Introduction
Atherosclerotic cardiovascular disease is the
leading cause of mortality worldwide (1,2).
The cardiomyocytes (CMs) in patients with acute myocardial
infarction (AMI) undergo cell death, alteration of cell cycle, and
abrupt mitochondrial oxidative metabolism in either ischemic or
hypoxic conditions (3-5). Therefore, antiplatelet agents,
anticoagulation agents, and emergent coronary revascularization are
often used to treat patients with acute coronary syndrome (6).
Apoptotic cell death is important in myocardial
damage following AMI, which may lead to malignant arrhythmia, heart
failure and cardiac death (7,8).
During hypoxia and reoxygenation injury, CMs exhibit typical
morphological characteristics of apoptotic nuclei, including
membrane blebs, myofibillary disarrangement, chromatin
condensation, and peripheral margination of mitochondria (3). Increased myocardial apoptosis has
also been found in autopsies of patients who have succumbed to AMI
and multi-vessel coronary artery diseases (9,10).
Furthermore, changes in several pro-apoptotic and anti-apoptotic
mediators have been noted in ischemic or hypoxic CMs in
vivo, in isolated animal hearts, and in humans (5,7,8,11,12).
Unlike traditional Sanger sequencing technology,
next-generation sequencing (NGS) provides rapid analyses of large
quantities of genomic information, including DNAs, mRNAs, microRNAs
(miRNAs), and non-coding RNAs (13,14). Using NGS in combination with
bioinformatics analyses, whole exon sequencing has been performed
in previous studies to identify a wide spectrum of genetic
mutations in several scientific fields (13,14). NGS has also been used to identify
novel genetic regulations in basic and clinical research (15,16), and it has been used in the genetic
diagnosis of congenital heart diseases and inherited cardiac
dysrhythmia (16,17).
Although several studies have investigated the
signaling pathways and cellular responses of CMs in ischemic or
hypoxic conditions, the regulation of genetic expression with
regard to hypoxia-related apoptosis is complex and remains to be
fully elucidated (18-20). Therefore, the present study was
designed to perform a comprehensive investigation of various
genetic expression changes in hypoxic human cardiomyocytes using
NGS and bioinformatic analyses.
Materials and methods
Cell culture
The AC16 human cardiomyocyte cell line, purchased
from EMD Millipore (Billerica, MA, USA), was cultured following
standard manufacturer protocol. The cells (1×106
cells/well) were seeded in 10% FBS (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and DMEM/F-12 medium
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and allowed to grow
overnight. The AC16 CMs were then incubated in either a normoxic
condition (37°C, 20% O2 and 5% CO2) or
hypoxic condition (37°C, 1% O2 and 5% CO2)
for 24 h. The hypoxic condition was maintained in a physiological
oxygen workstation (InvivO2 400; Baker Ruskinn, Sanford, ME,
USA).
Flow cytometry and detection of
apoptosis
The cells were analyzed using an Annexin V-FITC
Early Apoptosis Detection kit (Cell Signaling Technology, Inc.,
Danvers, MA, USA) and a BD Accuri C6 Plus flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA) as previously reported
(21).
RNA sequencing
Total RNAs from the normoxic and hypoxic AC16 CMs
were extracted with TRIzol reagent (Invitrogen, Thermo Fisher
Scientific, Inc.) following the manufacturer's standard protocol.
The purified RNA was measured at OD260nm with an ND-1000
spectrophotometer (Nanodrop Technologies; Thermo Fisher Scientific,
Inc., Wilmington, DE, USA) and qualitatively analyzed with an RNA
6000 LabChip kit (Agilent Technologies, Inc., Santa Clara, CA, USA)
and the Bioanalyzer 2100 (Agilent Technologies, Inc.).
Library preparation and deep sequencing were
performed according to the manufacturer's protocol (Illumina,
Welgene Biotechnology Company, Taipei, Taiwan), as described in our
previous reports (15,22). For small RNA sequencing, following
reverse transcription of total RNA, cDNA with sizes indicating
18-40-nucleotide RNA fragments (140-155 nucleotides in length with
both adapters) was extracted and sequenced on the Illumina
instrument (75 single-end cycles). Following trimming or removing
low-quality data using Trimmomatic (version 0.36) (23), the qualified reads were analyzed
using miRDeep2 software (version 2.0.0.8) (24) and the human genome from the
University of California Santa Cruz (UCSC) database (https://genome.ucsc.edu/). The miRNAs with low levels
[<1 normalized read per million (rpm)] in both hypoxic and
normoxic cells were excluded. For transcriptome sequencing, the
library constructed with the SureSelect Strand Specific RNA Library
Preparation kit (Agilent Technologies, Inc.) was sequenced with the
TruSeq SBS kit on the Solexa platform (Illumina NextSeq 500; 75
cycles, single-end). Following trimming or removing low-quality
data using Trimmomatics (23),
the qualified reads were analyzed using Cufflinks (25) and the Ensembl database (https://asia.ensembl.org/index.html). The genes
with low expression levels (<0.3 fragment per kilobase of
transcript per million mapped reads) in both hypoxic and normoxic
cells were excluded.
miRNA database analyses
The miRmap is a web database used to predict
putative genes targeted by candidate miRNAs and is available from
http://cegg.unige.ch/mirmap (26). In the present study, a search was
performed for putative targeted miRNAs from human species and
miRmap scores >99.0. A search was also performed for potential
miRNA interactions in the miRmap, TargetScan (http://www.targetscan.org/vert_71/) and miRDB
(http://www.mirdb.org/) databases.
Ingenuity® Pathway Analysis
(IPA)
IPA software (Ingenuity Systems, Redwood City, CA,
USA) integrates numerous results and performs multiple analyses
providing a comprehensive interpretation of a large quantity of
experimental data. In the present study, IPA analysis was performed
for the network analyses of candidate genes.
Gene expression omnibus (GEO) database
analysis
The GEO database (https://www.ncbi.nlm.nih.gov/geo/) is a useful web
database containing raw gene expression data from microarray
studies and NGS. The present study used a GEO array (GEO accession:
GDS3483) investigating the gene responses to hypoxia in primary
human pulmonary microvascular endothelial cells and cardiac
microvascular endothelial cells (27). Data were collected from the
cardiac microvascular endothelial cells under normoxia or hypoxia
(1% O2, 5% CO2, and 94% N2) over
various durations (3, 24 and 48 h). The raw data extracted from the
GEO database were re-plotted and statistically analyzed using
analysis of variance (ANOVA) followed by Dunnett's test using
GraphPad Prism® 7 software (GraphPad Software, Inc., La
Jolla, CA, USA).
Database for Annotation Visualization and
Integrated Discovery (DAVID) analysis
The DAVID (https://david.ncifcrf.gov/) database (28) is a useful tool for gene functional
classification. It integrates data from multiple functional
annotation databases, including Gene Ontology (GO) and Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathway. A list of genes
of interest is classified by clustering of related biological
functions, signaling pathways, or diseases by calculating the
similarity of global annotation profiles using an agglomeration
algorithm method. The functions of differentially expressed genes
were analyzed following methods used in our previous studies
(15,22).
Statistical analysis
The expression levels of the genes were compared
between cells treated with hypoxia for different lengths of time
(3, 24 and 48 h) and normoxic cells using ANOVA, followed by
Dunnett's test. P<0.05 (two-tailed) was considered to
indicate a statistically significant difference.
Results
Gene expression profiling and miRNA
changes in hypoxic AC16 CMs
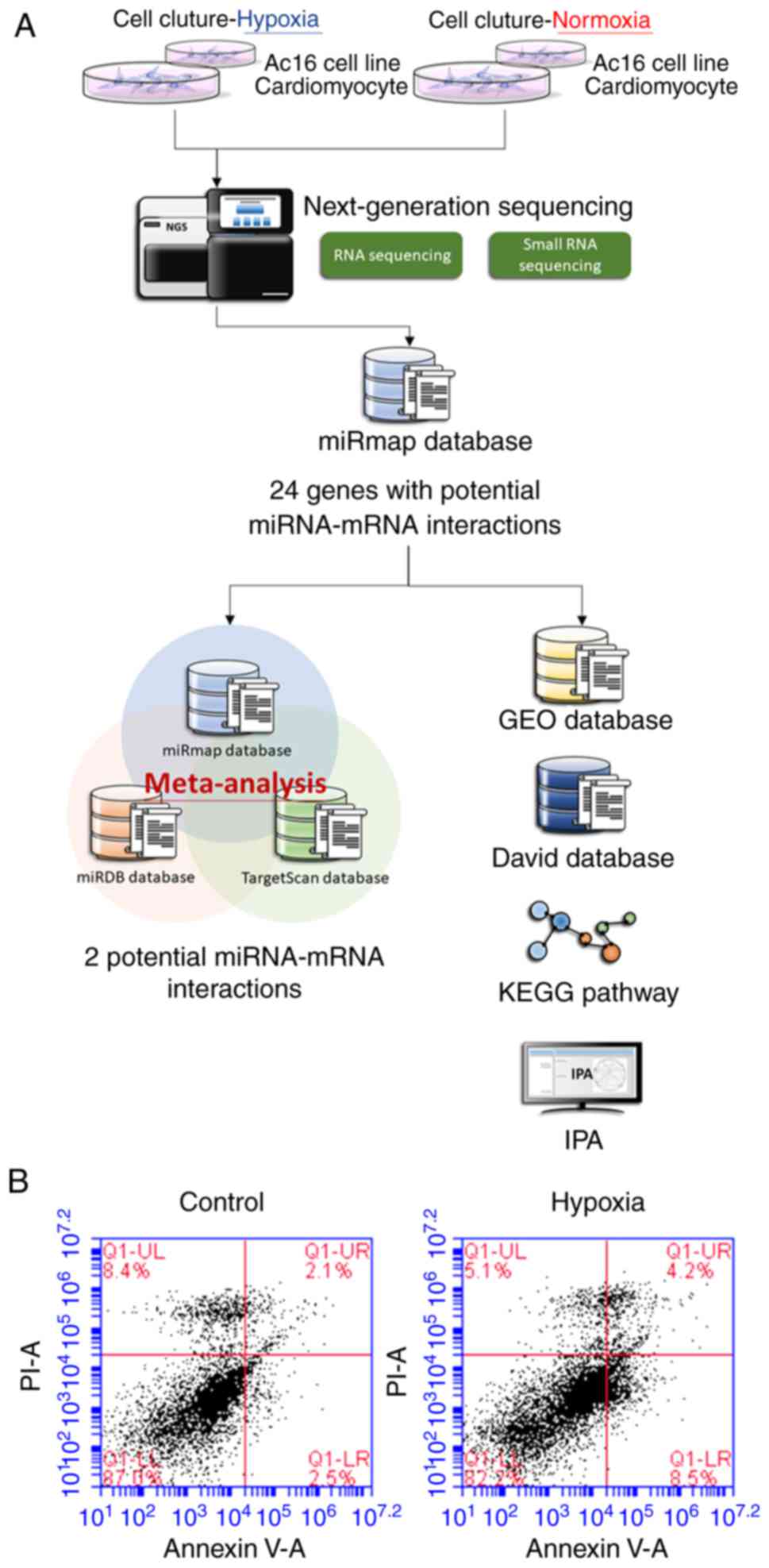
As shown in Fig.
1A, RNAs were extracted from the cells and sent for NGS
followed by bioinformatics analyses. The AC16 CMs incubated under
hypoxic conditions exhibited increased apoptosis compared with the
cells incubated under normoxic conditions (Fig. 1B).
A volcano plot of differentially upregulated (right
panel) and downregulated (left panel) gene expression in hypoxic,
vs. normoxic AC16 CMs is shown in Fig. 2A. Genes with −log10
(P-value) >1.3 and >2-fold changes (Fig. 2B) were selected for further
analyses. The small RNA-sequencing data generated with NGS were
analyzed to identify potentially significant changes in miRNA
profiles in the AC16 CMs under hypoxia, vs. normoxia. As shown in
Fig. 2C and D, 184 miRNAs were
identified with >2-fold changes (99 upregulated and 85
downregulated). Following selection of those with thresholds of
>1 rpm in both hypoxic and normoxic cells, 62 miRNAs were
identified, including 30 miRNAs with >2-fold upregulation and 32
miRNAs with >2-fold downregulation (Table I).
 | Table ImiRNAs with significant change in
hypoxic, vs. normoxic AC16 CMs. |
Table I
miRNAs with significant change in
hypoxic, vs. normoxic AC16 CMs.
| miRNA | Precursor | AC16 CMs hypoxia
| AC16 CMs normoxia
| Fold change | Up/down |
|---|
| seq (norm) | seq (norm) |
|---|
| hsa-miR-10b-3p | hsa-mir-10b | 2.81 | 1.03 | 2.73 | Up |
| hsa-miR-1276 | hsa-mir-1276 | 4.84 | 1.84 | 2.63 | Up |
| hsa-miR-142-5p | hsa-mir-142 | 6.08 | 2.18 | 2.79 | Up |
|
hsa-miR-181b-2-3p | hsa-mir-181b-2 | 8.33 | 3.33 | 2.50 | Up |
| hsa-miR-210-3p | hsa-mir-210 | 392.50 | 122.50 | 3.20 | Up |
| hsa-miR-210-5p | hsa-mir-210 | 23.52 | 8.49 | 2.77 | Up |
| hsa-miR-212-5p | hsa-mir-212 | 6.19 | 1.95 | 3.17 | Up |
| hsa-miR-224-3p | hsa-mir-224 | 4.61 | 1.15 | 4.01 | Up |
|
hsa-miR-26a-2-3p | hsa-mir-26a-2 | 7.43 | 2.75 | 2.70 | Up |
| hsa-miR-26b-3p | hsa-mir-26b | 10.80 | 4.47 | 2.42 | Up |
| hsa-miR-299-5p | hsa-mir-299 | 27.57 | 10.09 | 2.73 | Up |
|
hsa-miR-3200-3p | hsa-mir-3200 | 10.01 | 3.67 | 2.73 | Up |
| hsa-miR-330-3p | hsa-mir-330 | 9.79 | 4.70 | 2.08 | Up |
| hsa-miR-33a-3p | hsa-mir-33a | 3.15 | 1.26 | 2.50 | Up |
| hsa-miR-34b-3p | hsa-mir-34b | 13.39 | 5.28 | 2.54 | Up |
|
hsa-miR-365b-5p | hsa-mir-365b | 3.60 | 1.15 | 3.13 | Up |
|
hsa-miR-3944-5p | hsa-mir-3944 | 2.48 | 1.15 | 2.16 | Up |
| hsa-miR-454-5p | hsa-mir-454 | 14.40 | 5.73 | 2.51 | Up |
| hsa-miR-485-3p | hsa-mir-485 | 22.84 | 5.51 | 4.15 | Up |
| hsa-miR-486-3p | hsa-mir-486-1 | 8.78 | 2.64 | 3.33 | Up |
| hsa-miR-486-3p | hsa-mir-486-2 | 8.89 | 2.29 | 3.88 | Up |
| hsa-miR-494-3p | hsa-mir-494 | 18.00 | 8.83 | 2.04 | Up |
|
hsa-miR-548e-3p | hsa-mir-548e | 6.86 | 3.21 | 2.14 | Up |
|
hsa-miR-550a-3p | hsa-mir-550a-1 | 2.48 | 1.03 | 2.41 | Up |
|
hsa-miR-550a-3p | hsa-mir-550a-2 | 2.48 | 1.03 | 2.41 | Up |
|
hsa-miR-550a-3p | hsa-mir-550a-3 | 2.48 | 1.03 | 2.41 | Up |
| hsa-miR-5690 | hsa-mir-5690 | 5.51 | 2.41 | 2.29 | Up |
| hsa-miR-582-3p | hsa-mir-582 | 9.45 | 3.67 | 2.57 | Up |
| hsa-miR-590-3p | hsa-mir-590 | 15.42 | 7.34 | 2.10 | Up |
| hsa-miR-641 | hsa-mir-641 | 8.21 | 3.90 | 2.11 | Up |
| hsa-miR-766-3p | hsa-mir-766 | 10.69 | 5.05 | 2.12 | Up |
| hsa-miR-92b-5p | hsa-mir-92b | 11.59 | 5.51 | 2.10 | Up |
| hsa-miR-98-3p | hsa-mir-98 | 8.78 | 4.36 | 2.01 | Up |
|
hsa-miR-1249-3p | hsa-mir-1249 | 2.48 | 5.28 | −2.13 | Down |
| hsa-miR-1262 | hsa-mir-1262 | 1.35 | 3.56 | −2.64 | Down |
|
hsa-miR-1292-5p | hsa-mir-1292 | 1.24 | 2.52 | −2.03 | Down |
| hsa-miR-1303 | hsa-mir-1303 | 2.59 | 5.51 | −2.13 | Down |
|
hsa-miR-1306-5p | hsa-mir-1306 | 1.69 | 4.01 | −2.37 | Down |
| hsa-miR-134-3p | hsa-mir-134 | 1.58 | 3.44 | −2.18 | Down |
|
hsa-miR-1908-3p | hsa-mir-1908 | 1.01 | 2.29 | −2.27 | Down |
| hsa-miR-23a-5p | hsa-mir-23a | 5.63 | 14.68 | −2.61 | Down |
| hsa-miR-296-3p | hsa-mir-296 | 1.35 | 3.67 | −2.72 | Down |
| hsa-miR-29a-5p | hsa-mir-29a | 2.48 | 6.54 | −2.64 | Down |
|
hsa-miR-3130-3p | hsa-mir-3130-1 | 1.13 | 4.59 | −4.06 | Down |
|
hsa-miR-3130-3p | hsa-mir-3130-2 | 1.13 | 4.59 | −4.06 | Down |
| hsa-miR-3167 | hsa-mir-3167 | 2.93 | 6.08 | −2.08 | Down |
| hsa-miR-3176 | hsa-mir-3176 | 9.34 | 22.94 | −2.46 | Down |
| hsa-miR-33b-3p | hsa-mir-33b | 2.14 | 4.82 | −2.25 | Down |
| hsa-miR-3615 | hsa-mir-3615 | 4.95 | 15.71 | −3.17 | Down |
|
hsa-miR-3619-5p | hsa-mir-3619 | 1.13 | 4.01 | −3.55 | Down |
| hsa-miR-377-5p | hsa-mir-377 | 6.53 | 13.30 | −2.04 | Down | |
| hsa-miR-4454 | hsa-mir-4454 | 3.04 | 6.31 | −2.08 | Down | |
| hsa-miR-483-5p | hsa-mir-483 | 1.69 | 5.05 | −2.99 | Down | |
|
hsa-miR-487a-5p | hsa-mir-487a | 1.35 | 3.10 | −2.30 | Down | |
| hsa-miR-503-5p | hsa-mir-503 | 4.28 | 10.44 | −2.44 | Down | |
| hsa-miR-541-3p | hsa-mir-541 | 2.14 | 7.46 | −3.49 | Down | |
|
hsa-miR-548d-5p | hsa-mir-548d-1 | 2.59 | 11.70 | −4.52 | Down | |
|
hsa-miR-548d-5p | hsa-mir-548d-2 | 3.26 | 13.65 | −4.19 | Down | |
| hsa-miR-548n | hsa-mir-548n | 2.81 | 5.73 | −2.04 | Down | |
|
hsa-miR-548o-3p | hsa-mir-548o | 13.73 | 27.87 | −2.03 | Down | |
|
hsa-miR-548o-3p | hsa-mir-548o-2 | 13.73 | 27.87 | −2.03 | Down | |
|
hsa-miR-551b-3p | hsa-mir-551b | 1.13 | 2.87 | −2.54 | Down | |
|
hsa-miR-6840-5p | hsa-mir-6840 | 1.46 | 2.98 | −2.04 | Down | |
|
hsa-miR-6852-5p | hsa-mir-6852 | 2.03 | 4.36 | −2.15 | Down | |
| hsa-miR-877-5p | hsa-mir-877 | 9.00 | 18.24 | −2.03 | Down | |
| hsa-miR-887-5p | hsa-mir-887 | 1.01 | 3.10 | −3.07 | Down | |
|
hsa-miR-92a-1-5p | hsa-mir-92a-1 | 1.35 | 4.01 | −2.97 | Down | |
| hsa-miR-93-3p | hsa-mir-93 | 1.58 | 7.57 | −4.79 | Down | |
Identification of potential miRNA-mRNA
interactions in hypoxic AC16 CMs
The present study aimed to determine the potential
miRNA-mRNA interactions in hypoxic AC16 CMs. To achieve this, the
putative targets of the miRNAs with >2-fold changes were
searched (Fig. 2D) from the NGS
results using a miRmap database search for those with threshold
miRmap scores >99.0. The genes showing >2-fold changes
(Fig. 2B) were matched with these
putative targets, and, as shown in the intersection Venn diagram in
Fig. 2C, 24 genes were
potentially involved in miRNA-mRNA interactions (15 upregulated and
nine downregulated) (Table
II).
 | Table IIGenes selected by intersection
between RNA sequencing candidates and microRNA putative
targets. |
Table II
Genes selected by intersection
between RNA sequencing candidates and microRNA putative
targets.
| Official gene
symbol | Gene name | Log2
ratio (hypoxia/control) | Gene
expression | miRNA with putative
interaction |
|---|
| SLC6A13 | Solute carrier
family 6 member 13 | 11.77 | Up |
hsa-miR-3619-5p |
| HIF3A | Hypoxia inducible
factor 3 α subunit | 3.29 | Up | hsa-miR-1254, |
| | | |
hsa-miR-377-5p, |
| | | | hsa-miR-615-5p |
| EPB49 | Dematin actin
binding protein | 2.60 | Up | hsa-miR-1254 |
| APLN | Apelin | 2.16 | Up |
hsa-miR-3619-5p, |
| | | | hsa-miR-503-5p |
| ACVRL1 | Activin A receptor
like type 1 | 2.11 | Up |
hsa-miR-3619-5p |
| SLC2A5 | Solute carrier
family 2 member 5 | 1.98 | Up |
hsa-miR-3619-5p |
| STC1 | Stanniocalcin
1 | 1.77 | Up |
hsa-miR-541-3p, |
| | | | hsa-miR-615-5p |
| SLC6A8 | Solute carrier
family 6 member 8 | 1.64 | Up | hsa-miR-541-3p |
| TNS1 | Tensin 1 | 1.41 | Up | hsa-miR-3176 |
| CAMK1D | Calcium/calmodulin
dependent protein kinase ID | 1.24 | Up | hsa-miR-1254 |
| C1QL1 | Complement C1q like
1 | 1.20 | Up | hsa-miR-541-3p |
| GPR68 | G protein-coupled
receptor 68 | 1.14 | Up | hsa-miR-4741 |
| CD4 | CD4 molecule | 1.13 | Up | hsa-miR-4741 |
| MME | Membrane
metalloendopeptidase | 1.09 | Up | hsa-miR-1303 |
| BNIP3L | BCL2 interacting
protein 3 like | 1.08 | Up | hsa-miR-93-3p |
| APOL6 | Apolipoprotein
L6 | −1.09 | Down | hsa-miR-4421 |
| METTL7A | Methyltransferase
like 7A | −1.13 | Down | hsa-miR-4421 |
| TFRC | Transferrin
receptor | −1.17 | Down | hsa-miR-296-5p |
| BCL2 | BCL2, apoptosis
regulator | −1.26 | Down | hsa-miR-296-5p |
| CHAC1 | Chac glutathione
specific | −1.44 | Down | hsa-miR-3918 |
|
γ-glutamylcyclotransferase 1 | | | |
| DIO2 | Iodothyronine
deiodinase 2 | −1.48 | Down |
hsa-miR-4717-3p |
| SLC7A11 | Solute carrier
family 7 member 11 | −1.50 | Down |
hsa-miR-3129-3p, |
| | | |
hsa-miR-4768-5p, |
| | | |
hsa-miR-3074-5p, |
| | | | hsa-miR-590-3p |
| TMEM144 | Transmembrane
protein 144 | −1.84 | Down | hsa-miR-582-3p |
| HDAC11 | Histone deacetylase
11 | −2.11 | Down | hsa-miR-3918, |
| | | | hsa-miR-766-3p |
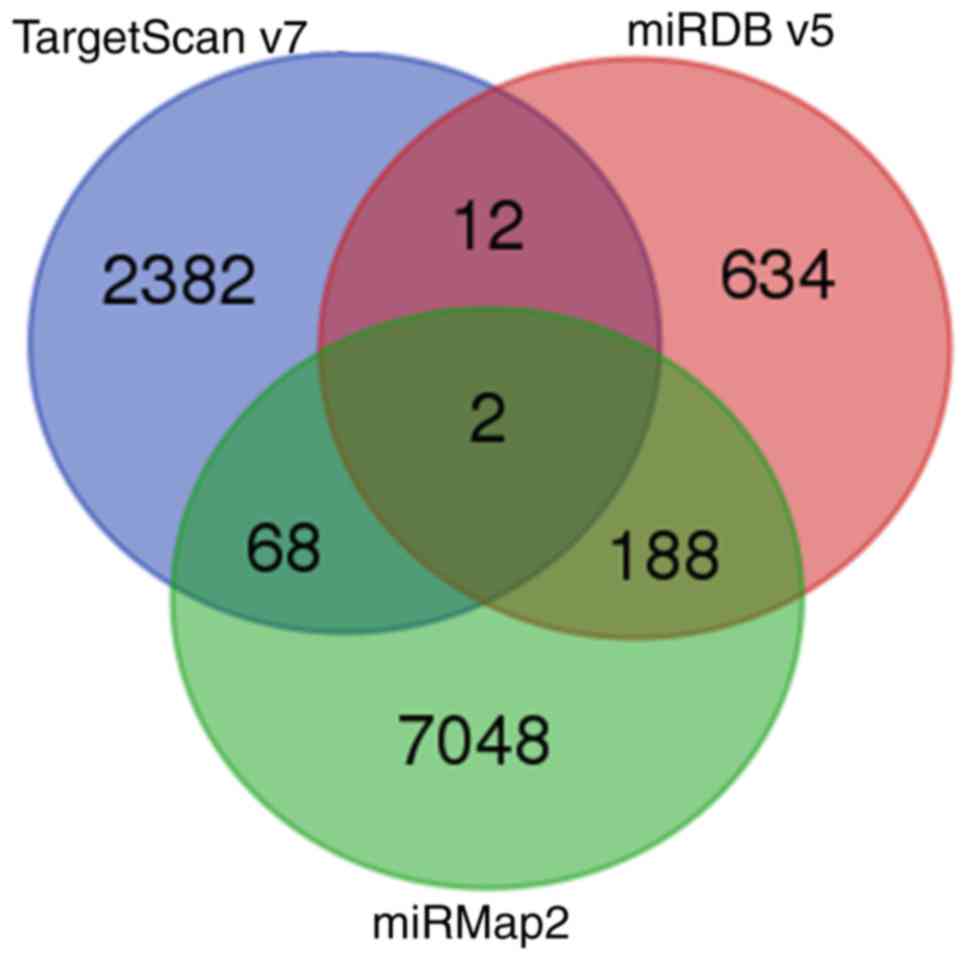
A search was also performed for the potential
miRNA-mRNA interactions of the miRNAs with >2-fold changes in
various miRNA target predicting databases. On combining the results
from miRmap, TargetScan and miRDB, two miRNA-mRNA interactions were
identified: hsa-miR-129-5p/CDC42EP3 and
hsa-miR-330-3p/HELZ (Fig.
3). However, although hsa-miR-129-5p and hsa-miR-330-3p were
significantly upregulated in hypoxic CMs, no significant
differences in the expression levels of CDC42EP3 or
HELZ were noted between the hypoxic and normoxic CMs.
Validation of the differentially
expressed genes in response to hypoxia
The 24 genes with potential miRNA-mRNA interactions
(Table II) were further compared
against associated array data obtained from the GEO database.
Briefly, the gene expression levels in cardiac microvascular
endothelial cells were extracted from the GEO database (GEO
accession: GDS3483) (27). The
expression levels of the genes were compared between cells treated
with hypoxia over different lengths of time (3, 24 and 48 h) and
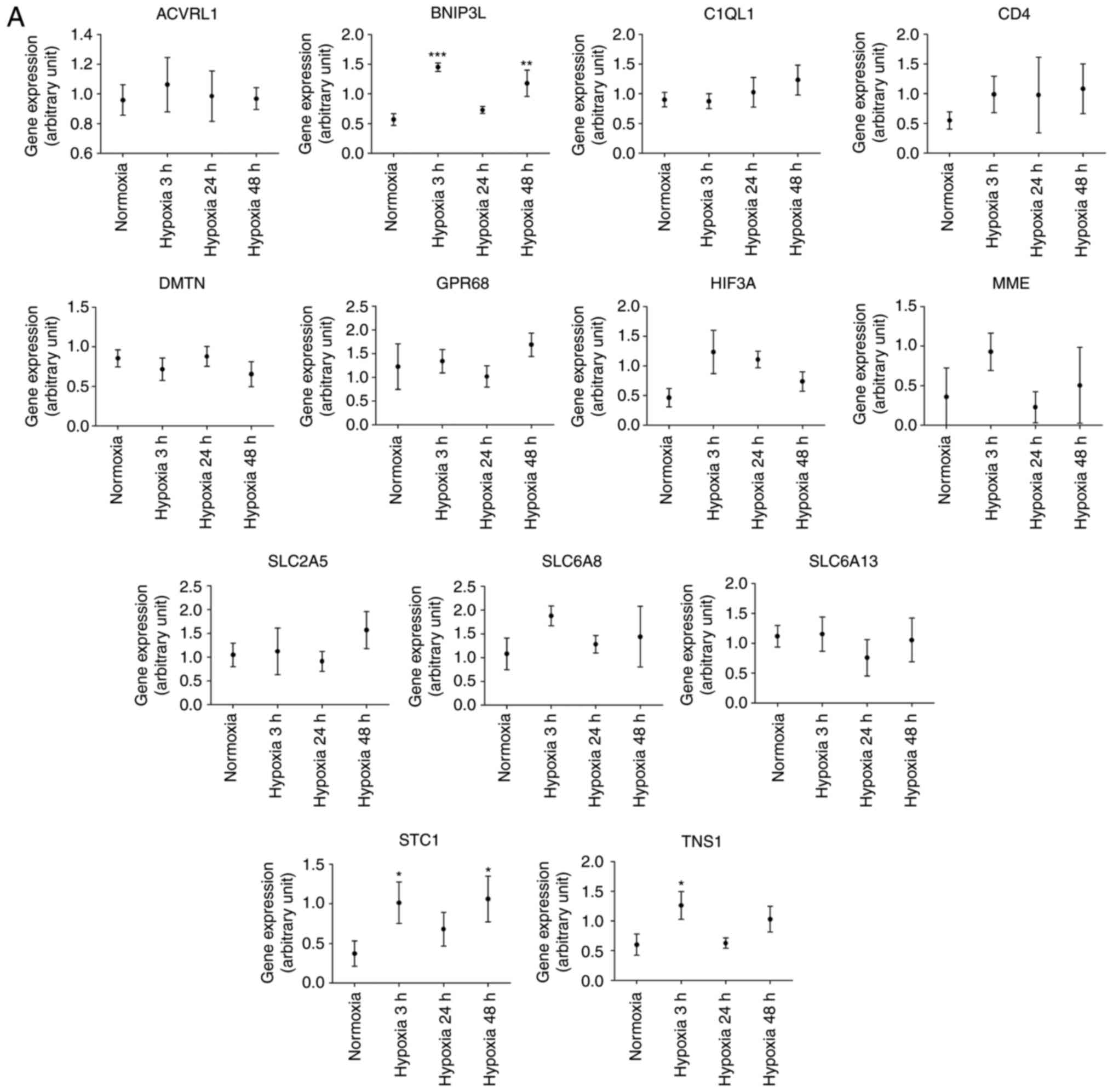
those of normoxic cells. The findings were partially similar to
those from the NGS CM data. In hypoxic cardiac microvascular
endothelial cells, tensin 1 (TNS1), B-cell lymphoma 2
(BCL2)/adenovirus E1B 19 kDa protein-interacting protein 3
(BNIP3L), and stanniocalcin 1 (STC1) were
significantly upregulated (Fig.
4A) and transferrin receptor (TFRC) and
methyltransferase like 7A (METTL7A) were significantly
downregulated (Fig. 4B). However,
unlike the findings based on NGS CM data, Calcium/calmodulin
dependent protein kinase 1D (CAMK1D) was significantly
downregulated.
GO annotations and KEGG pathway analyses
of DEGs in hypoxic AC16 CMs
Network analyses of the 24 genes with potential
miRNA-mRNA interactions were performed using IPA software. The top
three networks associated with genes targeted by miRNAs
differentially expressed in hypoxic AC16 CMs are listed in Table III. In network 1 (Fig. 5A), 14 targeted genes
(iodothyronine deiodinase 2, solute carrier family 2 member 5,
apolipoprotein L6, histone deacetylase 11, STC1, membrane
metalloendopeptidase (MME), chac glutathione specific
γ-glutamylcyclotransferase 1, apelin, TFRC, CD4 molecule,
hypoxia inducible factor 3 α subunit, BCL2, dematin actin
binding protein, and solute carrier family 6 member 13) were
associated with free radical scavenging, small molecule
biochemistry, and carbohydrate metabolism. In network 2 (Fig. 5B), seven targeted genes (solute
carrier family 7 member 11, activin A receptor like type 1,
BNIP3L, MME, METTL7A, TNS1, and G
protein-coupled receptor 68) were associated with cancer,
organismal injury and abnormalities, and cell death and survival.
In network 3, only CAMK1D was associated with carbohydrate
metabolism, small molecule biochemistry, and digestive system
development and function. Two genes (complement C1q like 1 and
transmembrane protein 144) were not involved in these networks.
 | Figure 5Networks analysis of the 24
differentially expressed genes with potential miRNA-mRNA
interactions in hypoxic AC16 CMs. The network analyses of the 24
differentially expressed genes with potential miRNA-mRNA
interactions in hypoxic AC16 CMs were investigated using
Ingenuity® Pathway Analysis software. (A) In network 1,
14 targeted genes (DIO2, SLC2A5, APOL6,
HDAC11, STC1, MME, CHAC1, APLN,
TFRC, CD4, HIF3A, BCL2, DMTN,
and SLC6A8) were associated with free radical scavenging,
small molecule biochemistry, and carbohydrate metabolism. (B) In
network 2, seven targeted genes (SLC7A11, ACVRL1,
BNIP3L, MME, METTL7A, TNS1, and
GPR68) were associated with cancer, organismal injury and
abnormalities, and cell death and survival. In network 3, only
CAMK1D was associated with carbohydrate metabolism, small
molecule biochemistry, and digestive system development and
function. CMs, cardiomyocytes; miRNA, microRNA. Diamonds,
horizontal ovals, vertical ovals, squares, rectangles, trapezoids,
and inverted triangles represent enzymes, transcription regulators,
transmembrane receptors, cytokines, ligand-dependent nuclear
receptors, transporters, and kinases, respectively. Solid and
dashed lines represent direct and indirect action, respectively.
Red and green symbols represent upregulated and downregulated
molecules, respectively. |
 | Table IIITop three networks associated with
genes targeted by microRNAs differentially expressed in hypoxic
AC16 cardiomyocytes. |
Table III
Top three networks associated with
genes targeted by microRNAs differentially expressed in hypoxic
AC16 cardiomyocytes.
| Network | Top diseases and
functions | Score | Focus
molecules | Molecules in
network |
|---|
| 1 | Free radical
scavenging, small molecule biochemistry, carbohydrate
metabolism | 32 | 14 | aAPLN, aAPOL6, aBCL2, CAT, aCD4, Cg, aCHAC1, CTNNB1, aDIO2, aDMTN, EPAS1,
F13A1, FBXO31, G0S2, aHDAC11, aHIF3A, IFNG,
IL17RB, LAP3, MAPK1, MICU1, aMME, NFkB(complex),
NRP2, SEC22B, SERPINB8, SLC16A3,
aSLC2A5, aSLC6A8, aSTC1, SULF2, aTFRC, TNF,
TNFSF4, UTP18 |
| 2 | Cancer, organismal
injury and abnormalities, cell death and survival | 13 | 7 | aACVRL1, BMP4, aBNIP3L, C5, CD74,
CREBBP, CXCL8, ESR1, F3, FOXO1,
GDF2, aGPR68,
ID1, Iga, IGF1, Igm, IKBKE,
ITGAM, KITLG, aMETTL7A, aMME, MMP14,
NCOR2, S100A6, SERPINB5, aSLC7A11, SMAD2,
SMARCA2, SMARCA4, SMARCE1, SOX2,
STAT3, TLR2, TLR4, aTNS1 |
| 3 | Carbohydrate
metabolism, small molecule biochemistry, digestive system
development and function | 2 | 1 | aCAMK1D, ERG, GCG,
INS |
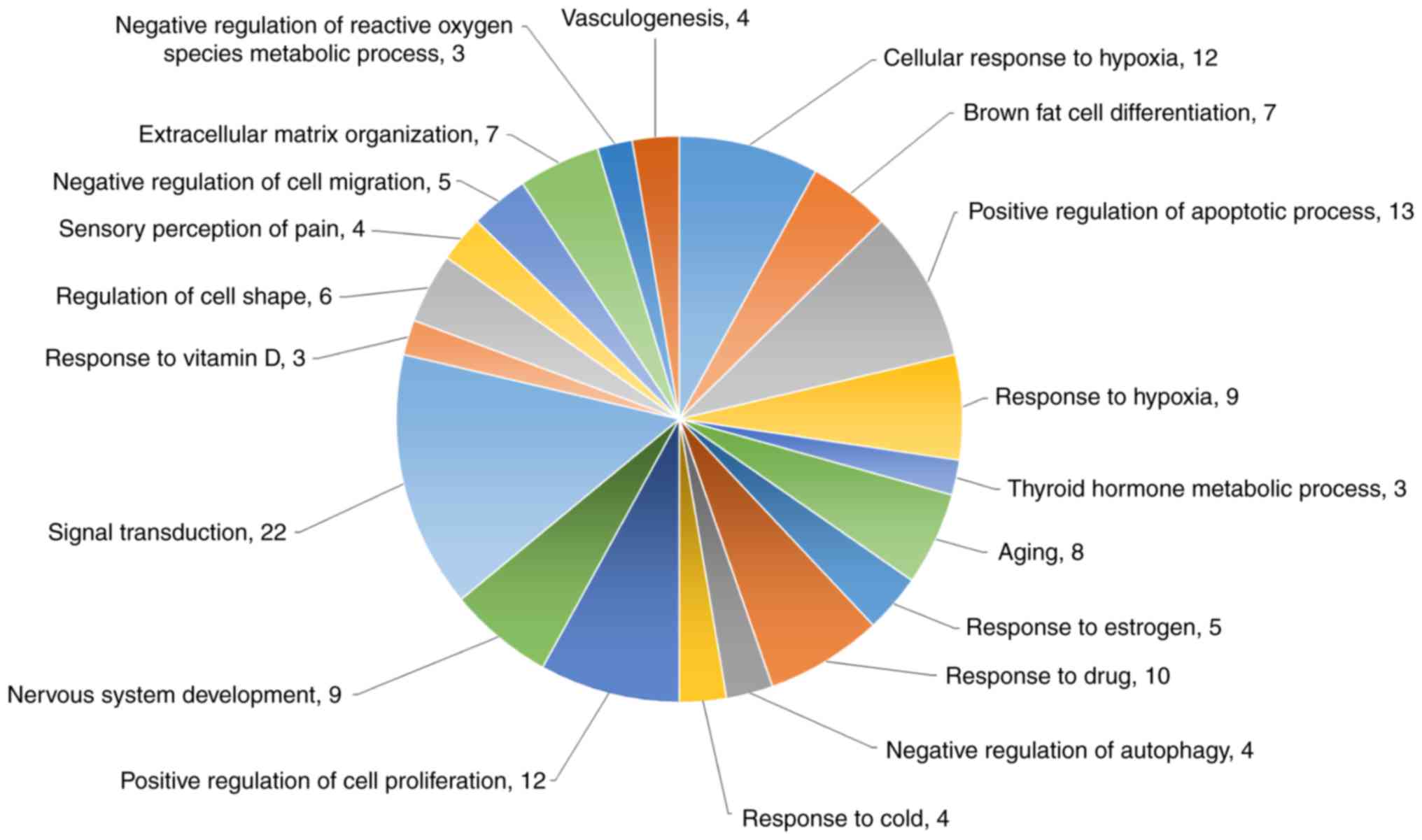
Functional analysis of the DEGs from mRNA sequencing
was also performed by DAVID biological process analysis (Fig. 6). The top four biological
processes of these genes were cellular response to hypoxia (12
genes), brown fat cell differentiation (seven genes), positive
regulation of apoptotic process (13 genes), and response to hypoxia
(nine genes). The protein-coding genes specifically associated with
apoptosis, cell proliferation inhibition, and cell cycle arrest
were also analyzed with a heatmap (Fig. 7).
The top 20 KEGG pathways of dysregulated genes were
identified using mRNA sequencing data (Table IV and Fig. 8). The legionellosis (fold
enrichment=7.11), regulation of lipolysis in adipocytes (fold
enrichment=6.86), and glycolysis/gluco-neogenesis (fold
enrichment=5.73) pathways were significantly enriched in the
hypoxic CMs.
 | Table IVKyoto Encyclopedia of Genes and
Genomes pathway analysis of the dysregulated genes (top 20)
identified from mRNA sequencing. |
Table IV
Kyoto Encyclopedia of Genes and
Genomes pathway analysis of the dysregulated genes (top 20)
identified from mRNA sequencing.
| Description | Count P | -value | Gene
upregulated | Gene
downregulated | Fold
enrichment |
|---|
| Legionellosis | 4 | 0.02 | ITGB2,
EEF1A2, BNIP3 | CXCL2 | 7.11 |
| Regulation of
lipolysis in adipocytes | 4 | 0.02 | AQP7,
PTGS2 | ADORA1,
PIK3R3 | 6.86 |
|
Glycolysis/gluconeogenesis | 4 | 0.03 | LDHA,
TPI1, PGK1 | LDHC | 5.73 |
| Cysteine and
methionine metabolism | 3 | 0.06 | LDHA | SDSL,
LDHC | 7.58 |
| Adrenergic
signaling in cardiomyocytes | 5 | 0.06 | CACNG6,
RAPGEF4, | BCL2,
PIK3R3 | 3.29 |
| | | PPP2R5B | | |
| Biosynthesis of
antibiotics | 6 | 0.07 | LDHA,
TPI1, PGK1, RGN | SDSL,
LDHC | 2.72 |
| HIF-1 signaling
pathway | 4 | 0.08 | VEGFA | TFRC,
BCL2, PIK3R3 | 3.92 |
| Insulin
resistance | 4 | 0.10 | PPP1R3B,
PPP1R3C | PPARGC1A,
PIK3R3 | 3.55 |
| Carbon
metabolism | 4 | 0.11 | TPI1,
PGK1, RGN | SDSL | 3.40 |
| Sphingolipid
signaling pathway | 4 | 0.13 | PPP2R5B | ADORA1,
BCL2 PIK3R3 | 3.20 |
| VEGF signaling
pathway | 3 | 0.13 | VEGFA,
PTGS2 | PIK3R3 | 4.72 |
| AMPK signaling
pathway | 4 | 0.13 | PFKFB4,
PPP2R5B | PPARGC1A,
PIK3R3 | 3.15 |
| Insulin signaling
pathway | 4 | 0.17 | PPP1R3B,
PPP1R3C | PPARGC1A,
PIK3R3 | 2.78 |
| Rap1 signaling
pathway | 5 | 0.17 | ITGB2,
VEGFA, RAPGEF4, | PIK3R3 | 2.29 |
| Biosynthesis of
amino acids | 3 | 0.18 | TPI1,
PGK1 | SDSL | 3.89 |
| Hepatitis B | 4 | 0.19 | | BCL2,
TLR3, PIK3R3, IFIH1 | 2.65 |
| Hematopoietic cell
lineage | 3 | 0.22 | MME,
CD4 | TFRC | 3.39 |
| Small cell lung
cancer | 3 | 0.22 | PTGS2 | BCL2,
PIK3R3 | 3.39 |
| Oxytocin signaling
pathway | 4 | 0.22 | PTGS2,
CACNG6, CAMK1D | PIK3R3 | 2.43 |
Discussion
Differential gene expression with altered signaling
pathways has been reported in CMs responding to hypoxic/ischemic
conditions (3,29,30). The reciprocal regulation of miRNAs
and
mRNA in human CMs from patients with heart failure
has also been investigated using microarray analysis (31). The present study comprehensively
surveyed the differential expression of mRNAs and miRNAs and
potential mRNA-miRNA interactions using NGS techniques and
bioinformatics.
miRNAs are short, small non-coding RNAs, which
regulate gene expression via several post-transcriptional processes
(32). Hypoxia is a stress
condition that may provoke multiple biological and molecular
regulatory networks associated with apoptosis, cell proliferation,
and alterations in metabolism (32). Previous studies have suggested
that miRNAs may potentially be used as novel diagnostic makers and
therapeutic targets for hypoxic CMs in clinical scenarios,
including AMI (33). The present
study found 62 miRNAs with >2-fold changes in hypoxic CMs. Based
on the miRNA-mRNA interactions predicted using miRmap, TargetScan
and miRDB databases, hsa-miR-129-5p and hsa-miR-330-3p were
identified as having the highest potential of becoming such
biomarkers. The overexpression of miR-129-5p inhibits cell
proliferation in smooth muscle cells, glioblastoma multiforme
cells, gastric cancer cells, and H9C2 CMs (34-37). Previous experimental studies have
noted wingless-type MMTV integration site family member 5A,
collagen, type I, a1, and cyclin-dependent kinase 6 to be the
targets of miR-129-5p (34-37). The overexpression of miR-330 has
been found to inhibit cell proliferation through B cell-specific
Moloney murine leukemia virus integration site 1, E2F transcription
factor 1 and musashi RNA binding protein 1 in osteosarcoma cells,
gastric cells, and prostate cancer cells, respectively (38-40). However, miR-330 has also found to
increase cell proliferation by regulating SH3-domain GRB2-like 2
and WNT signaling pathways in human glioblastoma cells and vascular
endothelial cells, respectively (41,42). Although the expression levels of
these genes exhibited no significant changes in AC16 CMs exposed to
hypoxia, the downstream regulatory mechanisms of hsa-miR-129-5p and
hsa-miR-330-3p in hypoxic CMs warrant further investigation.
The present study identified 24 genes with
significant changes and potential miRNA-mRNA interactions. These
genes were associated with important cellular functions, including
cell proliferation, apoptosis, and carbohydrate metabolism. Using
the gene expression profiles of cardiac microvascular endothelial
cells obtained from the GEO database, it was possible to validate
the significant upregulation of TNS1, BNIP3L and
STC1, and the significant downregulation of TFRC and
METTL7A in response to hypoxia. CAMK1D exhibited
paradoxical changes in different cells.
TNS1 is a focal adhesion molecule which serves as a
scaffold for cell mobility and adhesion through binding to
fibronectin and β1-integrin (43,44). The overexpression of TNS1
has been found to contribute to the invasion and metastasis of
breast cancer cells (45). TNS1
may also be involved in the micro-environmental changes that occur
in the myocardium during hypoxia.
Programmed cell death may occur in CMs via the
extrinsic cytokine death pathway or the intrinsic mitochondrial
pruning pathway (46,47). The BNIP3 and BNIP3-like (BNIP3L,
also known as NIP3-like protein X, NIP3L, or NIX), belong to the
pro-apoptotic Bcl-2 protein family and are mitochondrial stress
sensors (48). They can promote
mitophagy and autophagy in response to hypoxia (49,50). Upregulated BNIP3 and BNIP3L
trigger apoptosis in conditions which include myocardial
hypertrophy and are important in cardiac remodelling (51,52). Therefore, these pathways have been
investigated for their potential role in inhibiting ischemic CM
apoptosis (53). Similarly, the
present study found BNIP3L to be upregulated in hypoxic CMs
and cardiac microvascular endothelial cells.
STC1, an endogenous glycoprotein, has
anti-inflammatory and anti-oxidative effects through the uncoupling
protein (UCP) family in mice kidney and lung injuries (54,55). However, its main role in CMs
remains to be elucidated. STC1 has been found to be significantly
increased in patients with dilated cardiomyopathy, but normalizes
following treatment using a left ventricular assist device
(56). STC1 inhibits super-oxide
generation via the induction of UCP3 in CMs, which may ameliorate
angiotensin II-mediated cardiac injury (57). However, the cardiotoxic effects of
STC1 also appear to be mediated via mitochondrial injuries through
loss of integrity of the mitochondrial membrane, increased
mitochondrial calcium levels, and reactive oxygen species
production (58). In the present
study, STC1 was upregulated in hypoxic CMs and cardiac
microvascular endothelial cells. Whether this up regulation of
STC1 was associated with anti-oxidative effects or
pro-oxidative effects warrants further investigation.
The present study revealed significant
downregulation of TFRC and METTL7A in hypoxic CMs and
cardiac microvascular endothelial cells. Previous studies have
shown that either iron overloading or deficiency in CMs were
associated with cardiotoxicity and heart failure. In mice, the lack
of TFRC results in poor cardiac and mitochondrial function, and
early death (59). Decreased
expression of TFRC has been associated with reduced
pulmonary smooth muscle cell proliferation in hypoxia (60). METTL7A has been recognized
as a novel tumor suppressor gene (61). The actual role of the
downregulation of METTL7A in CMs and cardiac microvascular
endothelial cells in response to hypoxia remains uncertain and
requires further investigation.
CAMK1D is a key regulator of granulocyte function
and has been associated with newly-developed type 2 diabetes
mellitus in Japan (62,63). The overexpression of CAMK1D
results in increased cell proliferation and epithelial-mesenchymal
transition activity in breast epithelial cells (64). In the present study, it was found
that CAMK1D was significantly upregulated in hypoxic CMs but
significantly downregulated in hypoxic cardiac microvascular
endothelial cells. Although the reason for this paradoxical finding
remains to be fully elucidated, it was hypothesized that the
overexpression of CAMK1D in hypoxic CMs may be involved in
alterations in carbohydrate metabolism and cell detachment.
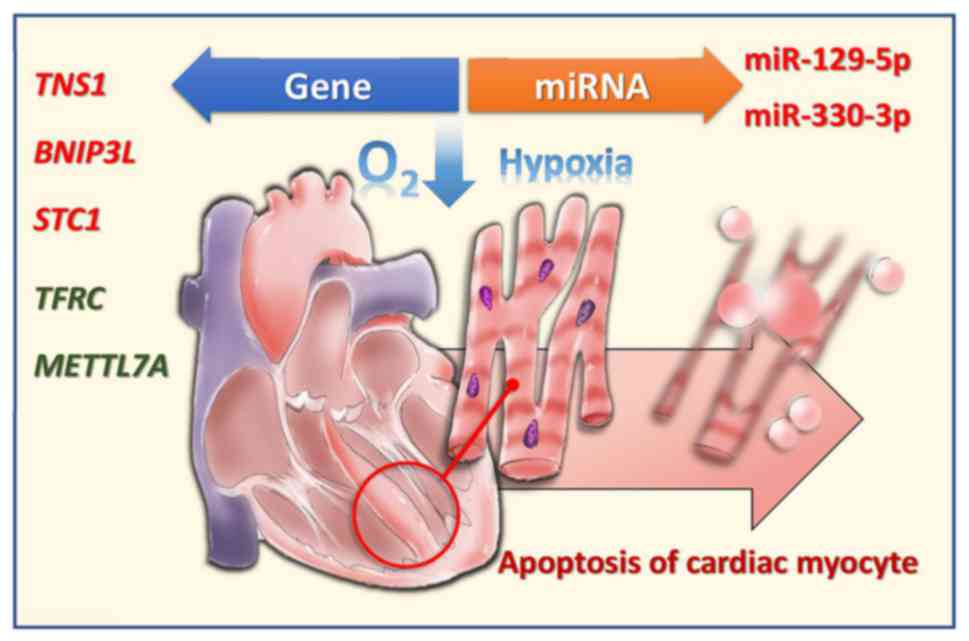
In conclusion, the present study reports on the
differentially upregulated/downregulated mRNAs and miRNAs in
hypoxic human CMs (Fig. 9). An
improved understanding of the mRNA and miRNA profiles, in addition
to the potential miRNA-mRNA interactions, in hypoxic CMs, enables
the development of novel diagnostic tools and therapeutic
strategies for hypoxic human CMs as is found in AMI.
Funding
The present study was supported by research grants
from the Ministry of Science and Technology (grant no. MOST
107-2320-B-037-011-MY3), Kaohsiung Medical University Hospital
(grant nos. KMUHS10701 and KMUHS10712), and the Kaohsiung Medical
University (grant no. KMU-DK108003).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HMS and PLK conceived the study. WHL, MJT, WAC, KFC,
and PLK analyzed and interpreted the data. LYW and HYW performed
the cell culture and flow cytometry. WHL, MJT and PLK were the
major contributors in writing the manuscript. WAC produced the
illustration. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors confirm that they have no competing
interests.
Acknowledgments
The authors thank the Center for Research Resources
and Development of Kaohsiung Medical University (Kaohsiung,
Taiwan).
References
|
1
|
Lee WH, Hsu PC, Chu CY, Su HM, Lee CS, Yen
HW, Lin TH, Voon WC, Lai WT and Sheu SH: Cardiovascular events in
patients with atherothrombotic disease: A population-based
longitudinal study in taiwan. PLoS One. 9:e925772014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Soler EP and Ruiz VC: Epidemiology and
risk factors of cerebral ischemia and ischemic heart diseases:
Similarities and differences. Curr Cardiol Rev. 6:138–149. 2010.
View Article : Google Scholar :
|
|
3
|
Kang PM, Haunstetter A, Aoki H, Usheva A
and Izumo S: Morphological and molecular characterization of adult
cardiomyocyte apoptosis during hypoxia and reoxygenation. Circ Res.
87:118–125. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun T, Dong YH, Du W, Shi CY, Wang K,
Tariq MA, Wang JX and Li PF: The Role of MicroRNAs in myocardial
infarction: From molecular mechanism to clinical application. Int J
Mol Sci. 18:pii: E745. 2017. View Article : Google Scholar
|
|
5
|
Adachi S, Ito H, Tamamori-Adachi M, Ono Y,
Nozato T, Abe S, Ikeda Ma, Marumo F and Hiroe M: Cyclin A/cdk2
activation is involved in hypoxia-induced apoptosis in
cardiomyocytes. Circ Res. 88:408–414. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ibanez B, James S, Agewall S, Antunes MJ,
Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA,
Halvorsen S, et al: 2017 ESC Guidelines for the management of acute
myocardial infarction in patients presenting with ST-segment
elevation: The Task Force for the management of acute myocardial
infarction in patients presenting with ST-segment elevation of the
European Society of Cardiology (ESC). Eur Heart J. 39:119–177.
2018. View Article : Google Scholar
|
|
7
|
Krijnen PA, Nijmeijer R, Meijer CJ, Visser
CA, Hack CE and Niessen HW: Apoptosis in myocardial ischaemia and
infarction. J Clin Pathol. 55:801–811. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takemura G and Fujiwara H: Morphological
aspects of apoptosis in heart diseases. J Cell Mol Med. 10:56–75.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Biondi-Zoccai GG, Abbate A, Vasaturo F,
Scarpa S, Santini D, Leone AM, Parisi Q, De Giorgio F, Bussani R,
Silvestri F, et al: Increased apoptosis in remote non-infarcted
myocardium in multivessel coronary disease. Int J Cardiol.
94:105–110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abbate A, Melfi R, Patti G, Baldi F,
D'Ambrosio A, Manzoli A, Baldi A and Di Sciascio G: Apoptosis in
recent myocardial infarction. Clin Ter. 151:247–251.
2000.PubMed/NCBI
|
|
11
|
Saraste A, Pulkki K, Kallajoki M,
Henriksen K, Parvinen M and Voipio-Pulkki LM: Apoptosis in human
acute myocardial infarction. Circulation. 95:320–323. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abbate A, Bussani R, Biondi-Zoccai GG,
Santini D, Petrolini A, De Giorgio F, Vasaturo F, Scarpa S,
Severino A, Liuzzo G, et al: Infarct-related artery occlusion,
tissue markers of ischaemia, and increased apoptosis in the
peri-infarct viable myocardium. Eur Heart J. 26:2039–2045. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
van Dijk EL, Auger H, Jaszczyszyn Y and
Thermes C: Ten years of next-generation sequencing technology.
Trends Genet. 30:418–426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mardis ER: Next-generation sequencing
platforms. Annu Rev Anal Chem (Palo Alto Calif). 6:287–303. 2013.
View Article : Google Scholar
|
|
15
|
Chen SC, Chen FW, Hsu YL and Kuo PL:
Systematic analysis of transcriptomic profile of renal cell
carcinoma under long-term hypoxia using next-generation sequencing
and bioinformatics. Int J Mol Sci. 18:2017. View Article : Google Scholar
|
|
16
|
Blue GM, Kirk EP, Giannoulatou E,
Dunwoodie SL, Ho JW, Hilton DC, White SM, Sholler GF, Harvey RP and
Winlaw DS: Targeted next-generation sequencing identifies
pathogenic variants in familial congenital heart disease. J Am Coll
Cardiol. 64:2498–2506. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lubitz SA and Ellinor PT: Next-generation
sequencing for the diagnosis of cardiac arrhythmia syndromes. Heart
Rhythm. 12:1062–1070. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xia P, Liu Y and Cheng Z: Signaling
Pathways in Cardiac Myocyte Apoptosis. Biomed Res Int.
2016:95832682016. View Article : Google Scholar
|
|
19
|
Chiong M, Wang ZV, Pedrozo Z, Cao DJ,
Troncoso R, Ibacache M, Criollo A, Nemchenko A, Hill JA and
Lavandero S: Cardiomyocyte death: Mechanisms and translational
implications. Cell Death Dis. 2:e2442011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Regula KM, Ens K and Kirshenbaum LA:
Inducible expression of BNIP3 provokes mitochondrial defects and
hypoxia-mediated cell death of ventricular myocytes. Circ Res.
91:226–231. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yen MC, Shih YC, Hsu YL, Lin ES, Lin YS,
Tsai EM, Ho YW, Hou MF and Kuo PL: Isolinderalactone enhances the
inhibition of SOCS3 on STAT3 activity by decreasing miR-30c in
breast cancer. Oncol Rep. 35:1356–1364. 2016. View Article : Google Scholar
|
|
22
|
Sheu CC, Tsai MJ, Chen FW, Chang KF, Chang
WA, Chong IW, Kuo PL and Hsu YL: Identification of novel genetic
regulations associated with airway epithelial homeostasis using
next-generation sequencing data and bioinformatics approaches.
Oncotarget. 8:82674–82688. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bolger AM, Lohse M and Usadel B:
Trimmomatic: A flexible trimmer for Illumina sequence data.
Bioinformatics. 30:2114–2120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Friedlander MR, Mackowiak SD, Li N, Chen W
and Rajewsky N: miRDeep2 accurately identifies known and hundreds
of novel microRNA genes in seven animal clades. Nucleic Acids Res.
40:37–52. 2012. View Article : Google Scholar :
|
|
25
|
Trapnell C, Roberts A, Goff L, Pertea G,
Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL and Pachter L:
Differential gene and transcript expression analysis of RNA-seq
experiments with TopHat and Cufflinks. Nat Protoc. 7:562–578. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vejnar CE and Zdobnov EM: MiRmap:
Comprehensive prediction of microRNA target repression strength.
Nucleic Acids Res. 40:11673–11683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Costello CM, Howell K, Cahill E, McBryan
J, Konigshoff M, Eickelberg O, Gaine S, Martin F and McLoughlin P:
Lung-selective gene responses to alveolar hypoxia: Potential role
for the bone morphogenetic antagonist gremlin in pulmonary
hypertension. Am J Physiol Lung Cell Mol Physiol. 295:L272–L284.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang DW, Sherman BT, Tan Q, Collins JR,
Alvord WG, Roayaei J, Stephens R, Baseler MW, Lane HC and Lempicki
RA: The DAVID Gene Functional Classification Tool: A novel
biological module-centric algorithm to functionally analyze large
gene lists. Genome Biol. 8:R1832007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kacimi R, Chentoufi J, Honbo N, Long CS
and Karliner JS: Hypoxia differentially regulates stress proteins
in cultured cardiomyocytes: Role of the p38 stress-activated kinase
signaling cascade, and relation to cytoprotection. Cardiovasc Res.
46:139–150. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Koeppen M, Lee JW, Seo SW, Brodsky KS,
Kreth S, Yang IV, Buttrick PM, Eckle T and Eltzschig HK:
Hypoxia-inducible factor 2-alpha-dependent induction of
amphiregulin dampens myocardial ischemia-reperfusion injury. Nat
Commun. 9:8162018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Matkovich SJ, Van Booven DJ, Youker KA,
Torre-Amione G, Diwan A, Eschenbacher WH, Dorn LE, Watson MA,
Margulies KB and Dorn GW II: Reciprocal regulation of myocardial
microRNAs and messenger RNA in human cardiomyopathy and reversal of
the microRNA signature by biomechanical support. Circulation.
119:1263–1271. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nallamshetty S, Chan SY and Loscalzo J:
Hypoxia: A master regulator of microRNA biogenesis and activity.
Free Radic Biol Med. 64:20–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ong SB, Katwadi K, Kwek XY, Ismail NI,
Chinda K, Ong SG and Hausenloy DJ: Non-coding RNAs as therapeutic
targets for preventing myocardial ischemia-reperfusion injury.
Expert Opin Ther Targets. 22:247–261. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Y, Liu Z, Zhou M and Liu C:
MicroRNA-129-5p inhibits vascular smooth muscle cell proliferation
by targeting Wnt5a. Exp Ther Med. 12:2651–2656. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zeng A, Yin J, Li Y, Li R, Wang Z, Zhou X,
Jin X, Shen F, Yan W and You Y: miR-129-5p targets Wnt5a to block
PKC/ERK/NF-kappaB and JNK pathways in glioblastoma. Cell Death Dis.
9:3942018. View Article : Google Scholar
|
|
36
|
Majumdar G and Raghow R: Trichostatin A
induces a unique set of microRNAs including miR-129-5p that blocks
cyclin-dependent kinase 6 expression and proliferation in H9c2
cardiac myocytes. Mol Cell Biochem. 415:39–49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Q and Yu J: MiR-129-5p suppresses
gastric cancer cell invasion and proliferation by inhibiting
COL1A1. Biochem Cell Biol. 96:19–25. 2018. View Article : Google Scholar
|
|
38
|
Zheng Z, Bao F, Chen X, Huang H and Zhang
X: MicroRNA-330-3p Expression Indicates Good Prognosis and
Suppresses Cell Proliferation by Targeting Bmi-1 in Osteosarcoma.
Cell Physiol Biochem. 46:442–450. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Guan A, Wang H, Li X, Xie H, Wang R, Zhu Y
and Li R: MiR-330-3p inhibits gastric cancer progression through
targeting MSI1. Am J Transl Res. 8:4802–4811. 2016.PubMed/NCBI
|
|
40
|
Lee KH, Chen YL, Yeh SD, Hsiao M, Lin JT,
Goan YG and Lu PJ: MicroRNA-330 acts as tumor suppressor and
induces apoptosis of prostate cancer cells through E2F1-mediated
suppression of Akt phosphorylation. Oncogene. 28:3360–3370. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Qu S, Yao Y, Shang C, Xue Y, Ma J, Li Z
and Liu Y: MicroRNA-330 is an oncogenic factor in glioblastoma
cells by regulating SH3GL2 gene. PLoS One. 7:e460102012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ren J, Ma R, Zhang ZB, Li Y, Lei P and Men
JL: Effects of microRNA-330 on vulnerable atherosclerotic plaques
formation and vascular endothelial cell proliferation through the
WNT signaling pathway in acute coronary syndrome. J Cell Biochem.
119:4514–4527. 2018. View Article : Google Scholar
|
|
43
|
Shih YP, Sun P, Wang A and Lo SH: Tensin1
positively regulates RhoA activity through its interaction with
DLC1. Biochim Biophys Acta. 1853:3258–3265. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bernau K, Torr EE, Evans MD, Aoki JK, Ngam
CR and Sandbo N: Tensin 1 Is essential for myofibroblast
differentiation and extracellular matrix formation. Am J Respir
Cell Mol Biol. 56:465–476. 2017. View Article : Google Scholar :
|
|
45
|
Zhan Y, Liang X, Li L, Wang B, Ding F, Li
Y, Wang X, Zhan Q and Liu Z: MicroRNA-548j functions as a
metastasis promoter in human breast cancer by targeting Tensin1.
Mol Oncol. 10:838–849. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dorn GW II: Mitochondrial pruning by Nix
and BNip3: An essential function for cardiac-expressed death
factors. J Cardiovasc Transl Res. 3:374–383. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ney PA: Mitochondrial autophagy: Origins,
significance, and role of BNIP3 and NIX. Biochim Biophys Acta.
1853:2775–2783. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chinnadurai G, Vijayalingam S and Gibson
SB: BNIP3 subfamily BH3-only proteins: Mitochondrial stress sensors
in normal and pathological functions. Oncogene. 27(Suppl 1):
S114–S127. 2008. View Article : Google Scholar
|
|
49
|
O'Sullivan TE, Johnson LR, Kang HH and Sun
JC: BNIP3- and BNIP3L-mediated mitophagy promotes the generation of
natural killer cell memory. Immunity. 43:331–342. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mazure NM and Pouyssegur J: Atypical
BH3-domains of BNIP3 and BNIP3L lead to autophagy in hypoxia.
Autophagy. 5:868–869. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yussman MG, Toyokawa T, Odley A, Lynch RA,
Wu G, Colbert MC, Aronow BJ, Lorenz JN and Dorn GW II:
Mitochondrial death protein Nix is induced in cardiac hypertrophy
and triggers apoptotic cardiomyopathy. Nat Med. 8:725–730. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Dorn GW II and Kirshenbaum LA: Cardiac
reanimation: Targeting cardiomyocyte death by BNIP3 and NIX/BNIP3L.
Oncogene. 27(Suppl 1): S158–S167. 2008. View Article : Google Scholar
|
|
53
|
Diwan A, Krenz M, Syed FM, Wansapura J,
Ren X, Koesters AG, Li H, Kirshenbaum LA, Hahn HS, Robbins J, et
al: Inhibition of ischemic cardiomyocyte apoptosis through targeted
ablation of Bnip3 restrains postinfarction remodeling in mice. J
Clin Invest. 117:2825–2833. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Pan JS, Huang L, Belousova T, Lu L, Yang
Y, Reddel R, Chang A, Ju H, DiMattia G, Tong Q and Sheikh-Hamad D:
Stanniocalcin-1 inhibits renal ischemia/reperfusion injury via an
AMP-activated protein kinase-dependent pathway. J Am Soc Nephrol.
26:364–378. 2015. View Article : Google Scholar :
|
|
55
|
Tang SE, Wu CP, Wu SY, Peng CK, Perng WC,
Kang BH, Chu SJ and Huang KL: Stanniocalcin-1 ameliorates
lipopolysac-charide-induced pulmonary oxidative stress,
inflammation, and apoptosis in mice. Free Radic Biol Med.
71:321–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sheikh-Hamad D, Bick R, Wu GY, Christensen
BM, Razeghi P, Poindexter B, Taegtmeyer H, Wamsley A, Padda R,
Entman M, et al: Stanniocalcin-1 is a naturally occurring L-channel
inhibitor in cardiomyocytes: Relevance to human heart failure. Am J
Physiol Heart Circ Physiol. 285:H442–H448. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu D, Huang L, Wang Y, Wang W, Wehrens
XH, Belousova T, Abdelrahim M, DiMattia G and Sheikh-Hamad D: Human
stanniocalcin-1 suppresses angiotensin II-induced superoxide
generation in cardiomyocytes through UCP3-mediated anti-oxidant
pathway. PLoS One. 7:e369942012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Guan J, Mishra S, Shi J, Plovie E, Qiu Y,
Cao X, Gianni D, Jiang B, Del Monte F, Connors LH, et al:
Stanniocalcin1 is a key mediator of amyloidogenic light chain
induced cardiotoxicity. Basic Res Cardiol. 108:3782013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Xu W, Barrientos T, Mao L, Rockman HA,
Sauve AA and Andrews NC: Lethal cardiomyopathy in mice lacking
transferrin receptor in the heart. Cell Rep. 13:533–545. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Naito Y, Hosokawa M, Sawada H, Oboshi M,
Hirotani S, Iwasaku T, Okuhara Y, Morisawa D, Eguchi A, Nishimura
K, et al: Transferrin receptor 1 in chronic hypoxia-induced
pulmonary vascular remodeling. Am J Hypertens. 29:713–718. 2016.
View Article : Google Scholar
|
|
61
|
Qi L, Song Y, Chan THM, Yang H, Lin CH,
Tay DJT, Hong H, Tang SJ, Tan KT, Huang XX, et al: An RNA
editing/dsRNA binding-independent gene regulatory mechanism of
ADARs and its clinical implication in cancer. Nucleic Acids Res.
45:10436–10451. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Cheng J, Tang L, Hong Q, Ye H, Xu X, Xu L,
Bu S, Wang Q, Dai D, Jiang D and Duan S: Investigation into the
promoter DNA methylation of three genes (CAMK1D, CRY2 and CALM2) in
the peripheral blood of patients with type 2 diabetes. Exp Ther
Med. 8:579–584. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Imamura M, Iwata M, Maegawa H, Watada H,
Hirose H, Tanaka Y, Tobe K, Kaku K, Kashiwagi A, Kawamori R, et al:
Genetic variants at CDC123/CAMK1D and SPRY2 are associated with
susceptibility to type 2 diabetes in the Japanese population.
Diabetologia. 54:3071–3077. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Bergamaschi A, Kim YH, Kwei KA, La Choi Y,
Bocanegra M, Langerød A, Han W, Noh DY, Huntsman DG, Jeffrey SS, et
al: CAMK1D amplification implicated in epithelial-mesenchymal
transition in basal-like breast cancer. Mol Oncol. 2:327–339. 2008.
View Article : Google Scholar
|