Introduction
Gastric adenocarcinoma is major type of the gastric
cancer (1) and in most cases, they
are detected in advanced stages. Despite the development of various
treatments (e.g., gastrectomy, chemotherapy, radiation therapy)
gastric adenocarcinoma ranks second in deaths caused by cancer,
although the incidence rate ranks fourth (2). Several drugs are being used in
gastric cancer therapy including 5-fluorouracil (5-FU) or its
analog capecitabine, BCNU (carmustine) as well as methyl-CCNU
(semustine), doxorubicin (Adriamycin), mitomycin C, cisplatin and
Taxotere (3,4). Although chemotherapy has been used
for a long time, there is no clear standard of care and since
gastric cancers are not particularly sensitive to these drugs,
chemotherapy is mostly used to reduce the size of the tumor before
surgery or used as adjuvant therapy (5). Since the selectivity of the drugs is
low, treatments typically include systemic toxicity (3). To reduce these side effects, further
studies are needed for safer and efficient treatment for gastric
cancer (5–7).
Stem cells have recently become of great interest
for researchers with the possibility of clinical use in cancer
treatment. While traditional chemotherapy involves administration
of manufactured drugs, genetically engineered stem cells (GESTECs)
induces cells to produce the therapeutic agent (8,9).
This technique enables one to replace damaged genes or insert
additional genes with a new function. For example, human neural
stem cells (hNSCs) are one of the candidate stem cells showing
therapeutic potential and tumor tropism for the treatment of
malignant tumors in the human brain including medulloblastomas and
gliomas (10–12). This supports the possibility of
using hNSCs as a gene carrier to the tumor site as well as a
tumor-specific enzyme/pro-drug system with concomitant prodrug
administration (13). HB1.F3 is an
immortalized hNSC derived from human fetal brain at 15 weeks of
gestation by an amphotropic, replication-incompetent retroviral
vector v-myc (14,15). Clonal HB1.F3.CD cells were derived
from parental HB1.F3 cells transfected with an Escherichia
coli (E. coli) cytosine deaminase (CD) gene (14). Additionally, clonal HB1.F3.CD.IFN-β
cells were derived from parental HB1.F3.CD cells and their cells
express both E. coli CD and human interferon-β (IFN-β) genes
(8). This clonally isolated,
multi-potent hNSC has the ability to self-renew and to
differentiate into cells of neuronal and glial lineages both in
vivo and in vitro (14).
The CD/5-fluorocytosine (5-FC) system is a
gene-directed enzyme/pro-drugs therapy (GEPT) (16–20)
which converts the non-toxic prodrug 5-FC into the cytotoxic
metabolite, 5-FU (21,22). 5-FU inhibits DNA synthesis in cells
and results in cytotoxicity (23,24).
This CD/5-FC GEPT system has been tested experimentally against
several types of tumors including colorectal and prostate cancers
(25–27).
In this study, we investigated the synergistic
effect of IFN-β with the CD/5-FC GEPT system. The proinflammatory
cytokine, IFN-β demonstrated antitumor activity by suppressing
angiogenesis, tumor growth and metastasis (28,29).
The use of this pro-drug seems to be less toxic compared to using
active anticancer drugs, but there is a difficulty in delivering
the converting enzymes to the exact tumor site for selective
activity. To reduce the side effect of therapeutic drugs and
increase their effect, many researchers are focusing on
gene-targeting therapy that selectively works on cancer cells
(30,31). Therefore, we investigated whether
the synergistic effect of the two systems can increase the
efficiency of the treatment for gastric cancer.
Its therapeutic capacity in brain tumors as well as
its tumor-tropic properties and migratory abilities makes GESTECs a
potential candidate for invasive tumors (10–12,32).
By delivering genes to selective tumor cells, GESTECs expressing
fusion genes (i.e., CD and IFN-β) may have a synergic antitumor
effect on gastric cancer cells.
Materials and methods
Cell culture
AGS, a human gastric adenocarcinoma cancer cell was
originally derived from fragments of a tumor from a patient (Korean
Cell Line Bank, Seoul, Korea). The cells were cultured in RPMI (PAA
Laboratories GmbH, Linz, Austria) supplemented with 10% (v/v) fetal
bovine serum (FBS; Hyclone Laboratories, Inc., Logan, UT, USA), 1%
HEPES (Invitrogen Life Technologies, Carlsbad, CA, USA), 1%
penicillin/streptomycin (Cellgro Mediatech, Inc., Manassan, VA,
USA) and 0.1% antimycoplasmal plasmocin (Invivogen, San Diego, CA,
USA) at 37°C in a humidified atmosphere of 5% CO2-95%
air. HB1.F3, HB1.F3.CD, HB1.F3.CD.IFN-β (Chungang Universuty,
Seoul, Korea) and the bovine fibroblast (Bovine FB) cells (Chungbuk
National University, Cheongju, Korea) were cultured in DMEM
(Hyclone Laboratories, Inc.) supplemented with 10% FBS, 1%
penicillin G and streptomycin, 1% HEPES and 0.1% plasmocin at 37°C
in a humidified atmosphere of 5% CO2-95% air. Cells were
trypsinized with 0.05% trypsin/0.02% EDTA (PAA Laboratories) in
Mg2+/Ca2+-free HBSS.
Reverse-transcription polymerase chain
reaction (RT-PCR)
According to recent findings, the tumor tropism of
the hNSCs are mediated by several chemoattractants and interaction
with their specific receptors including stem cell factor
(SCF)/c-Kit (33), stromal
cell-derived factor 1 (SDF-1)/CXC chemokine receptor 4 (CXCR4)
(34) and vascular endothelial
growth factor (VEGF)/VEGF receptors VEGFR1 and VEGFR2 (32). The presence of these
chemoattractants and related receptors in AGS were detected by
RT-PCR.
Extraction of RNA was performed using the TRIzol
reagent (Invitrogen Life Technologies). Using random primers,
single-stranded cDNA was synthesized from 1 μg of total RNA by
M-MLV RT (iNtRON Biotechnology, Sungnam, Kyeonggido, Korea). The
prepared cDNA from this procedure was used in the following PCR
reactions performed with 0.2 μmol/l of each sense and antisense
primers, 2.5 units of Taq polymerase (iNtRON Biotechnology), 0.2
mmol/l deoxynucleotide mix (iNtRON Biotechnology) and 10X PCR
buffer (iNtRON Biotechnology). PCR for these chemoattractant
factors (ligands and receptors) and glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) as a positive control was carried out for 30
cycles using PTC-100 (MJ Research, Inc., Waltham, MA, USA). PCR
cycles were composed of a denaturation reaction at 95°C for 30 sec,
annealing reaction at 58°C for 30 sec and extension reaction at
72°C for 30 sec. The results were analyzed on a 1.5% agarose gel
containing ethidium bromide (EtBr). The sense and antisense primers
and the predicted sizes of the RT-PCR reaction products are shown
in Table I.
 | Table IThe oligonucleotide sequences of the
primers used in this study and the predicted sizes of the PCR
products. |
Table I
The oligonucleotide sequences of the
primers used in this study and the predicted sizes of the PCR
products.
| mRNA | | Oligo-sequences
(5′-3′) | Expected size
(bp) |
|---|
| CD | Forward |
GCGCGAGTCACCGCCAGCCACACCACGGC | 559 |
| Reverse |
GTTTGTATTCGATGGCTTCTGGCTGC | |
| SCF | Forward |
ACTTGGATTCTCACTTGCATTT | 505 |
| Reverse |
CTTTCTCAGGACTTAATGTTGAAG | |
| c-Kit | Forward |
GCCCACAATAGATTGGTATTT | 570 |
| Reverse |
AGCATCTTTACAGCGACAGTC | |
| CXCR4 | Forward |
CTCTCCAAAGGAAAGCGCAGGTGGACAT | 558 |
| Reverse |
AGACTGTACACTGTAGGTGCTGAAATCA | |
| IFN-β | Forward |
AAAGAAGCAGCAATTTTCAG | 296 |
| Reverse |
TTTCTCCAGTTTTTCTTCCA | |
| VEGF | Forward |
AAGCCATCCTGTGTGCCCCTGATG | 377 |
| Reverse |
GCTCCTTCCTCCTGCCCGGCTCAC | |
| VEGFR2 | Forward |
ACGCTGACATGTACGGTCTAT | 438 |
| Reverse |
GCCAAGCTTGTACCATGTGAG | |
| GAPDH | Forward |
ATGTTCGTCATGGGTGTGAACCA | 351 |
| Reverse |
TGGCAGGTTTTTCTAGACGGCAG | |
Cell growth assay
To investigate the effect of 5-FC and 5-FU in
gastric adenocarcinoma cells (4,000 cells/well), AGS were seeded in
96-well plates and cultured in 0.1 ml medium with 5% FBS. After a
24-h pre-incubation, HB1.F3, HB1.F3.CD, and HB1.F3.CD.IFN-β cells
were added to the cultures in medium containing 5% FBS and
incubated for 24 h before treatment with 5-FC or 5-FU. On the day
of treatment, 5-FC and 5-FU (Sigma-Aldrich Corp., St. Louis, MO,
USA) were serially diluted with phosphate-buffered saline (PBS;
final concentration 100, 200, 300, 400 and 500 μg/ml) and the cells
were treated for 4 days. An MTT
[3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide]
assay was performed to measure cell viability on Day 7. MTT
solution (10 μl of stock at 5 mg/ml) was added to each well in the
plates and incubated for 4 h at 37°C. Supernatants were removed and
100 μl of dimethyl sulfoxide (DMSO, 99.0%; Junsei Chemical Co.,
Ltd., Tokyo, Japan) was added to each well to dissolve the
resultant formazan crystals. Optical densities were measured at 540
nm using an ELISA reader (VersaMan, Molecular Devices, CA, USA). An
MTT assay was carried out in duplicate.
To investigate the difference of cell growth and the
changes in the ratio of cancer cells to GESTECs, AGS (4,000
cells/well) were seeded in 96-well plates and cultured in 0.1 ml
medium with 5% FBS. After a 24-h pre-incubation, HB1.F3, HB1.F3.CD
or and HB1.F3.CD.IFN-β cells were added to the cultures in medium
containing 5% FBS separately at 8.0×103,
1.6×104 and 2.4×104 cells/well and incubated
for 24 h before treatment with 5-FC (Sigma-Aldrich Corp.). On the
day of treatment, cells were treated with 5-FC (final concentration
500 μg/ml) for 4 days. MTT assay was performed to measure cell
viability on Day 7. MTT solution (10 μl) was added to each well in
the plates and they were incubated for 4 h at 37°C. Supernatants
were removed and 100 μl of DMSO (Junsei Chemical Co., Ltd.) was
added to each well to dissolve the resultant formazan crystals.
Optical densities were measured at 540 nm using an ELISA reader
(VersaMan, Molecular Devices). The MTT assay was carried out in
duplicate.
In vitro migration assay
To investigate whether GESTECs are capable of
migrating to gastric cancer cells, AGS and bovine FB
(1×105 cells/well) were plated in 24-well plates and
incubated in RPMI and DMEM contained 10% FBS for 6 h at 37°C,
respectively. The cells were then incubated with new serum-free
media and incubated for 24 h. Transwell plates (8 μm; BD
Biosciences, Franklin Lakes, NJ, USA) coated with fibronectin (250
μg/ml; Sigma-Aldrich Corp.) were placed in the 24-well plates and
incubated overnight. Using a general protocol, 2 μM of
chloromethylbenzamido-1,1′-dioctadecyl-3,3,3′-tetramethyl-indocarbocyanine
perchlorate (CM-DiI; Invitrogen Life Technologies) was used to
label the HB1.F3, HB1.F3.CD or HB1.F3.CD.IFN-β cells
(1×105 cells/well) that were plated in the upper
chambers of the transwell plates and cultured in serum-free medium
for 24 h at 37°C. The next day, AGS and bovine FB were stained by
addition of a 200-ng/ml 4′,6-diamidino-2-phenylindole solution
(DAPI; Invitrogen, Lift Technologies) and the plate was incubated
for 10 min at 37°C. Each well was washed with PBS and the upper
side of the transwell membrane was then scraped to remove cells
that had not migrated into the membrane. Cells stained with CM-DiI
and DAPI were examined by fluorescence microscopy (IX71 Inverted
Microscope, Olympus, Japan).
Statistical analysis
The results of all cell growth assay experiments are
presented as means ± SD. One-way ANOVA was performed and a
P<0.05 was considered statistically significant.
Results
Confirmation of CD and IFN-β gene
expression in GESTECs
The expression of CD and IFN-β genes in HB1.F3,
HB1.F3.CD and HB1.F3.CD.IFN-β cells were confirmed by RT-PCR. mRNA
of the CD gene (559 bp) was confirmed in both HB1.F3.CD and
HB1.F3.CD.IFN-β cells demonstrating CD gene expression in HB1.F3.CD
and HB1.F3.CD.IFN-β cells (Fig.
1). In addition, the IFN-β gene (296 bp) was expressed in
HB1.F3.CD.IFN-β cells, but not in HB1.F3 and HB1.F3.CD cells
(Fig. 1). GAPDH was used as
positive control and found based on the presence of its 351 bp
cDNA. Results of RT-PCR were confirmed by 1.5% agarose gel
electrophoresis.
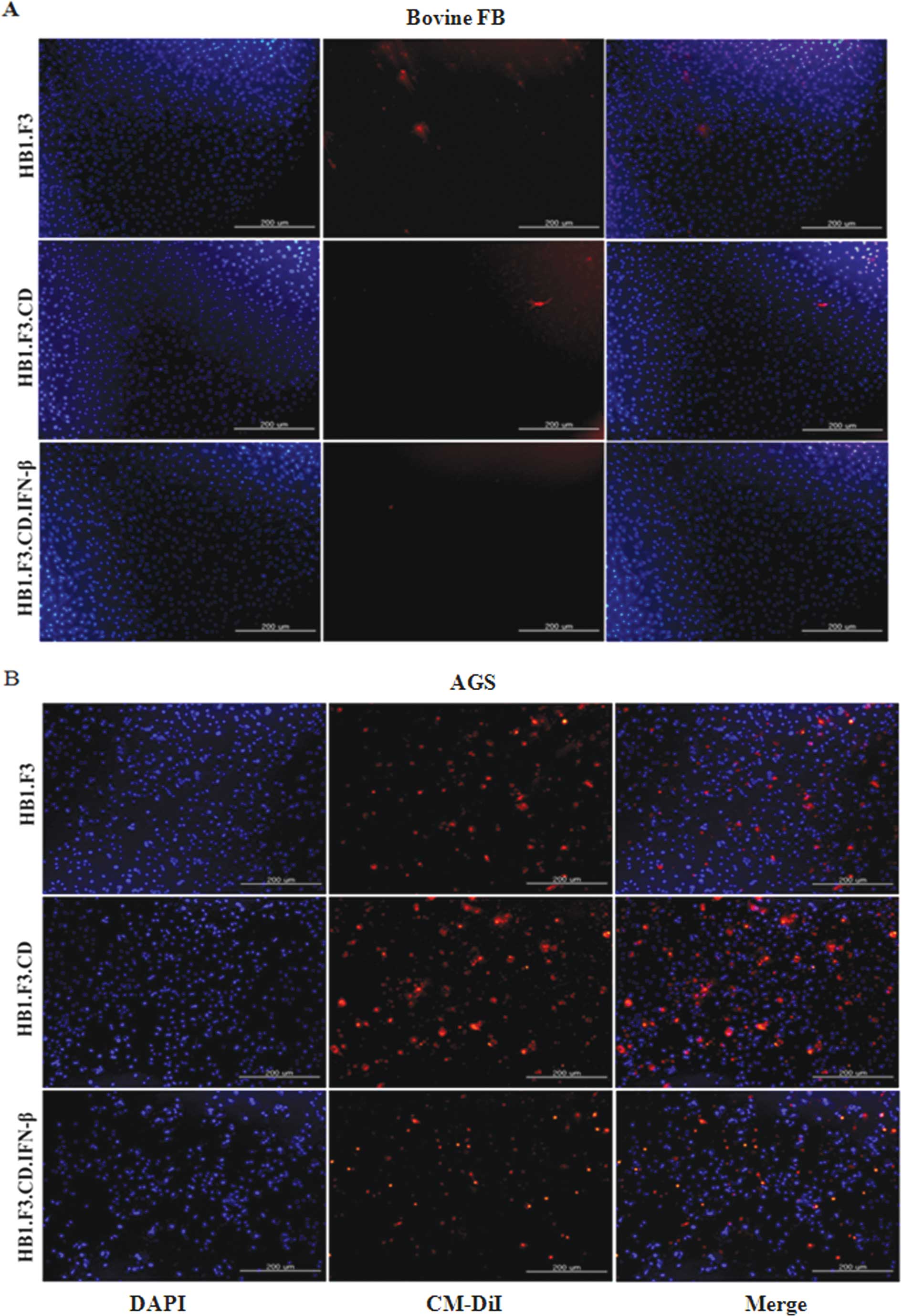
In vitro cell migration assay
To verify the migration capability of GESTECs toward
the AGS, a modified transwell migration assay was performed. Using
fluorescence microscopy, changes in CM-DiI stained hNSCs, HB1.F3,
HB1.F3.CD and HB1.F3.CD.IFN-β cells was performed. Compared with
DAPI-stained bovine FB as a control, AGS significantly increased
cell migration of the GESTECs (Fig.
2).
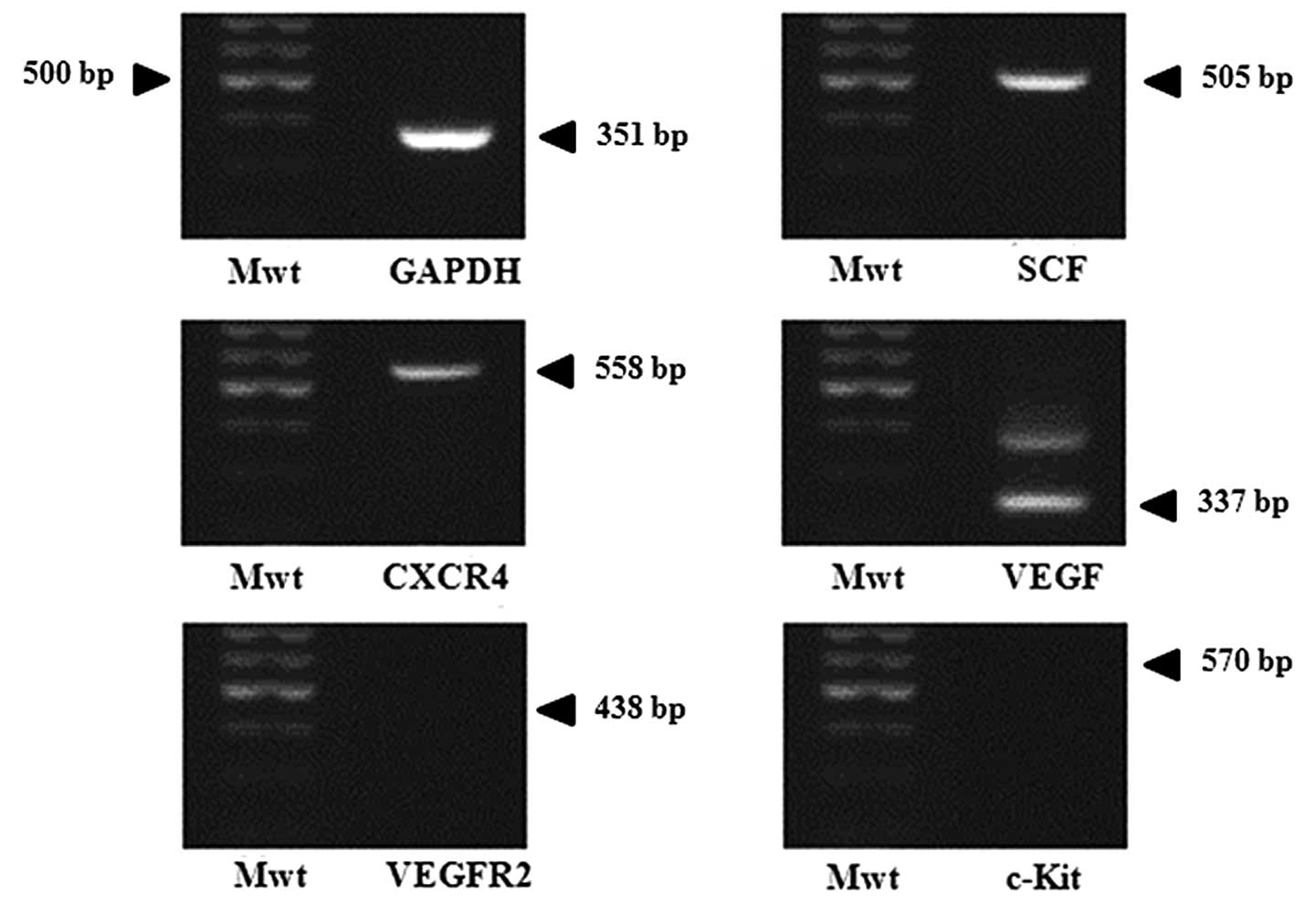
Confirmation of chemoattractant ligands
and receptors
To examine whether gastric cancer cells express
chemoattractant factors, RT-PCR for several chemoattractant ligands
and their related receptors were done in AGS. Results in Fig. 3 show the expression of SCF, CXCR4
and VEGF genes, but c-Kit and VEGFR2 were not expressed. According
to these findings, it can be assumed that AGS produces
chemoattractant molecules and related receptors which induce
migration of GESTECs.
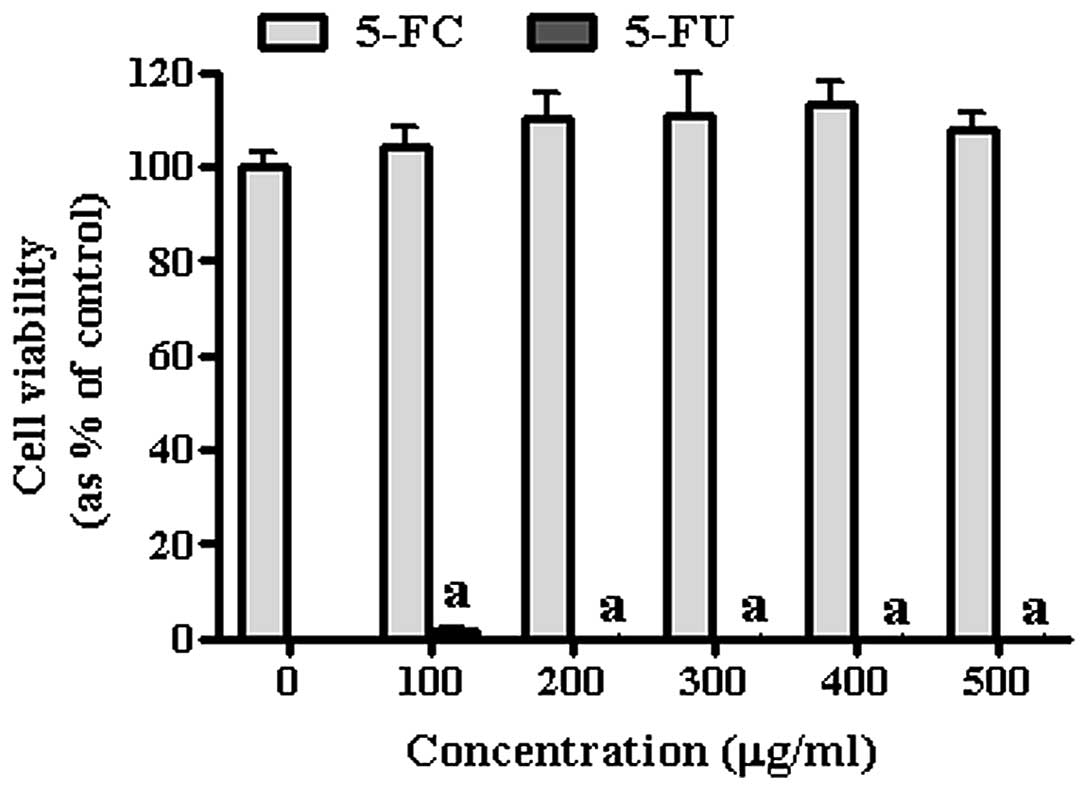
Effect of 5-FC/5-FU on gastric cancer
cells and GESTECs
To confirm the anticancer effect of HB1.F3,
HB1.F3.CD and HB1.F3.CD.IFN-β cells, cell viability assay was
conducted using a co-culture system and confirmed by MTT assay.
Prior to the co-culture experiment with of GESTECs, the effect of
the prodrug 5-FC and its active metabolite 5-FU on AGS are shown in
Fig. 4. According to these
results, 5-FC did not appear to effect the growth of the gastric
cancer cells. On the other hand, the growth inhibition effect of
5-FU was significant indicating AGS is highly sensitive to 5-FU,
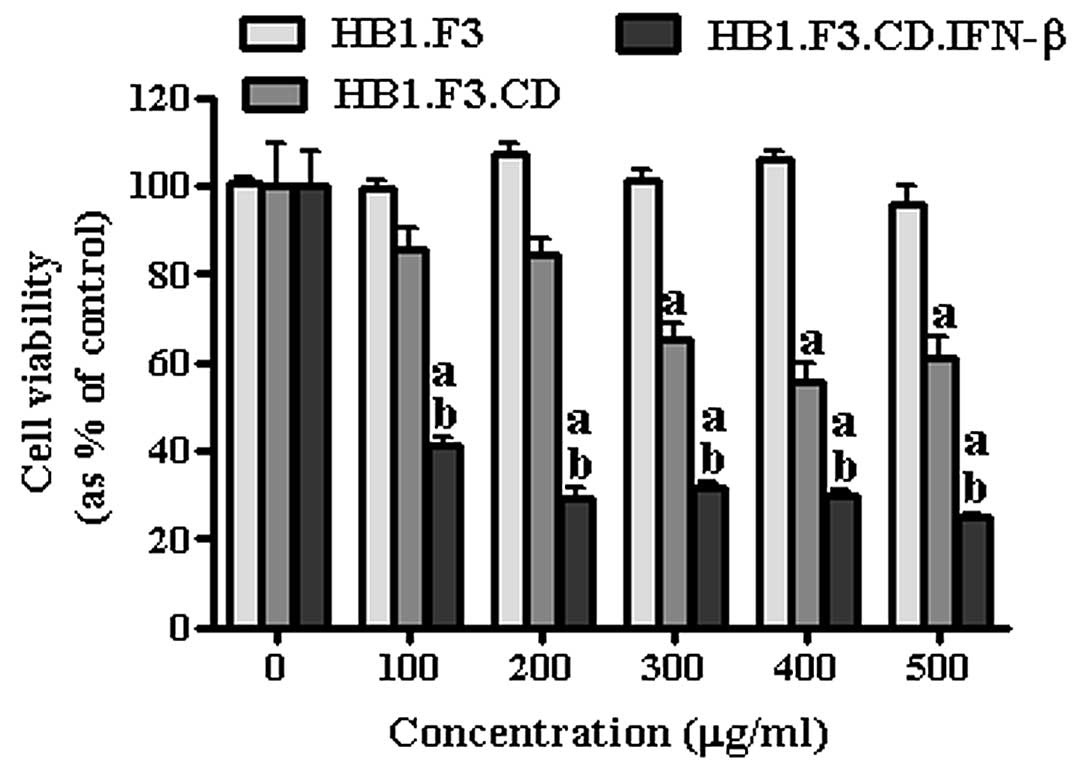
even at low concentration (100 μg/ml) (Fig. 4). To specifically determine the
prodrug conversion efficiency of GESTECs, AGS were co-cultured with
each stem cell treated by 5-FC at different concentrations (100,
200, 300, 400 and 500 μg/ml) (Fig.
5) and cell viability was measured. HB1.F3 cells, the
non-modified control NSC appeared not to inhibit cell growth at any
concentration, while HB1.F3.CD cells started to inhibit cancer cell
growth with 5-FC treatment reached 300 μg/ml. Impressively,
HB1.F3.CD.IFN-β cells showed significant inhibition at the lowest
5-FC concentration (100 μg/ml). In the presence of the GESTECs,
treatment of the 5-FC prodrug dose-dependently inhibited cancer
cell growth in HB1.F3.CD and HB1.F3.CD.IFN-β cells.
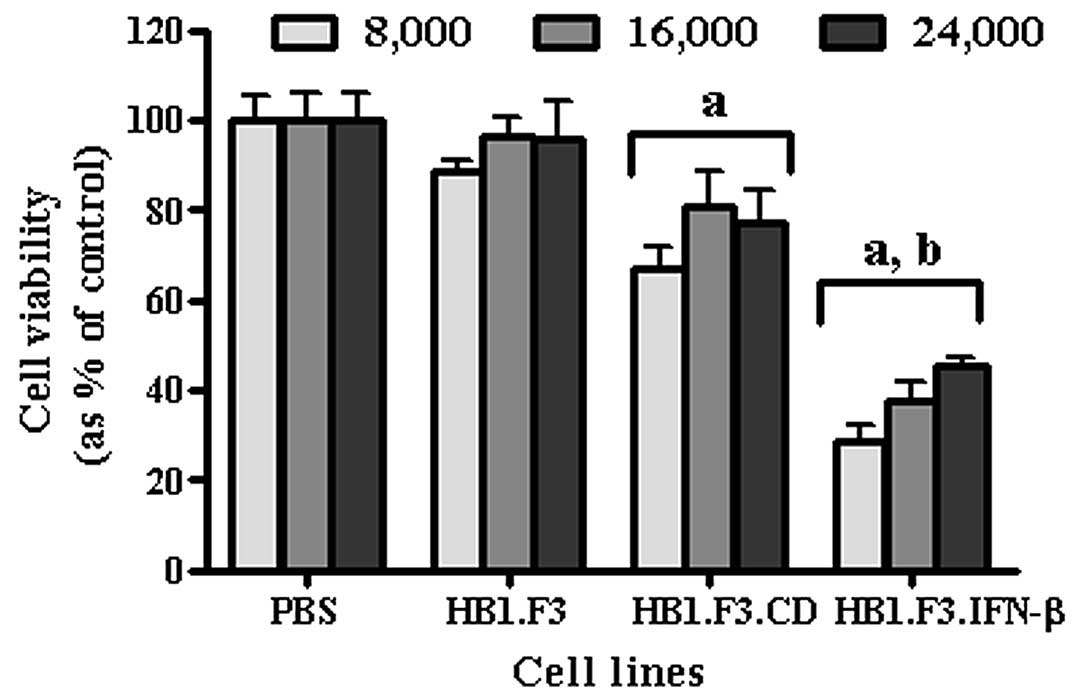
Furthermore, to verify whether the number of the
stem cells affected the intensity of anticancer effect in gastric
cancer cells, AGS (4.0×103 cells/well) were treated with
5-FC after co-culturing with different amounts of HB1.F3, HB1.F3.CD
and HB1.F3.CD.IFN-β cells (8.0×103, 1.6×104
and 2.4×104 cells/well) (Fig. 6). After 5-FC treatment, cell
viability was decreased in cells cultured with HB1.F3.CD and
HB1.F3.CD.IFN-β cells. Consistent with previous experiments, cancer
cell viability was significantly decreased when CD and IFN-β genes
were expressed together (HB1.F3.CD.IFN-β).
Discussion
This study is based on the theory that immortalized
GESTECs have potential for gene therapy and cell replacement
enabling treatment of neural disease and damage (14,35–40).
Among several GESTECs, the NSCs are able to migrate to brain tumor
sites and affect tumor growth both in vitro and in
vivo (11,12). In previous studies, using animal
models, it was shown that when tumor cells were treated with
HB1.F3.CD cells expressing the E. coli CD gene and systemic
5-FC administration together, the size of tumor cells were reduced
(30,31). At the same time when the tumor was
treated only with HB1.F3.CD cells or 5-FC separately, there was no
tumor cytotoxicity (12).
Additionally, a recent study confirmed that human IFN-β expressing
GESTEC, that is HB1.F3.CD.IFN-β cells, showed an anticancer effect
compared to HB1.F3 cells (41).
The therapeutic capability of CD gene/5-FC modified
GEPT system has been tested in several types of tumors including
breast, prostate and colon (20,25,42).
The anticancer application of GESTECs is not well investigated in
many other cancer cells. Therefore, in this study, we investigated
the effect of CD/CD plus IFN-β gene-expressing GESTECs in gastric
cancer cells.
First, we tested the direct cytotoxicity of the CD
gene/5-FC modified GEPT system with human IFN-β-expressing GESTECs.
5-FU, an inhibitor of thymidylate synthetase (43), has been used to treat cancer for
several decades; it causes side effects when administered
systemically which include myelosuppression and stomatitis which
develop serious complications (3,4).
Therefore, to reduce this unwanted effect, the non-toxic prodrug
converting E. coli CD system has recently received attention
from researchers. The CD enzyme, translated from the CD gene
converts non-toxic 5-FC into the cytotoxic 5-FU which inhibits cell
growth selectively in the site where the gene is expressed
(44). In our study, 5-FC-treated
HB1.F3.CD cells increased in number when co-cultured with AGS
indicating the use of this CD/5-FC GEPT system is possible after
the injection of stem cells.
According to earlier reports, it was shown that a
small number of CD-transfected cells can induce antitumor effects
through a bystander effect (45),
thus, we investigated whether the number of the GESTECs induce
affected gastric cancer cells differently. When increasing number
of the three stem cell lines (8.0×103,
1.6×104 and 2.4×104 cells/well) were cultured
with AGS and equally treated with 5-FC at 500 μg/ml, HB1.F3.CD
cells expressing the CD gene and HB1.F3.CD.IFN-β cells expressing
both the CD and IFN-β fusion genes appeared to show maximum cancer
cell growth inhibition starting at a 1:2 ratio of stem cells:AGS,
results with higher stem cells number showed similar inhibition
effects.
To examine if these gene expressing GESTECs are able
to migrate to gastric cancer cells, we performed a modified
transwell migration assay. Compared to bovine FB (i.e., control
cells), the migration of cells increased in AGS, indicating that
gastric cancer cells tend to secrete chemoattractant factors and
GESTECs respond to them. In addition, this migrating capability of
the parental HB1.F3 cells, was also shown in previous studies using
melanoma, glioma, neuroblastoma prostate and breast tumors
(11), indicating this cells line
possesses a tendency to migrate towards variable types of cancer
which can be an advantage for use as antitumor treatment.
Modified migration assay results made it possible to
assume that gastric cancer cells might produce chemoattractant
factors which induce the migration of HB1.F3.CD and HB1.F3.CD.IFN-β
cells to cancer cells, resulting in the delivery of therapeutic
genes to the tumor site. Several factors such as SCF, VEGF are
known to play a chemoattractive role in tumor cells (10,12,14–27,46–49),
but the details in gastric cancer cells are not clearly known.
Thus, we assayed for chemoattractant ligands and receptors in AGS
and found that SCF, CXCR4 and VEGF genes were expressed. Therefore,
these genes may be related in tumor tropism of GESTECs that
selectively deliver the suicide enzyme and anticancer cytokine
genes to the gastric cancer site. Further study is required to
confirm the role of these genes in the mechanism underlying tumor
cell recognition and/or tumor tropism by GESTECs.
As explained previously, we studied whether the CD
and IFN-β fusion genes can maximize the antitumor effect. Since the
mechanism of action of the two genes is different, a possible
synergistic effect with the fusion gene was likely. CD acts as a
pro-drug-activating enzyme (12)
and IFN-β can enhance anti-angiogenic effects and immune responses
(31,50). Results from this study showed that
HB1.F3.CD.IFN-β cells have significantly powerful antitumor effect
compared to HB1.F3.CD cells.
In conclusion, this study showed that the CD
gene/5-FC modified GEPT system with the human IFN-β GEPT system
resulted in marked growth inhibition in gastric cancer cells. In
addition, GESTECs expressing CD or CD with IFN-β genes may
selectively migrate toward gastric cancer cells. Therefore, it is
possible to consider that GESTECs expressing suicide genes with an
application of pro-drugs may have therapeutic potential for
treating gastric cancer, and that GESTECs expressing the CD and
IFN-β fusion gene has a synergic antitumor effect compared to
GESTECs expressing CD alone.
Acknowledgements
This study was supported by the National Research
Foundation of Korea (NRF) grant (no. 2011-0015385) funded by the
Korea government (MEST). In addition, this work was also supported
by Priority Research Centers Program through the NRF funded by the
Ministry of Education, Science and Technology (2009-0094035).
References
|
1
|
Isik M, Caner S, Metin Seker M, et al:
Gastric adenocarcinoma under the age of 40; more metastatic, less
differentiated. J BUON. 16:253–256. 2011.PubMed/NCBI
|
|
2
|
Blum M, Suzuki A and Ajani JA: A
comprehensive review of S-1 in the treatment of advanced gastric
adenocarcinoma. Future Oncol. 7:715–726. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fidan E, Fidan S, Yildiz B, et al: Bolus
fluorouracil induced syncope and pulseless ventricular tachycardia:
a case report. Hippokratia. 15:93–95. 2011.PubMed/NCBI
|
|
4
|
Longley DB, Harkin DP and Johnston PG:
5-Fluorouracil: mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luo XR, Li JS, Niu Y and Miao L: Targeted
killing effects of double CD and TK suicide genes controlled by
survivin promoter on gastric cancer cell. Mol Biol Rep.
38:1201–1207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anderson LM, Krotz S, Weitzman SA and
Thimmapaya B: Breast cancer-specific expression of the Candida
albicans cytosine deaminase gene using a transcriptional
targeting approach. Cancer Gene Ther. 7:845–852. 2000.PubMed/NCBI
|
|
7
|
Joo KM, Park IH, Shin JY, et al: Human
neural stem cells can target and deliver therapeutic genes to
breast cancer brain metastases. Mol Ther. 17:570–575. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Studeny M, Marini FC, Champlin RE,
Zompetta C, Fidler IJ and Andreeff M: Bone marrow-derived
mesenchymal stem cells as vehicles for interferon-β delivery into
tumors. Cancer Res. 62:3603–3608. 2002.
|
|
9
|
Zhang JF, Wei F, Wang HP, et al: Potent
anti-tumor activity of telomerase-dependent and HSV-TK armed
oncolytic adenovirus for non-small cell lung cancer in vitro and in
vivo. J Exp Clin Cancer Res. 29:522010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aboody KS, Brown A, Rainov NG, et al:
Neural stem cells display extensive tropism for pathology in adult
brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA.
97:12846–12851. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aboody KS, Bush RA, Garcia E, et al:
Development of a tumor-selective approach to treat metastatic
cancer. PLoS One. 1:e232006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim SK, Kim SU, Park IH, et al: Human
neural stem cells target experimental intracranial medulloblastoma
and deliver a therapeutic gene leading to tumor regression. Clin
Cancer Res. 12:5550–5556. 2006. View Article : Google Scholar
|
|
13
|
Kim KY, Kim SU, Leung PC, Jeung EB and
Choi KC: Influence of the prodrugs 5-fluorocytosine and CPT-11 on
ovarian cancer cells using genetically engineered stem cells:
tumor-tropic potential and inhibition of ovarian cancer cell
growth. Cancer Sci. 101:955–962. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim SU: Human neural stem cells
genetically modified for brain repair in neurological disorders.
Neuropathology. 24:159–171. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim SU, Nakagawa E, Hatori K, Nagai A, Lee
MA and Bang JH: Production of immortalized human neural crest stem
cells. Methods Mol Biol. 198:55–65. 2002.PubMed/NCBI
|
|
16
|
Evoy D, Hirschowitz EA, Naama HA, et al:
In vivo adenoviral-mediated gene transfer in the treatment of
pancreatic cancer. J Surg Res. 69:226–231. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hirschowitz EA, Ohwada A, Pascal WR, Russi
TJ and Crystal RG: In vivo adenovirus-mediated gene transfer of the
Escherichia coli cytosine deaminase gene to human colon
carcinoma-derived tumors induces chemosensitivity to
5-fluorocytosine. Hum Gene Ther. 6:1055–1063. 1995.PubMed/NCBI
|
|
18
|
Kanai F, Lan KH, Shiratori Y, et al: In
vivo gene therapy for α-fetoprotein-producing hepatocellular
carcinoma by adenovirus-mediated transfer of cytosine deaminase
gene. Cancer Res. 57:461–465. 1997.
|
|
19
|
Lan KH, Kanai F, Shiratori Y, et al:
Tumor-specific gene expression in carcinoembryonic antigen -
producing gastric cancer cells using adenovirus vectors.
Gastroenterology. 111:1241–1251. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Z, Shanmugam N, Katayose D, et al:
Enzyme/prodrug gene therapy approach for breast cancer using a
recombinant adenovirus expressing Escherichia coli cytosine
deaminase. Cancer Gene Ther. 4:113–117. 1997.PubMed/NCBI
|
|
21
|
Austin EA and Huber BE: A first step in
the development of gene therapy for colorectal carcinoma: cloning,
sequencing, and expression of Escherichia coli cytosine
deaminase. Mol Pharmacol. 43:380–387. 1993.PubMed/NCBI
|
|
22
|
Mullen CA, Kilstrup M and Blaese RM:
Transfer of the bacterial gene for cytosine deaminase to mammalian
cells confers lethal sensitivity to 5-fluorocytosine: a negative
selection system. Proc Natl Acad Sci USA. 89:33–37. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Etienne MC, Cheradame S, Fischel JL, et
al: Response to fluorouracil therapy in cancer patients: the role
of tumoral dihydropyrimidine dehydrogenase activity. J Clin Oncol.
13:1663–1670. 1995.PubMed/NCBI
|
|
24
|
Pinedo HM and Peters GF: Fluorouracil:
biochemistry and pharmacology. J Clin Oncol. 6:1653–1664.
1988.PubMed/NCBI
|
|
25
|
Chung-Faye GA, Chen MJ, Green NK, et al:
In vivo gene therapy for colon cancer using adenovirus-mediated,
transfer of the fusion gene cytosine deaminase and uracil
phosphoribosyltransferase. Gene Ther. 8:1547–1554. 2001. View Article : Google Scholar
|
|
26
|
Crystal RG, Hirschowitz E, Lieberman M, et
al: Phase I study of direct administration of a replication
deficient adenovirus vector containing the E. coli cytosine
deaminase gene to metastatic colon carcinoma of the liver in
association with the oral administration of the pro-drug
5-fluorocytosine. Hum Gene Ther. 8:985–1001. 1997.PubMed/NCBI
|
|
27
|
Freytag SO, Khil M, Stricker H, et al:
Phase I study of replication-competent adenovirus-mediated double
suicide gene therapy for the treatment of locally recurrent
prostate cancer. Cancer Res. 62:4968–4976. 2002.
|
|
28
|
Dong Z, Greene G, Pettaway C, et al:
Suppression of angiogenesis, tumorigenicity, and metastasis by
human prostate cancer cells engineered to produce interferon-β.
Cancer Res. 59:872–879. 1999.PubMed/NCBI
|
|
29
|
Rossiello F, De Cicco Nardone F and
Dell’Acqua S: Interferon-β increases the sensitivity of endometrial
cancer cells to cell-mediated cytotoxicity. Gynecol Oncol.
54:130–136. 1994.
|
|
30
|
Yi BR, Hwang KA, Kang NH, Kim SU, Jeung EB
and Choi KC: Antitumor therapeutic effects of cytosine deaminase
and interferon-β against endometrial cancer cells using genetically
engineered stem cells in vitro. Anticancer Res. 31:2853–2862.
2011.
|
|
31
|
Yi BR, O SN, Kang NH, et al: Genetically
engineered stem cells expressing cytosine deaminase and
interferon-β migrate to human lung cancer cells and have
potentially therapeutic anti-tumor effects. Int J Oncol.
39:833–839. 2011.
|
|
32
|
Schmidt NO, Przylecki W, Yang W, et al:
Brain tumor tropism of transplanted human neural stem cells is
induced by vascular endothelial growth factor. Neoplasia.
7:623–629. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun L, Lee J and Fine HA: Neuronally
expressed stem cell factor induces neural stem cell migration to
areas of brain injury. J Clin Invest. 113:1364–1374. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ehtesham M, Yuan X, Kabos P, et al: Glioma
tropic neural stem cells consist of astrocytic precursors and their
migratory capacity is mediated by CXCR4. Neoplasia. 6:287–293.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jeong SW, Chu K, Jung KH, Kim SU, Kim M
and Roh JK: Human neural stem cell transplantation promotes
functional recovery in rats with experimental intracerebral
hemorrhage. Stroke. 34:2258–2263. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim SU, Park IH, Kim TH, et al: Brain
transplantation of human neural stem cells transduced with tyrosine
hydroxylase and GTP cyclohydrolase 1 provides functional
improvement in animal models of Parkinson disease. Neuropathology.
26:129–140. 2006. View Article : Google Scholar
|
|
37
|
Meng XL, Shen JS, Ohashi T, Maeda H, Kim
SU and Eto Y: Brain transplantation of genetically engineered human
neural stem cells globally corrects brain lesions in the
mucopolysaccharidosis type VII mouse. J Neurosci Res. 74:266–277.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rosser AE, Zietlow R and Dunnett SB: Stem
cell transplantation for neurodegenerative diseases. Curr Opin
Neurol. 20:688–692. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ryu JK, Kim J, Cho SJ, et al: Proactive
transplantation of human neural stem cells prevents degeneration of
striatal neurons in a rat model of Huntington disease. Neurobiol
Dis. 16:68–77. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee ST, Chu K, Park JE, et al: Intravenous
administration of human neural stem cells induces functional
recovery in Huntington’s disease rat model. Neurosci Res.
52:243–249. 2005.PubMed/NCBI
|
|
41
|
Lee DH, Ahn Y, Kim SU, et al: Targeting
rat brainstem glioma using human neural stem cells and human
mesenchymal stem cells. Clin Cancer Res. 15:4925–4934. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Boucher PD, Im MM, Freytag SO and Shewach
DS: A novel mechanism of synergistic cytotoxicity with
5-fluorocytosine and ganciclovir in double suicide gene therapy.
Cancer Res. 66:3230–3237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hartmann KU and Heidelberger C: Studies on
fluorinated pyrimidines. XIII. Inhibition of thymidylate
synthetase. J Biol Chem. 236:3006–3013. 1961.PubMed/NCBI
|
|
44
|
Wei J, Wahl J, Knauss H, et al: Cytosine
deaminase/5-fluorocytosine gene therapy and Apo2L/TRAIL cooperate
to kill TRAIL-resistant tumor cells. Cancer Gene Ther. 14:640–651.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Huber BE, Austin EA, Richards CA, Davis ST
and Good SS: Metabolism of 5-fluorocytosine to 5-fluorouracil in
human colorectal tumor cells transduced with the cytosine deaminase
gene: significant antitumor effects when only a small percentage of
tumor cells express cytosine deaminase. Proc Natl Acad Sci USA.
91:8302–8306. 1994. View Article : Google Scholar
|
|
46
|
Saukkonen K and Hemminki A:
Tissue-specific promoters for cancer gene therapy. Expert Opin Biol
Ther. 4:683–696. 2004. View Article : Google Scholar
|
|
47
|
Tubiana M: Tumor cell proliferation
kinetics and tumor growth rate. Acta Oncol. 28:113–121. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Beppu K, Jaboine J, Merchant MS, Mackall
CL and Thiele CJ: Effect of imatinib mesylate on neuroblastoma
tumorigenesis and vascular endothelial growth factor expression. J
Natl Cancer Inst. 96:46–55. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sun L, Hui AM, Su Q, et al: Neuronal and
glioma-derived stem cell factor induces angiogenesis within the
brain. Cancer Cell. 9:287–300. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nakamizo A, Marini F, Amano T, et al:
Human bone marrow-derived mesenchymal stem cells in the treatment
of gliomas. Cancer Res. 65:3307–3318. 2005.PubMed/NCBI
|