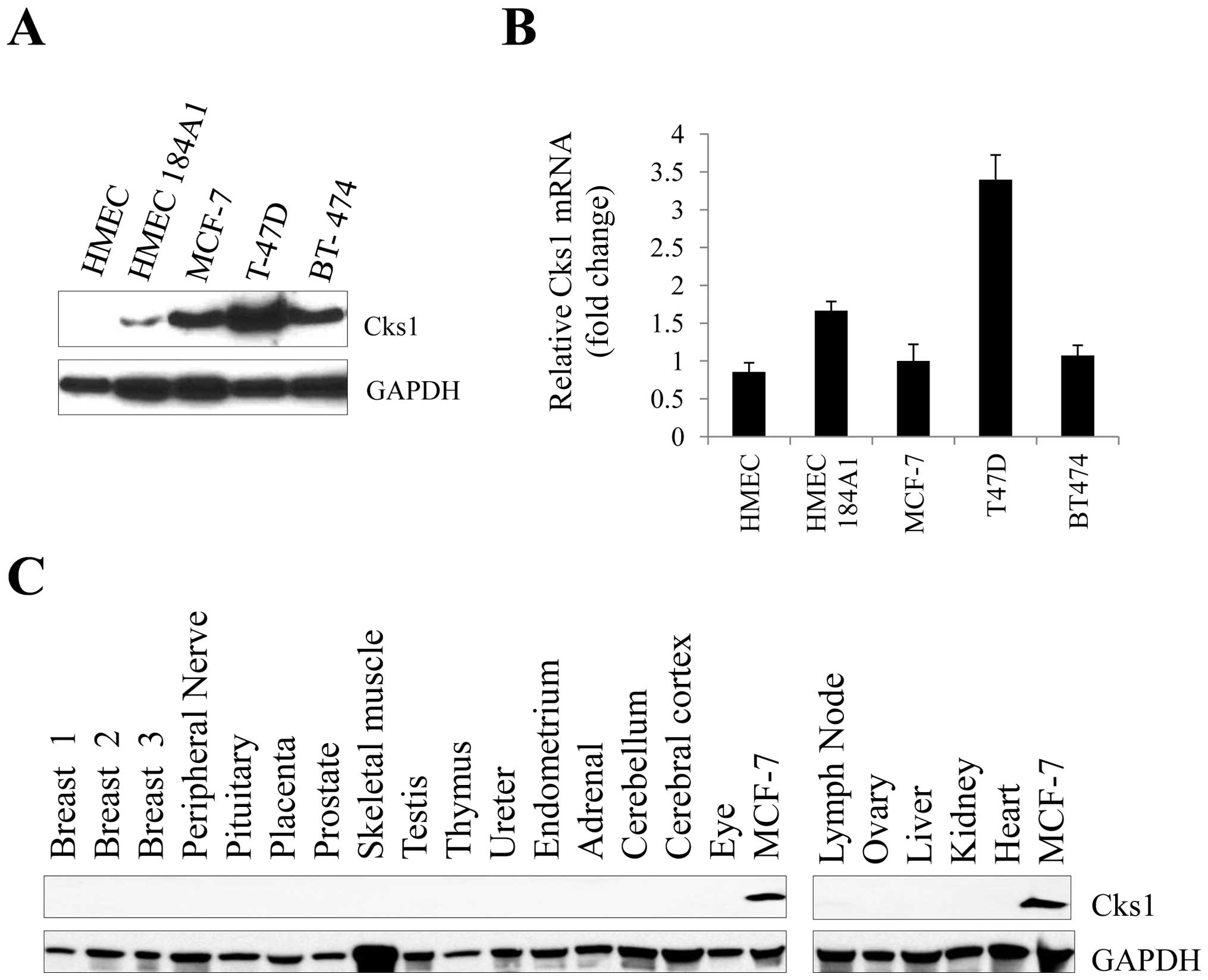
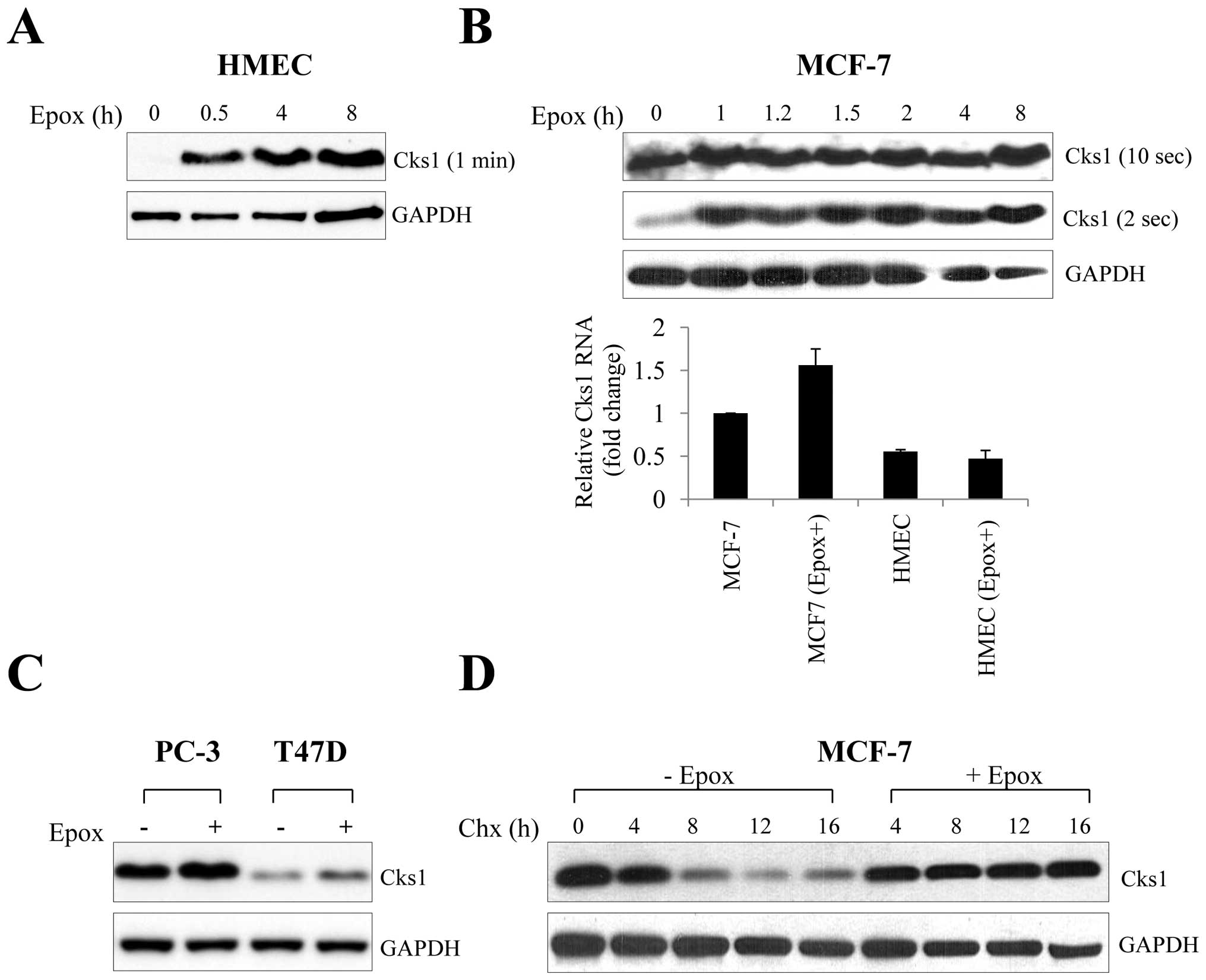
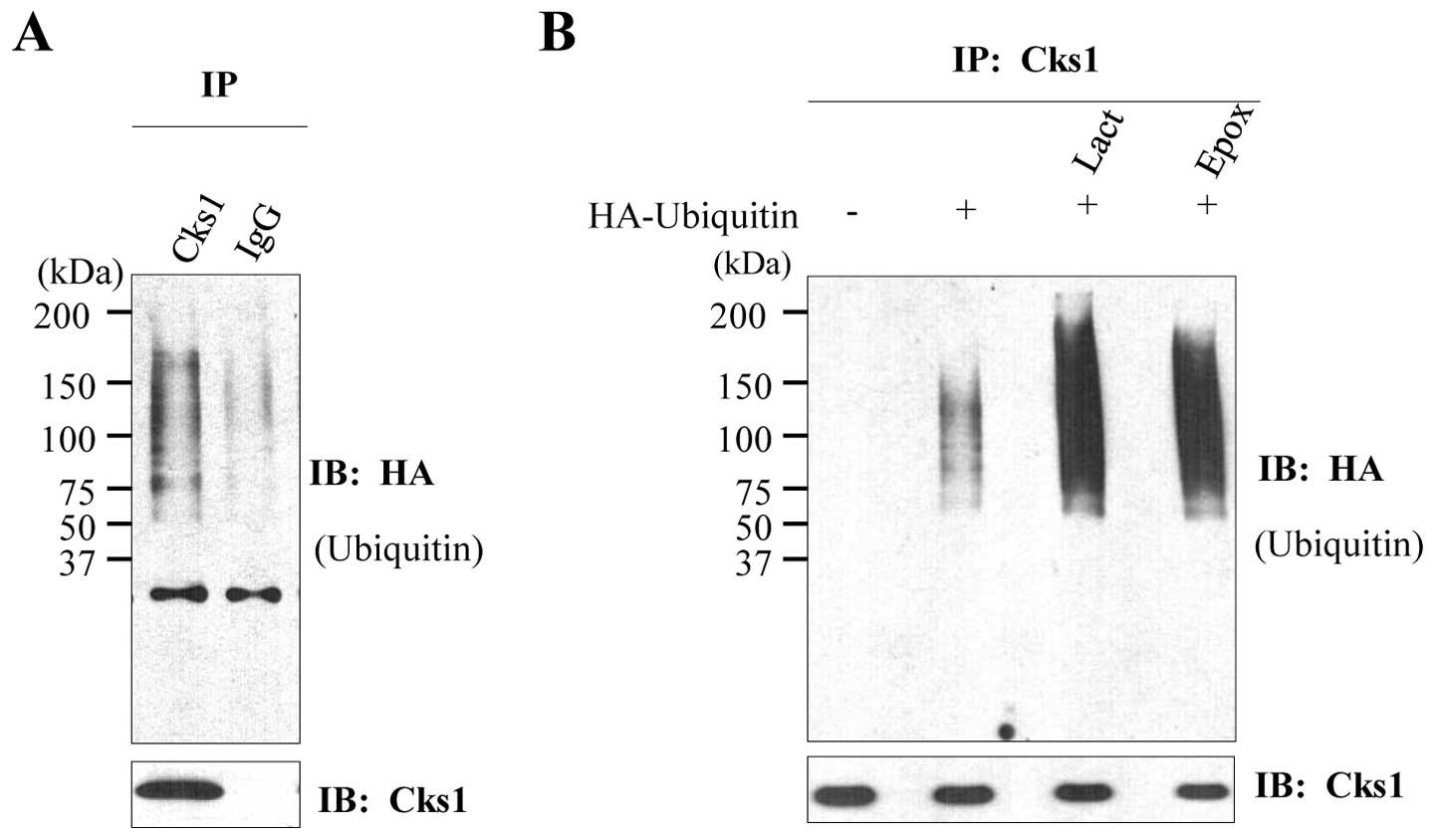
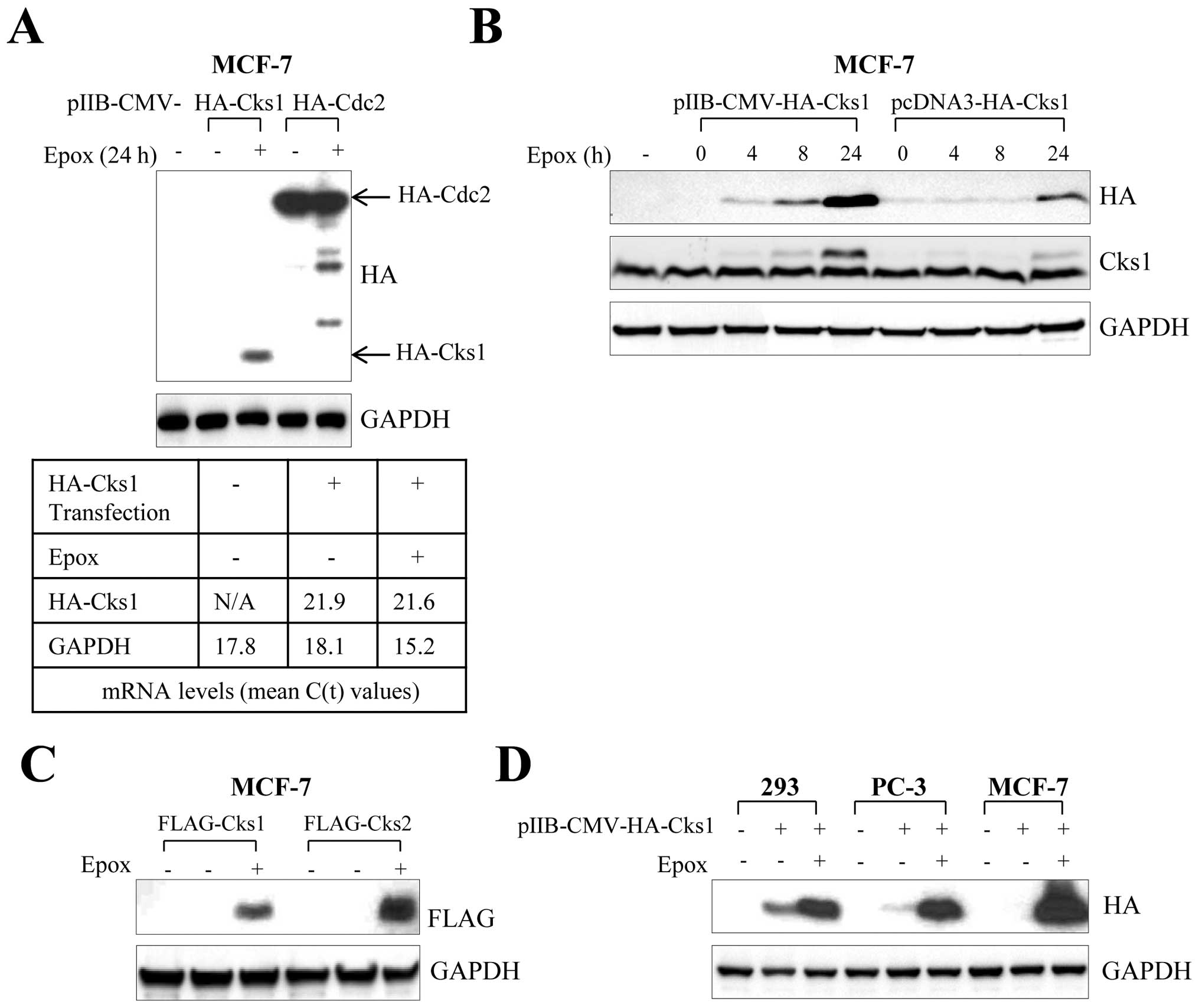
|
1
|
Khattar V and Thottassery JV: Cks1:
structure, emerging roles and implications in multiple cancers. J
Cancer Ther. 4:1341–1354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ganoth D, Bornstein G, Ko TK, Larsen B,
Tyers M, Pagano M and Hershko A: The cell-cycle regulatory protein
Cks1 is required for SCF(Skp2)-mediated ubiquitinylation of p27.
Nat Cell Biol. 3:321–324. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Spruck C, Strohmaier H, Watson M, et al: A
CDK-independent function of mammalian Cks1: targeting of SCF(Skp2)
to the CDK inhibitor p27Kip1. Mol Cell. 7:639–650. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Westbrook L, Manuvakhova M, Kern FG, Estes
NR, Ramanathan HN and Thottassery JV: Cks1 regulates cdk1
expression: a novel role during mitotic entry in breast cancer
cells. Cancer Res. 67:11393–11401. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hoellein A, Graf S, Bassermann F, et al:
Cks1 promotion of S phase entry and proliferation is independent of
p27Kip1 suppression. Mol Cell Biol. 32:2416–2427. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liberal V, Martinsson-Ahlzen HS, Liberal
J, Spruck CH, Widschwendter M, McGowan CH and Reed SI:
Cyclin-dependent kinase subunit (Cks) 1 or Cks2 overexpression
overrides the DNA damage response barrier triggered by activated
oncoproteins. Proc Natl Acad Sci USA. 109:2754–2759. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Westbrook L, Ramanathan HN, Isayeva T, et
al: High Cks1 expression in transgenic and carcinogen-initiated
mammary tumors is not always accompanied by reduction in
p27Kip1. Int J Oncol. 34:1425–1431. 2009.PubMed/NCBI
|
|
8
|
Slotky M, Shapira M, Ben-Izhak O, Linn S,
Futerman B, Tsalic M and Hershko DD: The expression of the
ubiquitin ligase subunit Cks1 in human breast cancer. Breast Cancer
Res. 7:R737–R744. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shapira M, Ben-Izhak O, Slotky M, Goldin
O, Lahav-Baratz S and Hershko DD: Expression of the ubiquitin
ligase subunit cyclin kinase subunit 1 and its relationship to
S-phase kinase protein 2 and p27Kip1 in prostate cancer.
J Urol. 176:2285–2289. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee SW, Kang SB, Lee DS and Lee JU: Akt
and Cks1 are related with lymph node metastasis in gastric
adenocarcinoma. Hepatogastroenterology. 60:932–937. 2013.PubMed/NCBI
|
|
11
|
Nagler RM, Ben-Izhak O, Ostrovsky D, Golz
A and Hershko DD: The expression and prognostic significance of
Cks1 in salivary cancer. Cancer Invest. 27:512–520. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Calvisi DF, Ladu S, Pinna F, et al: SKP2
and CKS1 promote degradation of cell cycle regulators and are
associated with hepatocellular carcinoma prognosis.
Gastroenterology. 137:1816–1826. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang H, Jiang N, Jiang H, Saha MN, Qi C,
Xu W and Reece D: CKS1B nuclear expression is inversely correlated
with p27Kip1 expression and is predictive of an adverse
survival in patients with multiple myeloma. Haematologica.
95:1542–1547. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pardo I, Lillemoe HA, Blosser RJ, et al:
Next-generation transcriptome sequencing of the premenopausal
breast epithelium using specimens from a normal human breast tissue
bank. Breast Cancer Res. 16:R262014. View
Article : Google Scholar
|
|
15
|
Hershko A and Ciechanover A: The ubiquitin
system for protein degradation. Annu Rev Biochem. 61:761–807. 1992.
View Article : Google Scholar
|
|
16
|
Hattori T, Kitagawa K, Uchida C, Oda T and
Kitagawa M: Cks1 is degraded via the ubiquitin-proteasome pathway
in a cell cycle-dependent manner. Genes Cells. 8:889–896. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bashir T, Dorrello NV, Amador V,
Guardavaccaro D and Pagano M: Control of the SCF (Skp2-Cks1)
ubiquitin ligase by the APC/C (Cdh1) ubiquitin ligase. Nature.
428:190–193. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hunter T: The age of crosstalk:
phosphorylation, ubiquitination, and beyond. Mol Cell. 28:730–738.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ungureanu D, Saharinen P, Junttila I,
Hilton DJ and Silvennoinen O: Regulation of Jak2 through the
ubiquitin-proteasome pathway involves phosphorylation of Jak2 on
Y1007 and interaction with SOCS-1. Mol Cell Biol. 22:3316–3326.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ali S, Nouhi Z, Chughtai N and Ali S:
SHP-2 regulates SOCS-1-mediated Janus kinase-2
ubiquitination/degradation downstream of the prolactin receptor. J
Biol Chem. 278:52021–52031. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He G, Zhang YW, Lee JH, et al:
AMP-activated protein kinase induces p53 by phosphorylating MDMX
and inhibiting its activity. Mol Cell Biol. 34:148–157. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nalavadi VC, Muddashetty RS, Gross C and
Bassell GJ: Dephosphorylation-induced ubiquitination and
degradation of FMRP in dendrites: a role in immediate early
mGluR-stimulated translation. J Neurosci. 32:2582–2587. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wolf-Yadlin A, Kumar N, Zhang Y, et al:
Effects of HER2 over-expression on cell signaling networks
governing proliferation and migration. Mol Syst Biol. 2:542006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hornbeck PV, Kornhauser JM, Tkachev S,
Zhang B, Skrzypek E, Murray B, Latham V and Sullivan M:
PhosphoSitePlus: a comprehensive resource for investigating the
structure and function of experimentally determined
post-translational modifications in man and mouse. Nucleic Acids
Res. 40:D261–D270. 2012.PubMed/NCBI
|
|
25
|
Wu F, Wang P, Young LC, Lai R and Li L:
Proteome-wide identification of novel binding partners to the
oncogenic fusion gene protein, NPM-ALK, using tandem affinity
purification and mass spectrometry. Am J Pathol. 174:361–370. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Olsen JV, Vermeulen M, Santamaria A, et
al: Quantitative phosphoproteomics reveals widespread full
phosphorylation site occupancy during mitosis. Sci Signal.
3:ra32010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shirayama M, Soto MC, Ishidate T, et al:
The conserved kinases CDK-1, GSK-3, KIN-19, and MBK-2 promote OMA-1
destruction to regulate the oocyte-to-embryo transition in C.
elegans. Curr Biol. 16:47–55. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arvai AS, Bourne Y, Hickey MJ and Tainer
JA: Crystal structure of the human cell cycle protein CksHs1:
single domain fold with similarity to kinase N-lobe domain. J Mol
Biol. 249:835–842. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Choudhary C, Kumar C, Gnad F, et al:
Lysine acetylation targets protein complexes and co-regulates major
cellular functions. Science. 325:834–840. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim W, Bennett EJ, Huttlin EL, et al:
Systematic and quantitative assessment of the ubiquitin-modified
proteome. Mol Cell. 44:325–340. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Holt LJ: Regulatory modules: coupling
protein stability to phosphoregulation during cell division. FEBS
Lett. 586:2773–2777. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hu Y, Sun H, Drake J, et al: From mice to
humans: identification of commonly deregulated genes in mammary
cancer via comparative SAGE studies. Cancer Res. 64:7748–7755.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kaneko T, Joshi R, Feller SM and Li SS:
Phosphotyrosine recognition domains: the typical, the atypical and
the versatile. Cell Commun Signal. 10:322012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Burshtyn DN, Yang W, Yi T and Long EO: A
novel phosphotyrosine motif with a critical amino acid at position
-2 for the SH2 domain-mediated activation of the tyrosine
phosphatase SHP-1. J Biol Chem. 272:13066–13072. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Songyang Z: Recognition and regulation of
primary-sequence motifs by signaling modular domains. Prog Biophys
Mol Biol. 71:359–372. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Heo WD, Inoue T, Park WS, Kim ML, Park BO,
Wandless TJ and Meyer T: PI(3,4,5)P3 and PI(4,5)P2 lipids target
proteins with polybasic clusters to the plasma membrane. Science.
314:1458–1461. 2006. View Article : Google Scholar : PubMed/NCBI
|