vasoactive intestinal peptide. The mouse groups were as follows:
No, negative control, mice treated with saline; Lop + Vehicle, mice
treated with Lop and the vehicle; Lop + NAR75, mice treated with
Lop and NAR at 75 mg/kg body weight; Lop + NAR150, mice treated
with Lop and NAR at 150 mg/kg body weight; Lop + NAR300, mice
treated with Lop and NAR at 300 mg/kg body weight. |
Histological alterations and the
expression levels of enteric nerves-related factors in the colons
of mice with Lop-induced constipation
As 300 mg/kg NAR exhibited the most potent laxative
effects in mice with Lop-induced constipation, the mice in the Lop
+ NAR300 group were used to analyze the mechanisms of action of NAR
on Lop-induced constipation in mice. The histological alterations
following treatment with NAR were investigated in the colons of
mice with Lop-induced constipation by H&E staining (Fig. 4A). The mice treated with Lop alone
exhibited a marked loss of epithelium of the colon, crypt damage
and goblet cell depletion compared with the No group (Fig. 4A, middle panel). Following
treatment with NAR, moderately destructed epithelial cells were
found when compared with the Lop + Vehicle group. The colons of
mice treated with 300 mg/kg NAR exhibited intact goblet cells and
epithelial cells, which were comparable to those of the No group
(Fig. 4A, right panel). Moreover,
enteric nerve-related factors, such as TRPV1, GDNF, BDNF and NOS
are important enteric nerve-related factors. The mRNA levels of
these factors were detected following treatment with NAR in mice
with Lop-induced constipation by RT-PCR analysis. The results
revealed that NAR altered the mRNA levels of TRPV1, GDNF, BDNF and
NOS. The mRNA expression levels of TRPV1 and NOS were increased by
Lop administration, whereas NAR significantly decreased the mRNA
levels of TRPV1 and NOS. The mRNA expression of GDNF and BDNF
showed opposite trends (Fig. 4B).
In addition, the protein expression levels of these enteric
nerve-related factors following treatment with NAR were also
examined in mice with Lop-induced constipation by western blot
analysis. As shown in Fig. 4C,
Lop induced a high level of TRPV1, as well as NOS, and the
downregulation of GDNF and BDNF; these effects were reversed by
treatment with 300 mg/kg NAR to a great degree (Fig. 4C). The quantitative value of gray
intensity analysis revealed that treatment with NAR inhibited the
protein levels of TRPV1 and NOS from 3.57±0.77 to 2.16±0.40 and
from 2.53±0.54 to 1.43±0.25, respectively compared with the Lop +
Vehicle group. NAR also increased the levels of GDNF and BDNF from
0.42±0.10 to 0.69±0.13 and 0.61±0.17 to 0.83±0.14, respectively,
compared with the Lop + Vehicle group (P<0.05; Fig. 4D). These results revealed that NAR
attenuated the disordered expression of enteric nerve-related
factors in mice with Lop-induced constipation.
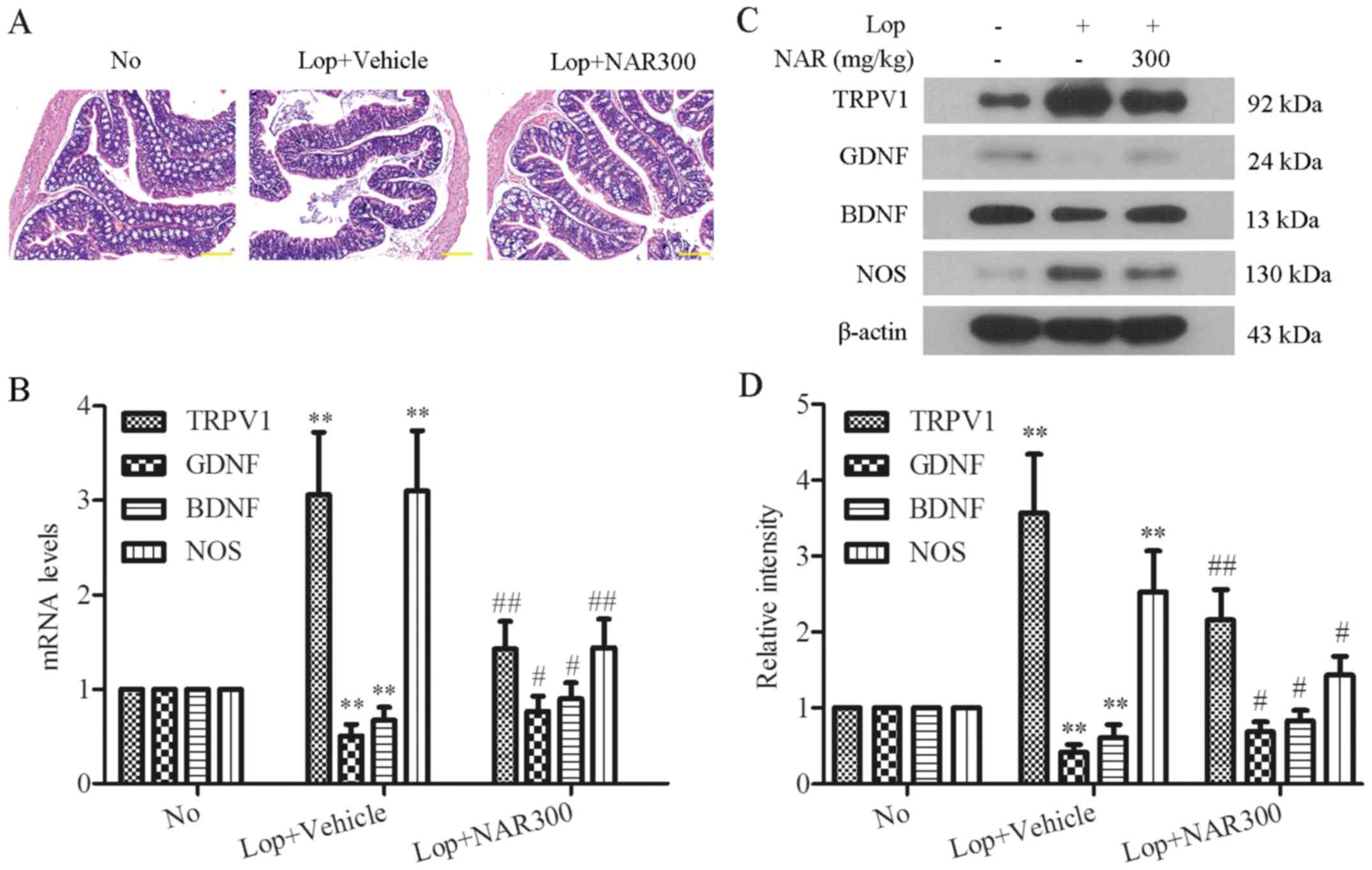 | Figure 4Histological alterations and the
expression levels of enteric nerve-related factors in the colons of
mice with Lop-induced constipation. (A) H&E-stained sections of
colon tissues of mice from the No, Lop + Vehicle, Lop + NAR75, Lop
+ NAR150 and Lop + NAR300 groups were observed using a light
microscope. Scale bar, 400 µM. (B) NAR regulated the mRNA
levels of enteric nerves-related factors in the colons of
Lop-induced constipated mice. Consitipation was induced by the
intragastric administration of Lop followed by treatment with or
without NAR. The mRNA levels of TRPV1, GDNF, BDNF and NOS in colons
of mice iwth Lop-induced constipation were analyzed by RT-PCR. (C)
The expression levels of enteric nerve-related factors in the
colons of mice with Lop-induced constipation. (C) The expression
levels of TRPV1, GDNF, BDNF and NOS were examined by western blot
analysis and the quantitative analysis of gray intensity was
calculated and shown in (D). **P<0.01 compared to the
No group; #P<0.05 and ##P<0.01 compared
to Lop + Vehicle group. Lop, loperamide; NAR, naringenin; TRPV1,
transient receptor potential cation channel subfamily V member 1;
GDNF, glial cell line-derived neurotrophic factor; BDNF,
brain-derived neurotrophic factor; NOS, nitric oxide synthase. The
mouse groups were as follows: No, negative control, mice treated
with saline; Lop + Vehicle, mice treated with Lop and the vehicle;
Lop + NAR75, mice treated with Lop and NAR at 75 mg/kg body weight;
Lop + NAR150, mice treated with Lop and NAR at 150 mg/kg body
weight; Lop + NAR300, mice treated with Lop and NAR at 300 mg/kg
body weight. |
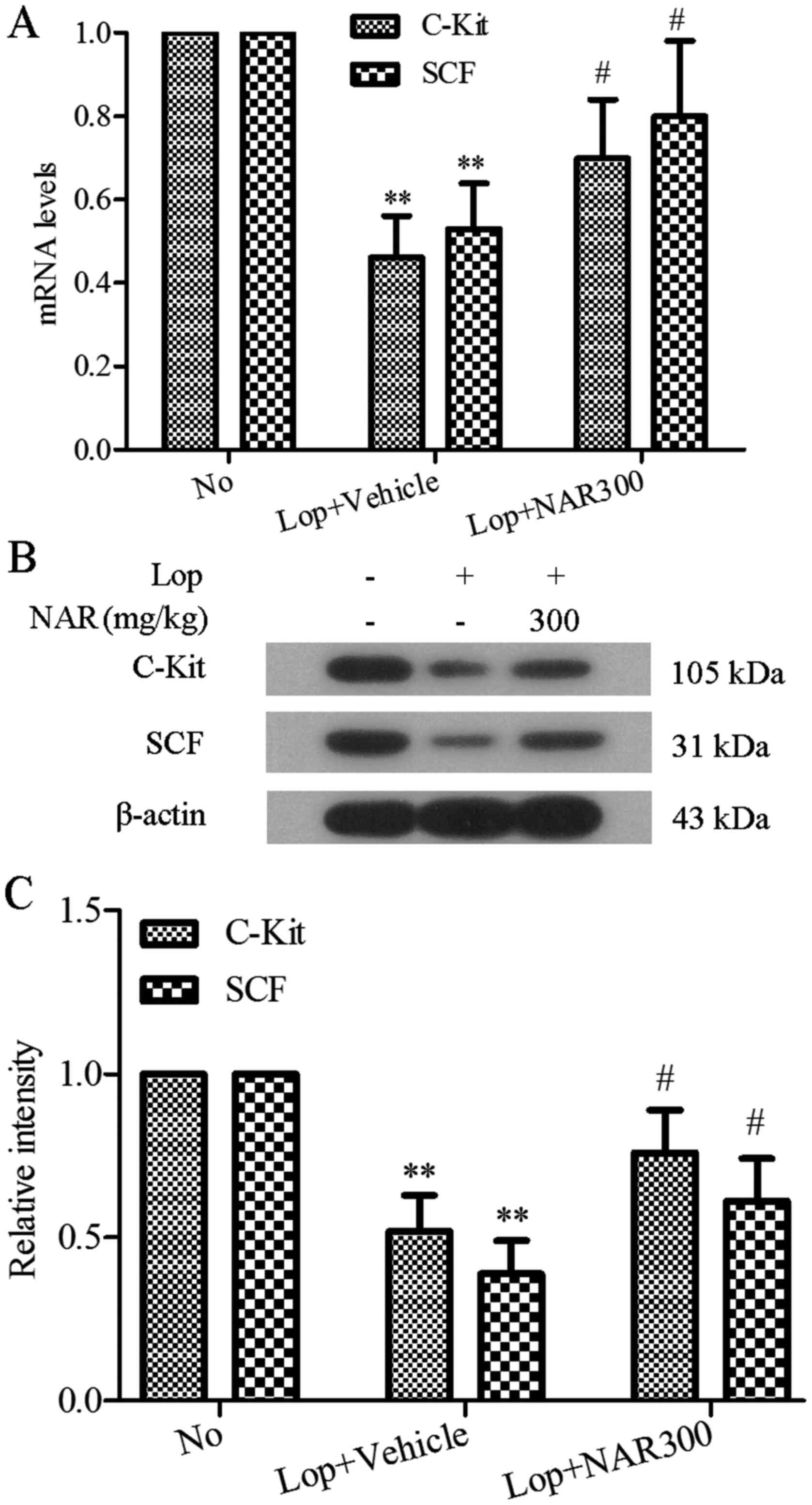
Effect of NAR on the expression levels of
c-Kit and SCF in the colons of mice with Lop-induced
constipation
ICCs have been reported to be involved in the
pathogenesis of a number of gastrointestinal motility dysfunctions,
including idiopathic STC (12).
c-Kit is recognized to be the specific marker of ICCs with its
receptor SCF that binds to it (13). In this study, to investigate the
effects of NAR on ICCs, the mRNA and protein expression levels of
c-Kit and SCF in colon tissues were determined by RT-PCR and
western blot analysis following treatment with NAR in mice with
Lop-induced constipation. As shown in Fig. 5A, Lop induced the downregulation
of both the c-Kit and SCF mRNA levels in colon tissues, whereas
treatment with NAR markedly altered the mRNA levels of c-Kit and
SCF (0.61±0.17 to 0.83±0.14). Identical to the RT-PCR results,
western blot analysis revealed that NAR also increased the c-Kit
and SCF expression levels which had been decreased by Lop, and the
levels returned to levels similar to those of the No group
(P<0.05; Fig. 5B). The
quantitative value of gray intensity analysis revealed that
treatment with NAR increased the c-Kit and SCF levels from
0.52±0.11 to 0.76±0.13 and 0.39±0.10 to 0.61±0.13, respectively,
compared with the Lop + Vehicle group (P<0.05; Fig. 5C).
NAR increases the production of AQP3 in
the colons of mice with Lop-induced constipation
AQP3 plays an important role in regulating water
transfer in the colon (40).
Thus, to examine the effect of NAR on the expression of AQP3 in the
colons of mice with Lop-induced constipation, the mRNA levels of
AQP3 in colon tissues were determined by RT-PCR analysis following
treatment with 300 mg/kg NAR. As shown in Fig. 6A, compared with the No group, Lop
decreased the mRNA level of AQP3 by half (from 1.00±0.00 to
0.51±0.13; P<0.05), whereas NAR increased it from 0.51±0.13 to
0.75±0.16 (P<0.05; Fig. 6A).
Western blot analysis was the used to examine the protein levels of
AQP3, which showed the same trend as the mRNA levels (Fig. 6B). The quantitative value of gray
intensity analysis revealed that treatment with NAR increased the
expression level of AQP3 from 0.31±0.07 to 0.65±0.13, compared with
the Lop + Vehicle group (P<0.01; Fig. 6C). Finally, IHC was used to
observe the alterations in the levels of AQP3 following treatment
with NAR in the colons of mice with Lop-induced constipation. The
data indicated that AQP3 was expressed mainly in the apical and
lateral mucosal epithelial cells in the colon tissues of healthy
mice. Lop decreased the expression of AQP3 both in the apical and
lateral mucosal epithelial cells of the colon tissue of constipated
mice. However, treatment with NAR attenuated this phenomenon. NAR
increased the expression of AQP3 which had been decreased by Lop.
These results suggest that NAR increases the expression of AQP3 in
the colons of mice with Lop-induced constipation.
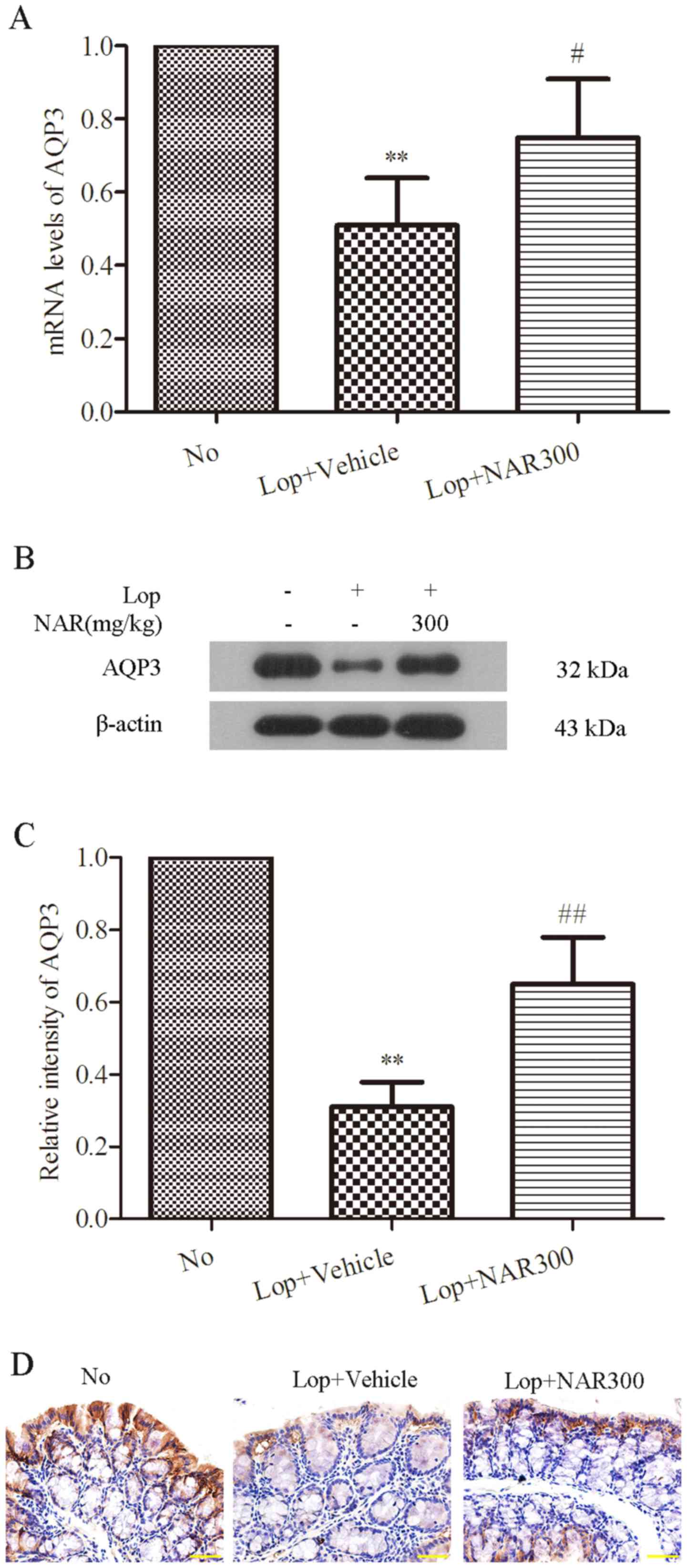 | Figure 6NAR increases the production of AQP3
in the colons of mice with of Lop-induced constipation. (A) NAR
upregulated the mRNA levels of AQP3 which were decreased by Lop in
the colons of constipated mice. The mRNA level of AQP3 in colons of
mice with Lop-induced constipation mice was examined by RT-PCR
analysis. (B) The expression level of AQP3 in the colons of
Lop-induced constipated mice. (C) The expression level of AQP3 was
examined by western blot analysis and the quantitative analysis of
gray intensity was calculated and shown in (C). (D) Protein
expression of AQP3 in the colons of mice Lop-induced constipation
detected by immunohistochemistry. Scale bar, 400 µM.
**P<0.01 compared to the No group;
#P<0.05 and ##P<0.01 compared to
Lop+Vehicle group. Lop, loperamide; NAR, naringenin; AQP3,
aquaporin 3. The mouse groups were as follows: No, negative
control, mice treated with saline; Lop + Vehicle, mice treated with
Lop and the vehicle; Lop + NAR75, mice treated with Lop and NAR at
75 mg/kg body weight; Lop + NAR150, mice treated with Lop and NAR
at 150 mg/kg body weight; Lop + NAR300, mice treated with Lop and
NAR at 300 mg/kg body weight. |
Discussion
Constipation is a chronic gastrointestinal disorder
characterized by symptoms, such as infrequent bowel movements,
difficulty during defecation and the sensation of incomplete bowel
evacuation (14) that causes
discomfort and affect the quality of life of patients (15). It may arise from a variety of
causes, including dietary habits, psychological stress and the use
of chemical compounds, such as morphine (16). NAR, as a flavonoid from citrus
fruits, has been reported to exert multiple effects, including
relieving constipation. However, the detailed mechanisms through
which NAR exerts laxative effects remain to be elucidated. Thus, in
this study, the laxative effects of NAR were evaluated in
Lop-induced constipated mice. We found that NAR relieved mice iwth
Lop-induced constipation based on the changes of fecal parameters
(numbers, weight and water content), the intestinal charcoal
transit ratio and the histological alteration. ELISA revealed that
NAR regulated the production of components related to
gastrointestinal metabolic in serum. The expression levels of
enteric nerves-related factors, c-Kit, SCF and AQP3 were examined
by western blot analysis and RT-PCR analysis, respectively. The
results of this study suggest that NAR relieves Lop-induced
constipation by upregulating the expression of AQP3.
Several chemical compounds, such as Lop and morphine
are widely used to induce constipation in laboratory animals. Among
these, Lop is well known to stimulate the extension of stool
evacuation time and to delay intestinal luminal transit through the
inhibition of water secretion (17), as well as smooth movement in the
intestinal wall (18,19). Furthermore, Lop has been used to
induce constipation in a variety of studies to determine the cause
of constipation and identify novel compounds with therapeutic
effects (15,20,21). In the present study, we used Lop
to induce constipation and observed the human-like symptoms of
constipation in ICR mice injected with Lop without any specific
problems.
Fecal parameters including fecal numbers, weight and
water contents and the intestinal charcoal transit ratio are
considered to be important factors for the evaluation of
constipation symptoms and the therapeutic effects of drugs. In our
study, the body weight did not differ between the experimental
groups, while stool-related factors, such as fecal number, weight
and water contents and the intestinal charcoal transit ratio were
shown to be markedly decreased in mice followng the administration
of Lop. Therefore, the observations indicated that a successful
model of constipation was established. However, these alterations
were significantly recovered by treatment with NAR. Treatment with
NAR induced an increase in fecal numbers, weight and water contents
and the intestinal charcoal transit ratio in a dose-dependent
manner (Figs. 1 and 2). Yang et al reported that NAR
produced a laxative effect and alleviated the symptoms of
Lop-induced constipation in rats (11). To the best of our knowledge, our
study demonstrated for the first time that NAR exerted a laxative
effect in mice with Lop-induced constipation.
On the other hand, we found that the serum levels of
MTL, Gas, ET, SP, AChE and VIP in mice iwth Lop-induced
constipation were lower than those in the No-treated mice. As
previously reported, MTL is the intestinal hormone which is
responsible for regulating the gastrointestinal motility in
response to the intake of food, as well as hunger stimuli (22). It has been indicated that the mean
function of MTL is to stimulate the production of pepsin and
increase the migrating motor complex (MMC) of gastrointestinal
motility (23). Gas has been
reported to be a peptide hormone that stimulates the secretion of
gastric acid (HCl) by the parietal cells of the stomach, promotes
pyloric sphincter relaxation and aids in gastric motility. It is
released by G cells in the pyloric antrum of the stomach, duodenum
and the pancreas (24). ET is a
peptide that constricts blood vessels and raises blood pressure. In
constipation, gastrointestinal disease occurs accompanied by not
also intestinal obstruction, but also by cardio-cerebrovascular
diseases in the elderly (25). In
our study, Lop suppressed the release of MTL, Gas as well as ET,
indicating constipation (23).
However, treatment with NAR increased the production of MTL, Gas
and ET in a dose-dependent manner. On the other hand, in patients
with constipation, abnormal neurotransmitters have been found in
the muscular layer of their intestinal walls, including the
deficiency of SP, which is recognized to contribute to peristalsis
(26). In the gastrointestinal
tract, as is known, muscle contraction, mucus secretion, as well as
myenteric nerve plexus are regulated by AChE (27). The dysfunction of the neuropeptide
VIP may initiate the functional changes observed in constipation
(28). In our study, we found a
significant decrease in the levels of SP, AChE and VIP in the Lop +
Vehicle group, whereas NAR increased these leels in a
dose-dependent manner (Fig. 3).
These data suggest that NAR relieves the symptoms of constipation
by increasing the serum levels of MTL, Gas, ET, SP, AChE and VIP
induced by Lop.
Constipation is associated with colonic
abnormalities. In our study, the surface mucus thickness in the
colonic lumen and the thickness of the colonic mucosa were shrunken
in the Lop + Vehicle group. Treatment with NAR restored the
thickness of the mucus and muscular layer compared with No group
(Fig. 4A). The data indicated
that histological changes were attenuated by NAR in the
gastrointestinal tract of mice with Lop-induced constipation.
It has been reported that TRPV1, which is involved
in the modulation and transmission of pain (nociception), as well
as the integration of diverse painful stimuli, regulates bowel
movements. Activated TRPV1 induces the release of the
neurotransmitter, which leads to the dysfunction of
gastrointestinal movement. High levels of TRPV1 expression are
always found in gastrointestinal damage (29). In this study, the constipated mice
had a much higher level of TRPV1, whereas NAR reduced the levels of
TRPV1 (Fig. 4C and D). GDNF
derived from intestinal smooth muscle cells is a key factor
influencing the structural and functional development of myenteric
neurons (30). The enhancement of
GDNF facilitates the repair of the damaged gastrointestinal tract,
which contributes to avoiding constipation (31). On the other hand, BDNF may play a
critical role in intestinal motility in constipation. It can alter
the gastrointestinal innervation structure and can lead to smooth
muscle secondary degeneration (32). NOS is a key factor which produces
endogenous NO that widely exists in gastrointestinal tissues and
plays an important role in modulating gastrointestinal movements.
The increasing level of NO has been shown to be involved in colonic
motility disorders of constipation (33). The downregulation of NOS can
reduce the production of NO, which is a feasible way to relieve
constipation (34). We found that
NAR increased the mRNA and protein expression levels of both GDNF
and BDNF in colon tissue and reduced the TRPV1 and NOS expression
levels in mice with Lop-induced constipation.
ICCs have been shown to be the pacemaker cells of
the intestine and have been implicated in the pathogenesis of a
number of gastrointestinal motility dysfunctions, including
idiopathic STC (35). A major
breakthrough in this field was the discovery that the tyrosine
kinase receptor, c-Kit, and its ligand, SCF, are critical in the
normal development, maturation, and maintenance of the phenotype of
ICCs, which can be reliably identified by c-Kit immunohistochemical
techniques. The blockade of c-Kit with neutralizing antibody can
induce the transdifferentiation of ICCs to a smooth muscle
phenotype, and intestinal slow waves disappear (36). Several studies have indicated that
the number of ICCs are decreased in the colons of patients with STC
(37–39). However, the mechanisms involved
are unknown. In our study, NAR significantly upregulated the levels
of both c-Kit and SCF in mice with Lop-induced constipation
(Fig. 5). These data thus
suggested that NAR increased the numbers of ICCs in mice with
Lop-induced constipation.
It is known that AQPs are primarily expressed in the
mucosal epithelial cells in the colon, in which AQP3 plays a
central role in water reabsorption across colonic surface cells
(40). Extensive research has
been conducted on AQP3. Kon et al reported that morphine
increases the AQP3 expression level in the colon, which promotes
water absorption from the luminal side to the vascular side and
causes constipation (41). In
this study, we found that NAR increased the mRNA and protein
expression levels of AQP3 in the colon, as shown by western blot
analysis. Furthermore, a positive correlation was observed between
this increase in the AQP3 level and the increase in fecal water
content (Figs. 1D and 6). In addition, NAR increased the level
of AQP3 both in apical and lateral mucosal epithelial cells in the
colons of mice with Lop-induced constipation. These results suggest
that NAR relieves the symptoms of constipation by increasing the
level of AQP3 in the colon, which is associoated with the
prevention of water reabsorption from the luminal side to the
vessel. However, the precise mechanisms through which NAR relieves
constipation in mice with Lop-induced constipation remain to be
further elucidated.
In conclusion, to the best of our knowledge, our
study demonstrates for the first time that NAR relieves the
symptoms of constipation, which is associated with the increased
expression of ICC markers (c-Kit and SCF) and AQP3 in mice with
Lop-induced constipation mice. Our results suggest the possible use
of NAR in the treatment of constipation.
References
|
1
|
Bassotti G and Blandizzi C: Understanding
and treating refractory constipation. World J Gastrointest
Pharmacol Ther. 5:77–85. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bassotti G: Understanding constipation
treatment: do we need to strain to obtain better results? Expert
Opin Drug Metab Toxicol. 9:387–389. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mostafa SM, Bhandari S, Ritchie G, Gratton
N and Wenstone R: Constipation and its implications in the
critically ill patient. Br J Anaesth. 91:815–819. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ouyang A and Locke GR 3rd: Overview of
neurogastroenterology-gastrointestinal motility and functional GI
disorders: classification, prevalence, and epidemiology.
Gastroenterol Clin North Am. 36:485–498. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang HL: Understanding the pathogenesis of
slow-transit constipation: one step forward. Dig Dis Sci.
60:2216–2218. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He CL, Burgart L, Wang L, Pemberton J,
Young-Fadok T, Szurszewski J and Farrugia G: Decreased interstitial
cell of cajal volume in patients with slow-transit constipation.
Gastroenterology. 118:14–21. 2000. View Article : Google Scholar
|
|
7
|
Andromanakos NP, Pinis SI and Kostakis AI:
Chronic severe constipation: current pathophysiological aspects,
new diagnostic approaches, and therapeutic options. Eur J
Gastroenterol Hepatol. 27:204–214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chan OT, Chiles L, Levy M, Zhai J, Yerian
LM, Xu H, Xiao SY, Soffer EE, Conklin JL, Dhall D, et al:
Smoothelin expression in the gastrointestinal tract: implication in
colonic inertia. Appl Immunohistochem Mol Morphol. 21:452–459.
2013. View Article : Google Scholar
|
|
9
|
Renugadevi J and Prabu SM: Cadmium-induced
hepatotoxicity in rats and the protective effect of naringenin. Exp
Toxicol Pathol. 62:171–181. 2010. View Article : Google Scholar
|
|
10
|
Ekambaram G, Rajendran P, Magesh V and
Sakthisekaran D: Naringenin reduces tumor size and weight lost in
N-methyl-N′-nitro-N-nitrosoguanidine-induced gastric carcinogenesis
in rats. Nutr Res. 28:106–112. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang ZH, Yu HJ, Pan A, Du JY, Ruan YC, Ko
WH, Chan HC and Zhou WL: Cellular mechanisms underlying the
laxative effect of flavonol naringenin on rat constipation model.
PLoS On. 3:pp. e33482008, View Article : Google Scholar
|
|
12
|
Yamamoto T, Watabe K, Nakahara M, Ogiyama
H, Kiyohara T, Tsutsui S, Tamura S, Shinomura Y and Hayashi N:
Disturbed gastrointestinal motility and decreased interstitial
cells of Cajal in diabetic db/db mice. J Gastroenterol Hepatol.
23:660–667. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chai Y, Huang Y, Tang H, Tu X, He J, Wang
T, Zhang Q, Xiong F, Li D and Qiu Z: Role of stem cell growth
factor/c-Kit in the pathogenesis of irritable bowel syndrome. Exp
Ther Med. 13:1187–1193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wald A: Chronic constipation: advances in
management. Neurogastroenterol Motil. 19:4–10. 2007. View Article : Google Scholar
|
|
15
|
Wintola OA, Sunmonu TO and Afolayan AJ:
The effect of Aloe ferox Mill. in the treatment of
loperamide-induced constipation in Wistar rats. BMC Gastroenterol.
10:952010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kakino M, Tazawa S, Maruyama H, Tsuruma K,
Araki Y, Shimazawa M and Hara H: Laxative effects of agarwood on
low-fiber diet-induced constipation in rats. BMC Complemen. Altern
Med. 10:682010.
|
|
17
|
Hughes S, Higgs NB and Turnberg LA:
Loperamide has antisecretory activity in the human jejunum in vivo.
Gut. 25:931–935. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamada K and Onoda Y: Comparison of the
effects of T-1815, yohimbine and naloxone on mouse colonic
propulsion. J Smooth Muscle Res. 29:47–53. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sohji Y, Kawashima K and Shimizu M:
[Pharmacological studies of loperamide, an anti-diarrheal agent.
II. Effects on peristalsis of the small intestine and colon in
guinea pigs (author's transl)]. Nihon Yakurigaku Zasshi.
74:155–163. 1978.In Japanese. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee HY, Kim JH, Jeung HW, Lee CU, Kim DS,
Li B, Lee GH, Sung MS, Ha KC, Back HI, et al: Effects of Ficus
carica paste on loperamide-induced constipation in rats. Food Chem
Toxicol. 50:895–902. 2012. View Article : Google Scholar
|
|
21
|
Kakino M, Izuta H, Ito T, Tsuruma K, Araki
Y, Shimazawa M, Oyama M, Iinuma M and Hara H: Agarwood induced
laxative effects via acetylcholine receptors on loperamide-induced
constipation in mice. Biosci Biotechnol Biochem. 74:1550–1555.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Zhao XR, Wang R, Qiu GQ and Zhang
M: Effect of Zhizhuwan on gastrointestinal peptide concentrations
in plasma of diabetic gastroenteropathy with constipation patients.
Zhongguo Zhong Yao Za Zhi. 33:2966–2968. 2008.In Chinese.
|
|
23
|
Suo H, Zhao X, Qian Y, Li G, Liu Z, Xie J
and Li J: Therapeutic effect of activated carbon-induced
constipation mice with Lactobacillus fermentum Suo on treatment.
Int J Mol Sci. 15:21875–21895. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Iijima K, Koike T, Abe Y and Shimosegawa
T: Cutoff serum pepsinogen values for predicting gastric acid
secretion status. Tohoku J Exp Med. 232:293–300. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fevang J, Ovrebø K, Myking O, Grong K and
Svanes K: Role of endothelin in the circulatory changes associated
with small bowel strangulation obstruction in pigs: effects of the
endothelin receptor antagonist bosentan. J Surg Res. 96:224–232.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yik YI, Farmer PJ, King SK, Chow CW,
Hutson JM and Southwell BR: Gender differences in reduced substance
P (SP) in children with slow-transit constipation. Pediatr Surg
Int. 27:699–704. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moriya R, Fujikawa T, Ito J, Shirakura T,
Hirose H, Suzuki J, Fukuroda T, Macneil DJ and Kanatani A:
Pancreatic polypeptide enhances colonic muscle contraction and
fecal output through neuropeptide Y Y4 receptor in mice.
Eur J Pharmacol. 627:258–264. 2010. View Article : Google Scholar
|
|
28
|
King SK, Sutcliffe JR, Ong SY, Lee M, Koh
TL, Wong SQ, Farmer PJ, Peck CJ, Stanton MP, Keck J, et al:
Substance P and vasoactive intestinal peptide are reduced in right
transverse colon in pediatric slow-transit constipation.
Neurogastroenterol Motil. 22:883–892. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Geppetti P and Trevisani M: Activation and
sensitisation of the vanilloid receptor: role in gastrointestinal
inflammation and function. Br J Pharmacol. 141:1313–1320. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rodrigues DM, Li AY, Nair DG and
Blennerhassett MG: Glial cell line-derived neurotrophic factor is a
key neurotrophin in the postnatal enteric nervous system.
Neurogastroenterol Moti. 23:e44–e56. 2011. View Article : Google Scholar
|
|
31
|
Saffrey MJ: Cellular changes in the
enteric nervous system during ageing. Dev Biol. 382:344–355. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen F, Yu Y, Wang P, Dong Y, Wang T, Zuo
X and Li Y: Brain-derived neurotrophic factor accelerates gut
motility in slow-transit constipation. Acta Physiol (Oxf). 212:pp.
226–238. 2014, View Article : Google Scholar
|
|
33
|
Peregud DI, Yakovlev AA, Stepanichev MY,
Onufriev MV, Panchenko LF and Gulyaeva V: Expression of BDNF and
TrkB phosphorylation in the rat frontal cortex during morphine
withdrawal are NO dependent. Cell Mol Neurobiol. 36:839–849. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tomita R, Igarashi S, Fujisaki S and
Tanjoh K: The effects of neurotensin in the colon of patients with
slow transit constipation. Hepatogastroenterology. 54:1662–1666.
2007.PubMed/NCBI
|
|
35
|
Xu J, Chen Y, Liu S and Hou X:
Electroacupuncture regulates apoptosis/proliferation of
intramuscular interstitial cells of cajal and restores colonic
motility in diabetic constipation rats. Evid Based Complement
Alternat Med. 584179:2013. View Article : Google Scholar
|
|
36
|
Farrugia G: Interstitial cells of Cajal in
health and disease. Neurogastroenterol Motil. 20:54–63. 2008.
View Article : Google Scholar
|
|
37
|
Yu CS, Kim HC, Hong HK, Chung DH, Kim HJ,
Kang GH and Kim JC: Evaluation of myenteric ganglion cells and
interstitial cells of Cajal in patients with chronic idiopathic
constipation. Int J Colorectal Dis. 17:253–258. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mostafa RM, Moustafa YM and Hamdy H:
Interstitial cells of Cajal, the Maestro in health and disease.
World J Gastroenterol. 16:3239–3248. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Parthasarathy G, Chen J, Chia N, O'Connor
HM, Gaskins HR and Bharucha AE: Reproducibility of assessing fecal
microbiota in chronic constipation. Neurogastroenterol Motil.
29:1–10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Silberstein C, Kierbel A, Amodeo G, Zotta
E, Bigi F, Berkowski D and Ibarra C: Functional characterization
and localization of AQP3 in the human colon. Braz J Med Biol Res.
32:1303–1313. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kon R, Ikarashi N, Hayakawa A, Haga Y,
Fueki A, Kusunoki Y, Tajima M, Ochiai W, Machida Y and Sugiyama K:
Morphine-induced constipation develops with increased aquaporin-3
expression in the colon via increased serotonin secretion. Toxicol
Sci. 145:337–347. 2015. View Article : Google Scholar : PubMed/NCBI
|