Introduction
Cardiovascular diseases are associated with high
morbidity and mortality rates worldwide. Restoring the blood supply
following myocardial ischemia may alleviate ischemic injury;
however, this process can promote damage to the ischemic
myocardium, which is identified as ischemia-reperfusion (I/R)
injury (1). It has been reported
that I/R injury induces damage associated with functional
impairment of the heart and arrhythmia (2). Therefore, it is important to
alleviate I/R injury when treating ischemic heart disease. In
clinical settings, drugs including diltiazem, are mainly used in
response to I/R injury. A previous study demonstrated that
apoptosis and inflammation are involved in the development of I/R
injuries (3), whereas
anti-inflammatory and antiapoptotic effects have been reported to
be associated with myocardial protection (4).
As a serine/threonine protein kinase of the Rho
family, Rho-associated protein kinase (ROCK) acts as a molecular
switch of the cellular signaling pathway, and may be activated by
RhoA. The RhoA/ROCK signaling pathway has been reported to be
involved in numerous diseases, including atherosclerosis, cardiac
hypertrophy, heart failure and diabetes-associated diseases. RhoA
also serves a central role in signal transduction. It has been
suggested that activation of the mitogen-activated protein kinase
(MAPK) and nuclear factor (NF)-κB signaling pathways is regulated
by RhoA/ROCK signaling (5). As a
selective Rho-kinase inhibitor,
(+)-(R)-trans-4-(1-aminoethyl)-N-(4-pyridyl) cyclohexane
carboxamide (Y-27632) has been used in numerous experiments
(6). Of note, Y-27632 has been
observed to exhibit protective effects against I/R cardiac injury
in animals (7). Y-27632 has also
been reported to suppress the apoptosis of human cardiac stem cells
(8). ROCK inhibitors have been
demonstrated to protect against I/R injury; however, the underlying
molecular mechanism requires further investigation. Therefore, the
present study aimed to investigate the pharmacological effects of
Y-27632 on I/R-induced heart injury and the potential underlying
mechanisms.
Materials and methods
Reagents
Y-27632 was purchased from Tocris Cookson, Ltd.
(Bristol, UK). All primary antibodies used in the present study,
including anti-RhoA, -ROCK1, -c-Jun N-terminal kinase (JNK),
-phosphorylated (P)-JNK, -extracellular signal-regulated kinase
(ERK), -P-ERK, -P-P38, -P38, -P-NF-κB, -NF-κB, -inhibitor of NF-κB
(IκB), -P-IκB, -B-cell lymphoma 2 (Bcl-2), -Bcl-2-associated X
(Bax), -Caspase-3, -Caspase-9 and -GAPDH, were obtained from Cell
Signaling Technology, Inc. (Danvers, MA, USA). ELISA kits for the
detection of interleukin (IL)-6, tumor necrosis factor (TNF)-α and
IL-1β were purchased by R&D Systems, Inc. (Minneapolis, MN,
USA). Creatine kinase (CK) and lactate dehydrogenase (LDH) assay
kits were obtained from MultiSciences (Lianke) Biotech Co., Ltd.
(Hangzhou, China).
Animals
Male Sprague-Dawley rats (8 weeks old; ~250-280 g)
were obtained from Beijing Vital River Laboratory Animal Technology
Co., Ltd. (Beijing, China) and housed together under a 12-h
light/dark cycle, with free access to food and water, at a constant
temperature of 22-24°C and relative humidity of 50±5%. All animal
treatments in the present study were performed humanely and
following the institutional and national guidelines for ethical
animal research. All experimental procedures were conducted
according to the guidelines and following approval from the Ethical
Committee of Experimental Animal Care at Wenzhou Medical University
(Wenzhou, China).
Myocardial I/R model
The rats were anesthetized via 2% isoflurane
inhalation prior to establishing the I/R rat model by ligation of
the left descending coronary artery, as previously described
(9). The rats were anesthetized
and restrained; the trachea was then incised and wrapped in catgut
for later use. An incision was then made in the skin at the
position of the heart, and the underlying ribs were exposed by
blunt dissection to provide convenient access during the heart
procedure. Following exposure of the heart, the left anterior
descending coronary artery was blocked with a specific suture. A
positive pressure respirator was applied to provide respiratory
function for the experimental rats during the procedure. Elevation
of the ST segment was used to confirm successful implementation of
the surgical model. Recovery of a strong, regular heart rhythm
indicated that the thoracic cavity was ready to be sutured. The
suture of rats in the sham group was not tied. The rats were
randomly divided into four groups: i) Sham; ii) I/R; iii) I/R +
diltiazem (Dil; I/R + 10 mg/kg Dil); and iv) I/R + Y-27632 (I/R + 5
mg/kg Y-27632). The rats in the Y-27632 and Dil groups were treated
continuously with Y-27632 or Dil for 5 days following I/R. At 24 h
following the final administration of Y-27632 or Dil, the rats were
sacrificed by CO2 asphyxiation with 20% volume
displacement/min; 8-ml blood samples and myocardial tissues were
then collected.
Langendorff-perfused heart model
Under anesthesia, the rats were administered with
heparin (250 U/kg) intraperitoneally and the heart was excised
following retrograde perfusion with complex Krebs-Henseleit (K-H)
buffer at a pressure of 70 cm H2O using the Langendorff
system. The heart and perfusate were maintained at 37°C with a
water jacket. Subsequently, K-H buffer was applied for the
perfusion of the isolated hearts under 80-90 mmHg. The rats were
then randomly divided into four groups: i) Control, hearts were
perfused for a stabilization period of 80 min; ii) I/R, hearts were
equilibrated for 20 min followed by global ischemia for 30 min. K-H
buffer was used for reperfusion for 30 min; iii) I/R + Dil; and iv)
I/R + Y-27632, hearts were processed according to the method of the
I/R group; however, reperfusion was conducted using Y-27632 (0.5
mg/kg) or Dil (0.2 mg/kg) dissolved K-H buffer for 30 min.
Coronary flow and myocardial
contractility analysis
Following reperfusion for various durations, the K-H
effluent flow was measured as the coronary flow; a flow rate of
>8 ml/min during the stabilization period was selected. Coronary
effluent was collected following downstream analysis. A portable
heart clip was used to record the contractile force of the
myocardium during the experiment.
Electrocardiogram (ECG)
In the present study, an ECG was generated using
needle electrodes and the BL-420 S biological function experiment
system (Chengdu TME Technology, Co., Ltd., Chengdu, China). ST
segment elevation on the ECG was considered to be an indicator of
ischemia.
Assessment of myocardial infarct
area
Triphenyltetrazolium (TTC) staining (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) was conducted to determine the
myocardial infarct size. Briefly, the hearts were incubated at
-20°C for 15 min. The hearts were cut into five parallel pieces and
placed in 1% TTC for 15 min at 37°C in the dark. The myocardial
infarct ratio [(infarct area/whole heart area) x percentage] was
calculated using image analysis software (Image-Pro Plus version
4.1; Media Cybernetics, Inc., Rockville, MD, USA).
Determination of inflammatory cytokines
in the serum
The levels of inflammatory markers, including IL-6,
IL-1β and TNF-α, in the serum were examined using mice ELISA kits
according to the manufacturer’s protocols.
Analysis of cardiac enzymes
The serum levels of CK and LDH were measured using
the commercial kits according to the manufacturer’s protocols.
Histological evaluation
The morphology of the myocardial tissues was
examined using hematoxylin and eosin (H&E) staining. Following
dehydration with 80, 90 and 100% ethanol and n-butanol, the
myocardial tissue was waxed in a 60°C wax box and then embedded in
paraffin. Tissue sections (5-µm) were dried at 45°C and
obtained from each paraffin block. The sections were heated at 60°C
for 1 h and dewaxed with xylene. Following hydration, the sections
were stained with H&E, dehydrated with gradient ethanol,
cleared with xylene and mounted with neutral gum. Images were
captured using a light microscope (BX41; Olympus Corporation,
Tokyo, Japan).
Western blotting
Total protein was extracted from heart samples and
analyzed by western blotting with the specific antibodies
described. Briefly, the samples were collected and homogenized, and
then lysed in extraction buffer for 1 h on ice. The total protein
content was quantified using a Bicinchoninic Acid protein assay kit
(Beyotime Institute of Biotechnology, Nanjing, China). Equal
quantities of protein (20 µg) were separated by 8-12%
SDS-PAGE and then electrotransferred onto polyvinylidene difluoride
(PVDF) membranes, which were blocked with skim milk at room
temperature for 2 h. Following blocking, the PVDF membranes were
incubated with specific antibodies against RhoA (1:1,000; cat. no.
2117), ROCK1 (1:1,000; cat. no. 4035), P-JNK (1:1,000; cat. no.
9255), JNK (1:1,000; cat. no. 9252, P-ERK (1:1,000; cat. no. 8544),
ERK (1:1,000; cat. no. 4348), P-P38 (1:1,000; cat. no. 4511), P38
(1:1,000; cat. no. 8690), P-NF-κB (1:1,000; cat. no. 3033), NF-κB
(1:1,000; cat. no. 8242), P-IκB (1:1,000; cat. no. 2859) IκB
(1:1,000; cat. no. 4812), Bax (1:1,000; cat. no. 2772), Bcl-2
(1:1,000; cat. no. 2764), Caspase-3 (1:1,000; cat. no. 9662),
Caspase-9 (1:1,000; cat. no. 9508) and GAPDH (1:2,000; cat. no.
5174; all from Cell Signaling Technology, Inc.) overnight at 4°C.
On the second day, the PVDF membranes were incubated with a
secondary antibody [horseradish peroxidase-labeled mouse
anti-rabbit immunoglobulin (Ig)-G (1:5,000; cat. no. 5127; Cell
Signaling Technology, Inc.); and horseradish peroxidase-labeled
rabbit anti-mouse IgG (1:5,000; cat. no. 58802; Cell Signaling
Technology, Inc.)], at room temperature for 1 h following three
washes with tris-buffered saline with Tween-20. Subsequently, the
immunoreactive bands were visualized with a gel imaging system
(Tanon 5200; Tanon Science and Technology Co., Ltd., Shanghai,
China).
Statistical analysis
The data are presented as the mean ± standard
deviation, and were analyzed by one-way analysis of variance
followed by Tukey’s post hoc test using GraphPad Prism software 6.0
(GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of Y-27632 on myocardial infarct
size
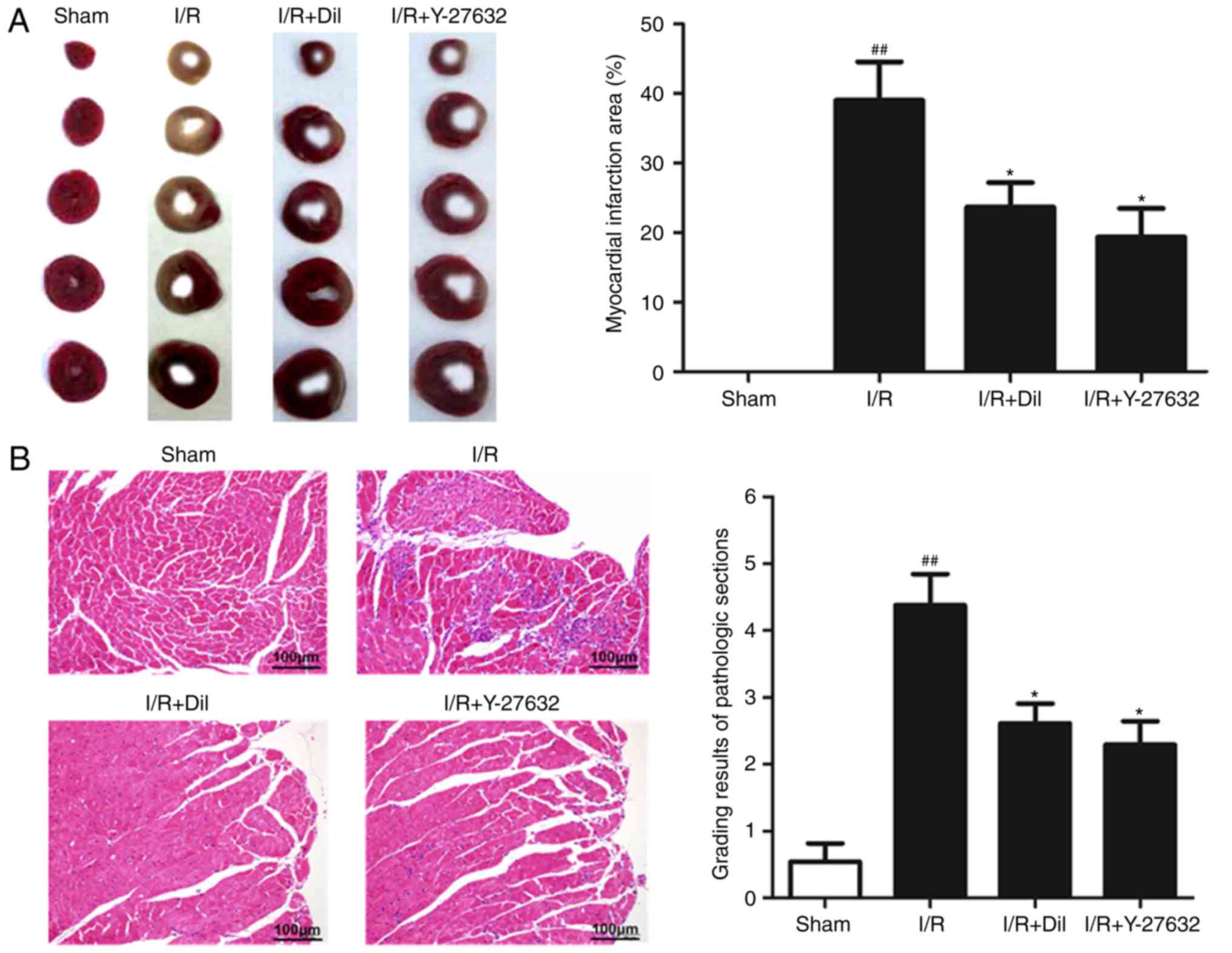
To determine whether Y-27632 exhibits myocardial
protective effects, the infarct size of rat hearts was analyzed by
TTC staining. As presented in Fig.
1A, compared with the sham group, I/R markedly increased the
infarct size, which was significantly reduced following treatment
with Y-27632 or Dil.
Effects of Y-27632 on myocardial
histology
To investigate the effects of Y-27632 on myocardial
injuries, histopathological alterations of the myocardial tissues
were analyzed. As presented in Fig.
1B, extensive inflammatory cells and the notable destruction of
myocardial cellular structure were observed in the I/R group
compared with the sham group. Additionally, treatment with Y-27632
or Dil markedly attenuated the histological alterations induced by
I/R.
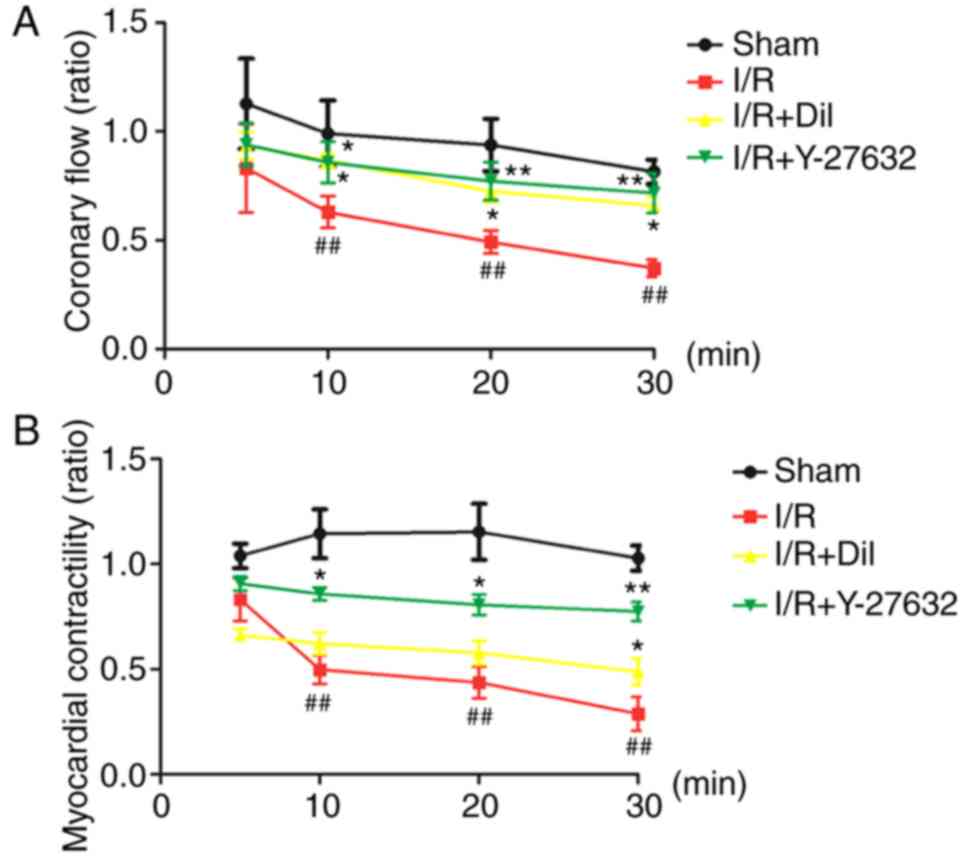
Effects of Y-27632 on coronary flow in
the I/R-induced isolated heart
As presented in Fig.
2A, the I/R group exhibited significant decreases in the ratio
of coronary flow compared with that in the sham group (P<0.01).
By contrast, treatment with Y-27632 or Dil led to notable increases
in the ratio of coronary flow.
Effects of Y-27632 on myocardial
contractility in the I/R-induced isolated heart
Analysis of the ratio of myocardial contractility of
isolated rat hearts revealed that the model group exhibited
significantly a reduced ratio of contractility following the
inhibition of K-H flow compared with that in the sham group
(P<0.01). By contrast, treatment with Y-27632 or Dil led to a
notable increase in the myocardial contractility (Fig. 2B).
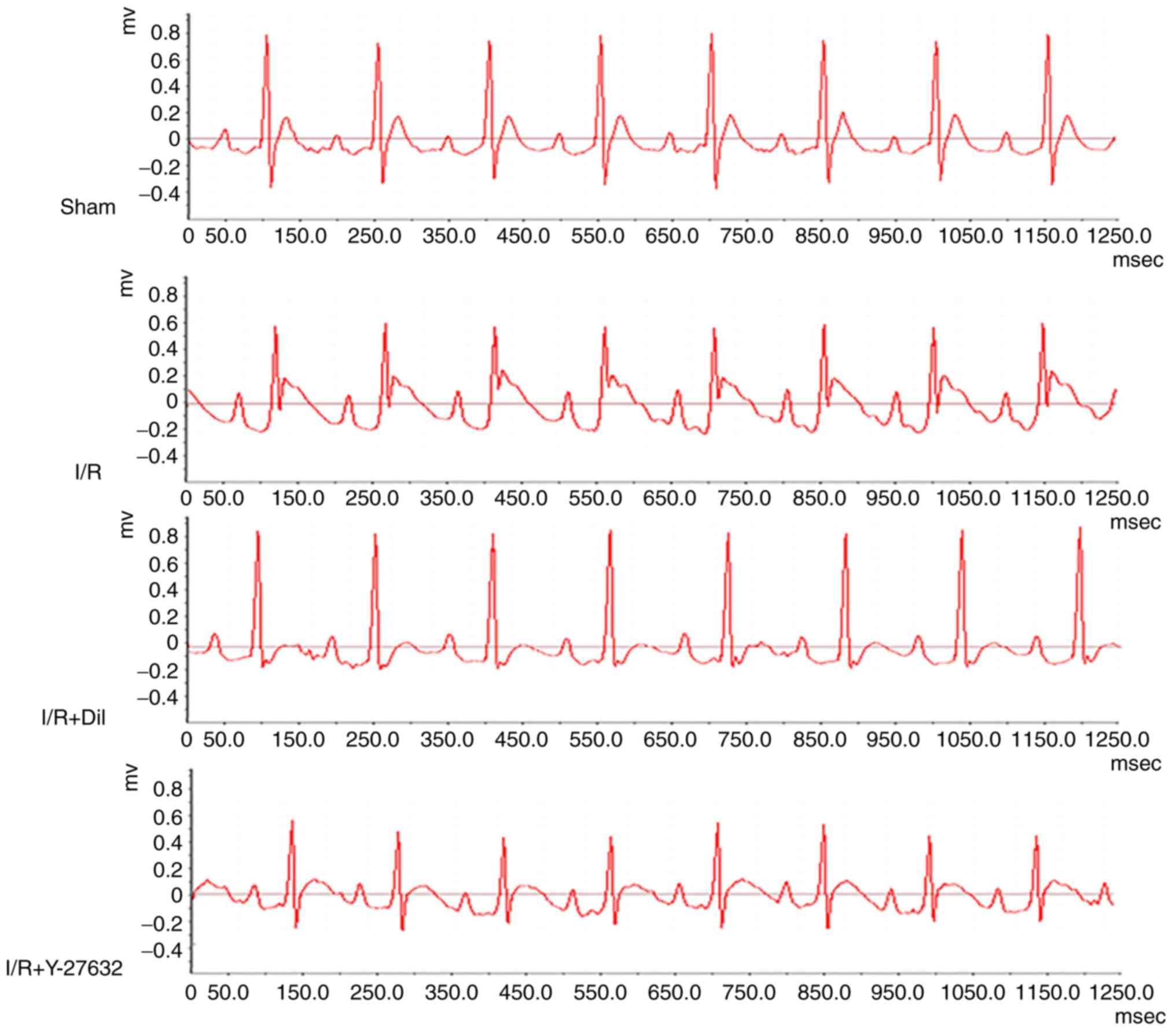
Effects of Y-27632 on ST-segment
elevation
As presented in Fig.
3, an increase in ST-segment and a decrease in R-amplitude were
observed in the I/R group compared with the sham group; however,
these effects were reversed following treatment with Y-27632 or
Dil.
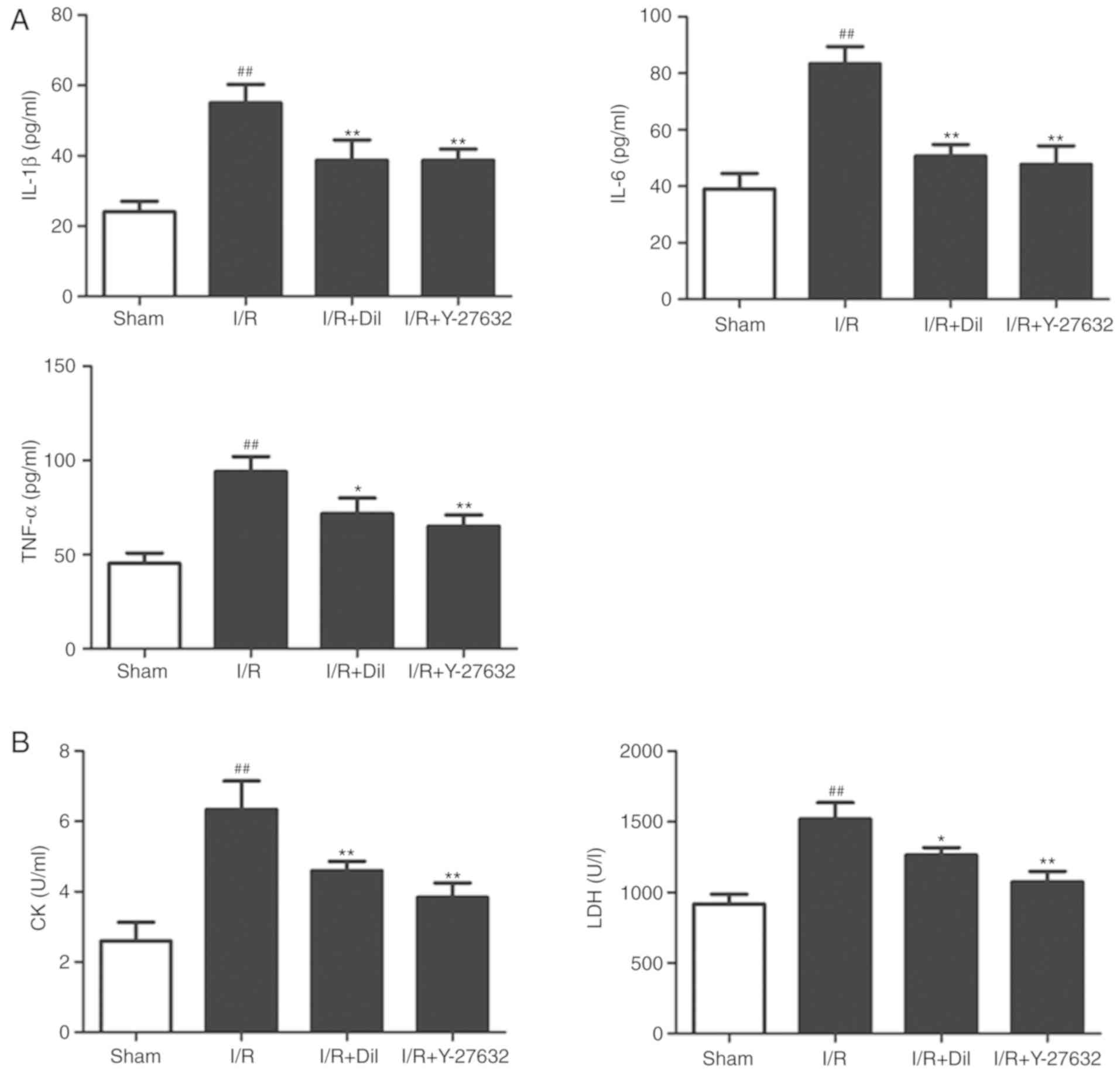
Effects of Y-27632 on inflammatory
cytokines in the serum
To investigate the potential anti-inflammatory
effects of Y-27632, the serum levels of IL-6, TNF-α and IL-1β were
measured by ELISA. As presented in Fig. 4A, the levels of IL-6, TNF-α and
IL-1β were significantly upregulated in the I/R model group
compared with those in the sham group. Treatment with Y-27632 or
Dil significantly reduced the levels of inflammatory cytokines
compared with those in the I/R group.
Effects of Y-27632 on cardiac
enzymes
As representative indicators of myocardial injury,
the levels of CK and LDH in the serum were analyzed. As presented
in Fig. 4B, the serum levels of
CK and LDH were significantly upregulated in the I/R group compared
with the those in the sham group (P<0.01, respectively). By
contrast, treatment with Y-27632 or Dil effectively suppressed the
levels of CK and LDH.
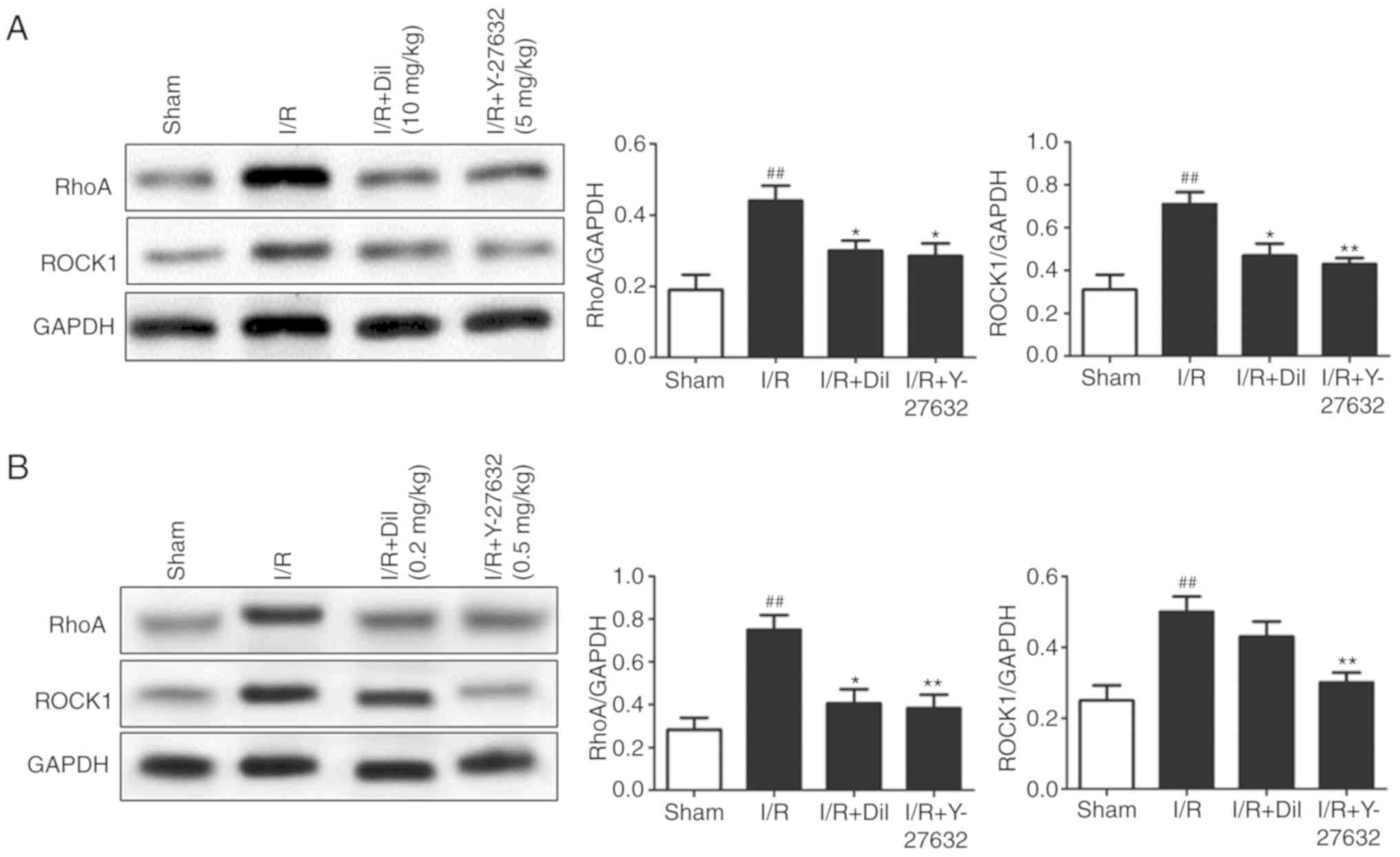
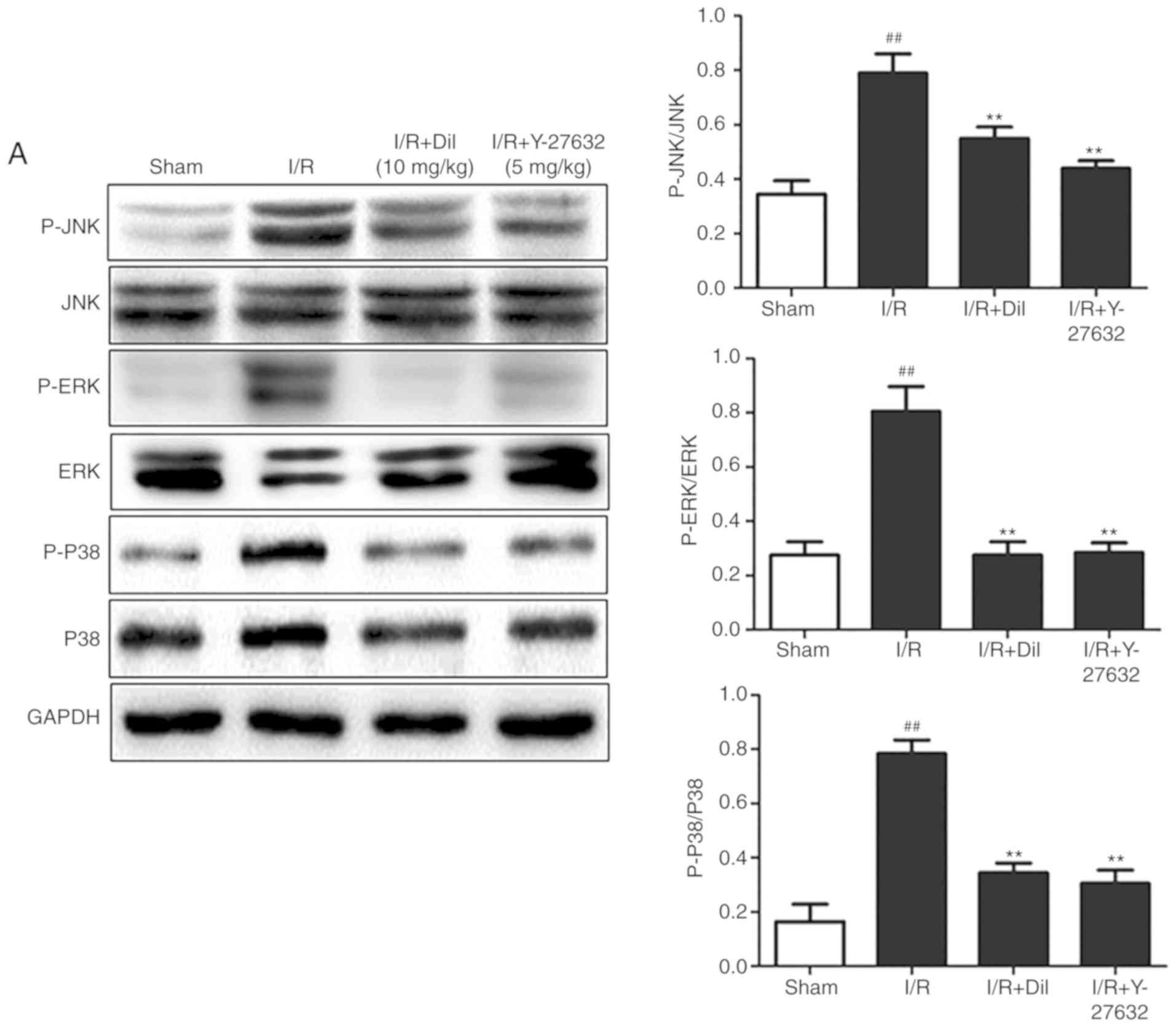
Effects of Y-27632 on the RhoA/ROCK and
MAPK/NF-κB signaling pathways, and apoptosis in the I/R-induced
heart
To determine the intracellular signaling pathway
underlying the myocardial protective effects of Y-27632 in I/R, the
RhoA/ROCK and MAPK/NF-κB signaling pathways, and apoptosis were
investigated. Upregulated expression levels of RhoA and ROCK1
(Fig. 5A and B) and increased
phosphorylation of ERK1/2, JNK and P38 proteins (Fig. 6A and B) were observed in the I/R
group; however, treatment with Y-27632 or Dil treatment notably
reversed the effects of I/R on the expression of the aforementioned
proteins.
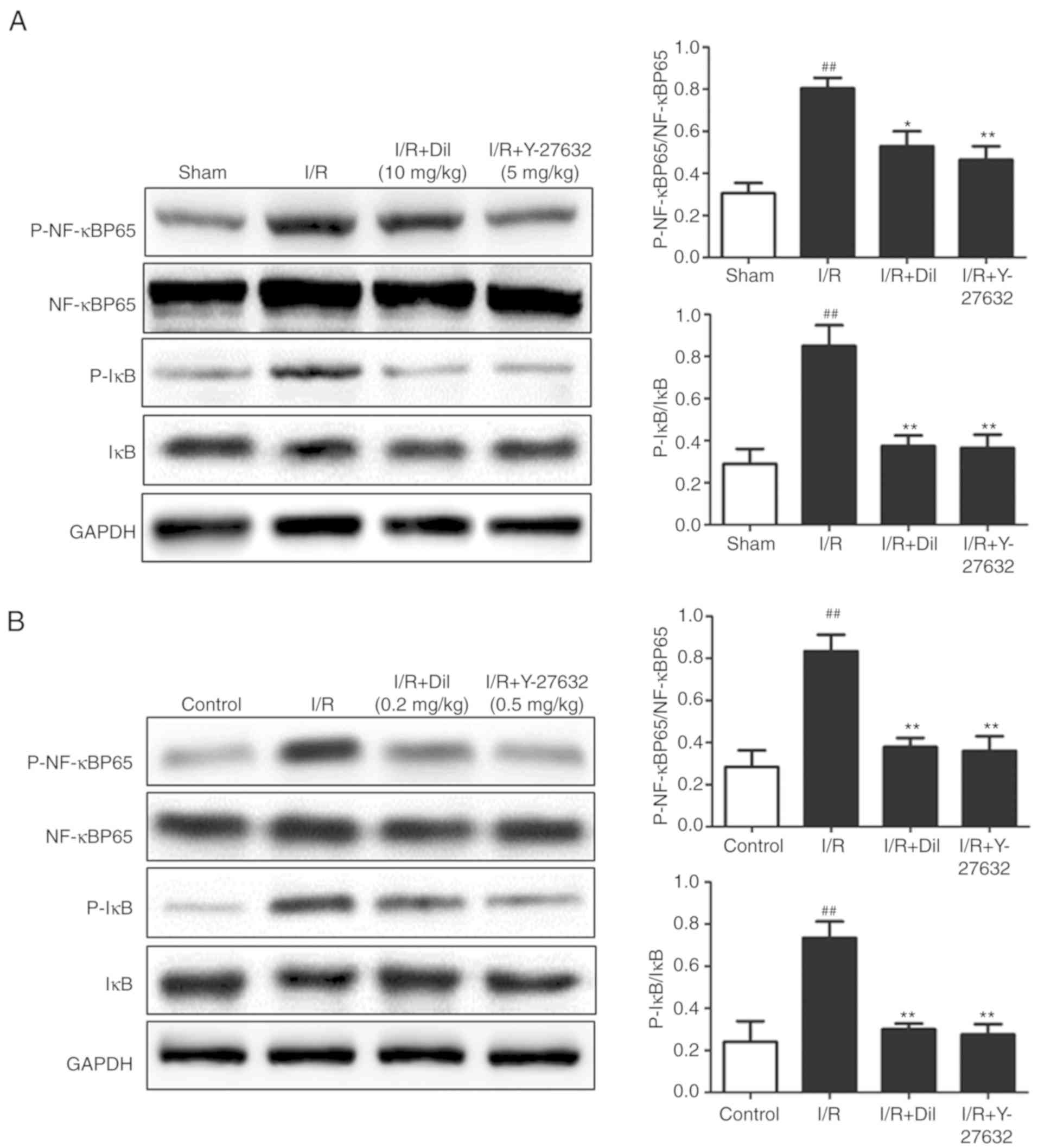
To further investigate the downstream mechanism
underlying the effects of Y-27632 on the I/R-induced heart in rats,
the expression levels of P-IκB and P-NF-κB were determined
(Fig. 7A and B). Western blot
analysis demonstrated upregulated expression of P-IκB and P-NF-κB
in the model group compared with the sham group. Following
treatment with Y-27632 or Dil, significantly suppressed expression
levels of P-IκB and P-NF-κB were detected. These results indicated
that the inhibition of ROCK1 may prevent the activation of
NF-κB.
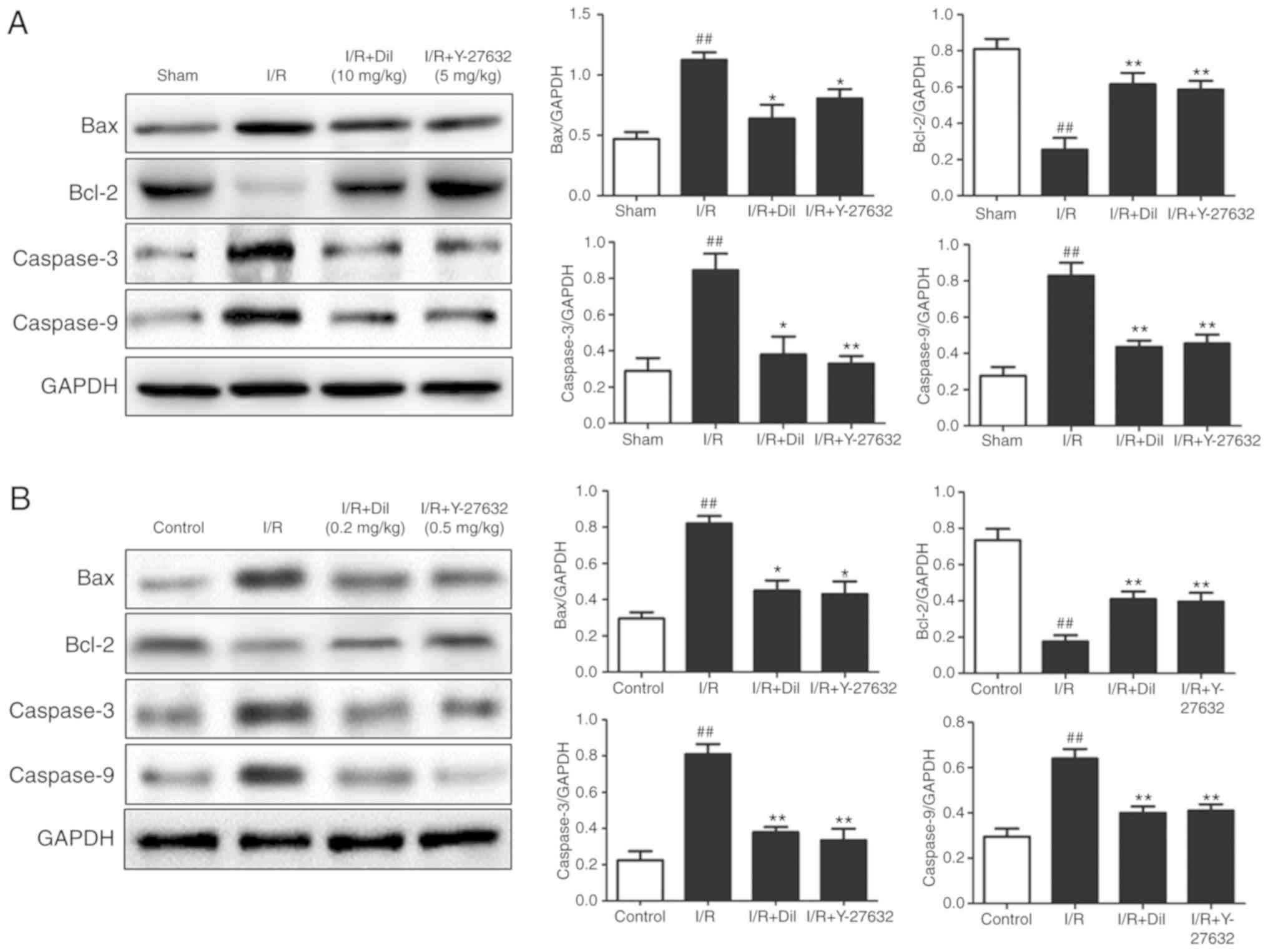
As presented in Fig.
8A and B, the expression levels of apoptosis-associated
proteins were detected to investigate the mechanism underlying the
effect of Y-27632 on cardiomyocyte apoptosis. I/R injury markedly
increased the expression of Bax, Caspase-3 and Caspase-9, which was
associated with reduced expression levels of Bcl-2. Treatment with
Y-27632 or Dil markedly reversed the effects of I/R injury on the
expression of the aforementioned proteins. These results indicated
that the inhibition of ROCK1 may suppress apoptosis.
Discussion
At present, the prevalence and mortality rates of
cardiovascular diseases have significantly increased with the aging
of populations and alterations in lifestyle (10). Cardiovascular diseases are the
main cause of mortality in the Chinese population and account for
>40% of the reported mortality rate, which may increase in the
next 10 years (11). The
therapeutic approaches and agents available have markedly improved;
however, the treatment of myocardial ischemia remains ineffective.
Therefore, further investigation into treating myocardial ischemia
is required. In the present study, the cardioprotective effects of
Y-27632 were determined via TTC and H&E staining, and analyzing
ST-segment elevations. These observations were supported by
analyzing coronary flow and myocardial contractility.
The Rho/ROCK signaling pathway has been associated
with the growth, proliferation and differentiation of cells, which
are widely involved in various pathophysiological processes. Rho A
is a small GTP-binding protein, whereas ROCK is the downstream
effector protein of Rho A (12).
ROCK is a serine/threonine protein kinase involved in the
regulation of numerous cellular functions, including migration,
contraction and adhesion. Blood pressure, cardiac hypertrophy,
heart failure and other cardiovascular diseases have been
associated with the activity of ROCK (13). As the downstream molecule of ROCK,
NF-κB is a nuclear transcription factor with multi-directional
regulation, and regulates the expression of genes associated with
immune, stress and inflammatory responses. It has been reported
that I/R of the heart can induce the overexpression of inflammatory
and apoptotic cytokines (14-16). Increasing evidence has indicated
that the Rho/ROCK/NF-κB signaling pathway serves important roles in
cardiomyocytes and experimental myocardial infarction (17-19). In the present study, it was
determined that Y-27632 successfully inhibited the Rho/ROCK
signaling pathway and may lead to suppression of the activation of
NF-κB.
MAPKs, including P38 MAPK, JNK and ERK, serve
crucial roles in cell signaling transduction (17). Upon stimulation by a variety of
stimuli, MAPKs can regulate the growth, proliferation,
differentiation and survival of cells. As widely known
stress-activated protein kinases, JNK and P38 MAPK promote cellular
survival or apoptosis (18). It
has been suggested that JNKs induce apoptosis caused by I/R,
inflammation or oxidative stress (19). Sustained activation of ERK1/2 in
I/R has been reported previously. Furthermore, ROCK has been
determined to be associated with the activation of MAPK in
cardiovascular diseases. The findings of the present study
suggested that the inhibition of ROCK effectively suppressed the
phosphorylation of MAPKs.
Myocardial I/R can induce apoptosis in heart
tissues. Myocardial infarction has been reported to increase cell
permeability, promote the caspase cascade and lead to the apoptosis
of cardiomyocytes (20). The
harmful stimulus of myocardial infarction promotes Caspase-9 via an
apoptotic signaling pathway and induces Caspase-3, a common
downstream effector involved in the apoptotic signaling pathway and
with a central role in this process. In addition, Bax and Bcl-2 of
the Bcl-2 family are the most important regulatory genes in
apoptosis. Numerous studies have suggested that Bcl-2, Bax,
Caspase-3 and Caspase-9 are involved in the pathogenesis of
myocardial I/R injury. The results of the present study revealed
that Y-27632 markedly prevented I/R-induced apoptosis in the rat
heart.
In conclusion, the present study demonstrated that
Y-27632, a ROCK inhibitor, markedly ameliorated myocardial
I/R-induced injury. The protective effects may be due to inhibited
MAPK/NF-κB signaling activation; thus, apoptosis and the
inflammatory response are suppressed. The findings of the present
study may provide novel insight into potential treatments for
cardiovascular diseases; however, further investigation is
required.
Acknowledgments
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81670225) and
Zhejiang Public Welfare Technology Application Research (grant no.
2013C33168).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
LYD, XXQ, YZ and SX contributed to the conception
and design of the study, carried out the animal experiments and
revised the manuscript. LYD, YZ and SX contributed to the
acquisition, analysis and interpretation of the data. LYD and SX
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All experimental procedures were approved by the
Ethical Committee of Experimental Animal Care at Wenzhou Medical
University (Wenzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hu Q, Wei B, Wei L, Hua K, Yu X, Li H and
Ji H: Sodium tanshinone IIA sulfonate ameliorates ischemia-induced
myocardial inflammation and lipid accumulation in Beagle dogs
through NLRP3 inflammasome. Int J Cardiol. 196:183–192. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhu L, Wei T, Gao J, Chang X, He H, Luo F,
Zhou R, Ma C, Liu Y and Yan T: The cardioprotective effect of
salidroside against myocardial ischemia reperfusion injury in rats
by inhibiting apoptosis and inflammation. Apoptosis. 20:1433–1443.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weinreuter M, Kreusser MM, Beckendorf J,
Schreiter FC, Leuschner F, Lehmann LH, Hofmann KP, Rostosky JS,
Diemert N, Xu C, et al: CaM Kinase II mediates maladaptive
post-infarct remodeling and pro-inflammatory chemoattractant
signaling but not acute myocardial ischemia/reperfusion injury.
EMBO Mol Med. 6:1231–1245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hua K, Sheng X, Li T, Wang LN, Zhang YH,
Huang ZJ and Ji H: The edaravone and 3-n-butylphthalide
ring-opening derivative 10b effectively attenuates cerebral
ischemia injury in rats. Acta Pharmacol Sin. 36:917–927. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fierro C, Novoa U, González V, Ocaranza MP
and Jalil JE: Simultaneous Rho kinase inhibition in circulating
leukocytes and in cardiovascular tissue in rats with high
angiotensin converting enzyme levels. Int J Cardiol. 215:309–317.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xue J, Fan B, Xing Q and Li T: Effect of
Y-27632 Rho-kinase inhibitor on proliferation and migration of
human glioblastoma cell. J Henan Univ Sci Technol. 28:247–252.
2010.
|
|
7
|
Ikeda F, Terajima H, Shimahara Y, Kondo T
and Yamaoka Y: Reduction of hepatic ischemia/reperfusion-induced
injury by a specific ROCK/Rho kinase inhibitor Y-27632. J Surg Res.
109:155–160. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kan L, Smith A, Chen M, Ledford BT, Fan H,
Liu Z and He JQ: Rho-associated kinase inhibitor (Y-27632)
attenuates doxorubicin-induced apoptosis of human cardiac stem
cells. PLoS One. 10:e01445132015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dong LY, Chen F, Xu M, Yao LP, Zhang YJ
and Zhuang Y: Quercetin attenuates myocardial ischemia-reperfusion
injury via downregulation of the HMGB1-TLR4-NF-kappaB signaling
pathway. Am J Transl Res. 10:1273–1283. 2018.
|
|
10
|
Bastien M, Poirier P, Lemieux I and
Després JP: Overview of epidemiology and contribution of obesity to
cardiovascular disease. Prog Cardiovasc Dis. 56:369–381. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gu D, Gupta A, Muntner P, Hu S, Duan X,
Chen J, Reynolds RF, Whelton PK and He J: Prevalence of
cardiovascular disease risk factor clustering among the adult
population of China: Results from the International Collaborative
Study of Cardiovascular Disease in Asia. Circulation. 112:658–665.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen T, Guo Q, Wang H, Zhang H, Wang C,
Zhang P, Meng S, Li Y, Ji H and Yan T: Effects of esculetin on
lipopolysaccharide (LPS)-induced acute lung injury via regulation
of RhoA/Rho kinase/NF-small ka, CyrillicB pathways in vivo and in
vitro. Free Radic Res. 49:1459–1468. 2015. View Article : Google Scholar
|
|
13
|
Ohki S, Iizuka K, Ishikawa S, Kano M,
Dobashi K, Yoshii A, Shimizu Y, Mori M and Morishita Y: A highly
selective inhibitor of Rho-associated coiled-coil forming protein
kinase, Y-27632, prolongs cardiac allograft survival of the
BALB/c-to-C3H/He mouse model. J Heart Lung Transplantat.
20:956–963. 2001. View Article : Google Scholar
|
|
14
|
Li C, Gao Y, Tian J, Shen J, Xing Y and
Liu Z: Sophocarpine administration preserves myocardial function
from ischemia- reperfusion in rats via NF-κB inactivation. J
Ethnopharmacol. 135:620–625. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin J, Wang H, Li J, Wang Q, Zhang S, Feng
N, Fan R and Pei J: κ-Opioid receptor stimulation modulates
TLR4/NF-κB signaling in the rat heart subjected to
ischemia-reperfusion. Cytokine. 61:842–848. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Henkel T, Machleidt T, Alkalay I, Krönke
M, Benneriah Y and Baeuerle PA: Rapid proteolysis of IκB-α is
necessary for activation of transcription factor NF-κB. Nature.
365:182–185. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gaestel M: MAPK-activated protein kinases
(MKs): Novel insights and challenges. Front Cell Dev Biol.
3:882016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Iliodromitis EK, Gaitanaki C, Lazou A,
Bofilis E, Karavolias GK, Beis I and Kremastinos DT: Dissociation
of stress-activated protein kinase (p38-MAPK and JNKs)
phosphorylation from the protective effect of preconditioning in
vivo. J Mol Cellular Cardiol. 34:1019–1028. 2002. View Article : Google Scholar
|
|
20
|
Wei Y, Xu M, Ren Y, Lu G, Xu Y, Song Y and
Ji H: The cardioprotection of dihydrotanshinone I against
myocardial ischemia-reperfusion injury via inhibition of
arachidonic acid ω-hydroxylase. Can J Physiol Pharmacol.
94:1267–1275. 2016. View Article : Google Scholar : PubMed/NCBI
|