Introduction
Sepsis, a systemic inflammatory the potential
prevention role of GDF-15 in sepsis, mice were intravenously
injected with GDF-15 (10 mg/kg) after being administered with LPS
(20 mg/kg) and D-GalN (700 mg/kg). Mice in the negative control
group were treated with vehicle (sterile PBS). All of the mice were
monitored for 36 h to assess their survival rates response syndrome
caused by severe microbial infection, remains the leading cause of
death in intensive care units worldwide (1,2).
Research efforts in the field of sepsis have focused primarily on
the innate immune system, and typically, have conceptually viewed
sepsis as a hyper-inflammation syndrome (3,4).
Acute liver injury, characterized by severe hepatic injury with
failure of hepatocyte function, is an important cause of morbidity
and mortality in patients with sepsis (5). The importance of macrophage
activation and endotoxin-mediated proinflammatory cytokine
production during liver injury is evident from numerous models of
acute and chronic liver diseases (6). Pro-inflammatory cytokines, such as
tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1, are
primarily involved in the promotion of inflammatory processes, and
have an important role in liver injury (7,8).
Lipopolysaccharide (LPS), a constituent of the outer
cell wall of gram-negative bacteria, has the ability to elicit a
severe inflammatory response in organisms, ultimately resulting in
systemic inflammatory response syndrome. Liver cells, including
Kupffer cells, hepatocytes and sinusoidal endothelial cells, can
take up circulating LPS, following both ex vivo exposure to
LPS and LPS injection (9).
D-galactosamine (D-GalN) can increase the sensitivity of liver
cells to LPS, and induce liver injury, as well as elevate serum
TNF-α levels abnormally in the presence of low-dose LPS (10). Therefore, the combined use of LPS
and D-GalN is used to establish a successful liver dysfunction
model (10).
Growth differentiation factor-15 (GDF-15), also
known as macrophage inhibitory cytokine-1 (MIC-1) and nonsteroidal
anti-inflammatory drug-activated gene-1 (NAG-1), is a divergent
member of the transforming growth factor (TGF) β family related to
immunosuppression, anti-apoptosis, anti-inflammation, growth
inhibition, and cancer cell invasion (11). Previous investigations have
suggested that GDF-15 has important roles in heart diseases. In
patients with atrial fibrillation, GDF-15 is an independent risk
indicator for major bleeding and all-cause mortality, although not
for stroke (12). In patients
with acute heart failure, enrolled in the RELAX in Acute Heart
Failure study, increases in GDF-15 levels, although not baseline
measurements, were related to a greater risk of adverse outcomes
(13). A previous experimental
study has demonstrated that GDF-15 deficiency augments inflammatory
responses, and exacerbates LPS-induced renal and cardiac injury,
while GDF-15 overexpression protects the kidney and heart from
LPS-induced organ dysfunction (14). The present study aimed to
determine whether GDF-15 participates in sepsis-induced acute liver
injury in mice, by establishing an experimental model for acute
liver injury using LPS and D-GalN. The histological changes,
inflammation status, and potential mechanism were investigated.
Materials and methods
Animals and experimental models
C57BL/6 male mice (5–6 weeks old, 20-22 g weight)
were obtained from Beijing HFK Bioscience Co., Ltd. (Beijing,
China). The animals were housed on 12-h light/dark cycles at 25°C.
Animals received standard animal rodent chow and water ad
libitum. Care of animals and the experimental protocols for
this study were approved by the Institutional Animal Use Committee
of the Southern Medical University (Guangzhou, China).
LPS (Escherichia coli 0111:B4), D-GalN and
curcumin were obtained from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany). Acute liver injury was induced by intraperitoneal
injection of 20 µg/kg body weight of LPS and 700 mg/kg body
weight of D-GalN. A total of 48 mice were randomly divided into 3
groups. Mice in the control group received injections of PBS alone.
Mice in the model group received LPS injections and 15 min later
were injected with D-GalN. Mice in the GDF-15 treatment group were
injected intravenously with GDF-15 [1 mg/kg body weight (15); purchased from Sino Bio, Beijing,
China] 10 min after the D-GalN injection. A total of 6 mice in each
group were anesthetized and sacrificed 6 h following LPS injection.
The liver tissue and blood serum were collected for further
analysis. A total of 10 mice in each group were used for survival
analysis for an additional 36 h.
Measurement of serum aminotransferase
activities
Serum aspartate aminotransferase (AST) and alanine
aminotransferase (ALT) activities were measured via the enzymatic
kinetic method, by using an automatic biochemistry analyzer
(SELECTA XL; Vital Scientific, Dieren, Netherlands) according to
the manufacturers' protocol.
Histological analysis
Liver tissues were fixed in 4% paraformaldehyde
solution at room temperature (22–25°C) for 48 h, embedded in
paraffin, and sectioned at 5-µm. Following dehydration,
sections were stained with hematoxylin and eosin (H&E) at room
temperature (22–25°C) according to the previously reported methods
(hematoxylin staining for 3 min and eosin staining for 1 min)
(16). Grading was adapted from
t'Hart et al (17) and
described as: 1, normal rectangular structure; 2, rounded
hepatocytes with an increase in the sinusoidal spaces; 3,
vacuolization; 4, nuclear picnosis; and 5, necrosis. Histological
evaluations of the damage scores were performed in a blinded manner
by 3 different observers.
ELISA
The IL-6 (cat. no. EMC004.96; NBS Biologicals, Ltd.,
Shenzhen, China; http://www.nbs-bio.com/), TNF-α (cat. no. EMC102a.96;
NBS Biologicals, Ltd.), IL-1β (cat. no. EMC001b.96; NBS
Biologicals, Ltd.), cyclo-oxygenase-2 (COX-2; cat. no. ab210574;
Abcam, Cambridge, UK) and monocyte chemoattractant protein-1
(MCP-1; cat. no. EMC113.96; NBS Biologicals, Ltd.) levels in the
serum, liver tissues and medium were determined by ELISA analysis,
following the instructions of the kit manufacturer.
Measurement of malondialdehyde (MDA)
MDA was quantified as thiobarbituric acid reactive
substances (TBARS), according to previously published methods
(18). Briefly, the weighed
samples were homogenized in 1 ml 5% trichloroacetic acid. The
samples were centrifuged (10,000 × g) at 4°C for 5 min and 250 ml
of the supernatant was reacted with the same volume of 20 mM
thiobarbituric acid for 35 min at 95°C, followed by 10 min at 4°C.
Sample fluorescence was measured using a spectrophotometric plate
reader at the wavelength of 545 nm.
Liver myeloperoxidase (MPO) assay
The liver MPO was determined as previously described
(19). Briefly, the liver tissue
was homogenized (50 mg/ml) in 0.5% hexadecyltrimethylammonium
bromide in 10 mM 3-(N-morpholino) propanesulfonic acid and
centrifuged (15,000 × g) at 4°C for 40 min. The suspension was then
sonicated 3 times for 30 sec at 1 min intervals. An aliquot of
supernatant was mixed with a solution of 1.6 mM
tetramethylbenzidine and 1 mM H2O2. The
activity was measured spectrophotometrically as the change in
absorbance at 37°C with a microplate reader (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The results are expressed as
units of MPO activity per gram of protein, as determined by the
Bradford assay (Beyotime Institute of Biotechnology, Beijing,
China).
Immunofluorescence staining
Liver tissues were fixed in 4% paraformaldehyde
solution at room temperature (22–25°C) for 48 h, embedded in
paraffin, and sectioned at 5-µm. Following dehydration and
antigen retrieval (3 min under high pressure), tissue samples were
blocked with goat serum at room temperature (22–25°C) for 15 min
and incubated with specific rabbit monoclonal antibody against
inducible nitric oxide synthase (iNOS; 1:200; cat. no. ab15323;
Abcam, Cambridge, UK) and rabbit monoclonal antibodies against CD68
(1:150; cat. no. ab125212; Abcam) at 4°C overnight. A secondary
antibody, either fluorescein isothiocyanate (FITC; 1:100; cat. no.
sc-2012) or cyanine (Cy) 3 (1:100; cat. no. sc-2010)-conjugated
anti-rabbit or anti-mouse IgG (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) was then added and incubated at 37°C for 1 h. The
nuclei were stained with DAPI (Beyotime Institute of
Biotechnology). Images were captured with a Nikon DX500 fluorescent
laser-scanning microscope (Nikon Corporation, Tokyo, Japan).
Cell culture and treatment
Kupffer cells were obtained from Jennio Bio
(Guangzhou, China), and they were confirmed by a short tandem
repeat analysis. The cells were routinely maintained in DMEM
(Invitrogen; Thermo Fisher Scientific, Inc.) with 10% fetal bovine
serum (FBS; Merck KGaA) and 1% antibiotic-antimitotic reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). For treatments,
Kupffer cells were plated in 6-well plates at 2×105 per
well. A total of 24 h post-plating, LPS (2 µg/ml) was added
to the cells, while sterile PBS was used as negative control.
GDF-15 (10 ng/ml) was added into the supernatant of LPS-treated
cells, as previous described (20). At 24 h post-treatment, the
supernatant and total protein were collected for ELISA and western
blotting analyses.
Western blotting analysis
Cells were collected and lysed with RIPA lysis
buffer (Beyotime Institute of Biotechnology) containing 1/100
protease inhibitor cocktail (Merck KGaA). Following centrifugation
at 12,000 × g, 4°C for 15 min, the proteins were collected and used
for concentration measurement with a BCA kit (Beyotime Institute of
Biotechnology). Total protein (20 µg) from each sample was
separated by SDS-PAGE (8 or 10%) and transferred to polyvinylidene
fluoride membranes (Merck KGaA) electro-phoretically. The membranes
were blocked with 5% nonfat milk in Tris-buffered saline containing
0.5% Tween-20 and then incubated overnight at 4°C with the
following primary antibodies from Cell Signaling Technology, Inc.
(Danvers, MA, USA): nuclear factor (NF)-κB p65 (1:800; cat. no.
8242), phosphorylated (p)-TGFβ-activated kinase 1 (TAK1; 1:1,200;
cat. no. 4505), TAK1 (1:800; cat. no. 9339), NF-κB p50 (1:1,000;
cat. no. 12540), p-NF-κB inhibitor α (IκBα; 1:800; cat. no. 5209),
IκBα (1:1,000; cat. no. 4814) and GAPDH (1:2,000; cat. no. 2118).
The membranes were then incubated with horseradish-peroxidase
conjugated anti-mouse (1:5,000; cat. no. ZDR5307) and anti-rabbit
IgG (1:5,000; cat. no. ZDR5306; both from Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China), for 1 h at room
temperature. The protein bands were developed using enhanced
chemiluminescence detection reagent (Merck KGaA) and quantified
using ImageJ (v1.8.0_112; National Institutes of Health, Bethesda,
MD, USA) (21). The relative
expression was normalized to GAPDH levels.
Flow cytometry
Cultured Kupffer cells were collected and stained
with unlabeled antibodies targeting iNOS (1:200; cat. no. ab15323;
Abcam) in PBS for 1 h at room temperature. After washing with PBS 3
times, a secondary FITC-conjugated anti-rabbit antibody (1:500;
cat. no. sc-2012; Santa Cruz Biotechnology, Inc.) was added for
further incubation at 4°C for 1 h in dark. The cells were analyzed
by flow cytometry on a BD FACSCalibur instrument. Data were
analyzed using FlowJo software (v10.2; FlowJo, LLC, Ashland, OR,
USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total mRNA was extracted from the cells using TRIzol
(Thermo Fisher Scientific, Inc.). Reverse transcription was
performed following the protocol of the PrimeScript RT reagent kit
with gDNA eraser (cat. no. RR047A; Takara Biotechnology Co., Ltd.,
Dalian, China). The Permix Taq kit (cat. no. RR066A; Takara
Biotechnology Co., Ltd.) was used for qPCR. All of the reactions
were performed using the CFX96 Touch Real-Time PCR detection system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The qPCR
conditions were as follows: Denaturation at 94°C for 2 min,
amplification for 30 cycles at 94°C for 0.5 min, annealing at 60°C
for 0.5 min and extension at 72°C for 1 min, followed by a terminal
elongation step at 72°C for 10 min. The threshold cycle (Cq) values
were determined by plotting the observed fluorescence against the
cycle number. Cq values were analyzed using the comparative
threshold cycle method and normalized to those of GAPDH (22). The relative gene expression levels
were estimated using the following formula: Relative expression = 2
- [Cq (iNOS) - Cq (GAPDH)]. The sequence of primers for
iNOS: Forward, 5′-GAGCGAGTTGTGGATTGTC-3′ and reverse,
5′-CTCCTTTGAGCCCTTTTGT-3′; for GAPDH: Forward,
5′-GGTCGGAGTCAACGGATTTGGTCG-3′ and reverse,
5′-CCTCCGACGCCTGCTTCACCAC-3′.
Statistical analysis
Data were expressed as means ± standard deviation.
All of the data were analyzed with SPSS 17.0 version (SPSS, Inc.,
Chicago, IL, USA). One-way analysis of variance followed by a
Dunnett's post hoc test for multiple comparisons and a students' t
test was used to compare two groups. P<0.05 was considered to
indicate a statistically significant difference. Three to five
random experiments were repeated.
Results
Protective effect of GDF-15 in
LPS/D-GalN-induced acute liver injury
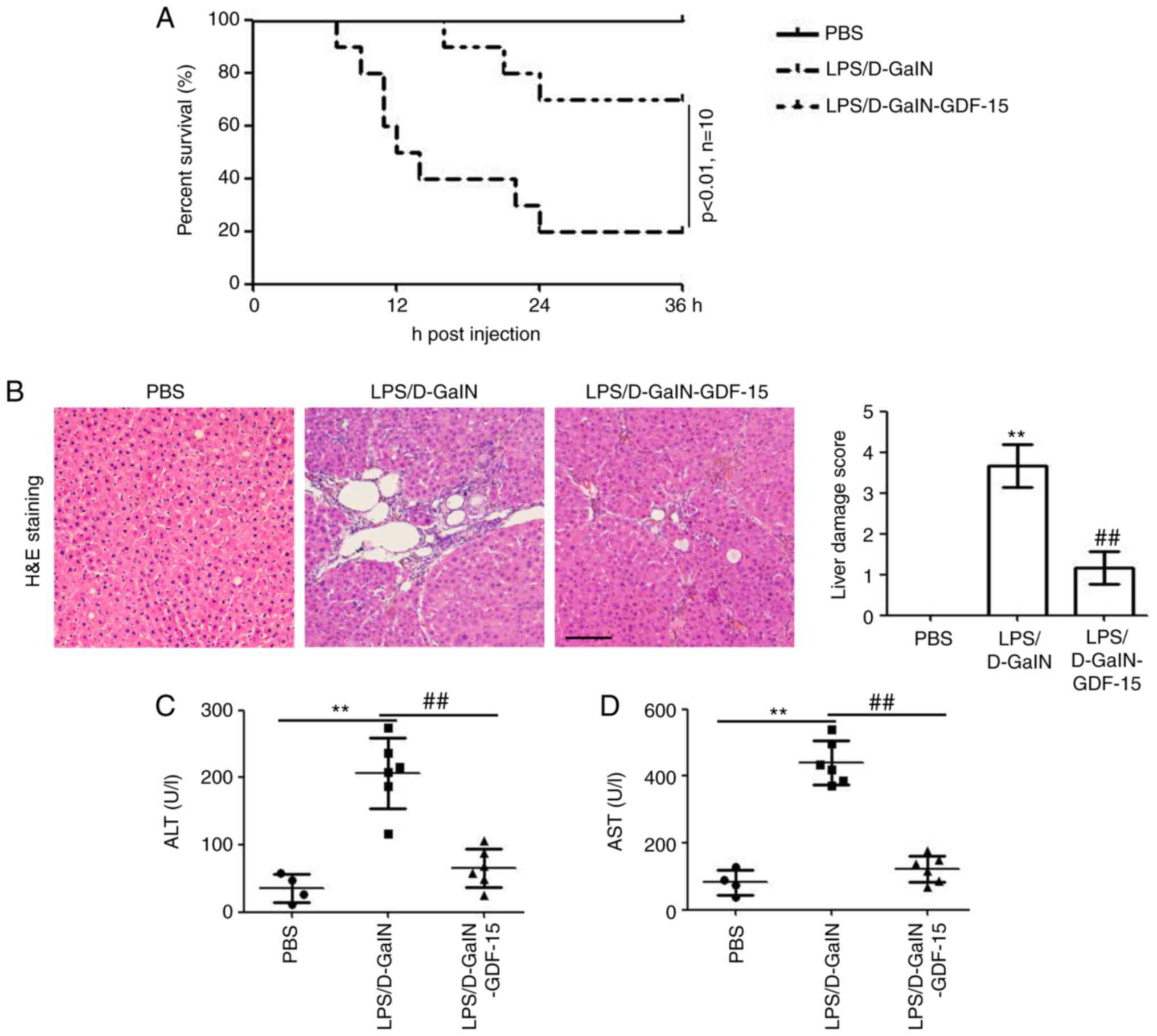
To evaluate. As illustrated in Fig. 1A, LPS/D-GalN administration
resulted in death in 80% of the mice death at 36 h compared with
the PBS injection group. However, GDF-15 treatment efficiently
enhanced the survival of mice (only 3 out of 10 mice were dead at
36 h post LPS/D-GalN injection; Fig.
1A). At 6 h post LPS/D-GalN injection, liver tissues from the
mice were used for histological examination. Results from H&E
staining identified hepatocyte necrosis and inflammatory cell
infiltration in the model group (LPS/D-GalN administration alone),
while these effects were markedly less evident in the GDF-15
treatment group (Fig. 1B). To
determine the effect of GDF-15 on liver injury induced by LPS/GalN,
the serum levels of AST and ALT were assessed. The levels of ALT
and AST were significantly increased by LPS/D-GalN administration
(Fig. 1C and D). However, GDF-15
treatment significantly decreased the activities of ALT and AST,
compared with the model group (Fig.
1C and D). Collectively, these results demonstrated the
preventative role of GDF-15 in LPS/D-GalN-induced acute liver
injury.
GDF-15 represses liver inflammation
induced by LPS/D-GalN
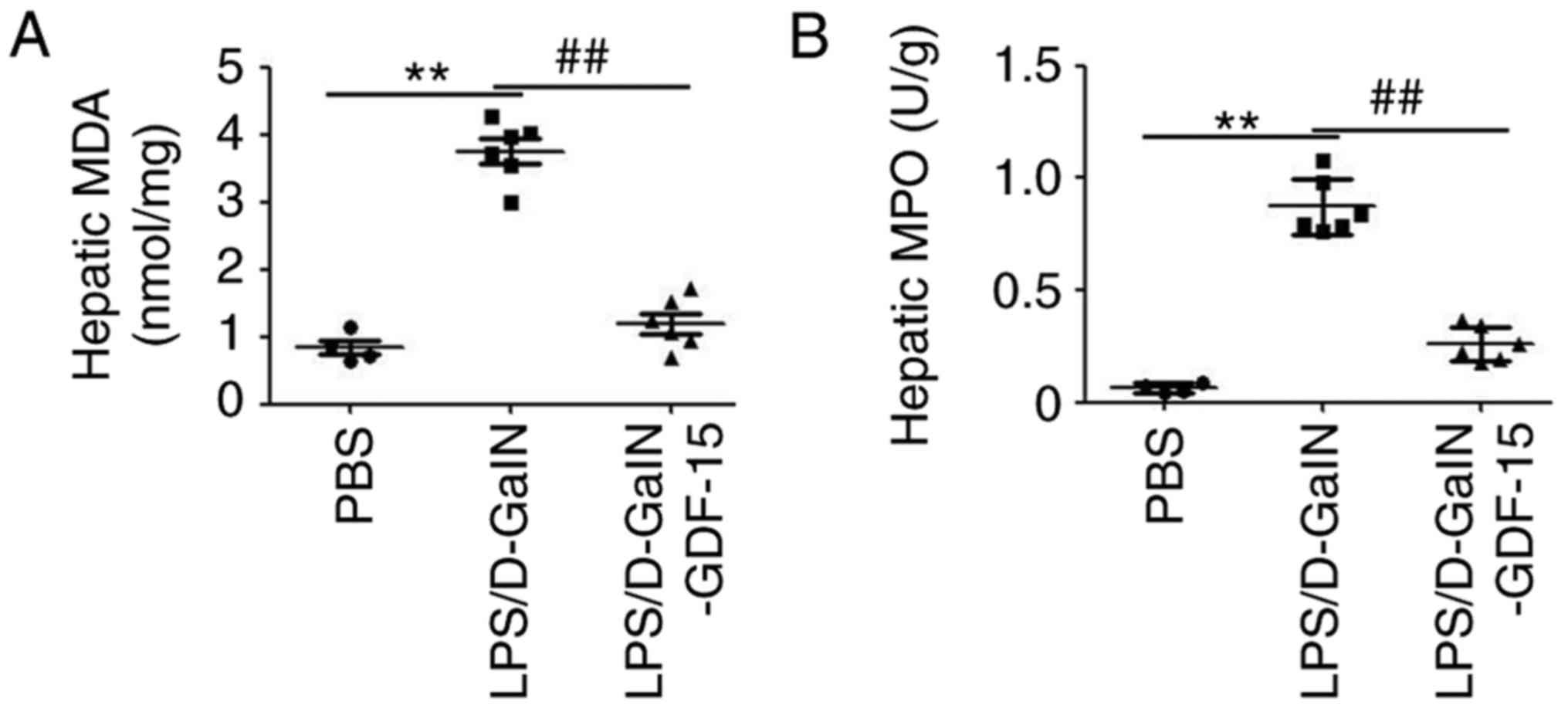
Subsequently, to determine the potential
anti-inflammatory effect of GDF-15, the levels of the pro-MDA and
MPO were monitored in liver. As presented in Fig. 2A, LPS/D-GalN administration
increased the amount of MDA compared with the control group.
However, compared with the model group, the content of MDA was
decreased in the GDF-15-treated mice (Fig. 2A). In addition, an MPO assay
demonstrated that GDF-15 dramatically attenuated LPS/D-GalN-induced
leukocyte infiltration in liver (Fig.
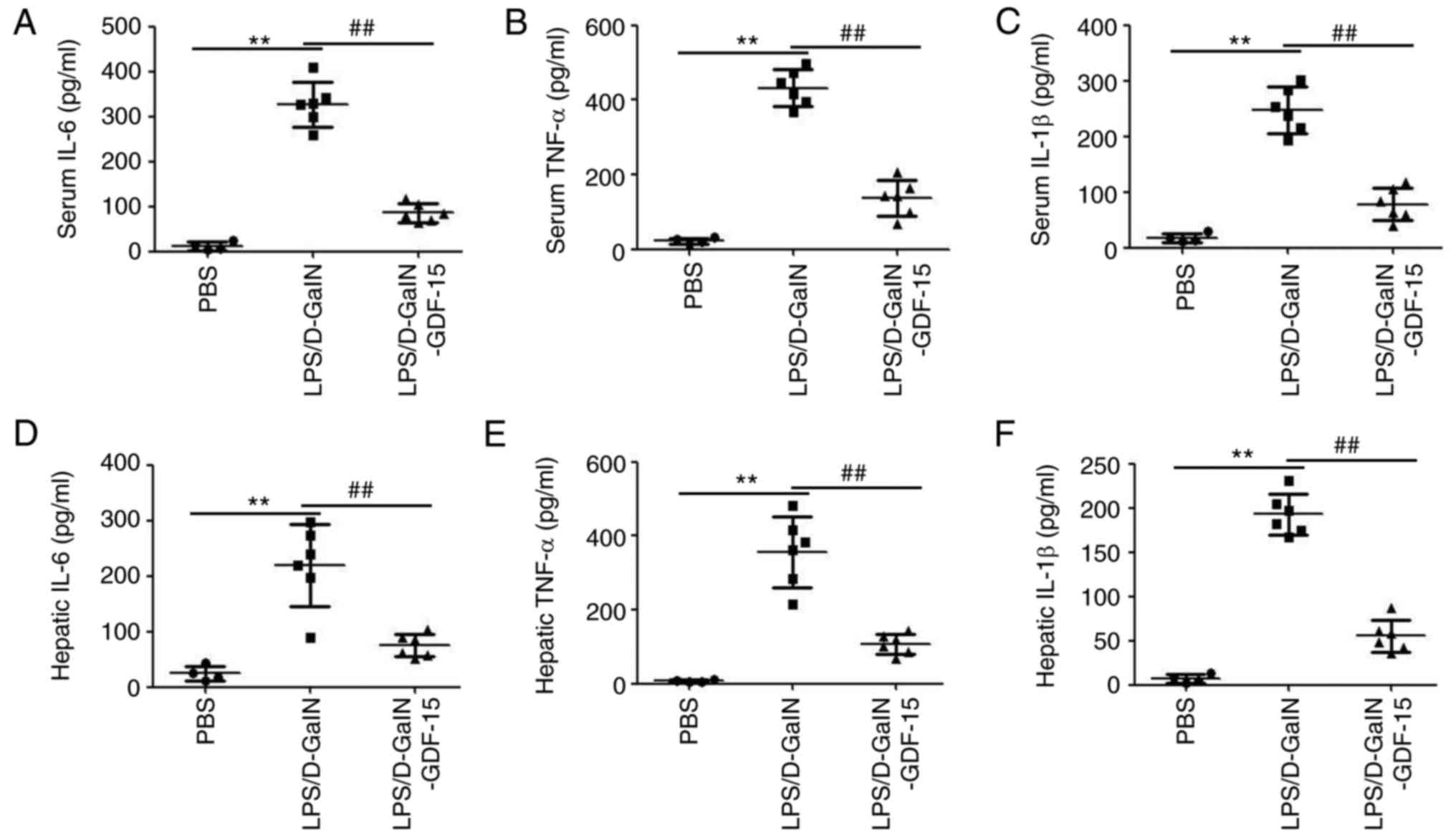
2B). ELISA analysis demonstrated the upregulation of
pro-inflammatory cytokines IL-6, TNF-α and IL-1β both in serum and
liver tissue of mice following LPS/D-GalN administration (Fig. 3). Furthermore, this increase in
serum and hepatic IL-6, TNF-α and IL-1β expression was prevented in
mice treated with GDF-15 (Fig.
3). These results indicated that GDF-15 had an obvious
anti-inflammatory activity in vivo.
GDF-15 inhibits LPS/D-GalN-induced iNOS
activation in liver
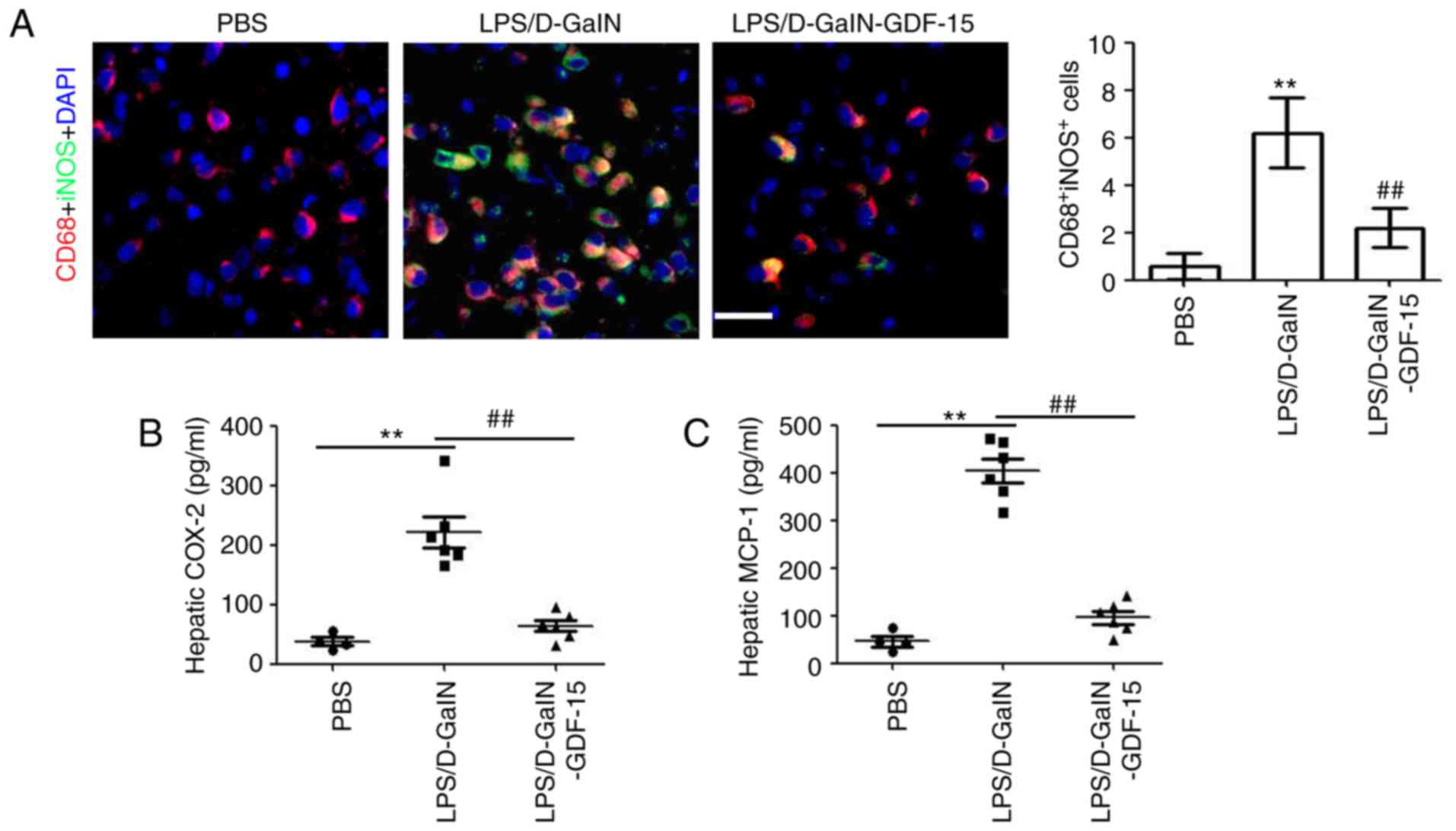
To determine the effect of GDF-15 on macrophage
activation, immunofluorescent staining was performed to detect
iNOS-positive macrophages in liver tissue. Immunofluorescent
staining of liver samples revealed that iNOS expression
colocalizing with CD68, a macrophage marker, was markedly increased
following LPS/D-GalN administration compared with the control group
(Fig. 4A). However, compared with
the model group, less iNOS/CD68-positive macrophages were observed
in the GDF-15-treated mice (Fig.
4A). Then, the levels of, the macrophage-associated
pro-inflammatory cytokines COX-2 and MCP-1 were determined. The
results demonstrated the increased expression of hepatic COX-2 and
MCP-1 in mice following LPS/D-GalN administration (Fig. 4B and C). Treatment with GDF-15,
however, inhibited the LPS/D-GalN-induced COX-2 and MCP-1
expression in liver (Fig. 4B and
C). Collectively, these results suggested that GDF-15 inhibited
the activation of pro-inflammatory macrophages in vivo.
GDF-15 attenuates LPS-induced
inflammation and iNOS activation in Kupffer cells
To investigate the anti-inflammatory mechanism of
GDF-15 in LPS/D-GalN-induced acute liver injury, GDF-15 was used to
treat Kupffer cells in vitro. ELISA analysis was performed
to determine the IL-6, TNF-α and IL-1β expression in the
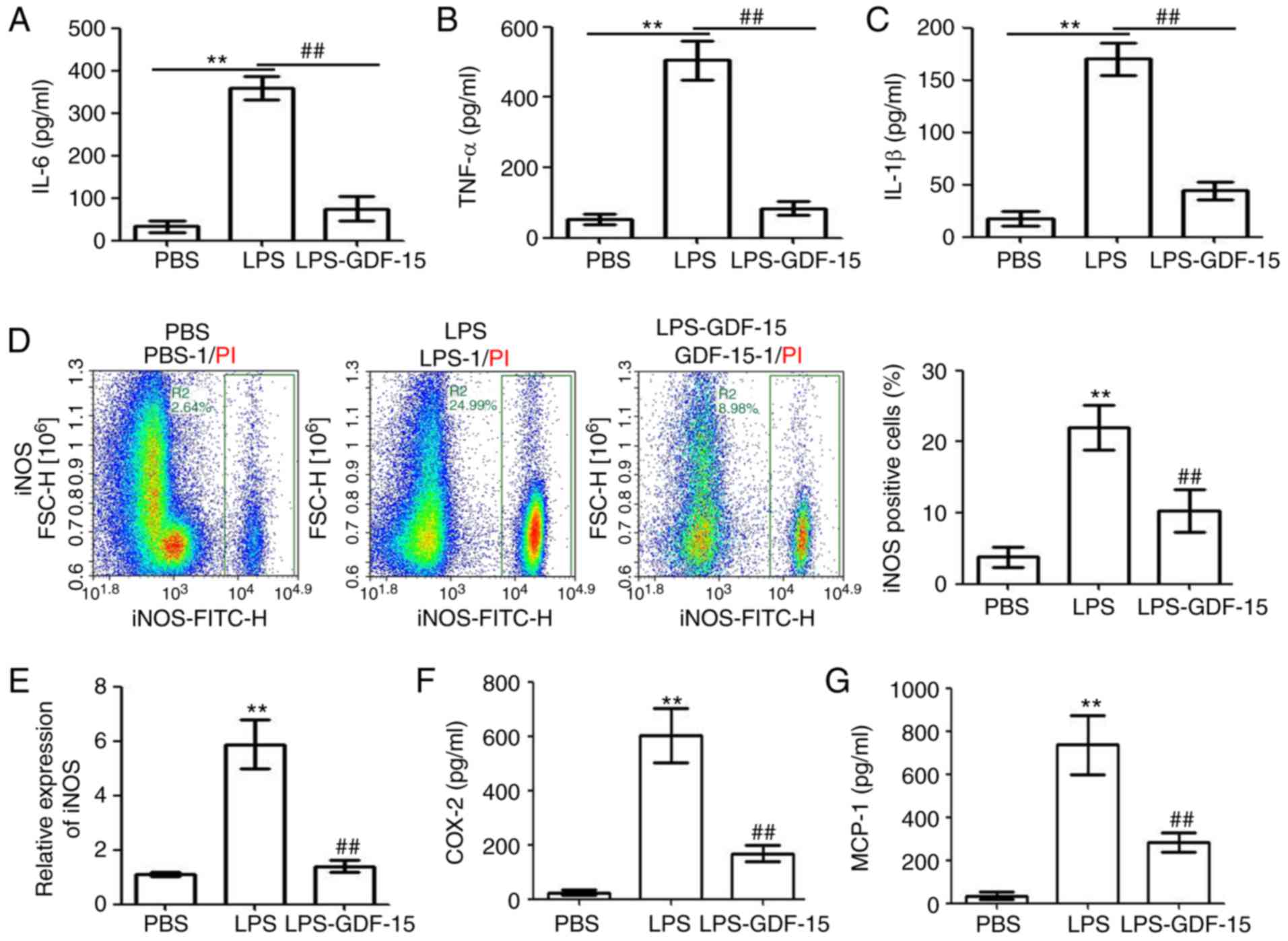
supernatant of the treated Kupffer cells. As presented in Fig. 5A–C, treatment of Kupffer cells
with LPS (2 µg/ml) significantly increased the levels of the
pro-inflammatory cytokines IL-6, TNF-α and IL-1β, while GDF-15
treatment significantly inhibited this effect. Flow cytometry
analysis indicated that LPS promoted the activation of
pro-inflammatory Kupffer cells (measured as the % of iNOS-positive
cells; Fig. 5D). However, the
LPS-induced activation of pro-inflammatory Kupffer cells was
significantly suppressed by GDF-15 treatment (Fig. 5D). RT-qPCR analysis also confirmed
the inhibiting effect of GDF-15 on LPS-induced iNOS upregulation at
the mRNA level (Fig. 5E).
Finally, LPS administration upregulated the levels of
macrophage-associated pro-inflammatory cytokines, COX-2 and MCP-1,
in the supernatant of Kupffer cells, and this effect was
significantly attenuated by GDF-15 treatment (Fig. 5F and G). These results indicated
that GDF-15 attenuated LPS-induced inflammation and iNOS activation
in Kupffer cells.
GDF-15 protects against LPS-induced NF-κB
pathway activation through regulating TAK1 phosphorylation in
Kupffer cells
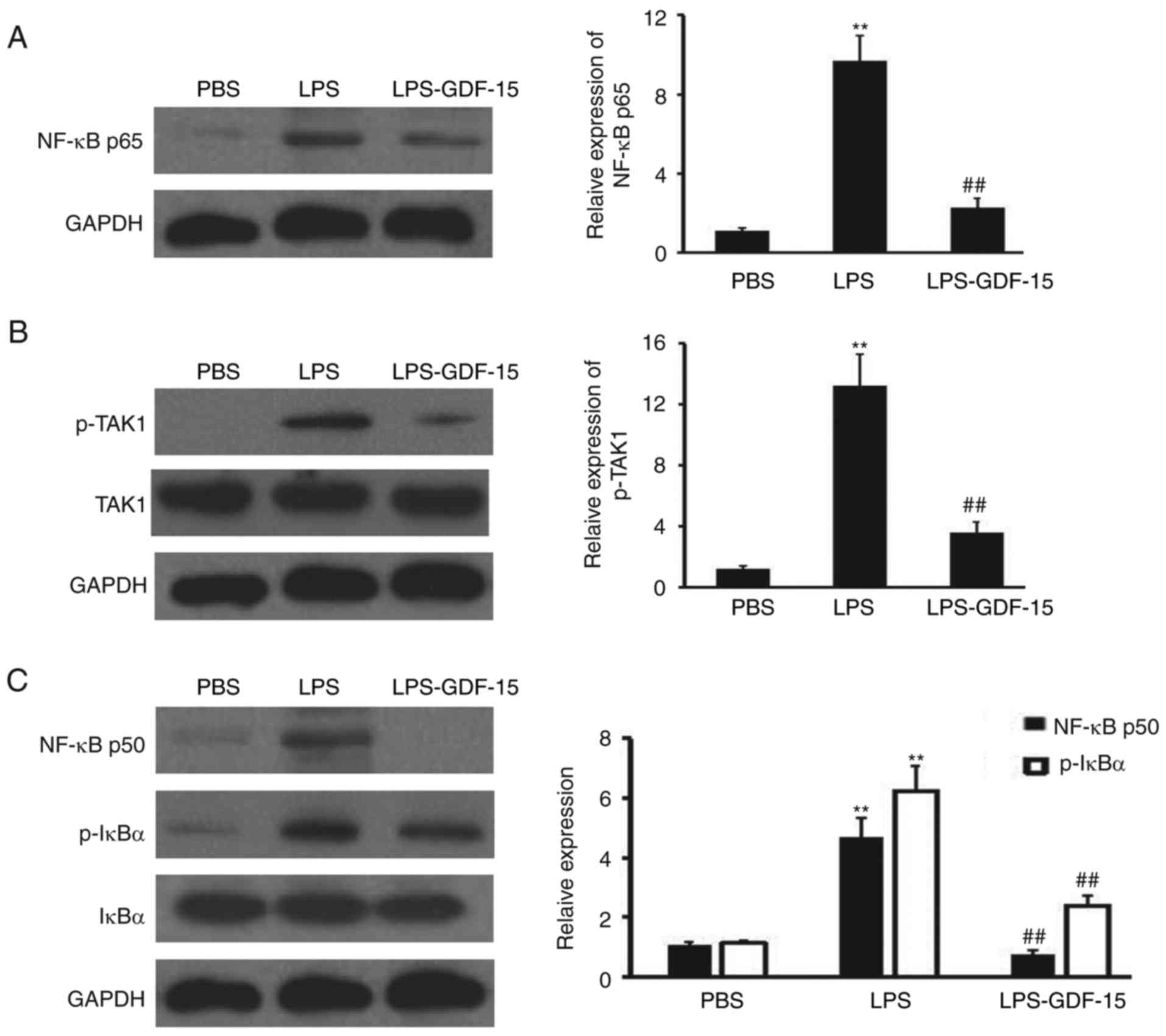
To further investigate the anti-inflammatory
mechanism of GDF-15 in Kupffer cells, western blotting analysis was
performed to detect NF-κB p65, p-TAK1, TAK, NF-κB p50, p-IκBα and
IκBα expression in Kupffer cells following LPS and GDF-15
treatment. As presented in Fig.
6A, NF-κB p65 expression was induced by LPS, compared with the
negative control group (PBS). However, GDF-15 dramatically impaired
the upregulation of NF-κB p65 (Fig.
6A), compared with the LPS group. GDF-15 also efficiently
prevented the activation of TAK1 (Fig. 6B), which has been demonstrated to
be the direct binding receptor of GDF-15 in macrophages (23). Furthermore, NF-κB p50 expression
and phosphorylation of IκBα was inhibited by GDF-15 teratment in
Kupffer cells, compared with the LPS alone treatment group
(Fig. 6C). Collectively, these
results suggested that GDF-15 protected LPS-induced NF-κB pathway
activation through regulating TAK1 phosphorylation in Kupffer
cells.
Discussion
The present study demonstrated that GDF-15 treatment
reduced LPS/D-GalN-induced acute liver injury in mice, suggesting
that GDF-15 was a preventive factor in the pathological process.
The mechanism underlying the role of GDF-15 in preventing
LPS/D-GalN-induced acute liver injury was further investigated.
Lower levels of inflammatory cytokines and numbers of inflammatory
macrophages (iNOS-positive) were measured in the GDF-15-treated
group compared with the model group. Investigations of the
molecular mechanism demonstrated that GDF-15 effectively protected
against LPS-induced NF-κB pathway activation, by regulating TAK1
phosphorylation in Kupffer cells. In conclusion, GDF-15 reduced the
activation of pro-inflammatory factors, and prevented
LPS/D-GalN-induced liver injury, most likely by disrupting TAK1
phosphorylation and consequently inhibiting the activation of the
IκBα/NF-κB pathway in the liver.
GDF-15 is a divergent member of the human TGF-β
superfamily showing similarity to both classical TGF-β isoforms and
bone morphogenetic proteins (BMPs) (24). In healthy individuals, GDF-15 is
strongly expressed in the placenta during pregnancy, and at
low-to-moderate levels, in the brain, liver, breast, colon, and
bone marrow (25). GDF-15
overexpression has been described in colorectal cancer and
malignant glioma (25-27). Additionally, serum GDF-15 levels
have been reported to correlate with heart failure (13), atrial fibrillation (12), atrial fibrosis (28), and cardiac injury (29). These previous findings possibly
indicated that GDF-15 has heterogeneous functions in different
diseases. Furthermore, a previous study indicated that
decompensated liver cirrhosis patients had increased serum GDF-15
levels compared with patients with compensated liver cirrhosis and
chronic hepatitis (30). Serum
GDF-15 levels are significantly increased in critically ill
patients, associated with sepsis, organ failure, and disease
severity (31). However, the
function of GDF-15 in sepsis remains unclear. The present study
demonstrated that decreased LPS/GalN-induced acute liver injury was
observed in mice injected with GDF-15, accompanied with lower serum
levels of AST and ALT. These findings suggested that GDF-15 is a
preventive factor in this pathological process and a potential
therapeutic agent for sepsis, although further research is required
to examine the clinical importance of GDF-15 in sepsis in
humans.
The inflammatory response following sepsis is
important for the induction of liver injury (32,33). Thus, perturbation of the induction
of the inflammatory response is a potential therapeutic strategy
for liver injury (7,34,35). Upon stimulation of
D-GalN-sensitized mice with LPS, the pro-inflammatory cytokines
IL-1, IL-6 and TNF-α are secreted, inducing hepatocellular
apoptosis, which has been identified as an early and possibly
causal event in LPS/D-GalN-induced liver failure (36,37). In the present study, the
inhibitory role of GDF-15 in LPS/D-GalN-induced inflammation was
demonstrated, evidenced by the low levels of hepatic MDA, MPO, and
pro-inflammatory cytokines in the mice treated with GDF-15. These
findings were consistent with those of a previous study by Kim
et al, reporting that transgenic mice expressing human
NAG-1/GDF-15 have less white adipose tissue, which may be
responsible for reduced inflammatory response to LPS (20).
Liver dysfunction following sepsis is an independent
risk factor for multiple organ dysfunction and sepsis-induced death
(32,38). Acting as a double-edged sword in
sepsis, liver-mediated immune response is responsible for
eliminating bacteria and toxins, although it also causes
inflammation, immunosuppression, and organ damage (32,38). As key components of the hepatic
innate immune system, Kupffer cells are postulated to have a
central role in the response to LPS and as mediators of LPS-induced
liver injury (39). Upon
stimulation by LPS, Kupffer cells secrete pro-inflammatory
molecules, including IL-1, IL-6, TNF-α, MCP-1 and COX-2 (40). In the present study, GDF-15 was
demonstrated to significantly inhibit in vivo and in
vitro inflammatory cyto-kine expression in murine Kupffer
cells, accompanied with a decrease in numbers of pro-inflammatory
macrophages. Further investigations of the molecular mechanism
revealed that GDF-15 effectively protected against the activation
of the NF-κB pathway, by regulating TAK1 phosphorylation in Kupffer
cells. These results were consistent with those of a previous
study, reporting that GDF-15 suppresses macrophage activity by
inhibiting TAK1 signaling to NF-κB (23). However, there were some findings
differing between the present study and this previous study, which
demonstrated that GDF-15 did not inhibit LPS-induced cytokine
expression in RAW264.7 cells or mouse Kupffer cells in vitro
(20). It can be speculated that
these discrepancies may result from the different treatment methods
used.
In summary, the main findings of the present study,
both in vitro and in vivo, demonstrated the
preventive role and mechanism of GDF-15 against LPS/D-GalN-induced
mortality, acute liver injury, and inflammatory response. Although
further research is required to examine the clinical importance of
the present findings, these results suggest a therapeutic potential
for GDF-15 in sepsis treatment.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of China (grant no. 81560031).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
ML was involved in the acquisition of the data. KS,
XWH and SMF were involved in the analysis and interpretation of the
data. QYZ was involved in the conception and design of the present
study and obtaining the funding.
Ethics approval and consent to
participate
The experimental protocols involving animals were
approved by the Institutional Animal Use Committee of the Southern
Medical University (Guangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Angus DC and van der Poll T: Severe sepsis
and septic shock. N Engl J Med. 369:840–851. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The third international consensus
definitions for sepsis and septic shock (Sepsis-3). JAMA.
315:801–810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cao Z and Robinson RA: The role of
proteomics in understanding biological mechanisms of sepsis.
Proteomics Clin Appl. 8:35–52. 2014. View Article : Google Scholar
|
|
4
|
Hamers L, Kox M and Pickkers P:
Sepsis-induced immunoparalysis: Mechanisms, markers, and treatment
options. Minerva Anestesiol. 81:426–439. 2015.
|
|
5
|
Chun K, Syndergaard C, Damas C, Trubey R,
Mukindaraj A, Qian S, Jin X, Breslow S and Niemz A: Sepsis pathogen
identification. J Lab Autom. 20:539–561. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tacke F and Zimmermann HW: Macrophage
heterogeneity in liver injury and fibrosis. J Hepatol.
60:1090–1096. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ge X, Feng Z, Xu T, Wu B, Chen H, Xu F, Fu
L, Shan X, Dai Y, Zhang Y and Liang G: A novel imidazopyridine
derivative, X22, attenuates sepsis-induced lung and liver injury by
inhibiting the inflammatory response in vitro and in vivo. Drug Des
Devel Ther. 10:1947–1959. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang C, Wang J, Chen Z, Wang Y and Zhang
W: 2-Phenylethynesulfonamide prevents induction of pro-inflammatory
factors and attenuates LPS-induced liver injury by targeting
NHE1-Hsp70 complex in mice. PLoS One. 8:e675822013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Scott MJ, Liu S, Shapiro RA, Vodovotz Y
and Billiar TR: Endotoxin uptake in mouse liver is blocked by
endotoxin pretreatment through a suppressor of cytokine
signaling-1-dependent mechanism. Hepatology. 49:1695–1708. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang JB, Wang HT, Li LP, Yan YC, Wang W,
Liu JY, Zhao YT, Gao WS and Zhang MX: Development of a rat model of
D-galactosamine/lipopolysaccharide induced hepatorenal syndrome.
World J Gastroenterol. 21:9927–9935. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Unsicker K, Spittau B and Krieglstein K:
The multiple facets of the TGF-β family cytokine
growth/differentiation factor-15/macrophage inhibitory cytokine-1.
Cytokine Growth Factor Rev. 24:373–384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hijazi Z, Oldgren J, Andersson U, Connolly
SJ, Eikelboom JW, Ezekowitz MD, Reilly PA, Yusuf S, Siegbahn A and
Wallentin L: Growth-differentiation factor 15 and risk of major
bleeding in atrial fibrillation: Insights from the randomized
evaluation of long-term anticoagulation therapy (RE-LY) trial. Am
Heart J. 190:94–103. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cotter G, Voors AA, Prescott MF, Felker
GM, Filippatos G, Greenberg BH, Pang PS, Ponikowski P, Milo O, Hua
TA, et al: Growth differentiation factor 15 (GDF-15) in patients
admitted for acute heart failure: Results from the RELAX-AHF study.
Eur J Heart Fail. 17:1133–1143. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abulizi P, Loganathan N, Zhao D, Mele T,
Zhang Y, Zwiep T, Liu K and Zheng X: Growth differentiation
factor-15 deficiency augments inflammatory response and exacerbates
septic heart and renal injury induced by lipopolysaccharide. Sci
Rep. 7:10372017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yan D, Liu HL, Yu ZJ, Huang YH, Gao D, Hao
H, Liao SS, Xu FY and Zhou XY: BML-111 protected LPS/D-GalN-induced
acute liver injury in rats. Int J Mol Sci. 17:pii: E1114. 2016.
View Article : Google Scholar
|
|
16
|
Dai L, Cui X, Zhang X, Cheng L, Liu Y,
Yang Y, Fan P, Wang Q, Lin Y, Zhang J, et al: SARI inhibits
angiogenesis and tumour growth of human colon cancer through
directly targeting cerulo-plasmin. Nat Commun. 7:119962016.
View Article : Google Scholar
|
|
17
|
t'Hart BA, Vervoordeldonk M, Heeney JL and
Tak PP: Gene therapy in nonhuman primate models of human autoimmune
disease. Gene Ther. 10:890–901. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mikic AN, Brkic S, Maric D, Sekulic B,
Cetkovic A and Mitic G: Thiobarbituric acid reactive substances as
marker of oxidative stress in pregnancies with pre-eclampsia. Med
Pregl. 64:377–380. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu MW, Liu R, Wu HY, Zhang W, Xia J, Dong
MN, Yu W, Wang Q, Xie FM, Wang R, et al: Protective effect of
Xuebijing injection on D-galactosamine- and
lipopolysaccharide-induced acute liver injury in rats through the
regulation of p38 MAPK, MMP-9 and HO-1 expression by increasing
TIPE2 expression. Int J Mol Med. 38:1419–1432. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim JM, Kosak JP, Kim JK, Kissling G,
Germolec DR, Zeldin DC, Bradbury JA, Baek SJ and Eling TE:
NAG-1/GDF15 transgenic mouse has less white adipose tissue and a
reduced inflammatory response. Mediators Inflamm. 2013:6418512013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH Image to ImageJ: 25 years of image analysis. Nat Methods.
9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods.
25:402–408. 2001. View Article : Google Scholar
|
|
23
|
Ratnam NM, Peterson JM, Talbert EE, Ladner
KJ, Rajasekera PV, Schmidt CR, Dillhoff ME, Swanson BJ, Haverick E,
Kladney RD, et al: NF-kappaB regulates GDF-15 to suppress
macrophage surveillance during early tumor development. J Clin
Invest. 127:3796–3809. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bootcov MR, Bauskin AR, Valenzuela SM,
Moore AG, Bansal M, He XY, Zhang HP, Donnellan M, Mahler S, Pryor
K, et al: MIC-1, a novel macrophage inhibitory cytokine, is a
divergent member of the TGF-beta superfamily. Proc Natl Acad Sci
USA. 94:11514–11519. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu DD and Mei YA: Effects of growth
differentiation factor-15 (GDF-15) on neurological systems,
cardiovascular diseases, and cancer progression. Sheng Li Xue Bao.
69:109–121. 2017.In Chinese. PubMed/NCBI
|
|
26
|
Sandor N, Schilling-Toth B, Kis E, Benedek
A, Lumniczky K, Safrany G and Hegyesi H: Growth differentiation
factor-15 (GDF-15) is a potential marker of radiation response and
radiation sensitivity. Mutat Res Genet Toxicol Environ Mutagen.
793:142–149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Unal B, Alan S, Bassorgun CI, Karakas AA,
Elpek GO and Ciftcioglu MA: The divergent roles of growth
differentiation factor-15 (GDF-15) in benign and malignant skin
pathologies. Arch Dermatol Res. 307:551–557. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou YM, Li MJ, Zhou YL, Ma LL and Yi X:
Growth differentiation factor-15 (GDF-15), novel biomarker for
assessing atrial fibrosis in patients with atrial fibrillation and
rheumatic heart disease. Int J Clin Exp Med. 8:21201–21207.
2015.
|
|
29
|
Kahli A, Guenancia C, Zeller M, Grosjean
S, Stamboul K, Rochette L, Girard C and Vergely C: Growth
differentiation factor-15 (GDF-15) levels are associated with
cardiac and renal injury in patients undergoing coronary artery
bypass grafting with cardiopulmonary bypass. PLoS One.
9:e1057592014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee ES, Kim SH, Kim HJ, Kim KH, Lee BS and
Ku BJ: Growth differentiation factor 15 predicts chronic liver
disease severity. Gut Liver. 11:276–282. 2017. View Article : Google Scholar :
|
|
31
|
Buendgens L, Yagmur E, Bruensing J,
Herbers U, Baeck C, Trautwein C, Koch A and Tacke F: Growth
differentiation factor-15 is a predictor of mortality in critically
Ill patients with sepsis. Dis Markers. 5271203:20172017.
|
|
32
|
Hoque R, Farooq A and Mehal WZ: Sterile
inflammation in the liver and pancreas. J Gastroenterol Hepatol.
28(Suppl 1): S61–S67. 2013. View Article : Google Scholar
|
|
33
|
Mehal WZ: The inflammasome in liver injury
and non-alcoholic fatty liver disease. Dig Dis. 32:507–515. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ambade A, Catalano D, Lim A and Mandrekar
P: Inhibition of heat shock protein (molecular weight 90 kDa)
attenuates proinflammatory cytokines and prevents
lipopolysaccharide-induced liver injury in mice. Hepatology.
55:1585–1595. 2012. View Article : Google Scholar :
|
|
35
|
Baranova IN, Souza AC, Bocharov AV,
Vishnyakova TG, Hu X, Vaisman BL, Amar MJ, Chen Z, Kost Y, Remaley
AT, et al: Human SR-BI and SR-BII potentiate
lipopolysaccharide-induced inflammation and acute liver and kidney
injury in mice. 196:3135–3147. 2016.
|
|
36
|
Liao WQ, Qi YL, Wang L, Dong XM, Xu T,
Ding CD, Liu R, Liang WC, Lu LT, Li H, et al: Recql5 protects
against lipopolysaccharide/D-galactosamine-induced liver injury in
mice. J Immunol. 21:10375–10384. 2015.
|
|
37
|
Zhang J, Xu L, Zhang L, Ying Z, Su W and
Wang T: Curcumin attenuates
D-galactosamine/lipopolysaccharide-induced liver injury and
mitochondrial dysfunction in mice. J Nutr. 144:1211–1218. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yan J and Li S and Li S: The role of the
liver in sepsis. Int Rev Immunol. 33:498–510. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dixon LJ, Barnes M, Tang H, Pritchard MT
and Nagy LE: Kupffer cells in the liver. Compr Physiol. 3:785–797.
2013.PubMed/NCBI
|
|
40
|
Tsutsui H and Nishiguchi S: Importance of
Kupffer cells in the development of acute liver injuries in mice.
Int J Mol Sci. 15:7711–7730. 2014. View Article : Google Scholar : PubMed/NCBI
|