Introduction
Chronic lymphocytic leukemia (CLL) belongs to the
group of hematological neoplasms with unknown etiology. Great
progress in cancer diagnostics and treatment has been made, but
this type of leukemia remains incurable. The simultaneous
coexistence of two populations of quiescent and cycling cells in
CLL treatment represents a special challenge (1–3).
It is accepted that an accumulation of genetic
aberrations or epigenetic modifications in neoplastic lymphocytes
could be related to heterogeneity in the clinical course of CLL and
transferred on response to therapy. Recently, a large number of
studies have been focused on the identification and evaluation of
factors which have an impact on treatment and reflect prognostic
value, e.g., genomic aberrations, alterations in miRNA level, and
epigenetic modifications (4–6).
Differences in the expression of factors regulating apoptosis and
cell signaling, as well as the microenvironment of malignant cells,
may also be responsible for the heterogeneity of leukemic cells and
their response to therapy (7–9).
At present, there is an increasing number of
treatment options, and a large number of agents with anticancer
potential are undergoing preclinical and clinical studies (10–12).
Among the new therapies for CLL, much attention is paid to agents
with the potential to turn on apoptosis (13). In light of the new therapeutic
options (e.g., immunochemotherapy, immunomodulators, kinase
inhibitors), and disease heterogeneity, one of the most pressing
issues is an elaboration of effective methods for determining the
individual sensitivity of CLL patients to potential therapeutic(s),
and the selection of optimal treatment for each patient (14,15).
The special importance of such approaches has been
confirmed by the data showing a profound immunological defect
reflected by hypogammaglobulinemia and elevated vulnerability to
patient infections associated with fludarabine and cyclophosphamide
administration (16). Therefore,
personalized therapy needs to be particularly addressed towards
‘refractory’ CLL patients (17).
The results of two single cases given in our earlier
report (18), together with the
combined results of 28 other cases presented in the current report,
confirm an association between the results of in vitro
testing (cytotoxicity and pro-apoptotic ability) of the tested
drugs and the subsequent clinical response to the applied
treatment.
Differential scanning calorimetry (DSC) is a
relatively fast thermal technique that provides data on physical
and energetic properties of cellular structures/compounds (19–21).
Earlier, it was documented that this method could be useful for the
monitoring of native chromatin in cell nuclei (19).
It has been reported by our laboratory that an
additional thermal transition occurs at 95±5°C in the DSC profiles
of nuclear fraction preparations obtained from the mononuclear
cells of patients in an advanced stage of CLL, along with the main
transition at 83±3°C characteristic for healthy donors (22,23).
Moreover, the obtained results also revealed that the decrease (or
even loss) of thermal transition at 95±5°C in thermal scans of
nuclear preparations of CLL cells, after both in vivo and
in vitro drug administration, are attributable to chromatin
fragmentation during apoptosis (23,24).
In the current study, the comparative cytometric
analysis of cell viability, apoptosis rate, DSC profiles of nuclear
preparations, and PARP-1 expression by western blotting in the
group of 28 patients were applied to examine the ability of
leukemic cells to enter apoptosis after their exposure to
cladribine or fludarabine combined with mafosfamide.
As described, a significant decrease or even
complete loss of thermal transition at 95±3°C was observed in DSC
scans of nuclear preparations when therapy was effective (22,24).
Moreover, our results reveal that a comparison of the DSC profiles
of nuclear preparations with the results of cell viability,
apoptosis rate, and proteolytic degradation of the PARP-1 display a
good predictive value for CLL cell sensitivity to anti-cancer
drugs. This prediction is of importance as it allows an opportunity
to choose the optimal therapy for patient avoiding ineffective
anticancer therapy.
Materials and methods
Ethics statement
The study was approved by the Local Ethics Committee
of the Medical University of Lodz (Lodz, Poland) (no.
RNN/143/10/KE); all patients signed a declaration of consent.
Patients, response criteria
In the current study the peripheral blood samples
from 28 randomized, untreated previously progressive CLL patients
(16 men, 12 women) with white blood cell counts ranging from 45 to
600×109/l were included (Table I). The immunophenotypic
characteristics of leukemic cells
(CD5+/CD19+/CD23+, presence on
cell surface immunoglobin κ or λ chains) were determined
cytometrically. The diagnosis of CLL and clinical staging
determination were established according to standard clinical,
immunological and cytological IWCLL criteria (25). The group included eligible patients
who underwent randomization (26).
 | Table IPatients’ characteristic features,
comparative results of in vivo and in vitro response
to treatment. |
Table I
Patients’ characteristic features,
comparative results of in vivo and in vitro response
to treatment.
| No. | Gender | Age | Stage of
disease | FISH | Vybrant | DSC | PARP-1
cleavage | Response in
vitro | Treatment in
vivo | Response to
treatment |
|---|
|
|
|
|---|
| CM | FM | CM | FM | CM | FM |
|---|
| 1 | M | 50 | III | del(11)(q22),
del(13)(q14) | ↓↓ | ↓↓ | ↓↓ | ↓↓ | + | + | SR | CC | PR |
| 2 | M | 60 | III | del(11)(q22),
del(13)(q14) | ↓↓ | ↓↓ | ↓ | ↓ | + | + | SR | CC | PR |
| 3 | M | 52 | II | del(11)(q22),
del(17)(p13) | → | → | → | ↓ | +/− | + | WR | CC | NR |
| 4 | F | 57 | IV | del(11)(q22),
del(13)(q14) | → | → | → | ↓ | 0 | 0 | WR | CC | NR |
| 5 | F | 56 | IV | del(13)(q14) | → | ↓ | ↓ | ↓↓ | +/− | +/− | WR | CC | NR |
| 6 | M | 69 | I | del(11)(q22),
del(13)(q14) | → | → | ↓ | → | nd | nd | MR | CC | CR |
| 7 | F | 80 | IV | normal | ↓ | ↓ | ↓↓ | ↓↓ | + | +/− | SR | CC | CR |
| 8 | F | 80 | I | del(17)(p13),
+12 | ↓ | ↓ | ↓ | ↓↓ | 0 | 0 | MR | CC | PR |
| 9 | M | 58 | III | del(13)(q14) | ↓↓ | ↓↓ | ↓ | | + | + | SR | CC | PR |
| 10 | M | 58 | III | ND | ↓↓ | → | ↓ | → | +/− | +/− | SR | CC | CR |
| 11 | F | 57 | II | Normal | ↓↓ | → | ↓↓ | ↓↓ | nd | nd | SR | CC | CR |
| 12 | F | 60 | III | ND | ↓ | → | ↓↓ | ↓ | + | 0 | SR | CC | CR |
| 13 | F | 51 | IV | del(11)(q22) | ↓ | ↓ | ↓ | ↓ | +/− | | MR | CC | CR |
| 14 | F | 55 | III | del(13)(q14) | ↓↓ | ↓ | ↓↓ | ↓↓ | + | +/− | SR | CC | CR |
| 15 | M | 52 | IV | Normal | ↓ | ↓ | ↓↓ | ↓↓ | + | + | SR | CC | CR |
| 16 | M | 61 | III | 12 | ↓ | ↓ | ↓↓ | ↓↓ | + | + | SR | CC | PR |
| 17 | M | 61 | IV | ND | ↓↓ | ↓↓ | ↓ | ↓↓ | +/− | +/− | MR | CC | NR |
| 18 | M | 69 | IV | ND | → | ↓↓ | → | ↓↓ | 0 | +/− | WR | CC | NR |
| 19 | M | 76 | I | del(13)(q14) | ↓ | ↓ | ↓↓ | ↓↓ | + | +/− | SR | CC | CR |
| 20 | M | 64 | I | del(11)(q22) | → | → | ↓↓ | → | +/− | | MR | CC | CR |
| 21 | M | 57 | I | del(11)(q22) | ↓ | ↓ | ↓ | ↓↓ | 0 | +/− | MR | FC | CR |
| 22 | F | 65 | 0 | ND | ↓ | ↓ | ↓↓ | ↓↓ | +/− | + | MR | FC | PR |
| 23 | F | 53 | II | del(11)(q22),
del(13)(q14) | ↓↓ | ↓↓ | ↓ | ↓↓ | +/− | +/− | SR | CC | CR |
| 24 | M | 71 | 0 | Normal | ↓↓ | ↓↓ | ↓ | ↓ | +/− | +/− | MR | FC | CR |
| 25 | F | 66 | I | del(11)(q22),
del(17)(p13) | ↓ | ↓↓ | ↓ | ↓↓ | nd | nd | MR | CC | CR |
| 26 | F | 71 | IV | del(13)(q14) | ↓ | ↓ | ↓ | ↓↓ | + | + | SR | CC | PR |
| 27 | M | 66 | IV | del(11)(q22),
del(13)(q14), +17 | ↓ | ↓ | ↓↓ | ↓ | +/− | +/− | MR | CC | PR |
| 28 | M | 58 | IV | Normal | ↓↓ | ↓↓ | ↓↓ | ↓↓ | + | + | SR | CC | PR |
Blood samples from the patients were collected
before administration with cladribine + cyclophosphamide (CC), or
fludarabine + cyclophosphamide (FC). The choice of therapeutic
schedules was made on accepted ECOG standards and as a result of
prognostic factor analysis. Drug combination was applied the next
day. Clinical response to the treatment after six cycles of drug
administration was evaluated by NCI-sponsored Working Group
criteria (25). Complete response
(CR), partial response (PR) or non-responder (NR) criteria have
been explained before (24,26).
Isolation of CLL cells
Peripheral blood mononuclear cell (PBMC) samples of
CLL patients were collected on EDTA. Mononuclear cells from blood
samples were separated using Histopaque-1077 (Sigma-Aldrich, St.
Louis, MO, USA) according to manufacturer’s instructions. The CLL
cell pellets were washed with phosphate-buffered saline (PBS),
resuspended in RPMI medium and divided for the planned
experiments.
Fluorescence in situ hybridization
(FISH)
FISH analysis was performed on the interphase nuclei
of leukemic cells from the blood of CLL patients before the onset
of treatment (27). The following
commercially available probes were used: LSI D13S319 (13q14.3)/LSI
13q34/CEP 12 probe, LSI p53 (17p13.1), LSI ATM (11q22.3) probe
(Vysis; Abbott Laboratories, Abbott Park, IL, USA). The estimated
cut-off levels were as follows: 8% for del(13)(q14.3), del(11)(q22.3) and del(17)(p13.1), and 5% for trisomy 12.
Signals were counted in 200 interphase nuclei for each sample.
In vitro treatment
PBMC samples from patients were resuspended at a
final density of 2.5–3.5×106 cells/ml in RPMI-1640
medium with 10% FCS, 2 mM L-glutamine, 100 U/ml penicillin, 100
μg/ml streptomycin and incubated with cladribine or fludarabine
with an active form of cyclophosphamide-mafosfamide for 48 h or
without any agents [controls (Ctr)] at concentrations described
previously (28,29). Control leukemic cells, as well as
the CLL cells were exposed to anticancer agents for 48 h at 37°C in
an atmosphere of 5% CO2.
Cladribine (Biodrybin) was from the Bioton
S.A./Institute of Biotechnology and Antibiotics (Warsaw, Poland),
fludarabine from Bayer Schering Pharma AG (Berlin, Germany). The
alkylating agent mafosfamide was donated by Baxter Oncology GmbH
(Frankfurt, Germany) or purchased from Niomech IIT GmbH (Bielefeld,
Germany).
Cell viability and determination of
apoptotic cell number
PBMC samples were incubated in culture medium only
(Ctr) or in drug supplementation as indicated. The level of viable,
as well as apoptotic cells after 48 h of drug treatment, was
assessed by flow cytometry using Vybrant Apoptosis Assay kit no. 4
(Molecular Probes, Inc., Eugene, OR, USA). PBMC samples were
analysed on LSR II Becton-Dickinson cytometer (Becton-Dickinson,
Franklin Lakes, NJ, USA).
Preparation of nuclear fraction and whole
cell lysates
Both, the pelleted control and drug-treated CLL cell
samples were rinsed with cold PBS and then suspended in isotonic
sucrose solution containing 5 mM MgCl2, 0.5% Triton
X-100, 50 mM Tris-HCl (pH 7.4) and protease inhibitors as
previously described (22). Cell
samples were homogenized in a Potter homogenizer, and centrifuged
at 800 × g for 8 min resulting in a crude nuclear pellet.
PBMC samples were lysed in buffer containing 10 mM
Tris-HCl (pH 7.5), 300 mM NaCl, 1% Triton X-100, 2 mM
MgCl2, 0.1 M dithiothreitol and protease inhibitor
cocktail (22).
Protein electrophoresis and
immunoblotting
Protein concentration was determined
colorimetrically (30). Protein
samples (40 μg) were separated on 8.0% SDS-polyacrylamide gels and
blotted onto Immobilon-P membrane. Equal protein loading and
protein transfer were confirmed by Ponceau S staining. To avoid
non-specific protein binding sites membranes were saturated with 5%
non-fat dry milk in TBS (10 mM Tris-HCl, pH 7.5, 150 mM NaCl) for
at least 1 h at room temperature. After extensive washing in TBS
containing 0.05% Tween-20 (TBST) blots were incubated with primary
antibodies specific to PARP-1 (sc-7150, 1:2,000), and actin
(sc-7210, 1:1,000) from Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA. The immune complexes were detected using alkaline
phosphatase (AP)-coupled secondary antibodies as previously
described (28).
DSC
Samples of the nuclear fraction isolated from PBMCs
incubated with or without anticancer agents were prepared for
calorimetric tests by the Almagor and Cole procedure (21,22).
The probes were transferred into sample pans and hermetically
sealed. Calorimetric experiments were performed on a Setaram TG-DSC
111 calorimeter (Setaram Instrumentation, Caluire, France) from 20
to 120°C at a scanning rate R (5°C/min, 0.083°C/sec) as reported
previously (24).
Monitoring of apoptosis and necrosis
The appearance of apoptotic and necrotic cells was
monitored under a fluorescence microscope (magnification, ×400,
Olympus IX70; Olympus, Tokyo, Japan). The cells after drug exposure
were washed and suspended in PBS at the concentration
1×106 cells/ml. Cell suspensions were incubated with
YO-PRO-1 and propidium iodide, and transferred onto microscopic
slides for examination. The fluorescent dyes vary in
characteristics and ability to penetrate cells. YO-PRO-1 passes
through the plasma membrane of apoptotic cells and labels them
selectively with green fluorescence, while the red-fluorescent
propidium iodide is permeant only to necrotic cells. Stained cells
were classified on the basis of their morphological and staining
characteristics as early, late apoptotic or necrotic (31).
Data analysis
Descriptive statistic analysis was used to summarise
patients’ clinical, laboratory and in vitro cladribine +
mafosfamide (CM)/fludarabine + mafosfamide (FM) treatment data. The
changes in cell viability, apoptosis rate, thermal transition at
95±5°C or PARP-1 proteolytic cleavage in respect to the clinical
response of patients were compared using Fisher’s exact test with
Bonferroni correction. The details of the statistical analyses are
included in the text and Fig.
1C.
Results
The results of in vitro leukemic cell
exposure to anticancer agents indicate their susceptibility to
anticancer drugs
Leukemic PBMCs obtained from blood of 28 randomized
patients scheduled for the first chemotherapy cycle were incubated
for 48 h with cladribine or fludarabine combined with mafosfamide
(CM or FM). The clinical characteristic of CLL patients, type of
treatment and response in vivo and in vitro are
presented in Table I.
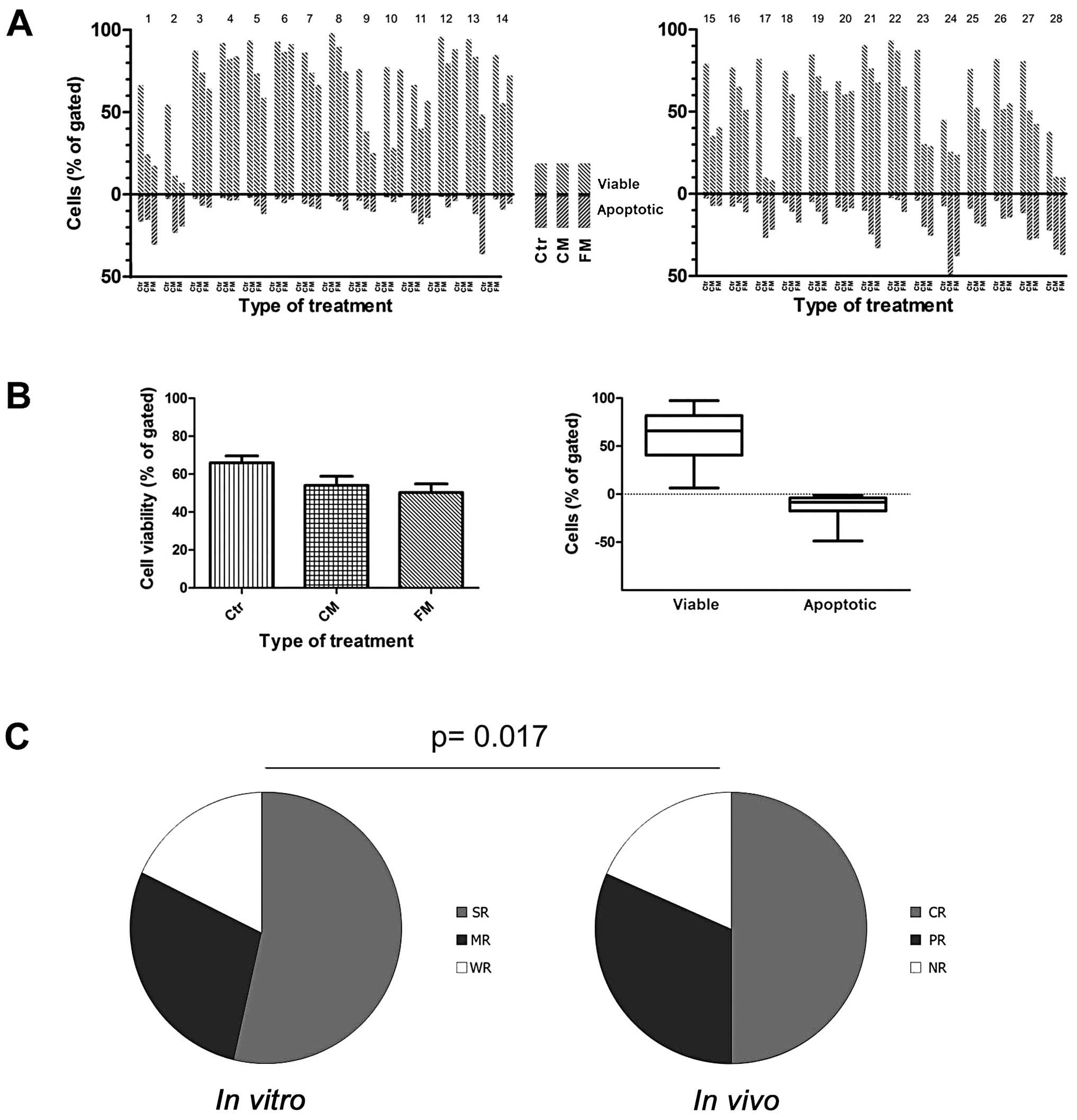
Exposure of PBMC samples to both anticancer drug
combinations reflects the differences in cell viability, as well as
apoptosis rate. Comparative analysis of the above studies showed a
different personal sensitivity and response of examined cells to
the used drug combinations (Figs.
1 and 2). Moreover, the
exposure of CLL cells to both investigated combinations usually
decreased the viable cells number, which was associated with an
elevation in apoptotic rate, but to a different extent. Among the
investigated patient samples, we did not obtain the same results
for a single patient. In leukemic cells isolated from blood of some
patients, apoptosis at high rate was rapidly induced, whereas in
other cases it was delayed and much weaker.
The comparison of the combined average results is
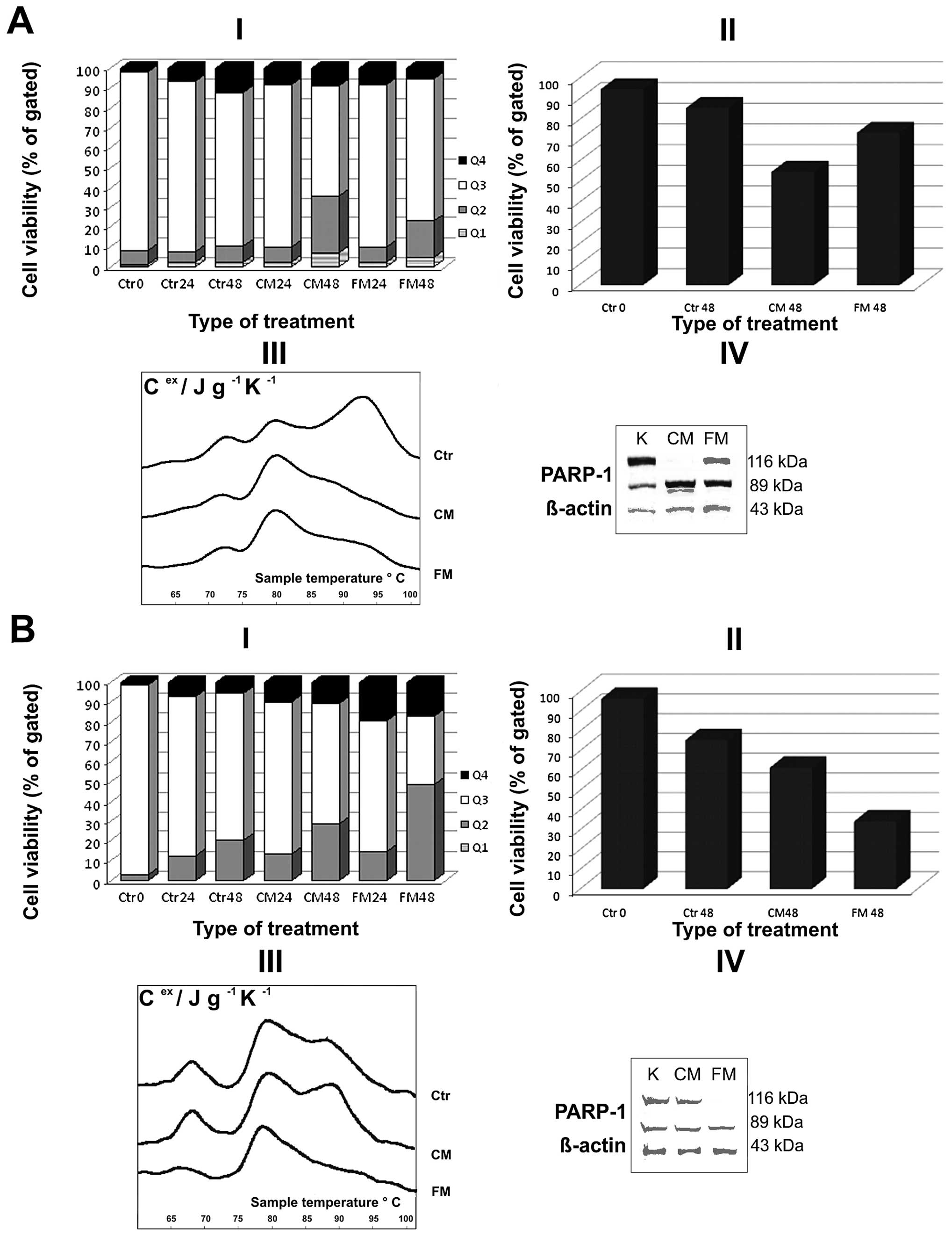
illustrated in Fig. 1A and B. The
diagrams in Fig. 2A and B show the
combined data of in vitro tests for CLL patients nos. 14 and
18, respectively. Patient no. 18 did not respond (NR) to CC therapy
applied in the clinic, but the results of in vitro tests
suggest that for this patient, FC would display a chance for better
response. The results for patient no. 14, who achieved a CR in
vivo, also in vitro tests show that this type of
treatment will be more profitable for this patient in comparison to
FM/FC. The results of in vitro tests demonstrate leukemic
patients’ personal predispositions that could be transferred to
treatment efficacy in vivo (compare Figs. 1 and 2).
Moreover, in the group of the studied blood samples
from CLL patients with sensitive and reactive leukemic cells, the
response to the treatment in vivo as well as to in
vitro conditions usually occurred. The second group showed a
weak response to the drugs used, which could be correlated with the
unsatisfactory response or even resistance of patients to therapy
in vivo. A third group of CLL cells displays a high
sensitivity to in vitro condition, which was concomitant
with high reduction of cell viability in control untreated cells
incubated for 48 h, as well as PARP-1 cleavage in control untreated
cells. For these cases, the additional analysis using supplementary
techniques are suggested to demonstrate the course of apoptosis
process.
Thermal transition at 95±5°C is
characteristic for nuclear samples from advanced stage of CLL
patients
A study from our laboratory has shown that in
advanced stages of CLL, the thermal profile of nuclei with an
additional thermal transition at 95±3°C is present in ~74% of cases
in advanced/aggressive disease (22). In the results presented here, all
control nuclear preparations of leukemic cells indicated thermal
transition at ~95°C. Moreover, in the majority of nuclear samples
isolated from PBMCs of advanced leukemia patients qualified to drug
administration (advanced stage of disease), this thermal transition
was usually dominant. Interestingly, the changes in thermal
profiles of nuclear preparations from leukemic PBMCs exposed to
studied drug combinations were correlated with viable cell
reduction, reflecting the potential efficacy of treatment.
To show that results of complementary tests closely
adhere to DSC profiles, we selected for presentation two exemplary
cases with different therapeutic outcomes: one illustrating a
positive response to CM and potential resistance to FM (patient no.
14; Table I, Figs. 1 and 2A), and the second one reflecting a weak
sensitivity to CM in comparison to FM (patient no. 18; Table I, Figs. 1 and 2B).
The thermal transition of exemplary nuclear fraction
(patient no. 14) at 95±5°C was reduced after leukemic cell exposure
to drugs for 48 h; the cells of this patient were characterized by
a higher apoptosis rate in respect to control cells (Fig. 2AI and II). No decrease of
transition at 95±5°C was observed in the cells treated with CM
(patient no. 18) that could be a reason of chromatin
hyper-condensation during PBMC cell exposure to drugs (Fig. 2BIII). While the changes in thermal
profiles directed towards decrease of thermal transition at 95±5°C
seem to be attributable to a degradation of chromatin during
apoptotic DNA fragmentation.
The comparative analyses of nuclear
fraction DSC scans with cell viability and apoptosis marker PARP-1
expression reflect leukemic cell sensitivity to anticancer
drugs
Using the complementary in vitro tests, we
assessed some parameters that simultaneously performed could
predict a potent efficacy of anticancer drugs in leukemic PBMCs. It
must be emphasized that the treatment response strongly varied
between individual patients (Fig. 1A
and B). Interestingly, for all studied 28 patients, the changes
in thermal profiles at 95±5°C of nuclear samples obtained from
PBMCs exposed to anticancer drugs correlated with the reduction of
viable cell percentage and elevation of apoptotic cell fraction
accompanied by the proteolysis in apoptotic marker PARP-1 (Fig. 2). In the group of examined 28
patients, 8 of them display a positive reaction (CR) in vivo
and strong reaction (SR) in vitro, while negative responses
were observed for 5 patients (3 of them in vivo and in
vitro). Similarly, in vivo and in vitro
correlations were seen for 4 patients reflecting PR. It must be
stated that sometimes patients did not respond the same in
vitro and in vivo. For five cases, there was an SR in
vitro followed by a PR in vivo, and for six others the
reaction to drug administration in vivo (CR) was stronger
than the reaction in vitro (MR) (Table I).
It must be underlined that we did not receive the
same results of four complementary tests for two individuals among
28 studied CLL patients. From the obtained results we have selected
two exemplary CLL patients, i.e., one responding to CM and
resistant to FM (no. 14), and the second one (no. 18) displaying a
weak response to CM treatment. As demonstrated in Figs. 1A and 2, personal differences and distinct cell
responses to in vitro CM and FM treatment in terms of cell
viability (I), and apoptosis rate (II) were observed. These data
were confirmed by the decrease of thermal transition at 95±5°C
after cell exposure to CM, and the even higher thermal profile in
the case of cells incubated with FM (patient no. 14; Fig. 2AIII). The obtained data were also
confirmed by strong proteolytic cleavage of PARP-1 in leukemic
cells incubated with CM (Fig.
2AIV). For patient no. 18, only a slight decrease of the living
cell numbers exposed to CM in comparison to control ones (Figs. 1A, B and 2BI, II) was observed (Figs. 1 and 2) and correlated with no changes in the
thermal profiles of nuclear fraction preparations. A slight PARP-1
cleavage, even in control untreated cells of this patient, was
observed (Fig. 2BIV).
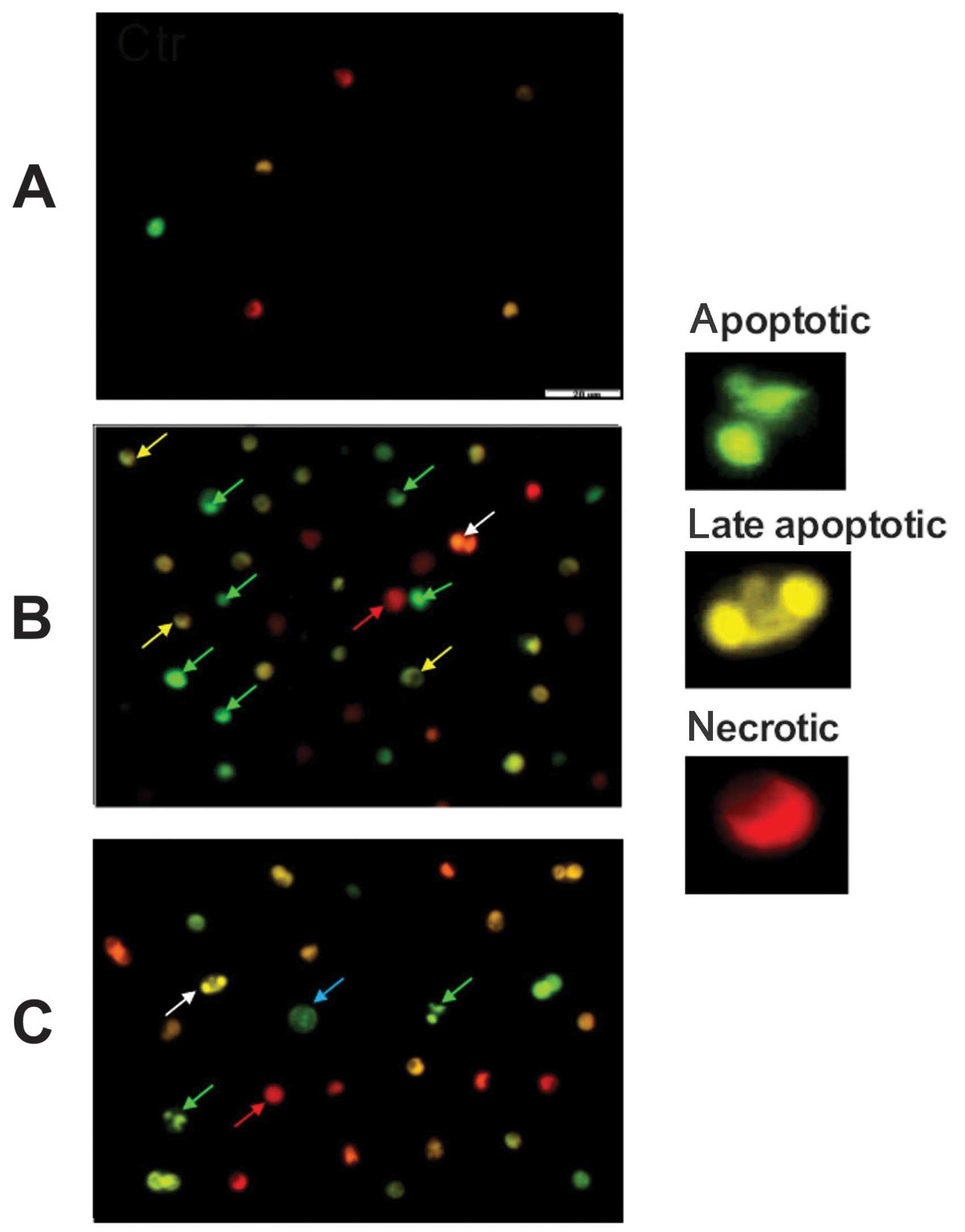
The apoptotic and necrotic morphological changes in
examined cells were monitored during cell incubations with
anticancer drugs (Fig. 3). The
cell suspensions were incubated with YO-PRO-1 and propidium iodide
and analyzed under fluorescence microscope (Olympus IX70) at
magnification ×400. Numerous shrunken cells with the typical
morphological features of early (green arrow) and late (yellow
arrow) apoptosis, i.e., chromatin condensation, marginalization
(semilunar shape) and nuclear fragmentation were seen after CM and
FM treatment. Some swollen enlarged cells typical for necrosis (red
arrow) and the cells with two nuclei (white arrow) were also
observed. In some cells, the beginning of nuclear changes were
visible as a condensed ring/sphere along the nuclear envelope (blue
arrow).
The stained cell areas observed after leukemic cell
incubations with drugs (patient no. 14) for 48 h chosen as an
example of anticancer drugs effect monitoring on leukemic cell
viability is illustrated in Fig.
3.
Finally, we addressed the question of whether
evaluation of thermal profile changes upon drug treatment of
leukemic cells might possess prognostic value. Interestingly, the
results of the in vitro experiments suggest that patient no.
14 should respond better to CM/CC treatment. During randomized
therapy option this patient was administered with CC, and after six
cycles of chemotherapy he reached complete remission. The results
for patient no. 18 indicated his very weak in vitro response
to CM in comparison with FM. This patient was administered with CC
and he did not respond (NR) to scheduled therapy (Table I).
The comparative statistical analysis using Fisher’s
exact test with Bonferroni correction between clinical patient
response (after six courses of therapy) and in vitro test
data reflects a statistical correlation (p=0.017) between in
vivo and in vitro response to the applied treatment
(Fig. 1C). Interestingly, we did
not find such correlation between the response to treatment versus
stage of disease (Rai staging), or cytogenetic aberration on 11q
and 13q chromosome.
As is shown in Table
I, among the 28 patients for whom cytogenetic assay was
performed, 25 underwent CC therapy and 12 reached CR (48%). The
deletion within chromosome 13 (13q14) was the most common genetic
abnormality in the investigated group (11/28, 39.28%). Among the
studied samples of CLL patients, only three cases of chromosomal 17
abnormality (17p13.1) were detected, and one of them reached
CR.
These results suggest that in vitro response
of leukemic cells to anticancer agents display predictive value and
could be helpful in the optimal therapeutic strategy selection for
individual patients in order to avoid ineffective therapy based on
purine analog combined with alkylating agent.
Discussion
Inherited genetic predispositions to CLL has
directed attention towards extensive studies on genetic alterations
which could disturb gene expression (6,32).
Studies looking for new genetic aberrations and epigenetic
modifications in chromatin structure have been performed (4–6). The
diversities in response to CLL therapy occur in respect to personal
genetic differences, having impact on cell signal transduction,
cell cycle alterations and disturbance of apoptosis. Sometimes,
because of personal variations in expression of some genes even
directed therapy towards cancer-related or specific markers could
not be fully effective. Personal diversities in response to
treatment or retreatment that introduce toxicity could direct into
myelodysplasia or secondary neoplasms (33,34).
More than 95% of leukemic cells in peripheral blood
of patients display hyper-condensed heterochromatin and are between
G0/G1 phase of the cell cycle. In more aggressive cases of disease,
the population of cycling cells induce another request for CLL
treatment directions. According to the theoretical predictions,
activity of drugs and their capability to induce apoptosis should
be the most effective manner for elimination of leukemic cells
(34,35). Standard therapies used in clinical
routine are based on the combination of purine analogs with
alkylating agent(s), which induce DNA damage (36). In light of published data, a
proportion of patients acquires resistance to the treatment
(14,37,38).
Thus, the optimization of drug(s) before their administration and
elaborating tailored therapy is a need for the group of
non-responding patients or those with high expression of
unfavorable prognostic factors.
In the current study, we evaluated in vitro
cytotoxicity, apoptosis induction potential and the changes in
chromatin conformation caused by cladribine or fludarabine combined
with mafosfamide to evaluate their potency for leukemic cell
elimination by apoptosis. As the consequence of two CLL cases
published recently (18), we
extended our study up to 28 patients. We used four experimental
approaches for monitoring, i.e., cell viability, rate of apoptosis,
DSC analysis of nuclear preparations, and expression of apoptotic
marker PARP-1. The obtained data revealed that in vitro
exposure of leukemic PBMCs to drug combinations before patient
treatment might indicate prognostic value. In the examined cases we
observed that patients whose PBMCs were insensitive to given drug
combinations in vitro did not respond to the treatment in
vivo either. The positive responses to drug combinations in
vitro, as the result of cell elimination by apoptosis, were in
most cases followed by CR to therapy in vivo. It must be
underlined that for some CLL patients whose cells indicate special
sensitivity to in vitro conditions individualized tumor
response testing suggested by Matutes et al (39) or cytotoxic tests performed
previously by Nagourney et al (40) could not be fully effective (compare
Fig. 1A and B).
Finally, the disappearance of some of the CLL cells
as a consequence of apoptosis induction after their exposure to
drugs was also confirmed by the changes in expression of certain
apoptosis-related proteins, for example PARP-1. A recently
published report concerning anticancer drug bioactivity on library
of approved anticancer drugs confirms CLL cell exceptionality, but
direct studies towards tailoring therapy for other types of
malignances in general are needed (41).
In summary, the results of our studies revealed that
determination of the in vitro CLL cell sensitivity based on
purine analogs and their combinations with alkylating agent would
be instrumental in the development of personalized therapy. It
should be stated that from our point of view in vitro study
gives the opportunity for CLL status analysis, before and during
drug application (18,22–24).
It reflects a special importance for a subset of patients resistant
to therapy, as well as for those heavily pre-treated or those in
weak condition.
Our current results for 28 patients confirm studies
published previously as a case report (18) suggesting that in vitro
incubations of leukemia cells with anticancer drugs is of
predictive value and would help to select the optimal therapeutic
strategy for individual patient in order to avoid ineffective
treatment.
Acknowledgements
Financial support: Polish National Science Centre
(Cracow, Poland) (Grant 2011/01/B/NZ/0102). We would like to thank
Wojciech Sobala for statistical analysis.
References
|
1
|
Oscier D, Dearden C, Erem E, et al:
Guidelines on the diagnosis, investigation and management of
chronic lymphocytic leukaemia. Br J Haematol. 159:541–564.
2012.PubMed/NCBI
|
|
2
|
Hallek M: Chronic lymphocytic leukemia:
2013 update on diagnosis, risk stratification and treatment. Am J
Hematol. 88:803–816. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Klein U and Dalla-Favera R: Germinal
centres: role in B-cell physiology and malignancy. Nat Rev Immunol.
8:22–33. 2008. View
Article : Google Scholar
|
|
4
|
Zenz T, Mertens D, Döhner H and
Stilgenbauer S: Importance of genetics in chronic lymphocytic
leukemia. Blood Rev. 25:131–137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rogalińska M and Kilianska ZM: Targeting
Bcl-2 in CLL. Curr Med Chem. 19:5109–5115. 2012. View Article : Google Scholar
|
|
6
|
Martin-Subero JI, López-Otín C and Campo
E: Genetic and epigenetic basis of chronic lymphocytic leukemia.
Curr Opin Hematol. 20:362–368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chiorazzi N: Implications of new
prognostic markers in chronic lymphocytic leukemia. Hematology Am
Soc Hematol Educ Program. 2012:76–87. 2012.PubMed/NCBI
|
|
8
|
Rodriquez-Vicente AE, Díaz MG and
Hernández-Rivas JM: Chronic lymphocytic leukemia: a clinical and
molecular heterogenous disease. Cancer Genet. 206:49–62. 2013.
View Article : Google Scholar
|
|
9
|
Oppezzo P and Dighiero G: Role of B-cell
receptor and the microenvironment in chronic lymphocytic leukemia.
Blood Cancer J. 3:e1492013. View Article : Google Scholar
|
|
10
|
Robak T: Application of new drugs in
chronic lymphocytic leukemia. Mediterr J Hematol Infect Dis.
2:e20100112010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rogalińska M and Kiliańska ZM: Potential
new agents for chronic lymphocytic leukemia treatment. Anticancer
Agents Med Chem. 10:666–682. 2010. View Article : Google Scholar
|
|
12
|
Qiu LN, Zhou YL, Wang ZN, Huang Q and Hu
WX: ZGDHu-1 promotes apoptosis of chronic lymphocytic leukemia
cells. Int J Oncol. 41:533–540. 2012.PubMed/NCBI
|
|
13
|
Chen R and Plunkett W: Strategy to induce
apoptosis and circumvent resistance in chronic lymphocytic
leukemia. Best Pract Res Clin Haemat. 23:155–166. 2010. View Article : Google Scholar
|
|
14
|
Bosanquet AG, Richards SM, Wade R, et al:
Drug cross-resistance and therapy-induced resistance in chronic
lymphocytic leukemia by an enhanced method of individualised tumour
response testing. Br J Haematol. 146:384–395. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rogalinska M, Blonski J, Góralski P, et
al: Usefulness of differential scanning calorimetry for monitoring
ex vivo the changes in responses of CLL cells to anticancer drugs:
development of personalized therapy. Blood (ASH Annual Meeting
abstracts). 116:46352010.
|
|
16
|
Riches JC, Ramsay AG and Gribben JG:
Immune dysfunction in chronic lymphocytic leukemia: the role for
immunotherapy. Curr Pharm Des. 18:3389–3398. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zenz T, Gribben JG, Hallek M, et al: Risk
categories and refractory CLL in the era of chemoimmunotherapy.
Blood. 119:4101–4107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rogalińska M, Franiak-Pietryga I, Błoński
JZ, et al: Toward personalized therapy for chronic lymphocytic
leukemia: DSC and cDNA microarray assessment of two cases. Cancer
Biol Ther. 14:6–12. 2013. View Article : Google Scholar
|
|
19
|
Balbi C, Abelmoschi ML, Gogioso L, et al:
Structural domains and conformational changes in nuclear chromatin:
a quantitative thermodynamic approach by differential scanning
calorimetry. Biochemistry. 28:3220–3227. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Allera C, Lazzarini G, Patrone E, et al:
The condensation of chromatin in apoptotic thymocytes shows a
specific structural change. J Biol Chem. 272:10817–10822. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Almagor M and Cole RD: Differential
scanning calorimetry of nuclei as a test for the effects of
anticancer drugs on human chromatin. Cancer Res. 49:5561–5566.
1989.PubMed/NCBI
|
|
22
|
Rogalińska M, Góralski P, Kobylińska A, et
al: Changes in leukemic cell nuclei revealed by differential
scanning calorimetry. Leuk Lymphoma. 46:121–128. 2005. View Article : Google Scholar
|
|
23
|
Góralski P, Rogalińska M, Błoński JZ, et
al: The differences in thermal profiles between normal and leukemic
cells exposed to anticancer drug evaluated by differential scanning
calorimetry. J Therm Anal Calorim. 118:1339–1344. 2014. View Article : Google Scholar
|
|
24
|
Rogalinska M, Goralski P, Wozniak K, et
al: Calorimetric study as a potential test for choosing treatment
of B-cell chronic lymphocytic leukemia. Leuk Res. 33:308–314. 2009.
View Article : Google Scholar
|
|
25
|
Cheson BD, Bennett JM, Grever M, et al:
National Cancer Institute-sponsored Working Group guidelines for
chronic lymphocytic leukemia: revised guidelines for diagnosis and
treatment. Blood. 87:4990–4997. 1996.PubMed/NCBI
|
|
26
|
Robak T, Blonski JZ, Gora-Tybor J, et al:
Cladribine alone and in combination with cyclophosphamide or
cyclophosphamide plus mitoxantrone in the treatment of progressive
chronic lymphocytic leukemia: report of a prospective, multicenter,
randomized trial of Polish Adult Leukemia Group (PALG CLL2). Blood.
108:473–479. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kotkowska A, Wawrzyniak E, Blonski JZ,
Robak T and Korycka-Wolowiec A: Chromosomal aberrations in chronic
lymphocytic leukemia detected by conventional cytogenetics with
DSP30 as a single agent: comparison with FISH. Leuk Res.
35:1032–1038. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rogalińska M, Błoński JZ, Komina O, et al:
R-roscovitine (Seliciclib) affects CLL cells more strongly than
combinations of fludarabine or cladribine with cyclophosphamide:
inhibition of CDK7 sensitizes leukemic cells to caspase-dependent
apoptosis. J Cell Biochem. 109:217–235. 2010. View Article : Google Scholar
|
|
29
|
Kobylinska A, Bednarek J, Blonski JZ, et
al: In vitro sensitivity of B-cell chronic lymphocytic leukemia to
cladribine and its combinations with mafosfamide and/or
mitoxantrone. Oncol Rep. 16:1389–1395. 2006.PubMed/NCBI
|
|
30
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
31
|
Gasiorowski B, Brokos A, Kulma A,
Ogorzałek A and Skórkowska K: A comparison of the methods applied
to detect apoptosis in genotoxically-damaged lymphocytes cultured
in the presence of four antimutagens. Cell Biol Mol Lett.
6:141–159. 2001.
|
|
32
|
Houlston RS, Catovsky D and Yuille MR:
Genetic susceptibility to chronic lymphocytic leukemia. Leukemia.
16:1008–1014. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Abrisqueta P, Crespo M and Bosch F:
Personalizing treatment for chronic lymphocytic leukemia. Expert
Rev Hematol. 4:27–35. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Robak T, Jamroziak K, Gora-Tybor J, et al:
Comparison of cladribine plus cyclophosphamide with fludarabine
plus cyclo-phospahamide as a first-line therapy for chronic
lymphocytic leukemia: a phase III randomized study by the Polish
Adult Leukemia Group (PALG-CLL3 study). J Clin Oncol. 28:1863–1869.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Decker T, Hipp S, Ringshausen I, et al:
Rapamycin-induced G1 arrest in cycling B-CLL cells is associated
with reduced expression of cyclin D3, cyclin E, cyclin A, and
survivin. Blood. 101:278–285. 2003. View Article : Google Scholar
|
|
36
|
Van den Neste E, Cardoen S, Offner F and
Bontemps F: Old and new insights into the mechanisms of action of
two nucleoside analogs active in lymphoid malignancies: Fludarabine
and cladribine (Review). Int J Oncol. 27:1113–1124. 2005.PubMed/NCBI
|
|
37
|
Wierda WG, Kipps TJ, Mayer J, et al:
Ofatumumab as single-agent CD20 immunotherapy in
fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol.
28:1749–1755. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sieklucka M, Pozarowski P, Bojarska-Junak
A, et al: Apoptosis in B-CLL: The relationship between higher ex
vivo spontaneous apoptosis before treatment in III–IV Rai stage
patients and poor outcome. Oncol Rep. 19:1611–1620. 2008.PubMed/NCBI
|
|
39
|
Matutes E, Bosanquet AG, Wade R, et al:
The use of individualized tumor response testing in treatment
selection: second randomization results from the LRF CLL4 trial and
the predictive value of the test at trial entry. Leukemia.
27:507–510. 2013. View Article : Google Scholar :
|
|
40
|
Nagourney RA, Evans SS, Messenger JC, Su
YZ and Weisenthal LM: 2 Chlorodeoxyadenosine activity and cross
resistance patterns in primary cultures of human hematologic
neoplasms. Br J Cancer. 67:10–14. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shen M, Zhang Y, Saba N, et al:
Identification of therapeutic candidates for chronic lymphocytic
leukemia from a library of approved drugs. PLoS One. 8:e752522013.
View Article : Google Scholar : PubMed/NCBI
|