Introduction
Current therapeutic options for cancer treatment,
including surgery, radiotherapy and chemotherapy, have made
advancements in recent years and the survival rate of patients with
cancer has gradually improved. However, these therapies remain far
from satisfactory in most cancers (1,2).
Therefore, the development of novel treatment modalities, including
antigen-specific cancer immunotherapies with peptide vaccines,
dendritic cell vaccines and adoptive cell transfer therapies, is
critical for the further advancement of effective cancer treatments
(3–5).
We found that glypican-3 (GPC3), which is an
oncofetal antigen that is overexpressed in human hepatocellular
carcinoma (HCC), was shown to be a useful target antigen for
immunotherapy in several studies (6–10).
Based on results obtained from preclinical studies, we conducted a
phase I clinical trial using a GPC3-derived peptide vaccine in 33
patients with advanced HCC. In almost all vaccinated patients, the
frequency of GPC3 peptide-specific CTLs increased after
vaccination. Furthermore, this was the first study to show that the
frequency of peptide-specific CTLs was correlated with overall
survival in patients with HCC receiving peptide vaccines (11,12).
Although the peptide vaccine is a potentially attractive treatment
modality, the antitumor effects of the peptide vaccine alone are
not dramatic in patients with advanced HCC. Therefore, the
establishment of an innovative strategy to enhance the power of
antigen-specific cancer immunotherapy is urgently required.
Cellular immunotherapy of solid and hematopoietic
malignancies is regarded as a promising approach to treat relapse
after or resistance to conventional treatments. The adoptive
transfer of autologous tumor-infiltrating lymphocytes (TILs)
results in objective cancer regression in 49 to 72% of patients
with metastatic melanoma (13).
However, due to the scarcity of TILs, this therapy is only possible
for a limited number of patients. It is difficult to isolate and
expand functionally active T cells. Development of a new method of
CTL expansion may be useful in addressing this problem.
It was recently reported that γδ T cells are
attractive mediators of cancer immunotherapy (14). Several clinical studies that
included manipulation of γδ T cells by aminobisphosphonate
administration or adoptive transfer of γδ T cells were performed
(15–17). γδ T cells recognize their targets
independently of major histocompatibility complex (MHC)-mediated
antigen presentation (18–21). Human γδ T cells kill a vast
repertoire of tumor cell lines and primary samples in vitro,
including leukemia, lymphoma, melanoma, neuroblastoma and multiple
types of carcinomas (22–25). In addition, human γδ T cells
mediate antibody-dependent cellular cytotoxicity (26,27).
On the other hand, activated human γδ T cells produce large amounts
of interferon-γ (28,29), a central cytokine in antitumor
immune responses. Moreover, it has been reported that zoledronate
stimulates proliferation of γδ T cells, which then stimulate CTLs
as antigen-presenting cells (APCs) (30–33).
Therefore, we have attempted to use γδ T cells as both effector
cells and APCs.
We report on the development of a more effective
adoptive immunotherapy. We investigated a new method to induce
expansion of γδ T cells and peptide-specific CTLs using
zoledronate.
Materials and methods
Patient samples
Patient blood samples were obtained during the
performance of clinical trials at National Cancer Center Hospital
East. We carried out two clinical trials involving GPC3-derived
peptide vaccine. The phase I trial was carried out among 33
patients with advanced or metastatic HCC from February, 2007 to
November, 2009 (11,12). The trial was registered with the
University Hospital Medical Information Network Clinical Trials
Registry (UMIN-CTR no. 000001395). We subsequently conducted a
phase II trial involving the GPC3-derived peptide vaccine as an
adjuvant therapy for patients with HCC. Forty patients with initial
HCC who had undergone surgery or radiofrequency ablation were
enrolled in this phase II trial (UMIN-CTR no. 000002614). These
patients were enrolled after providing a written informed consent.
Patients were intradermally injected with HLA-A24-restricted
GPC3298-306 (EYILSLEEL) or HLA-A2-restricted
GPC3144-152 (FVGEFFTDV) peptide vaccine emulsified with
incomplete Freund’s adjuvant (IFA, Montanide ISA-51VG; SEPPIC,
Paris, France). This study was approved by the Ethics Committee of
the National Cancer Center and conformed to the ethical guidelines
of the 1975 Declaration of Helsinki.
PBMCs
Peripheral blood (30 ml) was obtained from healthy
volunteers or patients at the times designated in the protocol
(before the first vaccination and 2 weeks after each vaccination).
Peripheral blood mononuclear cells (PBMCs) were isolated by
standard Ficoll density gradient centrifugation from buffy coats.
In this study, we used the remaining PBMCs after immunological
monitoring in the clinical trials.
Cell lines
The human liver cancer cell lines SK-Hep-1
(GPC3−, HLA-A*02:01/A*24:02) and SK-Hep-1/hGPC3
(GPC3+, HLA-A*02:01/A*24:02) were used as target cells.
SK-Hep-1/hGPC3 is an established stable GPC3-expressing cell line
transfected with a human GPC3 gene, and SK-Hep-1/vec is an
established counterpart cell line in which an empty vector was
transfected. T2 (HLA-A*02:01, TAP−) and T2A24
(HLA-A*02:01/A*24:02, TAP−) cells were pulsed with GPC3
peptide or human immunodeficiency (HIV) peptide at room temperature
for 1 h. They were conserved in our laboratory. Cells were cultured
at 37°C in RPMI-1640 or DMEM medium (Sigma-Aldrich, St. Louis, MO,
USA) supplemented with 10% fetal bovine serum, 100 U/ml penicillin,
and 100 μg/ml streptomycin in a humidified atmosphere containing 5%
CO2.
Synthetic peptides
The peptides used in this study were as follows:
HLA-A*02:01-restricted GPC3144-152 (FVGEFFTDV) peptide
(American Peptide Company, Sunnyvale, CA), HLA-A*24:02-restricted
GPC3298-306 (EYILSLEEL) peptide (American Peptide
Company), HLA-A*02:01-restricted cytomegalovirus
(CMV)495-503 (NLVPMVATV) peptide (ProImmune, Rhinebeck,
NY, USA), HLA-A*24:02-restricted CMV341-349 (QYDPVAALF)
peptide (ProImmune), and HLA-A*02:01-restricted HIV77-85
(SLYNTYATL) peptide (ProImmune). The peptides were dissolved and
diluted in 7% NaHCO3 or dimethyl sulfoxide.
Large-scale expansion using
zoledronate
PBMCs were cultured (2×106 cells/well)
with zoledronate (5 μM) (Novartis Pharma, Basel, Switzerland) and
CMV or GPC3 peptide (10 μM) in AIM-V medium (Gibco) supplemented
with 10% human AB serum (Sigma) and recombinant human interleukin
(IL)-2 (1,000 IU/ml) (Novartis Pharma) for 14 days. The stimulation
procedure was performed at 37°C and 5% CO2. Scale-up of
cells was performed in accordance with their growth.
Expansion of peptide-specific CTLs in the
absence of zoledronate
To obtain zoledronate-activated γδ T cells, PBMCs
were stimulated with zoledronate and IL-2 for 7 days. On day 7,
zoledronate-activated γδ T cells were sorted using FACSAria II.
CD8+ cells and γδ T cells without zoledronate activation
were sorted from non-cultured PBMCs using microbeads and FACSAria
II, respectively. Dendritic cells (DCs) were induced from
CD14+ cells using GM-CSF and IL-4. On day 5, DCs were
stimulated with TNF-α for 2 days. We used γδ T cells with or
without zoledronate activation and TNF-α-stimulated DCs as
stimulator cells. Stimulator cells were pulsed with CMV peptide (10
μM) for 1 h at room temperature. After washing out the peptide,
stimulator cells were co-cultured for 2 weeks with responder
CD8+ cells and the addition of IL-2 in the absence of
zoledronate. We compared the percentages of CMV peptide-specific
CTLs in responder CD8+ cells using dextramer assays.
In vitro stimulation of GPC3
peptide-specific CTL clones
GPC3 peptide-specific CTL clones were previously
generated by single cell sorting using a GPC3-dextramer or CD107a
antibody. CTL clones were stimulated as described previously
(34).
Dextramer staining and flow cytometry
analysis
The PBMCs were stained with CMV, GPC3 or HIV
Dextramer-RPE (Immudex, Copenhagen, Denmark) for 10 min at room
temperature and with anti-CD8-FITC (ProImmune) or anti-CD8-APC
(BioLegend, San Diego, CA), anti-CD45RA-FITC (BD Biosciences, San
Jose, CA, USA), and anti-CCR7-PerCP/Cy5.5 (BioLegend) for 20 min at
4°C. To detect γδ T cells, PBMCs were stained with
anti-TCR-Vγ9-FITC (Beckman Coulter, Erembodegem, Belgium) and
anti-CD3-PC5 (BioLegend) for 20 min at 4°C. γδ T cells, with or
without zoledronate activation, and TNF-DCs were stained with
anti-HLA-class I-FITC, anti-CD80-FITC, anti-CD83-FITC and
anti-CD86-PE (BD Biosciences) antibodies for 20 min at 4°C. Flow
cytometry analysis was carried out using FACSCanto II (BD
Biosciences).
Cytotoxicity assay
Cytotoxic activity against target cells was analyzed
using the Terascan VPC system (Minerva Tech, Tokyo, Japan) as
described previously (34). Target
cells were labeled with calcein AM (Dojindo, Kumamoto, Japan)
solution for 30 min at 37°C. The labeled cells were then incubated
with effector cells for 4 to 6 h. As effector cells,
CD8+ and CD8− T cells were isolated using
human CD8 microbeads (BD Bioscience) from PBMCs stimulated for 14
days. Assays were conducted in duplicate.
Transfer of effector cells to NOD/SCID
mice implanted with the GPC3+ or GPC3− cell
line
Female NOD/SCID (6–8 weeks old) were purchased from
Japan Charles River Laboratories (Yokohama, Japan). All animal
procedures were performed according to the guidelines for the
Animal Research Committee of the National Cancer Center, Japan. We
inoculated SK-Hep-1/hGPC3 or SK-Hep-1/vec cells subcutaneously into
the right flank of NOD/SCID mice. We intravenously injected the
CD8+ cells, CD8− cells, or both, as effector
cells. We injected PBS as a negative control. Before adoptive
transfer, we examined the percentage of CD8+ cells in
expanded cells using flow cytometry. The percentage of
CD8+ cells after expansion was ~25% of all cells. We
injected immune cells at this ratio. We injected 5×106
cells per mouse for the CD8+-cell-treatment group. We
injected 1.5×107 cells per mouse for the CD8−
cell treatment group and 2.0×107 cells per mouse for the
all cells treatment group. We performed adoptive cell transfer of
expanded cells using five mice per group. The tumor volume was
monitored and calculated using the following formula: tumor volume
(mm3) = a × b2 × 0.5, where a is the longest
diameter, b is the shortest diameter, and 0.5 is a constant to
calculate the volume of an ellipsoid.
Statistical analysis
The correlation between the number of GPC3-specific
CTLs and γδ T cells at days 0 and 14 was analyzed using the
Spearman’s rank correlation coefficient. Comparisons of tumor
volume at the last time point were performed using the Mann-Whitney
U test. Differences were considered significant at P<0.05.
Results
Zoledronate induces expansion of γδ T
cells and peptide-specific CTLs from PBMCs
To assess whether this new culture method can induce
the expansion of γδ T cells and peptide-specific CTLs, PBMCs were
stimulated once with zoledronate and an antigen-derived peptide.
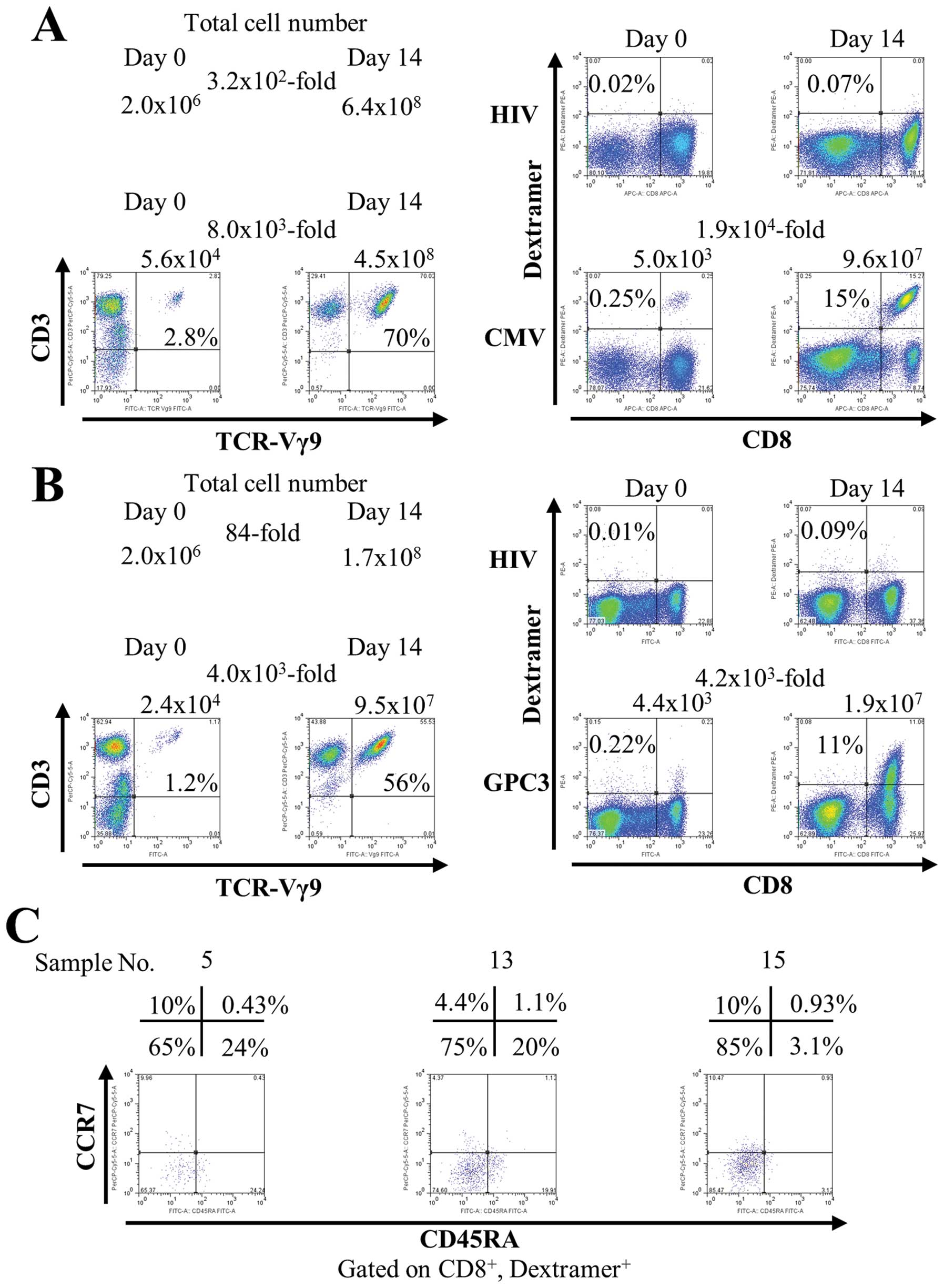
Fig. 1A shows the representative
data using PBMCs from a healthy volunteer. The number of total
cells increased 3.2×102-fold after 14 days (from
2.0×106 to 6.4×108). In flow cytometry
analysis, γδ T cells increased 8.0×103-fold after 14
days [from 5.6×104 (2.8%) to 4.5×108 (70%)].
Simultaneously with γδ T cells, CMV peptide-specific CTLs increased
1.9×104-fold after 14 days [from 5.0×103
(0.25%) to 9.6×107 (15%)]. Similar results were obtained
from three healthy subjects (data not shown).
Next, we investigated the capacity of this culture
method to induce expansion of CTLs specific for peptides derived
from the weakly immunogenic tumor-associated self-antigen GPC3.
PBMCs from vaccinated patients were stimulated once with
zoledronate and a GPC3-derived peptide. In Fig. 1B, the number of total cells
increased 84-fold after 14 days (from 2.0×106 to
1.7×108). γδ T cells increased 4.0×103-fold
after 14 days [from 2.4×104 (1.2%) to 9.5×107
(56%)]. GPC3 peptide-specific CTLs increased
4.2×103-fold after 14 days [from 4.4×103
(0.22%) to 1.9×107 (11%)]. In addition, during
expansion, GPC3 peptide-specific CTLs acquired mainly an effector
memory phenotype (CD45RA−, CCR7−) (Fig. 1C). One of the features of this
culture method is the rate of increase in the number of cells.
Table I shows the rate of increase
in the number of cells in 16 patients with HCC. We found that the
total cell number increased (range, 1.1–270-fold), γδ T cells
increased (range, 23–1.1×104-fold), and GPC3
peptide-specific CTLs increased (range, 24–1.7×105-fold)
after 14 days. These results suggest that peptide-specific CTLs
were successfully expanded with the proliferation of γδ T cells
from PBMCs by this new culture method.
 | Table IRate of increase in the number of
cells in 16 patients with HCC. |
Table I
Rate of increase in the number of
cells in 16 patients with HCC.
| | Total | γδ | GPC3 specific
CTLs |
|---|
| |
|
|
|
|---|
| Sample | HLA-A | Day 0 | Day 14 | The rate of
increase | Day 0 | Day 14 | The rate of
increase | Day 0a | Day 14b | The rate of
increase |
|---|
| 1 | 02:01 |
2.0×106 |
1.7×108 | 85 |
1.2×104 |
2.8×107 |
2.3×103 |
2.0×103 |
6.1×107 |
3.1×104 |
| 2 | 02:01 |
2.0×106 |
5.4×108 |
2.7×102 |
2.8×104 |
3.1×108 |
1.1×104 |
1.6×102 |
2.7×107 |
1.7×105 |
| 3 | 02:01 |
2.0×106 |
1.7×108 | 85 |
8.8×103 |
7.8×107 |
8.9×103 | 92 |
1.0×106 |
1.1×104 |
| 4 | 02:01 |
2.0×106 |
1.7×108 | 85 |
2.4×104 |
9.5×107 |
4.0×103 |
1.3×103 |
1.9×107 |
1.5×104 |
| 5 | 02:01 |
2.0×106 |
7.4×107 | 37 |
9.4×103 |
5.2×107 |
5.5×103 | 92 |
1.8×105 |
2.0×103 |
| 6 | 02:01 |
2.0×106 |
2.5×107 | 13 |
2.0×104 |
1.2×107 |
6.0×102 |
1.0×103 |
1.9×106 |
1.9×103 |
| 7 | 02:01 |
2.0×106 |
1.6×108 | 80 |
1.1×104 |
7.4×107 |
6.7×103 |
2.1×102 |
4.4×106 |
2.1×104 |
| 8 | 02:01 |
2.0×106 |
4.0×108 |
2.0×102 |
1.2×104 |
1.3×108 |
1.1×104 |
4.0×102 |
2.6×107 |
6.5×104 |
| 9 | 02:01 |
2.0×106 |
4.0×107 | 20 |
8.0×103 |
4.4×106 |
5.5×102 |
8.4×102 |
5.9×106 |
7.0×103 |
| 10 | 02:01 |
2.0×106 |
2.2×106 | 1.1 |
2.0×103 |
4.5×104 | 23 | 92 |
2.2×103 | 24 |
| 11 | 02:01 |
2.0×106 |
1.5×108 | 75 |
6.0×103 |
4.8×107 |
8.0×103 |
3.0×102 |
2.0×107 |
6.7×104 |
| 12 | 02:01 |
2.0×106 |
1.1×108 | 55 |
6.0×103 |
1.2×107 |
2.0×103 |
1.2×103 |
3.5×107 |
2.9×104 |
| 13 | 24:02 |
2.0×106 |
5.8×106 | 2.9 |
1.8×104 |
3.8×106 |
2.1×102 |
1.3×102 |
6.3×104 |
4.9×102 |
| 14 | 24:02 |
2.0×106 |
4.0×106 | 2 |
2.2×104 |
2.6×106 |
1.2×102 |
1.0×102 |
3.1×104 |
3.1×102 |
| 15 | 24:02 |
2.0×106 |
9.9×107 | 50 |
3.8×103 |
3.8×107 |
1.0×104 |
1.8×102 |
1.2×106 |
6.7×103 |
| 16 | 24:02 |
2.0×106 |
4.0×107 | 20 |
9.8×103 |
5.6×106 |
5.7×102 |
1.4×102 |
1.4×105 |
1.0×103 |
Efficiency of the culture method to
induce expansion of GPC3 peptide-specific CTLs
One of the problems of cell transfer therapy is that
it cannot predict cell growth prior to cell culture. Therefore, to
identify predicting factors, we investigated the ability of this
culture method to induce expansion of γδ T cells and GPC3
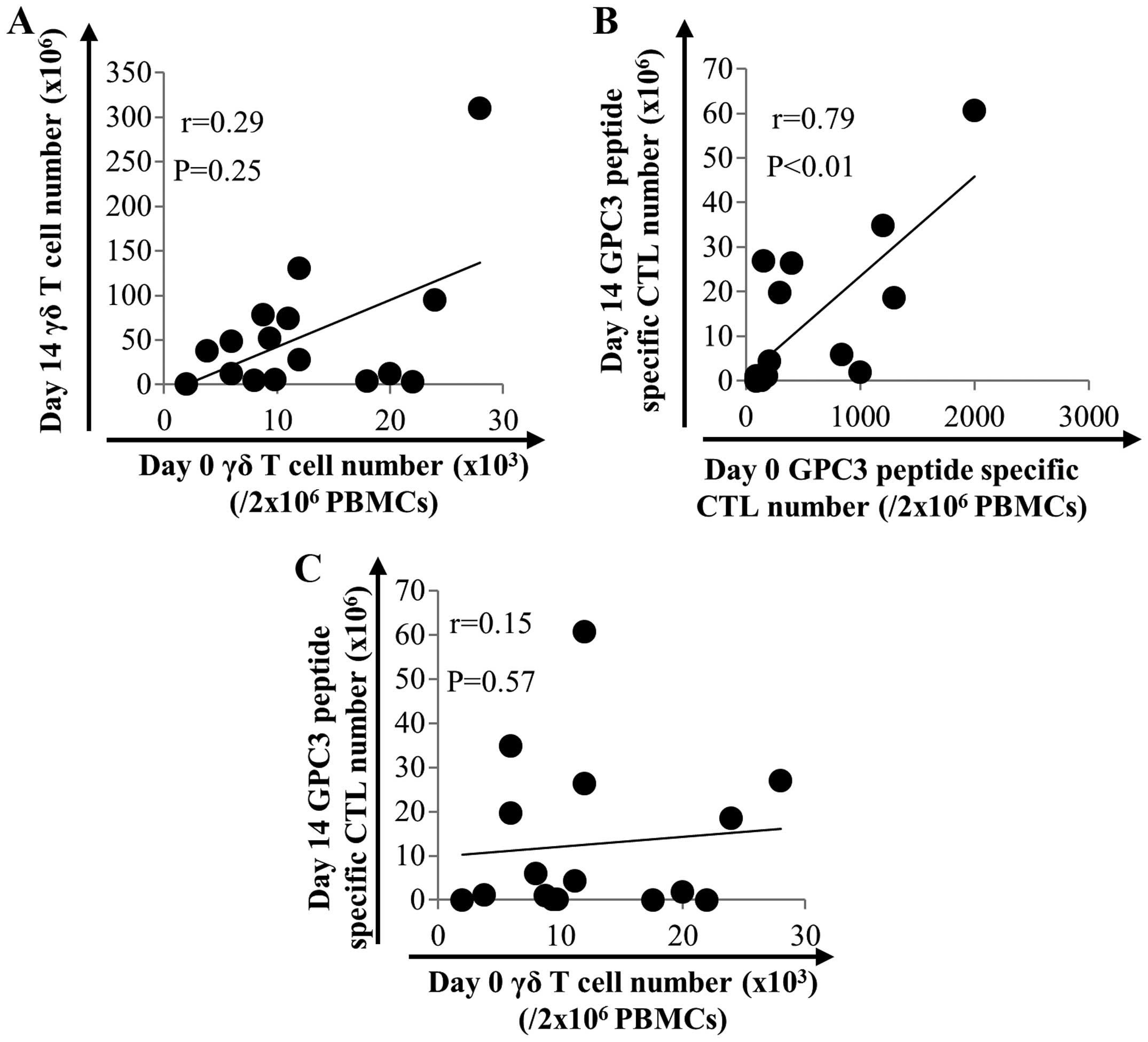
peptide-specific CTLs in 16 patients with HCC. As shown in Fig. 2, the number of γδ T cells after
expansion did not correlate with that before expansion (Fig. 2A). On the other hand, the number of
GPC3 peptide-specific CTLs after expansion correlated with that
before expansion (P<0.01, r=0.79) (Fig. 2B). This result indicates that the
number of GPC3 peptide-specific CTLs before expansion is a
predicting factor. We expected a positive correlation between the
number of γδ T cells and the number of GPC3 peptide-specific CTLs
after expansion. However, no such correlation was observed
(Fig. 2C).
Activated γδ T cells function as
antigen-presenting cells
To examine whether the expansion of peptide-specific
CTLs is enhanced by simultaneous activation/expansion of γδ T
cells, we expanded peptide-specific CTLs in the absence of
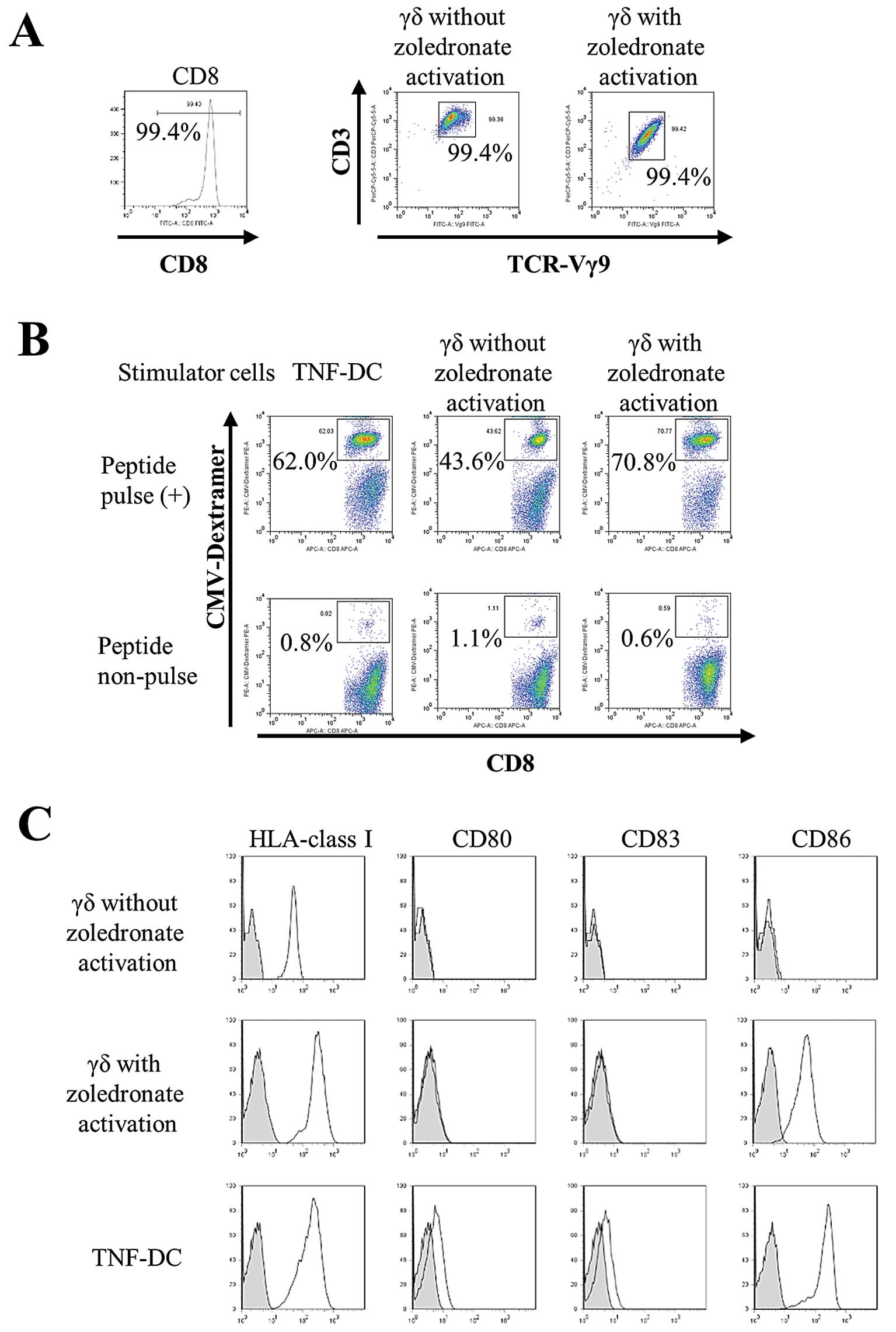
zoledronate. The purity of sorted CD8+ cells and γδ T
cells with or without zoledronate activation was greater than 99%
(Fig. 3A). The expansion of
peptide-specific CTLs stimulated by γδ T cells with zoledronate
activation (70.8%) was higher than by γδ T cells without
zoledronate activation (43.6%). Moreover, the CTL-expanding ability
of zoledronate-activated γδ T cells was comparable to that of
TNF-DCs (62.0%), which are known professional antigen-presenting
cells. These results indicate that zoledronate-activated γδ T cells
function as antigen-presenting cells in co-cultures in the absence
of zoledronate (Fig. 3B). We
compared cell surface expression of antigen-presenting molecules
and co-stimulatory molecules on γδ T cells (with or without
zoledronate activation) and TNF-DCs. All cells expressed HLA-class
I; however, γδ T cells without zoledronate activation did not
express co-stimulatory molecules. Furthermore, CD86 expression in
zoledronate-activated γδ T cells was comparable with that of
TNF-DCs (Fig. 3C). These results
indicate that γδ T cells activated by zoledronate acquire
antigen-presenting properties accompanied by CD86 expression.
Cytotoxic activity of expanded cells
We performed a cytotoxicity assay to assess the
peptide specificity and cytotoxic activity of expanded cells
against cancer cells. We used CD8+ and CD8−
cells that were isolated from cultured cells using CD8 microbeads
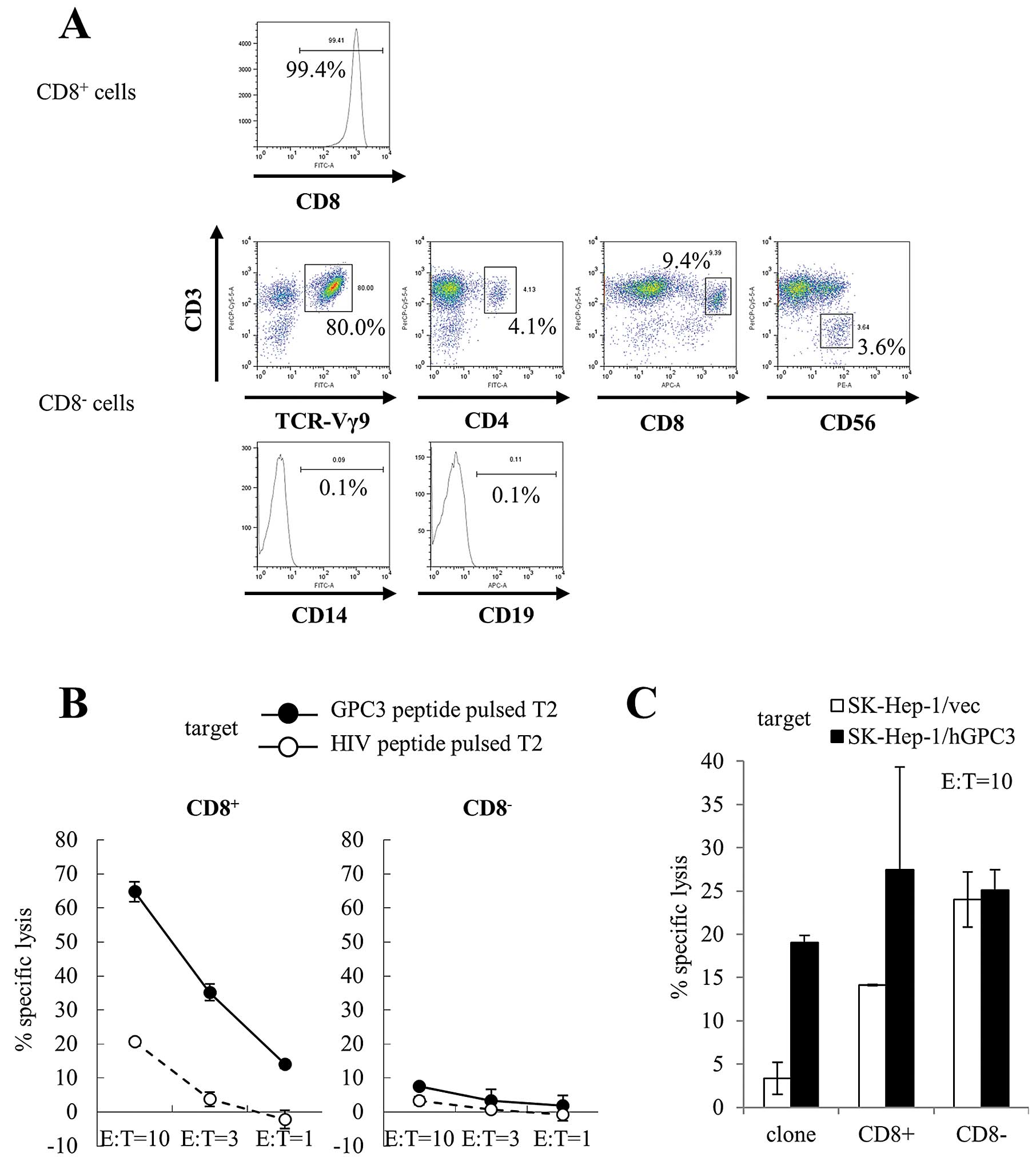
at day 14 as effector cells. The purity of CD8+ cells
was 99.4%. We performed further immunophenotyping of
CD8− cells. CD3+ Vg9+ cells were
80.0% of CD8− cells. CD8− cells also included
CD3+ CD4+ cells (4.1%), CD3+
CD8+ cells (9.4%), and CD3− CD56+
cells (NK cells; 3.6%). CD14+ cells (monocytes; 0.1%)
and CD19+ cells (B cells; 0.1%) were not observed in
CD8− cells. These results indicate that CD8−
cells were predominantly γδ T cells (Fig. 4A). Similar results were obtained
from four patients. CD8+ cells showed cytotoxicity
against T2 cells pulsed with GPC3 peptide, whereas CD8−
cells did not show cytotoxicity against T2 cells pulsed with both
GPC3 and HIV peptide (Fig. 4B).
Moreover, we used SK-Hep-1/hGPC3 cells as target cells; they were
transfected with the GPC3 gene and endogenously presented GPC3
peptide. CD8+ cells showed GPC3-specific cytotoxicity,
whereas CD8− cells showed cytotoxicity against SK-Hep-1
cells but did not show GPC3 specificity (Fig. 4C). We performed cytotoxicity assays
using expanded cells from four patients. Similar results were
obtained in three of the four patients. These results indicate that
CD8+ cells included mostly GPC3 peptide-specific CTLs
that had cytotoxic activity against cancer cells and endogenously
presented GPC3 peptide, and CD8− cells included mostly
γδ T cells that had cytotoxic activity against cancer cells.
Antitumor activity of γδ T cells and
GPC3-specific CTLs in vivo
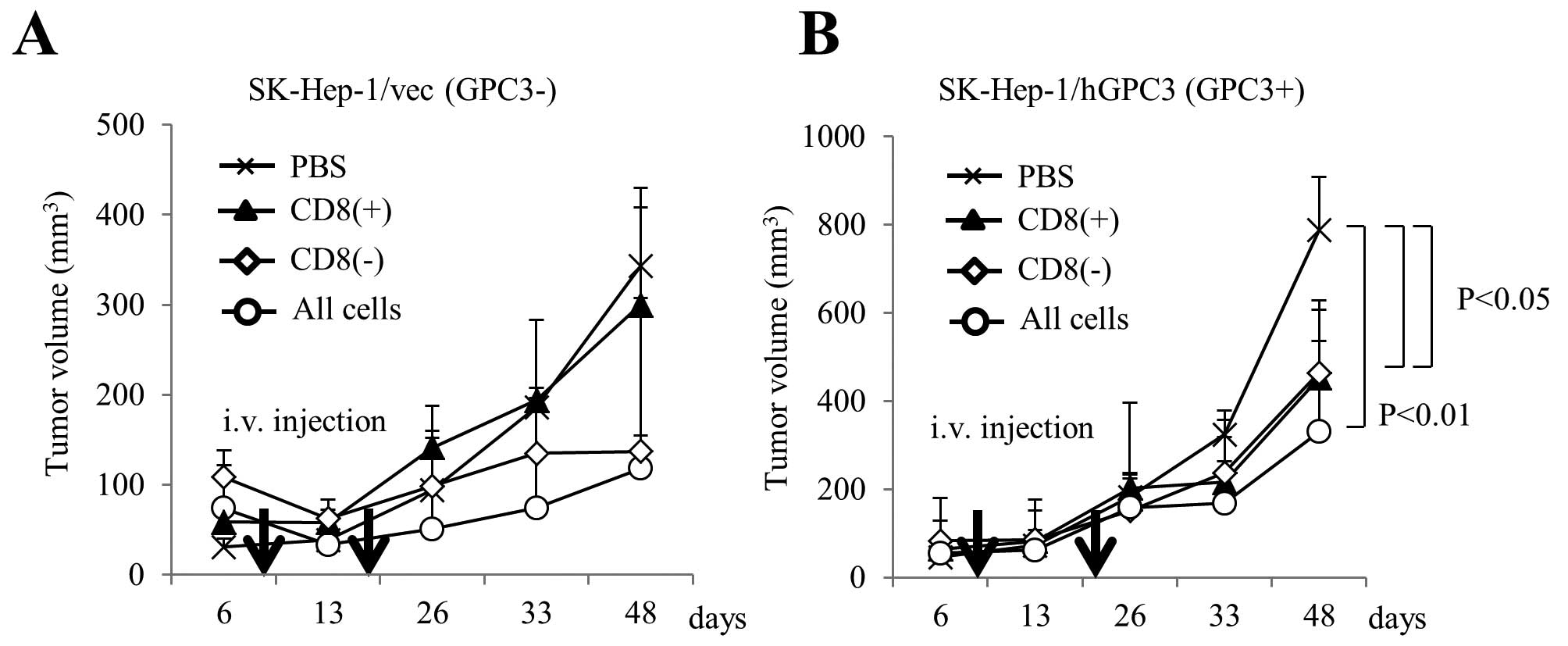
We performed adoptive cell transfer of expanded
cells in a mouse model. We subcutaneously inoculated SK-Hep-1/vec
(Fig. 5A) or SK-Hep-1/hGPC3
(Fig. 5B) cell lines into NOD/SCID
mice and intravenously injected effector cells twice. As effector
cells, we used CD8+ or CD8− cells that were
isolated from cultured cells using CD8 microbeads at day 14, and we
used all cells that included both CD8+ and
CD8− cells. As shown Fig.
5B, the growth of SK-Hep-1/hGPC3 treated with CD8+
or CD8− cells was significantly inhibited compared with
the negative control. In addition, treatment of all cells,
including both CD8+ and CD8− cells, tended to
show an additive inhibitory effect. On the other hand, the growth
of SK-Hep-1/vec that was inhibited in the treatment of
CD8− or all cells was not inhibited by treatment of
CD8+ cells (Fig. 5A).
These results indicate that cultured cells had antitumor effects
due to the respective CD8+ and CD8−
cells.
Discussion
Specific cellular immunotherapy of cancer requires
efficient generation and expansion of CTLs that recognize
tumor-associated antigens. ACT with TILs isolated from metastatic
melanoma lesions lead to objective tumor regression. However, TILs
can be exploited only in melanoma patients with resectable tumors
and from which T cells can be expanded ex vivo. An
alternative approach has been explored for patients with other
types of tumor using autologous lymphocytes isolated from
peripheral blood. Various clinical trials involving adoptively
transferred autologous T cells transduced with a TCR or chimeric
antigen receptors have been conducted (35–37).
Clinical trials using our culture method should be performed in the
future.
The standard approach to generating tumor-specific
CTLs is based on antigen presentation by dendritic cells (DCs).
Although DCs are the most efficient APCs known so far, serious
drawbacks to their use in adoptive immunotherapy exist, including
their scarcity in the peripheral blood, their limited expansion and
their functional heterogeneity. These limitations have motivated an
intense search for alternative sources of APCs. An
antigen-presenting function of γδ T cells was suggested by recent
observations that upon activation, these cells acquire phenotypic
and functional characteristics of professional APCs concomitant
with the capacity to induce primary CD4+ and
CD8+ T-cell responses to antigens (30–33).
To the best of our knowledge, this is the first report of the
simultaneous expansion of γδ T cells and antigen-specific CTLs from
the PBMCs of patients.
Most adoptive CTL transfer studies in patients with
tumors used approximately 108 to 1011 T
cells/m2 body surface area of the patient (38). The expansion of CTLs from PBMCs of
vaccinated patients with advanced HCC yields cell numbers
sufficient for adoptive transfer. Theoretically, the number of
GPC3-specific CTLs obtained for apheresis (10 L) is, at most,
1.5×1011 cells.
One reason for the scarcity of adoptive
immunotherapy is the individual variability in cell growth. In
vitro, it is difficult to adequately expand antigen-specific
CTLs in most patients with cancer. In addition, cell growth cannot
be predicted before culture. Therefore, to identify predicting
factors, we investigated the efficacy of this culture method in
inducing expansion of GPC3 peptide-specific CTLs in 16 patients
with HCC. The prediction of cell growth may enable the
implementation of personalized medicine.
In this study, we assessed the expansion of GPC3
peptide-specific CTLs using PBMCs from vaccinated patients with
HCC. GPC3 is also overexpressed in other malignant tumors, such as
melanoma, Wilms’ tumor, hepatoblastoma, yolk sac tumor, ovarian CCC
and lung squamous cell carcinoma (39–43).
Adoptive transfer of GPC3 peptide-specific CTLs may also be
available for other GPC3-expressing cancers.
This culture method has a limitation. We performed
this culture using PBMCs of the same person both before and after
vaccination. GPC3 peptide-specific CTLs could be induced from the
PBMCs of all patients after vaccinations. However, this method
failed to induce GPC3 peptide-specific CTLs from the PBMCs of
patients before vaccination. Similarly, GPC3 peptide-specific CTLs
could not be induced from the PBMCs of healthy donors (data not
shown). These results may have been caused by the low frequency of
cancer antigen-specific CTLs in peripheral blood before
vaccination. These results suggest that to increase GPC3
peptide-specific CTLs, vaccination is effective before cell
culture. On the other hand, with regard to CMV-derived peptide, CMV
peptide-specific CTLs could be induced with the proliferation of γδ
T cells from the PBMCs of healthy donors by this culture method.
This culture method may also be available for other antigens.
Adoptive cell transfer of all cells after expansion,
including both CTLs and γδ T cells, significantly inhibited tumor
growth in a mouse model. Tumor cells acquire various immune escape
mechanisms including loss of antigens or the HLA-class I molecule.
It may be effective to use both CTLs and γδ T cells because they
have different antigen recognition abilities. However, we did not
confirm that the results were due to either synergy or an additive
effect. Because activated γδ T cells produce large amounts of
interferon-γ, it may be a synergy effect. Analysis of the
mechanisms of the effectiveness of CTLs and γδ T cells is a future
challenge.
On the other hand, we previously reported that
intratumor peptide injection was an effective method of enhancing
tumor cell antigenicity and that it showed an induced
antigen-spreading effect in vivo (44,45).
Moreover, we are investigating the antitumor activity of γδ T cells
against HCC cells pretreated with zoledronate. The combination of
these pretreatments that enhance tumor cell antigenicity and
adoptive immunotherapy using CTLs and γδ T cells may be a useful
application for cancer therapy.
In conclusion, this study indicates that
simultaneous expansion of γδ T cells and peptide-specific CTLs
using zoledronate are useful for adoptive immunotherapy.
Acknowledgements
This study was supported in part by the Adaptable
and Seamless Technology Transfer Program from the Japan Science and
Technology Agency, as well as Health and Labor Science Research
Grants for Clinical Research and Third Term Comprehensive Control
Research for Cancer from the Ministry of Health, Labor and Welfare,
Japan.
References
|
1
|
Sant M, Capocaccia R, Coleman MP, et al:
Cancer survival increases in Europe, but international differences
remain wide. Eur J Cancer. 37:1659–1667. 2001. View Article : Google Scholar
|
|
2
|
Verdecchia A, Francisci S, Brenner H,
Gatta G, Micheli A, Mangone L and Kunkler I: Recent cancer survival
in Europe: a 2000-02 period analysis of EUROCARE-4 data. Lancet
Oncol. 8:784–796. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aarntzen EH, Figdor CG, Adema GJ, Punt CJ
and de Vries IJ: Dendritic cell vaccination and immune monitoring.
Cancer Immunol Immunother. 57:1559–1568. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rosenberg SA, Restifo NP, Yang JC, Morgan
RA and Dudley ME: Adoptive cell transfer: a clinical path to
effective cancer immunotherapy. Nat Rev Cancer. 8:299–308. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Perez SA, von Hofe E, Kallinteris NL,
Gritzapis AD, Peoples GE, Papamichail M and Baxevanis CN: A new era
in anticancer peptide vaccines. Cancer. 116:2071–2080.
2010.PubMed/NCBI
|
|
6
|
Nakatsura T, Yoshitake Y, Senju S, et al:
Glypican-3, overexpressed specifically in human hepatocellular
carcinoma, is a novel tumor marker. Biochem Biophys Res Commun.
306:16–25. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shirakawa H, Suzuki H, Shimomura M, et al:
Glypican-3 expression is correlated with poor prognosis in
hepatocellular carcinoma. Cancer Sci. 100:1403–1407. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakatsura T, Komori H, Kubo T, et al:
Mouse homologue of a novel human oncofetal antigen, glypican-3,
evokes T-cell-mediated tumor rejection without autoimmune reactions
in mice. Clin Cancer Res. 10:8630–8640. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Komori H, Nakatsura T, Senju S, et al:
Identification of HLA-A2- or HLA-A24-restricted CTL epitopes
possibly useful for glypican-3-specific immunotherapy of
hepatocellular carcinoma. Clin Cancer Res. 12:2689–2697. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Motomura Y, Ikuta Y, Kuronuma T, et al:
HLA-A2 and -A24-restricted glypican-3-derived peptide vaccine
induces specific CTLs: preclinical study using mice. Int J Oncol.
32:985–990. 2008.PubMed/NCBI
|
|
11
|
Sawada Y, Yoshikawa T, Nobuoka D, et al:
Phase I trial of a glypican-3-derived peptide vaccine for advanced
hepatocellular carcinoma: immunologic evidence and potential for
improving overall survival. Clin Cancer Res. 18:3686–3696. 2012.
View Article : Google Scholar
|
|
12
|
Sawada Y, Sakai M, Yoshikawa T, Ofuji K
and Nakatsura T: A glypican-3-derived peptide vaccine against
hepatocellular carcinoma. Oncoimmunology. 1:1448–1450. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rosenberg SA: Cell transfer immunotherapy
for metastatic solid cancer - what clinicians need to know. Nat Rev
Clin Oncol. 8:577–585. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gomes AQ, Martins DS and Silva-Santos B:
Targeting gamma-delta T lymphocytes for cancer immunotherapy: from
novel mechanistic insight to clinical application. Cancer Res.
70:10024–10027. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wilhelm M, Kunzmann V, Eckstein S, Reimer
P, Weissinger F, Ruediger T and Tony HP: Gammadelta T cells for
immune therapy of patients with lymphoid malignancies. Blood.
102:200–206. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sakamoto M, Nakajima J, Murakawa T, et al:
Adoptive immunotherapy for advanced non-small cell lung cancer
using zoledronate-expanded gammadelta T cells: a phase I clinical
study. J Immunother. 34:202–211. 2011. View Article : Google Scholar
|
|
17
|
Nakajima J, Murakawa T, Fukami T, et al: A
phase I study of adoptive immunotherapy for recurrent
non-small-cell lung cancer patients with autologous gammadelta T
cells. Eur J Cardiothorac Surg. 37:1191–1197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Champagne E: Gammadelta T cell receptor
ligands and modes of antigen recognition. Arch Immunol Ther Exp
(Warsz). 59:117–137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nedellec S, Bonneville M and Scotet E:
Human Vgamma9Vdelta2 T cells: from signals to functions. Semin
Immunol. 22:199–206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mookerjee-Basu J, Vantourout P, Martinez
LO, et al: F1-adenosine triphosphatase displays properties
characteristic of an antigen presentation molecule for
Vgamma9Vdelta2 T cells. J Immunol. 184:6920–6928. 2010. View Article : Google Scholar
|
|
21
|
Gober HJ, Kistowska M, Angman L, Jeno P,
Mori L and De Libero G: Human T cell receptor gammadelta cells
recognize endogenous mevalonate metabolites in tumor cells. J Exp
Med. 197:163–168. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gomes AQ, Correia DV, Grosso AR, et al:
Identification of a panel of ten cell surface protein antigens
associated with immunotargeting of leukemias and lymphomas by
peripheral blood gammadelta T cells. Haematologica. 95:1397–1404.
2010. View Article : Google Scholar
|
|
23
|
D’Asaro M, La Mendola C, Di Liberto D, et
al: V gamma 9V delta 2 T lymphocytes efficiently recognize and kill
zoledronate-sensitized, imatinib-sensitive, and imatinib-resistant
chronic myelogenous leukemia cells. J Immunol. 184:3260–3268.
2010.
|
|
24
|
Chargui J, Combaret V, Scaglione V, et al:
Bromohydrin pyrophosphate-stimulated Vgamma9delta2 T cells expanded
ex vivo from patients with poor-prognosis neuroblastoma lyse
autologous primary tumor cells. J Immunother. 33:591–598. 2010.
View Article : Google Scholar
|
|
25
|
Todaro M, D’Asaro M, Caccamo N, et al:
Efficient killing of human colon cancer stem cells by gammadelta T
lymphocytes. J Immunol. 182:7287–7296. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gertner-Dardenne J, Bonnafous C, Bezombes
C, et al: Bromohydrin pyrophosphate enhances antibody-dependent
cell-mediated cytotoxicity induced by therapeutic antibodies.
Blood. 113:4875–4884. 2009. View Article : Google Scholar
|
|
27
|
Capietto AH, Martinet L and Fournie J:
Stimulated gamma-delta T cells increase the in vivo efficacy of
trastuzumab in HER-2+ breast cancer. J Immunol.
187:1031–1038. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takahara M, Miyai M, Tomiyama M, Mutou M,
Nicol AJ and Nieda M: Copulsing tumor antigen-pulsed dendritic
cells with zoledronate efficiently enhance the expansion of tumor
antigen-specific CD8+ T cells via Vgamma9gammadelta T
cell activation. J Leukoc Biol. 83:742–754. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Correia DV, d’Orey F, Cardoso BA, et al:
Highly active microbial phosphoantigen induces rapid yet sustained
MEK/Erk- and PI-3K/Akt-mediated signal transduction in anti-tumor
human gammadelta T-cells. PLoS One. 4:e56572009. View Article : Google Scholar
|
|
30
|
Brandes M, Willimann K and Moser B:
Professional antigen-presentation function by human gammadelta T
cells. Science. 309:264–268. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moser B and Eberl M: Gammadelta T-APCs: a
novel tool for immunotherapy? Cell Mol Life Sci. 68:2443–2452.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Landmeier S, Altvater B, Pscherer S, et
al: Activated human gammadelta T cells as stimulators of specific
CD8+ T-cell responses to subdominant Epstein Barr virus
epitopes: potential for immunotherapy of cancer. J Immunother.
32:310–321. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Altvater B, Pscherer S, Landmeier S,
Kailayangiri S, Savoldo B, Juergens H and Rossig C: Activated human
gammadelta T cells induce peptide-specific CD8+ T-cell
responses to tumor-associated self-antigens. Cancer Immunol
Immunother. 61:385–396. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yoshikawa T, Nakatsugawa M, Suzuki S, et
al: HLA-A2-restricted glypican-3 peptide-specific CTL clones
induced by peptide vaccine show high avidity and antigen-specific
killing activity against tumor cells. Cancer Sci. 102:918–925.
2011. View Article : Google Scholar
|
|
35
|
Robbins PF, Morgan RA, Feldman SA, et al:
Tumor regression in patients with metastatic synovial cell sarcoma
and melanoma using genetically engineered lymphocytes reactive with
NY-ESO-1. J Clin Oncol. 29:917–924. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sadelain M, Brentjens R and Riviere I: The
basic principles of chimeric antigen receptor design. Cancer
Discov. 3:388–398. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Porter DL, Levine BL, Kalos M, Bagg A and
June CH: Chimeric antigen receptor-modified T cells in chronic
lymphoid leukemia. N Engl J Med. 365:725–733. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Weber J, Atkins M, Hwu P, Radvanyi L,
Sznol M and Yee C: White paper on adoptive cell therapy for cancer
with tumor-infiltrating lymphocytes: a report of the CTEP
subcommittee on adoptive cell therapy. Clin Cancer Res.
17:1664–1673. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nakatsura T, Kageshita T, Ito S, et al:
Identification of glypican-3 as a novel tumor marker for melanoma.
Clin Cancer Res. 10:6612–6621. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Saikali Z and Sinnett D: Expression of
glypican 3 (GPC3) in embryonal tumors. Int J Cancer. 89:418–422.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Toretsky JA, Zitomersky NL, Eskenazi AE,
et al: Glypican-3 expression in Wilms tumor and hepatoblastoma. J
Pediatr Hematol Oncol. 23:496–499. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Maeda D, Ota S, Takazawa Y, et al:
Glypican-3 expression in clear cell adenocarcinoma of the ovary.
Mod Pathol. 22:824–832. 2009.PubMed/NCBI
|
|
43
|
Aviel-Ronen S, Lau SK, Pintilie M, Lau D,
Liu N, Tsao MS and Jothy S: Glypican-3 is overexpressed in lung
squamous cell carcinoma, but not in adenocarcinoma. Mod Pathol.
21:817–825. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nobuoka D, Yoshikawa T, Takahashi M, et
al: Intratumoral peptide injection enhances tumor cell antigenicity
recognized by cytotoxic T lymphocytes: a potential option for
improvement in antigen-specific cancer immunotherapy. Cancer
Immunol Immunother. 62:639–652. 2013. View Article : Google Scholar
|
|
45
|
Nobuoka D, Yoshikawa T, Fujiwara T and
Nakatsura T: Peptide intra-tumor injection for cancer
immunotherapy: enhancement of tumor cell antigenicity is a novel
and attractive strategy. Hum Vaccin Immunother. 9:1234–1236. 2013.
View Article : Google Scholar : PubMed/NCBI
|