Introduction
Despite significant reductions in esophageal cancer
rates in association with lifestyle changes, esophageal cancer
mortality remains high worldwide, and incidence, especially that of
esophageal squamous cell carcinoma (ESCC) is increasing in China
(1,2). Chemotherapy is the most common
treatment option for improving the poor survival rate in advanced
esophageal cancer (3). The
first-line drugs in the clinical treatment of esophageal cancer
are, sequentially, cisplatin, 5-fluorouracil (5-FU), and paclitaxel
(3). However, single-agent
chemotherapy is not effective for esophageal cancer because of
natural resistance and the development of resistance (4). In esophageal cancer, many studies
have demonstrated that combination therapy is more effective than
single-drug therapy (3,5,6).
Several heat shock protein 90 (Hsp90) inhibitors
that bind to the N-terminal ATP pocket of Hsp90 have entered
clinical trials. Hsp90 is a crucial molecular chaperone for protein
folding and stabilization that is considered a promising target for
anticancer therapy (7). Hsp90 may
be a target for esophageal cancer treatment because it and its
client proteins are always overexpressed in several ESCC cell lines
and patient tissues (8).
Previously, we reported that the novel Hsp90 inhibitor BJ-B11 had
potent antitumor activity by inducing cell cycle arrest, apoptosis,
and autophagy in human ESCC Eca-109 cells (9). Liu et al reported that Hsp90
antisense RNA led to cell cycle changes and increased sensitivity
to various chemotherapeutic agents in the same cell line (10). Wu et al suggested that
17-AAG, a traditional Hsp90 inhibitor, effectively inhibited
proliferation and viability in other ESCC lines (11). Similarly, NVP-AUY922, another novel
Hsp90 inhibitor, was a potent inhibitor of proliferation in
esophageal cancer TE-4 cells (12). These reports provide a rationale
for current preclinical efforts targeting Hsp90 to treat ESCC.
SNX-2112, a selective Hsp90 inhibitor, has broad
antitumor activity and has entered phase I clinical trials in solid
tumors and lymphoma (13). In
previous studies, we identified the anticancer effect of SNX-2112
in breast cancer, hepatocellular carcinoma, and leukemia (14–16).
Recently, SNX-2112 was reported as being effective in combination
with cisplatin or paclitaxel in head and neck SCC (17). However, there have been no reports
on SNX-2112 in combination with 5-FU, another widely used
anticancer drug, in esophageal cancer. We examined the effects of
SNX-2112 combined with 5-FU in esophageal cancer Eca-109 cells by
detecting cell growth, cell cycle, apoptosis, and Hsp90 client
proteins. This study may provide guidance for the clinical
application of Hsp90 inhibitors.
Materials and methods
Reagents
SNX-2112 was synthesized as previously described in
our lab with >98.0% purity; we stored 10 mM SNX-2112 stock
solution in dimethyl sulfoxide (DMSO) at −20°C (18). We purchased 5-FU, 3-(4,
5-diethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) from
Sigma-Aldrich (St. Louis, MO, USA). An Annexin V-fluorescein
isothiocyanate/propidium iodide (FITC/PI) staining kit was
purchased from Beyotime (Haimen, China). RPMI-1640 medium and DMEM
were purchased from Gibco (Carlsbad, CA, USA). Heat-inactivated
fetal bovine serum (FBS) was provided by the Sijiqing Co.
(Hangzhou, China). Antibodies against caspase-3, caspase-8,
caspase-9, poly(ADP-ribose) polymerase (PARP), Akt, phosphorylated
(p)-Akt, inhibitor of κB kinase (IKK), extracellular
signal-regulated kinase (ERK)1/2, glycogen synthase
kinase (GSK)-3β, and glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) were purchased from Cell Signaling Technology (Beverly, MA,
USA).
Cell culture
We cultured Eca-109 and EC-9706 cells (Cell Bank of
the Chinese Academy of Sciences, Shanghai, China) in RPMI-1640 or
DMEM medium supplemented with 10% FBS and 100 U/ml
penicillin/streptomycin in 5% CO2 at 37°C.
Cell viability assay
Cell viability was assessed with the MTT assay.
Exponentially growing cells (~3,500/well in 100 μl medium) were
plated in 96-well culture plates, cultured overnight, and incubated
with a series of concentrations of SNX-2112 (0–2 μM) or 5-FU (0–100
μg/ml) for 48 h. After adding 10 μl MTT solution per well, the
plates were incubated at 37°C for 4 h, the medium removed, formazan
crystals solubilized in 100 μl DMSO/well, and the absorbance values
read at 570 nm. The inhibition ratio was calculated as follows:
(Acontrol - Atreated)/Acontrol ×
100%, where Atreated and Acontrol are the
absorbance of the treated and control groups after 48-h incubation,
respectively.
Calculation of the combination effect
index
We determined the inhibitory effects of SNX-2112 and
5-FU using the MTT assay. We used the combination index (CI)
described by Chou and Talalay for analysis (19), performed using CalcuSyn software
(BioSoft, Oxford, UK). CI<1 indicates synergism; CI=1 indicates
summation; CI>1 indicates antagonism (20).
Cell cycle analysis
Cells were exposed to 0.125 μM SNX-2112 or 25 μg/ml
5-FU alone or in combination for 48 h, harvested in cold
phosphate-buffered saline, fixed in 70% ethanol, stored overnight
at 4°C, and resuspended in 50 μg/ml PI staining reagent containing
100 μg/ml RNase and 0.1% Triton X-100 for 30 min in the dark. Cells
were analyzed by flow cytometry (FACSCalibur; Becton-Dickinson, San
Jose, CA, USA).
Quantitative real-time RT-PCR
(Q-PCR)
Total cellular RNA was extracted using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions. Quantitative RT-PCR was carried out
using a Chromo4 instrument (Bio-Rad, Richmond, CA, USA) and
SYBR® Premix Ex Taq™ kit (Takara Bio, Otsu, Japan) to
detect the mRNA level of cyclin D1, Cdk2, Cdk4, p53, Chk1 and Chk2,
with GAPDH as a normalizing control. The specific PCR primer
sequences of these genes were designed by Primer premier 5.0
software (Table I). Cycling
conditions were: 95°C for 20 sec to activate DNA polymerase,
followed by 40 cycles of 95°C for 10 sec, 55°C for 20 sec, and 65°C
for 30 sec. The relative changes in gene expression were calculated
with the 2−ΔΔCt method, where ΔΔCt = ΔCt (drugs treated)
- ΔCt (control) for RNA samples.
 | Table IQ-PCR primers. |
Table I
Q-PCR primers.
| Name | Forward primer (5′-
to 3′) | Reverse primer (5′-
to 3′) |
|---|
| Cyclin D1 |
GCCCTCGGTGTCCTACTTC |
CTCCTCCTCGCACTTCTGTT |
| Cdk2 |
TGCCTGATTACAAGCCAAGTT |
GAGTCGAAGATGGGGTACTGG |
| Cdk4 |
CAGCTACCAGATGGCACTTACA |
CAAAGATACAGCCAACACTCCA |
| Chk1 |
AAACATACCTCAACCCTTGGA |
CCTTTTTGCCCCTTTCTTG |
| Chk2 |
TTGGAAGTGGTGCCTGTG |
GGTCTGCCTCTCTTGCTGAA |
| p53 |
CTCCTCAGCATCTTATCCGAGT |
GCTGTTCCGTCCCAGTAGATTA |
| GAPDH |
AACGGATTTGGTCGTATTGGG |
TCGCTCCTGGAAGATGGTGAT |
Annexin V-FITC/PI analysis
Cells were exposed to the indicated concentrations
of SNX-2112 or 5-FU alone or in combination for 48 h, resuspended
in 500 μl incubation buffer containing Annexin V-FITC and PI,
incubated in the dark for 15 min, and analyzed by flow cytometry.
Data acquisition and analysis were performed using a FACSCalibur
flow cytometer with CellQuest software (Becton-Dickinson).
Mitochondrial membrane potential
assay
Cells were cultured on glass cover slips and treated
with SNX-2112 and 5-FU for 48 h, then incubated in complete medium
containing 10 μg fluorescent lipophilic cationic JC-1 dye for 20
min at 37°C in the dark. The stained cells were washed twice with
JC-1 buffer solution and examined by laser scanning confocal
microscopy. We also analyzed the cells by flow cytometry. Data
acquisition and analysis were performed in a FACSCalibur flow
cytometer using CellQuest software. The loss of mitochondrial
membrane potential (MMP) was quantified as the percentage of cells
expressing JC-1 monomer fluorescence.
Western blotting
Cell were treated with SNX-2112 and 5-FU for 48 h,
harvested, and lysed in sodium dodecyl sulfate (SDS) lysis buffer
(SDS:phenylmethylsulfonyl fluoride = 50:1) at 100°C for 20 min.
Lysates were clarified by centrifugation (12,000 rpm) at 4°C for 15
min and the supernatant was collected. Equal amount of lysate
(20–30 μg) was denatured in 5X SDS sample buffer, resolved with
6–15% SDS-polyacrylamide gel electrophoresis, transferred to
polyvinylidene difluoride membranes, blocked with 5% skimmed milk
in Tris-buffered saline containing 0.1% Tween-20 (TBST) at room
temperature for 1 h, and probed with primary antibody
(1:1,000–1:5,000) overnight at 4°C. The membranes incubated with
secondary antibody (1:5,000) for 1 h at room temperature. Protein
bands were visualized using an enhanced chemiluminescence kit
(Beyotime, Shanghai, China) and imaged by autoradiography. GAPDH
was used as the loading control.
Docking assay
The affinity of 5-FU and SNX-2112 for Hsp90 was
determined by molecular operating environment docking. We obtained
the crystal structure of Hsp90 from the Protein Data Bank (PDB
code: 3R92). The two drugs were docked to the binding pocket of
Hsp90 to check the fitness according to our previous methods. A
lower score indicated more favorable binding.
Statistical analysis
Data are expressed as the means ± SD. Differences
between two groups were analyzed using the Student’s t-test; groups
of three or more were analyzed using one-way analysis of variance
(multiple comparisons). P<0.05 and P<0.01 were considered
statistically significant. We performed statistical analyses using
SPSS 17.0 software (IBM, Armonk, NY, USA).
Results
Effects of SNX-2112 and 5-FU on Eca-109
cells
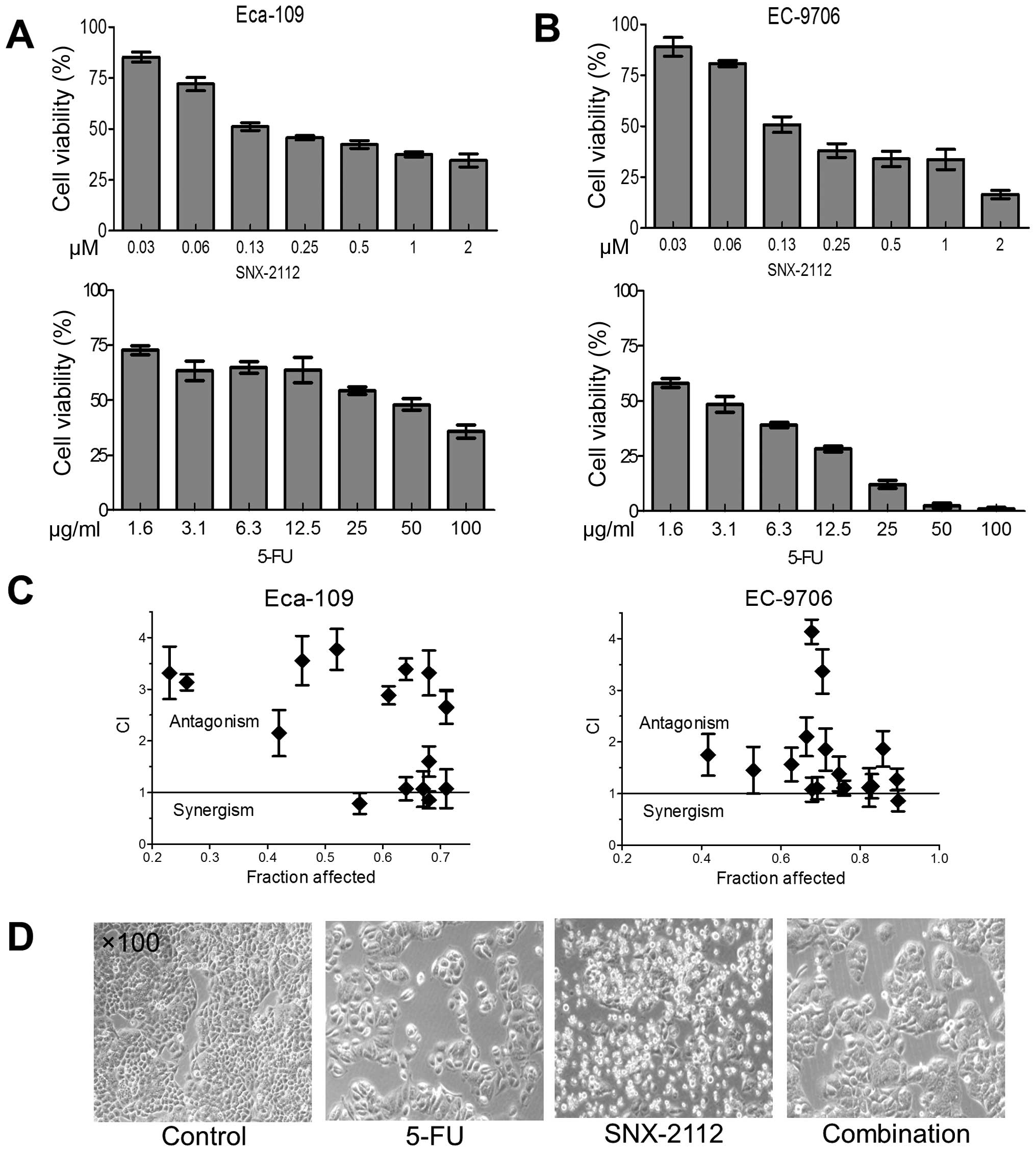
Initially, we examined the effects of SNX-2112 and
5-FU on Eca-109 and EC-9706 cell growth by MTT assay. Cells were
cultured in a range of concentrations of SNX-2112 or 5-FU for 48 h.
As expected, both SNX-2112 and 5-FU inhibited Eca-109 and EC-9706
cell growth in a dose-dependent manner. The median inhibitory
concentration (IC50) of SNX-2112 and 5-FU was 0.12±0.01
μM and 48±0.2 μg/ml in Eca-109 cells, respectively (Fig. 1A). Similarly, the IC50
of SNX-2112 and 5-FU was 0.13±0.02 μM and 3±0.2 μg/ml in EC-9706
cells, respectively (Fig. 1B).
Unexpectedly, there was an antagonistic effect in most cases in the
combination group (CI>1, Fig.
1C). The occurrences of strong antagonistic effect (CI>2)
were 62.5% in Eca-109 cells and 18.75% in EC-9706 cells,
respectively, suggesting that the antagonistic effect in Eca-109
cells was more obvious (Tables II
and III). The effect was most
obvious when 0.125 μM SNX-2112 was combined with 25 μg/ml 5-FU in
Eca-109 cells (CI=3.7, Table II
and Fig. 1D). Therefore, we used
0.125 μM SNX-2112 and 25 μg/ml 5-FU as the optimal concentrations
in Eca-109 cells for the remaining experiments.
 | Table IICombination effects of SNX-2112 and
5-FU on Eca-109 cells. |
Table II
Combination effects of SNX-2112 and
5-FU on Eca-109 cells.
| SNX-2112 (μM) | 5-FU (μg/ml) | Fa | CI |
|---|
| 0.03125 | 50 | 0.71 | 2.662±0.329 |
| 25 | 0.46 | 3.558±0.477 |
| 12.5 | 0.23 | 3.319±0.509 |
| 6.25 | 0.56 | 0.789±0.204 |
| 0.0625 | 50 | 0.71 | 2.647±0.318 |
| 25 | 0.68 | 1.601±0.296 |
| 12.5 | 0.64 | 1.074±0.226 |
| 6.25 | 0.26 | 3.136±0.154 |
| 0.125 | 50 | 0.68 | 3.317±0.436 |
| 25 | 0.52 | 3.772±0.396 |
| 12.5 | 0.67 | 1.068±0.347 |
| 6.25 | 0.42 | 2.151±0.447 |
| 0.25 | 50 | 0.64 | 3.389±0.209 |
| 25 | 0.61 | 2.884±0.175 |
| 12.5 | 0.71 | 1.074±0.375 |
| 6.25 | 0.68 | 0.859±0.167 |
 | Table IIICombination effects of SNX-2112 and
5-FU on EC-9706 cells. |
Table III
Combination effects of SNX-2112 and
5-FU on EC-9706 cells.
| SNX-2112 (μM) | 5-FU (μg/ml) | Fa | CI |
|---|
| 0.0625 | 25 | 0.71 | 3.366±0.429 |
| 12.5 | 0.67 | 2.102±0.377 |
| 6.25 | 0.68 | 1.079±0.234 |
| 3.125 | 0.42 | 1.75±0.404 |
| 0.125 | 25 | 0.89 | 1.276±0.213 |
| 12.5 | 0.71 | 1.853±0.407 |
| 6.25 | 0.63 | 1.563±0.326 |
| 3.125 | 0.53 | 1.453±0.454 |
| 0.25 | 25 | 0.68 | 4.355±0.236 |
| 12.5 | 0.83 | 1.143±0.234 |
| 6.25 | 0.76 | 1.107±0.145 |
| 3.125 | 0.70 | 1.102±0.214 |
| 0.5 | 25 | 0.86 | 1.869±0.345 |
| 12.5 | 0.90 | 0.865±0.209 |
| 6.25 | 0.82 | 1.118±0.375 |
| 3.125 | 0.75 | 1.38±0.334 |
5-FU blocked SNX-2112-induced G2/M
arrest
Cell cycle arrest is the basic mechanism of cancer
cell growth inhibition by Hsp90 inhibitors (16). To investigate the mechanism of 5-FU
antagonism of SNX-2112 further, we examined cell cycle distribution
with flow cytometry. There was 45.8% G2/M arrest and only 14.1%
G0/G1 arrest following SNX-2112 treatment, but there was only 1.5%
G2/M accumulation and the G0/G1 arrest increased to 48.2% in the
combination group (Fig. 2A). This
indicated that 5-FU completely recovered SNX-2112-induced G2/M
arrest and partly increased the G0/G1 arrest of SNX-2112. Further,
we found that the mRNA levels of cyclin D1, Cdk4 and Chk2 were
decreased in the combination group compared to treatment with
SNX-2112 alone (Fig. 2B), while
the mRNA levels of p53, Chk1 and Cdk2 did not change significantly
(Fig. 2C).
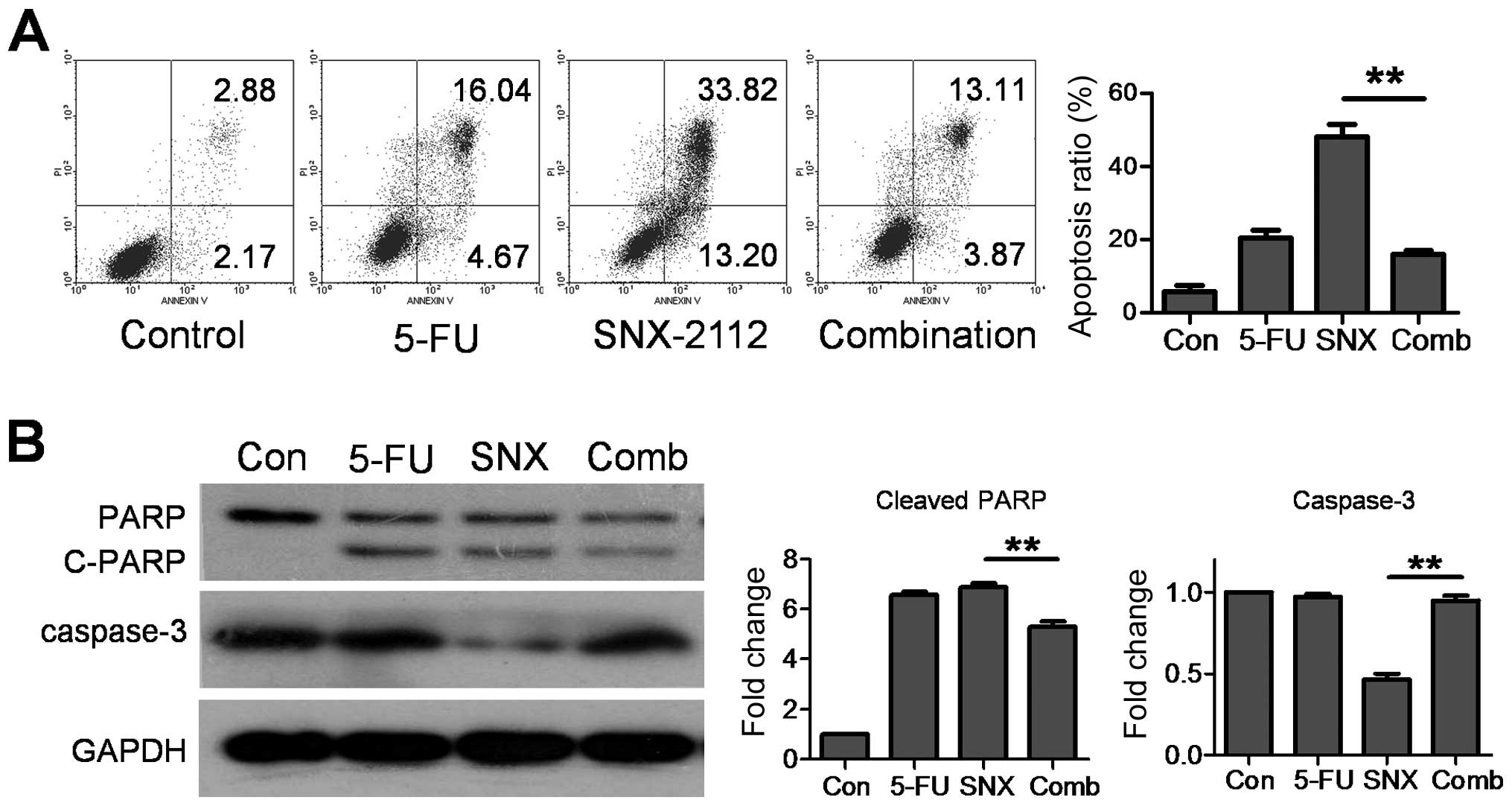
5-FU suppressed SNX-2112-induced
caspase-dependent apoptosis
To determine the effect of SNX-2112 plus 5-FU on
apoptosis, we examined the ratio of apoptosis using flow cytometry.
We found that SNX-2112 and 5-FU alone induced 47.02 and 20.71%
apoptosis, respectively; SNX-2112 plus 5-FU led to apoptosis
decreasing to 16.98% (Fig. 3A). We
then examined caspase-3 and PARP expression (indicators of
apoptosis). 5-FU suppressed the caspase-3 downregulation and PARP
cleavage induced by SNX-2112 (Fig.
3B). Taken together, our results indicate that 5-FU inhibits
SNX-2112-induced apoptosis and caspase activation in Eca-109
cells.
5-FU prevents the initial decrease in MMP
during SNX-2112-induced apoptosis
Mitochondria play a central role in determining cell
survival or response to diverse stimuli (21). The SNX-2112 and 5-FU combination
greatly suppressed the caspase-9 activity (a downstream indicator
of the mitochondrial apoptotic pathway) induced by SNX-2112, while
caspase-8 (a downstream indicator of the death receptor apoptotic
pathway) was inhibited slightly (Fig.
4A). To determine whether the mitochondria mediated the 5-FU
antagonism of SNX-2112-induced apoptosis, we evaluated MMP by JC-1
staining. Following SNX-2112 treatment, the red/green fluorescence
ratio was significantly decreased to 0.97, which 5-FU recovered to
1.45 (Fig. 4B). Flow cytometry
showed 39.1% JC-1 fluorescence following SNX-2112 treatment; that
of the combination group was 21.8% (Fig. 4C). These results show that 5-FU
partly reversed the MMP decline caused by SNX-2112, indicating that
5-FU antagonism of SNX-2112-induced apoptosis might occur through
mitochondrial repair.
 | Figure 4Effect of 5-FU and SNX-2112 on MMP in
Eca-109 cells. (A) Western blot assessment of caspase-9 and −8.
GAPDH was used as the protein loading control. (B) JC-1
fluorescence images of cells treated with SNX-2112, 5-FU, or
SNX-2112 and 5-FU. Red fluorescence indicates high membrane
potential; green fluorescence represents low membrane potential.
(C) Representative flow cytometric analyses of MMP in cells treated
with SNX-2112, 5-FU, or SNX-2112 and 5-FU; increased JC-1
expression indicates reduced MMP. Values are the mean ± SD of three
independent experiments. *P<0.05,
**P<0.01 compared with the SNX-2112 group. Con,
untreated control; SNX, SNX-2112; Comb, combination; C-caspase-8,
cleaved caspase-8; C-caspase-9, cleaved caspase-9. |
5-FU inhibits SNX-2112-induced
downregulation of Hsp90 client proteins
Previously, we found that SNX-2112 caused depletion
of Hsp90 client proteins such as Akt, p-Akt, IKK,
ERK1/2, and GSK-3β (18). To determine whether 5-FU
antagonized the anticancer effect of SNX-2112 on Hsp90 client
proteins, we investigated the expression of these proteins
following SNX-2112 and 5-FU treatment. The SNX-2112 and 5-FU
combination partly inhibited Akt, p-Akt, ERK1/2, and
GSK-3β depletion and recovered the SNX-2112-induced IKKα
downregulation completely (Fig.
5A). These results suggest that 5-FU attenuates
SNX-2112-induced downregulation of Hsp90 client proteins in Eca-109
cells.
 | Figure 5The safety and efficacy of the
combination of 5-FU and Hsp90 inhibitors need special attention in
cancer therapy. (A) Effects of SNX-2112, 5-FU, or SNX-2112 and 5-FU
on Hsp90 client proteins (Akt, p-Akt, IKKα, Erk1/2, and
GSK-3β) determined by western blotting. GAPDH was used as the
protein loading control. (B) 5-FU and SNX-2112 docking at the
N-terminal domain of Hsp90. The lowest-energy conformations are
shown; lower scores indicate more favorable binding. (C) Graphical
abstract. Values are the mean ± SD of three independent
experiments. *P<0.05, **P<0.01 compared
with the SNX-2112 group. Con, untreated control; SNX, SNX-2112;
Comb, combination. |
5-FU and SNX-2112 do not bind
competitively to the Hsp90 N-terminal ATP pocket
To study whether 5-FU binding to the N-terminal ATP
pocket of Hsp90 competed with that of SNX-2112, the fit was examine
by docking studies. A hydrogen bond residue (Phe-138) and a side
chain donor molecule (Lys-58) were in contact with SNX-2112; 5-FU
did not interact with Hsp90, and a major portion of Hsp90 was
exposed to the solvent. The scoring value of SNX-2112 and 5-FU was
-30.94 and −12.28 kcal/mol, respectively, indicating that 5-FU
could not compete with SNX-2112 to bind to the N-terminal ATP
pocket of Hsp90 (Fig. 5B).
Discussion
This study marks the first demonstration that the
combination of SNX-2112 with 5-FU exhibits antagonistic effects in
esophageal cancer cells. The antagonist effects were related to: i)
growth inhibition; ii) G2/M arrest; iii) caspase-dependent and
mitochondrial-mediated apoptosis; iv) Hsp90 client protein
expression. In most cases, however, the combination of Hsp90
inhibitors with chemotherapy drugs (such as SNX-2112 with
paclitaxel and cisplatin in head and neck SCC, ganetespib with the
taxanes paclitaxel and docetaxel in non-small cell lung cancer,
17-AAG with cisplatin in glioma) is synergistic (17,22,23).
From this point of view, the combination effect of SNX-2112 plus
5-FU is quite different from that of other combination therapies
based on Hsp90 inhibitors and chemotherapy drugs, and the
antagonistic effect in this exception catalyzed our efforts to
uncover the underlying mechanism.
SNX-2112 arrests the cell cycle at the G2/M phase in
various cancers (16,18). It has been established that
cyclins, cell cycle kinases [such as checkpoint proteins,
cyclin-dependent kinases (Cdks), and non-Cdk kinases], and other
cell cycle-related protein are responsible for cell cycle control.
For instance, Cdk4 is inactive in their monomeric form and
activated by cyclin D1, and the formation of Cdk4/cyclin D1
complexes plays an important role on the change of cell cycle
(24). Stepanova et al
reported that the inactivation of Hsp90 increased the complexes of
Cdk4/cyclin D1, but has no effect on Cdk2/cyclin E1 (25). These findings are consistent with
our study that the mRNA level of cyclin D1 and Cdk4 were decreased
in combination group compared to SNX-2112 treatment alone, with no
significant change of Cdk2. In addition, we found that 5-FU
completely reversed SNX-2112-induced G2/M arrest and partly
increased the G0/G1 arrest of SNX-2112. However, this contrasts
with the 5-FU enhancement (but not decrease) of G2/M cell
percentage that may antagonize the effect of celecoxib in breast
cancer (26). It remains unclear
why 5-FU has bidirectional effects in cell cycle arrest when
combined with different anticancer drugs.
We demonstrated that 5-FU suppressed
caspase-dependent and mitochondria-mediated apoptosis induced by
SNX-2112. Consistent with our previous studies, SNX-2112 activated
caspase-3, −8 and −9 and decreased MMP (14–16,18),
which 5-FU neutralized in this study. Mitochondria are involved in
many cellular processes, such as metabolism, signaling, cell
growth, and apoptosis (21).
Caspase-9 activation is significantly associated with mitochondrial
dysfunction, while caspase-8 activation is related to the death
receptor pathways (27). In our
protein assay, caspase-9 appeared more sensitive than caspase-8 to
5-FU treatment, suggesting that 5-FU antagonism of SNX-2112 is
mainly regulated by mitochondrial-dependent pathways.
The exact manner in which 5-FU decreases the
anticancer effect of the Hsp90 inhibitor SNX-2112 remains to be
determined. However, based on the literature and our findings, we
believe that there are at least two probabilities: i) 5-FU might
bind competitively to the N-terminal ATP pocket or another site of
Hsp90, altering Hsp90 conformation and function. However, it should
be noted that our molecular docking study only ruled out the
possibility of 5-FU indirect binding to the N-terminal ATP pocket
of Hsp90; ii) 5-FU recovers the downstream signaling pathways of
Hsp90 downregulated by SNX-2112. Our report proves that SNX-2112
suppresses the phosphatidylinositol 3-kinase (PI3K)/Akt and nuclear
factor κB (NF-κB) pathways (16,28).
It has been reported that 5-FU alone upregulates p-Akt and IKK
expression in cancer cells (29,30).
Therefore, we speculate that the recovery of the PI3K/Akt and NF-κB
pathways might be a possible mechanism of 5-FU antagonism of the
anticancer effect of SNX-2112.
In conclusion, the combination of SNX-2112 with 5-FU
exhibits antagonistic effects by reversing G2/M arrest, suppressing
caspase-dependent apoptosis, preventing the initial MMP decrease,
and decreasing the downregulation of Hsp90 client proteins
(Fig. 5C). Although further
demonstration of a more precise mechanism of 5-FU antagonism of
SNX-2112 is required in other cancer types, we suggest that the
SNX-2112 plus 5-FU combination should be used with special care in
clinical application.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant 81201727), the China
Postdoctoral Science Foundation (grants 2012M511882 and
2013T60827), the open project of State Key Laboratory of Molecular
Oncology (SKL-KF-2013-14), Guangdong Province and Ministry of
Education Ministry of Science and Technology Products Research
Combined Platform Project (grant 2010B091000013), and the Natural
Science Foundation of Guangdong Province (grant
S2012040006873).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
He YT, Hou J, Chen ZF, et al: Trends in
incidence of esophageal and gastric cardia cancer in high-risk
areas in China. Eur J Cancer Prev. 17:71–76. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tew WP, Kelsen DP and Ilson DH: Targeted
therapies for esophageal cancer. Oncologist. 10:590–601. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khushalani NI, Leichman CG, Proulx G, et
al: Oxaliplatin in combination with protracted-infusion
fluorouracil and radiation: report of a clinical trial for patients
with esophageal cancer. J Clin Oncol. 20:2844–2850. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Burmeister BH, Walpole ET, D’Arcy N, et
al: A phase II trial of chemoradiation therapy with weekly
oxaliplatin and protracted infusion of 5-fluorouracil for
esophageal cancer. Invest New Drugs. 27:275–279. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Juergens RA and Forastiere A: Combined
modality therapy of esophageal cancer. J Natl Compr Cancer Netw.
6:851–861. 2008.PubMed/NCBI
|
|
7
|
Neckers L: Hsp90 inhibitors as novel
cancer chemotherapeutic agents. Trends Mol Med. 8(Suppl 4):
S55–S61. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Faried A, Sohda M, Nakajima M, Miyazaki T,
Kato H and Kuwano H: Expression of heat-shock protein Hsp60
correlated with the apoptotic index and patient prognosis in human
oesophageal squamous cell carcinoma. Eur J Cancer. 40:2804–2811.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu KS, Zhang Y, Ding WC, et al: The
selective Hsp90 inhibitor BJ-B11 exhibits potent antitumor activity
via induction of cell cycle arrest, apoptosis and autophagy in
Eca-109 human esophageal squamous carcinoma cells. Int J Oncol.
41:2276–2284. 2012.
|
|
10
|
Liu XL, Xiao B, Yu ZC, et al:
Down-regulation of Hsp90 could change cell cycle distribution and
increase drug sensitivity of tumor cells. World J Gastroenterol.
5:199–208. 1999.PubMed/NCBI
|
|
11
|
Wu X, Wanders A, Wardega P, et al: Hsp90
is expressed and represents a therapeutic target in human
oesophageal cancer using the inhibitor
17-allylamino-17-demethoxygeldanamycin. Br J Cancer. 100:334–343.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bao XH, Takaoka M, Hao H-F, et al:
Antiproliferative effect of the HSP90 inhibitor NVP-AUY922 is
determined by the expression of PTEN in esophageal cancer. Oncol
Rep. 29:45–50. 2013.PubMed/NCBI
|
|
13
|
Rajan A, Kelly RJ, Trepel JB, et al: A
phase I study of PF-04929113 (SNX-5422), an orally bioavailable
heat shock protein 90 inhibitor, in patients with refractory solid
tumor malignancies and lymphomas. Clin Cancer Res. 17:6831–6839.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang X, Wang S, Liu Y, et al: The Hsp90
inhibitor SNX-2112 induces apoptosis of human hepatocellular
carcinoma cells: the role of ER stress. Biochem Biophys Res Commun.
446:160–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang R, Shao F, Liu Z, et al: The Hsp90
inhibitor SNX-2112, induces apoptosis in multidrug resistant
K562/ADR cells through suppression of Akt/NF-kappaB and disruption
of mitochondria-dependent pathways. Chem Biol Interact. 205:1–10.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang SX, Ju HQ, Liu KS, et al: SNX-2112, a
novel Hsp90 inhibitor, induces G2/M cell cycle arrest and apoptosis
in MCF-7 cells. Biosci Biotechnol Biochem. 75:1540–1545. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Friedman JA, Wise SC, Hu M, et al: HSP90
inhibitor SNX5422/2112 targets the dysregulated signal and
transcription factor network and malignant phenotype of head and
neck squamous cell carcinoma. Transl Oncol. 6:429–441. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu KS, Liu H, Qi JH, et al: SNX-2112, an
Hsp90 inhibitor, induces apoptosis and autophagy via degradation of
Hsp90 client proteins in human melanoma A-375 cells. Cancer Lett.
318:180–188. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chou T-C and Talalay P: Quantitative
analysis of dose-effect relationships: the combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chou T-C: Drug combination studies and
their synergy quantification using the Chou-Talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kroemer G, Galluzzi L and Brenner C:
Mitochondrial membrane permeabilization in cell death. Physiol Rev.
87:99–163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ohba S, Hirose Y, Yoshida K, Yazaki T and
Kawase T: Inhibition of 90-kD heat shock protein potentiates the
cytotoxicity of chemotherapeutic agents in human glioma cells. J
Neurosurg. 112:33–42. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Proia DA, Sang J, He S, et al: Synergistic
activity of the Hsp90 inhibitor ganetespib with taxanes in
non-small cell lung cancer models. Invest New Drugs. 30:2201–2209.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Casimiro MC, Crosariol M, Loro E, Li Z and
Pestell RG: Cyclins and cell cycle control in cancer and disease.
Genes Cancer. 3:649–657. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stepanova L, Leng X, Parker SB and Harper
JW: Mammalian p50Cdc37 is a protein kinase-targeting subunit of
Hsp90 that binds and stabilizes Cdk4. Genes Dev. 10:1491–1502.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
El-Awady RA, Saleh EM, Ezz M and Elsayed
AM: Interaction of celecoxib with different anti-cancer drugs is
antagonistic in breast but not in other cancer cells. Toxicol Appl
Pharmacol. 255:271–286. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Crow MT, Mani K, Nam Y-J and Kitsis RN:
The mitochondrial death pathway and cardiac myocyte apoptosis. Circ
Res. 95:957–970. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Okawa Y, Hideshima T, Steed P, et al:
SNX-2112, a selective Hsp90 inhibitor, potently inhibits tumor cell
growth, angiogenesis, and osteoclastogenesis in multiple myeloma
and other hematologic tumors by abrogating signaling via Akt and
ERK. Blood. 113:846–855. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fukuyama R, Ng KP, Cicek M, et al: Role of
IKK and oscillatory NFkappaB kinetics in MMP-9 gene expression and
chemoresistance to 5-fluorouracil in RKO colorectal cancer cells.
Mol Carcinog. 46:402–413. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Azuma M, Yamashita T, Aota K, Tamatani T
and Sato M: 5-Fluorouracil suppression of NF-KappaB is mediated by
the inhibition of IKappab kinase activity in human salivary gland
cancer cells. Biochem Biophys Res Commun. 282:292–296. 2001.
View Article : Google Scholar : PubMed/NCBI
|