1. Introduction
Prostaglandin E2 (PGE2) receptor 2 subtype (EP2) is
a G protein-coupled plasma membrane receptor for PGE2, which acts
through numerous signaling pathways to regulate various
physiological functions, including tumor occurrence, invasion and
metastasis, angiogenesis, chronic inflammation, tumor immunity and
cell apoptosis (1). Recently,
various studies have focused on identifying the specific EP2
receptors and signaling pathways that regulate the pleiotropic
activities of the cyclooxygenase-2 (COX-2)/PGE2/EP2 pathway
(2-5).
Over the past 10 years, COX-2 and its prostaglandin
products have attracted increasing attention due to their important
roles in the progression of tumors of the lung, head and neck,
prostate, colon, ovary, chest and liver (2,6–8).
However, inhibition of COX-2 using non-steroidal anti-inflammatory
drugs (NSAIDs) and specific COX-2 inhibitors is associated with
various side effects, including gastric ulcers and myocardial
infarction (9), which have
limited the use of these drugs (10). As a primary prostanoid derived
from COX-2, PGE2 can also promote the activities of tumor cells
(11); therefore, inhibition of
the biological activities of PGE2 at different levels may maintain
the anticancer properties of COX-2 inhibition and also help prevent
side effects (12). Among four
pharmacologically different G protein-coupled plasma membrane
receptors of PGE2, the EP2 subunit is an important mediator of
numerous physiological and pathological processes, and may be the
most useful targeted receptor in anticancer treatment (13). The present review aimed to
highlight the potential role of EP2 in cancer (Fig. 1).
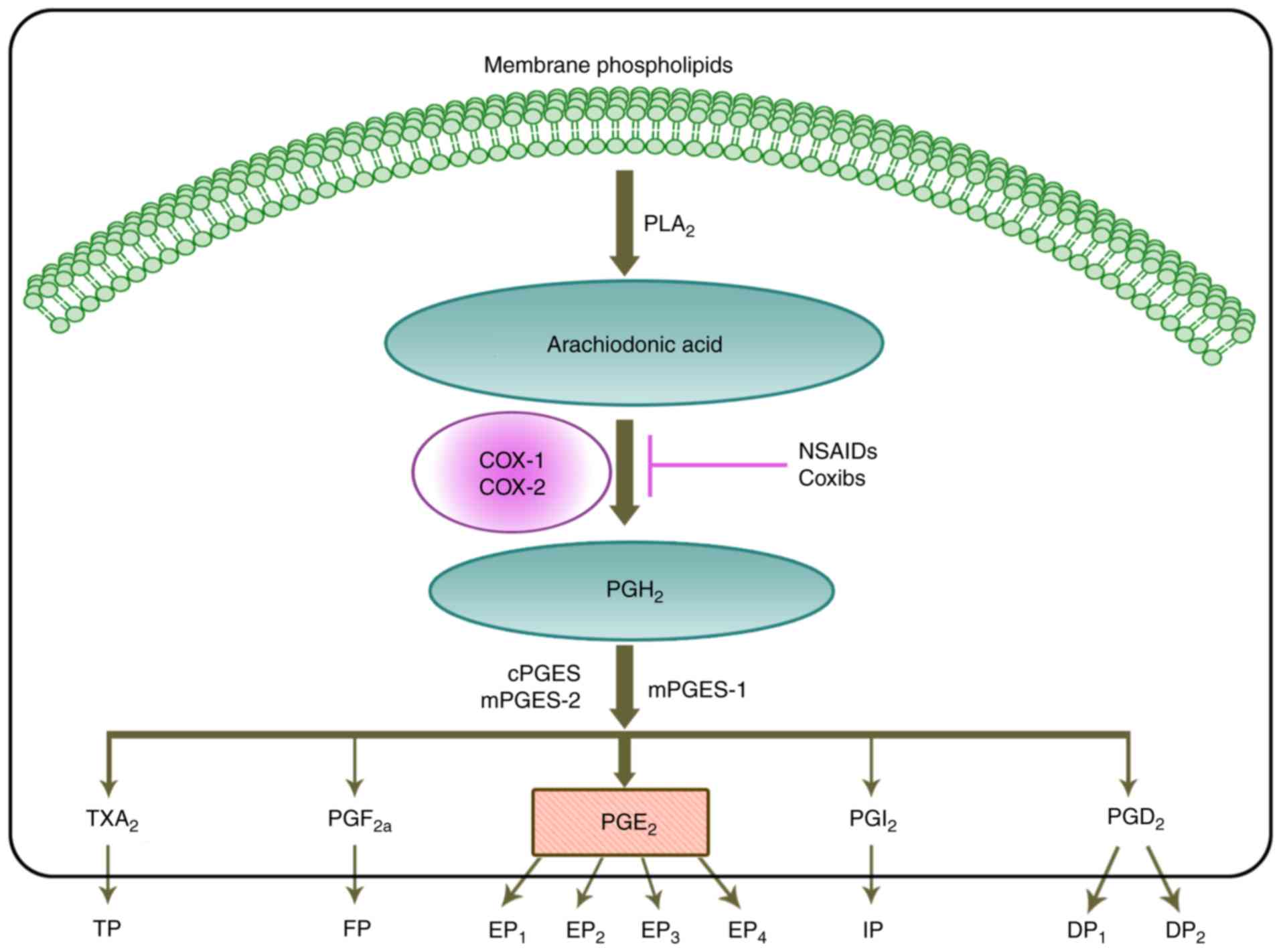 | Figure 1Biosynthesis of the prostaglandin EP2
receptor. Firstly, with action of PLA2 family members, arachidonic
acid is released from cell membranes and converted to PGH2 through
the activity of COX enzymes. PGH2 is rapidly converted to TXA2,
PGF2α, PGE2, PGI2 and PGD2 by one of three PGE2 synthases: cPGES,
mPGES-1 or mPGES-2. PGE2 signals through four G-protein coupled
receptors, namely EP1, EP2, EP3 and EP4. NSAIDs and Coxibs can
block the activity of COX enzymes, and inhibit the synthases of
PGE2. Therefore, they may suppress the pro-tumorigenic function of
PGE2. Alternatively, they may also suppress the activity of PGE2 by
blocking EP2. c, cytoplasmic; COX, cyclooxygenase; Coxibs, COX
inhibitors; DP, PGD2 receptor; EP, PGE2 receptor; FP, PGF receptor;
IP, PGI2 receptor; m, microsomal; NSAIDs, non-steroidal
anti-inflammatory drugs; PG, prostaglandin; PGES, PGE2 synthase;
PLA2, phospholipase A2; TP, thromboxane receptor; TXA2, thromboxane
2. |
2. Structure of the EP2 receptor
EP2 (53 kDa) (14)
is a PGE2 receptor encoded by the human PTGER2 gene. The PTGER2
gene contains two introns and three exons, and is located on human
chromosome 14 at position p22.1 (14q22.1) (15).
The human EP2 receptor consists of 358 amino acids,
whereas the mouse EP2 receptor consists of 632 amino acids
(16). It belongs to the family
of G protein-coupled receptors (GPCRs), which constitute a large
protein family of receptors that detect molecules outside the cell
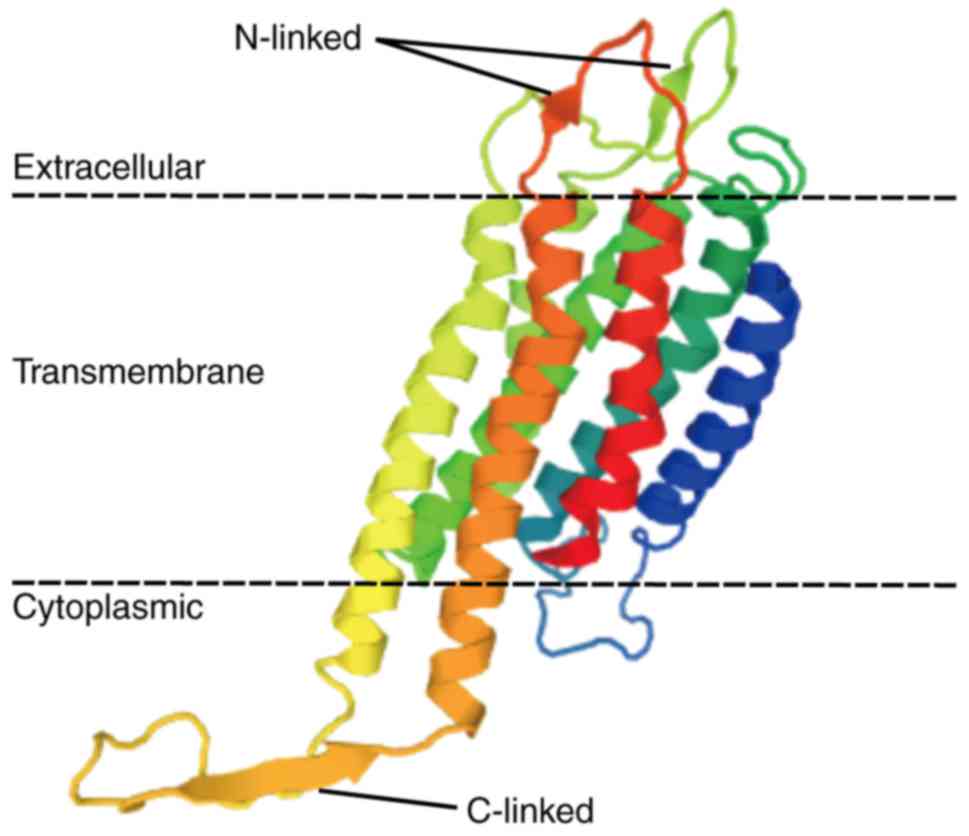
and activate internal cellular responses (17). EP2 is an integral membrane protein
that has an extracellular N-terminus and an intracellular
C-terminus (18,19). It has seven transmembrane (7-TM)
α-helices (TM-1 to TM-7) connected by three intracellular (IL-1 to
IL-3) and three extracellular (EL-1 to EL-3) loops (20). The EP2 is bound to a
heterotrimeric G protein complex consisting of the G stimulatory
(Gsub.) α and the tightly associated Gβγ
subunits (21,22). Binding of an agonist to EP2
results in activation of the Gsα subunit, which
regulates the cAMP-dependent pathway by stimulating the production
of cAMP from ATP (23) (Fig. 2). Both EP2 and EP4 are bound to
Gsα subunits (24).
3. Biological activity of the EP2
receptor
The expression of EP2 receptor EP2 is
widely distributed in humans (25)
The EP2 protein is expressed in the human small
intestine, lung, media of arteries and arterioles of the kidney,
thymus, uterus and cerebral cortex (26). In addition, its mRNA is widely
expressed in fibroblasts, aorta, the corpus cavernosum of the penis
and articular cartilage, among others (27-29) (Fig.
3). In rats, EP2 receptor protein and/or mRNA have been
detected in the lung, spleen, intestine, skin, kidney, liver, long
bones, and rather extensively throughout the brain and other parts
of the central nervous system (30). Therefore, the EP2 receptor appears
to serve a key role in biological development.
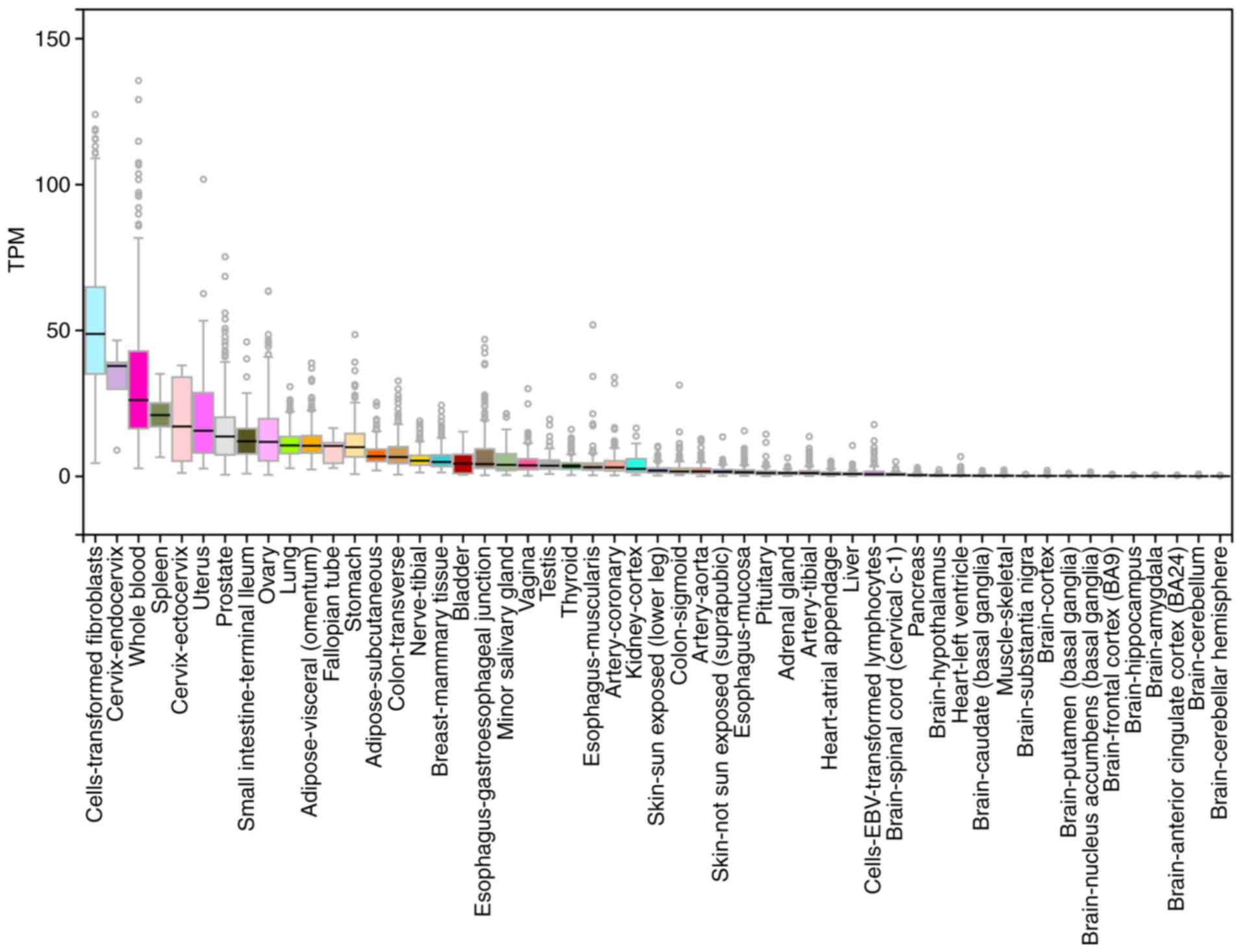 | Figure 3Expression of EP2 in humans. EP2 is
expressed in several types of human cells, including cells of the
small intestine, lung, media of arteries and arterioles of the
kidney, thymus, uterus, cerebral cortex, corpus striatum,
hippocampus, corneal epithelium, corneal choriocapillaries,
myometrium, eosinophils, sclera, articular cartilage, penile corpus
cavernosum and airway smooth muscle cells. Data source: GTEx
Analysis Release V7 (dbGaP Accession phs000424. v7.p2). EP,
prostaglandin E2 receptor 2 subtype. |
Desensitization
Activated GPCRs can be phosphorylated by G
protein-coupled receptor kinases (GRKs) (31), which modifies G protein-dependent
signaling by initiating receptor desensitization, internalization
and resensitization (32).
However, EP2 differs from all other prostaglandin receptors in that
it does not undergo homologous desensitization (33). When EP4 is expressed in Chinese
hamster ovary cells, EP4 receptors are found to undergo rapid
PGE2-induced desensitization, which is not observed with EP2
receptors (32). Due to its
failure to become desensitized, EP2 can act over more prolonged
periods of time compared with other prostaglandin receptors and,
therefore, may be able to contribute to more delayed and chronic
phases of cellular and tissue responses (34).
Positive feedback regulation
PGE2 signaling through EP2 can in turn boost
expression of COX-2 in polyp tissues (35), and it has been suggested that EP2
may regulate phosphorylated
(p)-phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K),
p-protein kinase B (Akt) and p-glycogen synthase kinase 3β (GSK-3β)
expression, and increase nuclear translocation of β-catenin in LoVo
cancer cells (36). The levels of
the cofactors lymphoid enhancer-binding factor-1 (LEF-1) and
transcription factor 4 (TCF-4) have also been reported to be
upregulated by EP2 in the nucleus, resulting in upregulation of
COX-2 expression (37). Using
genetically engineered animal models lacking EP2, or using EP2
antagonists in animals and animal and human tissues, it has been
reported that a lack of EP2 may downregulate the expression of
p-PI3K, p-Akt and p-GSK-3β, and reduce the levels of β-catenin and
cofactors LEF-1 and TCF-4 (5,38,39). Therefore, inhibition of EP2 may
reduce the proliferation and invasion of cancer cells.
4. Regulating the function of the EP2
receptor in cancer
The function of the EP2 receptor
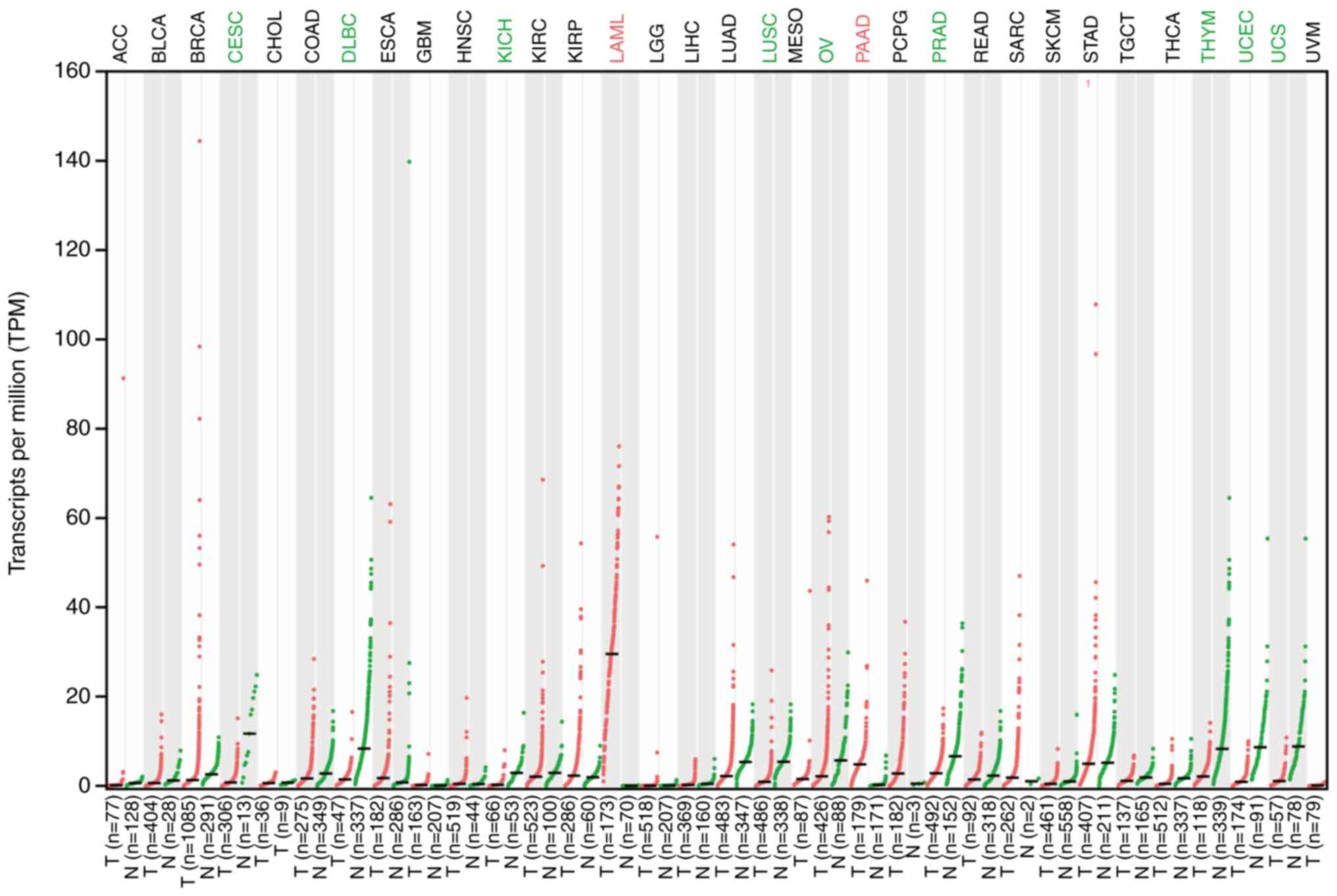
Numerous studies have demonstrated that EP2 is
abnormally expressed in cancer, including colon, prostate, liver
and breast cancer (5,40–43) (Fig.
4). Furthermore, EP2 is associated with poor survival in
chronic obstructive pulmonary disease (Fig. 5). The aberrant expression of EP2
has been found to be closely associated with factors associated
with cancer development, including chronic inflammation,
immunoregulation, angiogenesis, metastasis and multidrug
resistance.
EP2 induces chronic inflammation
It was recently demonstrated that inflammation
serves a key role in cancer, and ~60% of cancers are associated
with inflammation (44). The
inflammatory response may create a partial microenvironment that
promotes alterations in the genome and stimulates the formation of
tumors. Some tumor cells release cytokines and chemotactic factors
to attract monocytes and macrophages (45). The infiltrating macrophages in
turn secrete growth factors to promote tumor progression, recruit
secondary leukocytes, and enhance and maintain the interaction
between inflammatory and tumor cells (46).
As a main inflammatory mediator derived from COX-2,
PGE2 can induce several proinflammatory factors, including
cytokines, chemotactic factors, inducible nitric oxide synthase
(iNOS) and even COX-2 (47).
These factors can promote cell proliferation, survival,
angiogenesis, invasion, migration and metabolism (48). In addition, EP2 activation can
significantly induce the expression of proinflammatory factors,
such as interleukin (IL)-1β and IL-6 in tumor cells (42,49). IL-1β can promote tumor growth,
invasion and angiogenesis (50).
Under normal conditions, IL-6 levels are increased in patients with
several types of cancer, including prostate, colon, breast and
ovarian cancer (51). The PGE
signals initiated by EP2 or EP4 can exacerbate symptoms of
inflammation by increasing the expression levels of IL-23, and
decreasing the levels of IL-12 and IL-27 (52). PGE, together with IL-1β and IL-23,
promotes the differentiation and cytokine expression of T helper
(Th)17 cells (53). Recent
research has revealed that hepatic stellate cells increase the
numbers of Th17 cells and regulatory T cells via the PGE2/EP2
pathway (54). Hepatic Th17 and
regulatory T cell numbers are also increased in patients with
advanced-stage hepatitis B virus-associated liver fibrosis, which
potentially leads to hepatocellular carcinoma (55,56). In addition, PGE signals can affect
the skin microenvironment through enhancing blood flow by
regulating ultraviolet (UV)-induced acute skin inflammation
(57,58). As a result, small-molecule
antagonists of EP2 can mitigate chronic inflammation in tumor
tissues to provide an anti-inflammatory mechanism for the treatment
of cancer (56).
In colon tumors, EP2 is expressed by infiltrating
neutrophils and tumor-associated fibroblasts in the stroma
(39). The expression levels of
tumor necrosis factor-α (TNF-α), IL-6, chemokine (C-X-C motif)
ligand 1 (CXCL1), COX-2, and other proinflammatory genes acting
synergistically with TNF-α, are upregulated by EP2 (42,49). These results indicate that EP2 in
neutrophils and tumor-associated fibroblasts promotes colon
tumorigenesis by exerting a proinflammatory effect and regulating
the tumor microenvironment (59,60).
EP2 contributes to cancer immunotherapy
resistance
Since EP2 has an important role in the
differentiation of DCs (3), and
it also has an important role in the function and immunoregulation
of PGE2 suppression; therefore, the elimination of EP2 receptors
can suppress the growth of tumors and prolong survival (7). PGE2 contributes to immune evasion
and cancer immunotherapy resistance by suppressing the function of
macrophages, neutrophils and Th1 cells. PGE2 also markedly inhibits
the production of Th1 cytokines, including interferon-γ (IFN-γ),
TNF-α and IL-2 (61). The
immunoregulation of PGE2 is initiated through EP2 receptor
signaling. Activation of EP2 can downregulate the expression of
IFN-γ and TNF-α by immune cells, such as natural killer T cells,
neutrophilic granulocytes and macrophages (62), and adversely affects the
immunocompetence of these immune cells (47).
The signals initiated by the EP2 receptors can be
transduced by the same Gsα stimulating protein and the
concentration of cAMP in cells is increased by activation of EP2
(63). Cluster of differentiation
(CD)4+ Th cells are a key effector in the adaptive
immune system to control cancer (64) and the increase in cAMP is
associated with a decrease in Th1 cells and IFN-γ (65). In addition, PGE2 can suppress the
activities of natural killer cells and cytotoxic T lymphocytes,
which are part of antitumor immunity (66).
Apart from the direct suppression of immune cell
activities, EP2 signaling can promote the development of regulatory
T cells, which are efficient inhibitors of the immune system and
can suppress the activity of numerous immune cells, including DCs
(67). DCs have a key role in the
initiation of the tumor-specific immune response (52). The signals of EP2 (and EP4) not
only block DC activity, but can also block the generation of DCs,
resulting in development of the immuno-suppression of
myeloid-derived suppressor cells (68).
Knockout of the EP2 receptor can reduce tumor
progression and prolong the survival of mice injected with MC26 or
Lewis lung carcinoma cells (69).
This mechanism appears to be associated with the failure of PGE2 to
suppress differentiation of DCs, leading to induction of the
antitumor cytotoxic T-lymphocyte response (70). In a mixed lymphocyte model of the
cellular immune response, it was reported that EP2 and EP4 could
regulate the functions of antigens, indicating that EP2 receptors
can directly inhibit immune cell proliferation (71).
EP2 increases angiogenesis
EP2 can induce angiogenesis in cancer, whereas the
deletion of EP2 receptors can down-regulate the expression of
angiogenic factors, including vascular endothelial growth factor
(VEGF), and inhibit tumor angiogenesis (72). Apart from VEGF induction by EP2
activation, EP2 signaling in endothelial cells can regulate the
activity and survival of endothelial cells and promote tumor
angiogenesis in vivo (73). PGE2 signaling triggers hyperplasia
of the mammary gland and regulates VEGF induction in breast tumors
in mice (53). In addition, EP2
signaling can directly regulate tumor angiogenesis and survival by
enhancing the activity of epithelial cells (1,74).
It can also regulate hypertrophy and tumor invasion as a response
to UV stimulation and induce the growth of skin tumors (57). In addition, PGE2 facilitates tube
formation through EP2 signaling (23), indicating the involvement of EP2
in luteal angiogenesis and the progression of ovarian cancer
(75).
EP2 promotes tumor invasion and
metastasis
In addition to its association with angiogenesis and
immune suppression in cancer, a recent study demonstrated that EP2
receptor activation by PGE2 significantly enhances hepatocellular
carcinoma cell invasion and migration by upregulating the
expression levels of Snail (76).
It has also been demonstrated that treatment with various
concentrations of prostaglandin promotes the migratory ability of
human LoVo colon cancer cells via the EP2 receptor (40).
The PI3K signaling pathway has a key role in the
regulation of cell proliferation, differentiation, migration and
trafficking (77). The PI3K/Akt
cell survival pathway has been revealed to be upregulated by EP2
and EP4 activation (78,79), thereby upregulating the level of
matrix metalloproteinases, which has been observed in several types
of human cancer and regulates the efficacy of various therapies
(5). In breast cancer, EP2
receptors are also associated with metabolism, which may alter the
response of cells to transforming growth factor-β (TGF-β), which
can maintain the balance of tissues by inducing cell cycle arrest,
differentiation and apoptosis (80). However, during tumorigenesis,
genetic and epigenetic events convert TGF-β from a tumor suppressor
to a promoter of cell growth, invasion and metastasis (16). The altered response to TGF-β may
be attributed to the suppression of TGF-β-induced Smad2/3 nuclear
localization and signaling by PGE2, followed by uncoupling TGF-β
from activating Smad3 (16).
In addition, EP2 has been reported to regulate
metastasis via downregulation of solute carrier family 19 member 3
in triple-negative breast cancer (81). In addition, EP2 ablation
suppresses skin tumor development by limiting angiogenesis and
promoting apoptosis (82-84), whereas the overexpression of EP2
accelerates skin tumor development. EP2 also accelerates the
invasion of prostate tumor cells, which is inhibited by the EP2
antagonist, TG4-155 (85). In
laryngeal carcinoma, upregulated EP2 expression has been detected
in highly aggressive tumors, which are identified by deeper
invasion of the submucosa or cartilage (86).
EP2 promotes multidrug resistance (MDR)
in cancer
Epidermal growth factor receptor (EGFR) is also
involved in the pathogenesis and development of various types of
cancer (87). The activation of
EGFR accelerates the uncontrolled proliferation and metabolism of
cancer cells (88), whereas an
inhibitor of EGFR can be used to treat non-small cell lung cancer,
and pancreatic, breast and colon cancer (89). Despite the initial dynamic
response to these inhibitors, the majority of patients ultimately
develop resistance to therapy (90). It has been reported that PGE2
results in tyrosine kinase inhibitor resistance in some patients
with cancer through EP2 transactivation of EGFR (91). Although the potential underlying
mechanism remains unclear, accumulating evidence suggests that PGE2
is associated with MDR in cancer (92). However, clinical trials combining
specific COX-2 inhibitors, including celecoxib and aproxicoxib,
with EGFR inhibitors, such as erlotinib, have not produced
promising results. By contrast, they result in toxicity in a
proportion of patients (91,93). Therefore, more studies are
required to elucidate how to use the EP2 receptor as a target to
attenuate MDR in cancer.
5. Involvement of EP2 receptor signaling
pathways in cancer
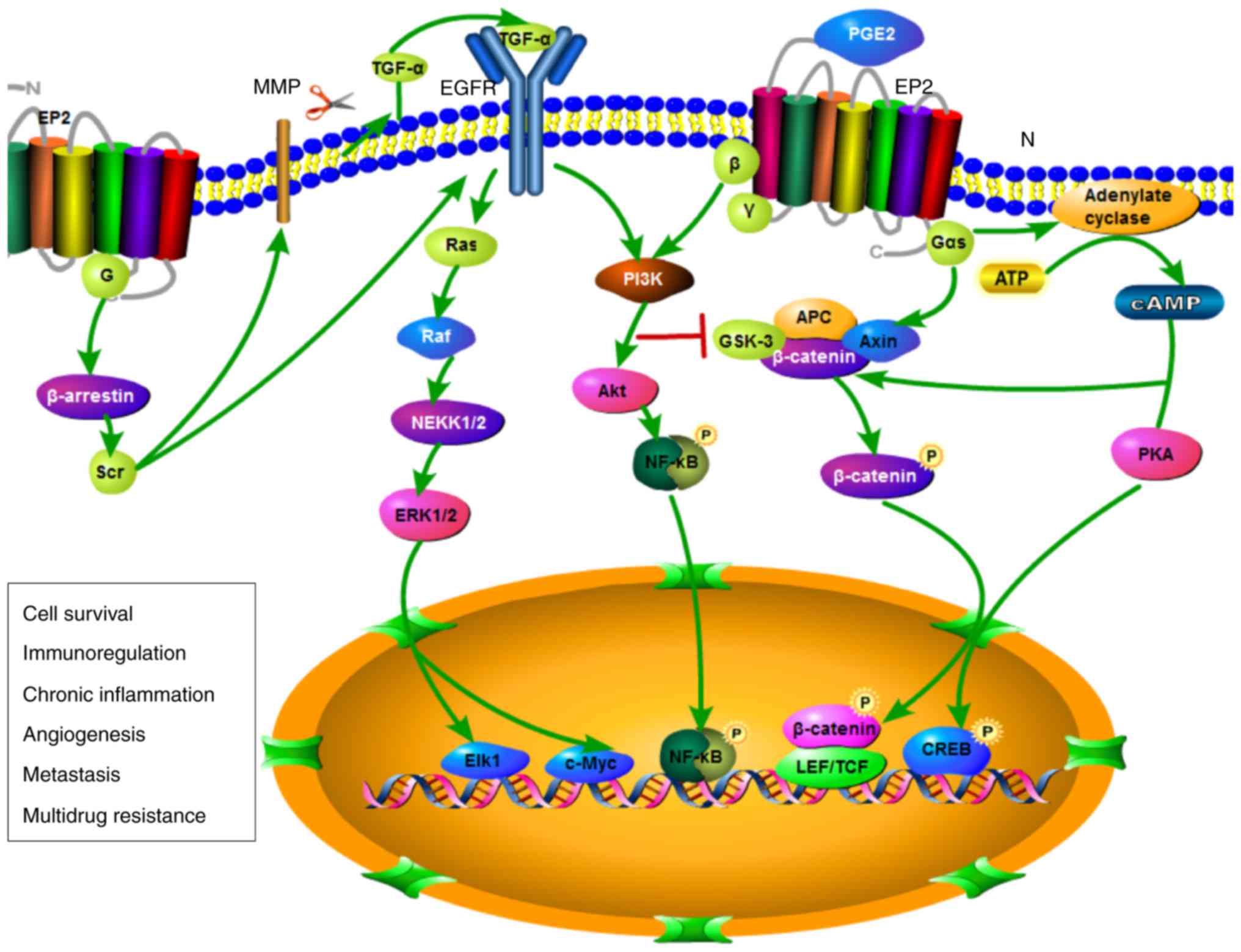
EP2 receptor signaling pathways
An increasing number of studies has demonstrated
that EP2 regulates cancer development various signaling pathways
(Fig. 6).
EP2 receptors mediate second messenger
signaling
As a Gs-coupled receptor, EP2 activation by PGE2 can
activate adenylate cyclase, thus resulting in an increase in cAMP
levels and protein kinase A (PKA) activation. In response to cAMP
binding, PKA activates and phosphorylates downstream transcription
factors, including cAMP response element-binding protein (CREB),
which regulate a wide range of biological processes. In cells
expressing EP2, 1 µM PGE272 is activated to form cAMP (94). In addition, aromatase-dependent
estrogen synthesis is associated with hormone-dependent breast
cancer (81), and EP2 can
regulate the cAMP/PKA/CREB pathway, in turn regulating cytochrome
P450 aromatase (75).
EP2-Gs-axin-pathway
EP2 can also activate the GSK3β and β-catenin
pathways, in turn increasing the transcription of several genes
associated with cancer, including c-myc, cyclin D1 and VEGF. When
PGE2 activates EP2, a Gs subunit directly binds with a
structural region, also referred to as the regulator of G protein
signaling (RGS). As a consequence, it can promote the release of
GSK-3β (95). Furthermore, EP2
receptors activate β/γ subunits to release Gαs subunits and
stimulate Akt by PI3K, resulting in the phosphorylation and
inactivation of GSK-3β (96).
However, this inactivation can lead to the accumulation of
β-catenin in the cytoplasm and migration to the cell nucleus, where
it can interact with TCF and LEF to activate genetic transcription
promoting tumor growth (57).
Castellone et al (39) reported that EP2 receptors are
involved in the PI3K/Akt and axin/β-catenin pathways activating
colon tumor growth. It was revealed that when the Gαs subunit was
bound to the RGS domain, free G protein β/γ subunits could
stimulate PI3K and Akt to activate β-catenin and proliferation of
DLD-1 cells, resulting in the mutation of adenomatous polyposis
coli (APC) genes. It appears that GPCR signaling pathways can
interact with APC-β-catenin-TCF. Numerous proteins, including Dsh,
axin, GSK-3β and APC, are involved in the Wnt pathway and can
interact with G proteins (39).
Crosstalk with other signaling
pathways
It has been demonstrated that EP2 can activate G
protein-independent signaling pathways through the formation of EP2
and β-arrestin complexes. β-arrestin serves as a regulator that
switches signals to G protein-independent signaling pathways
(97). It was recently reported
that EP2 can regulate β-arrestin signaling to initiate the PI3K,
Akt, Src, extracellular signal-regulated kinase (ERK), c-Jun
N-terminal kinase (JNK) and EGFR pathways; therefore, it may have
an important role in the proliferation and migration of cells.
Through PI3K and Akt, rather than through conventional cAMP
signaling, EP2 can inhibit the occurrence of tumor immunity
(98).
Previous studies have demonstrated that the
crosstalk between EP2 and EGF pathways increases the complexity of
the EP receptor pathways (97,98). EGFR is located on the surface of
cells and can activate EGF and TGF-α through binding with ligands.
EP receptors promote the transactivation of EGFR and involve
activation of c-Src genes. Phosphorylation can directly or
indirectly activate EGFR and EGFR ligands, in order to stimulate
the EGFR signaling network. Activation of EGFR can activate several
signal transduction pathways, including mitogen-activated protein
kinase, PI3K/Akt, signal transducer and activator of transcription
and phospholipase C, thus leading to the proliferation,
differentiation, migration and survival of cells. iNOS, ERK1/2 and
EP2 can be activated through indirect activation of EGFR to promote
the growth of squamous cell tumors (93). In addition, β-arrestin can cause
the phosphorylation of JNK, upregulate profilin 1, increase the
expression of f-actin, and promote the migration and proliferation
of tumor cells (99).
In conclusion, these signaling routes in the nucleus
promote the expression of genes associated with the growth,
survival, immune evasion, angiogenesis, infiltration and metabolism
of cancer cells.
6. Regulation of EP2 receptor signaling by
genetic engineering
Genetic ablation strategies and biochemistry studies
provide tools for elucidating EP2 signaling. In addition, studies
in EP2-knockout mice suggest that EP2 signaling has a key role in
cancer (59,60). A recent study on EP2-null mice
undergoing a two-stage chemical carcinogenesis protocol revealed
that EP2-null mice may develop fewer tumors (50%) and smaller
tumors compared with in wide-type mice. In addition, macrophage
infiltration was decreased, as was the expression of IL-1α in the
epidermis, and angiogenesis. Mice deficient in EP2 receptors also
exhibit low incidence rates of lung, skin and breast cancer
(59).
Gene knockout strategies have been used to study the
potential functions of EP2 in colon cancer. In a mouse model of
familial adenomatous polyposis, a genetic ablation in EP2 resulted
in an ~100% incidence of colon cancer. Although genetic ablation of
the EP2 receptor does not affect the formation of aberrant crypts,
it affects the formation of polyps and tumor angiogenesis through
Wnt/β-catenin pathway (59). In
addition, genetic ablation of EP2 reduces the size and number of
intestinal polyps in APC1309 mice, simulating the gene disruption
or inhibition of COX-2 in the same model. Similar to Min mice,
APCD716 mice also harbored a mutation in the same tumor suppression
gene, which may result in the development of colon cancer (39).
In order to investigate the major gene expression
alterations in tumor tissues, in a previous study, EP2-knockout
mice were implanted with EP2+/+ epithelial-like tumors.
As a result, tumor growth, acute inflammation and IL-6 expression
were suppressed in EP2-knockout mice. The expression of several
genes, including long non-coding RNAs, was also decreased in tumors
from the EP2-knockout mice (2).
7. Development of agonists, antagonists and
targeted drugs for EP2
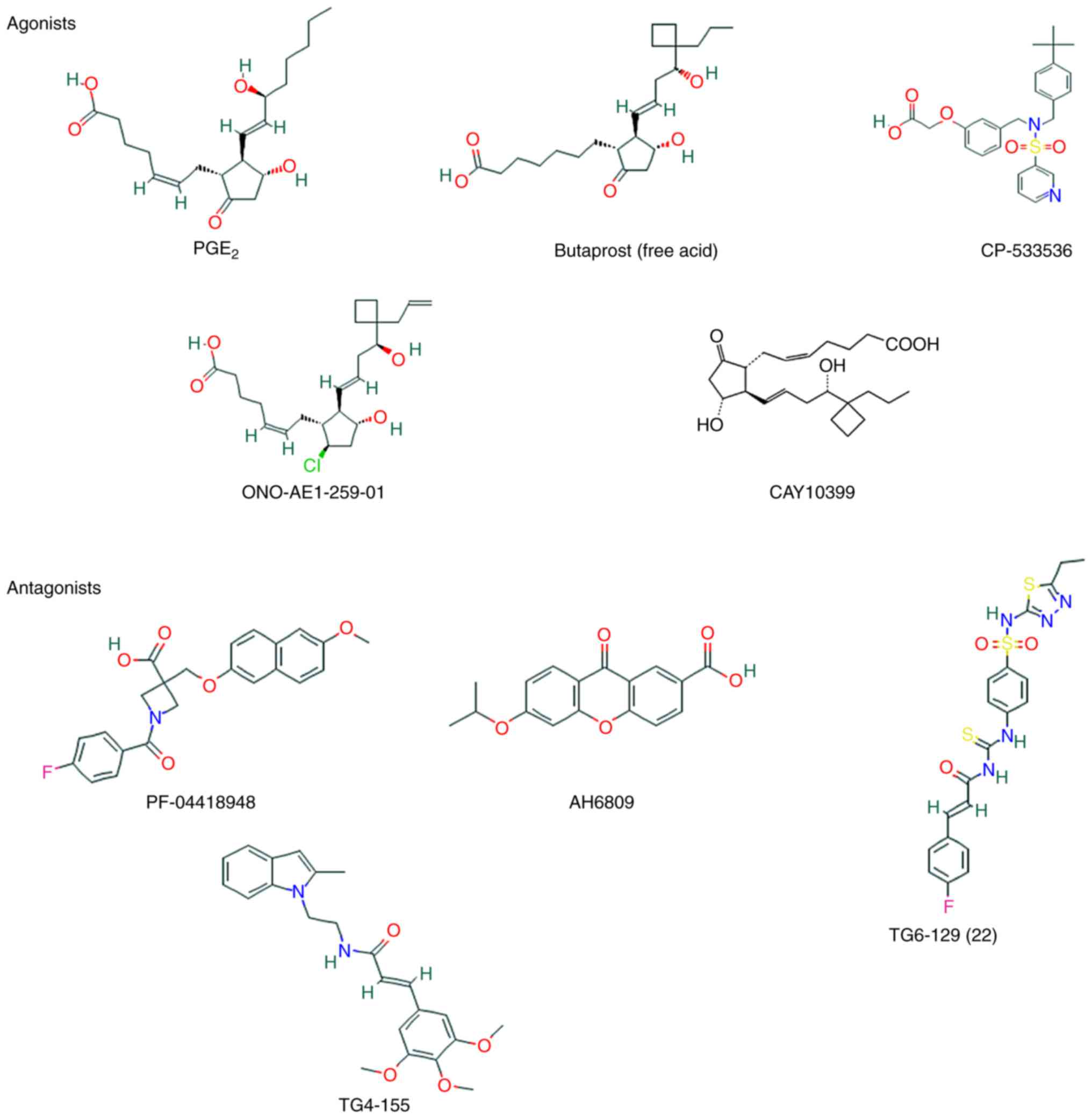
The following standard prostaglandins can activate
EP2, with a binding efficacy in the following order:
PGE2>PGF2α≥PGI2>PGD2 (100). Receptor-binding affinity,
expressed as the dissociation constant, is ~13 nM for PGE2 and ~10
nM for PGE1 for the human receptor, and ~12 nM for PGE2 for the
mouse receptor (101).
Apart from endogenous prostaglandins, three classes
of EP2 receptor agonists have been discussed. These agonists are
useful for the study of the function of EP2 and may be clinically
useful for the treatment of certain diseases, including glaucoma
and inflammatory bowel disease, and for the stimulation of hair
growth and the stimulation of hair to terminal hair transformation
(102). The first one comprises
ligands that are similar in structure to the endogenous ligand
PGE2. In order to enhance potency and selectivity, the ω-lipophilic
chain has been modified, such as in the free acid metabolite of
butaprost (102). However, in
binding studies, the selectivity of butaprost for EP2 was only
~18-fold of that for EP3 (103).
Due to its chemical instability and weak potency relative to PGE2,
ONO-AE1-259-01 (R=allyl; Ki=1.7 nM, EC50=1.8
nM) has been developed, introducing a 9β-chloro group in the place
of the C9-carbonyl moiety (104). The second class of agonists is a
series of pyridylsulfonamide derivatives, such as PF-04217329 and
CP-544326. The latter class is a set of pyridylaminoacetic acids,
one of which is TG3-95-1, which is only weakly active
(EC50=7.8 mM), but it may be worth mentioning as it
represents the only identified EP2-selective class of allosteric
potentiators. Apart from the natural agonist of EP2, a number of
PGE2 agonists, such as butaprost, CAY10399 and ONO-AE1-259-01, and
compounds with a non-prostanoid structure, such as CP-533536, can
also activate EP2 (105).
Due to the lack of selective antagonists for EP2,
the majority if studies focusing on the function of EP receptors in
cancer have been based upon genetic deletion and knockout studies
(106). Although the genetic
deletion of prostanoid receptors is very useful, it is
overcomplicated and may result in hypertension. Numerous
small-molecule ligands targeted to EP2 have been developed to
complement this strategy (107,108).
The non-selective EP receptor antagonist AH6809 has
been widely applied to explore the roles of PGE2/EP2 signaling
under normal and pathological conditions (109). Although AH6809 acts as an
antagonist of EP2, it may also serve as an antagonist of EP1 and
DP1 (110); it is neither
selective nor potent, and is therefore unsuitable for in
vivo studies (111).
However, it has been demonstrated that allosteric potentiators and
selective antagonists of the EP2 receptor with non-prostanoid
structure can explain the physiological functions of prostaglandin
receptors (112). These EP2
small-molecule modulators, such as PF-04418948 (Ki=16
nM), TG4-155 (Ki=9.9 nM), TG8-4 and TG6-129, which have
been used for studies in animal models of human diseases, enable
differentiation of EP2 from other prostanoid receptors (113). The increasing number of tools
for studying EP2 may enable a better understanding of the role of
this receptor under normal and pathological conditions (Fig. 7).
8. Conclusions and prospects
COX is a rate-limiting enzyme in biosynthetic
pathways. Although drugs targeting COX enzymes, such as NSAIDs or
specific COX-2 inhibitors, have been clinically used to treat
various diseases, they may be associated with numerous side
effects, including gastric ulcers and myocardial infarction.
Therefore, these adverse reactions limit the use of such drugs. In
the COX-2 downstream signaling pathway, EP2 is an important
mediator in several physiological and pathological events. It has
been demonstrated that EP2 can interact with G proteins through the
formation of EP2 and β-arrestin (β-inhibitory proteins act as
modulators), and signaling can be switched to the G
protein-independent pathway. The update on selective EP2
antagonists may be helpful in explaining the functions of EP2 to
supplement genetic knockout studies (114). Furthermore, nano-drug delivery
technology and EP2-targeted drugs may be applied in the treatment
of cancer. By establishing drug-loaded nanoparticles targeting EP2,
a novel nano-drug delivery system may be established to increase
drug targeting. In conclusion, studying EP2 may help elucidate the
mechanisms underlying cancer invasion and metastasis, angiogenesis,
chronic inflammation, tumor immunity and apoptosis, and may aid the
development of novel molecular targeting therapeutic
strategies.
Acknowledgments
The Genotype-Tissue Expression (GTEx) Project was
supported by the Common Fund of the Office of the Director of the
National Institutes of Health, and by NCI, NHGRI, NHLBI, NIDA,
NIMH, and NINDS. The data used for the analyses described in this
manuscript were obtained from: [https://www.gtexportal.org/home/] the GTEx Portal on
04/22/17 and dbGaP accession number phs000424.v7.p2 on 04/22/2017.
The RNA-Seq datasets used by GEPIA are based on the UCSC Xena
project (http://xena.ucsc.edu), which are
computed by a standard pipeline.
Funding
This study was supported by the International
Cooperation Key Project of the National Natural Science Foundation
of China (grant no. 81520108031 to Q.L.), the Science Foundation of
Shanghai Committee of Science Project (grant no. 14430722900), and
the Program for Outstanding Medical Academic Leader and Shanghai
Academic Research Leader (grant no. 16XD1403600).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XS compiled the information and wrote the review, QL
revised the manuscript critically for important intellectual
content.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cui FB, Huang DF, Zhang FL, Gao EY, Zhang
Y, Cao YM, Ding S, Wang Y, Cao QS and Cao XM: Investigation on the
regulatory effect of PGE2 on ESCC cells through the
trans-activation of EGFR by EP2 and the relevant mechanism. Eur Rev
Med Pharmacol Sci. 21:5668–5676. 2017.PubMed/NCBI
|
|
2
|
Asting AG, Iresjö BM, Nilsberth C, Smedh U
and Lundholm K: Host knockout of E-prostanoid 2 receptors reduces
tumor growth and causes major alterations of gene expression in
prostaglandin E2-producing tumors. Oncol Lett. 13:476–482. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Flórez-Grau G, Cabezón R, Borgman KJE,
España C, Lozano JJ, Garcia-Parajo MF and Benitez-Ribas D:
Up-regulation of EP2 and EP3 receptors in human tolerogenic
dendritic cells boosts the immunosuppressive activity of PGE2. J
Leukoc Biol. 102:881–895. 2017. View Article : Google Scholar
|
|
4
|
Maric J, Ravindran A, Mazzurana L,
Björklund ÅK, Van Acker A, Rao A, Friberg D, Dahlén SE, Heinemann
A, Konya V and Mjösberg J: Prostaglandin E2 suppresses human group
2 innate lymphoid cell function. J Allergy Clin Immunol.
141:1761–1773. 2018. View Article : Google Scholar :
|
|
5
|
Hsu HH, Lin YM, Shen CY, Shibu MA, Li SY,
Chang SH, Lin CC, Chen RJ, Viswanadha VP, Shih HN and Huang CY:
Prostaglandin E2-induced COX-2 expressions via EP2 and EP4
signaling pathways in human LoVo colon cancer cells. Int J Mol Sci.
18:pii: E1132. 2017. View Article : Google Scholar
|
|
6
|
Gong WH, Zhao N, Zhang ZM, Zhang YX, Yan L
and Li JB: The inhibitory effect of resveratrol on COX-2 expression
in human colorectal cancer: A promising therapeutic strategy. Eur
Rev Med Pharmacol Sci. 21:1136–1143. 2017.PubMed/NCBI
|
|
7
|
Wehbi VL and Taskén K: Molecular
mechanisms for cAMP-mediated immunoregulation in t cells-role of
anchored protein kinase a signaling units. Front Immunol.
7:2222016. View Article : Google Scholar
|
|
8
|
Shishikura K, Horiuchi T, Sakata N, Trinh
DA, Shirakawa R, Kimura T, Asada Y and Horiuchi H: Prostaglandin E2
inhibits neutrophil extracellular trap formation through production
of cyclic AMP. Br J Pharmacol. 173:319–331. 2016. View Article : Google Scholar
|
|
9
|
Lee SE, Lim C, Kim H and Cho S: A study of
the anti-inflammatory effects of the ethyl acetate fraction of the
methanol extract of forsythiae fructus. Afr J Tradit Complement
Altern Med. 13:102–113. 2016.
|
|
10
|
Xu L, Stevens J, Hilton MB, Seaman S,
Conrads TP, Veenstra TD, Logsdon D, Morris H, Swing DA, Patel NL,
et al: COX-2 inhibition potentiates antiangiogenic cancer therapy
and prevents metastasis in preclinical models. Sci Transl Med.
6:242ra2842014. View Article : Google Scholar
|
|
11
|
Al-Taei S, Salimu J, Spary LK, Clayton A,
Lester JF and Tabi Z: Prostaglandin E2-mediated
adenosinergic effects on CD14+ cells: Self-amplifying
immunosuppression in cancer. Oncoimmunology. 6:e12683082016.
View Article : Google Scholar
|
|
12
|
Fan Y, Wang Y and Wang K: Prostaglandin E2
stimulates normal bronchial epithelial cell growth through
induction of c-Jun and PDK1, a kinase implicated in oncogenesis.
Respir Res. 16:1492015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O'Callaghan G and Houston A: Prostaglandin
E2 and the EP receptors in malignancy: Possible therapeutic
targets? Br J Pharmacol. 172:5239–5250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kuo KT, Wang HW, Chou TY, Hsu WH, Hsu HS,
Lin CH and Wang LS: Prognostic role of PGE2 receptor EP2 in
esophageal squamous cell carcinoma. Ann Surg Oncol. 16:352–360.
2009. View Article : Google Scholar
|
|
15
|
Edwards TL, Shrubsole MJ, Cai Q, Li G, Dai
Q, Rex DK, Ulbright TM, Fu Z, Murff HJ, Smalley W, et al: A study
of prostaglandin pathway genes and interactions with current
nonsteroidal anti-inflammatory drug use in colorectal adenoma.
Cancer Prev Res (Phila). 5:855–863. 2012. View Article : Google Scholar
|
|
16
|
Tian M and Schiemann WP: PGE2 receptor EP2
mediates the antagonistic effect of COX-2 on TGF-beta signaling
during mammary tumorigenesis. FASEB J. 24:1105–1116. 2010.
View Article : Google Scholar :
|
|
17
|
Yu L, Wu WK, Li ZJ, Li HT, Wu YC and Cho
CH: Prostaglandin E(2) promotes cell proliferation via protein
kinase C/extracellular signal regulated kinase pathway-dependent
induction of c-Myc expression in human esophageal squamous cell
carcinoma cells. Int J Cancer. 125:2540–2546. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Trzaskowski B, Latek D, Yuan S,
Ghoshdastider U, Debinski A and Filipek S: Action of molecular
switches in GPCRs - theoretical and experimental studies. Curr Med
Chem. 19:1090–1109. 2012. View Article : Google Scholar :
|
|
19
|
King N, Hittinger CT and Carroll SB:
Evolution of key cell signaling and adhesion protein families
predates animal origins. Science. 301:361–363. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu X, Ahn S, Kahsai AW, Meng KC,
Latorraca NR, Pani B, Venkatakrishnan AJ, Masoudi A, Weis WI, Dror
RO, et al: Mechanism of intracellular allosteric β2AR antagonist
revealed by X-ray crystal structure. Nature. 548:480–484. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Engelhardt S and Rochais F: G proteins:
More than transducers of receptor-generated signals? Circ Res.
100:1109–1111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gilman AG: G proteins: Transducers of
receptor-generated signals. Annu Rev Biochem. 56:615–649. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kinoshita A, Higashino M, Yoshida K,
Aratani Y, Kakuuchi A, Hanada K, Takeda H, Naganawa A, Matsuya H
and Ohmoto K: Synthesis and evaluation of a potent, well-balanced
EP2/EP3 dual agonist. Bioorg Med Chem. 26:200–214. 2018. View Article : Google Scholar
|
|
24
|
Wang J, Zhang L, Kang D, Yang D and Tang
Y: Activation of PGE2/EP2 and PGE2/EP4 signaling pathways
positively regulate the level of PD-1 in infiltrating
CD8+ T cells in patients with lung cancer. Oncol Lett.
15:552–558. 2018.
|
|
25
|
Ota H, Katanosaka K, Murase S, Furuyashiki
T, Narumiya S and Mizumura K: EP2 receptor plays pivotal roles in
generating mechanical hyperalgesia after lengthening contractions.
Scand J Med Sci Sports. 28:826–833. 2018. View Article : Google Scholar
|
|
26
|
Wang S, Xie L, Zhang Y, Xu P and Liu A:
Expression of prostaglandin E2 receptors in acquired
middle ear cholesteatoma. Clin Exp Otorhinolaryngol. 11:17–22.
2018. View Article : Google Scholar
|
|
27
|
Dinç E, Dursun Ö, Yilmaz B, Vatansever M,
Sari AA, Yıldırım Ö and Adıgüzel U: Expression of prostaglandin E2
receptor subtypes in human pterygium and normal conjunctiva:
Immunohistochemical study. Int Ophthalmol. Jul 10–2017.Epub ahead
of print.
|
|
28
|
Yu Y and Chadee K: Prostaglandin E2
stimulates IL-8 gene expression in human colonic epithelial cells
by a posttranscriptional mechanism. J Immunol. 161:3746–3752.
1998.PubMed/NCBI
|
|
29
|
Nakayama T, Mutsuga N, Yao L and Tosato G:
Prostaglandin E2 promotes degranulation-independent release of
MCP-1 from mast cells. J Leukoc Biol. 79:95–104. 2006. View Article : Google Scholar
|
|
30
|
Weller CL, Collington SJ, Hartnell A,
Conroy DM, Kaise T, Barker JE, Wilson MS, Taylor GW, Jose PJ and
Williams TJ: Chemotactic action of prostaglandin E2 on mouse mast
cells acting via the PGE2 receptor 3. Proc Natl Acad Sci USA.
104:11712–11717. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Margeta-Mitrovic M, Jan YN and Jan LY: A
trafficking checkpoint controls GABA(B) receptor
heterodimerization. Neuron. 27:97–106. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Malty RH, Hudmon A, Fehrenbacher JC and
Vasko MR: Long-term exposure to PGE2 causes homologous
desensitization of receptor-mediated activation of protein kinase
A. J Neuroinflammation. 13:1812016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shibuya I, Setiadji SV, Ibrahim N,
Harayama N, Maruyama T, Ueta Y and Yamashita H: Involvement of
postsynaptic EP4 and presynaptic EP3 receptors in actions of
prostaglandin E2 in rat supraoptic neurones. J Neuroendocrinol.
14:64–72. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Su Y, Jackson EK and Gorelik E: Receptor
desensitization and blockade of the suppressive effects of
prostaglandin E(2) and adenosine on the cytotoxic activity of human
melanoma-infiltrating T lymphocytes. Cancer Immunol Immunother.
60:111–122. 2011. View Article : Google Scholar
|
|
35
|
Aoki T and Narumiya S: Prostaglandin
E2-EP2 signaling as a node of chronic inflammation in
the colon tumor microenvironment. Inflamm Regen. 37:42017.
View Article : Google Scholar
|
|
36
|
Salinas-Parra N, Reyes-Martinez C, Prieto
MC and Gonzalez AA: Prostaglandin E2 induces prorenin-dependent
activation of (Pro) renin receptor and upregulation of
cyclooxygenase-2 in collecting duct cells. Am J Med Sci.
354:310–318. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Byun JY, Youn YS, Lee YJ, Choi YH, Woo SY
and Kang JL: Interaction of apoptotic cells with macrophages
upregulates COX-2/PGE2 and HGF expression via a positive feedback
loop. Mediators Inflamm. 2014:4635242014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Baba Y, Nosho K, Shima K, Goessling W,
Chan AT, Ng K, Chan JA, Giovannucci EL, Fuchs CS and Ogino S:
PTGER2 overexpression in colorectal cancer is associated with
microsatellite instability, independent of CpG island methylator
phenotype. Cancer Epidemiol Biomarkers Prev. 19:822–831. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Castellone MD, Teramoto H, Williams BO,
Druey KM and Gutkind JS: Prostaglandin E2 promotes colon cancer
cell growth through a Gs-axin-beta-catenin signaling axis. Science.
310:1504–1510. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hsu HH, Chen MC, Day CH, Lin YM, Li SY, Tu
CC, Padma VV, Shih HN, Kuo WW and Huang CY: Thymoquinone suppresses
migration of LoVo human colon cancer cells by reducing
prostaglandin E2 induced COX-2 activation. World J Gastroenterol.
23:1171–1179. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lian S, Xia Y, Ung TT, Khoi PN, Yoon HJ,
Lee SG, Kim KK and Jung YD: Prostaglandin E2 stimulates
urokinase-type plasminogen activator receptor via EP2
receptor-dependent signaling pathways in human AGS gastric cancer
cells. Mol Carcinog. 56:664–680. 2017. View Article : Google Scholar
|
|
42
|
Merz C, von Mässenhausen A, Queisser A,
Vogel W, Andrén O, Kirfel J, Duensing S, Perner S and Nowak M: IL-6
overexpression in ERG-positive prostate cancer is mediated by
prostaglandin receptor EP2. Am J Pathol. 186:974–984. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zang S, Ma X, Wu Y, Liu W, Cheng H, Li J,
Liu J and Huang A: PGE2 synthesis and signaling in malignant
transformation and progression of human hepatocellular carcinoma.
Hum Pathol. 63:120–127. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Koh SJ, Kim JM, Kim IK, Ko SH and Kim JS:
Anti-inflammatory mechanism of metformin and its effects in
intestinal inflammation and colitis-associated colon cancer. J
Gastroenterol Hepatol. 29:502–510. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bonfill-Teixidor E, Otxoa-de-Amezaga A,
Font-Nieves M, Sans-Fons MG and Planas AM: Differential expression
of E-type prostanoid receptors 2 and 4 in microglia stimulated with
lipopolysaccharide. J Neuroinflammation. 14:32017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Huynh K: Inflammation: Targeting
inflammatory pathways to treat atherosclerosis and cancer. Nat Rev
Cardiol. 14:6292017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu Y, Fang S, Li X, Feng J, Du J, Guo L,
Su Y, Zhou J, Ding G, Bai Y, et al: Aspirin inhibits LPS-induced
macrophage activation via the NF-κB pathway. Sci Rep. 7:115492017.
View Article : Google Scholar
|
|
48
|
Kang X, Qiu J, Li Q, Bell KA, Du Y, Jung
DW, Lee JY, Hao J and Jiang J: Cyclooxygenase-2 contributes to
oxidopamine-mediated neuronal inflammation and injury via the
prostaglandin E2 receptor EP2 subtype. Sci Rep. 7:94592017.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gill SK, Yao Y, Kay LJ, Bewley MA,
Marriott HM and Peachell PT: The anti-inflammatory effects of PGE2
on human lung macrophages are mediated by the EP4 receptor. Br J
Pharmacol. 173:3099–3109. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kawahara K, Hohjoh H, Inazumi T, Tsuchiya
S and Sugimoto Y: Prostaglandin E2-induced inflammation: Relevance
of prostaglandin E receptors. Biochim Biophys Acta. 1851:414–421.
2015. View Article : Google Scholar
|
|
51
|
Jiang J, Quan Y, Ganesh T, Pouliot WA,
Dudek FE and Dingledine R: Inhibition of the prostaglandin receptor
EP2 following status epilepticus reduces delayed mortality and
brain inflammation. Proc Natl Acad Sci USA. 110:3591–3596. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hooper KM, Yen JH, Kong W, Rahbari KM, Kuo
PC, Gamero AM and Ganea D: Prostaglandin E2 Inhibition of IL-27
Production in Murine Dendritic Cells: A novel mechanism that
involves IRF1. J Immunol. 198:1521–1530. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li S, Xu X, Jiang M, Bi Y, Xu J and Han M:
Lipopolysaccharide induces inflammation and facilitates lung
metastasis in a breast cancer model via the prostaglandin E2-EP2
pathway. Mol Med Rep. 11:4454–4462. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li X, Su Y, Hua X, Xie C, Liu J, Huang Y,
Zhou L, Zhang M, Li X and Gao Z: Levels of hepatic Th17 cells and
regulatory T cells upregulated by hepatic stellate cells in
advanced HBV-related liver fibrosis. J Transl Med. 15:752017.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Fennekohl A, Lucas M and Püschel GP:
Induction by interleukin 6 of G(s)-coupled prostaglandin E(2)
receptors in rat hepatocytes mediating a prostaglandin
E(2)-dependent inhibition of the hepatocyte's acute phase response.
Hepatology. 31:1128–1134. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kabashima K, Nagamachi M, Honda T,
Nishigori C, Miyachi Y, Tokura Y and Narumiya S: Prostaglandin E2
is required for ultraviolet B-induced skin inflammation via EP2 and
EP4 receptors. Lab Invest. 87:49–55. 2007. View Article : Google Scholar
|
|
57
|
Prasad R and Katiyar SK: Ultraviolet
radiation-induced inflammation activates β-catenin signaling in
mouse skin and skin tumors. Int J Oncol. 44:1199–1206. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Prasad R and Katiyar SK: Prostaglandin E2
Promotes UV radiation-induced immune suppression through DNA
hyper-methylation. Neoplasia. 15:795–804. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ma X, Aoki T, Tsuruyama T and Narumiya S:
Definition of prostaglandin E2-EP2 signals in the colon tumor
microenvironment that amplify inflammation and tumor growth. Cancer
Res. 75:2822–2832. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Shehzad A, Ul Islam S, Lee J and Lee YS:
Prostaglandin E2 reverses curcumin-induced inhibition of survival
signal pathways in human colorectal carcinoma (HCT-15) cell lines.
Mol Cells. 37:899–906. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang D, Huang S, Yuan X, Liang J, Xu R,
Yao G, Feng X and Sun L: The regulation of the Treg/Th17 balance by
mesenchymal stem cells in human systemic lupus erythematosus. Cell
Mol Immunol. 14:423–431. 2017. View Article : Google Scholar :
|
|
62
|
Lejeune M, Moreau F and Chadee K: Loss of
EP2 receptor subtype in colonic cells compromise epithelial barrier
integrity by altering claudin-4. PLos One. 9:e1132702014.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Trésfier A, Musnier A, Landomiel F,
Bourquard T, Boulo T, Ayoub MA, León K, Bruneau G, Chevalier M,
Durand G, et al: G protein-dependent signaling triggers a
β-arrestin-scaffolded p70S6K/rpS6 module that controls 5'TOP mRNA
translation. FASEB J. 32:1154–1169. 2018. View Article : Google Scholar
|
|
64
|
Kaul V, Bhattacharya D, Singh Y, Van Kaer
L, Peters-Golden M, Bishai WR and Das G: An important role of
prostanoid receptor EP2 in host resistance to Mycobacterium
tuberculosis infection in mice. J Infect Dis. 206:1816–1825. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Qian X, Zhang J and Liu J: Tumor-secreted
PGE2 inhibits CCL5 production in activated macrophages through
cAMP/PKA signaling pathway. J Biol Chem. 286:2111–2120. 2011.
View Article : Google Scholar :
|
|
66
|
Leander R and Friedman A: Modulation of
the cAMP response by Gαi and Gβγ: A computational study of G
protein signaling in immune cells. Bull Math Biol. 76:1352–1375.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Sreeramkumar V, Hons M, Punzón C, Stein
JV, Sancho D, Fresno M and Cuesta N: Efficient T-cell priming and
activation requires signaling through prostaglandin E2 (EP)
receptors. Immunol Cell Biol. 94:39–51. 2016. View Article : Google Scholar
|
|
68
|
Mao Y, Sarhan D, Steven A, Seliger B,
Kiessling R and Lundqvist A: Inhibition of tumor-derived
prostaglandin-e2 blocks the induction of myeloid-derived suppressor
cells and recovers natural killer cell activity. Clin Cancer Res.
20:4096–4106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yang L, Yamagata N, Yadav R, Brandon S,
Courtney RL, Morrow JD, Shyr Y, Boothby M, Joyce S, Carbone DP and
Breyer RM: Cancer-associated immunodeficiency and dendritic cell
abnormalities mediated by the prostaglandin EP2 receptor. J Clin
Invest. 111:727–735. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Marin-Acevedo JA, Soyano AE, Dholaria B,
Knutson KL and Lou Y: Cancer immunotherapy beyond immune checkpoint
inhibitors. J Hematol Oncol. 11:82018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Fujimoto Y, Iwagaki H, Ozaki M, Ogino T,
Murata H, Sun DS, Sadamori H, Takahashi HK, Tanaka N and Yagi T:
Involvement of prostaglandin receptors (EPR2-4) in in vivo
immunosuppression of PGE2 in rat skin transplant model. Int
Immunopharmacol. 5:1131–1139. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Bonanno A, Albano GD, Siena L, Montalbano
AM, Riccobono L, Anzalone G, Chiappara G, Gagliardo R, Profita M
and Sala A: Prostaglandin E2 possesses different
potencies in inducing Vascular Endothelial Growth Factor and
Interleukin-8 production in COPD human lung fibroblasts.
Prostaglandins Leukot Essent Fatty Acids. 106:11–18. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wiktorowska-Owczarek A and Owczarek J: The
effect of hypoxia on PGE2-stimulated cAMP generation in HMEC-1.
Cell Mol Biol Lett. 20:213–221. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Aoyagi T, Newstead MW, Zeng X, Nanjo Y,
Peters-Golden M, Kaku M and Standiford TJ: Interleukin-36γ and
IL-36 receptor signaling mediate impaired host immunity and lung
injury in cytotoxic Pseudomonas aeruginosa pulmonary infection:
Role of prostaglandin E2. PLos Pathog. 13:e10067372017. View Article : Google Scholar
|
|
75
|
Takahashi T, Uehara H and Izumi K:
Inhibitory effect of soluble EP2 receptor on ovarian tumor growth
in nude mice and utility of TMPRSS4 as a combinatorial molecular
target. Int J Oncol. 43:416–424. 2013.PubMed/NCBI
|
|
76
|
Cheng SY, Zhang H, Zhang M, Xia SK, Bai
XM, Zhang L, Ma J, Rong R, Wang YP, Du MZ, et al: Prostaglandin
E2 receptor EP2 mediates Snail expression in
hepatocellular carcinoma cells. Oncol Rep. 31:2099–2106. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Sobhani N, Roviello G, Corona SP,
Scaltriti M, Ianza A, Bortul M, Zanconati F and Generali D: The
prognostic value of PI3K mutational status in breast cancer: A
meta-analysis. J Cell Biochem. 119:4287–4292. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ma W and St-Jacques B: Signalling
transduction events involved in agonist-induced PGE2/EP4 receptor
externalization in cultured rat dorsal root ganglion neurons. Eur J
Pain. 22:845–861. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Regan JW: EP2 and EP4 prostanoid receptor
signaling. Life Sci. 74:143–153. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Allison SE, Petrovic N, Mackenzie PI and
Murray M: Pro-migratory actions of the prostacyclin receptor in
human breast cancer cells that overexpress cyclooxygenase-2.
Biochem Pharmacol. 96:306–314. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Cheuk IW, Shin VY, Siu MT, Tsang JY, Ho
JC, Chen J, Tse GM, Wang X and Kwong A: Association of EP2 receptor
and SLC19A3 in regulating breast cancer metastasis. Am J Cancer
Res. 5:3389–3399. 2015.
|
|
82
|
Kim KM, Im AR, Kim SH, Hyun JW and Chae S:
Timosaponin AIII inhibits melanoma cell migration by suppressing
COX-2 and in vivo tumor metastasis. Cancer Sci. 107:181–188. 2016.
View Article : Google Scholar :
|
|
83
|
Rundhaug JE, Simper MS, Surh I and Fischer
SM: The role of the EP receptors for prostaglandin E2 in skin and
skin cancer. Cancer Metastasis Rev. 30:465–480. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Rundhaug JE and Fischer SM:
Cyclo-oxygenase-2 plays a critical role in UV-induced skin
carcinogenesis. Photochem Photobiol. 84:322–329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Singh T, Vaid M, Katiyar N, Sharma S and
Katiyar SK: Berberine, an isoquinoline alkaloid, inhibits melanoma
cancer cell migration by reducing the expressions of
cyclo-oxygenase-2, prostaglandin E2 and prostaglandin
E2 receptors. Carcinogenesis. 32:86–92. 2011. View Article : Google Scholar
|
|
86
|
Mochocki M, Morawski P, Kopta R,
Brzezinska-Blaszczyk E, Stasikowska O, Lewy-Trenda I; Student
Scientific Circle; Starska K: Expression of prostaglandin E2
prostanoid receptor EP2 and interleukin-1β in laryngeal
carcinoma-preliminary study. Contemp Oncol (Pozn). 19:113–119.
2015.
|
|
87
|
Cantaut-Belarif Y, Antri M, Pizzarelli R,
Colasse S, Vaccari I, Soares S, Renner M, Dallel R, Triller A and
Bessis A: Microglia control the glycinergic but not the GABAergic
synapses via prostaglandin E2 in the spinal cord. J Cell Biol.
216:2979–2989. 2017.PubMed/NCBI
|
|
88
|
de Almeida VH, de Melo AC, Meira DD, Pires
AC, Nogueira-Rodrigues A, Pimenta-Inada HK, Alves FG, Moralez G,
Thiago LS, Ferreira CG and Sternberg C: Radiotherapy modulates
expression of EGFR, ERCC1 and p53 in cervical cancer. Braz J Med
Biol Res. 51:e68222017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ochoa MC, Minute L, López A4, Pérez-Ruiz
E, Gomar C, Vasquez M, Inoges S, Etxeberria I, Rodriguez I, Garasa
S, et al: Enhancement of antibody-dependent cellular cytotoxicity
of cetuximab by a chimeric protein encompassing interleukin-15.
Oncoimmunology. 7:e13935972017. View Article : Google Scholar
|
|
90
|
Chen A, Ali N, Boasberg P and Ho AS:
Clinical remission of cutaneous squamous cell carcinoma of the
auricle with cetuximab and nivolumab. J Clin Med. 7:pii: E10. 2018.
View Article : Google Scholar
|
|
91
|
Fernández-Martínez AB and Lucio-Cazaña J:
Intracellular EP2 prostanoid receptor promotes cancer-related
phenotypes in PC3 cells. Cell Mol Life Sci. 72:3355–3373. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
López Bernal A, Rivera J, Europe-Finner
GN, Phaneuf S and Asbóth G: Parturition: Activation of stimulatory
pathways or loss of uterine quiescence? Adv Exp Med Biol.
395:435–451. 1995.PubMed/NCBI
|
|
93
|
Brocard E, Oizel K, Lalier L, Pecqueur C,
Paris F, Vallette FM and Oliver L: Radiation-induced PGE2 sustains
human glioma cells growth and survival through EGF signaling.
Oncotarget. 6:6840–6849. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Schmidt A, Sinnett-Smith J, Young S, Chang
HH, Hines OJ, Dawson DW, Rozengurt E and Eibl G: Direct
growth-inhibitory effects of prostaglandin E2 in pancreatic cancer
cells in vitro through an EP4/PKA-mediated mechanism. Surgery.
161:1570–1578. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Vaid M, Singh T, Prasad R, Kappes JC and
Katiyar SK: Therapeutic intervention of proanthocyanidins on the
migration capacity of melanoma cells is mediated through PGE2
receptors and β-catenin signaling molecules. Am J Cancer Res.
5:3325–3338. 2015.
|
|
96
|
Chang HH, Young SH, Sinnett-Smith J, Chou
CE, Moro A, Hertzer KM, Hines OJ, Rozengurt E and Eibl G:
Prostaglandin E2 activates the mTORC1 pathway through an
EP4/cAMP/PKA- and EP1/Ca2+-mediated mechanism in the human
pancreatic carcinoma cell line PANC-1. Am J Physiol Cell Physiol.
309:C639–C649. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Chun KS, Lao HC, Trempus CS, Okada M and
Langenbach R: The prostaglandin receptor EP2 activates multiple
signaling pathways and beta-arrestin1 complex formation during
mouse skin papilloma development. Carcinogenesis. 30:1620–1627.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Shu J, Zhang F, Zhang L and Wei W: G
protein coupled receptors signaling pathways implicate in
inflammatory and immune response of rheumatoid arthritis. Inflamm
Res. 66:379–387. 2017. View Article : Google Scholar
|
|
99
|
Yun SP, Ryu JM, Jang MW and Han HJ:
Interaction of profilin-1 and F-actin via a β-arrestin-1/JNK
signaling pathway involved in prostaglandin E(2)-induced human
mesenchymal stem cells migration and proliferation. J Cell Physiol.
226:559–571. 2011. View Article : Google Scholar
|
|
100
|
Narumiya S, Sugimoto Y and Ushikubi F:
Prostanoid receptors: Structures, properties, and functions.
Physiol Rev. 79:1193–1226. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Ganesh T: Prostanoid receptor EP2 as a
therapeutic target. J Med Chem. 57:4454–4465. 2014. View Article : Google Scholar :
|
|
102
|
Tani K, Naganawa A, Ishida A, Egashira H,
Sagawa K, Harada H, Ogawa M, Maruyama T, Ohuchida S, Nakai H, et
al: Design and synthesis of a highly selective EP2-receptor
agonist. Bioorg Med Chem Lett. 11:2025–2028. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Jadhav V, Jabre A, Lin SZ and Lee TJ: EP1-
and EP3-receptors mediate prostaglandin E2-induced constriction of
porcine large cerebral arteries. J Cereb Blood Flow Metab.
24:1305–1316. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Tani K, Naganawa A, Ishida A, Sagawa K,
Harada H, Ogawa M, Maruyama T, Ohuchida S, Nakai H, Kondo K and
Toda M: Development of a highly selective EP2-receptor agonist.
Part 1: Identification of 16-hydroxy-17, 17-trimethylene PGE2
derivatives. Bioorg Med Chem. 10:1093–1106. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Markovič T, Jakopin Ž, Dolenc MS and
Mlinarič-Raščan I: Structural features of subtype-selective EP
receptor modulators. Drug Discov Today. 22:57–71. 2017. View Article : Google Scholar
|
|
106
|
Yeo HS, Shehzad A and Lee YS:
Prostaglandin E2 blocks menadione-induced apoptosis through the
Ras/Raf/Erk signaling pathway in promonocytic leukemia cell lines.
Mol Cells. 33:371–378. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Tanné B, Bernier S and Dumais N: CCR7
receptor expression in Mono-MAC-1 cells: modulation by liver X
receptor α activation and prostaglandin E 2. Int J Inflam.
2015:2015712015. View Article : Google Scholar
|
|
108
|
Takahashi HK, Liu K, Wake H, Mori S, Zhang
J, Liu R, Yoshino T and Nishibori M: Prostaglandin E2 inhibits
advanced glycation end product-induced adhesion molecule
expression, cytokine production, and lymphocyte proliferation in
human peripheral blood mononuclear cells. J Pharmacol Exp Ther.
331:656–670. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Wang R, Zhang W, Dong Z, Qi Y, Hultström
M, Zhou X and Lai EY: c-Jun N-terminal Kinase mediates
prostaglandin-induced sympathoexcitation in rats with chronic heart
failure by reducing GAD1 and GABRA1 expression. Acta Physiol (Oxf).
219:494–509. 2017. View Article : Google Scholar
|
|
110
|
Cameron KO, Lefker BA, Ke HZ, Li M,
Zawistoski MP, Tjoa CM, Wright AS, DeNinno SL, Paralkar VM, Owen
TA, et al: Discovery of CP-533536: An EP2 receptor selective
prostaglandin E2 (PGE2) agonist that induces local bone formation.
Bioorg Med Chem Lett. 19:2075–2078. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Säfholm J, Dahlén SE and Adner M:
Antagonising EP1 and EP2 receptors reveal that the TP receptor
mediates a component of antigen-induced contraction of the guinea
pig trachea. Eur J Pharmacol. 718:277–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Yan G, Wang Q, Shi H, Han Y, Ma G, Tang C
and Gu Y: Regulation of rat intrapulmonary arterial tone by
arachidonic acid and prostaglandin E2 during hypoxia. PLoS One.
8:e738392013. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Kay LJ, Gilbert M, Pullen N, Skerratt S,
Farrington J, Seward EP and Peachell PT: Characterization of the EP
receptor subtype that mediates the inhibitory effects of
prostaglandin E2 on IgE-dependent secretion from human lung mast
cells. Clin Exp Allergy. 43:741–751. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
af Forselles KJ, Root J, Clarke T, Davey
D, Aughton K, Dack K and Pullen N: In vitro and in vivo
characterization of PF-04418948, a novel, potent and selective
prostaglandin EP2 receptor antagonist. Br J Pharmacol.
164:1847–1856. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45(W1):
W98–W102. 2017. View Article : Google Scholar : PubMed/NCBI
|