Introduction
Colorectal cancer is the third most common cancer
worldwide. More than 1 million people develop colorectal cancer.
Distant metastasis is one of the most important prognostic factors
for determining how patients will respond to colorectal cancer
treatment. Recently, the survival rates for colorectal cancer
patients have improved dramatically because of earlier diagnoses
and advances in anticancer therapy, such as molecular-targeted
agents. Prognostic improvement is anticipated even for colorectal
cancer patients who have peritoneal dissemination, particularly if
all of the nodes are surgically removed or if the patient is given
chemotherapy at an appropriate time (1–4).
However, diagnosing the peritoneal dissemination is difficult. The
peritoneal dissemination of small nodules is not detectable on
pre-operative computed tomography (CT) or
18F-fluoro-deoxy-glucose positron emission tomography
(FDG-PET). Bamba et al have reported that the sensitivity of
FDG-PET/CT for colorectal cancer peritoneal dissemination is 82.6%
(5). Therefore, small lesions can
only be diagnosed by intra-operative findings, or they are
sometimes missed during surgery. Therefore, the precise diagnosis
of peritoneal dissemination is necessary to determine the
appropriate treatment strategy for colorectal cancer.
In this study, we evaluated the usefulness of
photodynamic diagnosis (PDD) using 5-aminolevulinic acid (5-ALA) to
detect peritoneal dissemination of colorectal cancer. 5-ALA is a
natural precursor of the heme. In cancer cells, increased activity
of porphobilinogen deaminase and decreased activity of
ferrochelatase cause the intracellular accumulation of
protoporphyrin IX (PpIX) (6). PpIX
emits red fluorescence and peaks at 635 nm, with a blue-violet
light excitation of 405 nm. Based on these mechanisms, 5-ALA has
been used clinically as a photosensitizer in PDD in neurosurgery
and urology (7–10). Previous reports on 5-ALA have
demonstrated improved diagnostic performances in these fields.
Moreover, 5-ALA is also used in photodynamic therapy (PDT)
(11,12).
Recently, we reported on the efficacy of 5-ALA for
detecting lymph node metastasis of rectal cancer in mouse models
(13). Additionally, we previously
reported on the diagnostic usefulness of using 5-ALA for peritoneal
dissemination and lymph node metastasis in gastric cancer patients
(14,15). In this study, we applied this
method to fluorescent laparoscopy for detecting peritoneal
dissemination of human colorectal cancers, and we compared the
diagnostic accuracy of 5-ALA use with conventional laparoscopy in
the clinical setting.
Materials and methods
Cell line and cell culture
The human colorectal cancer cell line HT-29 was
used. HT-29 was cultured in McCoy’s medium with 10% fetal bovine
serum, 100 U/ml penicillin, and 100 μg/ml streptomycin at
37°C in a water-saturated atmosphere with 5% CO2/95%
air. The HT-29 cell line stably expressing enhanced green
fluorescent protein (EGFP) was established as previously described
(13). Briefly, HT-29 cells were
transiently transfected with an EGFP plasmid, and the successfully
transfected cells were then selected using 1 mg/ml G418 (Wako Pure
Chemical Industries, Osaka, Japan).
Animals
Five-week-old female BALB/c nude mice were used in
this study. All mice were housed in groups in plastic cages with
stainless-steel grid tops in an air-conditioned environment with a
12-h light-dark cycle. The mice were given ad libitum access
to food and water. All animal experiments were approved and
followed the institutional guidelines of the Kyoto Prefectural
University of Medicine.
Establishment of the mouse model of
peritoneal metastasis and fluorescent observation
An aliquot of 1×106 EGFP tagged HT-29
cells was injected into the peritoneal cavity of mice under general
anesthesia. After 2 weeks, the mice were intraperitoneally injected
with 5-ALA hydrochloride (Wako Pure Chemical Industries, Osaka,
Japan) at a dose of 250 mg/kg body weight. Six hours after 5-ALA
administration, the mice were euthanized and laparotomy was
performed. Metastatic nodules in the omentum were observed in white
light and fluorescence images. Fluorescence observation was
performed with a stereoscopic microscope (SZX12; Olympus, Tokyo,
Japan) equipped with a color CCD digital camera (DP71, Olympus) and
a mercury lamp (U-LH100HG; Olympus). We used a spectral analytic
system composed of a stereoscopic microscope equipped with an
intensified multi-channel spectrophotometer (MCPD-7000, Otsuka
Electronics, Osaka, Japan) for spectral analysis. PpIX images
(>430 nm, HQ430LP, Chroma Technology Corp., Rockingham, VT, USA)
were acquired by exciting at 405±20 nm (D405/20x, Chroma Technology
Corp.), and EGFP fluorescence images (510–530 nm) (GFPA cube,
Olympus) were acquired by exciting at 460–490 nm (GFPA cube,
Olympus); all images were recorded in the red, green and blue
format.
Enrolled patients
A clinical trial was conducted from March 2011 to
March 2013 with approval from the Ethics Committee of Kyoto
Prefectural University of Medicine, Kyoto, Japan. Twelve colorectal
cancer patients suspected of having serosal invasion (by
preoperative CT scanning) were included in the study. The patients
provided signed informed consent preoperatively. The exclusion
criteria included the presence of porphyria and obstruction of the
digestive tract. The clinical findings were categorized according
to the UICC 7th TNM classification.
Laparoscopic procedure
5-ALA hydrochloride (Cosmo Bio Co., Ltd., Tokyo,
Japan) dissolved in 20 ml of 50% glucose solution was orally
administered 3 h before surgery at a dose of 15–20 mg per kg body
weight, ≤1 g per patient. The system used for the fluorescence
laparoscopic analyses consisted of a laparoscopic videoscope
(OTV-Y0007, Olympus) equipped with a video system center (EVIS
EXCERAII CV-180, Olympus) and a xenon light source (EVIS EXCERAII
CLV-180, Olympus). At the start of the laparoscopic surgery, the
abdominal cavity was observed in white light and fluorescence
images with a long pass filter (>450 nm); images were taken
under excitation with blue-violet light (380–430 nm). Grossly
apparent peritoneal dissemination nodules were omitted from the
sampling.
The final four patients (nos. 9–12) were observed
with a D-LIGHT system (Karl Storz GmbH & Co., Tuttlingen,
Germany). They were excluded from the quantitative analysis.
Quantitative analysis
Fluorescence images were analyzed with image
analysis software (ImageJ 1.45s, National Institutes of Health,
Bethesda, MD, USA). The red value of the 24-bit RGB color image was
evaluated as a corresponding index for red fluorescence. The
maximum red value of each peritoneal metastatic nodule and
non-metastatic site of the abdominal wall, fat, and liver were
compared. The red/(red + green + blue) ratio was also evaluated to
correct for differences in the imaging conditions.
Results
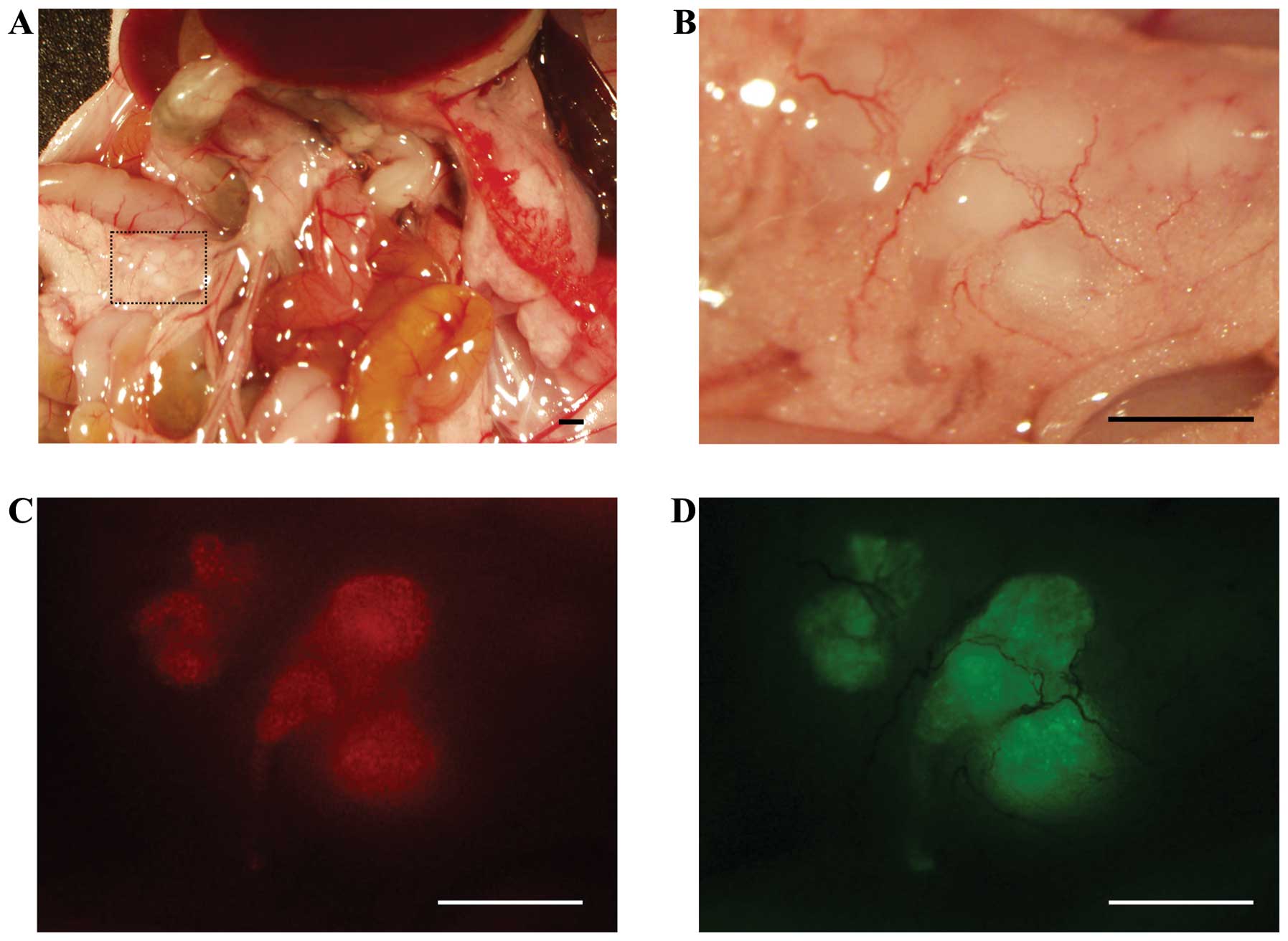
Mouse model
To investigate whether 5-ALA administration can
specifically visualize peritoneal disseminations, a mouse model of
human colon cancer was used. This model develops peritoneal
disseminations in the abdominal cavity, which are microscopically
visible within 2 weeks after the tumor implantation surgery. EGFP
fluorescent positive nodules were considered to be metastatic, and
5-ALA-induced red fluorescence colocalized with these nodules
(Fig. 1). Fluorescence spectra
with a peak of ∼635 nm were also observed in these nodules using a
spectral analytic system (data not shown).
Laparoscopic diagnosis using 5-ALA in
colorectal cancer patients
The details of the patient characteristics are
summarized in Table I. There were
9 men and 3 women (age range, 39–84 years). None of the enrolled
patients experienced any side effects from the 5-ALA
administration. All patients underwent laparoscopic observation
first. Four patients underwent sigmoidectomy; 3 patients underwent
right hemicolectomy; 1 patient underwent left hemicolectomy; 1
patient underwent transverse colectomy and 2 patients underwent
explorative laparotomy with ileostomy. Eight patients underwent
laparoscopic surgery, and 4 patients underwent laparotomy.
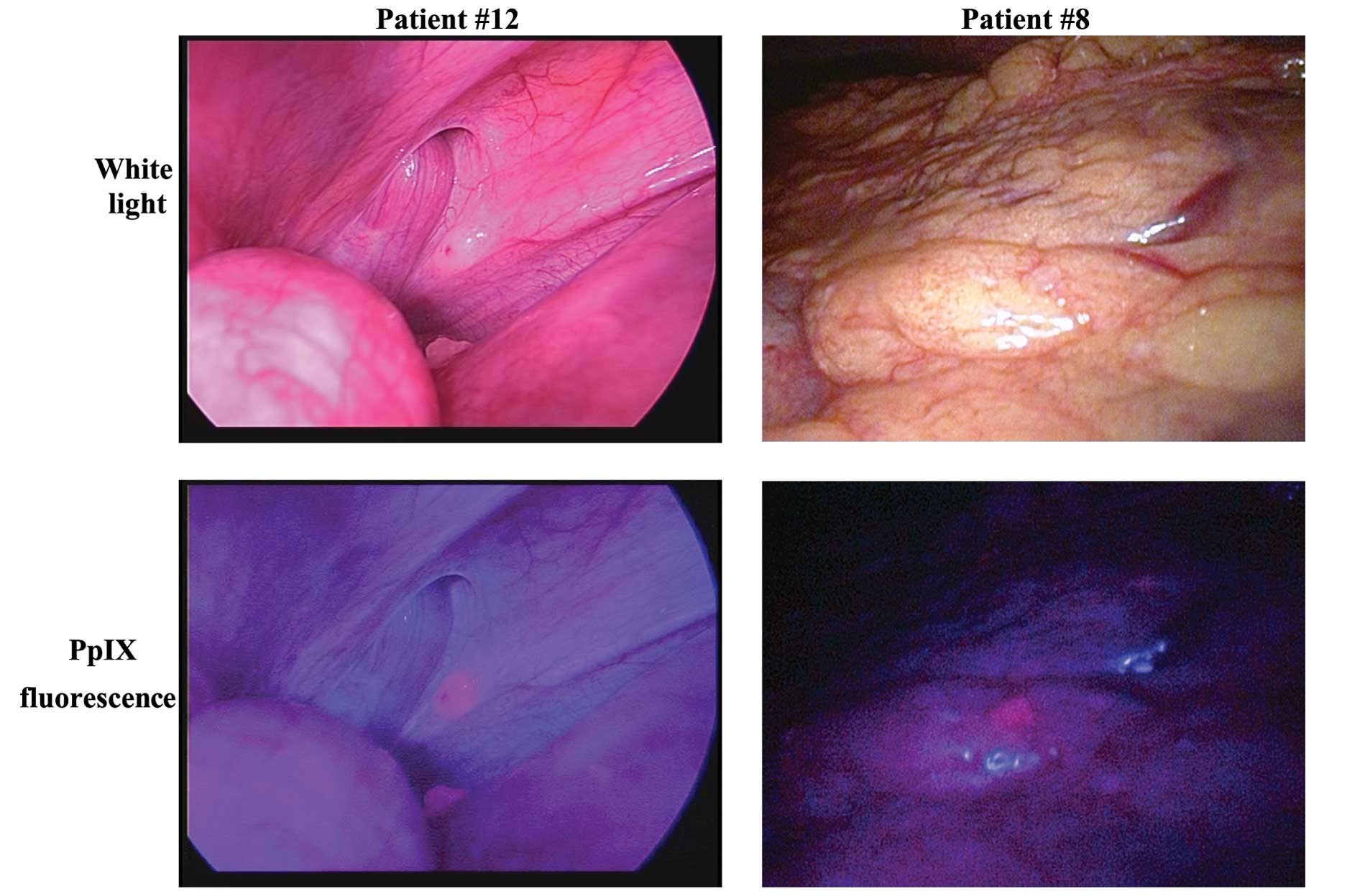
Peritoneal dissemination was observed in 8 patients with
conventional white light observation. All nodules suspected as
peritoneal dissemination by white light observation were similarly
detected in the fluorescence images (Fig. 2, left; this case was observed using
a D-LIGHT system). Some fluorescent nodules were biopsied and
pathologically confirmed as metastases (Table II). Liver metastases exposed on the
liver surfacppe were observed in 6 patients using white light
observation and fluorescence observation. In 1 patient, a small,
flat lesion that was invisible under white light observation was
only detectable by fluorescence imaging (Fig. 2, right). This nodule was biopsied
and pathologically diagnosed as a peritoneal metastasis. Among the
non-metastatic lesions, the fat tissue, liver, and bowel wall were
observed as slightly redder than the abdominal wall.
 | Table I.Enrolled patients. |
Table I.
Enrolled patients.
| Case | Gender | Age (years) | Tumor location | Histology | cT | cN | cM | Operation | |
|---|
| 1 | Male | 82 | S | tub2>por2 | 4a | 1b | 1a PER | Sigmoidectomy | Laparotomy |
| 2 | Male | 39 | S | tub1>tub2 | 4a | 1b | 1a PUL | Sigmoidectomy | Laparoscopic
surgery |
| 3 | Female | 47 | Ra | tub1 | 4a | 2a | 1a PER | Ileostomy | Laparotomy |
| 4 | Male | 43 | S | Por2>tub1 | 4a | 2b | 1b HEP LYM | Sigmoidectomy | Laparoscopic
surgery |
| 5 | Male | 66 | S | tub1 | 4a | 0 | 0 | Sigmoidectomy | Laparoscopic
surgery |
| 6 | Male | 62 | D | tub1>tub2 | 4a | 2a | 0 | Left
hemicolectomy | Laparoscopic
surgery |
| 7 | Male | 84 | S | tub1>tub2 | 4a | 2a | 1a HEP | Right
hemicolectomy | Laparoscopic
surgery |
| 8 | Male | 69 | S | tub1>tub2 | 4a | 2b | 1b HEP LYM OTH | Ileostomy | Laparoscopic
surgery |
| 9 | Female | 55 | A | tub2>tub1 | 4a | 1b | 1b HEP PUL | Right
hemicolectomy | Laparoscopic
surgery |
| 10 | Male | 72 | T, S, RS | tub1>tub2 | 4a | 2b | 1a PER | Hartmann | Laparotomy |
| 11 | Male | 74 | T | tub2>tub1 | 4a | 1b | 1a HEP | Right
hemicolectomy | Laparoscopic
surgery |
| 12 | Female | 45 | T | tub2>por2 | 4a | 1a | 1a OTH | Transverse
colectomy | Laparotomy |
 | Table II.Comparison of 5-ALA mediated
fluorescence laparoscopic imaging and pathological examination. |
Table II.
Comparison of 5-ALA mediated
fluorescence laparoscopic imaging and pathological examination.
| Examination by FL
| Pathology
|
|---|
| Case | Peritoneal
dissemination | Liver
metastasis | Depth of
invasion | Peritoneal
dissemintion |
|---|
| 1 | + | + | SE | + |
| 2 | + | + | SI (bladder) | + |
| 3 | + | − | | + |
| 4 | − | − | SE | − |
| 5 | − | − | SS | − |
| 6 | − | − | SS | − |
| 7 | + | + | SS | + |
| 8 | + | + | | + |
| 9 | − | + | SE | − |
| 10 | + | − | SS | + |
| 11 | + | + | SE | + |
| 12 | + | − | SE | + |
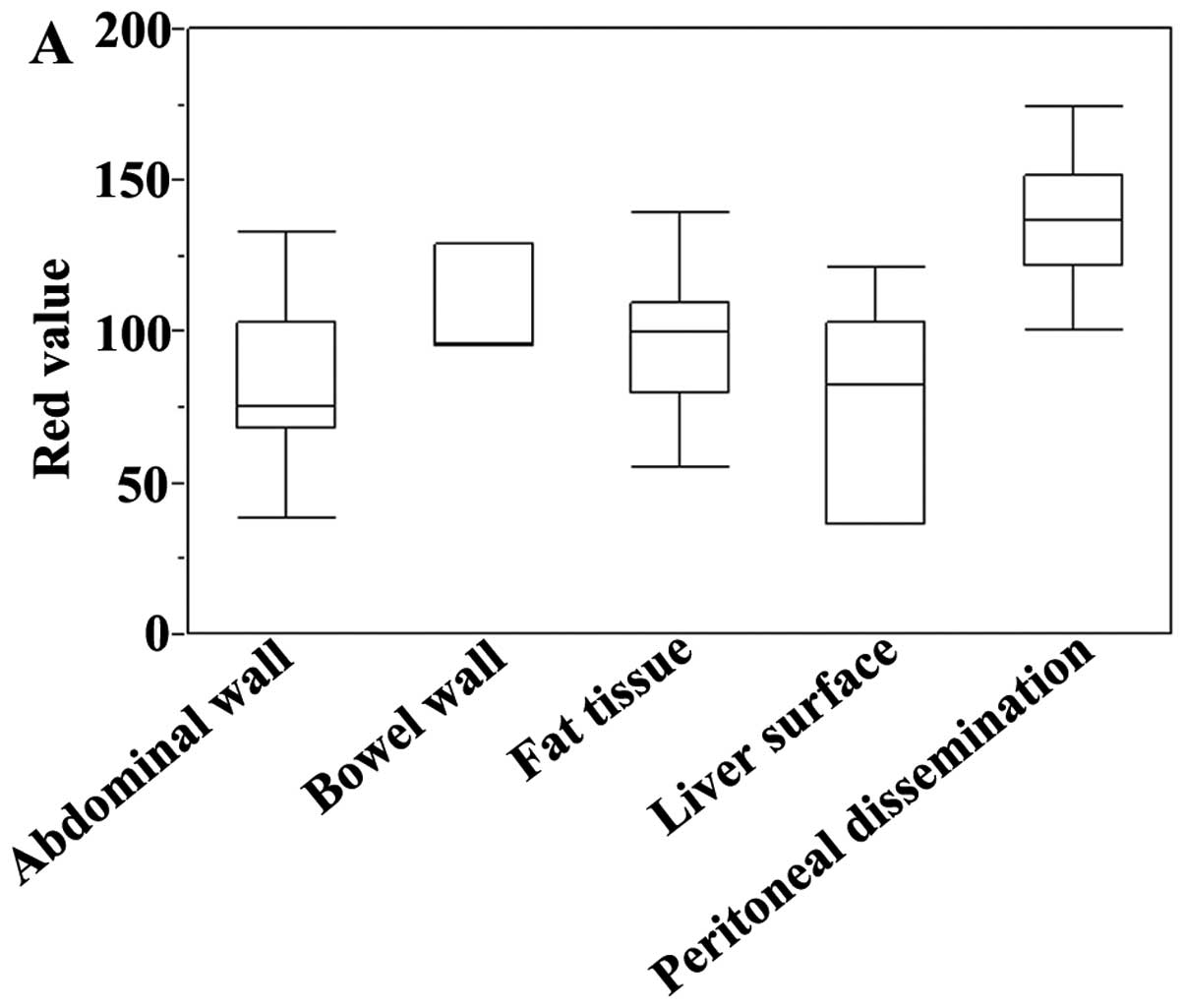
Quantitative analysis
The 5-ALA-induced fluorescence images were analyzed.
The maximum red value of each peritoneal metastatic nodule and
representative non-metastatic lesions (bowel wall, fat tissue, and
liver surface) were evaluated (Fig.
3A). The red value was higher in the metastatic nodules than in
the non-metastatic lesions, but there was some variability. The
red/(red + green + blue) ratio was significantly higher in the
metastatic nodules than in the abdominal wall (P<0.001), bowel
wall (P=0.0066), fat tissue (P=0.0057), and liver surface (P=
0.0014) (Fig. 3B). The ratio had
less variability than the absolute red value.
Discussion
Peritoneal dissemination is a form of colon cancer
metastasis. Its prognosis is poor, and it is difficult to diagnose.
Untreated peritoneal dissemination is associated with poor
survival, and systemic chemotherapy alone does not appear to yield
any clinically significant survival benefits (16,17).
Of the patients diagnosed with colorectal cancer metastases, ≤25%
have peritoneal dissemination without any other metastases
(18,19). Diagnosing minimal peritoneal
dissemination improves the prognosis because the patients undergo
chemotherapy treatment earlier. However, a diagnosis of peritoneal
dissemination is limited by the CT scanning, magnetic resonance
imaging (MRI), and PET-CT detection limits, as well as the
limitations of the surgeon’s gross inspection. Therefore, the
development of a new, accurate diagnostic method is needed.
Various cancer-specific fluorescent probes have been
developed. These probes have several advantages: they are minimally
invasive; the machine parts are compact, and they allow for
real-time diagnosis. However, clinically useful fluorescent probes
are limited. 5-ALA has few side effects and is a safe drug that has
previously been used to diagnose glioma and bladder cancer
(7–10). In urology, the sensitivity of
detecting dysplasia or early bladder cancer with fluorescence
cystoscopy (96.9%) is significantly higher than that for white
light cystoscopy (72.7%) (7).
Conventional white light cystoscopy does not provide adequate
information about the presence of ‘flat’ urothelial lesions, such
as carcinoma in situ, but fluorescence cystoscopy reveals
carcinoma lesions that do not look suspicious under white light
cystoscopy (8). In neurosurgery,
survival after surgery and radiotherapy for malignant glioma is
linked to the completeness of tumor removal. Nonetheless, it is
difficult to grossly differentiate malignant lesions from normal
brain tissues, and serious complications may occur after extensive
surgical removal. 5-ALA induced fluorescence can be used to
visualize malignant glioma intraoperatively, and it allows for
safer and more thorough tumor removal than conventional white light
surgical treatments (9,10). By applying 5-ALA-PDD, the exact
mapping of the malignant lesions and the visualization of less
visible lesions have improved the therapeutic effect (20). Although 5-ALA-PDD is a useful
method, no previous reports have used 5-ALA-PDD to diagnose
peritoneal metastasis in colorectal cancer.
In this study, all nodules suspected to be
peritoneal disseminations when they were observed by white light
also emitted 5-ALA-induced red fluorescence. Of those red nodules,
8 were biopsied and diagnosed as metastatic. Moreover, 1 small
nodule that was difficult to detect under white light was detected
by 5-ALA-PDD. 5-ALA-PDD improved the diagnostic accuracy of the
peritoneal dissemination of colorectal cancer by detecting small
and/or flat nodules that are invisible under white light
observation. Because 5-ALA-PDD allows for the diagnosis of
peritoneal dissemination in real time during surgery, without
requiring biopsy, it may be a useful diagnostic method for
completing resecting cancer lesions and decreasing the occurrence
of incomplete surgeries. 5-ALA-PDD is expected to be useful in
diagnosing peritoneal metastasis in the early stages, thereby
improving the treatment outcomes. Among the non-metastatic sites,
the bowel wall, liver surface, and fat tissue were grossly observed
as slightly reddish, but the suspected metastatic nodules in those
sites were redder, making them distinguishable from the surrounding
tissue. The reddish coloration of non-metastatic sites may be
caused by the physiological accumulation of PpIX and/or its optical
properties.
The evaluation of red fluorescence for 5-ALA-PDD has
a subjective component. Therefore, we used an RGB image analysis to
evaluate whether quantitative diagnosis is possible. We assigned
the red value as a corresponding index for the 5-ALA-induced
fluorescence intensity. The red value was significantly higher in
the peritoneal metastases than in the non-metastatic lesions, but
there was some variability because of the differences in the
imaging conditions, particularly the brightness. The red + green +
blue value corresponds to the brightness; thus, an evaluation of
the red/(red + green + blue) ratio could be used to more clearly
distinguish between peritoneal metastases and non-metastatic
lesions than an evaluation of the red value alone.
This study has some limitations. It was difficult to
inspect the entire peritoneal cavity with the rigid scope that we
used in this study. To resolve this issue, in the near future, we
will use a flexible scope that is capable of fluorescence
observation. Another problem is the undesirable effect of the
physiological accumulation of PpIX and autofluorescence of the
surrounding tissues. In this study, we could detect nodules on the
surface of fat tissue and liver, but the contrast was lower than
that for other sites. Tissue autofluorescence sometimes interferes
with the detection of PpIX fluorescence. If the autofluorescence of
the tissue adjacent to a tumor nodule is strong, the red
fluorescence of PpIX will not be detectable. New methods to reduce
these effects are required to improve the diagnostic accuracy of
5-ALA-PDD.
In conclusion, we observed better diagnostic
accuracy using 5-ALA-PDD compared to conventional laparoscopy in
patients with colorectal cancer. 5-ALA-PDD is a promising candidate
for diagnosing peritoneal dissemination in colorectal cancer.
Acknowledgements
The authors would like to thank Mr.
Motowo Nakajima and Toru Tanaka of the SBI ALA promo Corp. for
their helpful advice. This study was funded by SBI Pharmaceuticals
Co., Ltd.
References
|
1.
|
Sugarbaker PH: Peritonectomy procedures.
Ann Surg. 221:29–42. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Glehen O, Gilly FN, Boutitie F, Bereder
JM, Quenet F, Sideris L, Mansvelt B, Lorimier G, Msika S and Elias
D: Toward curative treatment of peritoneal carcinomatosis from
nonovarian origin by cytoreductive surgery combined with
perioperative intraperitoneal chemotherapy: a multi-institutional
study of 1,290 patients. French Surgical Association Cancer.
116:5608–5618. 2010.
|
|
3.
|
Brücher BL, Piso P, Verwaal V, Esquivel J,
Derraco M, Yonemura Y, Gonzalez-Moreno S, Pelz J, Königsrainer A,
Ströhlein M, Levine EA, Morris D, Bartlett D, Glehen O, Garofalo A
and Nissan A: Peritoneal carcinomatosis: cytoreductive surgery and
HIPEC - overview and basics. Cancer Invest. 30:209–224.
2012.PubMed/NCBI
|
|
4.
|
Koppe MJ, Boerman OC, Oyen WJG and
Bleichrodt RP: Peritoneal carcinomatosis of colorectal origin. Ann
Surg. 243:212–222. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Bamba Y, Itabashi M and Kameoka S:
Clinical use of PET/CT in peritoneal carcinomatosis from colorectal
cancer. Hepatogastroenterology. 59:1408–1411. 2012.PubMed/NCBI
|
|
6.
|
Ohgari Y, Nakayasu Y, Kitajima S, Sawamoto
M, Mori H, Shimokawa O, Matsui H and Taketani S: Mechanisms
involved in delta-aminolevulinic acid (ALA)-induced
photosensitivity of tumor cells: relation of ferrochelatase and
uptake of ALA to the accumulation of protoporphyrin. Biochem
Pharmacol. 71:42–49. 2005. View Article : Google Scholar
|
|
7.
|
Kriegmair M, Baumgartner R, Knuchel R,
Stepp H, Hofstadter F and Hofstetter A: Detection of early bladder
cancer by 5-aminolevulinic acid induced porphyrin fluorescence. J
Urol. 155:105–109. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Jichlinski P, Forrer M, Mizeret J,
Glanzmann T, Braichotte D, Wagnieres G, Zimmer G, Guillou L,
Schmidlin F, Graber P, van den Bergh H and Leisinger HJ: Clinical
evaluation of a method for detecting superficial surgical
transitional cell carcinoma of the bladder by light induced
fluorescence of protoporphyrin IX following the topical application
of 5-aminolevulinic acid: preliminary results. Lasers Surg Med.
20:402–408. 1997. View Article : Google Scholar
|
|
9.
|
Stummer W, Stocker S, Wagner S, Stepp H,
Fritsch C, Goetz C, Goetz AE, Kiefmann R and Reulen HJ:
Intraoperative detection of malignant gliomas by 5-aminolevulinic
acid-induced porphyrin fluorescence. Neurosurgery. 42:518–526.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Friesen SA, Hjortland GO, Madsen SJ,
Hirschberg H, Engebraten O, Nesland JM and Peng Q: 5-Aminolevulinic
acid-based photodynamic detection and therapy of brain tumors
(review). Int J Oncol. 21:577–582. 2002.PubMed/NCBI
|
|
11.
|
Hatakeyama T, Murayama Y, Komatsu S,
Shiozaki A, Kuriu Y, Ikoma H, Nakanishi M, Ichikawa D, Fujiwara H,
Okamoto K, Ochiai T, Kokuba Y, Inoue K, Nakajima M and Otsuji E:
Efficacy of 5-aminolevulinic acid-mediated photodynamic therapy
using light-emitting diodes in human colon cancer cells. Oncol Rep.
29:911–916. 2013.PubMed/NCBI
|
|
12.
|
Hino H, Murayama Y, Nakanishi M, Inoue K,
Nakajima M and Otsuji E: 5-Aminolevulinic acid-mediated
photodynamic therapy using light-emitting diodes of different
wavelengths in a mouse model of peritoneally disseminated gastric
cancer. J Surg Res. 185:119–126. 2013. View Article : Google Scholar
|
|
13.
|
Murayama Y, Harada Y, Imaizumi K, Dai P,
Nakano K, Okamoto K, Otsuji E and Takamatsu T: Precise detection of
lymph node metastases in mouse rectal cancer by using
5-aminolevulinic acid. Int J Cancer. 125:2256–2263. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Murayama Y, Ichikawa D, Koizumi N, Komatsu
S, Shiozaki A, Kuriu Y, Ikoma H, Kubota T, Nakanishi M, Harada Y,
Fujiwara H, Okamoto K, Ochiai T, Kokuba Y, Takamatsu T and Otsuji
E: Staging fluorescence laparoscopy for gastric cancer by using
5-aminolevulinic acid. Anticancer Res. 2:5421–5427. 2012.PubMed/NCBI
|
|
15.
|
Koizumi N, Harada Y, Murayama Y, Harada K,
Beika M, Yamaoka Y, Dai P, Komatsu S, Kubota T, Ichikawa D, Okamoto
K, Yanagisawa A, Otsuji E and Takamatsu T: Detection of meta-static
lymph nodes using 5-aminolevulinic acid in patients with gastric
cancer. Ann Surg Oncol. 20:3541–3548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Klaver YLB, Lemmens VEPP, Creemers GJ,
Rutten HJT, Nienhuijs SW and de Hingh IHJT: Population-based
survival of patients with peritoneal carcinomatosis from colorectal
origin in the era of increasing use of palliative chemotherapy. Ann
Oncol. 22:2250–2256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Verwaal VJ: Randomized trial of
cytoreduction and hyperthermic intraperitoneal chemotherapy versus
systemic chemotherapy and palliative surgery in patients with
peritoneal carcinomatosis of colorectal cancer. J Clin Oncol.
21:3737–3743. 2003. View Article : Google Scholar
|
|
18.
|
Maggiori L and Elias D: Curative treatment
of colorectal peritoneal carcinomatosis: current status and future
trends. Eur J Surg Oncol. 36:599–603. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Cao C, Yan TD, Black D and Morris DL: A
systematic review and meta-analysis of cytoreductive surgery with
perioperative intraperitoneal chemotherapy for peritoneal
carcinomatosis of colorectal origin. Ann Surg Oncol. 16:2152–2165.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Zöpf T, Schneider AR, Weickert U, Riemann
JF and Arnold JC: Improved preoperative tumor staging by
5-aminolevulinic acid induced fluorescence laparoscopy.
Gastrointest Endosc. 62:763–767. 2005.PubMed/NCBI
|