Introduction
African natives infrequently visit public health
services and drugs and therapies are usually too expensive for
their standard of living. Therefore, they rely heavily on
ethnomedicine and ancient traditions, preserved from the past, in
order to cure and prevent their pathologies (1,2). In
African communities, medicinal plant identification, processing and
administration to patients are commonly consigned to ‘traditional
healers’ who receive relative guidelines by their ancestors during
the dreams. These folkloric approaches are essentially based on the
use of plant extracts directly on wounds or as treatment by oral
ingestion (3). Many studies have
been performed to ascertain the scientific basis of African plant
biological effects. In particular, they have demonstrated how these
vegetal compounds were characterized by a great amount of secondary
metabolites with antiradical power (4–6).
Environmental conditions highly influence the levels and the
quality of these compounds that, in nature, are synthesized only by
plants, to protect themselves from biotic and abiotic factors or to
facilitate their propagation (7).
Since in the equatorial and tropical climates, UV exposure and
temperatures are extreme, African plant phyto-complexes have been
shown to be richer in antioxidant molecules than sub-tropical and
temperate ones (8). Alkaloids,
tannins, steroids, terpenoids, terpenes, flavonoids, simple
phenols, phenolic acids and glycosides have been recognized as the
principal metabolites whose powerful molecular activity has made
African plant extracts real alternative medicines, especially, on
arthritis, flu, diabetes, psoriasis, tuberculosis, ulcers,
infections, asthma, hypertension, central nervous system disorders,
laryngitis, liver disorders, bronchitis and also cancer (2,9,10).
After all, >50% of modern drugs are composed by molecules of
vegetal origin because of their inexpensive production costs, high
bioactive functions and low toxicity with respect to artificial
chemical substances (4). The
present study was conducted with the aim to find vegetal substances
able to represent efficient and alternative substitutions to the
actual tumor chemotherapeutics, reported to have serious
side-effects for patients (11).
In this study, different plant extracts, normally used in African
local tradition as natural drugs, were characterized by their free
radical scavenging activity and total phenolic content. The most
antioxidant ones were also evaluated for their antiproliferative
and differentiative effects on the B16F10 murine melanoma cell
line. The knowledge of the medicinal plant properties on biological
systems and the individuation of the molecular mechanisms that they
can activate are essential aspects for the identification and the
development of new drugs as well as to give scientific basis to
African medical culture.
Materials and methods
Plant material and extract
preparations
African plant materials (Table I) were collected, by an indigenous
‘traditional healers’, in the Cameroon forests (Central Africa) and
then dried, under the sun, until they were completely dehydrated.
Plant extracts were obtained in the laboratory according to African
tradition. Briefly, samples were ground with pestle, mortar and
liquid nitrogen and then boiled in bidistilled water (1 mg/ml) for
1 h. Extracts were filtered (0.22 μm) and finally stored at
−20°C.
 | Table IAfrican plant extracts. |
Table I
African plant extracts.
| Source | Section of the
plant | CODE |
|---|
| Moringa
oleifera Lam. - (Central region) | Leaves | MOC |
| Hibiscus
cannabinus L. | Leaves | HC |
| Mormodica
charantia L. | Leaves | MC |
| Zingiber
officinale Roscoe | Root | ZO |
| Pausinystalia
johimbe (K. Schum.) Pierre ex Beille | Cortex | PJ |
| Enantia
chlorantha Oliv. | Cortex | EC |
| Eremomastax
speciosa (Hochst.) Cufod. | Leaves | ES |
| Moringa
oleifera Lam. - (North region) | Leaves | MN |
| Moringa
oleifera Lam. - (North region) | Root | MNR |
| Moringa
oleifera Lam. - (North region) | Cortex | MNC |
| Moringa
oleifera Lam. - (North region) | Flowers | MNF |
| Moringa
oleifera Lam. - (North region) | Seeds | MNS |
| Aframomum
melegueta K. Schum. | Seeds | AM |
| Aframomum
pruinosum Gagnep. | Seeds | AP |
Total phenolic content
Total phenolic content was assessed by
Folin-Ciocalteau assay (12).
Briefly, 9 ml of ddH2O and 1 ml of Folin-Ciocalteau
reagent (Sigma-Aldrich) were added to 1 ml of each extract. After 5
min, the solution was supplemented with 10 ml of
Na2CO3 7% (w/v) and 4 ml of ddH2O,
vortexed and incubated at room temperature for 1 h in the dark.
Total phenolic concentration was detected by measuring the sample
absorbance at 760 nm (UV-visible spectrophotometer Cary 50, Bio
Varian), with respect to a caffeic acid calibration curve (20–100
mg/l). Results are expressed as μg of caffeic acid equivalents per
mg of dried sample (μg CAE/mg DW).
FRAP antiradical test
FRAP assay was performed as previously reported
(13). Plant extract (200 μl) was
mixed with 1.8 ml FRAP reagent (10 mM TPTZ in 40 mM HCl, 20 mM
FeCl3, 0.3 M acetate buffer pH 3.6; 1:1:10 v/v/v) and
placed in the dark, at 37°C for 10 min. This test evaluated the
capacity of natural antioxidants to reduce the colorless Fe III -
tripyridyltriazine compound (Merck) to the blue-colored Fe II form,
measuring sample absorbance change at 593 nm. Ascorbic acid (AA in
ddH2O) was used to obtain a standard solution (50–500
μM). Results are expressed as μg of ascorbic acid equivalents per
mg of dried sample (μg AA/mg DW).
DPPH antioxidant assay
According to Brand-Williams et al(14), plant extract antioxidant activity
was measured by the determination of its scavenging property
against the stable free radical 2,2-diphenyl-1-picrylhydrazyl
(DPPH, Merck). In brief, the absorbance decrease, at 517 nm, of a
100 μM DPPH methanolic solution was monitored, after 30 min of
sample addition. The antiradical effect of the extract is reported
as IC50 (sample concentration causing 50% of DPPH
activity reduction with respect to the control).
Cell culture, treatments, proliferation
assays and microscopic observations
Highly metastatic B16F10 murine melanoma cells were
grown and propagated in Dulbecco’s modified Eagle’s medium (D-MEM),
supplemented as reported in Gismondi et al(15), under standard culture conditions
(16). To study African plant
antiproliferative effects, melanoma cells were seeded in 35-mm
dishes and treated with vegetal extracts (2 mg of plant dried
weight per ml of cell culture media) for 24, 48 and 72 h (control
cells were treated with phosphate-buffered saline). Other treatment
concentrations were also tested (data not shown). Cell
proliferation and treatment cytotoxicity were evaluated by counting
cells, with a Neubauer modified chamber, after trypan blue staining
(1%, w/v). In addition, cell growth, measured as function of
mitochondrial activity, was analyzed by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
kit (Sigma). Microscopic observations were also performed on cells
by optical microscope (20X) (Nikon, TE2000-PFS).
Flow cytometry analysis
Cells were washed twice in phosphate-buffered saline
and fixed for 30 min at 4°C in cold methanol:acetone (4:1)
solution. Then, cells have been treated, at room temperature, for
20 min with RNase A (100 μg/μl) and for further 20 min with
propidium iodide (1 mg/ml), and analyzed by FACSCalibur instrument
(Becton-Dickinson) and the percentage of cells in the different
cell cycle phases was measured by CellQuest software.
Western blotting
Cells were harvested, resuspended in RIPA lysis
buffer, containing 1% protease inhibitor cocktail, and centrifuged
at 13,000 rpm for 30 min at 4°C. Protein concentration was
quantified by Bradford method (17), using bovine serum albumin as
standard. Proteins were separated on 12% sodium dodecyl
sulfate-polyacrylamide gel and transferred onto Protran
nitrocellulose membrane (Schleicher and Schuell). Blots were
incubated with the following primary antibodies: rabbit polyclonal
anti-β-actin (Sigma), mouse monoclonal anti-p53 (Santa Cruz), mouse
monoclonal anti-p27Kip1 (BD Pharmingen), mouse
monoclonal anti-p21WAF1/Cip1 (Sigma) and goat polyclonal
anti-MITF (microphthalmia-associated transcription factor, Santa
Cruz). Finally, primary antibodies were revealed using horseradish
peroxidase-conjugated anti-rabbit or anti-mouse or anti-goat
antibodies (Sigma) and an ECL chemiluminescence detection system
(Pierce). Signal detection and quantification was executed,
respectively, by VersaDoc Imaging System and Quantity One software
(Bio-Rad).
Tyrosinase activity and melanin
content
In vitro L-3,4-dihydroxyphenylalanine
(L-DOPA) oxidation by protein extracts was used as direct indicator
of cell tyrosinase activity, as previously described (18). In summary, treated and untreated
cells were resuspended in 10 ml of lysis buffer (50 mM sodium
phosphate buffer pH 6.8, Triton X-100 1% and 0.1 mM
phenylmethylsulfonyl fluoride) and frozen at −80°C for 30 min.
Then, cell extracts were centrifuged at 12000 rpm for 30 min at
4°C. The supernatant (±8 ml) was mixed with 2 ml of L-DOPA (2
mg/ml) and the absorbance at 492 nm of the solution was monitored,
after incubation for 1 h at 37°C, by the UV-visible
spectrophotometer, Cary 50 (Bio Varian). Intracellular melanin
quantification was measured and performed as suggested by Lotan and
Lotan (19). Briefly, cells were
harvested and lysed (Tris-HCl 50 mM pH 7.5, EDTA 2 mM, NaCl 150 mM,
Triton X-100 1%, protease inhibitor 1%). Then samples were
sonicated for 30 sec and centrifuged at 1400 rpm for 5 min. The
supernatant was used for sample protein quantization (17). The pellet was washed twice with 1
ml of ethanol:diethyl ether (1:1) and finally resuspended in
NH4OH 1 M at 37°C until it was completely dissolved. The
melanin amount in the solution was determined by analyzing the
absorbance value at 475 nm (UV-visible spectrophotometer Cary 50,
Bio Varian). Results are expressed as μg melanin per mg of cell
proteins.
Statistical analysis
All experiments were repeated in triplicate and the
relative results are shown as the mean ± standard error of the mean
(SEM) of the three independent measurements. Analysis of variance
was conducted using one-way ANOVA test with SPSS (ver.19 ita) for
Microsoft and the means were compared by Duncan tests. All p-values
were <0.05 versus vehicle control-treated cells.
Results
Secondary metabolites and antioxidant
properties
Fourteen African plants (Table I) were processed and subjected to
aqueous extraction, as described in Materials and methods. The
amount of phenolic compounds was measured in each extract by a
spectrophotometric analysis (Fig.
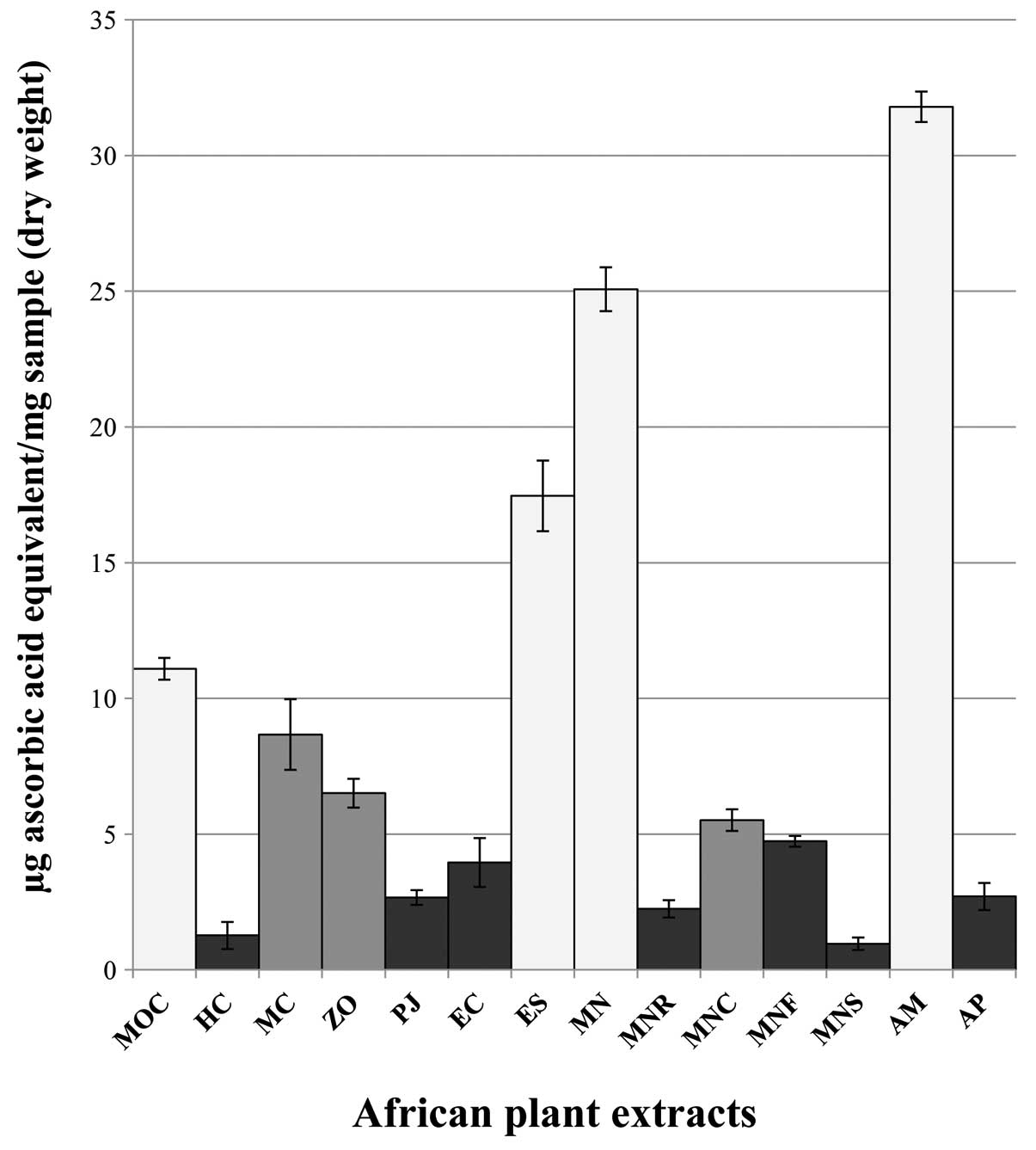
1). ES, MN, MOC, MC and AM samples showed the highest aromatic
secondary metabolite contents, respectively, 59.58, 55.83, 39.17,
33.33 and 32.92 μg CAE/mg DW. FRAP and DPPH assays were performed
in order to determine the antiradical power of Cameroon plant
extracts. The FRAP test (Fig. 2)
allowed us to separate samples in three principal clusters,
according to their antioxidant activity: the first group (including
HC, PJ, EC, MNR, MNF, MNS and AP samples) revealed a radical
scavenging property <5 μg AA/mg DW; the second cluster, made up
of MC, ZO and MNC specimens, evidenced intermediate values (between
5 and 10 μg AA/mg DW) whilst the best antioxidant extracts (MOC,
ES, MN and AM) exhibited a reducing activity >10 μg AA/mg DW. In
DPPH assay (Fig. 3), MOC, MC, MN,
ES, MNC, MNF and AM extracts were identified as the strongest
antiradical solutions. In particular, they respectively, presented
an IC50 value of 0.94, 0.80, 0.99, 0.36, 0.77, 0.61 and
0.55 μg extract per ml. In conclusion, in vitro tests showed
MN, ES and AM were the most antioxidant samples.
Effects on cell growth and
proliferation
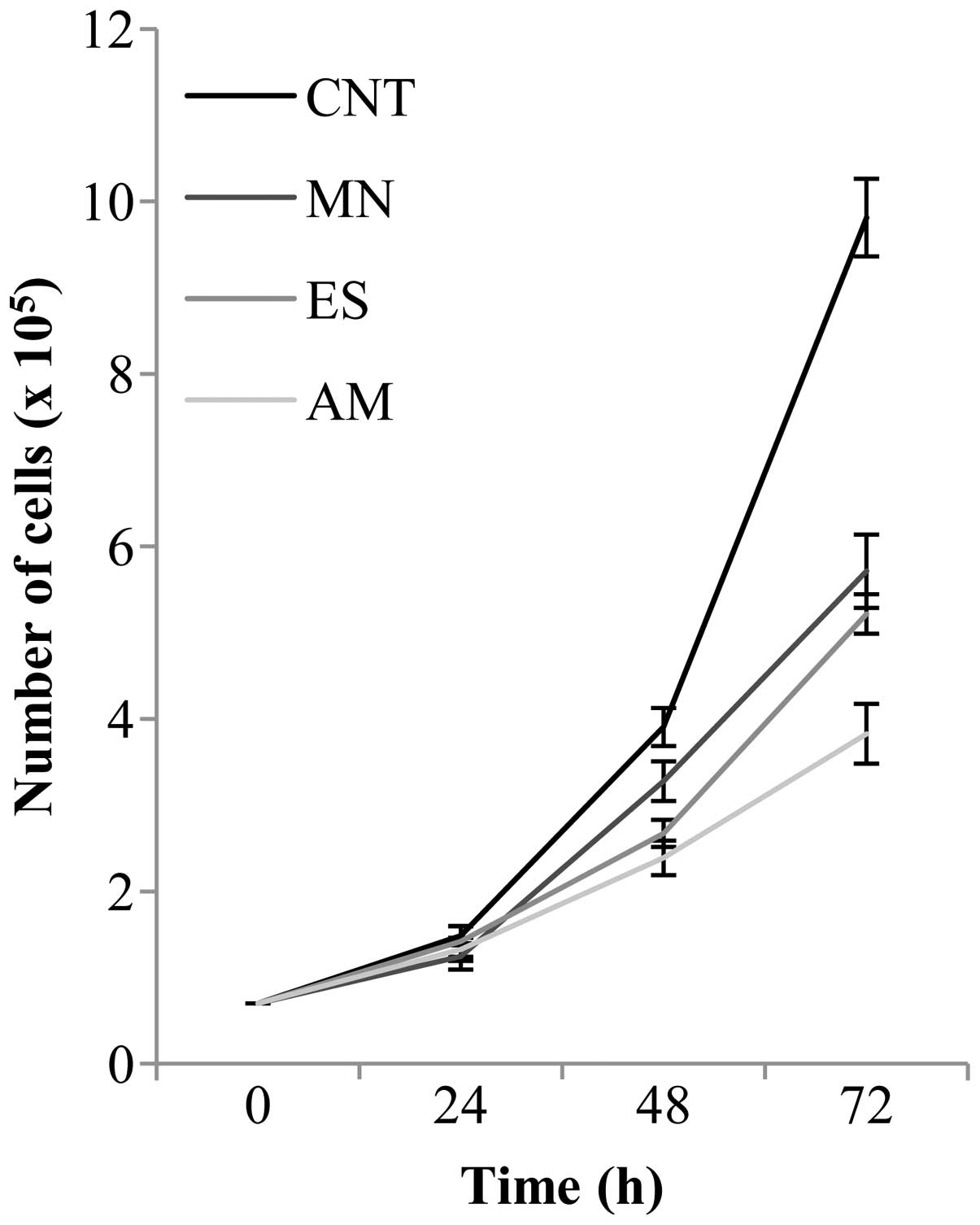
B16F10 murine melanoma cells were treated for 24, 48
and 72 h with 2 mg/ml of MN, ES and AM extracts in order to analyze
their effects on cell proliferation (Fig. 4). Proliferative tests were also
performed with other treatment concentrations but data are not
shown in this study because of irrelevance or the excessive
effects. Cells incubated for 24 h with African samples did not show
significant changes in cell growth, with respect to the control
(CNT). On the other hand, treatments with MN, ES and AM for 48 h
induced a reduction of cell proliferation, compared to control
cells, of ~16, 32 and 39%, respectively. After 72 h of incubation,
plant extracts caused the decrease of the number of cells of ~42
(MN), 47 (ES) and 61% (AM), with respect to the control. To
determine if natural solutions could be toxic for cells, trypan
blue exclusion test was also performed. As reported in Table II, treatments for 24 h did not
cause any cell injury: in all cases, toxicity was <2% with
respect to control cells. After 48 h, ES and AM solutions still
showed slight cytotoxicity (<6%) whilst MN extract was ~12%,
compared to control cells. Cells remained highly viable also after
72 h of contact with ES and AM extracts (only ~7% of toxicity was
detected); in contrast, MN treatment induced the death of ~22% of
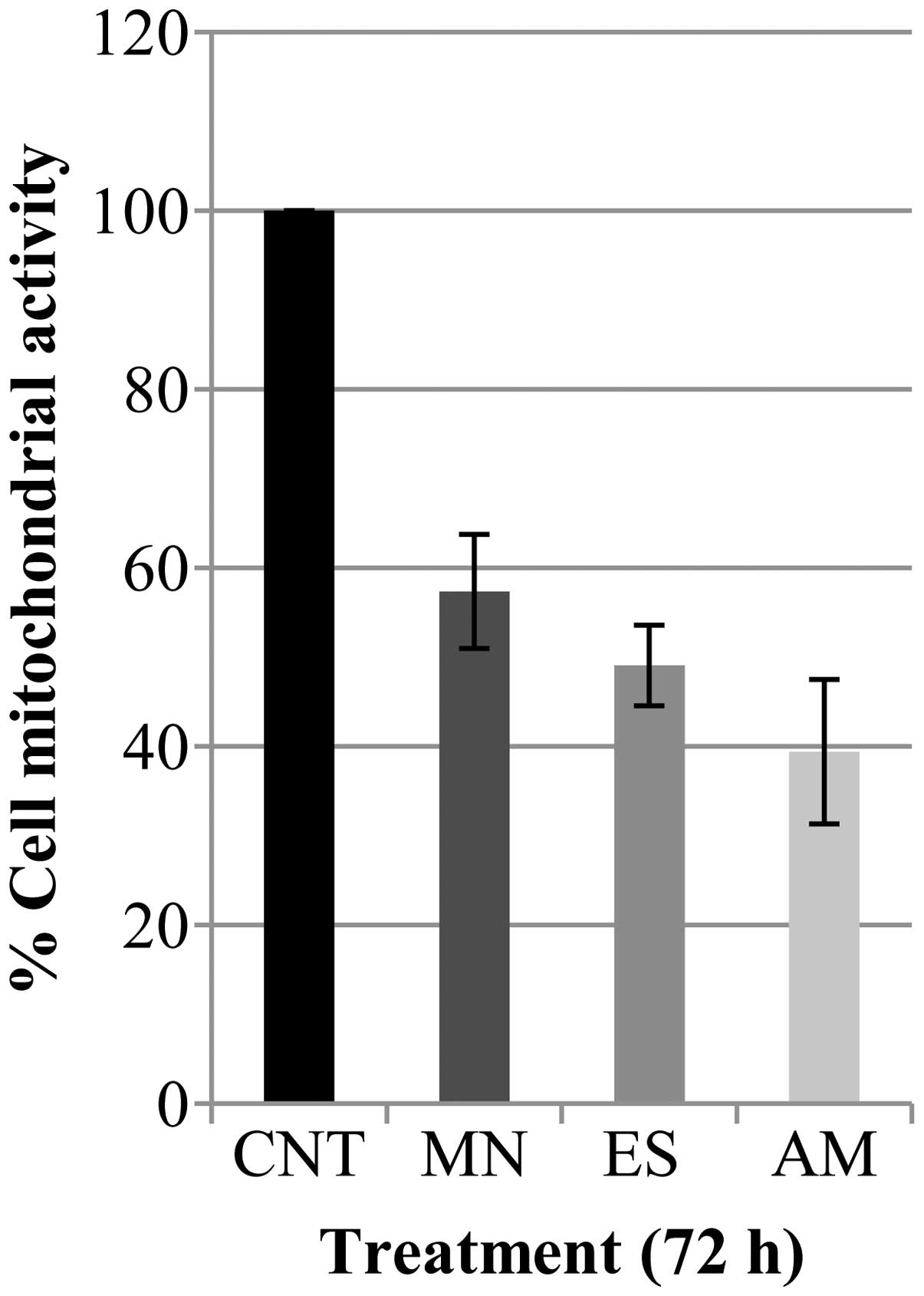
cells, with respect to the control. Cell proliferation, after
treatment with Cameroon plant extracts for 72 h, was further
monitored by MTT assay (Fig. 5).
MN, ES and AM treatments produced a decrease of cell growth of
42.7, 50.9 and 39.4%, respectively, compared to control cells.
 | Table IICytotoxicity percentage of African
extracts on B16F10 cells. |
Table II
Cytotoxicity percentage of African
extracts on B16F10 cells.
| Treatment
(hours) | CNT (%) | MN (%) | ES (%) | AM (%) |
|---|
| 24 | 5.3 | 7.1 | 5.7 | 6.5 |
| 48 | 4.1 | 16.5 | 9.5 | 9.8 |
| 72 | 4.4 | 26.3 | 10.8 | 11.2 |
Analysis of the cell cycle and related
proteins
In order to verify if the previous reduction of
B16F10 cell proliferation was associated with cell cycle
modifications, FACS analysis was carried out. After treatment for
72 h with the different African preparations, very contrasting cell
cycle profiles were revealed (Fig.
6), compared to the control (CNT). In particular, the amount of
apoptotic cells, that was minimal in the control (2.6%), increased
after MN treatment (21.2%) with respect to ES and AM, whose sub-G1
events were only 3.8 and 4.9%, respectively. As indicated in
Fig. 7, with respect to the
control, MN treatment produced an increase in G2/M phase (10.9%),
ES solution induced an accumulation of cells (13.4%) between the S
and G2/M peaks whilst AM extract clearly caused the arrest of the
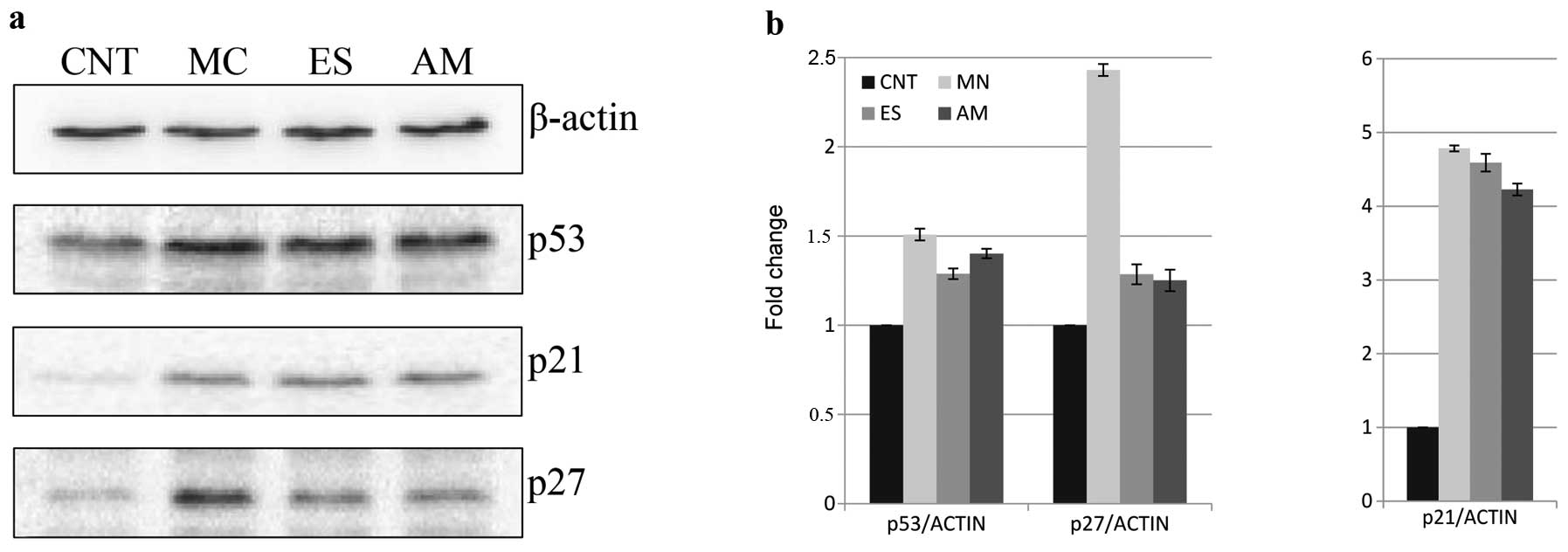
cell cycle in G1 phase (13.9%). Protein extraction was performed on
B16F10 cells and the principal cell cycle regulators were detected
by western blotting (Fig. 8). MN,
ES and AM solutions enhanced p53 levels 50.8, 28.8 and 40.1%, in
this order, with respect to the control. Similarly,
p21WAF1/Cip1 content was highly increased following the
treatments (>300% of the control). As a final point,
p27Kip1 amount was investigated in cell protein
extracts: the levels augmented of 29 and 25%, respectively, with ES
and AM extracts and, remarkably, of 143% following MN treatment,
with respect to control cells.
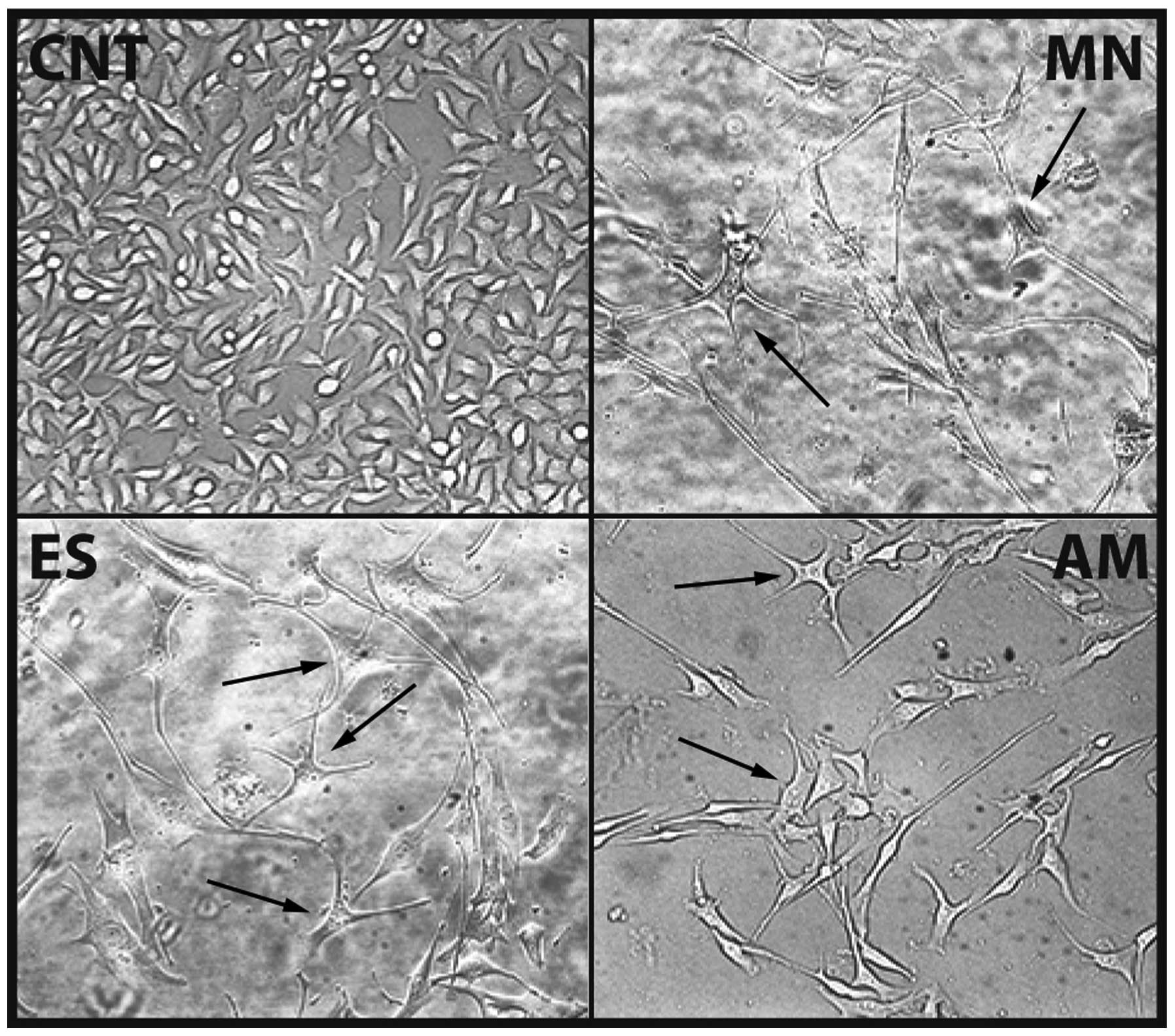
Differentiation induction
Differentiative properties of Cameroon plant
extracts on B16F10 cell line, were also checked. After treatment
for 72 h with MN, ES and AM extracts, with respect to the control,
cells evidenced great morphological changes and decrease of cell
density. Optical microscope images (Fig. 9) clearly showed how treated cells
(especially MN and ES) had developed cytoplasmic dendritic
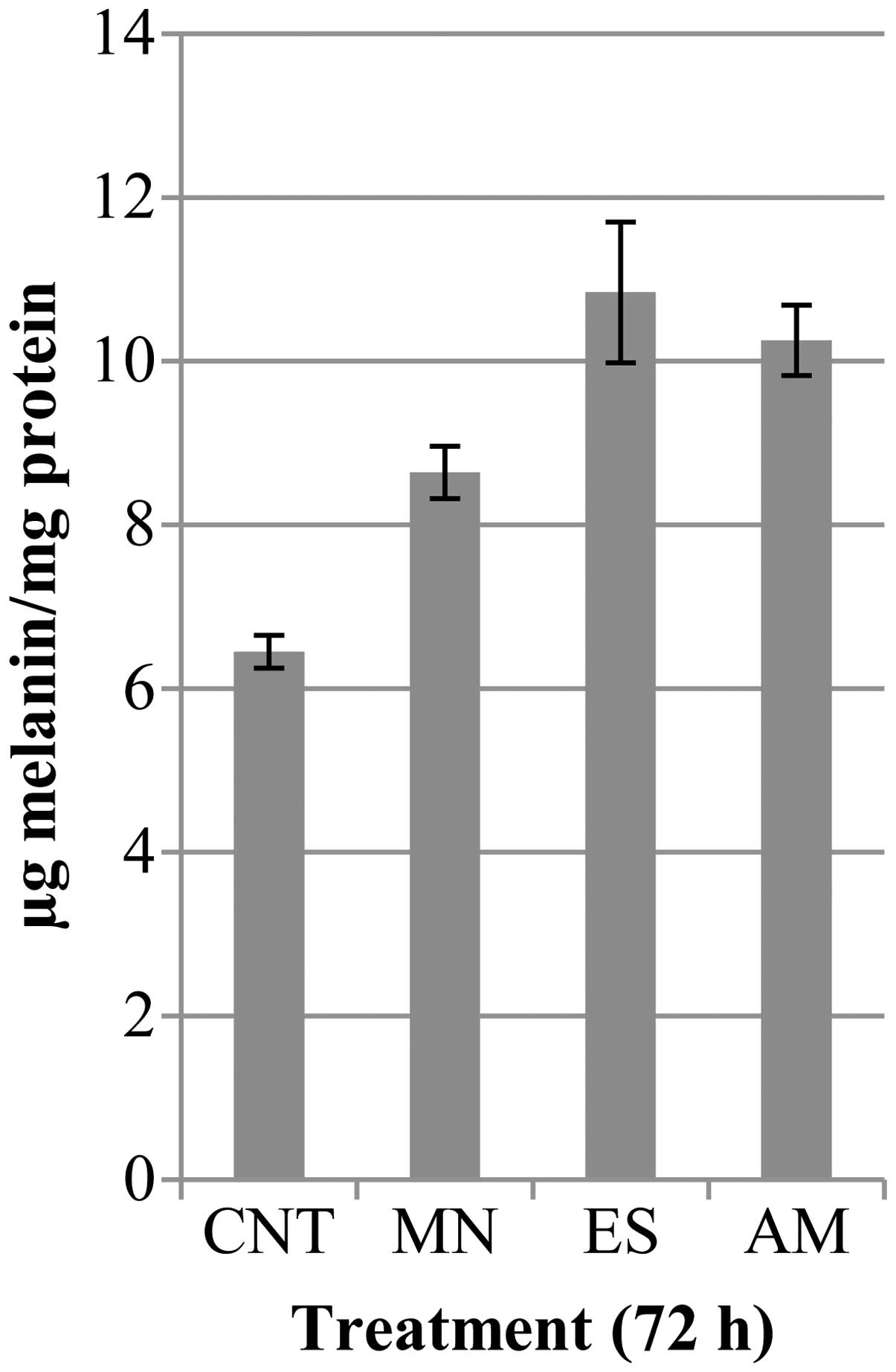
protrusions and acquired a star shape. Melanin amount (Fig. 10) and tyrosinase activity
(Fig. 11) were studied in cells
after exposure to African preparations for 72 h. With respect to
control cells, MN, ES and AM treatments, respectively, increased
cellular pigment levels of 33.9, 68 and 59% and enzyme activity of
1.6-, 2.2- and 2.1-fold. In addition, MITF protein was detected in
MN, ES and AM protein samples: respectively, western blot analysis
revealed an increase of the transcription factor 1.6-, 1.8- and
1.9-fold, compared to control cells, as shown in Fig. 12).
Discussion
The detection and characterization of plant
compounds have recently attracted research attention because of
their impact on human health and economy. The great biological
activity of these molecules and the low costs of their natural
synthesis are largely substituting the production of the modern
synthetic drugs (4). By contrast,
in Africa the use of plant extracts as medicine is an actual and
very common practice that hails from ancient rituals. Nevertheless,
Africans have often attributed and associated plant therapeutic
features to spiritualist agents (20). The aim of this study was to
investigate the health giving properties of African plant extracts
(Table I), collected from Cameroon
forests, that indigenous peoples daily employ in ethnomedicine. We
planned to identify the antineoplastic effects of plant sample
preparations on murine cancer cells. We decided to carry out plant
extracts by using hot water, even if a more organic and less polar
solvent would have had a greater capacity in the extraction of
plant compounds (21), in order to
obtain solutions that would be similar to the vegetal preparations
used by African natives. Oxidative and reducing processes are
essential for cell survival but when their equilibrium is
imbalanced cellular stability is altered. In particular, high
levels of reactive oxygen species (ROS) have been demonstrated able
to induce cell structural damage and apoptosis (22,23).
Plant molecules, in general, have been recognized as strong
antiradical compounds able to reduce cell oxidation: therefore, a
correct diet, rich in vegetables and fruits, is considered an
important factor for the prevention of several diseases, including
cancer, by its ability to prevent and to rescue oxidative stress
(24). Therefore, we begun this
research by analyzing sample antioxidant activity. Secondary
metabolites in plants can approximately range between 6.8 and 32.1
μg CAE/mg DW, although it highly depends on plant environmental and
physiological conditions (25). In
this study, ES and MN extracts were demonstrated to possess a total
phenolic content that abundantly exceeded these values (Fig. 1). Moreover, the same plant species
and AM sample also showed the strongest antioxidant properties
(Figs. 2 and 3), with respect to the other African
extracts and correlated literature data (26–28).
Another important result was that M. oleifera (North region)
leaves (MN) showed the most conspicuous quantity of free radical
scavenging molecules with respect to the other plant districts of
the same species (MNR, MNF, MNC and MNS samples). Over the past few
decades, African plant features have been largely described in
scientific publications (6,9,10).
In other reports, M. oleifera has been demonstrated a rich
source of ascorbic acid, oestrogenic substances, iron, calcium,
phosphorus, copper, vitamins, riboflavin, nicotinic acid, folic
acid, pyridoxine, β-carotene, proteins and essential amino acids
(29). Its extract also exhibit
antibacterial, antifungal, antihypertensive, diuretic,
hepatoprotective and cholesterol lowering activities (30,31).
Anticancer properties of this plant have been studied both in
vivo on mice and more rarely in vitro on tumor cell
lines (32,33). On the other hand, E.
speciosa and A. melegueta species have shown antifungal,
anti-inflammatory, antibacterical, gastro-protective and
fertilizing effects (34–36). However, no report has been clearly
focused on their antitumoral properties. Therefore, special core of
this study was the investigation of the effects and the molecular
mechanisms that MN, ES and AM preparations could activate and/or
regulate on the melanoma B16–F10 murine cell line. We evaluated the
effect of these solutions on cell viability. Trypan blue test
(Table II) showed that ES and AM
extracts had no toxic effects on cells, as expected because of
their simple processing in water and the naturalness of their
contents. Instead, surprisingly, MN treatment caused the death of
~22% of total cells, with respect to the control. Different plant
extracts contain diverse metabolite profiles (7) and, probably, MN phyto-complex
presented one or more compounds, absent in ES and AM, able to
induce cell instability and death (33). However, after treatment for
different times with MN, ES and AM extracts, cell proliferation
(Fig. 4) and growth (Fig. 5) were greatly reduced. Cell cycle
alteration and apoptosis inhibition are the principal
characteristics of cancer cells (37); therefore, FACS analysis was
performed to clarify if treated cells could undergo cell cycle
modifications in order to justify the decrease of cell
proliferation. As anticipated, samples produced different effects
on the cell cycle: MN induced a G2/M phase arrest, ES associated
the block of cells both in S phase and in G2/M and finally AM
caused a G0–G1 stop (Fig. 7).
Moreover, a large amount of apoptotic nuclei, detected by propidium
iodide staining, was only observed in the sub-G1 area of MN
specimen (Fig. 6); it confirmed
further the results obtained in the exclusion test (Table II). It is well-known that p53 is a
tumor suppressor protein: ~80% of cancer cells are characterized by
alterations in p53 gene or activity. When DNA damage occurs in
cells, p53 is activated and accumulated in the nucleus where it
promotes the transcription of different genes involved in DNA
repair, cell growth arrest and apoptosis thus preventing
cancinogenesis (38). Of the p53
targets, the CIP/KIP p21 and p27 cyclin-dependent kinase (CDK)
inhibitors are the most investigated genes because of their ability
to induce cell cycle arrest (39).
The study of these protein markers was complementary to
cytofluorimetric analysis and essential for clarifying the basal
molecular mechanisms that might have induced the inhibition of the
proliferation. We demonstrated that all treatments (MN, ES and AM)
enhanced p53, p21WAF1/Cip1 and p27Kip1
protein levels in B16F10 melanoma cells (Fig. 8). Probably, the African plant
extracts produced in cells induction of p53 that consequently
stimulated the p21WAF1/Cip1 and
p27Kip1-dependent cell cycle arrest. Different cell
cycle profiles (Fig. 7) could be
explained by a different activity of p21WAF1/Cip1 and
p27Kip1 in melanoma cells. Coqueret (39) reported that these two proteins
inhibit all CDK complexes, without a specific restriction for a
particular cell phase. Therefore, an increase in
p21WAF1/Cip1 or p27Kip1 can be easily
associated with both G0–G1 and S or G2-M phase block (40–42).
A remarkable increase of p27Kip1 level was detected in
an MN sample (Fig. 8). The
overexpression of this protein might be the reason, or the
consequence, of the activation of an apoptotic pathway in cells; in
fact, it is proved that a great p27Kip1 increment
triggers apoptosis in different cell lines (43,44).
This preliminary hypothesis that should be further confirmed by
other experiments, would explain the large amount of dead cells
detected only after treatment with MN (Figs. 6 and 7). Alteration in cell morphology, with
the development of dendritic protrusions, reduction of
proliferation and activation of melanogenesis have been considered
specific indicators of differentiation for melanoma cell lines
(45,46). The induction of this process in
B16F10, after treatment with Cameroon extracts, was firstly and
clearly suggested by cell acquisition of typical cytoplasmic
extensions (Fig. 9). In
literature, it has been well documented that p53 is a
transcriptional regulator in melanogenesis. Via
p21WAF1/Cip1, it positively regulates the promoter of
microphthalmia-associated transcription factor (MITF). The latter
plays a central role in the expression of melanocyte-specific genes
such as tyrosinase, the key enzyme of melanin synthesis (47). Melanin production (Fig. 10), tyrosinase activity (Fig. 11) and MITF levels (Fig. 12) were highly enhanced in all
samples. These results rigorously and scientifically confirmed the
antioxidant, antiproliferative and differentiative effects of the
MN, ES and AM extracts that commonly Cameroon indigenous people
adopt as food sources of medicinal compounds. By merging the
knowledge of African tradition and laboratory evidences it might
identify new nutraceutical products able to improve human health
and to prevent cancer.
References
|
1
|
Steenkamp V, Mathivha E, Gouws MC and van
Rensburg CEJ: Studies on antibacterial, antioxidant and fibroblast
growth stimulation of wound healing remedies from South Africa. J
Ethnopharmacol. 95:353–357. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ojewole JAO: Antinociceptive,
anti-inflammatory and antidiabetic properties of Hypoxis
hemerocallidea Fisch. & CA Mey (Hypoxidaceae) corm
[‘African Potato’] aqueous extract in mice and rats. J
Ethnopharmacol. 103:126–134. 2006.PubMed/NCBI
|
|
3
|
Soladoye MO, Amusa NA, Raji-Esan SO,
Chukwuma EC and Taiwo AA: Ethnobotanical survey of anti-cancer
plants in Ogun State, Nigeria. Ann Biol Res. 1:261–273. 2010.
|
|
4
|
Farombi OE: African indigenous plants with
chemotherapeutic potentials and biotechnological approach to the
production of bioactive prophylactic agents. Afr J Biotechnol.
2:662–671. 2003. View Article : Google Scholar
|
|
5
|
Scott G, Springfield EP and Coldrey N: A
pharmacognostical study of 26 South African plant species used as
traditional medicines. Pharm Biol. 42:186–213. 2004. View Article : Google Scholar
|
|
6
|
Atawodi SE: Antioxidant potential of
African medicinal plants. Afr J Biotechnol. 4:128–133. 2005.
|
|
7
|
Manach C, Scalbert A, Morand C, Remesy C
and Jimenez L: Polyphenols: food sources and bioavailability. Am J
Clin Nutr. 79:727–747. 2004.PubMed/NCBI
|
|
8
|
Abegaz BM, Ngadjui BT, Dango E and
Bezabith MT: Chemistry of the genus Dorstenia psiurus. Curr Org
Chem. 4:107–109. 2000. View Article : Google Scholar
|
|
9
|
Edeoga HO, Okwu DE and Mbaebie BO:
Phytochemical constituents of some Nigerian medicinal plants. Afr J
Biotechnol. 4:685–688. 2005. View Article : Google Scholar
|
|
10
|
Jiofack T, Fokunang C, Guedje N, Kemeuze
V, Fongnzossie E, Nkongmeneck BA, Mapongmetsem PM and Tsabang N:
Ethnobotanical uses of some plants of two ethnoecological regions
of Cameroon. AJPP. 3:664–684. 2009.
|
|
11
|
Steenkamp V and Gouws MC: Cytotoxicity of
six South African medicinal plant extracts used in the treatment of
cancer. S Afr J Bot. 72:630–633. 2006. View Article : Google Scholar
|
|
12
|
Singleton VL and Rossi JJA: Colorimetry of
total phenolics with phosphomolybdic-phosphotungstic acid reagents.
Am J Enol Viticul. 16:144–158. 1965.
|
|
13
|
Benzie IFF and Strain JJ: The ferric
reducing ability of plasma (FRAP) as a measure of antioxidant
power: the FRAP assay. Anal Biochem. 239:70–76. 1996. View Article : Google Scholar
|
|
14
|
Brand-Williams W, Cuvelier ME and Berset
C: Use of a free radical method to evaluate antioxidant activity.
Food Sci Technol. 28:25–30. 1995.
|
|
15
|
Gismondi A, Serio M, Canuti L and Canini
A: Biochemical, antioxidant and antineoplastic properties of
Italian saffron (Crocus sativus L.). AJPS. 3:1573–1580.
2012. View Article : Google Scholar
|
|
16
|
Fidler IJ: Selection of successive tumor
cell lines for metastasis. Nat New Biol. 242:148–149. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gismondi A, Lentini A, Tabolacci C,
Provenzano B and Beninati S: Transglutaminase-dependent
antiproliferative and differentiative properties of nimesulide on
B16–F10 mouse melanoma cells. Amino Acids. 38:257–262.
2010.PubMed/NCBI
|
|
19
|
Lotan R and Lotan D: Stimulation of
melanogenesis in a human melanoma cell by retinoids. Cancer Res.
40:3345–3350. 1980.PubMed/NCBI
|
|
20
|
Ndenecho EN: Herbalism and resources for
the development of ethnopharmacology in Mount Cameroon region.
AJPP. 3:78–86. 2009.
|
|
21
|
Anoosh E, Fathemeh S, Eradatmand AD and
Alireza H: Antioxidant activity of methanolic and aqueous extract
of Stachys inflate. Adv Environ Biol. 5:12562011.
|
|
22
|
Sengul M, Yildiz H, Gungor N, Cetin B,
Eser Z and Ercisli S: Total phenolic content, antioxidant and
antimicrobial activities of some medicinal plants. Pakistan J Pharm
Sci. 22:102–106. 2009.PubMed/NCBI
|
|
23
|
Ho SC and Chang PW: Inhibitory effects of
several spices on inflammation caused by advanced glycation end
products. AJPS. 3:995–1002. 2012. View Article : Google Scholar
|
|
24
|
Pan MH and Ho CT: Chemopreventive effects
of natural dietary compounds on cancer development. Chem Soc Rev.
37:2258–2574. 2008.
|
|
25
|
Bajpai M, Mishra A and Prakash D:
Antioxidant and free radical scavenging activities of some leafy
vegetables. Int J Food Sci Nutr. 56:473–481. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Siddhuraju P and Becker K: Antioxidant
properties of various solvent extracts of total phenolic
constituents from three different agroclimatic origins of drumstick
tree (Moringa oleifera Lam. ) leaves J Agr Food Chem.
51:2144–2155. 2003. View Article : Google Scholar
|
|
27
|
Sreelatha S and Padma PR: Antioxidant
activity and total phenolic content of Moringa oleifera
leaves in two stages of maturity. Plant Food Hum Nutr. 64:303–311.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moyo B, Oyedemi S, Masika PJ and Muchenje
V: Polyphenolic content and antioxidant properties of Moringa
oleifera leaf extracts and enzymatic activity of liver from
goats supplemented with Moringa oleifera leaves/sunflower
seed cake. Meat Sci. 91:441–447. 2012.PubMed/NCBI
|
|
29
|
Ferreira PMP, Farias DF, de Oliveira JTA
and de Carvalho AFU: Moringa oleifera: bioactive compounds
and nutritional potential. Revista de Nutrição - Campinas.
21:431–437. 2008.
|
|
30
|
Anwar F, Latif S, Ashraf M and Gilani AH:
Moringa oleifera: a food plant with multiple medicinal uses.
Phytother Res. 21:17–25. 2007. View
Article : Google Scholar
|
|
31
|
Chumarka P, Khunawat P, Sanvarinda Y,
Phornchirasilp S, Morales NP, Phivthongngam L, Ratanachamnong P,
Srisawat S and Pongrapeeporn KS: The in vitro and ex vivo
antioxidant properties, hypolipidaemic and antiatherosclerotic
activities of water extract of Moringa oleifera Lam. leaves
J Ethnopharmacol. 116:439–446. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Paliwal R, Sharma V, Pracheta A and Sharma
S, Yadav S and Sharma S: Anti-nephrotoxic effect of administration
of Moringa oleifera Lam in amelioration of DMBA-induced
renal carcinogenesis in Swiss albino mice. Biol Med Special Issue.
3:27–35. 2011.
|
|
33
|
Sreelatha S, Jeyachitra S and Padma PR:
Antiproliferation and induction of apoptosis by Moringa
oleifera leaf extract on human cancer cells. Food Chem Toxicol.
49:1270–1275. 2011. View Article : Google Scholar
|
|
34
|
Doherty VF, Olaniran OO and Kanife UC:
Antimicrobial activities of Aframomum Melegueta (Alligator
pepper). Int J Biol. 2:126–131. 2010.
|
|
35
|
Nwozo SO and Oyinloye BE: Hepatoprotective
effect of aqueous extract of Aframomum melegueta on
ethanol-induced toxicity in rats. Acta Biochim Pol. 58:355–358.
2011.PubMed/NCBI
|
|
36
|
Telefoa PB, Lienoua LL, Yemelea MD,
Lemfacka MC, Mouokeua C, Gokaa CS, Tagnea SR and Moundipab FP:
Ethnopharmacological survey of plants used for the treatment of
female infertility in Baham, Cameroon. J Ethnopharmacol.
136:178–187. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Surh YJ: Cancer chemoprevention with
dietary phytochemicals. Nat Rev Cancer. 3:768–780. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pitchakarn P, Suzuki S, Ogawa K, Pompimon
W, Takahashi S, Asamoto M, Limtrakul P and Shirai T: Induction of
G1 arrest and apoptosis in androgen-dependent human prostate cancer
by Kuguacin J, a triterpenoid from Momordica charantia leaf.
Cancer Lett. 306:142–150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Coqueret O: New roles for p21 and p27
cell-cycle inhibitors: a function for each cell compartment? Trends
Cell Biol. 13:65–70. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dozio E, Ruscica M, Passafaro L, Dogliotti
G, Steffani L, Pagani A, Demartini G, Esposti D, Fraschini F and
Magni P: The natural antioxidant alpha-lipoic acid induces
p27Kip1-dependent cell cycle arrest and apoptosis in
MCF-7 human breast cancer cells. Eur J Pharmacol. 641:29–34. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee YS, Choi KM, Choi MH, Ji SY, Lee S,
Sin DM, Oh KW, Lee YM, Hong JT, Yun YP and Yoo HS: Serine
palmitoyltransferase inhibitor myriocin induces growth inhibition
of B16F10 melanoma cells through G2/M phase arrest. Cell Prolif.
44:320–329. 2011. View Article : Google Scholar
|
|
42
|
Kim KN, Ahn G, Heo SJ, Kang SM, Kang MC,
Yang HM, et al: Inhibition of tumor growth in vitro and in vivo by
fucoxanthin against melanoma B16F10 cells. Environ Toxicol Phar.
35:39–46. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang P, Ma Q, Luo J, Liu B, Tan F, Zhang Z
and Chen Z: Nkx3.1 and p27KIP1 cooperate in
proliferation inhibition and apoptosis induction in human
androgen-independent prostate cancer cells. Cancer Invest.
27:369–375. 2009.
|
|
44
|
Indovina P, Giorgi F, Rizzo V, Khadang B,
Schenone S, Di Marzo D, Forte IM, Tomei V, Mattioli E, D’Urso V,
Grilli B, Botta M, Giordano A and Pentimalli F: New
pyrazolo[3,4-d]pyrimidine SRC inhibitors induce apoptosis in
mesothelioma cell lines through p27 nuclear stabilization.
Oncogene. 31:929–938. 2012.
|
|
45
|
Alesiani D, Cicconi R, Mattei M, Montesano
C, Bei R and Canini A: Cell cycle arrest and differentiation
induction by 5,7-dimethoxycoumarin in melanoma cell lines. Int J
Oncol. 32:425–434. 2008.PubMed/NCBI
|
|
46
|
Tabolacci C, Lentini A, Provenzano B,
Gismondi A, Rossi S and Beninati S: Similar antineoplastic effects
of nimesulide, a selective COX-2 inhibitor, and prostaglandin E1 on
B16–F10 murine melanoma cells. Melanoma Res. 20:273–279.
2010.PubMed/NCBI
|
|
47
|
Moleephan W, Wittayalertpanya S,
Ruangrungsi N and Limpanasithikul W: Effect of xanthoxylin on
melanin content and melanogenic protein expression in B16F10
melanoma. Asian Biomed. 6:413–422. 2012.
|