Introduction
Glioblastoma multiforme (GBM) is one of the most
malignant and aggressive tumors and has a very poor prognosis with
a mean survival time of <2 years even with the recent
development of temozolomide-based intensive treatment (1,2).
Therefore, a new therapeutic approach is urgently needed to control
recurrence and overcome resistance to treatment in glioblastoma
patients.
GBMs are composed of many types of cells expressing
astrocytic and neuronal lineage markers and generated from
multipotent stem cells. Recently, GBM stem-like cells (SC) were
successfully isolated from human resected tumors using serum-free
medium containing epidermal growth factor (EGF) and basic
fibroblast growth factor (bFGF) (3-5).
These cells share the properties of normal stem cells like
self-renewal and multi-lineage differentiation. The definition
according to some research groups is: i) the capability for
self-renewal; ii) multi-lineage differentiation; and iii) the
ability to regenerate GMB tumors histologically similar to the
original tumors in xenografts (6,7).
Based on these observations, it is worth attempting to develop
therapeutic agents for GMB-SC that affect cell proliferation and
resistance to chemo-radiation.
The activation of several signaling pathways
including receptor tyrosine kinase (8), Akt (9), MAPK (10), Wnt (11) and Notch and Hedgehog (12) pathways, is involved in the
progression of GBM. Importantly, constitutive activation of the
Janus kinase (JAK)/signal transducer and activator of transcription
(STAT) pathway contributes to the tumor progression by promoting
cell proliferation and the inhibition of apoptosis. The STAT
protein family is a group of transcription factors that play an
important role in relaying signals from growth factors and
cytokines (13). STAT3 is reported
to be involved in oncogenesis by upregulating the transcription of
several genes that control tumor cell survival, resistance to
apoptosis, cell cycle progression and angiogenesis. Targets of
STAT3 include Bcl-2, Bcl-xL, c-myc, cyclin D1, vascular endothelial
growth factor (VEGF) and human telomerase reverse
transcriptase.
The association between STAT3 signaling and GBM-SC
development has been investigated rigorously (14,15).
Sherry et al reported that genetic knockdown of stat3 using
short hairpin RNA inhibited proliferation and the formation of
neurospheres by GBM-SC, indicating that STAT3 can regulate the
growth and self-renewal of GBM-SC (14).
Considering STATs are a good target for cancer stem
cell therapy, several therapeutic agents including small molecules
have been demonstrated to show antitumor effects through the
regulation of GBM-SC. Previously, we identified a novel inhibitor
of STAT3 dimerization, STX-0119, which exhibited a potent antitumor
effect on a human lymphoma cell line with a highly activated STAT3
(16). In the present study, we
found that STX-0119 inhibited cell proliferation and the formation
of spheres in GBM-SC lines derived from human GBM tumors by
regulating STAT3 target genes and inducing apoptosis and suppressed
the growth of transplanted tumors of GBM-SC.
Materials and methods
Establishment of primary GB-stem cell
lines from GBM patients
GBM tumor samples were obtained from surgically
resected materials. The clinical research using tumor tissues from
GBM patients was approved by the Institutional Review Board of
Shizuoka Cancer Center, Shizuoka, Japan. All patients gave written
informed consent.
Tumors were dissociated by teasing with forceps to
make a single cell suspension and plated in 25-cm2
culture flasks in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma,
St. Louis, MO) supplemented with 10% fetal bovine serum (FBS,
Invitrogen), penicillin and streptomycin and gentamicin
(Invitrogen) for the serum-derived GB cell line. For GBM-SC
cultures, dissociated cells were plated in 6-well ultra-low
attachment plates (Corning Inc., Corning, NY) in serum-free DMEM
supplemented with EGF (Invitrogen) at 20 ng/ml, bFGF (Peprotech,
Rocky Hill, NJ) at 20 ng/ml, leukemia inhibitory factor (LIF,
Alomone Labs Ltd., Jerusalem, Israel) at 10 ng/ml and B27
(Invitrogen), referred to herein as the stem cell medium (SCM). The
cultures were passaged weekly after neurospheres formed.
U87 glioblastoma cell line was purchased from
American Type Culture Collection (ATCC, Manassas, VA) and
maintained in DMEM containing 10% FBS. The U87 stem cells were
cultured like the GBM-SC lines and after 20 passages in SCM were
used for experiments in vitro.
Among the GBM-SC lines, GB-SCC026 was used for
quantitative PCR and apoptosis assay after a 24-h exposure to
STX-0119, while GB-SCC010 and 026 cells were utilized in animal
experiments.
Antibodies and reagents
Antibodies against STAT3, phospho-specific STAT3
(Tyr705), cleaved caspase-3 and β-actin were purchased from Cell
Signaling Technology, Inc. (Danvers, MA) and Becton-Dickinson (BD)
Biosciences (Franklin Lakes, NJ) for western blotting (WB).
PE-labeled anti-CD133 antibody and FITC-labeled anti-CD44 antibody
were purchased from BD Biosciences and used for flow cytometry.
Chemicals
STX-0119 and the JAK-specific inhibitor WP1066 were
supplied by The Center for Drug Discovery, University of Shizuoka
(Shizuoka, Japan). These compounds were suspended and diluted in a
sterile 0.5% w/v methyl cellulose 400-cp solution (Wako, Tokyo,
Japan) or dissolved in a mixture of 20% dimethyl sulfoxide (DMSO)
(Wako) and 80% polyethylene glycol 300 (Wako) for use in animal
experiments.
Flow cytometry
The primary GBM-SC lines or U87 stem cells were
stained with the PE-labeled anti-CD133 antibody and FITC-labeled
anti-CD44 antibody or isotype control antibodies at a concentration
of 10 μg/ml. Stained cells were fixed with 0.5%
paraformaldehyde (Sigma-Aldrich) and analyzed on a flow cytometer
(FACSCalibur, BD).
Cell proliferation assay
Cell proliferation was examined using the WST-1
assay (Dojin Kagaku Corp., Japan) described previously (16). Briefly,
5×103–1×104 GBM-SC or U87 stem cells were
seeded into each well of a 96-well micro-culture plate (Corning,
NY) and compounds diluted with SCM (100-0.25 μM) were added.
After 4 days, the WST-1 substrate was added to the culture and
optical density (OD) was measured at 450 and 620 nm using an
immunoreader (Immuno Mini NJ-2300, Nalge Nunc International,
Roskilde, Denmark). The IC50 value was defined as the
dose needed for a 50% reduction in OD calculated from the survival
curve. Percent survival was calculated as follows: (mean OD of test
wells - mean OD of background wells) / (mean OD of control wells -
mean OD of background wells).
Sphere formation assay
GBM-SC were seeded in a 96-well micro-culture plate
at 500 per well and compounds diluted with SCM (100-0.25 μM)
were added. After a 7-day incubation at 37°C in a humidified 5%
CO2 atmosphere, the number of spheres with a diameter of
>50 μm was counted under a microscope.
Quantitative polymerase chain reaction
(qPCR) analysis
The real-time PCR analysis of stem cell and neuronal
markers and STAT3 target genes using the 7500 Real-Time PCR system
(Applied Biosystems, Foster, CA) was performed as described
previously. Briefly, all PCR primers (ABCB1, ALDH1A1, CD44, EGFR,
ESA, GFAP, KLF4, NANOG, NES, OLIG2, Oct3/4, CD133, SOX2, TGFBR2,
TUBB3, VIM for GB-SC markers; BCL2, Bcl-Xl, Survivin, Cyclin D1,
c-Myc, CXCL10, VEGFR2, MMP9, TGFB1, P53, VEGFA, VEGFC, HIF-1α for
STAT3 target genes) and TaqMan probes were purchased from Applied
Biosystems. GB-stem cell lines or U87 stem cells were treated with
STX-0119, WP1066 or DMSO for 24 h and total RNA was extracted.
Complementary DNA was synthesized from 100 ng of the total RNA and
quantitative PCR was carried using a TaqMan RNA-to-Ct 1-Step kit
(Applied Biosystems).
ELISA for human VEGF
VEGF levels in the supernatant of GBM-SC lines and
U87 stem cells treated with STX-0119 were measured using human
VEGF-specific ELISA. Cells were plated in 96-well ultralow cluster
plates (Corning Inc., Corning, NY) in SCM. After 1-day incubation,
cells were treated with STX-0119, WP1066 or DMSO for 24 h. Finally,
supernatants were collected and VEGF levels were measured.
Western blotting (WB)
GBM-SC or U87 stem cells were treated with STX-0119,
WP1066 or DMSO at various doses for 24–72 h in SCM. Cells were
lysed using RIPA buffer (Thermo Fisher Scientific Inc., Rockford,
IL) containing protease inhibitors and phosphatase inhibitors and
used for western blotting as described previously. Briefly, cell
lysate was subjected to SDS-PAGE with a 7.5% polyacrylamide
separating gel and then transferred to PVDF membranes. After
blocking, the membranes were incubated at 4°C overnight with the
primary antibody against STAT3, phosphospecific STAT3, cleaved
caspase-3 and β-actin (1:200–1:2,000) in blocking solution. After a
wash, the membranes were incubated for 1 h with horseradish
peroxidase (HRP)-conjugated anti-mouse IgG (1:5,000). Membranes
were treated with ECL plus reagent (GE Healthcare) and analyzed on
a chemiluminescence scanner (LAS-3000, Fujifilm, Tokyo, Japan).
Animal experiments
Immunodeficient male
NOD/Shi-Parkdcscid (NOD-scid) and
NOD/Shi-scid IL-2Rγnull (NOG) mice (5–6
weeks old) obtained from Nippon Clea (Tokyo, Japan) were used. They
were housed in a separate experimental room and given sterilized
food and water ad libitum. All animals were cared for and
used humanely according to guidelines for the welfare and use of
animals in cancer research, and procedures approved by the Animal
Care and Use Committee of Shizuoka Cancer Center Research
Institute.
The GBM-SC lines were re-suspended in RPMI-1640
medium (100 μl) containing Matrigel (BD Biosciences) at
1×106/ml and inoculated into the flank of
NOD-scid and NOG mice. To evaluate the activity against the
subcutaneous (s.c.) inoculated tumor cells, tumor volume was
calculated based on The National Cancer Institute formula as
follows: tumor volume (mm3) = length (mm) × [width
(mm)]2 × 1/2.
Immunohistochemistry
Three tumors generated in vivo from GBM-SC
lines were resected and fixed with formalin solution.
Hematoxylin-eosin staining was performed according to the
manufacturer’s instructions. A pathologist compared the
GBM-SC-derived tumor specimen to the surgically resected tumor and
made a diagnosis regarding the similarity of the tumors.
Statistical analysis
The statistical analysis was performed with
corrected p-values to compare with the untreated control using the
Steel multiple plus Kruskal-Wallis method and Mann-Whitney’s
rank-sum test.
Results
Establishment of primary GBM-SC lines
from GBM patients
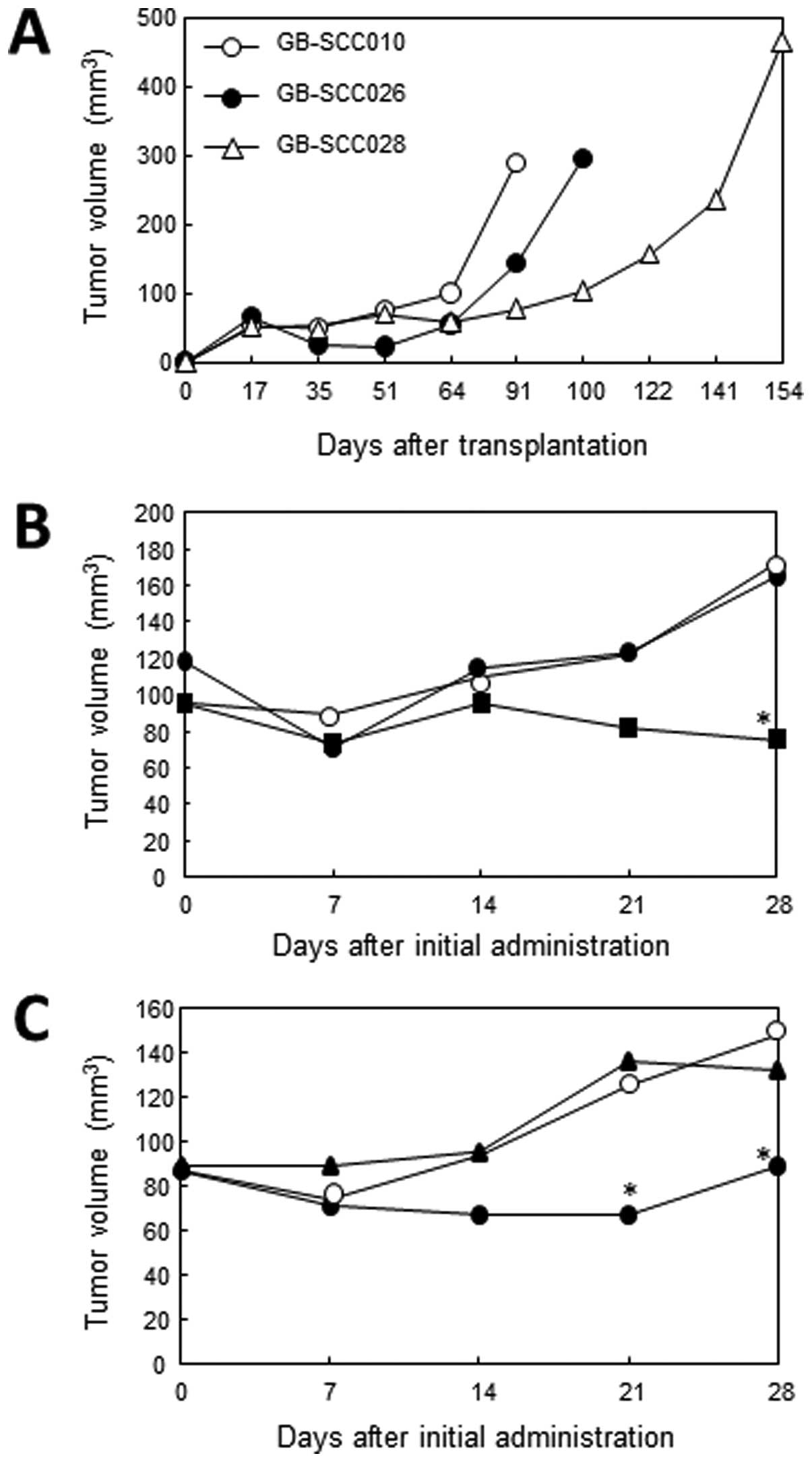
Three GBM-SC lines (GB-SCC010, 026, 028) were
established from 3 GBM patient-derived tumors (Fig. 1A). Each cell line showed a
neurosphere feature in SCM. On the other hand, the matched
traditionally grown cell line from serum-contained cultures showed
an adherent phenotype. Histological analysis of in vivo
tumors generated from GBM-SC lines demonstrated similar findings to
surgically resected tumors (Fig.
1B). All of the GBM-SC lines were shown to be positive for
CD133 stain using flow cytometry analysis (Table I).
 | Table I.Frequency of stem cell markers in
GB-SC lines. |
Table I.
Frequency of stem cell markers in
GB-SC lines.
| CD44+
(%) | CD133+
(%) |
CD44+/133+ (%) |
|---|
| GB-SCC010 | 48.3 | 10.6 | 10.4 |
| GB-SCC026 | 94.6 | 39.2 | 37.5 |
| GB-SCC028 | 82.8 | 34.4 | 33.7 |
| U87 stem cell | 93.8 | 72.4 | 71.6 |
STAT3 phosphorylation assay
The activation (phosphorylation) of STAT3 was
investigated in GBM-SC lines and matched serum-cultured cell lines
derived from three GBM patients. Constitutive STAT3 phosphorylation
was identified, but was stronger in the GBM-SC lines than
serum-cultured cell lines (Fig.
1C).
Expression of stem cell markers in
primary GB-SC lines
Quantitative PCR revealed stem cell-related marker
gene expression in a representative GBM-SC line, GB-SCC010
(Table II), which showed changes
in gene expression compared with the matched primary serum-cultured
cell line. The gene expression of stem cell-related markers CD133,
EGFR, MDR1, KLF4, Nanog, Nestin, Oct3/4, Olig2, Sox2, VEGFA and
vimentin was upregulated >10-fold compared to the serum-cultured
cell line. Impressively, the increase of MDR1 gene expression was
extraordinarily high, ∼200,000-fold. The stem cell-related marker
gene expression in the other 2 GBM-SC lines is shown in Table II. Taking the data from all three
GBM-SC lines into consideration, CD133, MDR1, Olig2, Sox2 and VEGFA
were remarkably upregulated, while TGFBR2 and VEGFR2 were
downregulated.
 | Table II.Frequency of stem cell-associated
markers in GB-SC lines. |
Table II.
Frequency of stem cell-associated
markers in GB-SC lines.
| Gene | GB-010 | GB-026 | GB-028 |
|---|
| ALDH1A1 | ND | 0.01 | 5.59 |
| CD133 | 14.1 | 27985 | 3831 |
| CD44 | 4.68 | 0.84 | 45.3 |
| c-Myc | 1.92 | 5.59 | 0.23 |
| EGFR | 48.7 | 0.17 | 25.3 |
| ESA | 11.7 | 0.51 | 49.8 |
| GFAP | 4.04 | 86.5 | 420 |
| KLF4 | 27.4 | 1.21 | 4.88 |
| MDR1 | 197220 | 18.8 | 783 |
| NANOG | 88.3 | 2.53 | 448 |
| Nestin | 44.7 | 2.65 | 398 |
| Oct4/5 | 69.6 | 4.87 | 41.3 |
| OLIG2 | 124 | 2035 | 56420 |
| SOX2 | 74.3 | 4635 | 693 |
| TGFBR2 | 0.03 | 0.02 | 0.06 |
| TUBB3 | 20.1 | 17.9 | 139 |
| VEGFA | 77.2 | 89.1 | 329 |
| VEGFR2 | 0.58 | 0.03 | 5.76 |
| Vimentin | 47.1 | 3.83 | 16.8 |
Cell proliferation assay
The growth inhibitory effect of STX-0119 on the
GBM-SC lines was moderate (IC50 15–44 μM) and
stronger than that of WP1066 in two cell lines (Fig. 2 and Table III). STX-0119 exhibited a stronger
inhibitory effect on GB-SCC026 stem cells than the others. On the
other hand, the effect of temozolomide was weak in all cell lines
(IC50 53–226 μM). The inhibitory effect on U87
stem cell growth did not differ between STX-0119 and WP1066.
Additionally, STX-0119 inhibited sphere formation at an
IC50 of <5 μM and had greater inhibitory
activity than WP1066.
 | Table III.Effect of STX-0119 and WP1066 on
GB-stem cell proliferation. |
Table III.
Effect of STX-0119 and WP1066 on
GB-stem cell proliferation.
| Cell line | Proliferation
(IC50 /μM)
| Sphere
(IC50 /μM)
|
|---|
| TMZ | STX-0119 | WP1066 | STX-0119 | WP1066 |
|---|
| GB-SCC010 | 226 | 36.1 | 44.8 | 3.8 | 7.8 |
| GB-SCC026 | 53.1 | 15.1 | >100 | 4.2 | 28.9 |
| GB-SCC028 | 167 | 44.5 | >100 | 5.2 | 50.0 |
| U87 parental | 45.2 | 6.6 | 2.1 | - | - |
| U87 stem cell | 66.7 | 31.4 | 10.6 | 2.4 | 2.2 |
Effect of STX-0119 on STAT3 target gene
expression and STAT3 phosphorylation
The effect of STX-0119 on STAT3 target gene
expression in a representative cell line, GB-SCC026, was analyzed
using real-time PCR. STX-0119 significantly inhibited c-myc gene
expression in a dose-dependent manner (Fig. 3A). In contrast, WP1066 did not
downregulate c-myc expression. C-myc expression in the other GBM-SC
lines was not effected by STX-0119 (data not shown). The expression
of other STAT3 targets such as Bcl-xL, survivin, cyclin D1, MMP9,
TGF-β1, VEGF was also suppressed by STX-0119 at 100 μM
(Fig. 3B). Interestingly, the
expression of VFGFR2 was remarkably inhibited by STX-0119. STAT3
phosphorylation was moderately down-regulated at 100 μM of
STX-0119 in the GB-SCC026 cell line (data not shown). Furthermore,
STX-0119 significantly inhibited the stem cell-associated gene
expression of CD44, Nanog, nestin and CD133 (Fig. 3C).
Induction of apoptosis by STX-0119 in
GBM-SC lines
The apoptosis induction in GB-SCC026 cell line and
U87 stem cell line was investigated using caspase-3 western
detection kit including the primary antibody against cleaved
caspase-3 (Cell Signaling). Apoptosis was remarkably identified
after a 24-h exposure by STX-0119 with the dose of >50 μM
(Fig. 4).
STX-0119 inhibits tumor growth in a
subcutaneous model of GBM-SC lines
Based on the growth of transplanted primary GBM-SC
lines, GB-SCC010 and 026 were shown to generate well-growing
tumors, used as a model of treatment with STX-0119 (Fig. 5A). STX-0119 at doses of 40 and 80
mg/kg suspended with methyl cellulose was orally administered to
NOD-scid and NOG mice bearing GBM tumors of >35
mm3. The administration of STX-0119 at 80 mg/kg for
three weeks caused a >50% inhibition of GB-SCC010-derived tumors
at day 28 (Fig. 5B) and
GB-SCC026-derived tumors at day 21, after the start of treatment
(Fig. 5C). In contrast, WP1066 did
not show an inhibitory effect on GB-SCC026 tumors. Additionally, no
significant side effects including weight loss were seen in
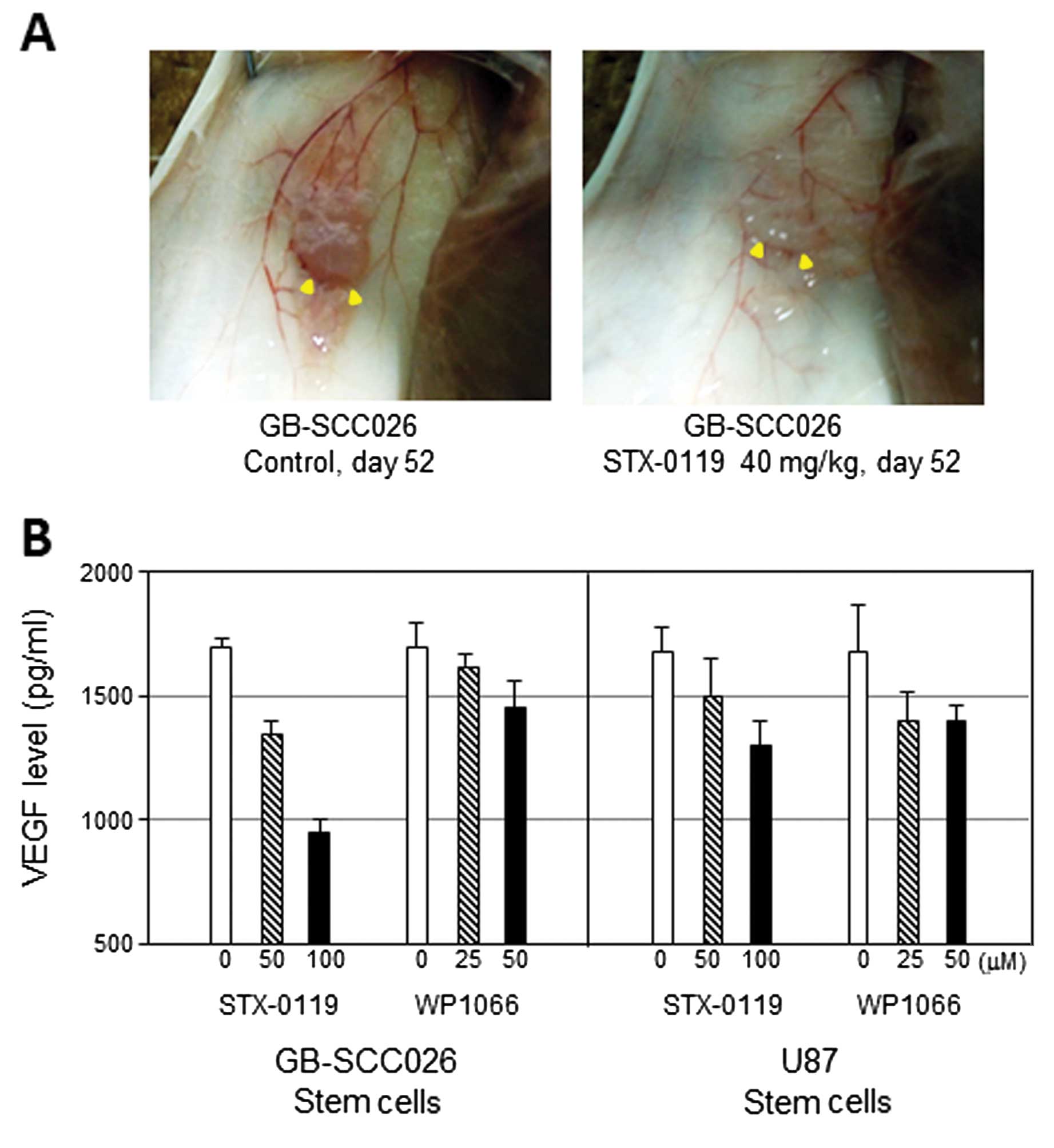
STX-0119-treated mice. Interestingly, it seemed that the
vascularity around the tumor decreased after the STX-0119
administration compared to the GB-SCC026T SC-derived tumor without
treatment (Fig. 6A).
Decrease of VEGF production from GBM-SC
lines treated with STX-0119
The VEGF levels in the supernatant of GBM-SC lines
treated with STX-0119 significantly decreased in a dose-dependent
manner (Fig. 6B). Specifically,
the reduction was more remarkable in GB-SCC026T SC than 010T SC and
U87 cells.
Discussion
Glioblastoma multiforme (GBM) is one of the most
malignant and aggressive tumors with a very poor prognosis. Despite
recent therapeutic advances, less than one-third of GBM patients
survive more than 2 years (1,2).
Thus, a novel therapeutic approach is urgently needed to control
recurrence and overcome resistance to treatment. In the present
study, we demonstrated that STX-0119 inhibited GBM-SC proliferation
in vitro and in vivo by downregulating the gene
expression of STAT3’s targets like BcX-L, cyclin D1, survivin,
c-myc, VEGF, MMP2 and HIF-1α and inducing apoptosis. Previously we
reported that STX-0119 significantly inhibited the growth of a
highly STAT3-phosphorylated lymphoma cell line in vitro and
in vivo by reducing expression c-myc, survivin, cyclin D1
and Bcl-xL and causing apoptosis. The IC50 value, 15–44
μM, in the present study regarding GBM-SC lines was higher
than that of lymphoma cell line reported previously. Importantly,
we demonstrated that higher STX-0119 level than IC50
value in mouse blood was obtained in vivo in a
pharmacokinetic study previously (17).
Since GBM-SC were identified as tumor-initiating
cells, substantial evidence has been emerged of specific features
in terms of gene signatures (6,7,18).
The criteria for GBM-SC are as follows: i) isolated and expanded
from GBM tumors in vitro as neurosphere cultures in
serum-free medium containing EGF and bFGF, ii) a capacity for
extended self-renewal, multi-lineage differentiation, iii) the
ability to reproduce the histology of human GBM tumors in
xenografts and iv) upregulation of drug-resistant and
anti-apoptotic genes in response to radiation or chemotherapy. Our
three GBM-SC lines met all these criteria. Additionally, we
established matched serum-derived cell lines from the same
patients. Interestingly, the expression of GBM stem cell markers
was weak and the maturation markers GFAP and neuron-specific class
III β-tublin were upregulated. Lee et al reported that stem
cell medium-derived GBM-SC had a specific gene expression signature
that more closely resembles the tumor of origin than do
serum-derived cell lines from the same tumor (7).
STAT3 is a member of a family of DNA-binding
molecules, which regulate numerous physiological and oncogenic
signaling pathways leading to target gene expression through
STAT3-SH2 dimerization and phosphorylation, promote cell
proliferation and induce anti-apoptotic activity, angiogenesis and
immunological regulation. Recent studies have showed that STAT3 is
constitutively activated in various types of cancers including
hematological and solid cancers. Experimental approaches for
blocking STAT3 signaling using small interfering RNA (siRNA), small
hairpin RNA (shRNA) and STAT3 antisense have been successful in
inhibiting cell proliferation in vitro (19) and tumor growth in vivo
(20).
As is the case with GB tumors, the constitutive
activation of the STAT3 pathway is linked to tumor promotion and
maintenance. With regard to GBM-SC signaling, the interaction
between STAT3 and other pathways including EGFR, Notch, Wnt,
Hedgehog, Akt, mTOR, olig2, PKC, MAPK, NF-κB and BMP4 was shown to
regulate self-renewal (21) and
astrocytic differentiation. Interestingly, STAT3 is involved in
both stem cell maintenance and astrocytic differentiation, which
could be important for therapeutics. Sherry et al (14) reported that the stat3 inhibition by
shRNA induced growth arrest and inhibition of sphere formation as
well as stem cell marker downregulation, which suggested that STAT3
contributes to the maintenance of stem-like characteristics. Our
observation in a quantitative PCR assay that one GBM-SC line showed
significant inhibition of stem cell markers and STAT3-target genes
on STX-0119 treatment, but another GBM-SC line did not, support
such a finding. These results might indicate that the dependency on
STAT3 differs among GBM-SC lines, perhaps leading to the difference
in response to the anti-STAT3 reagent STX-0119. First, it is
reasonable to assume that STX-0119-treated GBM-SC lines become
sensitive to apoptotic stimuli when they have differentiated. This
would be consistent with the observation that GBM-SC lines do not
undergo apoptosis upon STAT3 inhibition in stem cell medium unlike
serum-derived glioma cell lines. Additionally, other potential
functions of STAT3 in GBM-SC lines, as a tumor suppressor and
differentiation-inducing activity in astrocytes (22,23),
should be considered to clarify the various therapeutic effects of
STX-0119 including off target ones.
A second explanation for the heterogeneous response
to the anti-STAT3 agent is activation of Yamanaka factor-associated
signaling. Our observation regarding the downregulation of Yamanaka
factor expression in GBM-SC lines treated with STX-0119 revealed an
unexpected upregulation of KLF-4. The Oct-4 and Sox2 genes were
demonstrated to be associated with the maintenance of stemness in
glioma-initiating cells (24) and
Oct-4 and nanog have been linked to STAT3 signaling (25). Considering that KLF-4 is located
downstream of STAT3 and nanog, these observations indicate that
KLF-4 signaling is linked to pathways other than STAT3 and nanog.
Investigation of the cross talk between Yamanaka factor signaling
and the STAT3 pathway should facilitate the development of agents
against cancer stem cells. Another interesting point is that KLF-4
is reported to act as a tumor suppressor gene in colorectal cancer
cells in which loss of heterozygosity (LOH) and point mutations in
KLF-4 gene were identified (26).
This observation suggests that KLF-4 might differ from other
Yamanaka factors in terms of genetic function and upregulation of
KLF-4 is one of mechanisms for the antitumor effect of
STX-0119.
GMB-SC-targeted therapeutics such as small molecules
and other reagents have been developed. JAK2/STAT3 inhibitors,
AZD1480 (27), WP11193 (28) and LLL12 (29), were demonstrated to have inhibitory
effects on GB-SCs in vitro and in vivo mediated by
downregulation of GBM stem cell marker as well as STAT3 target
genes and induction of apoptosis in stem cells. Curcumin, a natural
compound, was shown to suppress tumor growth in vivo by
inducing autophagy (30). However,
anti-GBM-SC agents have yet to be successfully developed. One major
reason for this is the significant heterogeneity (31) and involvement of multi-pathway
signaling in stem cell growth (21). Novel biomarkers specific for GBM-SC
include protein phosphatase 2A (32), inhibitors of DNA binding proteins 2
and 4 (33), IL-13Rα2 (34), Girdin (35) and HIF-1α. These markers have also
been shown to be significant prognostic markers. Hypoxia in the
tumor microenvironment is crucial to the maintenance of GBM-SC and
HIF-1α may be a good GBM stem cell marker at tumor sites (36,37).
Additionally, TGF-β1 signaling (38) and EMT factors (39), closely involved in the growth and
maintenance of GBM-SC and tumor progression, might be potential
targets for treatment. Of note, HIF-1α and TGF-β1 expression was
downregulated in STX-0119-treated GBM-SC lines in our study,
suggesting these markers to be STAT3 pathway-linked GBM stem cell
markers. Another good prognostic marker regarding tumor recurrence
in GBM patients is the absence of a mesenchymal transition gene
signature (40).
In the present study, we demonstrated that the STAT3
inhibitor STX-0119 significantly inhibited stem cell proliferation
in vitro and tumor formation in vivo by
downregulating the gene expression of STAT3 targets and stem cell
markers. Collectively, combining STAT3 inhibitors with other
reagents targeting GBM-SC-specific molecules (receptor tyrosine
kinase inhibitors and anti-angiogenic agents) besides STAT3, may be
the basis for the next generation of GBM treatment. It is the most
important to control GBM recurrence after standard therapy and
prolong the relapse-free and overall survival of GBM patients.
Abbreviations:
|
STAT
|
signal transducer and activator of
transcription;
|
|
SH
|
Src homology;
|
|
DMSO
|
dimethyl sulfoxide;
|
|
JAK
|
Janus kinase;
|
|
T/C
|
tumor/control;
|
|
siRNA
|
small interfering RNA;
|
|
shRNA
|
small hairpin RNA
|
Acknowledgements
This study was supported by a grant
from the National Institute of Biomedical Innovation, Japan
(06-2).
References
|
1.
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A,
Lacombe D, Cairncross JG, Eisenhauer E and Mirimanoff RO:
Radiotherapy plus concomitant and adjuvant temozolomide for
glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Mirimanoff RO, Gorlia T, Mason W, Van den
Bent MJ, Kortmann RD, Fisherb, Reni M, Brandes AA, Curschmann J,
Villa S, Cairncross G, Allgerer A, Lacombe D and Stupp R:
Radiotherapy and temozolomide for newly diagnosed glioblastoma:
recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3
phase III randomized trial. J Clin Oncol. 24:2563–2569. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkeiman RM, Cusimano MD and Dirks PB:
Identification of human tumor initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Galli R, Binda E, Orfanelli U, Cipelletti
B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F and Vescovi
A: Isolation and characterization of tumorigenic, stem-like neural
precursors from human glioblastoma. Cancer Res. 64:7011–7021. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Singh SK, Clark ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
6.
|
Clark MF, Dick JE, Dirks PB, Eaves CJ,
Jamieson CH, Jones DL, Visvader J, Weissman IL and Wahl GM: Cancer
stem cells-perspectives on current status and future derections:
AACR workshop on cancer stem cells. Cancer Res. 66:9339–9344. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Lee J, Kotliarova S, Kotliarov Y, Li A, Su
Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, Park JK
and Fine HA: Tumor stem cells derived from glioblastomas cultures
in bFGF and EGF more closely mirror the phenotype and genotype of
primary tumors than do serum-cultured cell lines. Cancer Cells.
9:391–403. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Brennan C, Momota H, Hambardzumyan D,
Ozawa T, Tandon A, Pedraza A and Holland E: Glioblastoma subclasses
can be defined by activity among signal transduction pathways and
associated genomic alterations. PLoS One. 4:e77522009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Cheng CK, Fan QW and Weiss WA: PI3K
signaling in glioma-animal models and therapeutic challenges. Brain
Pathol. 19:112–120. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Demuth T, Reavie LB, Rennert JL, Nakada M,
Nakada S, Hoelzinger DB, Beaudry CE, Henrichs AN, Anderson EM and
Berens ME: MAP-ing glioma invasion: mitogen-activated protein
kinase 3 and p38 drive glioma invasion and progression and predict
patient survival. Mol Cancer Ther. 6:1212–1222. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Pu P, Zhang Z, Kang C, Jiang R, Jia Z,
Wang G and Jiang H: Downregulation of Wnt2 and beta-catenin by
siRNA suppresses malignant glioma cell growth. Cancer Gene Ther.
16:351–361. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Purow BW, Haque RM, Noel MW, Su Q, Burdick
MJ, Lee J, Sundaresan T, Pastorino S, Park JK, Mikolaenko I, Maric
D, Eberhart CG and Fine HA: Expression of Notch-1 and its ligands,
Delta-like-1 and Jagged-1, is critical for glioma cell survival and
proliferation. Cancer Res. 65:2353–2363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Zhong Z, Wen L and Darnell JE Jr: Stat3
and Stat4: members of the family of signal transducers and
activators of transcription. Proc Natl Acad Sci USA. 91:4806–4810.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Sherry MM, Reeves A, Wu JK and Cochran BH:
STAT3 is required for proliferation and maintenance of multipotency
in glioblastoma stem cells. Stem Cells. 27:2383–2392. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Li GH, Wei H, Lv SQ, Ji H and Wang DL:
Knockdown of STAT3 expression by RNAi suppresses growth and induces
apoptosis and differentiation in glioblastoma stem cells. Int J
Oncol. 37:103–110. 2010.PubMed/NCBI
|
|
16.
|
Ashizawa T, Miyata H, Ishii H, Oshita C,
Matsuno K, Masuda Y, Furuya T, Okawara T, Otsuka M, Ogo N, Asai A
and Akiyama Y: Antitumor activity of a novel small molecule STAT3
inhibitor against a human lymphoma cell line with high STAT3
activation. Int J Oncol. 38:1245–1252. 2011.PubMed/NCBI
|
|
17.
|
Matsuno K, Masuda Y, Uehara Y, Sato H,
Muroya A, Takahashi O, Yokotagawa T, Furuya T, Okawara T, Otsuka M,
Ogo N, Ashizawa T, Oshita C, Tai S, Ishii H, Akiyama Y and Asai A:
Identification of a new series of STAT3 inhibitors by virtual
screening. ACS Med Chem Lett. 1:371–375. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Carro MS, Lim WK, Alvarez MJ, Bollo RJ,
Zhao X, Snyder EY, Sulman EP, Anne SL, Doetsch F, Colman H,
Lasorella A, Aldape K, Califano A and Lavarone A: Transcriptional
network for mesenchymal transformation of brain tumors. Nature.
463:318–325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Gao L, Zhang L, Hu J, Li F, Shao Y, Zhao
Y, Kalvakolanu DV, Kopecko DJ, Zhao X and Xu DQ: Down-regulation of
signal transducer and activator of transcription 3 expression using
vector-based small interfering RNAs suppresses growth of human
prostate tumor in vivo. Clin Cancer Res. 11:6333–6341. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Ling X and Arlinghaus RB: Knockdown of
STAT3 expression by RNA interference inhibits the induction of
breast tumors in immunocompetent mice. Cancer Res. 65:2532–2536.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Ohka F, Natsume A and Wakabayashi T:
Current trends in targeted therapies for glioblastoma multiforme.
Neurol Res Int. 2012. View Article : Google Scholar
|
|
22.
|
Takizawa T, Nakashima K, Namihira M,
Ochiai W, Uemura A, Yanagisawa M, Fujita N, Nakao M and Taga T: DNA
methylation is a critical cell-intrinsic determinant of astrocyte
differentiation in the fetal brain. Dev Cell. 1:749–758. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
De la Iglesia N, Konopka G, Puram SV, Chan
JA, Bachoo RM, You MJ, Levy DE, Depinho RA and Bonni A:
Identification of a PTEN-regulated STAT3 brain tumor suppressor
pathway. Genes Dev. 22:449–462. 2008.PubMed/NCBI
|
|
24.
|
Ikushima H, Todo T, Ino Y, Takahashi M,
Saito N, Miyazaki K and Miyazono K: Glioma-initiating cells retain
their tumorigenicity through integration of the Sox axis and Oct4
protein. J Biol Chem. 286:41434–41441. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Guo Y, Mantel C, Hromas RA and Broxmeyer
HE: Oct-4 is critical for survival/antiapoptosis of murine
embryonic stem cells subjected to stress: effects associated with
Stat3/survivin. Stem Cells. 26:30–34. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Zhao W, Hisamuddin IM, Nandan MO, Babbin
BA, Lamb NE and Yang VW: Identification of Krüppel-like factor 4 as
a potential tumor suppressor gene in colorectal cancer. Oncogene.
23:395–402. 2004.
|
|
27.
|
McFarland BC, Ma JY, Langford CP,
Gillespie GY, Yu H, Zheng Y, Nozell SE, Huszar D and Benveniste EN:
Therapeutic potential of AZD1480 for the treatment of human
glioblastoma. Mol Cancer Ther. 10:2384–2393. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Sai K, Wang S, Balasubramaniyan V, Conrad
C, Lang FF, Aldape K, Szymanski S, Fokt I, Dasgupta A, Madden T,
Guan S and Chen Z: Induction of cell-cycle arrest and apoptosis in
glioblastoma stem-like cells by WP1193, a novel small molecule
inhibitor of the JAK2/STAT3 pathway. J Neurooncol. 107:487–501.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Ball S, Li C, Li PK and Lin J: The small
molecule, LLL12, inhibits STAT3 phosphorylation and induces
apoptosis in medulloblastoma and glioblastoma cells. PLoS One.
6:e188202011. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Zhuang W, Long L, Zheng B, Ji W, Yang N,
Zhang Q and Liang Z: Curcumin promotes differentiation of
glioma-initiating cells by inducing autophagy. Cancer Sci.
103:684–690. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Denysenko T, Gennero L, Roos MA, Melcarne
A, Juenemann C, Faccani G, Morra I, Cavallo G, Reguzzi S,
Pescarmona G and Ponzetto A: Glioblastoma cancer stem cells:
heterogeneity, microenvironment and related therapeutic strategies.
Cell Biochem Funct. 28:343–351. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Hofstetter CP, Burkhardt JK, Shin BJ,
Gursel DB, Mubita L, Gorrepati R, Brennan C, Holland EC and
Boockvar JA: Protein phosphatase 2A mediates dormancy of
glioblastoma multiforme-derived tumor stem-like cells during
hypoxia. PLoS One. 7:e300592012. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Wu Y, Richrad JP, Wang SD, Rath P, Laterra
J and Xia S: Regulation of glioblastoma multiforme stem-like cells
by inhibitor of DNA binding proteins and oligodendroglial
lineage-associated transcription factors. Cancer Sci.
103:1028–1037. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Nguyen V, Conyers JM, Zhu D, Gibo DM,
Dorsey JF, Debinski W and Mintz A: IL-13Rα2-targeted therapy
escapees: biologic and therapeutic implications. Transl Oncol.
4:390–400. 2011.
|
|
35.
|
Natsume A, Kato T, Kinjo S, Enomoto A,
Toda H, Shimato S, Ohka F, Motomura K, Kondo Y, Miyata T, Takahashi
M and Wakabayashi T: Girdin maintains the stemness of glioblastoma
stem cells. Oncogene. 31:2715–2724. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Pistollato F, Abbadi S, Rampazzo E,
Persano L, Della Puppa A, Frasson C, Sarto E, Scienza R, D’Avella D
and Basso G: Intratumoral hypoxic gradient drives stem cells
distribution and MGMT expression in glioblastoma. Stem Cells.
28:851–862. 2010.PubMed/NCBI
|
|
37.
|
Qiang L, Wu T, Zhang HW, Lu N, Hu R, Wang
YJ, Zhao L, Chen FH, Wang XT, You QD and Guo QL: HIF-1α is critical
for hypoxia-mediated maintenance of glioblastoma stem cells by
activating Notch signaling pathway. Cell Death Differ. 19:284–294.
2012.
|
|
38.
|
Ikushima H, Todo T, Ino Y, Takahashi M,
Miyazawa K and Miyazono K: Autocrine TGF-β signaling maintains
tumorigenicity of glioma-initiating cells through Sry-related
HMG-box factors. Cell Stem Cell. 5:504–514. 2009.
|
|
39.
|
Kaur H, Phillips-Mason PJ, Burden-Gulley
SM, Kerstetter-Fogle AE, Basilion JP, Sloan AE and Brady-Kalnay SM:
Cadherin-11, a marker of the mesenchymal phenotype, regulates
glioblastoma cell migration and survival in vivo. Mol Cancer Res.
10:293–304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Cheng WY, Kandel JJ, Yamshiro DJ, Canoll P
and Anastassiou D: A multi-cancer mesenchymal transition gene
expression signature is associated with prolonged time to
recurrence in glioblastoma. PLoS One. 7:e347052012. View Article : Google Scholar : PubMed/NCBI
|