In order to determine the function of a gene, the
gene can be inactivated by homologous recombination or by blocking
its messenger RNA through RNA interference (1). This approach can be applied in
cultured cells by transfection or in living organisms by
transgenesis (1). Recent advances
in genome editing allow the manipulation of any gene at its own
locus in a broad variety of species and tissues, including cultured
cells and animal organs. Genome editing is a powerful tool for
biomedical research and provides hope for correcting some inherited
diseases.
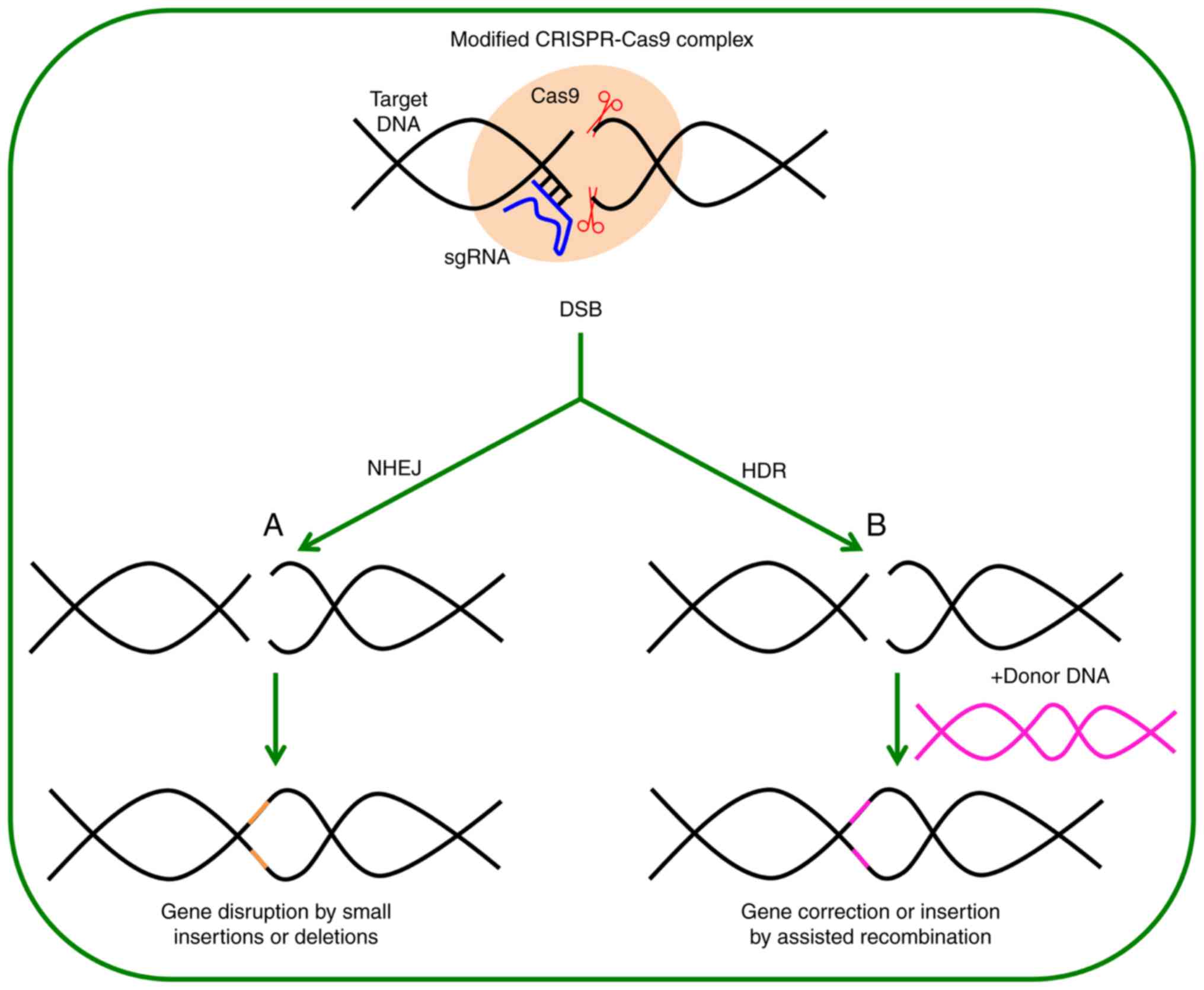
Genome editing is based on the use of highly
specific and programmable nucleases, which produce specific changes
in regions of interest in the genome by introducing double-strand
breaks (DSBs) that are later repaired by cellular mechanisms. These
repair mechanisms include the non-homologous end-joining (NHEJ)
that is prone to error, and the homology-directed repair (HDR) that
is error-free (Fig. 1). Repair
permits the generation of insertions, deletions or substitutions in
the target area (2-4). These mutations may interrupt,
eliminate or correct the defects in genes. The latter possibility
affords the ability to correct inherent errors in DNA that cause
diseases. The ‘programmable’ nucleases are mainly meganucleases
(5), zinc-finger nucleases
(6), transcription activator-like
effector nucleases (7), and the
CRISPR/Cas9 system [involving the Clustered Regularly Interspaced
Short Palindromic Repeats and nuclease(s) associated to the CRISPR
locus] (8). Usually, the
CRISPR/Cas9 system is comprised of a guide RNA (gRNA) that directs
the Cas9 nuclease to create a DSB in a specific place of the
genome. In the last decade, this system has gained wide acceptance
over other systems due to its simplicity, speed and efficiency for
modifying endogenous genes in any cell or target tissue, even in
the most traditionally difficult-to-treat organisms.
The eubacteria and archaea possess a defense system
that adapts, through RNA, to recognize and destroy external DNA and
RNA. This provides acquired immunity against invading plasmids and
viruses (9). This system is found
in approximately 50% of the bacterial genomes that are sequenced
and in 87% of the genomes of archaea (9-12).
It is also important in a range of additional functions, including
replicon divisions (13),
high-temperature adaptation (14), chromosomal rearrangements
(15) and DNA repair (16).
The type II CRISPR/Cas system is the most commonly
used system for genome editing, using the well-characterized Cas9
endonuclease of Streptococcus pyogenes. In the endogenous
system, the mature crRNA joins with a tracrRNA (small RNA that is
complementary to the CRISPR sequences) to form a tracrRNA:crRNA
complex, which guides the Cas9 to a target site. Thus, CRISPR/Cas9
performs sequence-specific cleavage by simple interaction of crRNA
by base pairing at the target site. After joining the target site,
the two DNA strands are cleaved by the nuclease domain of Cas9, a
HNH domain that cleaves the complementary target strand to the gRNA
and a RuvC-like nuclease domain, which cleaves the non-target
strand (33-35). The gRNA designed by Jinek et
al (8) in 2012 was a
chimerical RNA, which contains all the essential components of
crRNA and tracRNA to guide Cas9. Since then, multiple variants of
CRISPR/Cas9 have been developed, which recognize sequences of 18-24
nt of the gRNA, and 2-4 nt of protospacer adjacent motif (PAM) in
target sites (3,36). Therefore, CRISPR/Cas9 can
theoretically be directed to a specific sequence of DNA of 22-29
nt, which is unique in most of the genomes, although it has been
noted that CRISPR/Cas9 has a high-tolerance for non-specific mating
of base pairs between gRNA and its complementary target sequence.
This specificity is sensitive to numbers, position and distribution
of wrong interactions (3,8,28,29). For instance, the CRISPR/Cas9 of
Streptococcus pyogenes (SpCas9) tolerates up to six
imbalances of base pairs at target sites (8). The genome editing mediated by
CRISPR/Cas9 depends on the generation of the DSB and the subsequent
process of DNA repair. The DSB generated by the CRISPR/Cas9
triggers the process of cell repair in DNA, as a NHEJ, which is
prone to error and thus can produce mutations involving small
insertions and deletions (indels) in target sites, which can
interrupt or eliminate the function of the genes or the genomic
target elements (such as regulatory regions). Another repair
process that can also be triggered is the HDR error-free, which can
potentially correct innate disease-causing errors of DNA (genes or
regulatory elements) (37).
The specificity of CRISPR/Cas9, besides the
complementarity of the gRNA/target sequences, requires a PAM
sequence that is located immediately after the target sequence. The
reliance on the PAM sequence for the cleavage of the DNA restricts
the frequency of the cleavage sites in the genomes, thus target
sites are found more frequently for small PAM sequences than for
longer ones; consequently off-target cut sites are less likely to
exist for long PAM sequences than for short ones. The identified
PAM sequences vary between different microorganisms, and the
following sequences have been reported: 5′-NGG-3′ in
Streptococcus pyogenes (SpCas9) (8), 5′-NGGNG-3′ or 5′-NNAGAAW-3′ in
Streptococcus thermophiles (St1Cas9) (25,38,39), 5′-NNGRRT-3′ or 5′-NNGRR(N)-3′ in
Staphylococcus aureus (SaCas9) (40,41), 5′-NNNRRT-3′ or 5′-NNNNGMTT-3′ in
Neisseria meningitidis (NmCas9) (42), and 5′-NGG-3′ in Francisella
novivida (FnCpf1) (43,44), where N refers to every nucleotide,
R to purines A or G, M to nucleotide A or C, and W to weak bonds A
or T (3,11,36,45).
Although the DSB activity of CRISPR/Cas9 is based on
the complementarity of target sequences with the gRNAs of ~20 nt in
length, the system allows cleavage at genomic locations partially
complementary to the gRNA, because the gRNA allows mismatch
pairings between the DNA and gRNA (3). These off-target cuts of CRISPR/Cas9
are one of the biggest issues that currently remain unresolved
(causing certain undesirable consequences) and differ between
different target sites due to the diversity of nucleotide sequence
and the genomic context. The CRISPR/Cas9 system has been
implemented successfully for gene editing, and for the control of
different types of biological systems; however, there is little
evidence of the consequences of off-target genome editing Indeed,
there are only a few assays of genotoxicity based on cells in
culture that allow to quantify, stratify and help prevent
biological side effects of gene editing in a given target cell
population. Although a number of gene editing studies have reached
the phase of clinical trial, clinical evidence showing that gene
editing is truly capable of treating disorders is still scarce
(30-32).
Several methods have been proposed to optimize gRNA
design and minimize off-target cuts to reach the reliability and
specificity necessary for safety in therapeutics applications
(46). Identification of the
optimal gRNA among various candidates for a given target site, and
the localization of potential off-target sites can be supported by
different bioinformatics tools (47); for example: CRISPRdirect (48), E-CRISPR (49), WU-CRISPR (50), CRISPR gRNA design tool (https://www.atum.bio/eCom-merce/cas9/input), sgRNA
Designer (51), sgRNA Scorer 2.0
(52,53), CRISPRscan (54), CRISPR-ERA (55), CCtop (56), CRISPOR (57), Breaking-Cas (46), CHOPCHOP (58), CRISP MultiTarget (59), GT-Scan (60), ge-CRISPR (47), CRISPR Design (61), Cas-Designer (62), Cas-OFFinder (63), COSMID (64), DESKGEN Guide Picker (65), and CRISPR Genome Analyzer
(66) (Table I). These software programs have
different characteristics and applications. The optimal choice will
depend on the type of organism (eukaryotic or prokaryotic) for
which the gRNA is designed, the variety of PAM to be used, the size
of the DNA target, and the variety of recently-reported Cas-like
nucleases (46).
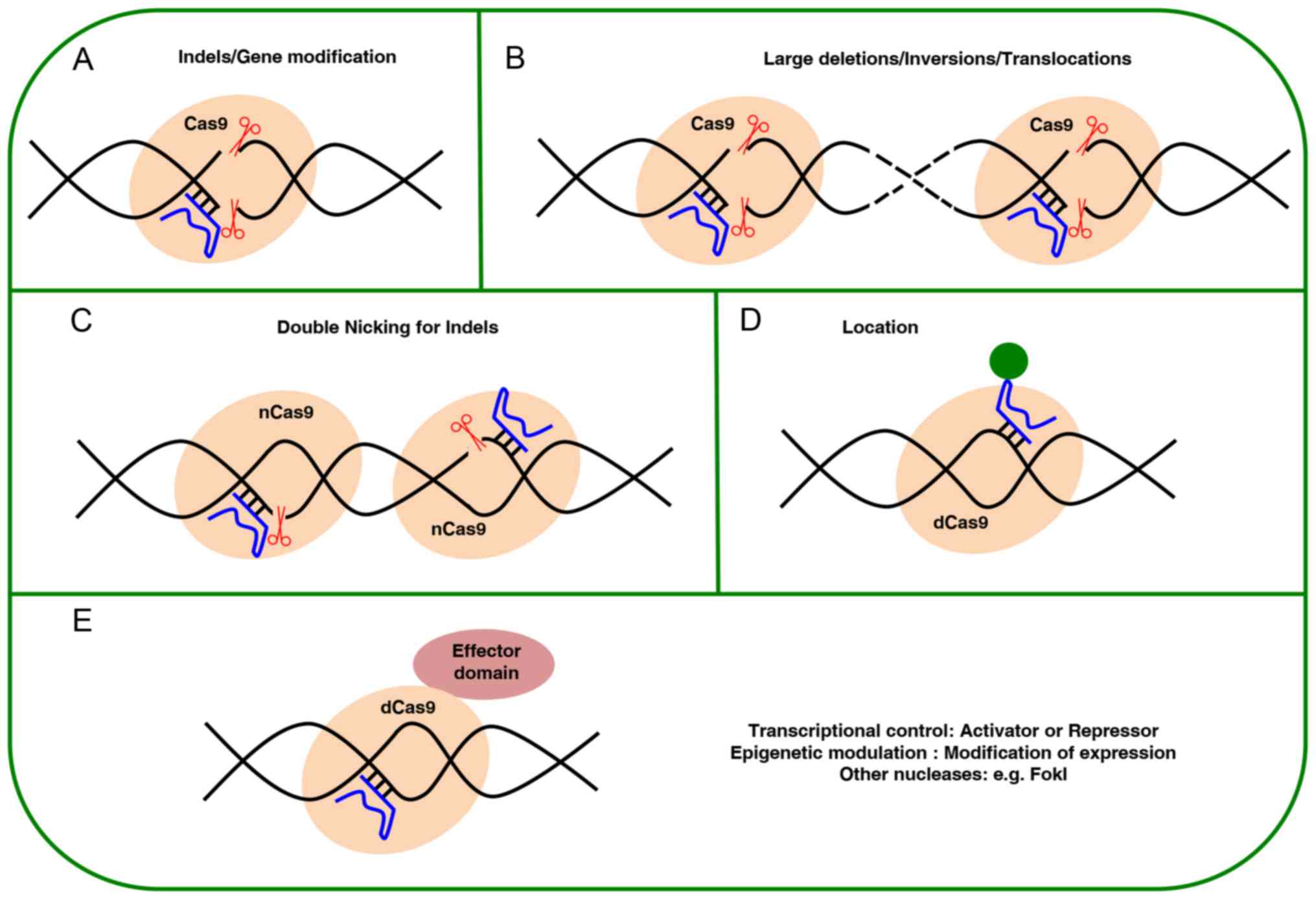
The design of variations in the traditional SpCas9
system has been examined for controlling its performance, such as
using an inducible system for temporary expression of the nuclease
(67). Another strategy to
decrease activity off-target is to replace the SpCas9 enzyme for
mutant nSpCas9 (nCas9), which cuts a single strand through the
inactivation of a nuclease domain RuvC or HNH. In this case, in
order to perform the DBS, two nCas9 are needed to target opposite
strands of DNA in close proximity (separated by no more than 100
bp), with each nCas9 guided by its own sgRNA that is able to cleave
only the strand complementary to the sgRNA (45,68). When two nCas9 are used to perform
a DSB, off-target activity is reduced by 50 to 1,500 times
(40,45,69,70). Furthermore, another strategy
consists in increasing the specificity by mitigating the helicase
activity (eSpCas9) to disrupt the off-target sites without
interfering with specificity and activity (71). Fusing the nuclease Fok1 with dead
SpCas9 (dSpCas9) also improves specificity; in this case, dSpCas9
lacks its cleavage activity by inactivation of its two-nuclease HNH
and RuvC domains. Consequently, the fusion forms RNA-guided FokI
nucleases, thus, the excision activity for the DSB will depend only
on the bounds of the two gRNAs to the DNA with a well-defined
spacing and orientation, allowing the dimerization of monomers
Fok1-dSCas9 to form a catalytically active Fok1 dimer, which
reduces the possibility that a suitable target sequence appears
again in the genome, and therefore improves specificity (72-74). Another modification is the binary
system Split-SpCas9 that uses the expression the nuclease lobe and
the α-helical domain independently. These two are naturally in the
enzyme Cas9 alone or attached to the DNA through gRNA. The domains
do not interact on their own, but the gRNA recruits all of them in
a ternary complex that recaps the activity of Cas9 and catalyzes
site-specific DNA cleavage. The uses of modified gRNA annul the
Cas9 activity dividing the dimer, which allows the development of
an inducible and adjustable dimerization system for genome editing
applications. This system has been tested in vitro and in
vivo in a mouse model (75,76). Among other modifications to date,
the SpCas9-cytidine deaminase can be found, which is a fusion of
dSpCas9 with the cytidine deaminase enzyme. This action by cytidine
deaminases, converts cytosine (C) to uracil (U), working on
single-stranded DNA accessible in the ternary complex between Cas9,
gRNA and the target DNA for the introduction of point mutations
(77).
Studies have identified other enzymes of the CRISPR
family, including enzymes encoded by Cas genes smaller than
SpCas9 (4.2 kb), such as SaCas9, St1Cas9, and
NmCas9 (3.2, 3.4, and 3.2 kb, respectively) (41,45). These enzymes would facilitate its
packaging into viral vectors (41,45). Another recently identified enzyme
called Cpf1 with shorter crRNA sequences can be used instead of
SpCas9 (43,78). Also, the most recently discovered
C2c2 (Cas13a) and C2c6 (Cas13b), which can cleave RNA (78-82). Cas13b has already been developed
as an RNA base editing technology, having been used as a tool for
RNA editing using catalytically-inactive Cas13b fused to the
adenosine deaminase domain of ADAR2 for programmable adenosine to
inosine replacement in transcripts (82).
The CRISPR/Cas9 system has an extraordinary
therapeutic potential for treating different diseases in which the
genetic cause of dysfunction is known, or for the study of these
diseases through the creation of cell or animal models. Therapy
based on genome editing can lead to the restoration of gene
function or compensation of the mutation. Single nucleotide
polymorphism (SNP) editing has been approached with different
strategies, such as: knocking out the gene that causes the disease
(88), introducing a protective
mutation (89) or adding a
therapeutic transgene (90). When
the disease is caused by a virus, cleavage of viral DNA can be
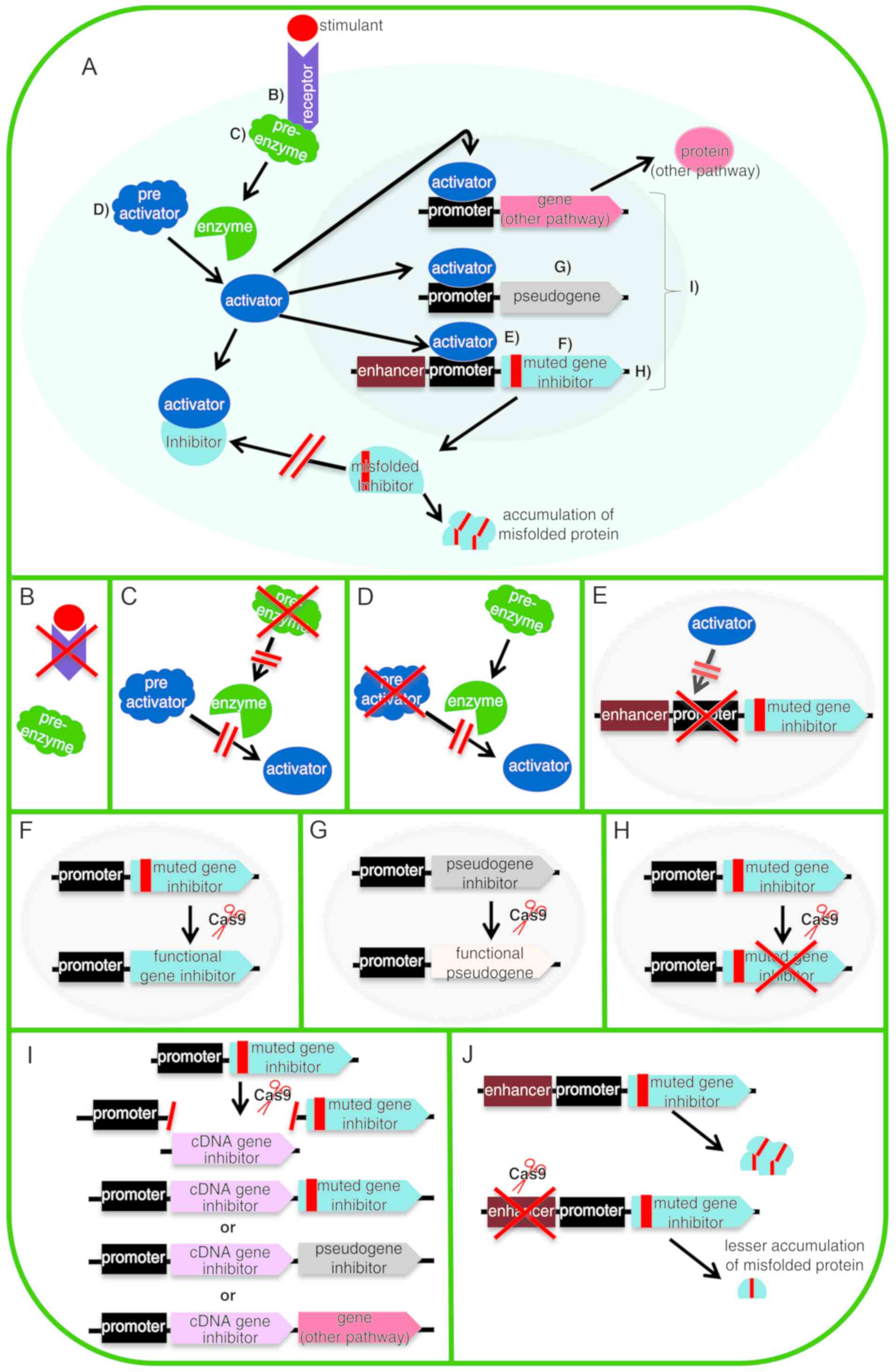
performed (70,91-94). Fig.
3 shows certain of the strategies already mentioned and others
that could be used to approach different diseases. The purpose of
this figure is to expand the panorama of the therapeutic
possibilities of CRISPR/Cas9. In the case that a mutation in a gene
is difficult to repair due to its genomic context, there may be a
pseudogene that could be activated to replace the mutated gene
(95). However, if the cause of
the disease is a protein that causes damage to the organism by its
anomalous characteristics (such as by misfolding and accumulation
in a tissue) (e.g. amyloidosis), its expression could be down
regulated at several points in its pathway of expression (96).
The homozygous ∆508 mutation in the cystic fibrosis
trans-membrane conductance regulator (CFTR) gene was
corrected using CRISPR/Cas9 in vitro in intestinal stem
cells of cystic fibrosis patients (97). Edited stem cells were
differentiated into intestinal organoids and exhibited a functional
CFTR product (97). CRISPR/Cas9
also shows potential in cases requiring a liver transplant, such as
drug therapy-refractory metabolic liver disorders (88,98). CRISPR has been used to suppress
genes, thus reprogramming the metabolic pathway, achieving a benign
phenotype with treatment of hereditary type I tyrosinemia in mice
(88). By eliminating the
4-hydroxyphenylpyruvate dioxygenase (HPD) gene in order to
inhibit the second step in tyrosine catabolism, the modified
hepato-cytes (FAH−/−/HPD−/−) exhibited a
growth advantage over the unedited hepatocytes
(FAH−/−/HPD+/+). In a number mice, the
replacement was almost complete (92-99%) in 8 weeks. The HPD
mutation increased tyrosine catabolism avoiding the pathologic
effect of FAH deficiency and the accumulation of tyrosine and toxic
metabolites, such as fumarylacetoacetate and succinylacetone in
hepatocytes, which in turn results in severe liver damage with
increased risk of hepatocarcinoma (88).
The CRISPR/Cas9 system can also be used as therapy
in Hirschsprung disease and the megacystis-microcolon-intestinal
hypoperistalsis syndrome (MMIHS), in which certain mutations were
identified through complete exome sequencing in patients diagnosed
with those diseases (99,100). In Hirschsprung disease, four
genes (DENND3, NCLN, NUP98 and TBATA) have been
linked to the neuronal processes shared by the central nervous
system and the enteric nervous system; this function was verified
in vivo through a gene knockout by CRISPR/Cas9 (99,100). A zebrafish model was used to
observe that the knockout of the afore mentioned genes resulted in
the loss of function and interruption of the development of the
enteric nervous system (loss of enteric neurons) causing a similar
Hirschsprung phenotype in vivo (99). The LMOD1 gene is involved
in establishing normal smooth muscle cytoskeletal-contractile
coupling, and a mutation causes MMIHS. Mice with knockout of
LMOD1 gene exhibited a similar phenotype to this syndrome
(protein levels and pathology consistent with MMIHS), displaying
beginning of bladder distention at 18.5 days of embryonic
development. In certain cases, the developing bladder expanded
until it encroached the abdominal cavity, while histological
analysis detected early onset thinning of the detrusor muscle of
the bladder of Lmod1−/− mice. These results suggest a
role for the LMOD1 gene in establishing normal smooth muscle
cytoskeletal-contractile coupling (100).
β-hemoglobinopathies, including sickle-cell disease
(SCD) and β-thalassemia, are caused by mutations in the β-globin
(HBB) gene, and affect millions of people worldwide. Several
studies have used CRISPR/Cas9 and donor sequences to achieve
homologous recombination in the HBB gene in induced
pluripotent stem cells (iPSCs) and hematopoietic stem cells (HSCs)
(101-104). For instance, efficient
correction of the Glu6Val mutation responsible of the SCD was
achieved using progenitor cells that were derived from patients and
differentiated to erythrocytes, resulting in the expression of the
mRNA of adult β-globin (HbA) and confirming the intact
transcriptional regulation of modified HBB alleles (101,102). Another genome editing strategy
to treat hemoglobinopathies is inserting a mutation to generate a
benign genetic condition. In the hereditary persistence of fetal
hemoglobin (HPFH), a benign genetic condition, the mutations
attenuate the shift of γ-globin to β-globin, causing a high level
of fetal hemoglobin expression (HbF) throughout life, which can
relieve the clinical signs of β-thalassemia or SCD. In a previous
study, CRISPR/Cas9 was used to mimic the HPFH mutation in promoters
of the HBG1 and HBG2 genes in human blood progenitor
cells. The edited progenitor cells produced red blood cells (RBCs)
with increased HbF levels that were sufficient to inhibit the
pathological hypoxia of RBCs found in SCD (105). Another strategy applied to
increase the HbF levels was CRISPR/Cas9 mutation of BCL11A
erythroid enhancer, a validated repressor of HbF and therapeutic
target for β-hemoglobin disorders (106). Furthermore, in β-thalassemia
caused by the expression-suppression point mutations or deletions
in the β-globin gene, effective correction of the HBB
mutations by CRISPR/Cas9 was achieved in iPSCs cells derived from a
patient, which differentiated in erythroblasts having restored the
expression of β-hemoglobin (103,104).
Finally, CRISPR/Cas9 has been applied in the
treatment of multiple other hematological diseases, such as
alloimmune bleeding disorders, including fetal and neonatal
alloimmune thrombocytopenia and post-transfusion purpura, to
transform Leu33+ megakaryocyte-like DAMI cells and iPSCs
to the Pro33 allotype, which is responsible for generating the
human platelet alloantigen 1a and 1b epitopes (107). Furthermore, this technology has
been used for treating Fanconi anemia by correcting point mutation
in patient-derived fibroblasts (108), as well as in hemophilia for the
restoration of factor VIII deficiency in mice (109-112). Notably, CRISPR/Cas9 has been
used for the generation of mutant pigs in the vWTF gene to
serve as an animal model for von Willebrand disease, or to
facilitate bleeding prior to the meat processing in the meat
industry (113).
Viruses are obligate intracellular pathogens that
infect cells through specific receptors and depend on cellular
components of the host for their replication. Upon entering the
cell, the viral genome is reproduced, transcribed and translated to
complete its life cycle (114).
A number of genomes of DNA viruses and retroviruses are integrated
into the cellular genome. When a virus infects a human, it can
cause severe disease with high mortality, morbidity and/or
subsequent transmission to other people. Certain viral infections
can be reduced by vaccination immunity, while this is not possible
for others. Viral infections that require more attention due to
their nature and social impact are those caused by the human
papilloma virus (HPV), the human immunodeficiency virus (HIV) and
the hepatitis B virus (HBV).
HPV16 expresses variants of the viral oncoproteins
E6 and E7, which are tightly linked to the development and
maintenance of malignant phenotypes that can result in cervical
cancer (93), the second most
frequent cause of cancer in women worldwide (92). CRISPR/Cas9 has been used alone or
in combination with other treatments in in vitro and in
vivo studies to combat HPV16 and HPV18 etiologic factors of
cervical cancer (92,93,115). CRISPR-Cas9 targeting HPV16 and
HPV18 oncogenes E6 and E7 in cervical carcinoma cell lines,
including HeLa and SiHa cells, led to the arrest of the cell cycle
and eventual death of the malignant cells (115). In another study, mutation of the
HPV16 E6 and E7 viral oncogenes inhibited tumor growth in
vivo, demonstrating that treatment with CRISPR/Cas9 works as a
therapy with cisplatin, one of the first-line treatments most
commonly used as a chemotherapeutic agent (93).
In HIV, the chemokine receptor 5 (CCR5) works as an
essential co-receptor to HIV-1; thus, loss of CCR5 receptor
function protects against viral infection. CRISPR/Cas9 was used to
edit the CCR5 gene in CD4+ cells (116) and in human iPSCs (hiPSCs) cells
(89). The mutant hiPSCs
differentiated into macrophages that became resistant to trophic
CCR5 HIV-1 virus (89).
The persistence of covalently closed circular DNA
(cccDNA) of HBV is a major barrier to antiviral therapy for chronic
hepatitis B eradication. A cure would require the elimination of
persistent cccDNA or removal of the hepatocytic viral load
(91). With specific gRNAs
against the HBV in multiple studies, the CRISPR/Cas9 system
significantly reduced the production of HBV core and surface
proteins in Huh-7 (70,91), HepG2 (117), HepG2.2.15 (117,119) and HepG2-H1.3 cell lines
(119), which were transfected
with a HBV expression vector. Furthermore, in a mouse model, this
system cleaved the intrahepatic plasmid containing the HBV genome
and facilitated its clearance in vivo, resulting in a
reduction of the serum antigen surface levels. This suggests that
the CRISPR/Cas9 system was able to disrupt HBV both in vitro
and in vivo, indicating its potential in the eradication of
persistent infection by this virus (91,118,119). CRISPR/Cas9 has not only been
used in the treatment of this virus, but also in the study of
cellular mechanisms that lead to carcinogenesis (120).
In the case of hepatitis C virus (HCV) that causes
chronic hepatitis, liver cirrhosis and hepatocellular carcinomas,
CRISPR/Cas9 has been used in the identification of critical host
components for HCV infections (121). In addition, the Epstein-Barr
virus (EBV) establishes a persistent infection throughout life in
90-95% of the adults. Although it does not cause disease in healthy
carriers, the infection is etiologically associated with lymphoid
and epithelial neoplasias, such as Burkitt’s lymphoma, Hodgkin’s
disease and nasopharyngeal carcinoma (122). The CRISPR/Cas9 system has been
used in vitro against EBV in the Raji cell line,
demonstrating a marked reduction in proliferation and viral load,
as well as restoring the apoptotic pathway in cells subsequent to
treatment (123). Another
strategy applied to this virus was the removal of the BART promoter
region (558 nt), which is one of two clusters that codes for 22
differentially expressed pre-microRNAs during latent EBV infection
and is believed to be involved in epithelial cell transformation
(122). This region was deleted
in specific human epithelial cell lines with latent EBV infection,
including nasopharyngeal carcinoma C666-1 cells. This was tested in
order to determine if it is required for infection and
transformation of epithelial cells, resulting in the loss of BART
miRNA expression and activity. It was identified that EBV
performance with pBART deletion was lower in comparison with that
of WT virus measured in Raji cells, indicating the importance of
miR-BARTs in the viral infection of epithelial cells, and that it
will be of great interest to investigate whether they are
particularly required for the infection and transformation of
epithelial cells (122).
In the case of diseases transmitted by a vector,
CRISPR/Cas9 has been used for the study of gene function and for
gene drive to eradicate important vector-transmitted diseases, such
as dengue, chikungunya, yellow fever and malaria (124,125).
In order to investigate the function of specific
genes, studies have been conducted on the Plasmodium sp.
genome, the parasite that causes malaria (125-127). Since the parasite resides in the
RBCs, the transfection efficiency is lower as it has to cross four
membranes (the RBC membrane, parasitophorous membrane, parasite
cytoplasm membrane and parasite nuclear membrane). However, using
CRISPR/Cas9 technology, 100% efficiency of gene deletion, 22-45%
efficiency in tagging and 25% efficiency in nucleotide replacement
were achieved, which is a significant advance in new studies of
this parasite (125).
Furthermore, CRISPR/Cas9 can be directed to the primary vector that
transmits the disease, such as Aedes aegypti, which is the
primary vector of several viruses. In this study, CRISPR/Cas9 was
used to research the genetic and neurological basis of innate
chemosensory behavior to achieve stable and precise
loss-of-function mutations in five genes (124).
CRISPR/Cas9-mediated gene drive involves
stimulating biased inheritance of particular alleles, such as gene
knockouts, gene replacements and genetic transformations, in order
to alter entire populations of organisms. This methodology is
important since several species harm human interests, including
human health or agriculture (128). CRISPR/Cas9 as part of a gene
drive tool can be used to provide a deleterious trait (such as
distorted sex ratio, reduced fertility and chemical sensitivity)
(129). For instance, in
Anopheles gambiae, the main vector for malaria, the
CRISPR/Cas9 system was used to confer a recessive female-sterility
phenotype upon disruption of three target genes: AGAP005958,
AGAP011377 and AGAP007280 (130). This study demonstrated that
disrupting AGAP007280 gene alone was sufficient for a
successful gene drive targeting female reproduction in an insect
population (130).
Cancer is a group of diseases characterized by
multiple genetic and epigenetic alterations in oncogenes and tumor
suppressor genes. Experimentation to manipulate normal and
cancerous cell genomes is vital for modeling the disease, as well
as for the systematic study of genes involved in the process of
initiation, progression and therapeutic response of cancer.
The fast modeling of genetic events has taken on a
major relevance due to the need to elucidate the importance of
genetic alterations present in human tumors, detected by
large-scale sequencing of the cancer genome. This is necessary to
discern between passenger mutations that are assumed to not
directly affect the tumorigenic process, and those that directly or
indirectly induce mutations that promote the transformation of
normal cells into cancer cells by mutating oncogenes (promoting
gain of function) and/or inactivation of tumor suppressor genes
(promoting loss of function) (131).
Although important, prior to CRISPR, the generation
of animal models to test anti-cancer agents and undetected drug
resistance mechanisms was an expensive and slow process (131). To provide a flexible and
effective method to investigate the somatic alterations of loss of
function, and their influence in tumorigenesis, CRISPR/Cas9 was
used for the disruption of somatic genes, by the individual
deletion of PTCH1 or the triple deletion of TRP53,
PTEN and NF1 genes in the mouse brain, resulting in the
development of medulloblastoma and glioblastoma, respectively
(134). In some instances,
chromosomal rearrangements serve a central role in the pathogenesis
of human cancer, such as the oncogenic fusion between the
echinoderm microtubule-associated protein like 4 (EML4) gene
and anaplastic lymphoma kinase (ALK) gene. The resulting
EML4-ALK oncogene is detected in a subset of human non-small
cell lung cancer (NSCLC), and this is clinically relevant since it
imparts sensitivity to inhibitors of ALK. CRISPR/Cas9 was used to
generate a mouse model of lung cancer driven by the EML4-ALK
gene rearrangement. The resulting tumors harbored the
EML4-ALK inversion, expressed the EML4-ALK fusion
gene, presented typical histopathological and molecular
characteristics of human ALK+ NSCLC, and responded to
treatment with ALK inhibitors (135).
The wide range of rheumatic diseases extends from
rare monogenic auto-inflammatory diseases to complex polygenic
autoimmune diseases (136). High
levels of IL-1 characterize monogenic autoinflammatory diseases.
Genetically, certain of these syndromes result from mutations in
the NLRP3 gene, with autosomal dominant inheritance and
variable penetrance (136,137). In the complex polygenic
autoimmune diseases, such as rheumatoid arthritis (RA), multiple
genetic factors and environmental triggers are involved; for
instance, the heritability of RA is considered to be ~65% (138). A number of these diseases are
potential clinical targets for CRISPR/Cas9, where this technology
could be used for in vitro and in vivo functional
genomics studies to elucidate the single and combined role of
single nucleotide polymorphisms identified by association studies
at the genomic level through the rapid creation of cellular and
animal models. Additionally, this technology can be applied in
individualized therapy as a tool to correct mutations, in
strategies adapted for each patient.
In this sense, CRISPR/Cas9 has been used for the
creation of a rat chondrosarcoma cell line that stably expresses
Cas9 for the study of complex interactions that regulate function,
differentiation and chondrocyte homeostasis, and to examine the
role of genes associated with cartilage degenerating diseases
(139). CRISPR/Cas9 can
accelerate the in vitro and in vivo functional
elucidation of the role played by genes in the inflammatory and
bone components of these diseases. In this regard, CRISPR/Cas9
technology was applied to a murine macrophage cell line to
demonstrate that the RAS-GRP3 gene limits the inflammatory
response by activating Rap1 (140). In another study, microRNA
(miR)-155 was shown to exert pro-inflammatory and
pro-osteoclastogenic effects through the CRISPR/Cas9-induced
mutation of the miR-155 binding site to the SHIP1 gene, a
negative inflammation regulator (141). In addition, CRISPR/Cas9
technology may also incorporate a cell therapy strategy, for
instance in RA, a disease characterized by deregulated responses to
pro-inflammatory cytokines such as IL-1 and tumor necrosis factor-α
(TNF-α). CRISPR/Cas9 was used to program murine induced iPSCs with
the ability to respond to an inflammatory stimulus with potent and
autonomously regulated production of anti-cytokines. TNF-a and IL-1
are two of the most potent stimulators of CCL2 gene
expression. If an antagonistic gene to TNF-α or IL-1 is placed
under the control of the CCL2 promoter, it will respond to
cytokine levels and cause a self-regulated inflammatory process.
This allows the control of the cell expression of biological
therapies (90).
Primary immunodeficiency (PID) is a group of
heterogeneous and rare chronic diseases, in which part of the
immune system functions inappropriately or does not function at
all. They are caused by numerous genetic defects, of which >230
have been identified with very variable clinical manifestations.
Among the PID diseases, severe combined immunodeficiency (SCID)
results in a blockage of T cell development with an additional
primary or secondary defect in B cells, and natural killer (NK)
cells may or may not be affected. The most common form of SCID is
the X chromosome-linked syndrome X-SCID, which is caused by
mutations in the gene that encode the γ receptor of IL-2 (namely
the IL2RG gene) (142).
PID, including SCID, can be treated by allogeneic
transplantation of healthy HSCs; however, histocompatibility
problems may be faced, together with the risk of acquisition of
transmitted diseases (143).
These problems can be overcome by the correction of the patient’s
own HSCs through inserting a copy of the functional gene by a viral
vector. Although there are success cases in clinical trials,
serious complications may also occur due to viral integration close
to oncogenes and hence, their activation (144,145). A promising alternative is the
use of CRISPR/Cas9 to induce a targeted homologous repair, having a
clear advantage over traditional gene therapy. Alternatively, in
certain immune deficiencies, there is loss-of-function or
gain-of-function in genes subjected to a strict control of
expression. Examples included the X-linked agammaglobulinemia and
the signal transducer and activator of transcription 3 (STAT3)
loss-of-function, as well as the STAT3 and STAT1 gain-of-function,
or activated PI3K-δ syndrome. These diseases ideally require the
correction of the existing gene to restore normal function or
regulation, which may be achieved by CRISPR/Cas9 (146).
It is important to mention that the therapeutic
efficacy of gene editing depends on several factors, including
editing efficiency, which varies significantly depending on the
cell type, senescence status, and cell cycle status of the target.
Other factors that also influence therapeutic efficiency include
cell aptitude, which refers to the feasibility of reaching a
therapeutic modification threshold, and the efficient delivery of
programmable nuclease system to the target tissue, which is only
considered to be effective if the programmable nuclease system
arrives safely and efficiently to the nucleus of the target cell.
Finally, the specificity of the editing is another important
factor, which refers to only editing the target DNA without
affecting any other genes (4,147). Since hematopoietic cells are the
most common target in immunological diseases, autologous
hematopoietic stem cell transplantation is a viable grafting method
of cells that are already corrected ex vivo by CRISPR/Cas9,
replacing all or part of the hematopoietic stem cell compartment
(146).
CRISPR/Cas9 has been used to generate ‘knockout’
models that had not been susceptible to efficient genetic
modification for the study of immune system diseases, such as
rabbits with knockout of 1-5 genes at the same time (IL2RG,
RAG1, RAG2, TIKI1, and ALB) with efficiencies ranging
from 100% (for 1 gene) to 33.3% (for 5 genes) by microinjection
into pronuclear stage embryos cytoplasm (151). Rabbits with IL2RG and
RAG1 gene knockout are an important animal model of immune
deficiencies characterized by the absence of mature T, B and NK
cells (151). Other model
examples involve hamsters carrying knockouts of the STAT2
gene for the study of viral infections, of the KCNQ1 gene
for cardiovascular function investigation and of the
PPP1R12C gene for transgenic integration (152). In addition, pigs carrying
knockout of the gene coding for the JH region of the IgM heavy
chain, which is crucial for the development and differentiation of
B cells, resulted in piglets lacking antibody-producing B cells.
The generation of a B cell-deficient mutant is the first step in
producing human antibody repertoires in large animal models
(153).
Regarding the use of CRISPR/Cas9 to investigate
allergic diseases, this technology has been used to examine the
role of certain genes, such as the MUC18 gene, which is also
known as CD146 or melanoma cell adhesion molecule. Through
CRISPR/Cas9, the knockout of MUC18 gene was conducted in
human nasal airway epithelial cells, which is the first line of
defense against environmental factors, such as pathogens and
pollution. This led to a reduced response of IL-8 following
stimulation of the Toll-like receptor agonist, suggesting a
pro-inflammatory role of MUC18 gene in response to bacterial
or viral stimulation (154).
Another example is the use of this technology in X-linked hyper IgM
syndrome, where CRISPR/Cas9 was used to correct mutations in the
CD40 ligand (155). Furthermore,
CRISPR/Cas9 was used to induce recombination of IgH chain class
changes in desired subclasses in murine and human B cells. It was
also used to produce Fab fragments instead of the whole IgH
molecule in mouse hybridoma cells, with the aim of a more careful
scrutiny of Ig subclasses and novel methods for accessible
production of Fab fragments for research or therapeutic uses
(156).
Monkeys serve as one of the most valuable animal
models for the development of therapeutic strategies due to their
close similarities with humans (157). CRISPR/Cas9 was successfully used
in the generation of monkeys with knockout in the NR0B1,
PPAR-γ and RAG1 genes (158), being able to serve as models of
X-linked adrenal hypoplasia congenita and hypogonadotropic
hypogonadism (159),
lipodystrophy metabolism (160),
insulin sensitivity, obesity and inflammatory disease (161). Furthermore, monkey knockout
models of RAG gene could be used in regenerative medicine,
allograft and xenograft transplantation, and reconstitution
experiments associated with the immune system (162).
The CRISPR/Cas9 system has been applied in cellular
and animal models to study and search treatments for different
neurological disorders, such as Parkinson’s disease (163). Mutation in PARK2 or
PINK1 genes leads to early onset Parkinson’s disease, as an
autosomal recessive disease in humans (164). The PARK2 gene encodes a
protein called parkin, which is a component of the multiprotein
complex E3 ubiquitin ligase, while the PINK1 gene encodes
for the PTEN-induced putative kinase 1, a mitochondrial
serine/threonine kinase protein. The CRISPR/Cas9 system has also
been applied in amyotrophic lateral sclerosis (165,166), Huntington’s disease (167), schizophrenia (168) and autism (168). It has also been applied in
movement diseases, such as Duchenne muscular dystrophy (169-172), in metabolic diseases, such as
type I diabetes (173) and
hypercholesterolemia (174), and
in inherited diseases that affect vision, such as retinitis
pigmentosa (175), among
numerous others.
Authors wish to acknowledge Tecnologico de
Monterrey and Mission XXI for their financial support regarding the
publication charges.
Not applicable.
DRRR reviewed the basic science literature and
wrote the manuscript; MAGE reviewed the clinical issues; RRS and
MDLGR supported DRRR in revisions of the manuscript; HABS conceived
the project and supervised the writing of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
The authors would like to thank Alec Christopher
Escalante Gomez (School of Nutrition of the Universidad Autónoma de
Nuevo León, Monterrey) for aid in the translation of this review
and Jessica García for valuable suggestions.
|
1
|
Im W, Moon J and Kim M: Applications of
CRISPR/Cas9 for gene editing in hereditary movement disorders. J
Mov Disord. 9:136–143. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gaj T, Gersbach CA and Barbas CF III: ZFN,
TALEN, and CRISPR/Cas-based methods for genome engineering. Trends
Biotechnol. 31:397–405. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sander JD and Joung JK: CRISPR-Cas systems
for editing, regulating and targeting genomes. Nat Biotechnol.
32:347–355. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cox DBT, Platt RJ and Zhang F: Therapeutic
genome editing: Prospects and challenges. Nat Med. 21:121–131.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Epinat JC, Arnould S, Chames P, Rochaix P,
Desfontaines D, Puzin C, Patin A, Zanghellini A, Pâques F and
Lacroix E: A novel engineered meganuclease induces homologous
recombination in yeast and mammalian cells. Nucleic Acids Res.
31:2952–2962. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Urnov FD, Rebar EJ, Holmes MC, Zhang HS
and Gregory PD: Genome editing with engineered zinc finger
nucleases. Nat Rev Genet. 11:636–646. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miller JC, Tan S, Qiao G, Barlow KA, Wang
J, Xia DF, Meng X, Paschon DE, Leung E, Hinkley SJ, et al: A TALE
nuclease architecture for efficient genome editing. Nat Biotechnol.
29:143–148. 2011. View Article : Google Scholar
|
|
8
|
Jinek M, Chylinski K, Fonfara I, Hauer M,
Doudna JA and Charpentier E: A programmable dual-RNA-guided DNA
endonuclease in adaptive bacterial immunity. Science. 337:816–821.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Horvath P and Barrangou R: CRISPR/Cas, the
immune system of bacteria and archaea. Science. 327:167–170. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sorek R, Kunin V and Hugenholtz P:
CRISPR-a widespread system that provides acquired resistance
against phages in bacteria and archaea. Nat Rev Microbiol.
6:181–186. 2008. View Article : Google Scholar
|
|
11
|
Singh V, Braddick D and Dhar PK: Exploring
the potential of genome editing CRISPR-Cas9 technology. Gene.
599:1–18. 2017. View Article : Google Scholar
|
|
12
|
Makarova KS, Wolf YI, Alkhnbashi OS, Costa
F, Shah SA, Saunders SJ, Barrangou R, Brouns SJ, Charpentier E,
Haft DH, et al: An updated evolutionary classification of
CRISPR-Cas systems. Nat Rev Microbiol. 13:722–736. 2015. View Article : Google Scholar
|
|
13
|
Mojica FJ, Ferrer C, Juez G and
Rodríguez-Valera F: Long stretches of short tandem repeats are
present in the largest replicons of the Archaea Haloferax
mediterranei and Haloferax volcanii and could be involved in
replicon partitioning. Mol Microbiol. 17:85–93. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Riehle MM, Bennett AF and Long AD: Genetic
architecture of thermal adaptation in Escherichia coli. Proc Natl
Acad Sci USA. 98:525–530. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
DeBoy RT, Mongodin EF, Emerson JB and
Nelson KE: Chromosome evolution in the Thermotogales: Large-scale
inversions and strain diversification of CRISPR sequences. J
Bacteriol. 188:2364–2374. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Makarova KS, Aravind L, Grishin NV,
Rogozin IB and Koonin EV: A DNA repair system specific for
thermophilic Archaea and bacteria predicted by genomic context
analysis. Nucleic Acids Res. 30:482–496. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ishino Y, Shinagawa H, Makino K, Amemura M
and Nakata A: Nucleotide sequence of the iap gene, responsible for
alkaline phosphatase isozyme conversion in Escherichia coli, and
identification of the gene product. J Bacteriol. 169:5429–5433.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mojica FJ, Díez-Villaseñor C, Soria E and
Juez G: Biological significance of a family of regularly spaced
repeats in the genomes of Archaea, Bacteria and mitochondria. Mol
Microbiol. 36:244–246. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jansen R, Embden JD, Gaastra W and Schouls
LM: Identification of genes that are associated with DNA repeats in
prokaryotes. Mol Microbiol. 43:1565–1575. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mojica FJ, Díez-Villaseñor C,
García-Martínez J and Soria E: Intervening sequences of regularly
spaced prokaryotic repeats derive from foreign genetic elements. J
Mol Evol. 60:174–182. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bolotin A, Quinquis B, Sorokin A and
Ehrlich SD: Clustered regularly interspaced short palindrome
repeats (CRISPRs) have spacers of extrachromosomal origin.
Microbiology. 151:2551–2561. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pourcel C, Salvignol G and Vergnaud G:
CRISPR elements in Yersinia pestis acquire new repeats by
preferential uptake of bacteriophage DNA, and provide additional
tools for evolutionary studies. Microbiology. 151:653–663. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barrangou R, Fremaux C, Deveau H, Richards
M, Boyaval P, Moineau S, Romero DA and Horvath P: CRISPR provides
acquired resistance against viruses in prokaryotes. Science.
315:1709–1712. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brouns SJJ, Jore MM, Lundgren M, Westra
ER, Slijkhuis RJH, Snijders APL, Dickman MJ, Makarova KS, Koonin EV
and van der Oost J: Small CRISPR RNAs guide antiviral defense in
prokaryotes. Science. 321:960–964. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Garneau JE, Dupuis MÈ, Villion M, Romero
DA, Barrangou R, Boyaval P, Fremaux C, Horvath P, Magadán AH and
Moineau S: The CRISPR/Cas bacterial immune system cleaves
bacteriophage and plasmid DNA. Nature. 468:67–71. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deltcheva E, Chylinski K, Sharma CM,
Gonzales K, Chao Y, Pirzada ZA, Eckert MR, Vogel J and Charpentier
E: CRISPR RNA maturation by trans-encoded small RNA and host factor
RNase III. Nature. 471:602–607. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sapranauskas R, Gasiunas G, Fremaux C,
Barrangou R, Horvath P and Siksnys V: The Streptococcus
thermophilus CRISPR/Cas system provides immunity in Escherichia
coli. Nucleic Acids Res. 39:9275–9282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mali P, Yang L, Esvelt KM, Aach J, Guell
M, DiCarlo JE, Norville JE and Church GM: RNA-guided human genome
engineering via Cas9. Science. 339:823–826. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cong L, Ran FA, Cox D, Lin S, Barretto R,
Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA and Zhang F:
Multiplex genome engineering using CRISPR/Cas systems. Science.
339:819–823. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cornu TI, Mussolino C and Cathomen T:
Refining strategies to translate genome editing to the clinic. Nat
Med. 23:415–423. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cyranoski D: CRISPR gene-editing tested in
a person for the first time. Nature. 539:479. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cyranoski D: Chinese scientists to pioneer
first human CRISPR trial. Nature. 535:476–477. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shub DA, Goodrich-Blair H and Eddy SR:
Amino-acid-sequence motif of group I intron endonucleases is
conserved in open reading frames of group II introns. Trends
Biochem Sci. 19:402–404. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Al-Attar S, Westra ER, van der Oost J and
Brouns SJ: Clustered regularly interspaced short palindromic
repeats (CRISPRs): The hallmark of an ingenious antiviral defense
mechanism in prokaryotes. Biol Chem. 392:277–289. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wright AV, Nuñez JK and Doudna JA: Biology
and applications of CRISPR systems: Harnessing nature’s toolbox for
genome engineering. Cell. 164:29–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang F, Wen Y and Guo X: CRISPR/Cas9 for
genome editing: Progress implications and challenges. Hum Mol
Genet. 23:R40–R46. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Canver MC, Bauer DE and Orkin SH:
Functional interrogation of non-coding DNA through CRISPR genome
editing. Methods. 121–122. 118–129. 2017.
|
|
38
|
Karvelis T, Gasiunas G, Miksys A,
Barrangou R, Horvath P and Siksnys V: crRNA and tracrRNA guide
Cas9-mediated DNA interference in Streptococcus thermophilus. RNA
Biol. 10:841–851. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gasiunas G, Barrangou R, Horvath P and
Siksnys V: Cas9-crRNA ribonucleoprotein complex mediates specific
DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci
USA. 109:E2579–E2586. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Friedland AE, Baral R, Singhal P, Loveluck
K, Shen S, Sanchez M, Marco E, Gotta GM, Maeder ML, Kennedy EM, et
al: Characterization of Staphylococcus aureus Cas9: A smaller Cas9
for all-in-one adeno-associated virus delivery and paired nickase
applications. Genome Biol. 16:2572015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ran FA, Cong L, Yan WX, Scott DA,
Gootenberg JS, Kriz AJ, Zetsche B, Shalem O, Wu X, Makarova KS, et
al: In vivo genome editing using Staphylococcus aureus Cas9.
Nature. 520:186–191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hou Z, Zhang Y, Propson NE, Howden SE, Chu
LF, Sontheimer EJ and Thomson JA: Efficient genome engineering in
human pluripotent stem cells using Cas9 from Neisseria
meningitidis. Proc Natl Acad Sci USA. 110:15644–15649. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen F, Ding X, Feng Y, Seebeck T, Jiang Y
and Davis GD: Targeted activation of diverse CRISPR-Cas systems for
mammalian genome editing via proximal CRISPR targeting. Nat Commun.
8:149582017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Price AA, Sampson TR, Ratner HK, Grakoui A
and Weiss DS: Cas9-mediated targeting of viral RNA in eukaryotic
cells. Proc Natl Acad Sci USA. 112:6164–6169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Murovec J, Pirc Ž and Yang B: New variants
of CRISPR RNA-guided genome editing enzymes. Plant Biotechnol J.
15:917–926. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Oliveros JC, Franch M, Tabas-Madrid D,
San-León D, Montoliu L, Cubas P and Pazos F:
Breaking-Cas-interactive design of guide RNAs for CRISPR-Cas
experiments for ENSEMBL genomes. Nucleic Acids Res. 44:W267–W271.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kaur K, Gupta AK, Rajput A and Kumar M:
ge-CRISPR-an integrated pipeline for the prediction and analysis of
sgRNAs genome editing efficiency for CRISPR/Cas system. Sci Rep.
6:308702016. View Article : Google Scholar
|
|
48
|
Naito Y, Hino K, Bono H and Ui-Tei K:
CRISPRdirect: Software for designing CRISPR/Cas guide RNA with
reduced off-target sites. Bioinformatics. 31:1120–1123. 2015.
View Article : Google Scholar :
|
|
49
|
Heigwer F, Kerr G and Boutros M: E-CRISP:
Fast CRISPR target site identification. Nat Methods. 11:122–123.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wong N, Liu W and Wang X: WU-CRISPR:
Characteristics of functional guide RNAs for the CRISPR/Cas9
system. Genome Biol. 16:2182015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Doench JG, Hartenian E, Graham DB, Tothova
Z, Hegde M, Smith I, Sullender M, Ebert BL, Xavier RJ and Root DE:
Rational design of highly active sgRNAs for CRISPR-Cas9-mediated
gene inactivation. Nat Biotechnol. 32:1262–1267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chari R, Mali P, Moosburner M and Church
GM: Unraveling CRISPR-Cas9 genome engineering parameters via a
library-on-library approach. Nat Methods. 12:823–826. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chari R, Yeo NC, Chavez A and Church GM:
sgRNA scorer 2.0: A species-independent model to predict
CRISPR/Cas9 activity. ACS Synth Biol. 6:902–904. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Moreno-Mateos MA, Vejnar CE, Beaudoin JD,
Fernandez JP, Mis EK, Khokha MK and Giraldez AJ: CRISPRscan:
Designing highly efficient sgRNAs for CRISPR-Cas9 targeting in
vivo. Nat Methods. 12:982–988. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liu H, Wei Z, Dominguez A, Li Y, Wang X
and Qi LS: CRISPR-ERA: A comprehensive design tool for
CRISPR-mediated gene editing, repression and activation.
Bioinformatics. 31:3676–3678. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Stemmer M, Thumberger T, Del Sol, Keyer M,
Wittbrodt J and Mateo JL: CCTop: An intuitive, flexible and
reliable CRISPR/Cas9 target prediction tool. PLoS One.
10:e01246332015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Haeussler M, Schönig K, Eckert H,
Eschstruth A, Mianné J, Renaud JB, Schneider-Maunoury S, Shkumatava
A, Teboul L, Kent J, et al: Evaluation of off-target and on-target
scoring algorithms and integration into the guide RNA selection
tool CRISPOR. Genome Biol. 17:1482016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Montague TG, Cruz JM, Gagnon JA, Church GM
and Valen E: CHOPCHOP: A CRISPR/Cas9 and TALEN web tool for genome
editing. Nucleic Acids Res. 42:W401–W407. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Prykhozhij SV, Rajan V, Gaston D and
Berman JN: CRISPR multitargeter: A web tool to find common and
unique CRISPR single guide RNA targets in a set of similar
sequences. PLoS One. 10:e01193722015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
O’Brien A and Bailey TL: GT-Scan:
Identifying unique genomic targets. Bioinformatics. 30:2673–2675.
2014. View Article : Google Scholar :
|
|
61
|
Hsu PD, Scott DA, Weinstein JA, Ran FA,
Konermann S, Agarwala V, Li Y, Fine EJ, Wu X, Shalem O, et al: DNA
targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol.
31:827–832. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Park J, Bae S and Kim JS: Cas-Designer: A
web-based tool for choice of CRISPR-Cas9 target sites.
Bioinformatics. 31:4014–4016. 2015.PubMed/NCBI
|
|
63
|
Bae S, Park J and Kim JS: Cas-OFFinder: A
fast and versatile algorithm that searches for potential off-target
sites of Cas9 RNA-guided endonucleases. Bioinformatics.
30:1473–1475. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Cradick TJ, Qiu P, Lee CM, Fine EJ and Bao
G: COSMID: A web-based tool for identifying and validating
CRISPR/Cas off-target sites. Mol Ther Nucleic Acids. 3:e2142014.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hough SH, Kancleris K, Brody L,
Humphryes-Kirilov N, Wolanski J, Dunaway K, Ajetunmobi A and
Dillard V: Guide Picker is a comprehensive design tool for
visualizing and selecting guides for CRISPR experiments. BMC
Bioinformatics. 18:1672017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Güell M, Yang L and Church GM: Genome
editing assessment using CRISPR genome analyzer (CRISPR-GA).
Bioinformatics. 30:2968–2970. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Cao J, Wu L, Zhang SM, Lu M, Cheung WK,
Cai W, Gale M, Xu Q and Yan Q: An easy and efficient inducible
CRISPR/Cas9 platform with improved specificity for multiple gene
targeting. Nucleic Acids Res. 44:e1492016.PubMed/NCBI
|
|
68
|
Ran FA, Hsu PD, Lin CY, Gootenberg JS,
Konermann S, Trevino AE, Scott DA, Inoue A, Matoba S, Zhang Y and
Zhang F: Double nicking by RNA-guided CRISPR Cas9 for enhanced
genome editing specificity. Cell. 154:1380–1389. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Sato T, Sakuma T, Yokonishi T, Katagiri K,
Kamimura S, Ogonuki N, Ogura A, Yamamoto T and Ogawa T: Genome
editing in mouse spermatogonial stem cell lines Using TALEN and
double-nicking CRISPR/Cas9. Stem Cell Reports. 5:75–82. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Sakuma T, Masaki K, Abe-Chayama H, Mochida
K, Yamamoto T and Chayama K: Highly multiplexed
CRISPR-Cas9-nuclease and Cas9-nickase vectors for inactivation of
hepatitis B virus. Genes Cells. 21:1253–1262. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Slaymaker IM, Gao L, Zetsche B, Scott DA,
Yan WX and Zhang F: Rationally engineered Cas9 nucleases with
improved specificity. Science. 351:84–88. 2016. View Article : Google Scholar :
|
|
72
|
Tsai SQ, Wyvekens N, Khayter C, Foden JA,
Thapar V, Reyon D, Goodwin MJ, Aryee MJ and Joung JK: Dimeric
CRISPR RNA-guided FokI nucleases for highly specific genome
editing. Nat Biotechnol. 32:569–576. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhang H, Yan Z, Li M, Peabody M and He TC:
CRISPR clear? Dimeric Cas9-Fok1 nucleases improve genome-editing
specificity. Genes Dis. 1:6–7. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Guilinger JP, Thompson DB and Liu DR:
Fusion of catalytically inactive Cas9 to FokI nuclease improves the
specificity of genome modification. Nat Biotechnol. 32:577–582.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wright AV, Sternberg SH, Taylor DW, Staahl
BT, Bardales JA, Kornfeld JE and Doudna JA: Rational design of a
split-Cas9 enzyme complex. Proc Natl Acad Sci USA. 112:2984–2989.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Chew WL, Tabebordbar M, Cheng JK, Mali P,
Wu EY, Ng AH, Zhu K, Wagers AJ and Church GM: A multifunctional
AAV-CRISPR-Cas9 and its host response. Nat Methods. 13:868–874.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Komor AC, Kim YB, Packer MS, Zuris JA and
Liu DR: Programmable editing of a target base in genomic DNA
without double-stranded DNA cleavage. Nature. 533:420–424. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Murugan K, Babu K, Sundaresan R, Rajan R
and Sashital DG: The revolution continues: Newly discovered systems
expand the CRISPR-Cas toolkit. Mol Cell. 68:15–25. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Shmakov S, Abudayyeh OO, Makarova KS, Wolf
YI, Gootenberg JS, Semenova E, Minakhin L, Joung J, Konermann S,
Severinov K, et al: Discovery and functional characterization of
diverse class 2 CRISPR-Cas systems. Mol Cell. 60:385–397. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Abudayyeh OO, Gootenberg JS, Konermann S,
Joung J, Slaymaker IM, Cox DB, Shmakov S, Makarova KS, Semenova E,
Minakhin L, et al: C2c2 is a single-component programmable
RNA-guided RNA-targeting CRISPR effector. Science. 353:aaf55732016.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Abudayyeh OO, Gootenberg JS,
Essletzbichler P, Han S, Joung J, Belanto JJ, Verdine V, Cox DBT,
Kellner MJ, Regev A, et al: RNA targeting with CRISPR-Cas13.
Nature. 550:280–284. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Cox DBT, Gootenberg JS, Abudayyeh OO,
Franklin B, Kellner MJ, Joung J and Zhang F: RNA editing with
CRISPR-Cas13. Science. 358:1019–1027. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Balboa D, Weltner J, Eurola S, Trokovic R,
Wartiovaara K and Otonkoski T: Conditionally stabilized dCas9
activator for controlling gene expression in human cell
reprogramming and differentiation. Stem Cell Reports. 5:448–459.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Konermann S, Brigham MD, Trevino AE, Joung
J, Abudayyeh OO, Barcena C, Hsu PD, Habib N, Gootenberg JS,
Nishimasu H, et al: Genome-scale transcriptional activation by an
engineered CRISPR-Cas9 complex. Nature. 517:583–588. 2015.
View Article : Google Scholar :
|
|
85
|
Piatek A, Ali Z, Baazim H, Li L, Abulfaraj
A, Al-Shareef S, Aouida M and Mahfouz MM: RNA-guided
transcriptional regulation in planta via synthetic dCas9-based
transcription factors. Plant Biotechnol J. 13:578–589. 2015.
View Article : Google Scholar
|
|
86
|
Fu Y, Rocha PP, Luo VM, Raviram R, Deng Y,
Mazzoni EO and Skok JA: CRISPR-dCas9 and sgRNA scaffolds enable
dual-colour live imaging of satellite sequences and repeat-enriched
individual loci. Nat Commun. 7:117072016. View Article : Google Scholar :
|
|
87
|
Qin P, Parlak M, Kuscu C, Bandaria J, Mir
M, Szlachta K, Singh R, Darzacq X, Yildiz A and Adli M: Live cell
imaging of low- and non-repetitive chromosome loci using
CRISPR-Cas9. Nat Commun. 8:147252017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Pankowicz FP, Barzi M, Legras X, Hubert L,
Mi T, Tomolonis JA, Ravishankar M, Sun Q, Yang D, Borowiak M, et
al: Reprogramming metabolic pathways in vivo with CRISPR/Cas9
genome editing to treat hereditary tyrosinaemia. Nat Commun.
7:126422016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Kang H, Minder P, Park MA, Mesquitta WT,
Torbett BE and Slukvin II: CCR5 disruption in induced pluripotent
stem cells using CRISPR/Cas9 provides selective resistance of
immune cells to CCR5-tropic HIV-1 virus. Mol Ther Nucleic Acids.
4:e2682015. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Brunger JM, Zutshi A, Willard VP, Gersbach
CA and Guilak F: Genome engineering of stem cells for autonomously
regulated, closed-loop delivery of biologic drugs. Stem Cell
Reports. 8:1202–1213. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Lin SR, Yang HC, Kuo YT, Liu CJ, Yang TY,
Sung KC, Lin YY, Wang HY, Wang CC, Shen YC, et al: The CRISPR/Cas9
system facilitates clearance of the intrahepatic HBV templates in
vivo. Mol Ther Nucleic Acids. 3:e1862014. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhen S, Hua L, Takahashi Y, Narita S, Liu
YH and Li Y: In vitro and in vivo growth suppression of human
papillomavirus 16-positive cervical cancer cells by CRISPR/Cas9.
Biochem Biophys Res Commun. 450:1422–1426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhen S, Lu JJ, Wang LJ, Sun XM, Zhang JQ,
Li X, Luo WJ and Zhao L: In vitro and in vivo synergistic
therapeutic effect of cisplatin with human papillomavirus16 E6/E7
CRISPR/Cas9 on cervical cancer cell line. Transl Oncol. 9:498–504.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Das D, Smith N, Wang X and Morgan IM: The
deacetylase SIRT1 regulates the replication properties of human
papilloma-virus 16 E1 and E2. J Virol. 91:e00102-e001172017.
View Article : Google Scholar
|
|
95
|
Merling R, Kuhns D, Sweeney C, Wu X,
Burkett S, Chu J, Lee J, Koontz S, Di Pasquale G, Afione S, et al:
Gene-edited pseudogene resurrection corrects p47 phox-deficient
chronic granulomatous disease. Blood Adv. 1:270–278. 2016.
View Article : Google Scholar
|
|
96
|
Paquet D, Kwart D, Chen A, Sproul A, Jacob
S, Teo S, Olsen KM, Gregg A, Noggle S and Tessier-Lavigne M:
Efficient introduction of specific homozygous and heterozygous
mutations using CRISPR/Cas9. Nature. 533:125–129. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Schwank G, Koo BK, Sasselli V, Dekkers JF,
Heo I, Demircan T, Sasaki N, Boymans S, Cuppen E, van der Ent CK,
et al: Functional repair of CFTR by CRISPR/Cas9 in intestinal stem
cell organoids of cystic fibrosis patients. Cell Stem Cell.
13:653–658. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Yin H, Xue W, Chen S, Bogorad RL,
Benedetti E, Grompe M, Koteliansky V, Sharp PA, Jacks T and
Anderson DG: Genome editing with Cas9 in adult mice corrects a
disease mutation and phenotype. Nat Biotechnol. 32:551–553. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Gui H, Schriemer D, Cheng WW, Chauhan RK,
Antiňolo G, Berrios C, Bleda M, Brooks AS, Brouwer RW, Burns AJ, et
al: Whole exome sequencing coupled with unbiased functional
analysis reveals new Hirschsprung disease genes. Genome Biol.
18:482017. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Halim D, Wilson MP, Oliver D, Brosens E,
Verheij JB, Han Y, Nanda V, Lyu Q, Doukas M, Stoop H, et al: Loss
of LMOD1 impairs smooth muscle cytocontractility and causes
megacystis microcolon intestinal hypoperistalsis syndrome in humans
and mice. Proc Natl Acad Sci USA. 114:E2739–E2747. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Dever DP, Bak RO, Reinisch A, Camarena J,
Washington G, Nicolas CE, Pavel-Dinu M, Saxena N, Wilkens AB,
Mantri S, et al: CRISPR/Cas9 β-globin gene targeting in human
haematopoietic stem cells. Nature. 539:384–389. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ou Z, Niu X, He W, Chen Y, Song B, Xian Y,
Fan D, Tang D and Sun X: The combination of CRISPR/Cas9 and iPSC
technologies in the gene therapy of human β-thalassemia in mice.
Sci Re. 6:324632016.
|
|
103
|
Xie F, Ye L, Chang JC, Beyer AI, Wang J,
Muench MO and Kan YW: Seamless gene correction of β-thalassemia
mutations in patient-specific iPSCs using CRISPR/Cas9 and piggyBac.
Genome Res. 24:1526–1533. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Xu P, Tong Y, Liu XZ, Wang TT, Cheng L,
Wang BY, Lv X, Huang Y and Liu DP: Both TALENs and CRISPR/Cas9
directly target the HBB IVS2 654 (C > T) mutation in
β-thalassemia-derived iPSCs. Sci Rep. 5:120652015. View Article : Google Scholar
|
|
105
|
Traxler EA, Yao Y, Wang YD, Woodard KJ,
Kurita R, Nakamura Y, Hughes JR, Hardison RC, Blobel GA, Li C and
Weiss MJ: A genome-editing strategy to treat β-hemoglobinopathies
that recapitulates a mutation associated with a benign genetic
condition. Nat Med. 22:987–990. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Canver MC, Smith EC, Sher F, Pinello L,
Sanjana NE, Shalem O, Chen DD, Schupp PG, Vinjamur DS, Garcia SP,
et al: BCL11A enhancer dissection by Cas9-mediated in situ
saturating mutagenesis. Nature. 527:192–197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zhang N, Zhi H, Curtis BR, Rao S, Jobaliya
C, Poncz M, French DL and Newman PJ: CRISPR/Cas9-mediated
conversion of human platelet alloantigen allotypes. Blood.
127:675–680. 2016. View Article : Google Scholar :
|
|
108
|
Osborn MJ, Gabriel R, Webber BR, DeFeo AP,
McElroy AN, Jarjour J, Starker CG, Wagner JE, Joung JK, Voytas DF,
et al: Fanconi anemia gene editing by the CRISPR/Cas9 system. Hum
Gene Ther. 26:114–126. 2015. View Article : Google Scholar :
|
|
109
|
Park CY, Kim DH, Son JS, Sung JJ, Lee J,
Bae S, Kim JH, Kim DW and Kim JS: Functional correction of large
factor VIII gene chromosomal inversions in hemophilia a
patient-derived iPSCs using CRISPR-Cas9. Cell Stem Cell.
17:213–220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zhang H and McCarty N: CRISPR-Cas9
technology and its application in haematological disorders. Br J
Haematol. 175:208–225. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Chen J, Jiang N, Wang T, Xie G, Zhang Z,
Li H, Yuan J, Sun Z and Chen J: DNA shuffling of uricase gene leads
to a more ‘human like’ chimeric uricase with increased uricolytic
activity. Int J Biol Macromol. 82:522–529. 2016. View Article : Google Scholar
|
|
112
|
Guan Y, Ma Y, Li Q, Sun Z, Ma L, Wu L,
Wang L, Zeng L, Shao Y, Chen Y, et al: CRISPR/Cas9-mediated somatic
correction of a novel coagulator factor IX gene mutation
ameliorates hemophilia in mouse. EMBO Mol Med. 8:477–488. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Hai T, Teng F, Guo R, Li W and Zhou Q:
One-step generation of knockout pigs by zygote injection of
CRISPR/Cas system. Cell Res. 24:372–375. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Puschnik AS, Majzoub K, Ooi YS and Carette
JE: A CRISPR toolbox to study virus-host interactions. Nat Rev
Microbiol. 15:351–364. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Kennedy EM, Kornepati AVR, Goldstein M,
Bogerd HP, Poling BC, Whisnant AW, Kastan MB and Cullen BR:
Inactivation of the human papillomavirus E6 or E7 gene in cervical
carcinoma cells by using a bacterial CRISPR/Cas RNA-guided
endonuclease. J Virol. 88:11965–11972. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Wang W, Ye C, Liu J, Zhang D, Kimata JT
and Zhou P: CCR5 gene disruption via lentiviral vectors expressing
Cas9 and single guided RNA renders cells resistant to HIV-1
infection. PLoS One. 9:e1159872014. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Zhen S, Hua L, Liu YH, Gao LC, Fu J, Wan
DY, Dong LH, Song HF and Gao X: Harnessing the clustered regularly
inter-spaced short palindromic repeat (CRISPR)/CRISPR-associated
Cas9 system to disrupt the hepatitis B virus. Gene Ther.
22:404–412. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Ramanan V, Shlomai A, Cox DBT, Schwartz
RE, Michailidis E, Bhatta A, Scott DA, Zhang F, Rice CM and Bhatia
SN: CRISPR/Cas9 cleavage of viral DNA efficiently suppresses
hepatitis B virus. Sci Rep. 5:108332015. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Karimova M, Beschorner N, Dammermann W,
Chemnitz J, Indenbirken D, Bockmann JH, Grundhoff A, Lüth S,
Buchholz F, Schulze zur Wiesch J and Hauber J: CRISPR/Cas9
nickase-mediated disruption of hepatitis B virus open reading frame
S and X. Sci Rep. 5:137342015. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Qi Xu Y, Luo Y, Yang J, Xie J, Deng Q, Su
C, Wei N, Shi W, Xu DF, et al: Hepatitis B virus X protein
stimulates proliferation, wound closure and inhibits apoptosis of
HuH-7 cells via CDC42. Int J Mol Sci. 18:E5862017. View Article : Google Scholar
|
|
121
|
Ren Q, Li C, Yuan P, Cai C, Zhang L, Luo
GG and Wei W: A dual-reporter system for real-time monitoring and
high-throughput CRISPR/Cas9 library screening of the hepatitis C
virus. Sci Rep. 5:88652015. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Yuen KS, Chan CP, Wong N-HM, Ho CH, Ho TH,
Lei T, Deng W, Tsao SW, Chen H, Kok KH and Jin DY:
CRISPR/Cas9-mediated genome editing of Epstein-Barr virus in human
cells. J Gen Virol. 96:626–636. 2015. View Article : Google Scholar
|
|
123
|
Wang J and Quake SR: RNA-guided
endonuclease provides a therapeutic strategy to cure latent
herpesviridae infection. Proc Natl Acad Sci USA. 111:13157–13162.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Kistler KE, Vosshall LB and Matthews BJ:
Genome engineering with CRISPR-Cas9 in the mosquito Aedes aegypti.
Cell Rep. 11:51–60. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Zhang C, Xiao B, Jiang Y, Zhao Y, Li Z,
Gao H, Ling Y, Wei J, Li S, Lu M, et al: Efficient editing of
malaria parasite genome using the CRISPR/Cas9 system. MBio.
5:e01414-142014. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Wagner JC, Platt RJ, Goldfless SJ, Zhang F
and Niles JC: Efficient CRISPR/Cas9-mediated genome editing in P.
falciparum. Nat Methods. 11:915–918. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Ghorbal M, Gorman M, Macpherson CR,
Martins RM, Scherf A and Lopez-Rubio JJ: Genome editing in the
human malaria parasite Plasmodium falciparum using the crisPr-cas9
system. Nat Biotechnol. 32:819–821. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Burt A: Site-specific selfish genes as
tools for the control and genetic engineering of natural
populations. Proc Biol Sci. 270:921–928. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Webber BL, Raghu S and Edwards OR:
Opinion: Is CRISPR-based gene drive a biocontrol silver bullet or
global conservation threat? Proc Natl Acad Sci USA.
112:10565–10567. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Hammond A, Galizi R, Kyrou K, Simoni A,
Siniscalchi C, Katsanos D, Gribble M, Baker D, Marois E, Russell S,
et al: A CRISPR-Cas9 gene drive system targeting female
reproduction in the malaria mosquito vector Anopheles gambiae. Nat
Biotechnol. 34:78–83. 2016. View Article : Google Scholar :
|
|
131
|
Sánchez-Rivera FJ and Jacks T:
Applications of the CRISPR-Cas9 system in cancer biology. Nat Rev
Cancer. 15:387–395. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Kawamura N, Nimura K, Nagano H, Yamaguchi
S, Nonomura N and Kaneda Y: CRISPR/Cas9-mediated gene knockout of
NANOG and NANOGP8 decreases the malignant potential of prostate
cancer cells. Oncotarget. 6:22361–22374. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
García-Tuñón I, Hernández-Sánchez M,
Ordoñez JL, Alonso-Pérez V, Álamo-Quijada M, Benito R, Guerrero C,
Hernández-Rivas JM and Sánchez-Martín M: The CRISPR/Cas9 system
efficiently reverts the tumorigenic ability of BCR/ABL in vitro and
in a xenograft model of chronic myeloid leukemia. Oncotarget.
8:26027–26040. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Zuckermann M, Hovestadt V, Knobbe-Thomsen
CB, Zapatka M, Northcott PA, Schramm K, Belic J, Jones DT, Tschida
B, Moriarity B, et al: Somatic CRISPR/Cas9-mediated tumour
suppressor disruption enables versatile brain tumour modelling. Nat
Commun. 6:73912015. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Maddalo D, Manchado E, Concepcion CP,
Bonetti C, Vidigal JA, Han YC, Ogrodowski P, Crippa A, Rekhtman N,
de Stanchina E, et al: In vivo engineering of oncogenic chromosomal
rearrangements with the CRISPR/Cas9 system. Nature. 516:423–427.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Pathak S, McDermott MF and Savic S:
Autoinflammatory diseases: Update on classification diagnosis and
management. J Clin Pathol. 70:1–8. 2017. View Article : Google Scholar
|
|
137
|
Sá DC: Inflammasomes and dermatology. An
Bras Dermatol. 91:566–578. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Kim K, Bang SY, Lee HS and Bae SC: Update
on the genetic architecture of rheumatoid arthritis. Nat Rev
Rheumatol. 13:13–24. 2017. View Article : Google Scholar
|
|
139
|
Yang M, Zhang L, Stevens J and Gibson G:
CRISPR/Cas9 mediated generation of stable chondrocyte cell lines
with targeted gene knockouts; analysis of an aggrecan knockout cell
line. Bone. 69:118–125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Tang S, Chen T, Yu Z, Zhu X, Yang M, Xie
B, Li N, Cao X and Wang J: RasGRP3 limits toll-like
receptor-triggered inflammatory response in macrophages by
activating Rap1 small GTPase. Nat Commun. 5:46572014. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Jing W, Zhang X, Sun W, Hou X, Yao Z and
Zhu Y: CRISPR/CAS9-mediated genome editing of miRNA-155 inhibits
proinflammatory cytokine production by RAW264.7 cells. Biomed Res
Int. 2015.326042:2015.
|
|
142
|
Ott de Bruin LM, Volpi S and Musunuru K:
Novel genome-editing tools to model and correct primary
immunodeficiencies. Front Immunol. 6:2502015. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Cowan MJ, Neven B, Cavazanna-Calvo M,
Fischer A and Puck J: Hematopoietic stem cell transplantation for
severe combined immunodeficiency diseases. Biol Blood Marrow
Transplant. 14(1 Suppl 1): S73–S75. 2008. View Article : Google Scholar
|
|
144
|
Deakin CT, Alexander IE and Kerridge I:
Accepting risk in clinical research: Is the gene therapy field
becoming too risk-averse. Mol Ther. 17:1842–1848. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Kohn DB and Kuo CY: New frontiers in the
therapy of primary immunodeficiency: From gene addition to gene
editing. J Allergy Clin Immunol. 139:726–732. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Goodman MA, Moradi Manesh D, Malik P and
Rothenberg ME: CRISPR/Cas9 in allergic and immunologic diseases.
Expert Rev Clin Immunol. 13:5–9. 2017. View Article : Google Scholar
|
|
147
|
Wang M, Glass ZA and Xu Q: Non-viral
delivery of genome-editing nucleases for gene therapy. Gene Ther.
24:144–150. 2017. View Article : Google Scholar
|
|
148
|
Chang CW, Lai YS, Westin E,
Khodadadi-Jamayran A, Pawlik KM, Lamb LS Jr, Goldman FD and Townes
TM: Modeling human severe combined immunodeficiency and correction
by CRISPR/Cas9-enhanced gene targeting. Cell Rep. 12:1668–1677.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Flynn R, Grundmann A, Renz P, Hänseler W,
James WS, Cowley SA and Moore MD: CRISPR-mediated genotypic and
phenotypic correction of a chronic granulomatous disease mutation
in human iPS cells. Exp Hematol. 43:838–848. e32015. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Wrona D, Siler U and Reichenbach J:
CRISPR/Cas9-generated p47(phox)-deficient cell line for chronic
granulomatous disease gene therapy vector development. Sci Rep.
7:441872017. View Article : Google Scholar
|
|
151
|
Yan Q, Zhang Q, Yang H, Zou Q, Tang C, Fan
N and Lai L: Generation of multi-gene knockout rabbits using the
Cas9/gRNA system. Cell Regen (Lond). 3:122014.
|
|
152
|
Fan Z, Li W, Lee SR, Meng Q, Shi B, Bunch
TD, White KL, Kong IK and Wang Z: Efficient gene targeting in
golden Syrian hamsters by the CRISPR/Cas9 system. PLoS One.
9:e1097552014. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Chen F, Wang Y, Yuan Y, Zhang W, Ren Z,
Jin Y, Liu X, Xiong Q, Chen Q, Zhang M, et al: Generation of B
cell-deficient pigs by highly efficient CRISPR/Cas9-mediated gene
targeting. J Genet Genomics. 42:437–444. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Chu HW, Rios C, Huang C,
Wesolowska-Andersen A, Burchard EG, O’Connor BP, Fingerlin TE,
Nichols D, Reynolds SD and Seibold MA: CRISPR-Cas9-mediated gene
knockout in primary human airway epithelial cells reveals a
proinflammatory role for MUC18. Gene Ther. 22:822–829. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Kuo CY, Hoban MD, Joglekar AV and Kohn DB:
Site specific gene correction of defects in CD40 ligand using the
Crispr/Cas9 genome editing platform. J Allergy Clin Immunol.
135:AB172015. View Article : Google Scholar
|
|
156
|
Cheong TC, Compagno M and Chiarle R:
Editing of mouse and human immunoglobulin genes by CRISPR-Cas9
system. Nat Commun. 7:109342016. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Rodriguez-Sanchez IP, Guindon J, Ruiz M,
Tejero ME, Hubbard G, Martinez-de-Villarreal LE, Barrera-Saldaña
HA, Dick EJ Jr, Comuzzie AG and Schlabritz-Loutsevitch NE: The
endocannabinoid system in the baboon (Papio spp.) as a complex
framework for developmental pharmacology. Neurotoxicol Teratol.
58:23–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Niu Y, Shen B, Cui Y, Chen Y, Wang J, Wang
L, Kang Y, Zhao X, Si W, Li W, et al: Generation of gene-modified
cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell
embryos. Cell. 156:836–843. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Kang Y, Zheng B, Shen B, Chen Y, Wang L,
Wang J, Niu Y, Cui Y, Zhou J, Wang H, et al: CRISPR/Cas9-mediated
Dax1 knockout in the monkey recapitulates human AHC-HH. Hum Mol
Genet. 24:7255–7264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Kim S, Huang LW, Snow KJ, Ablamunits V,
Hasham MG, Young TH, Paulk AC, Richardson JE, Affourtit JP,
Shalom-Barak T, et al: A mouse model of conditional lipodystrophy.
Proc Natl Acad Sci USA. 104:16627–16632. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Croasdell A, Duffney PF, Kim N, Lacy SH,
Sime PJ and Phipps RP: PPARγ and the innate immune system mediate
the resolution of inflammation. PPAR Res. 2015.549691:2015.
|
|
162
|
Huang J, Guo X, Fan N, Song J, Zhao B,
Ouyang Z, Liu Z, Zhao Y, Yan Q, Yi X, et al: RAG1/2 knockout pigs
with severe combined immunodeficiency. J Immunol. 193:1496–1503.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Chen Y, Xiong M, Dong Y, Haberman A, Cao
J, Liu H, Zhou W and Zhang SC: Chemical control of grafted human
PSC-derived neurons in a mouse model of Parkinson’s disease. Cell
Stem Cell. 18:817–826. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Zhou X, Xin J, Fan N, Zou Q, Huang J,
Ouyang Z, Zhao Y, Zhao B, Liu Z, Lai S, et al: Generation of
CRISPR/Cas9-mediated gene-targeted pigs via somatic cell nuclear
transfer. Cell Mol Life Sci. 72:1175–1184. 2015. View Article : Google Scholar
|
|
165
|
Wang L, Yi F, Fu L, Yang J, Wang S, Wang
Z, Suzuki K, Sun L, Xu X, Yu Y, et al: CRISPR/Cas9-mediated
targeted gene correction in amyotrophic lateral sclerosis patient
iPSCs. Protein Cell. 8:365–378. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Bhinge A, Namboori SC, Zhang X, VanDongen
AMJ and Stanton LW: Genetic correction of SOD1 mutant iPSCs reveals
ERK and JNK activated AP1 as a driver of neurodegeneration in
amyotrophic lateral sclerosis. Stem Cell Reports. 8:856–869. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Merienne N, Vachey G, de Longprez L,
Meunier C, Zimmer V, Perriard G, Canales M, Mathias A, Herrgott L,
Beltraminelli T, et al: The self-inactivating KamiCas9 system for
the editing of CNS disease genes. Cell Rep. 20:2980–2991. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Page SC, Hamersky GR, Gallo RA, Rannals
MD, Calcaterra NE, Campbell MN, Mayfield B, Briley A, Phan BN,
Jaffe AE and Maher BJ: The schizophrenia- and autism-associated
gene, transcription factor 4 regulates the columnar distribution of
layer 2/3 prefrontal pyramidal neurons in an activity-dependent
manner. Mol Psychiatry. 23:304–315. 2018. View Article : Google Scholar
|
|
169
|
Long C, Amoasii L, Mireault AA, McAnally
JR, Li H, Sanchez-Ortiz E, Bhattacharyya S, Shelton JM, Bassel-Duby
R and Olson EN: Postnatal genome editing partially restores
dystrophin expression in a mouse model of muscular dystrophy.
Science. 351:400–403. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Long C, McAnally JR, Shelton JM, Mireault
AA, Bassel-Duby R and Olson EN: Prevention of muscular dystrophy in
mice by CRISPR/Cas9-mediated editing of germline DNA. Science.
345:1184–1188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Nelson CE, Hakim CH, Ousterout DG, Thakore
PI, Moreb EA, Castellanos Rivera RM, Madhavan S, Pan X, Ran FA, Yan
WX, et al: In vivo genome editing improves muscle function in a
mouse model of Duchenne muscular dystrophy. Science. 351:403–407.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Tabebordbar M, Zhu K, Cheng JKW, Chew WL,
Widrick JJ, Yan WX, Maesner C, Wu EY, Xiao R, Ran FA, et al: In
vivo gene editing in dystrophic mouse muscle and muscle stem cells.
Science. 351:407–411. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Ackermann AM, Zhang J, Heller A, Briker A
and Kaestner KH: High-fidelity Glucagon-CreER mouse line generated
by CRISPR-Cas9 assisted gene targeting. Mol Metab. 6:236–244. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Jarrett KE, Lee CM, Yeh YH, Hsu RH, Gupta
R, Zhang M, Rodriguez PJ, Lee CS, Gillard BK, Bissig KD, et al:
Somatic genome editing with CRISPR/Cas9 generates and corrects a
metabolic disease. Sci Rep. 7:446242017. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Mookherjee Yu W, Chaitankar S, Hiriyanna
V, Kim S, Brooks JW, Ataeijannati M, Sun Y, Dong X, Li LT, et al:
Nrl knockdown by AAV-delivered CRISPR/Cas9 prevents retinal
degeneration in mice. Nat Commun. 8:147162017. View Article : Google Scholar : PubMed/NCBI
|