Introduction
Angiogenesis, the formation of new microvessels from
the pre-existing blood vasculature, is a highly regulated process
that involves the activation, growth and migration of endothelial
cells and capillary morphogenesis (1,2).
Although angiogenesis is an essential process in embryonic vascular
development, organ regeneration and wound healing, a variety of
pathological diseases, including cancer, rheumatoid arthritis and
diabetic retinopathy, also involve angiogenic events (1,3).
Therefore, the identification of anti-angiogenic agents with new
mechanisms of action would be an attractive strategy for studying
angiogenic processes and providing potential lead candidates for
the development of new drugs associated with angiogenesis (4). There is increasing evidence that
reactive oxygen species (ROS) are involved in the regulation of
angiogenesis. Although high levels of ROS may result in oxidative
damage to various cellular components and, finally, in cell
apoptosis (5), low levels of ROS
have been demonstrated to be involved in signal transduction
cascades that regulate endothelial cell growth, migration and
organization into tubular network structures, which are critical
steps in angiogenesis (6,7). The ROS-induced apoptotic pathway is
also considered the intrinsic or mitochondrial pathway, in which
intrinsic death stimuli directly or indirectly activate the
mitochondrial pathway (8). The
mitogen-activated protein kinase (MAPK) and AKT/mTOR signaling
pathways play important roles in the regulation of many cellular
processes, including cell growth and proliferation, differentiation
and apoptosis (9). Previous
studies have also indicated that ROS can induce or mediate the
activation of MAPK signaling pathways (10) and MAPK signaling proteins are
involved in growth arrest and apoptosis via ROS generation
(11).
Embryonic stem (ES) cells are pluripotent cells
established from the inner cell mass of blastocysts and have the
potential to differentiate into many cell types, such as
hematopoietic cells, neuronal cells, cardiomyocytes, muscle cells,
epithelial cells and endothelial cells (12,13).
Therefore, ES cells are considered a useful tool for the study of
angiogenesis, including the processes of angioblast
differentiation, proliferation, migration, endothelial cell-cell
adhesion and vascular morphogenesis (12,14,15).
One promising approach for differentiating ES cells into
endothelial cells is the use of embryoid bodies (EBs), which are
embryo-like aggregates of ES cells. Therefore, the ES/EB system
represents a good in vitro model for the study of
vasculogenesis as well as angiogenesis (16). In addition, ES-derived EB cells
closely resemble their in vivo counterparts and thus provide
a useful in vitro model for the study of specific cell
signaling systems (17,18). Recently, we also demonstrated the
usefulness of mES-derived endothelial cell systems in the
evaluation of the effect of 5-FU on vasculogenesis and the
anti-angiogenic effects of natural product-derived compounds
(19,20).
Magnolol is a neolignan from the bark of Magnolia
obovata Thunberg (Magnoliaceae), which has traditionally been
used to treat gastrointestinal tract disorders in Asian countries
(21,22). A variety of pharmacological
activities of magnolol have been reported, including
anti-inflammatory (23),
anti-oxidant (24), anticancer and
anti-angiogenic effects. The anticancer activity of magnolol was
found to be able to suppress the proliferation of cancer cells by
inhibiting DNA synthesis and inducing apoptosis (25–27).
However, the precise mechanism of action for the anti-angiogenic
activity of magnolol remains to be elucidated.
In the present study, we demonstrated that magnolol
might be able to suppress angiogenesis through the inhibition of
ROS-mediated MAPK and AKT/mTOR signaling in mES/EB-derived
endothelial-like cells.
Materials and methods
Reagents and antibodies
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT), dimethyl sulfoxide (DMSO), gelatin and
HRP-conjugated anti-mouse and anti-rabbit antibodies were purchased
from Sigma-Aldrich (St. Louis, MO, USA). Collagen type I was
purchased from BD Biosciences (San Jose, CA, USA). Phospho-specific
anti-p38(Thr180/Tyr182),
anti-JNK(Thr183/Tyr185), anti-PI3K,
anti-PDK1, anti-AKT(Ser473), anti-mTOR(Ser2448),
anti-p70S6K(Thr389),
anti-4EBP1(Thr37/Thr46) and procaspase-3
antibodies, the MAPK inhibitors; SB203580, PD98059 and SP600125;
and the PI3K inhibitor LY294002 were purchased from Cell Signaling
Technology (Danvers, MA, USA). The HRP-conjugated β-actin,
phospho-ERK(Thr202/Tyr204), Bcl-xL, Bax and
PECAM antibodies were purchased from Santa Cruz Biotechnology
(Santa Cruz, CA, USA). 2′7′-Dichlorofluorescein diacetate
(H2DCFDA) and Alexa Fluor 594-labeled chicken anti-rat
IgG were purchased from Molecular Probes (Invitrogen, Carlsbad, CA,
USA). Magnolol (purity >98%, Fig.
1A) isolated from the bark of Magnolia obovata was
provided by Dr K. Bae (28). The
compound was dissolved in 100% DMSO. A 100-mM stock solution of
magnolol was prepared and stored at −20°C until use.
Cell culture
Mouse D3 ES cells (ATCC cat. no.
CRL-1934, Rockville, MD, USA) were co-cultured with mitomycin
C-treated mouse embryonic fibroblasts in high-glucose DMEM
(Invitrogen) containing 10% fetal bovine serum (Hyclone, Ogden, UT,
USA), 1,000 U/ml leukemia inhibitory factor (LIF) (Chemicon,
Temecula, CA, USA) and basic ES medium components [50 U/ml
penicillin and 50 μg/ml streptomycin (Invitrogen), 1%
non-essential amino acids (Invitrogen) and 0.1 mM β-mercaptoethanol
(Invitrogen)]. The hanging drop method (20 μl per drop;
1×105 cells/ml) was used to induce differentiation as
previously described (20,29). The EBs were formed by incubating
the hanging drop cultures for 3 days. The resulting EBs were
transferred onto gelatin-coated chamber slides (Nunc, Denmark) or
60-mm dishes to allow attachment. Endothelial cell differentiation
was induced in EBs by switching the culture conditions to medium
containing EBM-2, 5% FBS, a growth factor cocktail and ascorbic
acid (EGM2-MV Bullet kit; Lonza, Walkersville, MD, USA). The cell
culture and endothelial differentiation conditions for the mES
cells followed the protocol in our previous report (20,30).
Growth inhibition assay
The growth inhibition activity in cultured
mES-derived endothelial-like cells was determined using MTT assays
as previously described (20,30).
Briefly, cells were seeded at a density of 5,000 cells/well into
96-well plates. On differentiation day 10, the cells were exposed
to various concentrations of magnolol for 24 h. After incubation,
MTT solution was added and the cells were incubated for an
additional 4 h. The resulting formazan was dissolved in DMSO and
the absorbance was detected at 570 nm with a VersaMax ELISA
microplate reader (Molecular Devices, Sunnyvale, CA, USA). After
treatment of cells with magnolol for 24 h at differentiation day
10, the cytotoxicity of magnolol was tested using the CytoTox
96® Non-Radioactive Cytotoxicity assay (Promega,
Madison, WI, USA). The effects on cell viability and the
cytotoxicity activity of magnolol were calculated as percentages
relative to the solvent-treated control.
Immunocytochemistry
Cells were plated onto confocal dishes and induced
to differentiate into endothelial-like cells by incubation in EGM-2
medium for 10 days. The mES/EB-derived endothelial-like cells were
incubated with magnolol with or without 5 mM NAC for 24 h. After
incubation, the cells were fixed with 4% paraformaldehyde. The
cells were blocked with blocking solution containing 1% BSA/PBS for
30 min and then incubated with rat anti-mouse PECAM (1:200) (Santa
Cruz) overnight at 4°C. After being washed, the cells were
incubated with Alexa Fluor 594-labeled chicken anti-rat IgG
(1:1,000) (Invitrogen). After staining, the cover slips were
mounted with medium containing DAPI (Vector Laboratories,
Burlingame, CA, USA). Confocal microscopy was performed using a
Zeiss Model 710 (Carl Zeiss, Jena, Germany).
RNA isolation and reverse
transcription-polymerase chain reaction (RT-PCR)
Cells were dissolved using TRIzol®
(Invitrogen) and total RNA was extracted according to the
manufacturer’s protocol. The total cellular RNA was quantified
using a NanoDrop spectrophotometer (NanoDrop Technologies, Inc.,
Wilmington, DE, USA). Reverse transcription was performed using 2
μg of purified total RNA and the SuperScript First-Strand
Synthesis System (Invitrogen). The synthesized cDNAs were amplified
by PCR. The primers used for RT-PCR are listed in Table I. The thermal cycling parameters
were as follows: 5 min at 94°C, 30 amplification cycles
(denaturation at 94°C for 30 sec, annealing at 55–60°C for 30 sec
and extension at 72°C for 30 sec) and a final extension at 72°C for
5 min. The amplified products were separated on 1.5% agarose gels.
The gels were stained with SYBR® Gold staining solution
(Invitrogen) and visualized by UV transillumination (GelDoc™ XR,
BioRad Molecular Imager).
 | Table I.Sequences of the primer pairs of
specific target genes used in RT-PCR. |
Table I.
Sequences of the primer pairs of
specific target genes used in RT-PCR.
| Gene | Primer
sequences | Product size
(bp) |
|---|
| PECAM | F
5′-CCATCATGGGAGGTGATGAA-3′ | |
| R
5′-GATACGCCATGCACCTTCAC-3′ | 278 |
| GAPDH | F
5′-GGAGCCAAAAGGGTCATCAT-3′ | |
| R
5′-GTGATGGCATGGACTGTGGT-3′ | 212 |
Flow cytometric analysis of
apoptosis
To determine the level of apoptosis following
magnolol exposure for 24 h during the differentiation of mES cells
into endothelial cells, the Annexin V-fluorescein isothiocyanate
(FITC) apoptosis detection kit (BD Pharmingen) was used. In this
assay, Annexin V-FITC binds to phosphatidylserine, which
translocates to the outer leaflet of the plasma membrane during the
early stages of cell apoptosis. Therefore, the apoptotic cells were
specifically stained with Annexin V-FITC, whereas the necrotic
cells were doubly stained with both Annexin V-FITC and PI. The
cells were suspended in binding buffer at a final cell
concentration of 1×105 cells/ml and incubated with both
Annexin V-FITC and PI for 25 min in the dark. The DNA contents of
the stained cells were analyzed using CellQuest Software and a FACS
Vantage SE flow cytometer (Becton-Dickinson, Germany).
Quantification of reactive oxygen species
production
The intracellular ROS levels were measured using the
fluorescent dye H2DCFDA (Molecular Probes). Cells were
induced to differentiate into endothelial-like cells by incubation
in EGM-2 medium for 10 days. The mES/EB-derived endothelial-like
cells were incubated with magnolol for 24 h. Then, the cells were
washed twice, stained with 20 μM H2DCFDA
(Molecular Probes) for 30 min and washed twice. The resulting
compound, 2′7′-dichlorofluorescein diacetate (H2DCFDA),
reacts with ROS to form a fluorescent compound, dichlorofluorescin
(DCF). The amount of intracellular DCF was measured using flow
cytometer (Becton-Dickinson).
Measurement of the mitochondrial membrane
potential
Rhodamine 123 was used to assess the mitochondrial
membrane potential. After treatment with magnolol for 24 h, the
cells were washed with 1X PBS and incubated with 1 μl/ml
Rhodamine 123 for 60 min at room temperature in the dark. The cells
were washed with 1X PBS, stained with 1 μg/ml PI solution
and analyzed by flow cytometer (Becton-Dickinson). In this
experiment, verapamil (20 μM) was used as a positive
control.
Western blot analysis
Cells were treated with magnolol for 24 h. Harvested
cells were lysed in protein extraction solution (Intron
Biotechnology, Inc., Kyunggi, Korea) containing protease inhibitors
and phosphatase inhibitors for 10 min at 4°C. The total protein
concentration in the supernatants was measured by the Bradford
assay. After heating at 95°C for 5 min, total protein samples (40
μg) were subjected to 6–15% SDS-PAGE. The proteins were
transferred onto PVDF membranes (Millipore, Bedford, MA, USA) at
100 V for 60–100 min. The membranes were incubated with 5% BSA in
TBST (TBS with 0.05% Tween-20) for 30 min at room temperature and
then with primary antibodies diluted (1:200–1:1,000) in 5% BSA in
TBST overnight at 4°C. The membranes were washed three times with
TBST and incubated with the corresponding secondary antibodies.
Protein bands were detected using an enhanced chemiluminescence
detection kit (Intron Biotechnology, Inc.) and a LAS-1000 Imager
(Fuji Film Corp., Tokyo, Japan).
3-Dimensional type I collagen sprouting
angiogenesis model
The three-dimensional tube formation and sprouting
angiogenesis model were performed in type I collagen (16,20,31).
Briefly, EBs were formed by incubating hanging drop cultures for 3
days and then the EBs were cultured in suspension in EGM-2 medium
for 7 days. The EBs were embedded in type I collagen and incubated
in EGM-2 medium at 37°C. Vascular sprouting was induced by
incubation with magnolol for 4 days. The morphology of the vascular
sprouts was analyzed using a phase contrast microscope (Nikon,
Eclipse TE 2000-U, Tokyo, Japan).
Statistical analyses
Data are presented as the mean ± SD for the
indicated number of independently performed experiments.
Statistical significance (P<0.05) was determined using Student’s
t-test for paired data. Statistical calculations were performed
using SPSS for Windows Version 10.0 (SPSS, Chicago, IL, USA).
Results
Effects of magnolol on the growth of
mES/EB-derived endothelial-like cells
To determine whether magnolol affects the growth of
mES/EB-derived endothelial-like cells, mES/EB-derived
differentiated cells (differentiation day 10) were treated with
magnolol (0–100 μM) for 24 h and then cell growth and
cytotoxicity were analyzed using the MTT and LDH leakage assays,
respectively. As shown in Fig. 1B,
the growth inhibition effect of magnolol on the differentiated
cells was concentration-dependent. The relatively low
concentrations of magnolol (≤25 μM) did not inhibit the
growth of the endothelial cells, but concentrations of magnolol
>50 μM significantly inhibited the growth of these cells
(P<0.01). Further experiments confirmed that the growth
inhibition activity of concentrations >50 μM magnolol was
associated with the level of cytotoxicity determined by the LDH
leakage assay (Fig. 1C).
Inhibition of vessel formation in the
mES/EB-derived endothelial-like cells by magnolol
Platelet endothelial cell adhesion molecule (PECAM)
is considered a cell surface marker for endothelial cells during
angiogenesis because PECAM is expressed by endothelial cells and
hematopoietic cells (32). To
determine whether magnolol inhibits microvessel formation in the
mES/EB-derived endothelial-like cells, the cells were treated with
magnolol (0–20 μM) for 24 h and then the expression of the
endothelial biomarker PECAM was analyzed. As shown in Fig. 2A, the differentiated (day 10)
mES/EB-derived cells exhibited remarkable expression of PECAM, but
treatment with magnolol suppressed the mRNA expression of PECAM in
a concentration-dependent manner. Magnolol also markedly suppressed
the expression of the PECAM protein (Fig. 2B), indicating that magnolol
significantly suppressed the expression of PECAM at both the mRNA
and protein levels (P<0.05, P<0.01, respectively). The effect
of magnolol on the expression of PECAM was also assessed using
immunofluorescence in a two-dimensional (2-D) culture of
mES/EB-derived differentiated cells. As shown in Fig. 2C, the microvessels generated from
the differentiation of mES/EB-derived cells clearly expressed
PECAM, but treatment with magnolol (20 μM) significantly
suppressed the expression of PECAM in the differentiated
endothelial cells. These results suggest that the anti-angiogenic
activity of magnolol is associated with the suppression of PECAM
expression. The anti-angiogenic activity of magnolol was also
assessed based on the inhibition of vascular tube formation in a
three-dimensional (3-D) collagen gel system. In this experiment,
vascular tube formation was induced by incubating mES-derived EBs
in a type I collagen gel containing EGM-2 medium as previously
described by Kim et al (20). As shown in Fig. 2D-a, the morphology of the vascular
sprouts formed by EB-derived endothelial-like cells was observed
after 4 days of induction. Accelerated vascular tube sprouting and
an extensive network of cellular outgrowths were observed in the
vehicle-treated control cells (Fig.
2D-a) and magnolol effectively blocked tube sprouting and the
formation of cellular networks (Fig.
2D-b and -c). These findings suggest that magnolol inhibits
vessel formation in mES/EB-derived endothelial-like cells in both
2-D and 3-D culture systems.
Induction of apoptosis by magnolol
We next determined whether the inhibition of both
tube sprouting and the formation of cellular networks by magnolol
is associated with apoptosis in mES/EB-derived endothelial-like
cells. Flow cytometric analysis revealed that magnolol treatment
resulted in Annexin V-positive cells in a concentration-dependent
manner (Fig. 3A). In particular,
the percentage of apoptotic cells among the cells treated with 20
μM magnolol (18.7%) was significantly higher than the
percentage of the vehicle-treated control cells (2.6%) (P<0.01).
To confirm the association between magnolol treatment and
apoptosis, the effects of magnolol on the modulation of apoptotic
regulatory molecules were evaluated. As shown in Fig. 3B, the proapoptotic protein Bax was
upregulated and the anti-apoptotic protein Bcl-xL was downregulated
by magnolol treatment. In addition, the activation of procaspase-3
was also induced by magnolol, as confirmed by the increase in the
intensity of the cleaved caspase-3 band along with the decrease in
the intensity of the procaspase-3 band in the western blotting.
These findings suggest that magnolol may induce apoptosis of
mES/EB-derived endothelial-like cells by upregulating the
proapoptotic protein Bax and subsequently activating caspase-3.
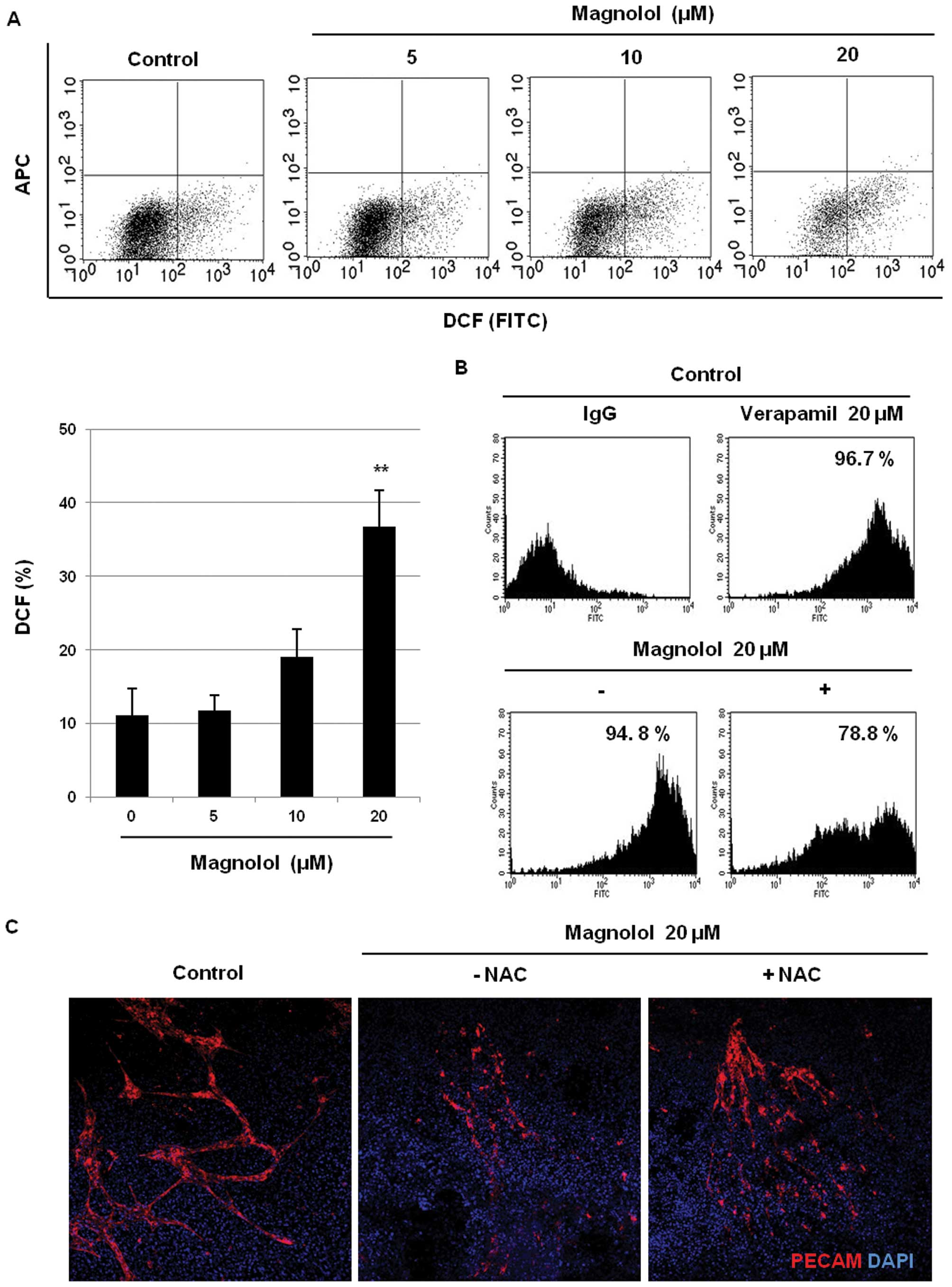
Effects of magnolol on ROS production and
the mitochondrial membrane potential
To further investigate whether the generation of
intracellular ROS is involved in the induction of apoptosis by
magnolol, the effects of magnolol on the ROS levels were determined
using an oxidant-sensitive fluorescent dye in mES/EB-derived
endothelial-like cells. Consistent with previous reports (33,34),
endogenous production of ROS was detected in mES/EB-derived
endothelial-like cells. As shown in Fig. 4A, however, magnolol (20 μM)
significantly increased the intracellular ROS levels (cells
exhibited much higher fluorescence intensities) relative to those
of the vehicle-treated control cells (P<0.01). We next examined
whether the induction of ROS production by magnolol was related to
the modulation of the mitochondrial membrane potential in the
cells. As shown in Fig. 4B, the
active accumulation of Rhodamine 123 was detected in the control
cells and the positive control (verapamil-treated) cells. However,
treatment with magnolol (20 μM) remarkably decreased the
uptake of Rhodamine 123 dye in the cells, suggesting that magnolol
affects the mitochondrial membrane potential as the result of
mitochondrial dysfunction caused by oxidative stress. In addition,
the involvement of oxidative stress induced by magnolol in
vasculogenesis was also confirmed using the anti-oxidant
N-acetyl-L-cysteine (NAC). As shown in Fig. 4C, the suppression of PECAM
expression by magnolol (20 μM) was recovered by co-treatment
with NAC (5 mM) and magnolol in mES/EB-derived endothelial-like
cells. These findings suggest that oxidative stress induced by
magnolol in part triggers apoptosis as the result of mitochondrial
dysfunction in mES/EB-derived endothelial-like cells.
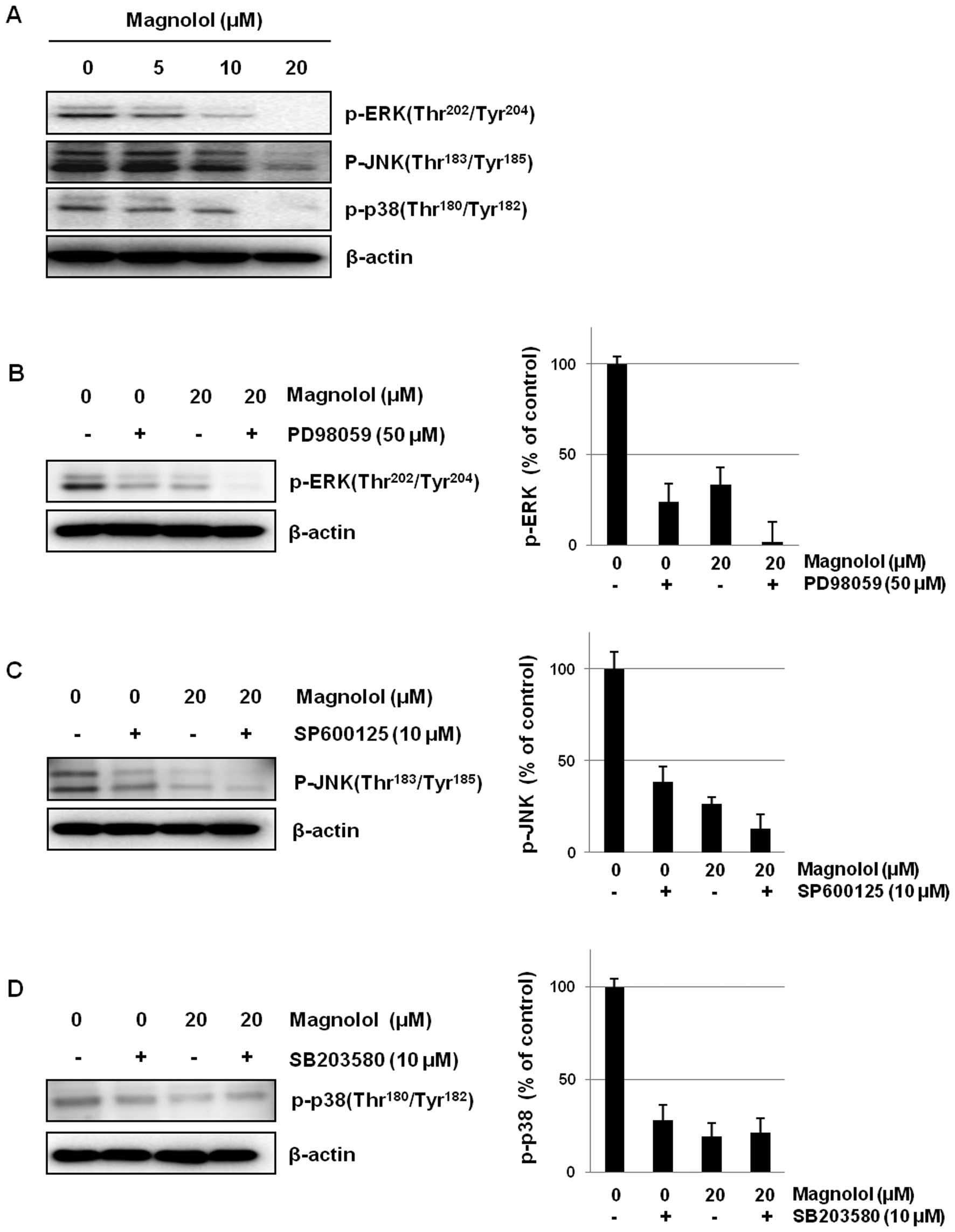
Effects of magnolol on MAP kinase
signaling
To further clarify whether the anti-angiogenic
activity of magnolol is also mediated by signaling molecules
related to cell proliferation, the effects of magnolol on MAPK
signaling were determined in mES/EB-derived endothelial-like cells.
Magnolol down-regulated the activation of MAP kinases including
ERK, JNK and p38 in mES/EB-derived endothelial-like cells (Fig. 5A). The association with MAPK
signaling was also confirmed using the inhibitors PD98059 (ERK
inhibitor), SP600125 (JNK inhibitor) and SB203580 (p38 inhibitor).
As shown in Fig. 5B–D, magnolol
suppressed the activation of MAPKs to an extent similar to that of
each kinase inhibitor in mES/EB-derived endothelial-like cells. In
addition, the co-treatment of cells with an ERK or JNK inhibitor
and magnolol (20 μM) enhanced the suppression of ERK and JNK
activation.
Effects of magnolol on the PI3K/AKT/mTOR
signaling pathway
Accumulating evidence suggests that the
PI3K/AKT/mTOR signaling pathway plays an important role in the
vasculogenesis and angiogenesis of endothelial cells (35,36).
To investigate whether magnolol affects this signaling pathway, the
constitutive activation of PI3K/AKT/mTOR expression was evaluated
in mES/EB-derived endothelial-like cells. As shown in Fig. 6A, the constitutive activation of
both PI3K and PDK was downregulated by treatment with magnolol in a
concentration-dependent manner. In addition, the downregulation of
AKT activation by magnolol led to the subsequent suppression of
signaling by mTOR and its downstream effectors, including S6K and
4EBP1, in mES/EB-derived endothelial-like cells. We also found that
co-treatment with the AKT inhibitor LY294002 and magnolol
synergistically suppressed the activation of AKT (Fig. 6B).
Discussion
Because angiogenesis is associated with many
pathological diseases, including cancer, diabetic retinopathy and
arthritis, anti-angiogenic agents with unique mechanisms of action
might be potential candidates to treat or prevent these diseases
(37,38). In the present study, we
demonstrated that magnolol exerts an anti-angiogenic activity
involving ROS-mediated apoptosis and the suppression of the
AKT/mTOR signaling pathway in mES/EB-derived endothelial-like
cells. A variety of assay systems have been developed to evaluate
the anti-angiogenic activity of test compounds (15,20).
In particular, the utility of stem cells has been highlighted in
recent reports (14,15). Recently, we also employed mouse
embryonic stem cells and demonstrated that mES/EB-derived
endothelial-like cells are a useful in vitro model system
for the evaluation of the anti-angiogenic activity of natural
products (20). Established 2-D
and 3-D culture systems using mES/EB-derived endothelial-like cells
were used to assess the characteristics of endothelial cells based
on the expression of PECAM and the formation of vascular networks.
Magnolol effectively inhibited vascular tube formation and
suppressed PECAM expression and vessel sprouting in the established
endothelial cell systems.
The induction of apoptosis is considered a plausible
anti-angiogenic mechanism (39–41).
In the present study with mES-derived EBs, we found that magnolol
might be able to induce apoptosis in mES/EB-derived
endothelial-like cells. The induction of apoptosis by magnolol was
confirmed by the increased percentage of Annexin V-positive cells
and the enhanced expression of the pro-apoptotic protein Bax. These
events subsequently activated caspase-3, thus leading to the
induction of apoptosis in the endothelial-like cells (Fig. 3). These findings suggest that the
induction of apoptosis by magnolol is in part associated with its
anti-angiogenic activity.
Accumulating evidence suggests that reactive oxygen
species (ROS) are important mediators in apoptosis. Increased
intra-cellular levels of ROS lead to the activation of apoptosis
(42). Based on the finding that
magnolol induces apoptosis in mES/EB-derived endothelial-like
cells, the association between ROS and the anti-angiogenic activity
of magnolol was determined. The cells were analyzed using an
oxidant-sensitive fluorescence dye (H2DCFDA) and
magnolol treatment (20 μM) significantly enhanced the
production of intracellular ROS in mES/EB-derived differentiated
endothelial-like cells. These results indicate that the apoptosis
induced by magnolol is due in part to the increased intracellular
levels of ROS in the cells. In addition, mitochondria play a
crucial role in metabolism and the cell cycle and they coordinate
extrinsic and intrinsic signals that affect proliferation,
differentiation and cell death. Mitochondria are the major
organelles that produce intracellular ROS and mitochondrial
dysfunction plays a key role in apoptosis (43). It is also known that increased ROS
levels are able to reduce the mitochondrial membrane potential,
thus leading to the activation of apoptotic pathways. The Rhodamine
123 dye was employed to assess the membrane potential and we found
that magnolol effectively decreased the mitochondrial membrane
potential, suggesting that the induction of apoptosis by magnolol
is correlated with the increase in intracellular ROS production and
the decrease in the mitochondrial membrane potential in
mES/EB-derived endothelial-like cells. The association between ROS
and the anti-angiogenic activity of magnolol was also partly
confirmed by the analysis of PECAM expression when the cells were
co-treated with the antioxidant NAC. The treatment with NAC
alleviated the ROS-mediated suppression of PECAM expression induced
by magnolol, indicating that the anti-angiogenic potential of
magnolol might be in part due to the regulation of ROS production
in mES/EB-derived endothelial-like cells.
Previous studies have demonstrated that the MAPK
signaling pathway is highly involved in cell growth (44) and/or the regulation of the cell
cycle (45). In our experiments,
magnolol suppressed the activation of MAPKs such as ERK, JNK and
p38 and these findings were confirmed using MAPK inhibitors in
mES/EB-derived endothelial-like cells. It is well-known that AKT
and its downstream signaling partners, including mTOR, are
activated during angiogenesis (46). AKT is a serine/threonine kinase
that plays a central role in a range of cellular functions,
including cell growth, proliferation, migration, protein synthesis,
transcription, survival and angiogenesis (9,47).
The mTOR kinase, a central regulator of cell metabolism, growth,
proliferation and survival, is also activated during various
cellular processes, such as tumor initiation, progression and
angiogenesis (35). Indeed, the
activation of the AKT/mTOR signaling pathway in endothelial cells
promotes the survival of these cells when cultured in vitro
(36). In the present study, we
found that magnolol suppressed the phosphorylation of AKT, mTOR,
S6K and 4EBP1 in mES/EB-derived endothelial-like cells. These
suppressive effects of magnolol might be an additional mechanism of
action explaining this compound’s anti-angiogenic activity in
mES/EB-derived endothelial-like cells.
In conclusion, the present findings demonstrate for
the first time that magnolol inhibits angiogenesis in mES/EB-
derived endothelial-like cells by regulating ROS-mediated apoptosis
and inhibiting the PI3K/AKT/mTOR signaling pathway.
Acknowledgements
This study was supported by the
National Research Foundation grant funded by the Korean Government
(MEST) (no. 2009-0083533).
References
|
1.
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Holderfield MT and Hughes CC: Crosstalk
between vascular endothelial growth factor, notch and transforming
growth factor-beta in vascular morphogenesis. Circ Res.
102:637–652. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Volz KS, Miljan E, Khoo A and Cooke JP:
Development of pluripotent stem cells for vascular therapy. Vascul
Pharmacol. 56:288–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Xu Z, Fang S, Zuo Y, et al: Combination of
pigment epithelium-derived factor with radiotherapy enhances the
antitumor effects on nasopharyngeal carcinoma by downregulating
vascular endothelial growth factor expression and angiogenesis.
Cancer Sci. 102:1789–1798. 2011. View Article : Google Scholar
|
|
5.
|
Sauer H, Gunther J, Hescheler J and
Wartenberg M: Thalidomide inhibits angiogenesis in embryoid bodies
by the generation of hydroxyl radicals. Am J Pathol. 156:151–158.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Abid MR, Kachra Z, Spokes KC and Aird WC:
NADPH oxidase activity is required for endothelial cell
proliferation and migration. FEBS Lett. 486:252–256. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
van Wetering S, van Buul JD, Quik S, et
al: Reactive oxygen species mediate Rac-induced loss of cell-cell
adhesion in primary human endothelial cells. J Cell Sci.
115:1837–1846. 2002.PubMed/NCBI
|
|
8.
|
Turrens JF: Mitochondrial formation of
reactive oxygen species. J Physiol. 552:335–344. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Yap TA, Garrett MD, Walton MI, Raynaud F,
de Bono JS and Workman P: Targeting the PI3K-AKT-mTOR pathway:
progress, pitfalls and promises. Curr Opin Pharmacol. 8:393–412.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
McCubrey JA, Lahair MM and Franklin RA:
Reactive oxygen species-induced activation of the MAP kinase
signaling pathways. Antioxid Redox Signal. 8:1775–1789. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
El-Najjar N, Chatila M, Moukadem H, et al:
Reactive oxygen species mediate thymoquinone-induced apoptosis and
activate ERK and JNK signaling. Apoptosis. 15:183–195. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Risau W, Sariola H, Zerwes HG, et al:
Vasculogenesis and angiogenesis in embryonic-stem-cell-derived
embryoid bodies. Development. 102:471–478. 1988.PubMed/NCBI
|
|
13.
|
Wang R, Clark R and Bautch VL: Embryonic
stem cell-derived cystic embryoid bodies form vascular channels: an
in vitro model of blood vessel development. Development.
114:303–316. 1992.PubMed/NCBI
|
|
14.
|
Li X and Claesson-Welsh L: Embryonic stem
cell models in vascular biology. J Thromb Haemost (Suppl). 1:53–56.
2009. View Article : Google Scholar
|
|
15.
|
Descamps B and Emanueli C: Vascular
differentiation from embryonic stem cells: novel technologies and
therapeutic promises. Vascul Pharmacol. 56:267–279. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Kawamura H, Li X, Goishi K, et al:
Neuropilin-1 in regulation of VEGF-induced activation of
p38MAPK and endothelial cell organization. Blood.
112:3638–3649. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Han HJ, Heo JS and Lee YJ:
Estradiol-17beta stimulates proliferation of mouse embryonic stem
cells: involvement of MAPKs and CDKs as well as protooncogenes. Am
J Physiol Cell Physiol. 290:C1067–C1075. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Heo JS, Lee YJ and Han HJ: EGF stimulates
proliferation of mouse embryonic stem cells: involvement of
Ca2+ influx and p44/42 MAPKs. Am J Physiol Cell Physiol.
290:C123–C133. 2006.PubMed/NCBI
|
|
19.
|
Kim GD, Rhee GS, Chung HM, Chee KM and Kim
GJ: Cytotoxicity of 5-fluorouracil: effect on endothelial
differentiation via cell cycle inhibition in mouse embryonic stem
cells. Toxicol In Vitro. 23:719–727. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Kim GD, Bae SY, Park HJ, Bae K and Lee SK:
Honokiol inhibits vascular vessel formation of mouse embryonic stem
cell-derived endothelial cells via the suppression of PECAM and
MAPK/mTOR signaling pathway. Cell Physiol Biochem. 30:758–770.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Ho JW and Jie M: Pharmacological activity
of cardiovascular agents from herbal medicine. Cardiovasc Hematol
Agents Med Chem. 5:273–277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Amblard F, Govindarajan B, Lefkove B, et
al: Synthesis, cytotoxicity and antiviral activities of new
neolignans related to honokiol and magnolol. Bioorg Med Chem Lett.
17:4428–4431. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park J, Lee J, Jung E, et al: In vitro
antibacterial and anti-inflammatory effects of honokiol and
magnolol against Propionibacterium sp. Eur J Pharmacol.
496:189–195. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Tsai YC, Cheng PY, Kung CW, et al:
Beneficial effects of magnolol in a rodent model of endotoxin
shock. Eur J Pharmacol. 641:67–73. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Park JB, Lee MS, Cha EY, et al:
Magnolol-induced apoptosis in HCT-116 colon cancer cells is
associated with the AMP-activated protein kinase signaling pathway.
Biol Pharm Bull. 35:1614–1620. 2012.PubMed/NCBI
|
|
26.
|
Chuang TC, Hsu SC, Cheng YT, et al:
Magnolol down-regulates HER2 gene expression, leading to inhibition
of HER2-mediated metastatic potential in ovarian cancer cells.
Cancer Lett. 311:11–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Lin SY, Liu JD, Chang HC, Yeh SD, Lin CH
and Lee WS: Magnolol suppresses proliferation of cultured human
colon and liver cancer cells by inhibiting DNA synthesis and
activating apoptosis. J Cell Biochem. 84:532–544. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Park EJ, Zhao YZ, Na M, et al: Protective
effects of honokiol and magnolol on tertiary butyl hydroperoxide-
or D-galactosamine-induced toxicity in rat primary hepatocytes.
Planta Med. 69:33–37. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Heuer J, Bremer S, Pohl I and Spielmann H:
Development of an in vitro embryotoxicity test using murine
embryonic stem cell cultures. Toxicol In Vitro. 7:551–556. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Scholz G, Pohl I, Genschow E, Klemm M and
Spielmann H: Embryotoxicity screening using embryonic stem cells in
vitro: correlation to in vivo teratogenicity. Cells Tissues Organs.
165:203–211. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Jakobsson L, Kreuger J, Holmborn K, et al:
Heparan sulfate in trans potentiates VEGFR-mediated angiogenesis.
Dev Cell. 10:625–634. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Newman PJ: The biology of PECAM-1. J Clin
Invest. 99:3–8. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Sauer H, Rahimi G, Hescheler J and
Wartenberg M: Effects of electrical fields on cardiomyocyte
differentiation of embryonic stem cells. J Cell Biochem.
75:710–723. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Sauer H and Wartenberg M: Reactive oxygen
species as signaling molecules in cardiovascular differentiation of
embryonic stem cells and tumor-induced angiogenesis. Antioxid Redox
Signal. 7:1423–1434. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Karar J and Maity A: PI3K/AKT/mTOR pathway
in angiogenesis. Front Mol Neurosci. 4:512011. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Olsson AK, Dimberg A, Kreuger J and
Claesson-Welsh L: VEGF receptor signalling-in control of vascular
function. Nat Rev Mol Cell Biol. 7:359–371. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Sato Y: The vasohibin family: a novel
family for angiogenesis regulation. J Biochem. 153:5–11. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Frampton JE: Ranibizumab: in diabetic
macular oedema. Drugs. 72:509–523. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Hong SW, Jung KH, Lee HS, et al: SB365
inhibits angiogenesis and induces apoptosis of hepatocellular
carcinoma through modulation of PI3K/Akt/mTOR signaling pathway.
Cancer Sci. 103:1929–1937. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Hong SW, Jung KH, Lee HS, et al: Apoptotic
and anti-angiogenic effects of Pulsatilla koreana extract on
hepatocellular carcinoma. Int J Oncol. 40:452–460. 2012.PubMed/NCBI
|
|
41.
|
Nishikawa T, Tsuno NH, Okaji Y, et al: The
inhibition of autophagy potentiates anti-angiogenic effects of
sulforaphane by inducing apoptosis. Angiogenesis. 13:227–238. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Xu HL, Tang W, Du GH and Kokudo N:
Targeting apoptosis pathways in cancer with magnolol and honokiol,
bioactive constituents of the bark of Magnolia officinalis. Drug
Discov Ther. 5:202–210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Xia Z, Dickens M, Raingeaud J, Davis RJ
and Greenberg ME: Opposing effects of ERK and JNK-p38 MAP kinases
on apoptosis. Science. 270:1326–1331. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
McCubrey JA, Steelman LS, Chappell WH, et
al: Roles of the Raf/MEK/ERK pathway in cell growth, malignant
transformation and drug resistance. Biochim Biophys Acta.
1773:1263–1284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Cho DH, Choi YJ, Jo SA, et al:
Troglitazone acutely inhibits protein synthesis in endothelial
cells via a novel mechanism involving protein phosphatase
2A-dependent p70 S6 kinase inhibition. Am J Physiol Cell Physiol.
291:C317–C326. 2006. View Article : Google Scholar
|
|
47.
|
Manning BD and Cantley LC: AKT/PKB
signaling: navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|